Abstract
As early and effective antiretroviral therapy has become more widespread, HIV has transitioned from a progressive, fatal disease to a chronic, manageable disease marked by elevated risk of chronic comorbid diseases, including cardiovascular diseases (CVDs). Rates of myocardial infarction, heart failure, stroke, and other CVD manifestations, including pulmonary hypertension and sudden cardiac death, are significantly higher for people living with HIV than for uninfected control subjects, even in the setting of HIV viral suppression with effective antiretroviral therapy. These elevated risks generally persist after demographic and clinical risk factors are accounted for and may be partly attributed to chronic inflammation and immune dysregulation. Data on long-term CVD outcomes in HIV are limited by the relatively recent epidemiological transition of HIV to a chronic disease. Therefore, our understanding of CVD pathogenesis, prevention, and treatment in HIV relies on large observational studies, randomized controlled trials of HIV therapies that are underpowered to detect CVD end points, and small interventional studies examining surrogate CVD end points. The purpose of this document is to provide a thorough review of the existing evidence on HIV-associated CVD, in particular atherosclerotic CVD (including myocardial infarction and stroke) and heart failure, as well as pragmatic recommendations on how to approach CVD prevention and treatment in HIV in the absence of large-scale randomized controlled trial data. This statement is intended for clinicians caring for people with HIV, individuals living with HIV, and clinical and translational researchers interested in HIV-associated CVD.
Keywords: AHA Scientific Statements, cardiovascular diseases, HIV, preventive medicine
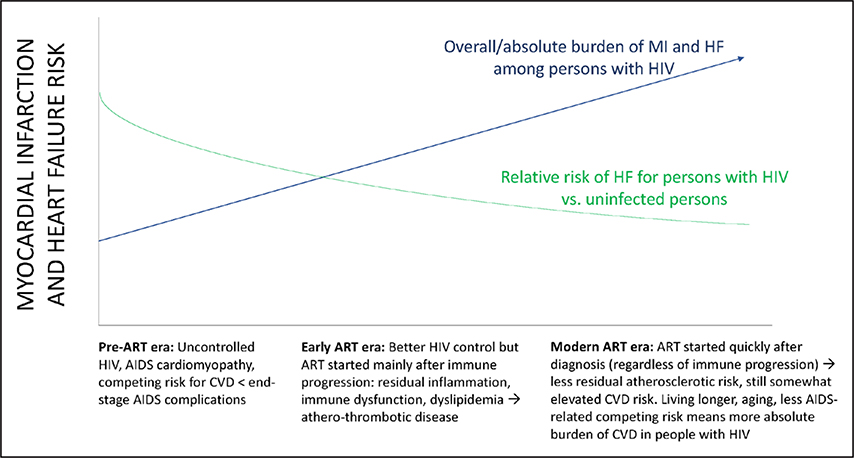
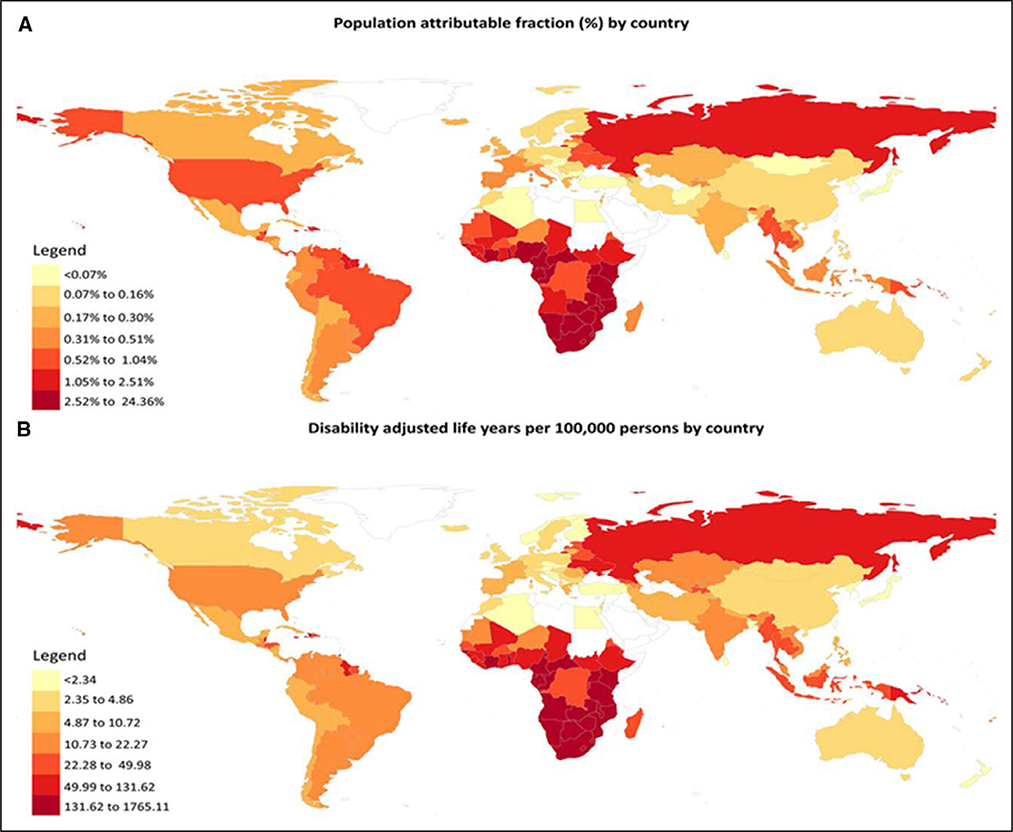
With contemporary antiretroviral therapy (ART), people living with HIV (PLWH) are living longer1 and experiencing a rising burden of cardiovascular diseases (CVDs).2,3 Relative risks of various CVD manifestations are generally 1.5- to 2-fold greater for PLWH compared with uninfected individuals.4 Although the relative risk has decreased with effective ART, there is a large and rising absolute burden of CVD among PLWH (conceptual model in Figure 1).2–4 In a meta-analysis of 793 635 individuals with a total of 3.5 million person-years of follow-up, the global burden of HIV-associated CVD tripled over the past 2 decades and accounted for 2.6 million disability-adjusted life-years per year, with the greatest impact in Sub-Saharan Africa and the Asia-Pacific regions (Figure 2).4 PLWH have an excess risk of myocardial infarction (MI),5,6 ischemic stroke,7,8 heart failure (HF),9,10 pulmonary hypertension,11,12 and venous thrombosis.13,14 Underlying mechanisms likely include an interplay among traditional risk factors, HIV-specific factors (eg, chronic immune activation/inflammation),15,16 ART-related dyslipidemia and other metabolic comorbidities,17,18 behavioral factors (eg, smoking),5,19 and disparities in access to or receipt of care.20–22
Figure 1. Conceptual model of the changing epidemiology of myocardial infarction (MI) and heart failure (HF) risk in HIV.
ART indicates antiretroviral therapy; and CVD, cardiovascular disease.
Figure 2. Global burden of atherosclerotic cardiovascular disease in people living with HIV.
A, Population-attributable fraction by country and (B) disability-adjusted life-years per 100000 people by country. Reprinted from Shah et al.4 Copyright © 2018, American Heart Association, Inc.
HIV-ASSOCIATED ATHEROSCLEROTIC CVD: MI AND STROKE
Over the past decade, a variety of studies from around the world have reported an excess risk of MI among PLWH compared with uninfected people. The risk ranges from a 50% relative risk increase to a doubling of risk.5,23–25 Regardless of study, HIV-related viremia and immune dysfunction are associated with higher MI risks.5,25–27 Several studies have found that lower CD4 count is associated with higher MI risks5,25–27; similarly, a lower CD4/CD8 ratio is associated with more coronary atherosclerosis.28 Moreover, PLWH who achieve sustained HIV viral suppression5 or have few, if any, cardiovascular risk factors23 have higher MI risks than people without HIV infection. This excess MI risk may be greater among women living with HIV/AIDS.24,29 PLWH also have significantly elevated risks for stroke. In HIV-endemic populations in Sub-Saharan Africa, HIV is the leading risk factor for stroke in young cohorts, with a population-attributable fraction of almost 50%.30 Women with HIV may be at particularly elevated risk compared with uninfected women.31 Both immunosuppression and HIV viremia appear to be risk factors: Both lower CD4 count and higher levels of HIV viremia are associated with greater stroke risk.7,30,32–34 Coinfection with HIV and hepatitis C (versus HIV infection alone) may increase stroke risk further.35
HIV-ASSOCIATED HF
Given the excess risk of coronary heart disease, it is not surprising that PLWH also have elevated HF risks, with current estimates ranging from a 1.5- to 2-fold greater risk for HF among PLWH compared with uninfected individuals after adjustment for relevant confounders.9,10,36,37 However, this excess risk is not entirely attributable to MI; after adjustment for prior MI, PLWH still have a >1.5-fold higher hazard for HF than uninfected individuals.10 This risk extends to both HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (EF). Similar to MI risks, unsuppressed HIV viral load and lower CD4 count are associated with higher HF risks for PLWH.10,38 The epidemiology and characteristics of HF in HIV are discussed in greater detail in the HF Pathogenesis and Presentation in HIV section of this document.
OTHER MANIFESTATIONS OF CVD
In contrast to the evidence linking HIV infection to MI, HF, and stroke, there are fewer studies of atrial fibrillation, sudden cardiac death, and peripheral artery disease. However, 1 study reported that PLWH have a 4-fold greater rate of sudden cardiac death compared with expected rates in the general population.39 Low CD4 counts (<200 cells/mm3) are associated with elevated incidence40 and prevalence41 of atrial fibrillation among PLWH, but it is unclear whether PLWH without detectable HIV viremia or immune compromise have elevated atrial fibrillation risks. Similarly, several studies have reported that PLWH have an excess risk of peripheral artery disease compared with uninfected individuals.42,43 HIV-related pulmonary arterial hypertension has been well described since the 1990s and is considered to be in group 1 of the World Health Organization classification of pulmonary hypertension.44–49 The prevalence of HIV-associated pulmonary arterial hypertension is considerably higher than pulmonary arterial hypertension in the general population,50 and ART has not changed this epidemiology.51 Elevated pulmonary artery systolic pressure has also been reported in HIV.11,52 As a result of limited data, treatment goals are similar to those of other pulmonary arterial hypertension subgroups.53 Finally, given the confluence of sleep disorders, particularly obstructive sleep apnea, with CVD,54 it is also worth noting that HIV is associated with sleep impairment in general55–57 and that obstructive sleep apnea may be underdiagnosed among PLWH.58,59
PATHOPHYSIOLOGY AND PRESENTATION OF ATHEROSCLEROTIC CVD AND HF IN HIV
After 2 decades of progress in studying the elevated risks for CVD among PLWH, the underlying mechanisms and biology of this process still remain incompletely defined. Furthermore, delineating risk that is attributable to HIV disease itself versus ART versus traditional risk factors is challenging because many of these factors are interrelated. The pathophysiology of HIV-associated CVD is multifactorial and includes the interplay among traditional risk factors, exposure to ART and virological suppression, and chronic inflammation/immune activation that persists in the setting of treated HIV in the face of an aging HIV population. An older person with a history of HIV for decades likely has a distinct risk profile for CVD compared with a newly diagnosed individual who was started on newer ART immediately. PLWH have high rates of traditional risk factors, including dyslipidemia, metabolic disease, smoking, hypertension, and substance use, as described in the sections below. Aside from traditional risk factors, HIV-specific issues are implicated in CVD and include ART, chronic inflammation, and immune activation in the setting of treated and suppressed HIV disease. Imaging techniques have provided valuable insight into CVD onset and progression in HIV.
Atherosclerosis Pathophysiology in HIV: Chronic Inflammation and Immune Activation
Numerous studies have demonstrated that chronic inflammation and immune activation are abnormal in the setting of treated HIV infection60–64 and, in turn, are strongly predictive of mortality, non-AIDS events,65 and CVD.66–69 Higher levels of several inflammatory markers, including IL (interleukin)-6 and soluble tumor necrosis factor receptors α−1 and α−2, have been associated with coronary atherosclerosis in HIV.70,71 Arterial inflammation as assessed by fluorodeoxyglucose positron emission tomography/computed tomography (CT) is higher in the setting of HIV72–74 and relates to circulating inflammatory markers. Likewise, elevated levels of monocyte activation markers, including soluble CD163 and soluble CD14, are associated with coronary atherosclerosis and carotid plaque progression.75–77 However, it is worth noting that a recent systematic review did not identify a clear association between inflammatory markers and surrogate CVD outcomes in HIV.78 Whether this is the result of insufficient follow-up data in the studies reviewed (the vast majority of which were cross-sectional) or a lack of a strong association is not clear.
Although treatment with ART reduces levels of circulating inflammation markers, many markers of inflammation remain elevated with viral suppression in PLWH relative to uninfected individuals.79 Furthermore, several studies have included PLWH who are able to maintain an undetectable HIV RNA level despite not being on ART (elite controllers) and demonstrated heightened levels of subclinical vascular disease79,80 and clinical events in this population relative to uninfected control subjects.81 Inflammation is also elevated with hepatitis C coinfection and, if left untreated, may contribute to the development of atherosclerosis.82 Lymph node activity is even more pronounced in individuals with treated HIV compared with uninfected people and is closely linked to HIV disease characteristics, suggesting that distinct patterns of immune activation exist73 and that interventions to reduce HIV reservoirs may not predictably affect arterial inflammation. Despite the fact that the SMART study (Strategies for Management of Antiretroviral Therapy) demonstrated the key role of chronic inflammation in HIV-associated CVD >10 years ago,83 effective interventions designed to lower inflammation in treated HIV have been elusive.
Large studies with hard clinical end points of common cardioprotective therapies such as statins and aspirin have not been completed in HIV. Small therapeutic studies of statins and aspirin targeting inflammation/coagulation (eg, hsCRP [high-sensitivity C-reactive protein], IL-6, and D-dimer) among PLWH or HIV-specific therapies have not reported consistent results.84–86 Alterations in gut permeability and subsequent microbial translocation result in downstream chronic inflammation and immune activation. Therapeutic interventions that have targeted the gut, including rifaximin,87 sevelamer,88 and mesalamine,89 have not consistently reduced circulating inflammatory markers or markers of immune activation. In the general population, a monoclonal antibody to IL-1β had no impact on low-density lipoprotein (LDL) cholesterol (LDL-C) but significantly reduced inflammatory markers cardiovascular events90 and, in an analysis of nonprimary end points, also reduced lung cancer mortality.91 Individuals whose hsCRP was reduced by <2 mg/L had a 25% reduction in major CVD events, a 31% reduction in CVD mortality, and a 31% reduction in all-cause mortality.92 In a small study of treated PLWH, IL-1β inhibition significantly reduced IL-6, hsCRP, and arterial and bone marrow inflammation.93 In contrast, among ART-treated PLWH, low-dose methotrexate did not affect inflammatory markers but reduced levels of CD8+ T cells and T-cell activation.94
Atherosclerosis Pathophysiology in HIV: Metabolic Contributors
HIV infection is associated with metabolic complications, including dyslipidemia, insulin resistance, and body composition changes, which can contribute to CVD. Initially, dyslipidemia in HIV was characterized by increased triglyceride levels, thought to be related to immunodeficiency in the pre-ART era.95 Later, specific ART medications, including several protease inhibitors (PIs) and efavirenz, a non–nucleoside reverse transcriptase inhibitor (NRTI), were associated with dyslipidemia (particularly elevated triglyceride levels).96–99 However, current first-line ART regimens have minimal lipid effects.100 Over time, additional research has suggested that inflammation and other factors may contribute to an atherogenic dyslipidemia, with low high-density lipoprotein cholesterol and increased oxidized LDL-C in association with increased innate immune activation.101 In contrast, overall levels of LDL-C are often not elevated in PLWH; levels can be low with initial infection and related inflammation and then return to normal levels with improved health on ART.102 Dyslipidemia in HIV may contribute to elevated atherosclerotic CVD (ASCVD) risk and has been shown to contribute independently to ASCVD among PLWH.103 The specific contributions of dyslipidemia to ASCVD events in HIV are an important area for future investigation.
Insulin resistance and diabetes mellitus are also seen with increasing frequency in HIV.104 Prevalence estimates range up to 26% and 47% in Sub-Saharan Africa for diabetes mellitus and prediabetes mellitus, respectively.99 Mechanisms may relate to effects of specific ART on glucose translocation,105 inflammation, and lipodystrophy. Diabetes mellitus has been linked to ASCVD in HIV such that PLWH with diabetes mellitus have a 2.4-fold increased risk of coronary heart disease events.106
Body composition changes are common in HIV. Patients presenting in the initial era of ART often demonstrated relative loss of subcutaneous fat and gain in abdominal visceral fat.107 The changes in fat distribution were often heterogeneous and frequently were associated with insulin resistance and deposition of ectopic adipose in the liver and muscle. Multiple factors contributed to these changes, including effects of ART. Use of specific thymidine NRTIs is associated with subcutaneous fat loss and deposition of ectopic adipose tissue in the liver and muscle, as well as arterial inflammation.108 Early PI therapy was associated with increased abdominal fat gain.
In the modern ART era, this phenotype has changed, and recent work has focused on dysfunctional subcutaneous fat, related in part to the effect of HIV on peroxisome proliferator-activated receptor-γ and Dicer, as well as other mechanisms.109,110 With increasingly effective ART, gains in both subcutaneous and visceral fat are often seen with the initiation of ART, regardless of regimen,111 and rates of generalized obesity are increasing among PLWH.112 Changes in body composition, including excess visceral adipose tissue, have been linked to overall mortality.113 These changes have been associated with increased coronary plaque, including both noncalcified and calcified plaque.114,115
Atherosclerosis Pathophysiology in HIV: ARTs
ART is a critical component of ASCVD prevention because treatment interruption and uncontrolled HIV viremia are associated with elevated risk of MI.5,83 There were too few ASCVD events in the START trial (Strategic Timing of AntiRetroviral Treatment) to definitively answer whether immediate ART for all PLWH reduces ASCVD risk,116 and changes in ASCVD risk factors levels were not clearly positive or negative.117 Thus, the impact of early ART on ASCVD is uncertain.
Certain antiretroviral drugs and drug classes have been associated with elevated risk of ASCVD events, most notably among people with higher levels of traditional risk factors. PIs were first associated with MI in the landmark D:A:D study (Data Collection on Adverse Events of Anti-HIV Drugs),118 in which each year of cumulative PI use was associated with a 10% greater risk of MI, even after adjustment for cholesterol changes caused by PIs. Analyses from the D:A:D cohort of atazanavir and darunavir, 2 current-generation PIs in widespread clinical use, suggest that ritonavir-boosted or unboosted atazanavir is not associated with increased risk, whereas cumulative exposure to ritonavir-boosted darunavir is associated with progressively increasing risk for CVD.119,120 Rates of carotid intima-media thickness (IMT) progression were also noted to be slower with boosted atazanavir compared with darunavir and ritonavir in a randomized trial, with some of the proposed benefit thought to be the result of the bilirubin-increasing effect of atazanavir.121 In contrast, the association of higher bilirubin with lower mortality in a Veterans Affairs study was not mediated by atazanavir use,122 and another mechanistic clinical trial showed mixed effects of atazanavir on surrogate CVD risk markers (reduced oxidative stress but increased von Willebrand factor and no effect on endothelial function).123 It appears that the association of PIs with ASCVD events is a class effect, with atazanavir being the exception.
NRTIs have also evolved over time, with newer generations of these drugs having fewer metabolic side effects and presumed less mitochondrial toxicity124; however, abacavir is a widely used NRTI that has been associated with increased risk of MI in observational studies. The association of current or recent abacavir use with MI was first described in the D:A:D cohort in 2008,125 with subsequent D:A:D analyses demonstrating that the hazard ratio did not change substantially although abacavir use among individuals at high CVD risk decreased over time.126 Similar associations of abacavir with CVD risk have been shown in the Kaiser Permanente California health system127 and NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design).128 Possible mechanisms of risk include endothelial dysfunction,129 vascular inflammation, and platelet hyperreactivity.130 Despite guideline recommendations to avoid abacavir or to use it with caution in patients at high CVD risk,131 the issue remains controversial because of meta-analyses (US Food and Drug Administration and industry funded) that demonstrated no significant effect of abacavir on MI among generally low-risk individuals in shorter-duration clinical trials.132,133
The population-level impact of ART toxicities on ASCVD risk among PLWH may be relatively low134 and may be further attenuated by the use of antiplatelet agents and statins among high-risk individuals.135 The decision of what ART to start and whether to switch because of comorbidities or side effects is complex and depends on many factors, including cardiovascular risk assessment, HLA-B*57:01 testing (for hypersensitivity to abacavir), HIV resistance testing, medication compliance, pregnancy/child-bearing age, and other comorbidities such as bone and renal disease.
Atherosclerosis Pathophysiology in HIV: Hypertension, Smoking, and Other Factors
Other traditional risk factors, including hypertension and cigarette smoking, play an important role in the pathogenesis of ASCVD in PLWH. A meta-analysis of 63 554 participants from studies published from 2011 to 2016 estimated hypertension prevalence to be 35% for PLWH on ART and 13% for ART-naïve PLWH.136 Although untreated HIV is typically associated with lower blood pressure, resulting perhaps from uncontrolled inflammation and periseptic states of vascular permeability,136–138 studies are inconsistent on whether individuals with treated HIV have a higher prevalence of hypertension compared with uninfected individuals.139–141 Mechanisms of hypertension in PLWH may include chronic inflammation and activation of the renin-angiotensin-aldosterone system.141,142 Overt hypertension, prehypertension, and borderline hypertension (systolic blood pressure, 120–140 mm Hg) were associated with greater risk for acute MI in VACS (Veterans Aging Cohort Study), but there was no evidence that this association was stronger among PLWH compared with uninfected people.140
On a population-level scale, smoking may be the most important modifiable CVD risk factor among PLWH. Smoking is highly prevalent among PLWH (42% were current smokers and 20% were former smokers in a nationally representative US sample143) and is strongly associated with coronary artery plaque and MI.144,145 In 1 study, the population-attributable fraction for MI associated with ever smoking was 72% for PLWH compared with 24% for general population control subjects.144 These data underscore the critical public health importance of including smoking cessation as a cornerstone of any efforts related to CVD prevention in HIV.
Heavy alcohol use, although not generally considered a traditional atherosclerotic risk factor, may contribute disproportionately to CVD among PLWH.19 In addition to (and often in conjunction with) substance use disorders, mood and anxiety disorders are quite common among PLWH146–148 and may contribute to elevated CVD risk (including MI149 and HF36). PLWH also have low levels of physical and cardiorespiratory fitness, which are associated with vascular dysfunction, inflammation, and risk for CVD, as well as all-cause mortality, in patients with HIV (and in the general population).150–153
Atherosclerosis Pathophysiology in HIV: Insights From Imaging
PLWH have more subclinical atherosclerosis relative to those who are uninfected as measured with a variety of imaging modalities. Carotid ultrasound studies have demonstrated that PLWH have more carotid plaque and higher IMT compared with uninfected individuals in cross-sectional and longitudinal studies.66,154,155 The pattern of atherosclerosis progression in the carotid artery has been demonstrated to be particularly marked in the bifurcation region.66 Whereas some studies of carotid IMT have not found an association with HIV,156 the majority of studies demonstrate significantly higher IMT for PLWH than uninfected control subjects.157
Imaging with noncontrast CT allows measurement of coronary artery calcium (CAC), which has been shown to progress more rapidly in PLWH compared with HIV-negative individuals.158 Coronary CT angiography provides visualization of both calcified and noncalcified components of atherosclerotic plaque. HIV is associated with a greater prevalence and extent of noncalcified plaque159–161 and with coronary artery remodeling.162,163 Both of these atherosclerotic features predispose to plaque rupture and may represent a phenotype of elevated risk associated with HIV infection. Treatment with statin therapy reduced noncalcified plaque volume and high-risk plaque features relative to placebo in a small pilot study.84 The effects of statin therapy on coronary atherosclerosis in PLWH are being comprehensively evaluated in a substudy of the ongoing REPRIEVE trial (Randomized Trial to Prevent Vascular Events in HIV; URL: ClinicalTrials.gov. Unique identifier: NCT02344290).
Arterial inflammation can be measured with 18F-fluorodeoxyglucose positron emission tomography imaging of the aorta relative to venous background. PLWH have greater aortic arterial inflammation than uninfected individuals with similar cardiovascular risk factors.72 Arterial inflammation is associated with soluble CD163, a marker of monocyte activation, with visceral fat, and with high-risk coronary atherosclerotic plaque.164 Arterial inflammation is a process that appears to be independent of HIV disease activity as measured by inflammation seen in the lymph nodes.73
Stroke Pathophysiology and Presentation in HIV
The phenotypes of extracranial (eg, carotid and vertebral) and intracranial (eg, middle cerebral and perforator) arteries are distinct and differ in their degree of media and adventitia thickness and presence or absence of dura mater. The additional barrier from the blood-brain interface, which is relevant to intracranial arteries, prevents systemic infection and freely circulating antibodies in the brain. However, HIV infection manipulates this barrier and enters an immune-naïve brain early during primary infection.165 These characteristics influence how extracranial and intracranial arteries respond to vascular risk factors and inflammation. Common pathways of atherosclerosis and CVD events relate more closely to extracranial (eg, proximal carotid arteries and aorta) than intracranial arterial causes of ischemic stroke.166
The body of evidence on the pathogenesis of HIV infection and CVD pertains largely to extracranial disease.143,167,168 However, more than one-third of ischemic stroke in HIV is the result of intracranial disease.169,170 Advanced HIV infection is associated with secondary causes of ischemic stroke such as opportunistic infection (eg, varicella zoster) and coagulopathy.169,170 Once an individual is on ART, HIV-associated vasculopathy, which encompasses several subtypes (eg, atherosclerosis, HIV-associated vasculitis, nonatherosclerotic vasculopathy, and small vessel disease), becomes more prevalent.169–171 Knowledge about intracranial HIV-associated vasculopathy is sparse. However, emerging data suggest that vessel wall remodeling occurs through neuroinflammation and that this may be independent of atherosclerosis.172,173 Furthermore, as the immune system recovers, thinning and erosion of intracranial arteries ensue.174 The latter is in keeping with the first 6 months of ART in immunosuppressed patient being associated with a high risk of stroke.30 Although neuroinflammation appears to be an important factor, it remains unclear whether this occurs in conjunction with or independently of atherosclerosis and warrants further investigation. Nevertheless, as pharmacological strategies evolve for CVD prevention in HIV (ranging from statins with demonstrated stroke prevention benefit175 to newer anti-inflammatory drugs), consideration for those that cross the blood-brain barrier will be critical in addressing the burden of intracranial arterial disease in the future.
HF Pathogenesis and Presentation in HIV
Myocardial dysfunction and HF have been known complications of HIV since the first reports of AIDS cardiomyopathy in the 1980s.176–178 Cases of AIDS cardiomyopathy in the pre-ART era were common and marked by progressive viremia and immune dysfunction, opportunistic infection, and global ventricular dysfunction, with myocarditis commonly seen on pathology.178–180 As HIV has evolved from a fatal disease marked by severe immune compromise and viremia to a chronic, manageable disease marked by inflammation and variable immune dysfunction, the pathophysiology and characteristics of myocardial dysfunction and HF in HIV have likewise evolved. Diastolic dysfunction and HF associated with coronary artery disease have become more common among PLWH.181 A seminal contemporary study from VACS demonstrated that PLWH followed up since 2003 had significantly higher risks than uninfected individuals for HF overall.10 After adjustment for possible confounders, PLWH had significantly higher risks for each HF phenotype analyzed: HF with reduced EF, HFpEF, and borderline HFpEF. Among PLWH, the most common incident HF cases were HF with reduced EF (40%), followed by HFpEF (30%), then borderline HFpEF (15%), and then HF with unknown EF (15%). As expected, worse HIV viremia and related immune dysfunction were associated with the highest risks for HF in this study and another that used physician-adjudicated HF end points.38 Nevertheless, PLWH with viral control (HIV viral RNA <500 copies/mL) and minimal immune compromise (CD4 ≥500 cells/mm3) were still significantly more likely than uninfected individuals to have HF.10 The greater HF risks among PLWH remained after restriction to nonhypertensive people without documented alcohol, tobacco, or cocaine abuse and after adjustment for MI. This suggests that the higher risk for HF in HIV is not solely attributable to substance abuse or MI, although residual confounding from drug use is possible given underreporting by patients. Studies in different cohorts and settings have likewise demonstrated elevated rates of HF in PLWH.29,182 In regions of the world where HIV disease control rates are low, the pattern of HIV-associated HF still resembles the pre-ART epidemiology.183 The prognosis of HF in HIV also may be worse, with a recent small study finding significantly higher rates of HF hospitalization and mortality among women with HIV compared with uninfected women.184
In light of the still-evolving epidemiology, data investigating mechanisms and phenotypes of HIV-associated HF in the modern ART era are limited. However, several cross-sectional studies have indicated that subclinical myocardial disease is particularly prevalent among PLWH. On cardiac magnetic resonance imaging and CT, PLWH have more myocardial fibrosis and steatosis than uninfected control subjects; these subclinical abnormalities are closely associated with myocardial injury and mechanical dysfunction among PLWH.185–187 PLWH with a history of advanced immune suppression are at higher risk of left ventricular hypertrophy and diastolic dysfunction than PLWH with preserved immune function.188 Likewise, PLWH have a substantially higher prevalence of diastolic dysfunction and higher left ventricular mass index than uninfected people on echocardiography, independently of demographics and cardiovascular risk factors.189
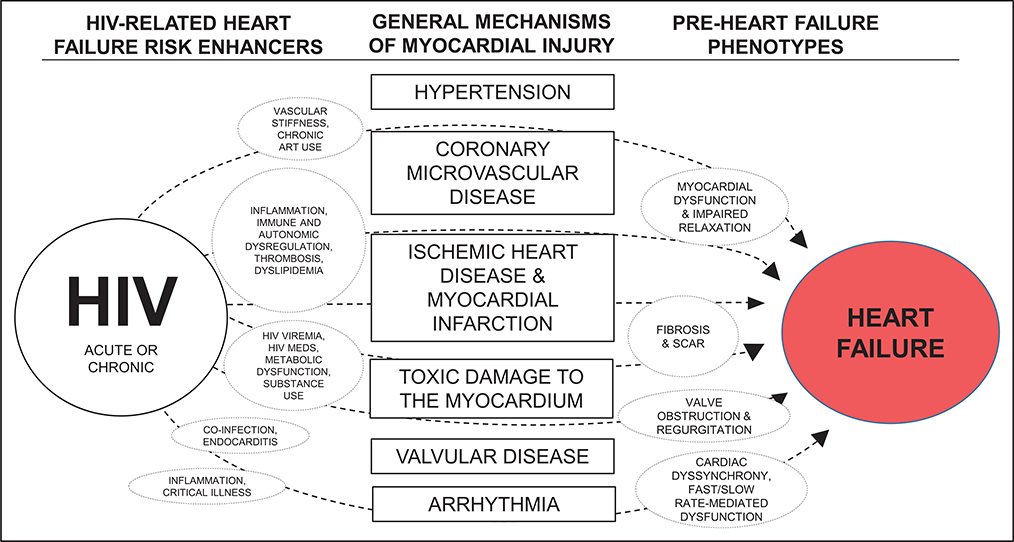
The pathophysiology underlying subclinical myocardial dysfunction and overt HF in HIV is less clearly defined. A substantial portion of PLWH in low- and middle-income countries (and some in high-income countries) are not on ART, have uncontrolled HIV, and remain at risk for severe myocardial inflammation, fibrosis, and systolic dysfunction characteristic of AIDS cardiomyopathy.178,179,190 For PLWH on ART with viral control, several mechanisms may predispose them to myocardial dysfunction and HF (Figure 3). Given the predilection of PLWH for atherosclerosis, thrombosis, and MI, myocardial fibrosis and scar resulting from MI may explain some of the higher HF risks in HIV. This may be particularly true if the myocardium of PLWH is highly vulnerable to ischemia191 and MI, resulting in larger areas of scar and dysfunction after MI for PLWH, as a study of PLWH and uninfected people who underwent coronary angiography and subsequent cardiac magnetic resonance imaging suggested.192 Furthermore, microvascular dysfunction is a contributor to diastolic dysfunction and HFpEF193 and may play a role given the association of HIV-related inflammation and immune dysfunction with microvascular disease.194
Figure 3. Proposed mechanisms of myocardial dysfunction and heart failure in HIV.
ART indicates antiretroviral therapy.
Nonvascular mechanisms are also implicated in HIV-associated myocardial dysfunction and HF. Substance use is a common cause of cardiomyopathy and HF in general and is common in HIV (particularly alcohol, methamphetamine, and cocaine). However, this is unlikely to be the primary driver of HF in HIV, particularly in light of the aforementioned VACS analysis demonstrating greater HF risks in HIV after the analyses were restricted to people without substance use.10 Cardiac arrhythmias contribute to myocardial dysfunction and may be particularly common in HIV. PLWH appear to have a several-fold greater risk of sudden death than uninfected people, although the extent to which malignant arrhythmias drive these sudden deaths is not known.39 Although worse HIV disease severity is associated with atrial arrhythmias among PLWH,40,41 atrial arrhythmias were no more common among PLWH than in uninfected individuals after adjustment for demographics and CVD risk factors in a recent analysis.41
Whether specific antiretrovirals predispose to or protect from HF remains controversial. The mitochondrial toxicity of some older-generation NRTIs (zalcitabine, didanosine, stavudine, and zidovudine) led to concerns related to cardiomyopathy development,124,195 whereas concerns are more mixed for contemporary NRTIs: Abacavir has inconsistently been associated with elevated MI (but not necessarily HF) risk,196,197 whereas tenofovir disoproxil fumarate was associated with a lower HF risk among veterans with HIV.198 Data are lacking on the associations of other specific antiretrovirals with HF. In any case, the cardiovascular and general health benefits of taking ART clearly outweigh the risks according to large, seminal studies of ART strategy favoring early and continuous ART.83,116
CVD RISK ASSESSMENT IN HIV*
CVD risk assessment in HIV is challenging given the relatively recent evolution of HIV as a chronic disease and the resulting dearth of long-term data on CVD incidence in the modern ART era.199 In general, the purpose of predicting disease risk is to inform the risk-benefit calculus of different preventive interventions. Theoretically, the higher the person’s risk is for a particular disease, whether over the next 5 or 10 years or the course of a lifetime, the greater the absolute risk reduction (benefit) from therapy is, and the higher tolerance is for some amount of risk from the intervention. The 2013 and 2018 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on CVD risk assessment200 and lipid-lowering therapy201,202 applied this principle, defining groups of adults in whom the benefit of statin therapy generally outweighs the risks resulting from elevated absolute risks for ASCVD: ≥21 years of age with clinical ASCVD (known coronary artery disease or stroke) and/or significantly elevated LDL-C (≥190 mg/dl) and 40 to 75 years old with diabetes mellitus and/or ≥7.5% 10-year ASCVD risk according to the ACC/AHA ASCVD Risk Calculator.203 The European Society of Cardiology applied a similar central principle in its 2016 guideline on CVD prevention, although with a different risk prediction model.204 Although differences certainly exist among CVD risk prediction equations, they are broadly similar in that they predict a person’s risk of CVD on the basis of risk factor levels known to be associated with CVD.205–208
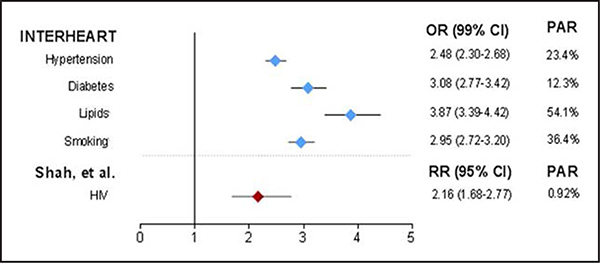
Traditional ASCVD risk factors such as age, diabetes mellitus, current smoking, hypertension, and dyslipidemia are associated with elevated ASCVD risk among PLWH just as they are in the general population.5,24,34 Similar to traditional ASCVD risk factors, HIV infection is associated with elevated ASCVD risk (Figure 5),209 particularly for PLWH with low current or nadir CD4 count (especially <200 cells/mm3)5,25,26,210 or a history of sustained untreated HIV.5,210 No general population risk assessment models have focused on PLWH. The D:A:D study used a large, primarily white European211 cohort to derive a CVD risk prediction model among PLWH.212,213 The D:A:D model incorporates traditional ASCVD risk factors in addition to certain HIV-specific factors associated with CVD (CD4 count, cumulative exposure to PIs and NRTIs, and current abacavir use). Several studies have evaluated CVD risk estimation models derived from the general population or the D:A:D model among PLWH using HIV cohorts in the United States (multicenter214–216 and single center217), Europe,218,219 and Sub-Saharan Africa.220 Although the individual conclusions on model performance in these studies varied slightly depending in large part on the validation cohort used, the CVD risk prediction models performed similarly overall, with apparent underestimation of CVD risk among PLWH.214,216,217 Therefore, a clear best risk estimation model for HIV has not been identified.
Figure 5. Cardiovascular risk of HIV compared with traditional risk factors.
OR indicates odds ratio; PAR, population-attributable risk; and RR, relative risk. Reprinted from Hsue and Waters.209
The presence and extent of subclinical atherosclerosis can be used to refine CVD risk, especially in those considered to be at intermediate risk.19 CAC, measured from noncontrast CT scans, is a potent predictor of coronary heart disease events and has been studied extensively in the general population.20 However, assessment of CAC alone may not reflect underlying coronary artery disease among PLWH because they have more noncalcified plaque than uninfected individuals, and this can be detected only with coronary CT angiography.161 CT angiography is not recommended for screening in asymptomatic individuals. The ability of CAC to discriminate risk for coronary heart disease events in PLWH has not been determined; however, those with CAC (particularly if extensive) can be presumed to be at elevated risk. Carotid IMT is also a predictor of future MI and stroke in the general population221 and has been associated with mortality in HIV.222,223
In the absence of robust data on the adjunctive value of subclinical imaging and biomarker levels for ASCVD risk stratification among PLWH, it is reasonable to consider selected ASCVD risk enhancers identified in the 2018 ACC/AHA cholesterol clinical practice guidelines as likely ASCVD risk enhancers in HIV (Figure 4).202 These include early family history of MI or stroke (men, age <55 years; women, age <65 years), persistently elevated LDL-C ≥160 mg/dL (≥4.1 mmol/L), chronic kidney disease, preeclampsia or premature menopause, subclinical atherosclerosis on imaging (including CAC), and high levels of selected biomarkers associated with elevated ASCVD risk independently of traditional risk factors (Lp(a) [lipoprotein(a)], hsCRP, and apoB [apolipoprotein B]).202 Unlike the 2018 ACC/AHA guidelines, we did not include elevated triglycerides as a significant ASCVD risk enhancer in HIV because studies in large HIV cohorts demonstrated that triglyceride levels, which are often labile and sensitive to ART changes in HIV, either were not predictive of CVD end points independently of other traditional CVD risk factors or were associated with marginally elevated ASCVD risk.224–226 At present, there are insufficient data to recommend routine measurement of subclinical atherosclerosis on imaging or inflammatory biomarkers because the additive value of these measurements for CVD risk stratification in HIV is unclear. Nevertheless, if already measured, atherosclerosis on imaging and elevated levels of Lp(a), hsCRP, or apoB suggest higher ASCVD risk and may warrant more aggressive strategies for ASCVD prevention (Figure 4).
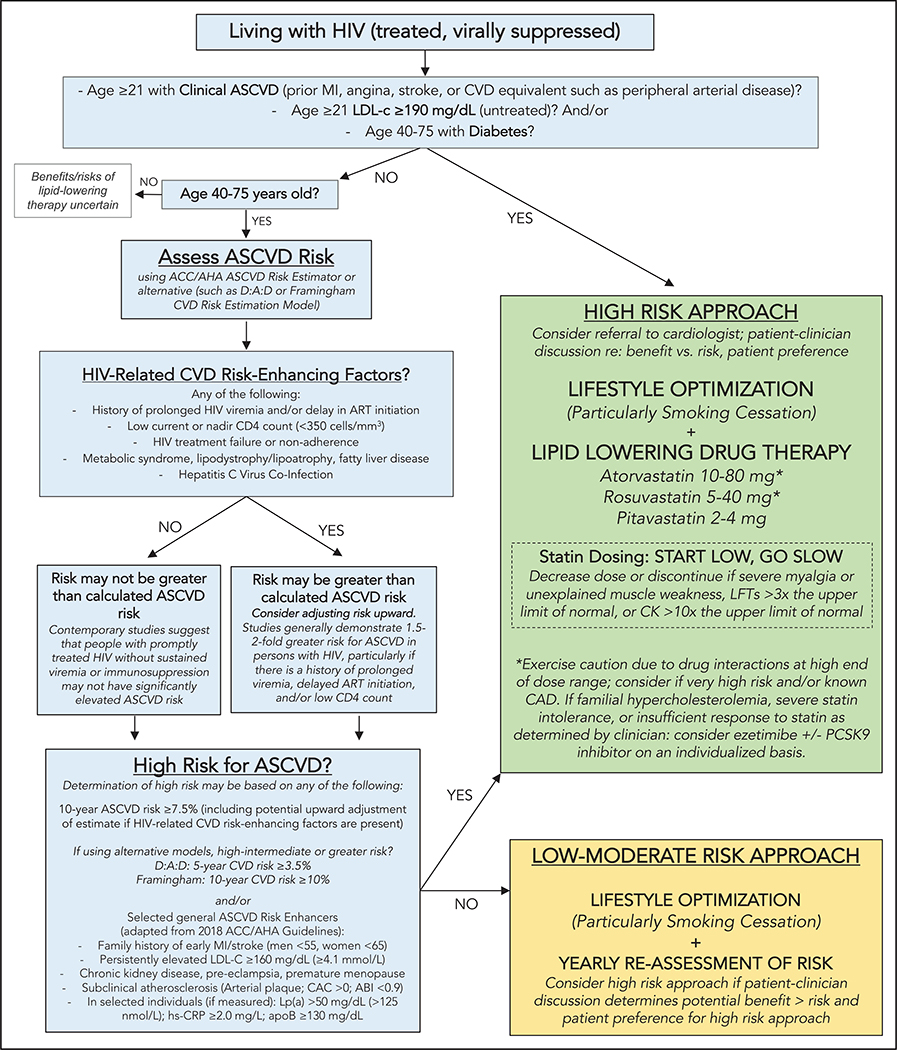
Figure 4. Pragmatic approach to atherosclerotic cardiovascular disease (ASCVD) risk assessment and prevention in treated HIV infection.
This figure applies to people with treated HIV. For people with uncontrolled HIV, the first priority is appropriate HIV therapy to achieve viral suppression per the HIV provider. Thresholds based on findings of elevated CVD risk at current or nadir CD4 count <200, <350, and <500 cells/mm3 in Silverberg et al,25 Lichtenstein et al,26 and Triant et al.27 Hazard ratios and incidence rate ratios of 1.4 to 2.1 for myocardial infarction (MI) for people living with HIV (PLWH) vs uninfected people demonstrated in Freiberg et al,5 Triant et al,24 and Silverberg et al.25 Hazard ratio of stroke for PLWH vs uninfected people was 1.40 in Chow et al.8 ABI indicates ankle-brachial index; ACC/AHA, American College of Cardiology/American Heart Association; apoB, apolipoprotein B; ART, antiretroviral therapy; CAC, coronary artery calcium; CAD, coronary artery disease; CK, creatine kinase; CVD, cardiovascular disease; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; hsCRP, high sensitivity C-reactive protein; LFT, liver function test; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein A; and PCSK9, proprotein convertase subtilisin-kexin type 9.
PREVENTION AND TREATMENT OF HIV-ASSOCIATED ASCVD AND HF
Lifestyle Optimization
As in the general population,200–202 adherence to a healthy lifestyle is an essential first step for primary and secondary prevention of CVD among PLWH. Smoking cessation is of paramount importance given the high prevalence of smoking among PLWH143 and the clear role of smoking in atherosclerosis and MI.144,145 (An extensive library of resources for patients and providers to approach smoking cessation can be found online.227,228) Limiting alcohol consumption is likewise important given the potential disproportionate contribution of alcohol to CVD in HIV.19 Although it is clear that heavy alcohol consumption has adverse effects on CVD and other disease end points,229 there is debate about whether a “healthy” level of alcohol consumption exists; some large analyses suggested a cardioprotective effect of light to moderate alcohol consumption (<100 g/wk [<7 drinks/wk]),230,231 whereas others found no benefit and perhaps elevated HF and stroke risks for light to moderate alcohol consumption.232,233 Regular physical activity is also an essential aspect of lifestyle optimization in HIV given the associations of physical inactivity with poor health and adherence in HIV and, conversely, the improvement in inflammation and cardiometabolic health with increasing physical activity in HIV.226,234,235 (HIV-specific resources for exercise and physical activity can be found online.236,237) A randomized trial of sedentary PLWH at high risk for CVD demonstrated feasibility of a lifestyle-focused behavioral intervention to reduce sweetened beverage consumption and weight, although there was no significant effect of the intervention on physical activity levels.238 Absent HIV-specific data on optimal diets to prevent CVD, adherence to ACC/AHA dietary guidelines is recommended. This dietary approach emphasizes vegetables, fruits, legumes, healthy protein sources (fish/seafood, nuts, low-fat poultry, and low-fat dairy), whole grains, and nontropical vegetable oils while limiting intake of sweets, sugar-sweetened and artificially sweetened beverages (associated with coronary plaque burden in HIV239), and red meats.202
Pharmaco-Prevention of Coronary Artery Disease in HIV
Primary prevention to reduce the risk of ASCVD is an important goal for PLWH. Statins significantly reduce CVD events in patients without HIV with increased inflammation and low levels of LDL-C.240 As discussed, PLWH often present with normal LDL but increased systemic and arterial inflammation72 and persistent immune activation despite successful ART.241 Traditional CVD risk factors, particularly smoking, are also more common and should be targeted in HIV.242
Statin use in HIV is complicated by potential drug interactions, although newer statin and ART therapies appear to have more benign drug-drug interaction profiles.243 Potent cytochrome P450 (CYP) inhibitors such as ritonavir and cobicistat interact with specific statins with significant CYP metabolism.244 Simvastatin and lovastatin are extensively metabolized by the CYP system and can have levels increased >500% when coadministered with CYP inhibitors; accordingly, they should be avoided in HIV.244–246 Pravastatin and pitavastatin are least likely to interact with ART because of minimal CYP metabolism, whereas atorvastatin and rosuvastatin, the 2 highest-intensity statins, with LDL-C lowering of >50% at the highest commonly prescribed doses, have modest interactions with ART.244,245 A comprehensive guide to HIV medications and drug-drug interactions may be found online.247
In terms of clinical adverse events, observational cohorts have shown that most statins (simvastatin and lovastatin excluded) can be safely prescribed for PLWH with lipid-lowering effects similar to those for people without HIV.248,249 A caveat to this may be people >75 years of age, for whom there are conflicting data on net statin benefits in the general population.250–252 As in the general population, vitamin D deficiency also is associated with statin intolerance in HIV.253 In a randomized study among hypercholesterolemic PLWH, pitavastatin lowered LDL more than pravastatin, and neither was associated with increases in glucose, an important consideration for PLWH.254 In this study, pitavastatin also lowered soluble CD14, oxidized LDL, and Lp-PLA2 (lipoprotein-associated phospholipase 2), important markers of innate immune function and arterial inflammation, but did not significantly lower IL-6 or hsCRP.255 A randomized controlled trial of rosuvastatin 10 mg versus placebo among PLWH demonstrated a reduction in some markers of inflammation, monocyte activation markers, and vascular inflammation with rosuvastatin.256,257 There was a significant increase relative to placebo in insulin resistance but no significant difference in fasting glucose, hemoglobin A1c, or the incidence of diabetes mellitus.258 However, other studies have not shown effects on specific inflammatory indexes, including IL-6, hsCRP, and D-dimer.84,85 Efficacy data for the primary prevention of ASCVD are not yet available, and statins may be underused in HIV.215,259,260
To address this knowledge gap, the National Institutes of Health launched REPRIEVE, a randomized, placebo-controlled, 7,500-person global trial to test a primary prevention strategy in HIV.261,262 REPRIEVE includes patients at low to moderate risk and assesses whether treatment with pitavastatin will prevent adjudicated major adverse cardiovascular events. REPRIEVE will also assess the degree to which changes in lipids, immune activation, and inflammation contribute to this effect. Furthermore, little is known about the differential effects of statins in women with HIV, but immune activation is higher among women.159 This knowledge gap is being investigated in REPRIEVE, which has enrolled a high percentage of female PLWH.
In addition to statin therapy, other strategies to potentially reduce CVD risk in HIV include antithrombotic agents, which may be underused in HIV263 but have not yet been assessed in prospective studies powered to evaluate CVD events. Given the prothrombotic milieu common in HIV,61,264–266 inconsistent findings related to aspirin effects on inflammation and endothelial dysfunction in HIV,86,267 and the tradeoff seen between reduced vascular events and increased bleeding with aspirin for primary ASCVD prevention in non-HIV populations,268 further studies are needed to elucidate the role of antithrombotic therapy for ASCVD prevention in HIV. Diabetes mellitus and hypertension should be managed as recommended for the general population because there are insufficient data to recommend a divergent approach in HIV.
A practical expert consensus approach to ASCVD risk assessment and primary prevention in HIV that is based on available (albeit incomplete) evidence is provided in Figure 4.
Acute Coronary Syndromes and Secondary Prevention of Coronary Artery Disease
PLWH who experience an acute coronary syndrome such as ST-segment–elevation and non–ST-segment–elevation MI tend to have lower overall coronary plaque burden,269 more single-vessel disease,270 lower TIMI (Thrombolysis in Myocardial Infarction) risk,270 and a higher likelihood of proximal lesions than uninfected individual.271 A meta-analysis272 of 6 studies273–278 conducted between 2003 and 2015 suggests that after percutaneous coronary intervention, PLWH have similar mortality, cardiac death, recurrent MI, target vessel revascularization, target lesion revascularization, major adverse cardiac events, and stroke (pooled hazard ratios, 1.13–1.47; all P>0.15) compared with uninfected control subjects over 1 to 3 years of follow-up. Despite this and further evidence that drug-eluting stents (compared with bare metal stents) are associated with better outcomes among PLWH,278,279 PLWH were less likely to undergo percutaneous coronary intervention and less likely to receive drug-eluting stents after acute MI compared with uninfected control subjects in a propensity-matched analyses of the US Nationwide Inpatient Sample.279 Furthermore, women with HIV appear to be less likely than men with HIV to receive invasive cardiac procedures.280 In the nonacute setting, however, 1 single-center study showed that PLWH were more likely to receive percutaneous coronary intervention after an abnormal stress test.191 As with other aspects of CVD among PLWH, inflammation and immune activation appear to be important drivers of restenosis risk after stent placement.270,281 For those with more advanced disease or complex anatomy, coronary artery bypass graft surgery appears to be safe and effective for PLWH without advanced immunosuppression, with similar inpatient mortality and only modestly higher rates of postoperative blood transfusions (adjusted odds ratio, 1.19 [95% CI, 1.01–1.40]).282,283 However, rates of longer-term major adverse cardiac events after coronary artery bypass graft surgery may be higher for PLWH compared with uninfected individuals.283
Although aggressive secondary ASCVD prevention measures are indicated for PLWH, uptake has not been consistent. Compared with uninfected individuals, PLWH are less frequently prescribed high-intensity statin after acute coronary syndrome (15% versus 45%), and LDL reduction 6 months after the acute coronary syndrome event is lower.284 Similarly, 57% of PLWH with prior CVD events did not meet guideline-recommended blood pressure targets in a Dutch study.285 With regard to aspirin use, a similar difference in secondary prevention exists, with only 52% of PLWH with coronary disease on aspirin compared with 65% of uninfected people in a large urban health system.263
Nonstatin Strategies to Prevent ASCVD and to Reduce Inflammation in HIV: Investigational Approaches
Both initiation of ART60 and early initiation of ART286 lower inflammation in HIV, but levels remain high compared with levels in uninfected people. Similarly, switching ART regimens (namely a PI-based to an integrase inhibitor–based strategy) or intensifying ART does not appear to significantly reduce inflammatory markers.287–290 These findings suggest that alternative and adjunctive approaches may be needed to reduce excess ASCVD risk in HIV.
In recent years, several studies of lipid-lowering therapies added to a background of statin therapy have demonstrated that aggressive LDL-C lowering in populations at high ASCVD risk reduces cardiovascular events.291,292 The benefit of aggressive LDL-C lowering is demonstrated by data from 14 large statin studies in the non-HIV population (Cholesterol Treatment Trialists’ Collaborators) in which each 38.6-mg/dL reduction in LDL-C translated to a reduction in cardiovascular events by 22%.293 PCSK9 (proprotein convertase subtilsin-kexin type 9) binds and degrades LDL receptors, leading to an increase in LDL-C.294 PCSK9 inhibitors are monoclonal antibodies with minimal significant drug-drug interactions identified thus far that reduce LDL-C by ≈60% even in the setting of high-intensity statin therapy.292 Two PCSK9 inhibitors are approved by the US Food and Drug Administration for individuals with heterozygous familial cholesterolemia or clinical ASCVD on maximally tolerated statins who require additional LDL-C lowering. Among uninfected people with ASCVD, PCSK9 inhibitor therapy in addition to statin therapy reduced clinical events by 15% (P<0.001).292 A longer study demonstrated that PCSK9 inhibitor therapy reduced rates of major adverse cardiovascular events significantly overall and reduced mortality among individuals with an LDL-C ≥100 mg/dL.295 PCSK9 levels are higher in PLWH than in uninfected person, particularly in the setting of hepatitis C virus coinfection, and are increased in parallel with inflammatory markers such as IL-6.296 This relationship may be more pronounced among individuals who are ART naïve.297 A clinical trial investigating the impact of PCSK9 inhibitor therapy on lipids, inflammatory markers, and subclinical ASCVD (including noncalcified plaque and arterial inflammation) in HIV is currently being conducted (EPIC-HIV study [Effect of PCSK9 Inhibition on Cardiovascular Risk in Treated HIV Infection]; URL: ClinicalTrials.gov. Unique identifier: NCT03207945). Future studies are needed to evaluate the impact of PCSK9 inhibition on clinical events in HIV.
As discussed, chronic inflammation and immune activation remain elevated in effectively treated HIV infection60 and are strongly predictive of non-AIDS events, including CVD and mortality.67,68 In particular, IL-6 and D-dimer are strongly associated with mortality in HIV.64 In the non-HIV population with known CVD and hsCRP ≥2 mg/L, treatment with a monoclonal antibody targeting IL-1β (canakinumab) significantly reduced IL-6 and hsCRP and led to a significantly lower rate of recurrent cardiovascular events but also increased the rate of fatal infections in CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study).90 Treatment with canakinumab was also associated with a significant reduction in incident lung cancer and lung cancer mortality, although this was not the primary study end point.91 Individuals who achieved an on-treatment hsCRP <2 mg/L had relative reductions in both cardiovascular mortality and all-cause mortality by 31% compared with placebo92; a similar finding was demonstrated for individuals with on-treatment IL-6 <1.65 ng/L.298 Conversely, in the CIRT trial (Cardiovascular Inflammation Reduction Trial) of uninfected individuals with stable atherosclerosis, low-dose methotrexate did not reduce inflammatory biomarkers or CVD events.299 In this study, methotrexate was associated with elevations in liver transaminases and reductions in leukocyte counts. In light of the positive results from CANTOS (with the notable exception of increased fatal infections) and the null findings from CIRT, further study is needed to define the role of targeted therapies to reduce inflammation and CVD risk in HIV.
Stroke Prevention and Therapy in HIV
Although the risk for ischemic stroke is elevated in HIV,8 data on pharmacotherapy for stroke prevention in HIV are sparse. Whereas systemic atherosclerosis is a likely driver of the elevated stroke risk in HIV, atrial fibrillation (which may be more common overall in HIV but not after adjustment for common CVD risk factors)41 accounts for 30% of ischemic stroke among PLWH.169 Using risk stratification tools, for example, the CHA2DS2-VASc and HAS-BLED scores, to estimate cardioembolic stroke and hemorrhagic complications of antithrombotic therapy, respectively, is an important step to rationalize primary and secondary intervention in general and perhaps in HIV.300 However, the reliability of these scores in PLWH is unclear,300 as are the safety of anticoagulants (particularly direct-acting oral anticoagulants) and their interactions with ART.168,301
There are no trials on the safety and efficacy of thrombolysis in HIV infection, but retrospective studies have shown that the risk of death with thrombolysis was similar for PLWH and uninfected individuals.302,303 Endovascular treatment is now routine in acute stroke treatment304; 1 case series, with limited safety data, showed successful endovascular treatment in patients with HIV-associated vasculopathy.305 HIV-associated vasculitis may manifest as acute stroke, driven by inflammation alone or secondary infection. This may occur soon after the patient starts ART, suggesting an immune reconstitution inflammatory syndrome.170 Optimal workup, including cerebral angiogram and lumbar puncture, if safe, is essential in diagnosing this potentially treatable pathogenesis.171 High-dose corticosteroids may be necessary in patients with immune reconstitution inflammatory syndrome with impending brain herniation and helpful in less severe cases with symptomatic central nervous system inflammation.306
HF Diagnosis and Treatment
The uncertainty about the mechanisms and course of HF in HIV precludes evidence-based recommendations on HIV-specific HF diagnosis and treatment. There are insufficient data to suggest approaches to HF workup and therapy differing from those used in the general population. However, given the elevated risks for HF in HIV, it would be reasonable for clinicians engaged in the care of PLWH to have a high index of suspicion for HF in the setting of possible HF symptoms, along with a low threshold to pursue noninvasive diagnostic testing (such as echocardiography) in the setting of such symptoms or cumulative exposures to high-risk features (eg, high cumulative viremia, low CD4 count, or substance abuse). Furthermore, given the importance of left ventricular EF as a predictor of sudden cardiac death for PLWH307 (as in the general population), it is reasonable to follow general population indications for implantable cardioverter-defibrillator therapy in PLWH. For PLWH with end-stage HF requiring advanced therapies, HIV should not be considered a contraindication to transplantation or left ventricular assist device implantation given the longer life expectancy of PLWH, which is approaching that of uninfected people.308
DISPARITIES IN CARE AND PLWH AS A VULNERABLE POPULATION
PLWH represent a vulnerable and often stigmatized population that faces structural and economic barriers to optimal healthcare services.309 Understanding and addressing CVD in PLWH necessitates recognizing the systematic barriers that perpetuate disparities in care delivery. (A comprehensive review of HIV-associated CVD in resource-limited settings is beyond the scope of this statement; however, given the clear importance of this topic, we have included a brief discussion here.)
Many factors exacerbate vulnerability for PLWH, including education level, residential location, healthcare literacy, disenfranchisement from the healthcare system, cognitive impairment, injection drug use, internalized and anticipated stigma, gait and mobility impairment, frailty, depression, and social isolation. These factors can be intensified by disparities in care according to individual factors such as age, race, ethnicity, and sex, as well as factors associated with high HIV transmission rates such as homosexual contact between men, heterosexual contact among black women, and injection drug use.310
There are well-documented disparities in care for CVD among PLWH. PLWH have fewer clinic visits that meet guideline-directed medical therapy for aspirin therapy (5.1% versus 13.8%) and use of statins (23.6% versus 35.8%).21 Data from a large cohort of PLWH in Europe demonstrated that women are less likely to receive lipid-lowering therapy, antihypertensive medications, angiotensin-converting enzyme inhibitors, and invasive cardiovascular procedures after MI.311 Data from the Veteran’s Health Administration Corporate Data Warehouse also demonstrate that blood pressure, diabetes mellitus, and lipid management are worse in black compared with white PLWH.312 Black and Hispanic PLWH have among the highest estimated 10-year risk of ASCVD compared with other racial and ethnic groups.214,313 Substance-related disorders also impair CVD care in PLWH; they are associated with less appropriate statin use (23% versus 40%) compared with those without substance-related disorders.314 Such disparities in CVD prevention for PLWH portend greater risk for MI.315 Furthermore, major depressive disorder is particularly common in HIV and associated with elevated risk for HF in HIV.36
Geographic factors also affect CVD prevention and management. MI, stroke, and stroke mortality rates are up to 4 times higher in the South compared with other regions in the United States.316–318 HIV prevalence is highest in the South, with significant racial disparities in incidence and prevalence.319 Black men and women have lower rates of HIV viral suppression, which predisposes to more inflammation and CVD. On a global landscape, 67% of all PLWH reside in Sub-Saharan Africa. A large systematic review and meta-analysis of longitudinal studies of CVD in HIV infection examined CVD rates among PLWH worldwide.4 Between 1990 and 2015, the global population-attributable fraction of CVD caused by HIV tripled from 0.36% to 0.92%. There was marked regional variation, with most cardiovascular disability-adjusted life-years lost in the Sub-Saharan Africa (0.87 million) and Asia-Pacific (0.39 million) regions.
Addressing Disparities: Opportunities for Positive Impact
Given the physiological, socioeconomic, and geographic factors that make PLWH particularly vulnerable to the onset and progression of CVD, there is considerable room for improvement in CVD prevention and treatment. The investment in HIV care and research over the years has resulted in a growing infrastructure and strategies that can be leveraged for optimal CVD prevention and management. The HIV treatment cascade and the global 90–90-90 initiatives aim to maximize diagnosis, treatment, and viral suppression.320 Expanding the HIV treatment cascade for the prevention of non-AIDS comorbidities is a necessary extension of the treatment cascade paradigm.321 Empowered PLWH are also driving the conversation about CVD prevention and management (see the Data Supplement: HIV, Aging, and the Patient’s Perspective) through the use of publicly available resources such as the National AIDS Treatment Advocacy Project.322
Targeted attention and investment are needed. The quality of our care for HIV is further limited by shortcomings in the US healthcare reimbursement system. Healthcare providers are often unable to spend the time required to understand the problems facing the aging HIV population. PLWH who also have CVD often need longer visit times,323 care coordination, and multidisciplinary team engagement. There are many opportunities for implementation research aimed at leveraging the HIV care infrastructure to deliver integrated cardiovascular preventive and therapeutic care for PLWH.324 Such structures could include improving health insurance access to specialists, strengthening specialist referral pathways, nurse management, clinical pharmacist engagement,325 team-based approaches,326 electronic medical record–based approaches to targeting high-risk patients, colocated clinics, and other approaches that consider the specific vulnerabilities in this population.
Models of integration of primary care and HIV services have been demonstrated to be feasible and effective,327 but little information is available on cost or effectiveness of specific approaches in the United States. A research agenda has been suggested for Sub-Saharan Africa that prioritizes developing evidence-based service delivery models, generating data through informatics platforms and research, and advancing research-informed policy, among other cross-cutting health system issues. The impact of interventions to reduce the burden of CVD in PLWH in the United States likewise needs to be evaluated and optimized. This will require continued funding support from the National Institutes of Health and public-private partnerships, including support from industry to study the effects of emerging therapies.
CONCLUSIONS AND FUTURE DIRECTIONS
Although much progress has been made over the past decade in understanding HIV-associated CVD, considerable gaps exist, and much work remains to be done in the future. Even with effective HIV viral suppression, inflammation and immune dysregulation appear to increase risks for MI, stroke, and HF. Several studies have analyzed the pathophysiology of atherosclerosis in HIV, but relatively few have been devoted to understanding thrombosis and HF. Therefore, further studies of the pathophysiology of thrombosis and HF in HIV are sorely needed. Similarly important is the lack of large-scale clinical trials on CVD prevention and treatment in HIV; these trials are necessary for informed decision-making and effective CVD prevention and treatment in the aging HIV population. In the meantime, a reasonable approach may be to consider PLWH at particularly elevated CVD risk and therefore more likely to benefit from CVD-preventive therapy if risk-enhancing factors that are related to HIV (eg, low current or nadir CD4 count or a history of prolonged viremia) or are more general (eg, family history of premature ASCVD, chronic kidney disease, or atherosclerosis on imaging) are present. Future studies should also address gaps in implementation to ensure that PLWH who are at risk for CVD or have existing CVD are identified and provided appropriate CVD care. If these steps are taken, perhaps we can reverse the trend of the growing burden of CVD in HIV.
Supplementary Material
Disclosures
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Matthew J. Feinstein | Northwestern University | AHA (research support)†; NIH (research support)† | None | None | None | None | None | None |
| Priscilla Y. Hsue | San Francisco General Hospital | NIH (grant support)†; Regeneron/Sanofi (study drug and placebo provided for trial)*; Novartis (study drug and placebo provided for trial)* | None | None | None | None | Gilead*; Merck* | None |
| Laura A. Benjamin | University of Liverpool Institute of Infection and Global Health | None | None | None | None | None | None | None |
| Gerald S. Bloomfield | Duke Clinical Research Institute, Duke Global Health Institute and Duke University Medical Center | NIH (research grant R01MD013493)* | None | None | None | None | None | None |
| Judith S. Currier | Center for AIDS Research and Education | NIH grant (steering committee of REPRIEVE trial)*; Theratechnologies (PI of clinical trial at UCLA, ended June 2018)* | None | None | None | None | None | None |
| Matthew S. Freiberg | Vanderbilt University | NIH (grants studying HIV and CVD)† | None | None | None | None | None | None |
| Steven K. Grinspoon | Massachusetts General Hospital | Gilead†; KOWA†; Navidea*; Theratechnologies* (all to institution); NIH (P30 Center Grant)† | None | None | None | None | Theratechnologies* | None |
| Jules Levin | National AIDS Treatment Advocacy Project | None | None | None | None | None | None | None |
| Chris T. Longenecker | Case Western Reserve University School of Medicine | Gilead Sciences (ISR)† | None | None | None | None | None | None |
| Wendy S. Post | Johns Hopkins University, Johns Hopkins Hospital | NIH (to Hopkins)† | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Edgar Overton | University of Alabama at Birmingham | None | None | None | None | None | Merck*; ViiV* | None |
| Virginia A. Triant | Massachusetts General Hospital–Harvard Medical School | NIH (co-PI of R01 on cardiovascular risk prediction in HIV)† | None | None | None | None | None | None |
| Allison R. Webel | Case Western Reserve University | Gilead Sciences (My university received a research grant for our work from Gilead)† | None | Association of Nurses in AIDS Care* | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on February 5, 2019, and the American Heart Association Executive Committee on February 19, 2019. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or e-mail kelle.ramsay@wolterskluwer.com.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIR.0000000000000695.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Note: The sections on CVD risk assessment and prevention in HIV are accompanied by Figure 4, which provides a pragmatic approach to ASCVD risk assessment and prevention in treated HIV infection based on available evidence. The lack of data related to HF risk assessment in HIV and adjudicated HF events in HIV precludes informed discussions of HF risk assessment here.
REFERENCES
- 1.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 2.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, Freiberg MS, Lloyd-Jones DM. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117:214–220. doi: 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16 [DOI] [PubMed] [Google Scholar]
- 4.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, Rajagopalan S, Kottilil S, Nair H, Newby DE, McAllister DA, Mills NL. Global burden of atherosclerotic cardiovascular disease in people living with the HIV: a systematic review and meta-analysis. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, Burkholder GA, Mathews WC, Silverberg MJ, Sterling TR, Heckbert SR, Budoff MJ, Van Rompaey S, Delaney JAC, Wong C, Tong W, Palella FJ, Elion RA, Martin JN, Brooks JT, Jacobson LP, Eron JJ, Justice AC, Freiberg MS, Klein DB, Post WS, Saag MS, Moore RD, Crane HM. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75:568–576. doi: 10.1097/QAI.0000000000001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, Rimland D, Bidwell Goetz M, Butt AA, Rodriguez-Barradas MC, Gibert C, Leaf D, Brown ST, Samet J, Kazis L, Bryant K, Freiberg MS; Veterans Aging Cohort Study. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–1940. doi: 10.1212/WNL.0000000000001560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez-Barradas MC, Lim J, Kazis LE, Gottlieb S, Justice AC, Freiberg MS. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brittain EL, Duncan MS, Chang J Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Goetz M, Akgun K, Crothers K, Zola C, Kim J, Gibert C, Pisani M, Morris A, Hsue P, Tindle HA, Justice A, Freiberg M. Increased echocardiographic pulmonary pressure in HIV-infected and uninfected individuals in the Veterans Aging Cohort Study. Am J Respir Crit Care Med. 2018;197:923–932. doi: 10.1164/rccm.201708-1555OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA. 2008;299:324–331. doi: 10.1001/jama.299.3.324 [DOI] [PubMed] [Google Scholar]
- 13.Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med. 2004;116:420–423. doi: 10.1016/j.amjmed.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 14.Baker JV. Chronic HIV disease and activation of the coagulation system. Thromb Res. 2013;132:495–499. doi: 10.1016/j.thromres.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Parrinello CM, Hunt P, Deeks SG, Hodis HN. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811 [DOI] [PubMed] [Google Scholar]
- 18.Lundgren JD. Combination antiretroviral therapy and the risk of myocardial infarction: the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. N Engl J Med. 2003;349:1993–2003.14627784 [Google Scholar]
- 19.Freiberg MS, McGinnis KA, Kraemer K, Samet JH, Conigliaro J, Curtis Ellison R, Bryant K, Kuller LH, Justice AC; VACS Project Team. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53:247–253. doi: 10.1097/QAI.0b013e3181c6c4b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freiberg MS, Leaf DA, Goulet JL, Goetz MB, Oursler KK, Gibert CL, Rodriguez-Barradas MC, Butt AA, Justice AC. The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med. 2009;24:334–340. doi: 10.1007/s11606-008-0891-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, Mafi JN. Disparities in the quality of cardiovascular care between HIV-infected versus HIV-uninfected adults in the United States: a cross-sectional study. J Am Heart Assoc. 2017;6:e007107. doi: 10.1161/JAHA.117.007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkholder GA, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO, Saag MS, Willig JH. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55:1550–1557. doi: 10.1093/cid/cis752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, Rimland D, Bedimo R, Goetz MB, Rodriguez-Barradas MC, Crane HM, Gibert CL, Brown ST, Tindle HA, Warner AL, Alcorn C, Skanderson M, Justice AC, Freiberg MS. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–216. doi: 10.1097/QAI.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP Jr, Klein DB. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–166. doi: 10.1097/QAI.0000000000000009 [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, Wood K, Holmberg SD, Brooks JT; HIV Outpatient Study (HOPS) Investigators. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144 [DOI] [PubMed] [Google Scholar]
- 27.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, Budoff M, Butt AA, Gibert C, Goetz MB, Gottdiener J, Gottlieb S, Justice AC, Leaf D, McGinnis K, Rimland D, Rodriguez-Barradas MC, Sico J, Skanderson M, Tindle H, Tracy RP, Warner A, Freiberg MS. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. doi: 10.1161/JAHA.114.001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, Hopkins M, Emsley HC, Bryer A, Faragher B, Heyderman RS, Allain TJ, Solomon T. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86:324–333. doi: 10.1212/WNL.0000000000002278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow FC, Regan S, Zanni MV, Looby SE, Bushnell CD, Meigs JB, Grinspoon SK, Feske SK, Triant VA. Elevated ischemic stroke risk among women living with HIV infection. AIDS. 2018;32:59–67. doi: 10.1097/QAD.0000000000001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole JW, Pinto AN, Hebel JR, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Price TR, Sloan MA, Stern BJ, Wityk RJ, Wozniak MA, Kittner SJ. Acquired immunodeficiency syndrome and the risk of stroke. Stroke. 2004;35:51–56. doi: 10.1161/01.STR.0000105393.57853.11 [DOI] [PubMed] [Google Scholar]
- 33.Marcus JL, Leyden WA, Chao CR, Chow FC, Horberg MA, Hurley LB, Klein DB, Quesenberry CP Jr, Towner WJ, Silverberg MJ. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–1919. doi: 10.1097/QAD.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 34.Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS. 2014;28:2573–2577. doi: 10.1097/QAD.0000000000000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvaro-Meca A, Berenguer J, Díaz A, Micheloud D, Aldámiz-Echevarría T, Fanciulli C, Resino S. Stroke in HIV-infected individuals with and without HCV coinfection in Spain in the combination antiretroviral therapy era. PLoS One. 2017;12:e0179493. doi: 10.1371/journal.pone.0179493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, Gibert CL, Rimland D, Rodriguez-Barradas MC, Leaf DA, Bedimo RJ, Gottdiener JS, Kop WJ, Gottlieb SS, Budoff MJ, Khambaty T, Tindle HA, Justice AC, Freiberg MS. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132:1630–1638. doi: 10.1161/CIRCULATIONAHA.114.014443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, Sanders JM, Sinha A, Nance RM, Achenbach CJ, Christopher Delaney JA, Heckbert SR, Shah SJ, Hanna DB, Hsue PY, Bloomfield GS, Longenecker CT, Crane HM, Lloyd-Jones DM. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. 2018;7:e009985. doi: 10.1161/JAHA.118.009985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steverson AB, Pawlowski AE, Schneider D, Nannapaneni P, Sanders JM, Achenbach CJ, Shah SJ, Lloyd-Jones DM, Feinstein MJ. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol. 2017;24:1746–1758. doi: 10.1177/2047487317732432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, Shlipak MG. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61:2288–2295. doi: 10.1016/j.jacc.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 41.Sanders JM, Steverson AB, Pawlowski AE, Schneider D, Achenbach CJ, Lloyd-Jones DM, Feinstein MJ. Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus-infected persons and matched uninfected controls. PLoS One. 2018;13:e0194754. doi: 10.1371/journal.pone.0194754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckman JA, Duncan MS, Alcorn CW, So-Armah K, Butt AA, Goetz MB, Tindle HA, Sico JJ, Tracy RP, Justice AC, Freiberg MS. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation. 2018;138:255–265. doi: 10.1161/CIRCULATIONAHA.117.032647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Periard D, Cavassini M, Taffé P, Chevalley M, Senn L, Chapuis-Taillard C, de Vallière S, Hayoz D, Tarr PE; Swiss HIV Cohort Study. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46:761–767. doi: 10.1086/527564 [DOI] [PubMed] [Google Scholar]
- 44.Barnett CF, Hsue PY. Human immunodeficiency virus-associated pulmonary arterial hypertension. Clin Chest Med. 2013;34:283–292. doi: 10.1016/j.ccm.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coplan NL, Shimony RY, Ioachim HL, Wilentz JR, Posner DH, Lipschitz A, Ruden RA, Bruno MS, Sherrid MV, Gaetz H. Primary pulmonary hypertension associated with human immunodeficiency viral infection. Am J Med. 1990;89:96–99. [DOI] [PubMed] [Google Scholar]
- 46.Himelman RB, Dohrmann M, Goodman P, Schiller NB, Starksen NF, Warnock M, Cheitlin MD. Severe pulmonary hypertension and cor pulmonale in the acquired immunodeficiency syndrome. Am J Cardiol. 1989;64:1396–1399. [DOI] [PubMed] [Google Scholar]
- 47.Pellicelli AM, Barbaro G, Palmieri F, Girardi E, D’Ambrosio C, Rianda A, Barbarini G, Frigiotti D, Borgia MC, Petrosillo N. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology. 2001;52:31–41. doi: 10.1177/000331970105200105 [DOI] [PubMed] [Google Scholar]
- 48.Petitpretz P, Brenot F, Azarian R, Parent F, Rain B, Herve P, Simonneau G. Pulmonary hypertension in patients with human immunodeficiency virus infection: comparison with primary pulmonary hypertension. Circulation. 1994;89:2722–2727. [DOI] [PubMed] [Google Scholar]
- 49.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. [DOI] [PubMed] [Google Scholar]
- 50.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC [DOI] [PubMed] [Google Scholar]
- 51.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22(suppl 3):S35–S40. doi: 10.1097/01.aids.0000327514.60879.47 [DOI] [PubMed] [Google Scholar]
- 52.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, Ellman AB, Huang L, Dollard SC, Martin JN. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–833. doi: 10.1097/QAD.0b013e3282f7cd42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(suppl):D73–D81. doi: 10.1016/j.jacc.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 54.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906 [DOI] [PubMed] [Google Scholar]
- 55.Allavena C, Guimard T, Billaud E, de la Tullaye S, Reliquet V, Pineau S, Hüe H, Supiot C, Chennebault JM, Michau C, Hitoto H, Vatan R, Raffi F. Prevalence and risk factors of sleep disturbances in a large HIV-infected adult population. J Int AIDS Soc. 2014;17(suppl 3):19576. doi: 10.7448/IAS.17.4.19576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang X, Li H, Meyers K, Xia W, Meng Z, Li C, Bai J, He S, Cai W, Huang C, Liu S, Wang H, Ling X, Ma P, Tan D, Wang F, Ruan L, Zhao H, Wei H, Liu Y, Yu J, Lu H, Wang M, Zhang T, Chen H, Wu H. Burden of sleep disturbances and associated risk factors: a cross-sectional survey among HIV-infected persons on antiretroviral therapy across China. Sci Rep. 2017;7:3657. doi: 10.1038/s41598-017-03968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Aouizerat BE. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med. 2012;8:67–75. doi: 10.5664/jcsm.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goswami U, Baker JV, Wang Q, Khalil W, Kunisaki KM. Sleep apnea symptoms as a predictor of fatigue in an urban HIV clinic. AIDS Patient Care STDS. 2015;29:591–596. doi: 10.1089/apc.2015.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunisaki KM, Akgün KM, Fiellin DA, Gibert CL, Kim JW, Rimland D, Rodriguez-Barradas MC, Yaggi HK, Crothers K. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med. 2015;16:105–113. doi: 10.1111/hiv.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD; INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786 [DOI] [PubMed] [Google Scholar]
- 63.Hunt PW, Landay AL, Sinclair E, Martinson JA, Hatano H, Emu B, Norris PJ, Busch MP, Martin JN, Brooks C, McCune JM, Deeks SG. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One. 2011;6:e15924. doi: 10.1371/journal.pone.0015924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen CJ, Phillips A, Lundgren JD, Neaton JD; INSIGHT SMART/ESPRIT/SILCAAT Study Group. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One. 2016;11:e0155100. doi: 10.1371/journal.pone.0155100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem Av, de Wolf F, Hallett TB; ATHENA Observational Cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–818. doi: 10.1016/S1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, Maka K, Martin JN, Ganz P, Deeks SG. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1:jah3–e000422. doi: 10.1161/JAHA.111.000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Tracy RP, Rhame F, Henry K, Neaton JD. Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One. 2012;7:e46894. doi: 10.1371/journal.pone.0046894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordell AD, McKenna M, Borges ÁH, Duprez D, Neuhaus J, Neaton JD; INSIGHT SMART, ESPRIT Study Groups; SILCAAT Scientific Committee. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:e000844. doi: 10.1161/JAHA.114.000844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsue PY, Tawakol A. Inflammation and fibrosis in HIV: getting to the heart of the matter. Circ Cardiovasc Imaging. 2016;9:e004427. doi: 10.1161/CIRCIMAGING.116.004427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, Palella F, Witt MD, McConnell MV, Kingsley L, Post WS. Inflammatory markers associated with subclinical coronary artery disease: the Multicenter AIDS Cohort Study. J Am Heart Assoc. 2016;5:e003371. doi: 10.1161/JAHA.116.003371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30:2065–2074. doi: 10.1097/QAD.0000000000001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, Pampaloni M, Rupert A, Hsu D, Sereti I, Fromentin R, Chomont N, Ganz P, Deeks SG, Hsue PY. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. 2017;2:163–171. doi: 10.1001/jamacardio.2016.4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Longenecker CT, Sullivan CE, Morrison J, Hileman CO, Zidar DA, Gilkeson R, O’Donnell J, McComsey GA. The effects of HIV and smoking on aortic and splenic inflammation. AIDS. 2018;32:89–94. doi: 10.1097/QAD.0000000000001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, Cohen MH, Gange SJ, Haberlen SA, Heath SL, Lazar JM, Liu C, Mack WJ, Ofotokun I, Palella FJ, Tien PC, Witt MD, Landay AL, Kingsley LA, Tracy RP, Kaplan RC. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis. 2017;215:1352–1361. doi: 10.1093/infdis/jix082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr, Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, Tracy RP, Brown TT, Post WS. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein-Grobusch K. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One. 2017;12:e0169986. doi: 10.1371/journal.pone.0169986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN, Deeks SG. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, Abbara S, Grinspoon SK. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crowell TA, Gebo KA, Blankson JN, Korthuis PT, Yehia BR, Rutstein RM, Moore RD, Sharp V, Nijhawan AE, Mathews WC, Hanau LH, Corales RB, Beil R, Somboonwit C, Edelstein H, Allen SL, Berry SA; HIV Research Network. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211:1692–1702. doi: 10.1093/infdis/jiu809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ Jr, Thio CL, Seaberg EC. A cross-sectional study of the association between chronic hepatitis C virus infection and subclinical coronary atherosclerosis among participants in the Multicenter AIDS Cohort Study. J Infect Dis. 2016;213:257–265. doi: 10.1093/infdis/jiv396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strategies for Management of Antiretroviral Therapy (SMART) Study Group,El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fätkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 84.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209:1156–1164. doi: 10.1093/infdis/jiu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Brien MP, Hunt PW, Kitch DW, Klingman K, Stein JH, Funderburg NT, Berger JS, Tebas P, Clagett B, Moisi D, Utay NS, Aweeka F, Aberg JA. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis. 2017;4:ofw278. doi: 10.1093/ofid/ofw278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, Taiwo B, Margolis DM, Jacobson JM, Landay AL, Wilson CC; A5286 Team. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy: ACTG A5286. J Infect Dis. 2015;211:780–790. doi: 10.1093/infdis/jiu515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL, Douek DC, Tressler R, Read SW, Wilson CC, Deeks SG, Lederman MM, Gandhi RT; AIDS Clinical Trials Group A5296 Team. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210:1549–1554. doi: 10.1093/infdis/jiu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Somsouk M, Dunham RM, Cohen M, Albright R, Abdel-Mohsen M, Liegler T, Lifson J, Piatak M, Gorelick R, Huang Y, Wu Y, Hsue PY, Martin JN, Deeks SG, McCune JM, Hunt PW. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PLoS One. 2014;9:e116306. doi: 10.1371/journal.pone.0116306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 91.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- 92.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 93.Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, Ganz P, Ridker PM, Deeks SG, Tawakol A. IL-1β inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. 2018;72:2809–2811. doi: 10.1016/j.jacc.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, Daar ES, Havlir D, Yeh E, Tawakol A, Lederman M, Currier JS, Stein JH. Safety and impact of low-dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS Clinical Trials Group Study A5314 [published online September 14, 2018]. Clin Infect Dis. doi: 10.1093/cid/ciy781. https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciy781/5096825?redirectedFrom=fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. [DOI] [PubMed] [Google Scholar]
- 96.Tashima KT, Bausserman L, Alt EN, Aznar E, Flanigan TP. Lipid changes in patients initiating efavirenz- and indinavir-based antiretroviral regimens. HIV Clin Trials. 2003;4:29–36. doi: 10.1310/hct.2003.4.1.004 [DOI] [PubMed] [Google Scholar]
- 97.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–2056. [DOI] [PubMed] [Google Scholar]
- 98.Murphy RL, Sanne I, Cahn P, Phanuphak P, Percival L, Kelleher T, Giordano M. Dose-ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral-naive subjects: 48-week results. AIDS. 2003;17:2603–2614. doi: 10.1097/01.aids.0000096930.51231.5d [DOI] [PubMed] [Google Scholar]
- 99.Njuguna B, Kiplagat J, Bloomfield GS, Pastakia SD, Vedanthan R, Koethe JR. Prevalence, risk factors, and pathophysiology of dysglycemia among people living with HIV in Sub-Saharan Africa. J Diabetes Res. 2018;2018:6916497. doi: 10.1155/2018/6916497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, Sax PE, Smith DM, Thompson MA, Buchbinder SP, Del Rio C, Eron JJ Jr, Fätkenheuer G, Günthard HF, Molina JM, Jacobsen DM, Volberding PA. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379–396. doi: 10.1001/jama.2018.8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, Hoffmann U, Grinspoon SK, Lo J. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS. 2016;30:583–590. doi: 10.1097/QAD.0000000000000946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978 [DOI] [PubMed] [Google Scholar]
- 103.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD; Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- 104.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179 [DOI] [PubMed] [Google Scholar]
- 105.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200 [DOI] [PubMed] [Google Scholar]
- 106.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, Monforte AD, Kirk O, Fontas E, Dabis F, Law MG, Lundgren JD, Friis-Møller N. Diabetes mellitus, pre-existing coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation. 2009;119:805–811. doi: 10.1161/CIRCULATIONAHA.108.790857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joy T, Keogh HM, Hadigan C, Dolan SE, Fitch K, Liebau J, Johnsen S, Lo J, Grinspoon SK. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008;47:174–184. doi: 10.1097/QAI.0b013e31815b0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Srinivasa S, Fitch KV, Torriani M, Zanni MV, Defilippi C, Christenson R, Maehler P, Looby SE, Lo J, Grinspoon SK. Relationship of visceral and subcutaneous adipose depots to markers of arterial injury and inflammation among individuals with HIV. AIDS. 2019;33:229–236. doi: 10.1097/QAD.0000000000002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agarwal N, Iyer D, Patel SG, Sekhar RV, Phillips TM, Schubert U, Oplt T, Buras ED, Samson SL, Couturier J, Lewis DE, Rodriguez-Barradas MC, Jahoor F, Kino T, Kopp JB, Balasubramanyam A. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci Transl Med. 2013;5:213ra164. doi: 10.1126/scitranslmed.3007148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Torriani M, Srinivasa S, Fitch KV, Thomou T, Wong K, Petrow E, Kahn CR, Cypess AM, Grinspoon SK. Dysfunctional subcutaneous fat with reduced dicer and brown adipose tissue gene expression in HIV-infected patients. J Clin Endocrinol Metab. 2016;101:1225–1234. doi: 10.1210/jc.2015-3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dubé MP, Kelesidis T, Rothenberg J, Stein JH, Brown TT. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62:853–862. doi: 10.1093/cid/ciw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson-Paul AM, Wei SC, Mattson CL, Robertson M, Hernandez-Romieu AC, Bell TK, Skarbinski J. Obesity among HIV-infected adults receiving medical care in the United States: data from the Cross-Sectional Medical Monitoring Project and National Health and Nutrition Examination Survey. Medicine (Baltimore). 2015;94:e1081. doi: 10.1097/MD.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, Shlipak MG, Grunfeld C; Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–1414. doi: 10.1097/QAD.0b013e32834884e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fitch KV, Lo J, Abbara S, Ghoshhajra B, Shturman L, Soni A, Sacks R, Wei J, Grinspoon S. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr. 2010;55:495–499. doi: 10.1097/QAI.0b013e3181edab0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palella FJ Jr, McKibben R, Post WS, Li X, Budoff M, Kingsley L, Witt MD, Jacobson LP, Brown TT. Anatomic fat depots and coronary plaque among human immunodeficiency virus-infected and uninfected men in the Multicenter AIDS Cohort Study. Open Forum Infect Dis. 2016;3:ofw098. doi: 10.1093/ofid/ofw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baker JV, Sharma S, Achhra AC, Bernardino JI, Bogner JR, Duprez D, Emery S, Gazzard B, Gordin J, Grandits G, Phillips AN, Schwarze S, Soliman EZ, Spector SA, Tambussi G, Lundgren J; INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) START (Strategic Timing of Antiretroviral Treatment) Study Group. Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc. 2017;6:e004987. doi: 10.1161/JAHA.116.004987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DAD Study Group, Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte Ad, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 119.Monforte Ad, Reiss P, Ryom L, El-Sadr W, Dabis F, De Wit S, Worm SW, Law MG, Weber R, Kirk O, Pradier C, Phillips AN, Lundgren JD, Sabin CA. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS. 2013;27:407–415. doi: 10.1097/QAD.0b013e32835b2ef1 [DOI] [PubMed] [Google Scholar]
- 120.Ryom L, Lundgren JD, El-Sadr W, Reiss P, Kirk O, Law M, Phillips A, Weber R, Fontas E, d’ Arminio Monforte A, De Wit S, Dabis F, Hatleberg CI, Sabin C, Mocroft A; D:A:D Study Group. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV. 2018;5:e291–e300. doi: 10.1016/S2352-3018(18)30043-2 [DOI] [PubMed] [Google Scholar]
- 121.Brown TT, Moser C, Currier JS, Ribaudo HJ, Rothenberg J, Kelesidis T, Yang O, Dubé MP, Murphy RL, Stein JH, McComsey GA. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis. 2015;212:1241–1249. doi: 10.1093/infdis/jiv194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marconi VC, Duncan MS, So-Armah K, Re VL 3rd, Lim JK, Butt AA, Goetz MB, Rodriguez-Barradas MC, Alcorn CW, Lennox J, Beckman JA, Justice A, Freiberg M. Bilirubin is inversely associated with cardiovascular disease among HIV-positive and HIV-negative individuals in VACS (Veterans Aging Cohort Study). J Am Heart Assoc.2018;7:e007792. doi: 10.1161/JAHA.117.007792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beckman JA, Wood BR, Ard KL, Price CN, Solomon DA, Zuflacht JP, Milian J, Prenner JC, Sax PE. Conflicting effects of atazanavir therapy on atherosclerotic risk factors in stable HIV patients: a randomized trial of regimen switch to atazanavir. PLoS One. 2017;12:e0181993. doi: 10.1371/journal.pone.0181993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gardner K, Hall PA, Chinnery PF, Payne BA. HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies. Toxicol Pathol. 2014;42:811–822. doi: 10.1177/0192623313503519 [DOI] [PubMed] [Google Scholar]
- 125.D:A:D Study Group, Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, D’Arminio Monforte A, Friis-Møller N, Kirk O, Pradier C, Weller I, Phillips AN, Lundgren JD. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multicohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sabin CA, Reiss P, Ryom L, Phillips AN, Weber R, Law M, Fontas E, Mocroft A, de Wit S, Smith C, Dabis F, d’Arminio Monforte A, El-Sadr W, Lundgren JD; D:A:D Study Group. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. doi: 10.1186/s12916-016-0588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marcus JL, Neugebauer RS, Leyden WA, Chao CR, Xu L, Quesenberry CP Jr, Klein DB, Towner WJ, Horberg MA, Silverberg MJ. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr. 2016;71:413–419. doi: 10.1097/QAI.0000000000000881 [DOI] [PubMed] [Google Scholar]
- 128.Elion RA, Althoff KN, Zhang J, Moore RD, Gange SJ, Kitahata MM, Crane HM, Drozd DR, Stein JH, Klein MB, Eron JJ, Silverberg MJ, Mathews WC, Justice AC, Sterling TR, Rabkin CS, Mayor AM, Klein DB, Horberg MA, Bosch RJ, Eyawo O, Palella FJ Jr; North American AIDS Cohort Collaboration on Research and Design of IeDEA. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr. 2018;78:62–72. doi: 10.1097/QAI.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsue PY, Hunt PW, Wu Y, Schnell A, Ho JE, Hatano H, Xie Y, Martin JN, Ganz P, Deeks SG. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009;23:2021–2027. doi: 10.1097/QAD.0b013e32832e7140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alvarez A, Orden S, Andújar I, Collado-Diaz V, Núñez-Delgado S, Galindo MJ, Estrada V, Apostolova N, Esplugues JV. Cardiovascular toxicity of abacavir: a clinical controversy in need of a pharmacological explanation. AIDS. 2017;31:1781–1795. doi: 10.1097/QAD.0000000000001547 [DOI] [PubMed] [Google Scholar]
- 131.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US Department of Health and Human Services. https://aidsinfo.nih.gov/contentfiles/adultandadolescentgl003093.pdf. Accessed July 18, 2018. [Google Scholar]
- 132.Nan C, Shaefer M, Urbaityte R, Oyee J, Hopking J, Ragone L, Perger T, Win B, Vangerow H, McCoig C, Vannappagari V. Abacavir use and risk for myocardial infarction and cardiovascular events: pooled analysis of data from clinical trials. Open Forum Infect Dis. 2018;5:ofy086. doi: 10.1093/ofid/ofy086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ding X, Andraca-Carrera E, Cooper C, Miele P, Kornegay C, Soukup M, Marcus KA. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61:441–447. doi: 10.1097/QAI.0b013e31826f993c [DOI] [PubMed] [Google Scholar]
- 134.Smit M, van Zoest RA, Nichols BE, Vaartjes I, Smit C, van der Valk M, van Sighem A, Wit FW, Hallett TB, Reiss P; Netherlands AIDS Therapy Evaluation in The Netherlands (ATHENA) Observational HIV Cohort. Cardiovascular disease prevention policy in human immunodeficiency virus: recommendations from a modeling study. Clin Infect Dis. 2018;66:743–750. doi: 10.1093/cid/cix858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabin CA, Ryom L, d’Arminio Monforte A, Hatleberg CI, Pradier C, El-Sadr W, Kirk O, Weber R, Phillips AN, Mocroft A, Bonnet F, Law M, de Wit S, Reiss P, Lundgren JD; D:A:D Study Group. Abacavir use and risk of recurrent myocardial infarction. AIDS. 2018;32:79–88. doi: 10.1097/QAD.0000000000001666 [DOI] [PubMed] [Google Scholar]
- 136.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11:530–540. doi: 10.1016/j.jash.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 137.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, Fitzgerald DW, Kataraihya JB. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. doi: 10.1186/s12916-014-0125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feinstein MJ, Bogorodskaya M, Bloomfield GS, Vedanthan R, Siedner MJ, Kwan GF, Longenecker CT. Cardiovascular complications of HIV in endemic countries. Curr Cardiol Rep. 2016;18:113. doi: 10.1007/s11886-016-0794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gelpi M, Afzal S, Lundgren J, Ronit A, Roen A, Mocroft A, Gerstoft J, Lebech AM, Lindegaard B, Kofoed KF, Nordestgaard BG, Nielsen SD. Higher risk of abdominal obesity, elevated low-density lipoprotein cholesterol, and hypertriglyceridemia, but not of hypertension, in people living with human immunodeficiency virus (HIV): results from the Copenhagen Comorbidity in HIV Infection Study. Clin Infect Dis. 2018;67:579–586. doi: 10.1093/cid/ciy146 [DOI] [PubMed] [Google Scholar]
- 140.Armah KA, Chang CC, Baker JV, Ramachandran VS, Budoff MJ, Crane HM, Gibert CL, Goetz MB, Leaf DA, McGinnis KA, Oursler KK, Rimland D, Rodriguez-Barradas MC, Sico JJ, Warner AL, Hsue PY, Kuller LH, Justice AC, Freiberg MS; Veterans Aging Cohort Study (VACS) Project Team. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58:121–129. doi: 10.1093/cid/cit652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: novel pathophysiologic mechanisms. Hypertension. 2018;72:44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, Lo J, Adler GK, Grinspoon SK. RAAS activation is associated with visceral adiposity and insulin resistance among HIV-infected patients. J Clin Endocrinol Metab. 2015;100:2873–2882. doi: 10.1210/jc.2015-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mdodo R, Frazier E, Mattson C, Sutton M, Brooks J, Skarbinski J. Cigarette smoking among HIV+ adults in care: Medical Monitoring Project, US, 2009. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. [Google Scholar]
- 144.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60:1415–1423. doi: 10.1093/cid/civ013 [DOI] [PubMed] [Google Scholar]
- 145.Kelly SG, Plankey M, Post WS, Li X, Stall R, Jacobson LP, Witt MD, Kingsley L, Cox C, Budoff M, Palella FJ Jr. Associations between tobacco, alcohol, and drug use with coronary artery plaque among HIV-infected and uninfected men in the Multicenter AIDS Cohort Study. PLoS One. 2016;11:e0147822. doi: 10.1371/journal.pone.0147822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malee KM, Mellins CA, Huo Y, Tassiopoulos K, Smith R, Sirois PA, Allison SM, Kacanek D, Kapetanovic S, Williams PL, Grant ML, Marullo D, Aidala AA; Pediatric HIVAIDS Cohort Study (PHACS). Prevalence, incidence, and persistence of psychiatric and substance use disorders among mothers living with HIV. J Acquir Immune Defic Syndr. 2014;65:526–534. doi: 10.1097/QAI.0000000000000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chibanda D, Benjamin L, Weiss HA, Abas M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J Acquir Immune Defic Syndr. 2014;67(suppl 1):S54–S67. doi: 10.1097/QAI.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 148.Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the southeastern United States. J Acquir Immune Defic Syndr. 2006;42:298–306. doi: 10.1097/01.qai.0000219773.82055.aa [DOI] [PubMed] [Google Scholar]
- 149.Khambaty T, Stewart JC, Gupta SK, Chang CH, Bedimo RJ, Budoff MJ, Butt AA, Crane H, Gibert CL, Leaf DA, Rimland D, Tindle HA, So-Armah KA, Justice AC, Freiberg MS. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus-infected adults: Veterans Aging Cohort Study. JAMA Cardiol. 2016;1:929–937. doi: 10.1001/jamacardio.2016.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vancampfort D, Mugisha J, Rosenbaum S, Firth J, De Hert M, Probst M, Stubbs B. Cardiorespiratory fitness levels and moderators in people with HIV: a systematic review and meta-analysis. Prev Med. 2016;93:106–114. doi: 10.1016/j.ypmed.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 151.Greene M, Covinsky K, Astemborski J, Piggott DA, Brown T, Leng S, Galai N, Mehta SH, Guralnik J, Patel KV, Kirk GD. The relationship of physical performance with HIV disease and mortality. AIDS. 2014;28:2711–2719. doi: 10.1097/QAD.0000000000000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dirajlal-Fargo S, Webel AR, Longenecker CT, Kinley B, Labbato D, Sattar A, McComsey GA. The effect of physical activity on cardiometabolic health and inflammation in treated HIV infection. Antivir Ther. 2016;21:237–245. doi: 10.3851/IMP2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Webel AR, Perazzo J, Longenecker CT, Jenkins T, Sattar A, Rodriguez M, Schreiner N, Josephson RA. The influence of exercise on cardiovascular health in sedentary adults with human immunodeficiency virus. J Cardiovasc Nurs. 2018;33:239–247. doi: 10.1097/JCN.0000000000000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, Anastos K, Gange SJ, Landay AL, Lazar JM, Palella FJ, Tien PC, Witt MD, Xue X, Young MA, Kaplan RC, Kingsley LA. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61:640–650. doi: 10.1093/cid/civ325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, Ho JE, Selby V, Madden E, Martin JN, Deeks SG, Ganz P, Waters DD. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol. 2012;109:742–747. doi: 10.1016/j.amjcard.2011.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanna DB, Guo M, Bůžková P, Miller TL, Post WS, Stein JH, Currier JS, Kronmal RA, Freiberg MS, Bennett SN, Shikuma CM, Anastos K, Li Y, Tracy RP, Hodis HN, Delaney JA, Kaplan RC. HIV Infection and carotid artery intima-media thickness: pooled analyses across 5 cohorts of the NHLBI HIV-CVD Collaborative. Clin Infect Dis. 2016;63:249–256. doi: 10.1093/cid/ciw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stein JH, Hsue PY. Inflammation and arterial injury in individuals with human immunodeficiency virus infection. JAMA Cardiol. 2016;1:481–482. doi: 10.1001/jamacardio.2016.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kingsley LA, Deal J, Jacobson L, Budoff M, Witt M, Palella F, Calhoun B, Post WS. Incidence and progression of coronary artery calcium in HIV-infected and HIV-uninfected men. AIDS. 2015;29:2427–2434. doi: 10.1097/QAD.0000000000000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parra S, Coll B, Aragonés G, Marsillach J, Beltrán R, Rull A, Joven J, Alonso-Villaverde C, Camps J. Nonconcordance between subclinical atherosclerosis and the calculated Framingham Risk Score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x [DOI] [PubMed] [Google Scholar]
- 161.Post WS, Budoff M, Kingsley L, Palella FJ Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–1272. doi: 10.1097/QAD.0b013e32835eca9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller PE, Haberlen SA, Metkus T, Rezaeian P, Palella F, Kingsley LA, Witt MD, George RT, Jacobson LP, Brown TT, Budoff M, Post WS. HIV and coronary arterial remodeling from the Multicenter AIDS Cohort Study (MACS). Atherosclerosis. 2015;241:716–722. doi: 10.1016/j.atherosclerosis.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S, Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–171. doi: 10.1097/QAI.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams DW, Anastos K, Morgello S, Berman JW. JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J Leukoc Biol. 2015;97:401–412. doi: 10.1189/jlb.5A0714-347R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bae HJ, Yoon BW, Kang DW, Koo JS, Lee SH, Kim KB, Lee J, Roh JK. Correlation of coronary and cerebral atherosclerosis: difference between extracranial and intracranial arteries. Cerebrovasc Dis. 2006;21:112–119. doi: 10.1159/000090209 [DOI] [PubMed] [Google Scholar]
- 167.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11:878–890. doi: 10.1016/S1474-4422(12)70205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Benjamin L, Khoo S. HIV infection and stroke. Handb Clin Neurol. 2018;152:187–200. doi: 10.1016/B978-0-444-63849-6.00015-3 [DOI] [PubMed] [Google Scholar]
- 169.Chow FC, Price RW, Hsue PY, Kim AS. Greater risk of stroke of undetermined etiology in a contemporary HIV-Infected cohort compared with uninfected individuals. J Stroke Cerebrovasc Dis. 2017;26:1154–1160. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benjamin LA, Allain TJ, Mzinganjira H, Connor MD, Smith C, Lucas S, Joekes E, Kampondeni S, Chetcuti K, Turnbull I, Hopkins M, Kamiza S, Corbett EL, Heyderman RS, Solomon T. The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. J Infect Dis. 2017;216:545–553. doi: 10.1093/infdis/jix340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Benjamin LA, Bryer A, Lucas S, Stanley A, Allain TJ, Joekes E, Emsley H, Turnbull I, Downey C, Toh CH, Brown K, Brown D, Ison C, Smith C, Corbett EL, Nath A, Heyderman RS, Connor MD, Solomon T. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm. 2016;3:e254. doi: 10.1212/NXI.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gutierrez J, Menshawy K, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Metalloproteinases and brain arterial remodeling among individuals with and those without HIV infection. J Infect Dis. 2016;214:1329–1335. doi: 10.1093/infdis/jiw385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gutierrez J, Menshawy K, Gonzalez M, Goldman J, Elkind MS, Marshall R, Morgello S. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS. 2016;30:415–423. doi: 10.1097/QAD.0000000000000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hunter MD, Shenoy A, Dwork A, Elkind MSV, Marshall R, Mohr JP, Morgello S, Gutierrez J. Brain vascular intima vulnerability among HIV-positive and negative individuals. AIDS. 2018;32:2209–2216. doi: 10.1097/QAD.0000000000001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. [DOI] [PubMed] [Google Scholar]
- 176.Cohen IS, Anderson DW, Virmani R, Reen BM, Macher AM, Sennesh J, DiLorenzo P, Redfield RR. Congestive cardiomyopathy in association with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:628–630. doi: 10.1056/NEJM198609043151007 [DOI] [PubMed] [Google Scholar]
- 177.Klima M, Escudier SM. Pathologic findings in the hearts of patients with acquired immunodeficiency syndrome. Tex Heart Inst J. 1991;18:116–121. [PMC free article] [PubMed] [Google Scholar]
- 178.Levy WS, Simon GL, Rios JC, Ross AM. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Am J Cardiol. 1989;63:86–89. [DOI] [PubMed] [Google Scholar]
- 179.De Castro S, d’Amati G, Gallo P, Cartoni D, Santopadre P, Vullo V, Cirelli A, Migliau G. Frequency of development of acute global left ventricular dysfunction in human immunodeficiency virus infection. J Am Coll Cardiol. 1994;24:1018–1024. [DOI] [PubMed] [Google Scholar]
- 180.Herskowitz A, Vlahov D, Willoughby S, Chaisson RE, Schulman SP, Neumann DA, Baughman KL. Prevalence and incidence of left ventricular dysfunction in patients with human immunodeficiency virus infection. Am J Cardiol. 1993;71:955–958. [DOI] [PubMed] [Google Scholar]
- 181.Shah MR, Wong RP. The changing paradigm of HIV-related heart failure. Glob Heart. 2015;10:241–244. doi: 10.1016/j.gheart.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 182.Al-Kindi SG, ElAmm C, Ginwalla M, Mehanna E, Zacharias M, Benatti R, Oliveira GH, Longenecker CT. Heart failure in patients with human immunodeficiency virus infection: epidemiology and management disparities. Int J Cardiol. 2016;218:43–46. doi: 10.1016/j.ijcard.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail. 2015;3:579–590. doi: 10.1016/j.jchf.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Janjua SA, Triant VA, Addison D, Szilveszter B, Regan S, Staziaki PV, Grinspoon SA, Hoffmann U, Zanni MV, Neilan TG. HIV infection and heart failure outcomes in women. J Am Coll Cardiol. 2017;69:107–108. doi: 10.1016/j.jacc.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, Looby SE, Hoffmann U, Zanni M, Grinspoon SK. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic HIV-infected individuals. AIDS. 2016;30:2205–2214. doi: 10.1097/QAD.0000000000001186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719 [DOI] [PubMed] [Google Scholar]
- 187.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, Schmidt N, Hur J, Sibley CT, Bluemke DA, Hadigan C. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212:1544–1551. doi: 10.1093/infdis/jiv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Okeke NL, Alenezi F, Bloomfield GS, Dunning A, Clement ME, Shah SH, Naggie S, Velazquez EJ. Determinants of left ventricular hypertrophy and diastolic dysfunction in an HIV clinical cohort. J Card Fail. 2018;24:496–503. doi: 10.1016/j.cardfail.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, Martin JN, Deeks SG, Bolger AF. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hansen BF. Pathology of the heart in AIDS: a study of 60 consecutive autopsies. APMIS. 1992;100:273–279. [DOI] [PubMed] [Google Scholar]
- 191.Feinstein MJ, Poole B, Engel Gonzalez P, Pawlowski AE, Schneider D, Provias TS, Palella FJ, Achenbach CJ, Lloyd-Jones DM. Differences by HIV serostatus in coronary artery disease severity and likelihood of percutaneous coronary intervention following stress testing. J Nucl Cardiol. 2018;25:872–883. doi: 10.1007/s12350-016-0689-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Feinstein MJ, Mitter SS, Yadlapati A, Achenbach CJ, Palella FJ Jr, Gonzalez PE, Meyers S, Collins JD, Shah SJ, Lloyd-Jones DM. HIV-related myocardial vulnerability to infarction and coronary artery disease. J Am Coll Cardiol. 2016;68:2026–2027. doi: 10.1016/j.jacc.2016.07.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. doi: 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sinha A, Ma Y, Scherzer R, Li D, Ganz P, Deeks SG, Hsue PY. Role of T-cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J Am Heart Assoc. 2016;5:e004243. doi: 10.1161/JAHA.116.004243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Balcarek K, Venhoff N, Deveaud C, Beauvoit B, Bonnet J, Kirschner J, Venhoff AC, Lebrecht D, Walker UA. Role of pyrimidine depletion in the mitochondrial cardiotoxicity of nucleoside analogue reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2010;55:550–557. doi: 10.1097/QAI.0b013e3181f25946 [DOI] [PubMed] [Google Scholar]
- 196.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. doi: 10.1097/COH.0000000000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, Monforte AD, Friis-Møller N, Kirk O, Fontas E, Weller I, Phillips A, Lundgren J. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897 [DOI] [PubMed] [Google Scholar]
- 198.Chen R, Scherzer R, Hsue PY, Jotwani V, Estrella MM, Horberg MA, Grunfeld C, Shlipak MG. Association of tenofovir use with risk of incident heart failure in HIV-infected patients. J Am Heart Assoc. 2017;6:e005387. doi: 10.1161/JAHA.116.005387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Feinstein MJ. Cardiovascular disease risk assessment in HIV: navigating data-sparse zones. Heart. 2016;102:1157–1158. doi: 10.1136/heartjnl-2016-309752 [DOI] [PubMed] [Google Scholar]
- 200.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75]. Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 201.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PWF. 2013 ACC/ AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:e396]. Circulation. 2014;129(suppl 2)S1–45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 202.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol [published online ahead of print November 10, 2018]. Circulation. doi: 10.1161/CIR.0000000000000625. https://www.ahajournals.org/doi/abs/10.1161/CIR.0000000000000625. [DOI]
- 203.American Heart Association. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/ASCVDRiskCalculator/UCM_457698_. Accessed February 5, 2019.
- 204.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the Prospective Cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–315. [DOI] [PubMed] [Google Scholar]
- 206.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM; SCORE Project Group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 207.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 208.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 209.Hsue PY, Waters DD. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation. 2018;138:1113–1115. doi: 10.1161/CIRCULATIONAHA.118.036211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D; French Hospital Database on HIV-ANRS CO4. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f [DOI] [PubMed] [Google Scholar]
- 211.Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD; DAD Study Group. Cardiovascular disease risk factors in HIV patients: association with antiretroviral therapy: results from the DAD Study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1 [DOI] [PubMed] [Google Scholar]
- 212.Friis-Møller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, De Wit S, Monforte AD, Kirk O, Fontas E, Sabin C, Phillips A, Lundgren J, Law M; D:A:D Study Group. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-Collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214–223. doi: 10.1177/2047487315579291 [DOI] [PubMed] [Google Scholar]
- 213.Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, Phillips A, Sabin CA, Lundgren JD, Law MG; DAD Study Group. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150 [DOI] [PubMed] [Google Scholar]
- 214.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, Budoff MJ, Mathews WC, Kitahata MM, Saag MS, Eron JJ, Moore RD, Achenbach CJ, Lloyd-Jones DM, Crane HM. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2:155–162. doi: 10.1001/jamacardio.2016.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Clement ME, Park LP, Navar AM, Okeke NL, Pencina MJ, Douglas PS, Naggie S. Statin utilization and recommendations among HIV- and HCV-infected veterans: a cohort study. Clin Infect Dis. 2016;63:407–413. doi: 10.1093/cid/ciw289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Thompson-Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr, Skarbinski J, Chmiel JS, Hart R, Wei SC, Loustalot F, Brooks JT, Buchacz K. Cardiovascular disease risk prediction in the HIV Outpatient Study. Clin Infect Dis. 2016;63:1508–1516. doi: 10.1093/cid/ciw615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, D’Agostino RB Sr. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137:2203–2214. doi: 10.1161/CIRCULATIONAHA.117.028975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, Atherosclerotic Cardiovascular Disease Risk Score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17:289–297. doi: 10.1111/hiv.12300 [DOI] [PubMed] [Google Scholar]
- 219.Raggi P, De Francesco D, Manicardi M, Zona S, Bellasi A, Stentarelli C, Carli F, Beghetto B, Mussini C, Malagoli A, Guaraldi G. Prediction of hard cardiovascular events in HIV patients. J Antimicrob Chemother. 2016;71:3515–3518. doi: 10.1093/jac/dkw346 [DOI] [PubMed] [Google Scholar]
- 220.Mosepele M, Hemphill LC, Palai T, Nkele I, Bennett K, Lockman S, Triant VA. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) atherosclerotic cardiovascular disease (ASCVD) risk score among HIV-infected patients in Sub-Saharan Africa. PLoS One. 2017;12:e0172897. doi: 10.1371/journal.pone.0172897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 222.Hanna DB, Moon JY, Haberlen SA, French AL, Palella FJ Jr, Gange SJ, Witt MD, Kassaye S, Lazar JM, Tien PC, Feinstein MJ, Kingsley LA, Post WS, Kaplan RC, Hodis HN, Anastos K. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS. 2018;32:2393–2403. doi: 10.1097/QAD.0000000000001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mangili A, Polak JF, Quach LA, Gerrior J, Wanke CA. Markers of atherosclerosis and inflammation and mortality in patients with HIV infection. Atherosclerosis. 2011;214:468–473. doi: 10.1016/j.atherosclerosis.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(suppl 2):S79–S85. doi: 10.1097/QAI.0b013e318186519c [DOI] [PubMed] [Google Scholar]
- 225.Worm SW, Kamara DA, Reiss P, Kirk O, El-Sadr W, Fux C, Fontas E, Phillips A, D’Arminio Monforte A, De Wit S, Petoumenos K, Friis-Mller N, Mercie P, Lundgren JD, Sabin C. Elevated triglycerides and risk of myocardial infarction in HIV-positive persons. AIDS. 2011;25:1497–1504. doi: 10.1097/QAD.0b013e32834917c6 [DOI] [PubMed] [Google Scholar]
- 226.d’Ettorre G, Ceccarelli G, Giustini N, Mastroianni CM, Silvestri G, Vullo V. Taming HIV-related inflammation with physical activity: a matter of timing. AIDS Res Hum Retroviruses. 2014;30:936–944. doi: 10.1089/AID.2014.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.US Department of Health and Human Services. BeTobaccoFree.gov website. https://betobaccofree.hhs.gov/. Accessed February 5, 2019.
- 228.Centers for Disease Control and Prevention. Tips from former smokers. https://www.cdc.gov/tobacco/campaign/tips/index.html. Accessed February 5, 2019.
- 229.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M, Degenhardt L. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113:1905–1926. doi: 10.1111/add.14234 [DOI] [PubMed] [Google Scholar]
- 230.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054 [DOI] [PubMed] [Google Scholar]
- 231.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG 2nd, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després JP, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J; Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. doi: 10.1016/S0140-6736(18)30134-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang X, Rana P, Leong DP, Dagenais G, Seron P, Rosengren A, Schutte AE, Lopez-Jaramillo P, Oguz A, Chifamba J, Diaz R, Lear S, Avezum A, Kumar R, Mohan V, Szuba A, Wei L, Yang W, Jian B, McKee M, Yusuf S; PURE Investigators. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386:1945–1954. doi: 10.1016/S0140-6736(15)00235-4 [DOI] [PubMed] [Google Scholar]
- 234.Jaggers JR, Prasad VK, Dudgeon WD, Blair SN, Sui X, Burgess S, Hand GA. Associations between physical activity and sedentary time on components of metabolic syndrome among adults with HIV. AIDS Care. 2014;26:1387–1392. doi: 10.1080/09540121.2014.920075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Blashill AJ, Mayer KH, Crane H, Magidson JF, Grasso C, Mathews WC, Saag MS, Safren SA. Physical activity and health outcomes among HIV-infected men who have sex with men: a longitudinal mediational analysis. Ann Behav Med. 2013;46:149–156. doi: 10.1007/s12160-013-9489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.HIV.gov website. https://www.hiv.gov/. Accessed February 5, 2019.
- 237.US National Library of Medicine. Living with HIV/AIDS: exercise and physical fitness. https://aids.nlm.nih.gov/topic/1141/living-with-hiv-aids/1146/exercise-and-physical-fitness. Accessed February 5, 2019.
- 238.Webel AR, Moore SM, Longenecker CT, Currie J, Horvat Davey C, Perazzo J, Sattar A, Josephson RA. Randomized controlled trial of the SystemCHANGE intervention on behaviors related to cardiovascular risk in HIV+ adults. J Acquir Immune Defic Syndr. 2018;78:23–33. doi: 10.1097/QAI.0000000000001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hall LN, Sanchez LR, Hubbard J, Lee H, Looby SE, Srinivasa S, Zanni MV, Stanley TL, Lo J, Grinspoon SK, Fitch KV. Aspartame intake relates to coronary plaque burden and inflammatory indices in human immunodeficiency virus. Open Forum Infect Dis. 2017;4:ofx083. doi: 10.1093/ofid/ofx083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 241.Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, Feldpausch MN, Martin A, Melbourne K, Triant VA, Suchindran S, Lee H, Hoffmann U, Williams KC, Tawakol A, Grinspoon SK. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016;1:474–480. doi: 10.1001/jamacardio.2016.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lesko CR, Keil AP, Moore RD, Chander G, Fojo AT, Lau B. Measurement of current substance use in a cohort of HIV-infected persons in continuity HIV care, 2007–2015. Am J Epidemiol. 2018;187:1970–1979. doi: 10.1093/aje/kwy092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mosepele M, Molefe-Baikai OJ, Grinspoon SK, Triant VA. Benefits and risks of statin therapy in the HIV-infected population. Curr Infect Dis Rep. 2018;20:20. doi: 10.1007/s11908-018-0628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Myerson M, Malvestutto C, Aberg JA. Management of lipid disorders in patients living with HIV. J Clin Pharmacol. 2015;55:957–974. doi: 10.1002/jcph.473 [DOI] [PubMed] [Google Scholar]
- 245.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015;115:1760–1766. doi: 10.1016/j.amjcard.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 246.Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM. Pharmacokinetic interactions between nelfinavir and 3-hydroxy3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob Agents Chemother. 2001;45:3445–3450. doi: 10.1128/AAC.45.12.3445-3450.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.US Department of Health and Human Services. AIDS info. https://aidsinfo.nih.gov/. Accessed February 5, 2019.
- 248.Silverberg MJ, Leyden W, Hurley L, Go AS, Quesenberry CP Jr, Klein D, Horberg MA. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150:301–313. [DOI] [PubMed] [Google Scholar]
- 249.Singh S, Willig JH, Mugavero MJ, Crane PK, Harrington RD, Knopp RH, Kosel BW, Saag MS, Kitahata MM, Crane HM. Comparative effectiveness and toxicity of statins among HIV-infected patients. Clin Infect Dis. 2011;52:387–395. doi: 10.1093/cid/ciq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Han BH, Sutin D, Williamson JD, Davis BR, Piller LB, Pervin H, Pressel SL, Blaum CS; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med. 2017;177:955–965. doi: 10.1001/jamainternmed.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979–1981. doi: 10.1161/CIRCULATIONAHA.117.028271 [DOI] [PubMed] [Google Scholar]
- 253.Calza L, Magistrelli E, Colangeli V, Borderi M, Contadini I, Bon I, Re MC, Viale P. Significant association between statin-associated myalgia and vitamin D deficiency among treated HIV-infected patients. AIDS. 2017;31:681–688. doi: 10.1097/QAD.0000000000001397 [DOI] [PubMed] [Google Scholar]
- 254.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV. 2017;4:e284–e294. doi: 10.1016/S2352-3018(17)30075-9 [DOI] [PubMed] [Google Scholar]
- 255.Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, McCurdy Pate M, Aberg JA, Zanni MV, Grinspoon SK. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. 2017;31:797–806. doi: 10.1097/QAD.0000000000001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Erlandson KM, Jiang Y, Debanne SM, McComsey GA. Rosuvastatin worsens insulin resistance in HIV-infected adults on antiretroviral therapy. Clin Infect Dis. 2015;61:1566–1572. doi: 10.1093/cid/civ554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kelly SG, Krueger KM, Grant JL, Penugonda S, Feinstein MJ, Taiwo BO, Achenbach CJ. Statin prescribing practices in the comprehensive care for HIV-infected patients. J Acquir Immune Defic Syndr. 2017;76:e26–e29. doi: 10.1097/QAI.0000000000001454 [DOI] [PubMed] [Google Scholar]
- 260.Monroe AK, Fu W, Zikusoka MN, Jacobson LP, Witt MD, Palella FJ, Kingsley LA, Post WS, Brown TT. Low-density lipoprotein cholesterol levels and statin treatment by HIV status among Multicenter AIDS Cohort Study men. AIDS Res Hum Retroviruses. 2015;31:593–602. doi: 10.1089/AID.2014.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23:146–149. [PMC free article] [PubMed] [Google Scholar]
- 262.Mitka M Exploring statins to decrease HIV-related heart disease risk. JAMA. 2015;314:657–659. doi: 10.1001/jama.2015.5498 [DOI] [PubMed] [Google Scholar]
- 263.Suchindran S, Regan S, Meigs JB, Grinspoon SK, Triant VA. Aspirin use for primary and secondary prevention in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients. Open Forum Infect Dis. 2014;1:ofu076. doi: 10.1093/ofid/ofu076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon DE, Paton NI, Neaton JD; INSIGHT SMART Study Group. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marcantoni E, Allen N, Cambria MR, Dann R, Cammer M, Lhakhang T, O’Brien MP, Kim B, Worgall T, Heguy A, Tsirigos A, Berger JS. Platelet transcriptome profiling in HIV and ATP-binding cassette subfamily C member 4 (ABCC4) as a mediator of platelet activity. JACC Basic Transl Sci. 2018;3:9–22. doi: 10.1016/j.jacbts.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schechter ME, Andrade BB, He T, Richter GH, Tosh KW, Policicchio BB, Singh A, Raehtz KD, Sheikh V, Ma D, Brocca-Cofano E, Apetrei C, Tracy R, Ribeiro RM, Sher A, Francischetti IMB, Pandrea I, Sereti I. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med 2017;9:eaam5441. doi: 10.1126/scitranslmed.aam5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Gettenberg G, Cavanagh K, Aberg JA, Bhardwaj N, Berger JS. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013;63:280–288. doi: 10.1097/QAI.0b013e31828a292c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 269.O’Dwyer EJ, Bhamra-Ariza P, Rao S, Emmanuel S, Carr A, Holloway CJ. Lower coronary plaque burden in patients with HIV presenting with acute coronary syndrome. Open Heart. 2016;3:e000511. doi: 10.1136/openhrt-2016-000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, Waters DD. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–319. doi: 10.1161/01.CIR.0000114520.38748.AA [DOI] [PubMed] [Google Scholar]
- 271.Theodoropoulos K, Mennuni MG, Sartori S, Meelu OA, Yu J, Baber U, Stefanini GG, Mastoris I, Moreno P, Dangas GD, Mehran R, Sharma SK, Kini AS. Quantitative angiographic characterisation of coronary artery disease in patients with human immunodeficiency virus (HIV) infection undergoing percutaneous coronary intervention. EuroIntervention. 2017;12:1757–1765. doi: 10.4244/EIJ-D-15-00409 [DOI] [PubMed] [Google Scholar]
- 272.Bundhun PK, Pursun M, Huang WQ. Does infection with human immunodeficiency virus have any impact on the cardiovascular outcomes following percutaneous coronary intervention? A systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17:190. doi: 10.1186/s12872-017-0624-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Boccara F, Teiger E, Cohen A, Ederhy S, Janower S, Odi G, Di Angelantonio E, Barbarini G, Barbaro G. Percutaneous coronary intervention in HIV infected patients: immediate results and long term prognosis. Heart. 2006;92:543–544. doi: 10.1136/hrt.2005.068445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Badr S, Minha S, Kitabata H, Fatemi O, Torguson R, Suddath WO, Satler LF, Pichard AD, Waksman R. Safety and long-term outcomes after percutaneous coronary intervention in patients with human immunodeficiency virus. Catheter Cardiovasc Interv. 2015;85:192–198. doi: 10.1002/ccd.25466 [DOI] [PubMed] [Google Scholar]
- 275.Boccara F, Mary-Krause M, Teiger E, Lang S, Lim P, Wahbi K, Beygui F, Milleron O, Gabriel Steg P, Funck-Brentano C, Slama M, Girard PM, Costagliola D, Cohen A; Prognosis of Acute Coronary Syndrome in HIV-infected patients (PACS) Investigators. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J. 2011;32:41–50. doi: 10.1093/eurheartj/ehq372 [DOI] [PubMed] [Google Scholar]
- 276.Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, Touzery C, Hamblin J, Gudjoncik A, Cottin Y, Quantin C. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127:1767–1774. doi: 10.1161/CIRCULATIONAHA.113.001874 [DOI] [PubMed] [Google Scholar]
- 277.Matetzky S, Domingo M, Kar S, Noc M, Shah PK, Kaul S, Daar E, Cercek B. Acute myocardial infarction in human immunodeficiency virus-infected patients. Arch Intern Med. 2003;163:457–460. [DOI] [PubMed] [Google Scholar]
- 278.Ren X, Trilesskaya M, Kwan DM, Nguyen K, Shaw RE, Hui PY. Comparison of outcomes using bare metal versus drug-eluting stents in coronary artery disease patients with and without human immunodeficiency virus infection. Am J Cardiol. 2009;104:216–222. doi: 10.1016/j.amjcard.2009.03.036 [DOI] [PubMed] [Google Scholar]
- 279.Singh V, Mendirichaga R, Savani GT, Rodriguez AP, Dabas N, Munagala A, Alfonso CE, Cohen MG, Elmariah S, Palacios IF. Coronary revascularization for acute myocardial infarction in the HIV population. J Interv Cardiol. 2017;30:405–414. doi: 10.1111/joic.12433 [DOI] [PubMed] [Google Scholar]
- 280.Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, De Wit S, Dabis F, Pradier C, d’Arminio Monforte A, Kovari H, Law M, Lundgren JD, Sabin CA; Data Collection of Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Gender differences in the use of cardiovascular interventions in HIV-positive persons; the D:A:D Study. J Int AIDS Soc. 2018;21:e25083. doi: 10.1002/jia2.25083 [DOI] [Google Scholar]
- 281.Schneider S, Spinner CD, Cassese S, Promny D, Hapfelmeier A, Byrne RA, Baumann M, Jäger H, Steinlechner E, Laugwitz KL, Kastrati A. Association of increased CD8+ and persisting C-reactive protein levels with restenosis in HIV patients after coronary stenting. AIDS. 2016;30:1413–1421. doi: 10.1097/QAD.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 282.Robich MP, Schiltz N, Johnston DR, Mick S, Tse W, Koch C, Soltesz EG. Outcomes of patients with human immunodeficiency virus infection undergoing cardiovascular surgery in the United States. J Thorac Cardiovasc Surg. 2014;148:3066–3073. doi: 10.1016/j.jtcvs.2014.07.074 [DOI] [PubMed] [Google Scholar]
- 283.Boccara F, Cohen A, Di Angelantonio E, Meuleman C, Ederhy S, Dufaitre G, Odi G, Teiger E, Barbarini G, Barbaro G; French Italian Study on Coronary Artery Disease in AIDS Patients (FRISCA-2). Coronary artery bypass graft in HIV-infected patients: a multicenter case control study. Curr HIV Res. 2008;6:59–64. [DOI] [PubMed] [Google Scholar]
- 284.Boccara F, Miantezila Basilua J, Mary-Krause M, Lang S, Teiger E, Steg PG, Funck-Brentano C, Girard PM, Costagliola D, Cohen A, Guiguet M; PACS-HIV Investigators (Prognosis of Acute Coronary Syndrome in HIV-infected patients). Statin therapy and low-density lipoprotein cholesterol reduction in HIV-infected individuals after acute coronary syndrome: results from the PACS-HIV lipids substudy. Am Heart J. 2017;183:91–101. doi: 10.1016/j.ahj.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 285.van Zoest RA, van der Valk M, Wit FW, Vaartjes I, Kooij KW, Hovius JW, Prins M, Reiss P; AGEhIV Cohort Study Group. Suboptimal primary and secondary cardiovascular disease prevention in HIV-positive individuals on antiretroviral therapy. Eur J Prev Cardiol. 2017;24:1297–1307. doi: 10.1177/2047487317714350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Baker JV, Sharma S, Grund B, Rupert A, Metcalf JA, Schechter M, Munderi P, Aho I, Emery S, Babiker A, Phillips A, Lundgren JD, Neaton JD, Lane HC; INSIGHT START (Strategic Timing of AntiRetroviral Treatment) Study Group. Systemic inflammation, coagulation, and clinical risk in the START Trial. Open Forum Infect Dis. 2017;4:ofx262. doi: 10.1093/ofid/ofx262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rasmussen TA, McMahon JH, Chang JJ, Audsley J, Rhodes A, Tennakoon S, Dantanarayana A, Spelman T, Schmidt T, Kent SJ, Morcilla V, Palmer S, Elliott JH, Lewin SR. The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: a randomised, placebo-controlled, double-blind trial. Lancet HIV. 2018;5:e221–e230. doi: 10.1016/S2352-3018(18)30040-7 [DOI] [PubMed] [Google Scholar]
- 288.Martínez E, Larrousse M, Podzamczer D, Pérez I, Gutiérrez F, Loncá M, Barragán P, Deulofeu R, Casamitjana R, Mallolas J, Pich J, Gatell JM; BICOMBO Study Team. Abacavir-based therapy does not affect biological mechanisms associated with cardiovascular dysfunction. AIDS. 2010;24:F1–F9. doi: 10.1097/QAD.0b013e32833562c5 [DOI] [PubMed] [Google Scholar]
- 289.Ribaudo HJ, Benson CA, Zheng Y, Koletar SL, Collier AC, Lok JJ, Smurzynski M, Bosch RJ, Bastow B, Schouten JT; ACTG A5001/ALLRT Protocol Team. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011;52:929–940. doi: 10.1093/cid/ciq244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, Dube MP, Murphy R, Yang OO, Currier JS, McComsey GA. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651–660. doi: 10.1093/cid/civ327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 292.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 293.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 294.Bergeron N, Phan BA, Ding Y, Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132:1648–1666. doi: 10.1161/CIRCULATIONAHA.115.016080 [DOI] [PubMed] [Google Scholar]
- 295.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, Chiang CE, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Kimura T, Kiss RG, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Tricoci P, Xavier D, Zeiher AM, Steg PG, for the ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2019;73:387–396. doi: 10.1016/j.jacc.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 296.Kohli P, Ganz P, Ma Y, Scherzer R, Hur S, Weigel B, Grunfeld C, Deeks S, Wasserman S, Scott R, Hsue PY. HIV and hepatitis C-coinfected patients have lower low-density lipoprotein cholesterol despite higher proprotein convertase subtilisin kexin 9 (PCSK9): an apparent “PCSK9-lipid paradox.” J Am Heart Assoc. 2016;5:e002683. doi: 10.1161/JAHA.115.002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Boccara F, Ghislain M, Meyer L, Goujard C, Le May C, Vigouroux C, Bastard JP, Fellahi S, Capeau J, Cohen A, Cariou B; ANRS-COPANA Study Group. Impact of protease inhibitors on circulating PCSK9 levels in HIV-infected antiretroviral-naive patients from an ongoing prospective cohort. AIDS. 2017;31:2367–2376. doi: 10.1097/QAD.0000000000001633 [DOI] [PubMed] [Google Scholar]
- 298.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310 [DOI] [PubMed] [Google Scholar]
- 299.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380:752–762. doi: 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chau KH, Scherzer R, Grunfeld C, Hsue PY, Shlipak MG. CHA2DS2-VASc score, warfarin use, and risk for thromboembolic events among HIV-infected persons with atrial fibrillation. J Acquir Immune Defic Syndr. 2017;76:90–97. doi: 10.1097/QAI.0000000000001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.West TA, Perram J, Holloway CJ. Use of direct oral anticoagulants for treatment of atrial fibrillation in patients with HIV: a review. Curr Opin HIV AIDS. 2017;12:554–560. doi: 10.1097/COH.0000000000000412 [DOI] [PubMed] [Google Scholar]
- 302.Sweeney EM, Thakur KT, Lyons JL, Smith BR, Willey JZ, Cervantes-Arslanian AM, Hickey MK, Uchino K, Haussen DC, Koch S, Schwamm LH, Elkind MS, Shinohara RT, Mateen FJ. Outcomes of intravenous tissue plasminogen activator for acute ischaemic stroke in HIV-infected adults. Eur J Neurol. 2014;21:1394–1399. doi: 10.1111/ene.12506 [DOI] [PubMed] [Google Scholar]
- 303.AbdelRazek MA, Gutierrez J, Mampre D, Cervantes-Arslanian A, Ormseth C, Haussen D, Thakur KT, Lyons JL, Smith BR, O’Connor O, Willey JZ, Mateen FJ. Intravenous thrombolysis for stroke and presumed stroke in human immunodeficiency virus-infected adults: a retrospective, multicenter US study. Stroke. 2018;49:228–231. doi: 10.1161/STROKEAHA.117.019570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 305.Delgado Almandoz JE, Crandall BM, Fease JL, Scholz JM, Anderson RE, Kadkhodayan Y, Tubman DE. Successful endovascular treatment of three fusiform cerebral aneurysms with the Pipeline Embolization Device in a patient with dilating HIV vasculopathy. J Neurointerv Surg. 2014;6:e12. doi: 10.1136/neurintsurg-2012-010634.rep [DOI] [PubMed] [Google Scholar]
- 306.Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneap Minn). 2012;18(6 Infectious Disease):1319–1337. doi: 10.1212/01.CON.0000423849.24900.ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Moyers BS, Secemsky EA, Vittinghoff E, Wong JK, Havlir DV, Hsue PY, Tseng ZH. Effect of left ventricular dysfunction and viral load on risk of sudden cardiac death in patients with human immunodeficiency virus. Am J Cardiol. 2014;113:1260–1265. doi: 10.1016/j.amjcard.2013.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Uriel N, Nahumi N, Colombo PC, Yuzefpolskaya M, Restaino SW, Han J, Thomas SS, Garan AR, Takayama H, Mancini DM, Naka Y, Jorde UP. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant. 2014;33:924–930. doi: 10.1016/j.healun.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 309.Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108:e1–e11. doi: 10.2105/AJPH.2017.304246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gayles TA, Kuhns LM, Kwon S, Mustanski B, Garofalo R. Socioeconomic disconnection as a risk factor for increased HIV infection in young men who have sex with men. LGBT Health. 2016;3:219–224. doi: 10.1089/lgbt.2015.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, de Wit S, Dabis F, Pradier C, Monforte Ad, Rickenbach M, Law M, Lundgren J, Sabin C. Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. J Int AIDS Soc. 2014;17(suppl 3):19516. doi: 10.7448/IAS.17.4.19516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Richardson KK, Bokhour B, McInnes DK, Yakovchenko V, Okwara L, Midboe AM, Skolnik A, Vaughan-Sarrazin M, Asch SM, Gifford AL, Ohl ME. Racial disparities in HIV care extend to common comorbidities: implications for implementation of interventions to reduce disparities in HIV care. J Natl Med Assoc. 2016;108:201–210.e3. doi: 10.1016/j.jnma.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 313.Ramírez-Marrero FA, De Jesús E, Santana-Bagur J, Hunter R, Frontera W, Joyner MJ. Prevalence of cardiometabolic risk factors in Hispanics living with HIV. Ethn Dis. 2010;20:423–428. [PMC free article] [PubMed] [Google Scholar]
- 314.Bednasz C, Luque AE, Zingman BS, Fischl MA, Gripshover BM, Venuto CS, Gu J, Feng Z, DiFrancesco R, Morse GD, Ma Q. Lipid-lowering therapy in HIV-infected patients: relationship with antiretroviral agents and impact of substance-related disorders. Curr Vasc Pharmacol. 2016;14:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hatleberg CI, Ryom L, El-Sadr W, Smith C, Weber R, Reiss P, Fontas E, Dabis F, Law M, Monforte Ad, De Wit S, Mocroft A, Phillips A, Lundgren JD, Sabin C; D:A:D Study Group. Improvements over time in short-term mortality following myocardial infarction in HIV-positive individuals. AIDS. 2016;30:1583–1596. doi: 10.1097/QAD.0000000000001076 [DOI] [PubMed] [Google Scholar]
- 316.Esenwa C, Ilunga Tshiswaka D, Gebregziabher M, Ovbiagele B. Historical slavery and modern-day stroke mortality in the United States Stroke Belt. Stroke. 2018;49:465–469. doi: 10.1161/STROKEAHA.117.020169 [DOI] [PubMed] [Google Scholar]
- 317.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Ahmed A, Frishman WH, Fonarow GC. Regional variation across the United States in management and outcomes of ST-elevation myocardial infarction: analysis of the 2003 to 2010 Nationwide Inpatient Sample database. Clin Cardiol. 2014;37:204–212. doi: 10.1002/clc.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e1–e473. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 319.Centers for Disease Control and Prevention (CDC). HIV in the Southern United States. 2016. https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf. Accessed February 5, 2019.
- 320.90–90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 321.Longenecker CT. Vascular disease and aging in HIV: time to extend the treatment cascade. Vasc Med. 2018;23:476–477. doi: 10.1177/1358863X18789767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.National AIDS Treatment Advocacy Project website. http://www.natap.org. Accessed February 5, 2019.
- 323.Palma AM, Rabkin M, Simelane S, Gachuhi AB, McNairy ML, Nuwagaba-Biribonwoha H, Bongomin P, Okello VN, Bitchong RA, El-Sadr WM. A time-motion study of cardiovascular disease risk factor screening integrated into HIV clinic visits in Swaziland. J Int AIDS Soc. 2018;21:e25099. doi: 10.1002/jia2.25099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vorkoper S, Kupfer LE, Anand N, Patel P, Beecroft B, Tierney WM, Ferris R, El-Sadr WM; HIV/NCD Project. Building on the HIV chronic care platform to address noncommunicable diseases in Sub-Saharan Africa: a research agenda. AIDS. 2018;32(suppl 1):S107–S113. doi: 10.1097/QAD.0000000000001898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cope R, Berkowitz L, Arcebido R, Yeh JY, Trustman N, Cha A. Evaluating the effects of an interdisciplinary practice model with pharmacist collaboration on HIV patient co-morbidities. AIDS Patient Care STDS. 2015;29:445–453. doi: 10.1089/apc.2015.0018 [DOI] [PubMed] [Google Scholar]
- 326.Bloch M, Jayewardene A, Vincent T, Linton N, Quan D, Gowers A. Effectiveness of a team intervention in reducing modifiable cardiovascular disease risk in HIV-infected subjects on antiretroviral therapy. J Int AIDS Soc. 2014;17(suppl 3):19546. doi: 10.7448/IAS.17.4.19546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Edwards JK, Bygrave H, Van den Bergh R, Kizito W, Cheti E, Kosgei RJ, Sobry A, Vandenbulcke A, Vakil SN, Reid T. HIV with non-communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010–2013. Trans R Soc Trop Med Hyg. 2015;109:440–446. doi: 10.1093/trstmh/trv038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.