ABSTRACT
At present, bacteria continue to threaten human health, and the resistance of bacteria to antibiotics continues to increase, so the development of new antibacterial agents and antibacterial materials is increasingly important to ensure human health. In this paper, three polyether biguanide compounds with high antibacterial properties were synthesized by reacting polyetheramine T403 with o-tolylbiguanide, m-tolylbiguanide and p-tolylbiguanide (o-TTB, m-TTB and p-TTB), respectively. The antimicrobial performance of polyether biguanide against E. coli and S. aureus was evaluated using a minimum inhibitory concentration method, and the results showed that the synthesized polyether biguanide exhibited efficient and broad-spectrum antimicrobial effects. Among them, o-tolyl biguanide derivative o-TTB showed the best antimicrobial performance, with minimum inhibitory concentrations of 20 and 15 μg/mL against E. coli and S. aureus, respectively. Then, epoxy resin E51 was cured using the obtained TTB as a curing agent to prepare an epoxy resin with antibacterial properties. The inhibition of the growth of S. aureus by the cured o-TTB/E51 resin was investigated by incubating the cured epoxy resin with bacteria, and the results showed that the cured resin had a significant inhibitory effect on the growth of bacteria. The non-isothermal curing kinetics of the o-TTB/E51 system were investigated by differential scanning calorimetry (DSC) to determine the optimized curing reaction temperature, curing kinetic parameters and curing kinetics equation.
KEYWORDS: Antibacterial agent, biguanide, epoxy resin, antibacterial materials, curing
Introduction
Infection of humans, animals and plants with harmful bacteria can lead to illnesses and serious damage to human health, while pathogenic bacteria are ubiquitous in nature, they spread widely and survive under mild conditions, making it challenging to eliminate them [1–3]. Some highly toxic pathogens that spread in medical institutions and public places can also form biofilms. Biofilms allow microorganisms to be embedded in the membrane network structure to provide protection for the microbial population and hinder the penetration of most antibiotics. Biofilms can also produce extracellular polymers to protect the biofilm, which stabilizes the film matrix over time [4]. The adhesion of microorganisms to biological materials on medical devices and the subsequent formation of bacterial membranes are the main reason for infections. In hospitals, many people die every year due to medical device infections. Not only are they harmful to human health, but microorganisms can grow and reproduce, which destroys the structure of material, causing deterioration, corrosion, damage and other bad effects, which can lead to significant property loss and sometimes loss of life [1–5]. With further developments in society, people continue to pay more attention to quality of life, and solving the hazards caused by microorganisms has become a growing concern. The development and use of antibacterial substances have become more frequent and been applied in various fields.
Research on cationic antibacterial agents is rapidly developing. Guanidine compounds can be ionized in water, thereby inducing positive charges, which then contact the negatively charged bacteria through electrostatic attraction, destroy the bacterial cell membrane, and kill the bacteria. This avoids the development of drug resistance, and the positively charged guanidine compounds do not interact with neutral human cells, so they can selectively kill bacteria and exhibit low toxicity to humans [6–10]. Guanidine functionalized polymers have been extensively studied, the guanidine group exists in the polymer main chain or in the side chain, and these polymers exhibited effective broad-spectrum antibacterial activities and have great potential in the fields of antibacterial or antifouling coatings, membranes and so on [11–18].
Antibacterial materials are functional materials that can kill bacteria or inhibit the growth of bacteria while maintaining the functionality of the original material. Due to their special functionality, antibacterial materials have been used in a wide range of applications, and development of antibacterial polymers, such as antibacterial epoxy resins, polyurethane, and acrylic resin and so on is of great value in improving human health and reducing bacterial infections [11–28]. In general, the antibacterial activity of resin materials is realized by adding antibacterial agent, such as ZnO, silver or copper and so on, in the polymer matrix or using metal-chelated resins [29,30]. However, the leaching of the antibacterial agent causes decrease in antibacterial activity, in addition, adding metal is expensive and leaching metal ions results in the environmental problem. Therefore, developing non-leaching resin materials in which antibacterial functional groups are covalently bonded to the matrix is of great value to conquer these problems.
Epoxy resin (EP) is one of the most important polymers and widely used in coatings, food, medical and composite materials. They exhibit many advanced properties such as high strength, excellent adhesion to various substrates, low shrinkage, ease of cure and good chemical resistance due to a three-dimensional cross-linked structure [31–33]. Guanidine compound is one kind of the commonly used curing agents for epoxy resin [34–41], meanwhile, it exhibits good antibacterial properties in many occasions, which inspired us to use biguanide compounds to prepare epoxy resins with antibacterial functionality. In addition, the curing process of EP clearly influences the mechanical performance of the cured resin. Kinetic models developed from kinetic analysis of DSC data have been widely applied to the curing of epoxy resins. Under the nonisothermal condition, the Kissinger or Crane equations can be used to give the activation energy (Ea), reaction order and Arrhenius pre-exponential factor, and finally the curing temperature can be calculated by extrapolating method [34–41].
In this paper, three-branched polyether biguanide compounds with high antibacterial performance were synthesized by reacting polyetheramine T403 with o-tolylbiguanide, m-tolylbiguanide and p-tolylbiguanide (o-TTB, m-TTB and p-TTB), respectively. Meanwhile, epoxy resin E51 was cured using the obtained compound as a curing agent to prepare an epoxy resin with antibacterial properties (TTB/E51). The antimicrobial performance of biguanide derivatives against E. coli and S. aureus was evaluated by the method of minimum inhibitory concentration, and the influence of the position of the substituent on the aromatic ring on the antibacterial performance was investigated. The growth inhibition of S. aureus by the TTB/E51 curing compounds was investigated by incubating the cured epoxy resin with bacteria, and the non-isothermal curing kinetics of the TTB/E51 compounds were investigated by differential scanning calorimetry (DSC).
Experimental
Materials
o-, m- and p-Tolyl biguanide and polyetheramine T403 were supplied by Saen Chemical Technology (Shanghai) Co., Ltd., China. 2, 3, 5-Triphenyltetrazolium chloride (TTC), magnesium sulfate anhydrous, NaCl, and 2, 2’-Azobis (2-methylpropionitrile) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., China. Solvents, agar, beef, PBS buffer, and other solvents were supplied by Chemical Reagent Co., Ltd., China. The bacterial strains of Gram-positive S. aureus and Gram-negative E. coli were provided by Shanghai Luwei Technology Co., Ltd., China.
Synthesis of polyether biguanide (TTB)
TTB was synthesized according to the previous report by an optimized method [14–16]. The general synthesis process is as follows:
2.28 g (0.01 mol) of o-tolyl biguanide hydrochloride and 1.5 mL (0.003 mol) of polyether amine T403 were placed in a flask. 50 mL of N, N-dimethyl acetamide (DMAc) was added as solvent and the reaction was carried out at 150°C for 24 h. The solution was evaporated to dryness, and a dark brown viscous liquid was obtained. Then, it was dissolved in water, pH was adjusted to 10 by using NaOH aq (1 M). The precipitate after filtration was removed, and the filtrate was collected and evaporated to obtain a yellow viscous liquid o-tolyl polyether biguanide o-TTB (2.87 g, yield: 88.0%).
m-TTB and p-TTB were synthesized according to the above steps except that the raw materials of biguanide were replaced with o-tolyl biguanide and p-tolyl biguanide respectively.
o-TTB: 1H-NMR (400 MHz, DMSO-d6): 7.66 (br, C = NH), 7.15–7.51 (br, Ph-H), 3.1–3.8 (br, CH2,CH and C-NH-C), 2.19 (s, Ph-CH3), 1.26 (br, CH2-CH3), 1.04 (br, O-CH-CH3), 0.77 (s, CH2-CH3). IR (cm−1, KBr) 3321 cm−1 and 3158 cm−1 (N-H), 2771 cm−1 (CH3), 1563 (N-H), 1661 cm−1 (C = N), 1258 cm−1 (C-N), 1104 cm−1 (C-O-C).
m-TTB: 1H-NMR (400 MHz, DMSO-d6): 7.64 (br, C = NH), 6.87–7.27 (br, Ph-H), 3.1–3.4 (br, CH2,CH and C-NH-C), 2.22 (s, Ph-CH3), 1.28 (br, CH2-CH3), 1.01 (br, O-CH-CH3), 0.74 (s, CH2-CH3). IR (cm−1, KBr) 3320 cm−1 and 3172 cm−1 (N-H), 2772 cm−1 (CH3), 1563 (N-H), 1655 cm−1 (C = N), 1258 cm−1 (C-N), 1104 cm−1 (C-O-C).
p-TTB: 1H-NMR (400 MHz, DMSO-d6): 7.68 (br, C = NH), 7.03–7.24 (br, Ph-H), 3.1–3.4 (br, CH2,CH and C-NH-C), 2.00 (s, Ph-CH3), 1.28 (br, CH2-CH3), 0.99 (br, O-CH-CH3), 0.67 (s, CH2-CH3). IR (cm−1, KBr) 3320 cm−1 and 3172 cm−1 (N-H), 2772 cm−1 (CH3), 1563 (N-H), 1655 cm−1 (C = N), 1258 cm−1 (C-N), 1104 cm−1 (C-O-C).
Characterization
1 H NMR (400 MHz) spectra were recorded on a JEOL LEOLEX-400 spectrometer. The surface functional groups and chemical compositions of the products were investigated using Fourier transform infrared spectroscopy (FT-IR, Nicolet Avatar-330) by the KBr pellet pressing method. Bacterial growth was investigated by a microplate reader (Mindray MR-96A).
Minimum inhibitory concentration (MIC) of samples by TTC colorimetry
TTC (2, 3, 5-triphenyltetrazolium chloride) is a commonly used REDOX indicator in biochemical experiments. After contact with live bacteria, TTC is reduced by enzymes to red TPF (1, 3, 5-tamamidine), which is used for bacterial staining to detect the survival of live bacteria. MIC refers to the minimum concentration of antibacterial agents needed to inhibit the growth of experimental strains. Gram-positive (S. aureus) and Gram-negative (E. coli) were cultured in liquid medium on a logarithmic scale, and the bacterial solution was diluted to a concentration of 106–107 CFU/mL. One hundred μL of liquid medium containing 0.02% TTC was added to each well of the 96-well plate, and then the compound (100 μL) was added, followed by two-fold dilution and three parallel experiments. Finally, 100 μL diluted bacterial solution was added to each well of the 96-well plate. The negative (only add the liquid medium without adding the bacterial solution) and positive blanks (only add the bacterial solution without adding the test compound) were tested. Finally, the 96-well plates were placed into a 37°C environment and incubated (Bacillus subtilis) for 72 hours and 24 hours (other bacteria), respectively, and the color changes of the plates were observed. The TTC will show red coloration when exposed to live bacteria, and the concentration without red color is the minimum inhibitory concentration compared to the control group.
Evaluation of antibacterial performance of o-TTB cured E51 epoxy resin
o-TTB/E51 epoxy resin was prepared according to the following method: a certain amount of biguanide derivative and epoxy resin was mixed well according to the stoichiometric ratio of active hydrogen/epoxy group 1:1. It was coated on a Teflon plate with a thickness of about 1 mm and placed in a vacuum drying oven at 60°C for degassing for 1 h, and then the resin was cured at 120°C for 2 h and then at 180°C for 1 h in the air. The cured film was peeled off and then thoroughly washed with ethanol in ultrasound. Finally, the film was dried in vacuum at room temperature.
The cultured S. aureus was diluted with nutrient broth, and the absorbance at 630 nm (OD630) was measured with an enzyme-labeled instrument. Then, the bacterial solution was diluted until its absorbance was about 0.1. Ten mL of bacteria solution was added into sterilized test tubes, leaving one as a blank control; 0.5 g of epoxy resin cured by different concentrations of TTB was added into the remaining test tubes, then sealed and incubated in a gas-bath thermostat oscillator at 37°C at 140 rpm for 0 h, 1 h, 2 h, 4 h, 6 h, and 8 h. The absorbance of 0.1 mL of the sample was measured at 630 nm using an enzyme-labeled instrument.
Results and discussion
Antibacterial activity of polyether biguanide
The antibacterial activity of o-TTB, m-TTB and p-TTB against Gram-negative E. coli and Gram-positive S. aureus was tested using the minimum inhibitory concentration (MIC) method Figure 1 and 2(Figure 3), and the MIC of each TTB was determined and compared with the MICs of T403 and tolylbiguanide as synthetic raw materials (Table 1).
Figure 1.
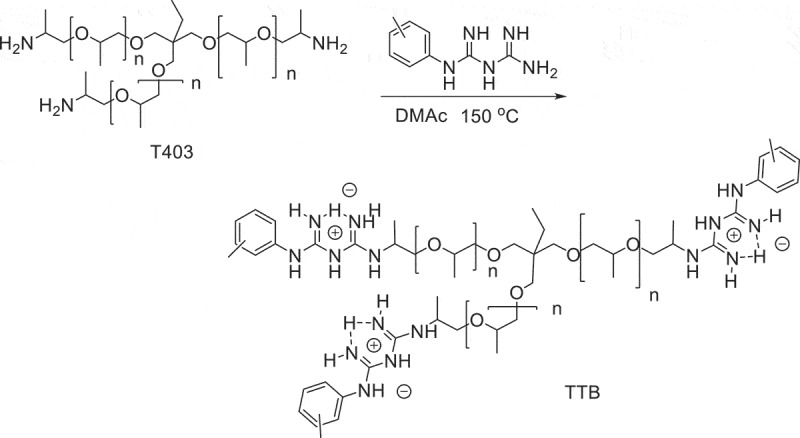
Synthesis of polyether biguanide TTB (o-, m- and p-TTB)
Figure 2.
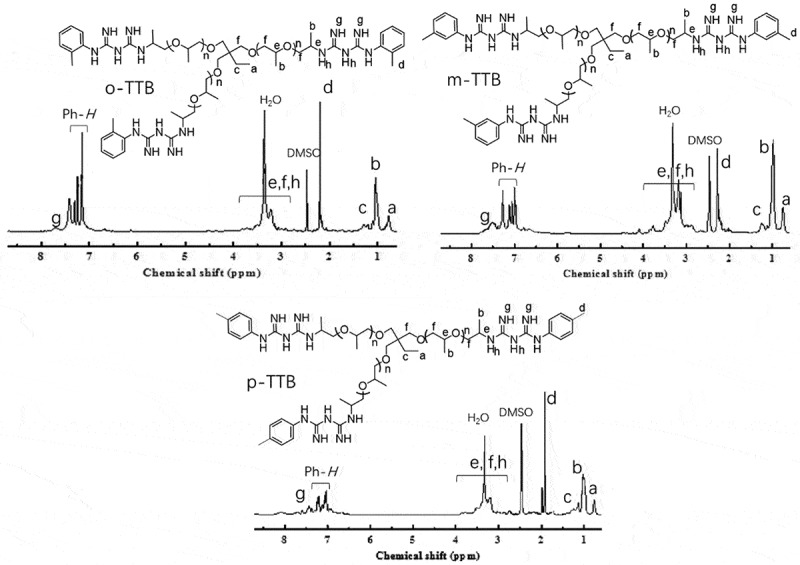
1H-NMR spectrum of TTB (DMSO-d6, 25°C, 400 MHz)
Figure 3.
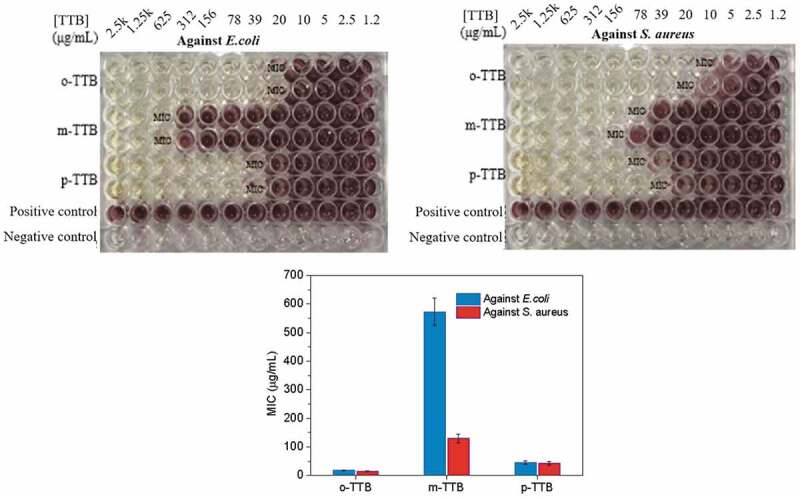
Antibacterial effects of o-TTB, m-TTB and p-TTB on E. coli and S. aureus
Table 1.
Average MIC of TTB to E. coli and S. aureus
| compound | |
MIC (μg/mL) |
|
|
|---|---|---|---|---|
| E. coli | SE | S. aureus | SE | |
| o-tolyl biguanide | 1458 | 190 | 729 | 95 |
| m-tolyl biguanide | 1458 | 190 | 1458 | 190 |
| p-tolyl biguanide | 2083 | 241 | 1458 | 190 |
| T403 | 729 | 95 | 677 | 115 |
| o-TTB | 18.3 | 1.52 | 15 | 2.04 |
| m-TTB | 573 | 47.6 | 130 | 15.0 |
| p-TTB | 45.5 | 5.9 | 42.3 | 7.10 |
| SE: standard error | ||||
According to (Table 1), both phenyl biguanide and polyetheramine T403 as raw materials showed weak antibacterial properties, while the synthesized polyether biguanide showed excellent antibacterial performance. The antibacterial effect of TTB on S. aureus was slightly better than that on E. coli, which might be due to the thinner cell wall of Gram-positive S. aureus. o-TTB showed the best antibacterial performance, where the MICs to E. coli and S. aureus were 20 μg/mL and 15 μg/mL, respectively. p-TTB showed slight lower antibacterial performance than o-TTB with MIC values to E. coli and S. aureus of 39 and 58 μg/mL, respectively. m-TTB’s antibacterial performance was relatively low, and the MIC values to E. coli and S. aureus were 625 μg/mL and 117 μg/mL, respectively. These results indicate that TTB, especially o-TTB, has a broad spectrum and high antimicrobial performance.
The antibacterial mechanism of biguanide antibacterial agents is generally considered to be a cationic antibacterial mechanism. Biguanide is a positively charged cationic group, while the surface of the bacteria is negatively charged. The positively charged biguanide group is easy to adsorb on the surface of the bacteria through electrostatic interactions, thereby altering the electrochemical properties of the membrane surface. At the same time, it exchanges with cations such as Ca 2+ and Mg 2+ in the cell membrane, destroying the homeostasis of bacteria. Further, due to the thin peptidoglycan layer between the inner and outer membranes of the bacteria, the hydrophobic alkyl groups in TTB penetrate more easily penetrate the cell membrane, resulting in perforation, leakage of intracellular material, and microbial cell death [17,18]. The excellent antimicrobial properties of TTB may be attributed to its branched structure. Polyetheramine T403 is a branched polymer with three -NH2 end groups that react with phenyl biguanide. Therefore, the synthesized TTB has three phenyl biguanide groups in the molecular structure (Figure 1Figure 2), which greatly increase the local density of functional groups and the number of cationic charges. Compared with free the cations, this branch structure is more favorable to the adsorption of cations on the surface of bacteria in kinetics. Therefore, TTB can more effectively interact with the negatively charged cell membrane of bacteria. TTB has three biguanide groups with a large positive charge density, which makes it easier to interact with negatively charged bacteria, thereby killing them. At the same time, polyether biguanide has good water solubility, which also facilitates its contact with bacteria. This leads to the efficient and broad-spectrum antibacterial activity of TTB. In addition, the poorer antibacterial activity of m-TTB was thought to be caused by the different electron distribution in the benzene ring. The methyl group at o- or p-position enhances the electron density on the carbon connected to the biguanide group, which may be conductive to the stability of the positive charge on the biguanide group and caused the higher antibacterial activity of o-and p-TTB.
DSC curve analysis
The curing reaction of epoxy resin is a complex chemical process. The thermal analysis of the epoxy resin curing process can help to obtain the kinetic data of the curing process and determine the curing temperature, curing time, curing rate and other parameters for the theoretical guidance of the curing study. Among the three types of TTB, o-TTB exhibits the best antimicrobial performance, and guanidine as a raw material can be used as a curing agent for epoxy resin. Therefore, to prepare epoxy resin with antimicrobial functionality, the curing behavior of the TTB/E51 system with o-TTB as a curing agent has been investigated below.
(Figure 4) shows the DSC curves of o-TTB/E51 at heating rates of 5 K/min, 10 K/min, 15 K/min, and 20 K/min, respectively. As the heating rate increases, the exothermic peak of the system moves to the high-temperature region, and the characteristic temperature also increases, which indicates that when the heating rate is low, the resin curing system has sufficient reaction time, and as the heating rate increases, thermal effects increase and the thermal inertia of the system becomes larger, resulting in a larger temperature difference and the exothermic peak gradually entering the high-temperature region. Isothermal curing temperature (Table 3) was obtained by extrapolation from a linear fit of experimentally obtained characteristic temperatures (Table 2 and Figure 5).
Figure 4.
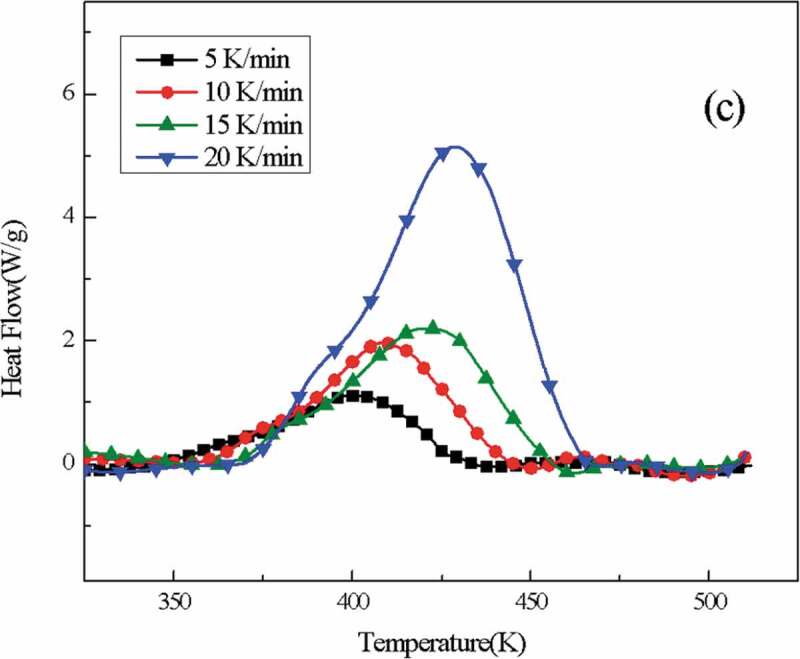
DSC curves of o-TTB/E51 at different heating rates
Table 3.
Optimized curing temperatures of the o-TTB/E51 system
| System | Tf/°C | Tp/°C | Ti/°C |
|---|---|---|---|
| o-TTB/E51 | 198.67 | 140.45 | 76.65 |
Table 2.
Tf, Tp, Ti and enthalpy of o-TTB/E51under different heating rate
| Heating rate (K/min) | Ti (K) | Tp (K) | Tf (K) | Enthalpy (J/g) |
|---|---|---|---|---|
| 5 | 354.98 | 417.93 | 476.98 | 151.0 |
| 10 | 364.24 | 428.64 | 488.80 | 164.7 |
| 15 | 369.80 | 434.39 | 497.10 | 166.4 |
| 20 | 374.56 | 439.40 | 502.13 | 151.8 |
Tf: final temperature; Tp: peak temperature; Ti: initial temperature
Figure 5.
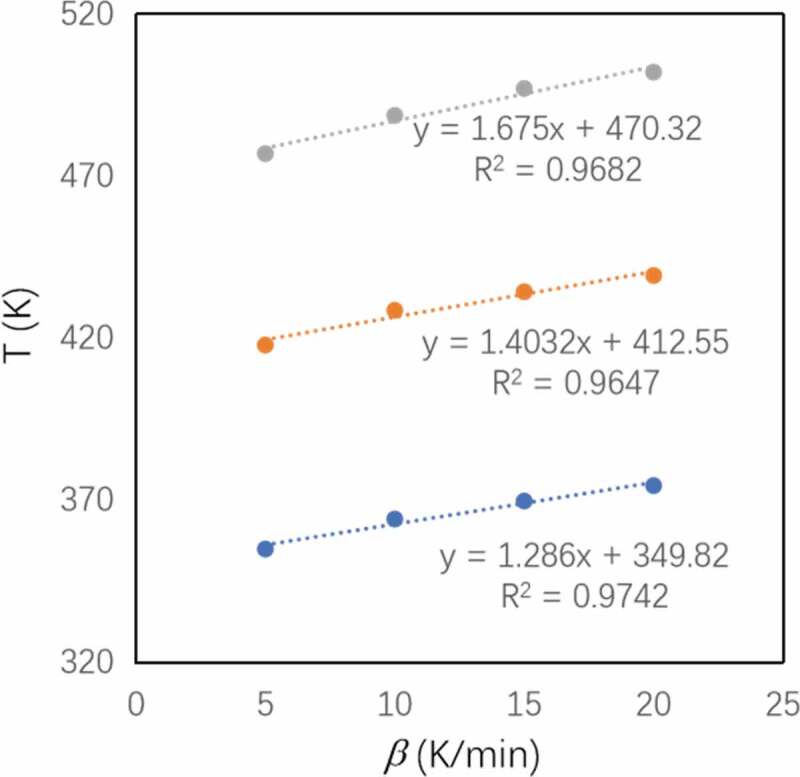
The fitting for the determination of optimized curing temperature (Tf, Tp and Ti) of o-TTB/E51
Curing kinetic parameters
The Kissinger equation was used to determine the apparent activation energy (Ea) of the curing kinetics [34,37], where Ea is the minimum energy required for a curing reaction to occur, indicating the degree of difficulty with which the reaction occurs, and is one of the most important factors influencing the rate of the reaction.
The Kissinger equation is as follows:
| (1) |
where β is heating rate (K/min), Tpi is peak temperature (K), Ea is the apparent activation energy (J/mol), R is the molar gas constant (8.314 J/molK), and A is pre-exponential factor (s−1).
The Crane equation [38] is used to determine the reaction order (n) of the curing kinetics, where n represents the complexity of the curing reaction.
The Crane equation is as follows:
| (2) |
where β is heating rate (K/min), Tp is peak temperature (K), Ea is the apparent activation energy (J/mol), n is the reaction order, and R is the molar gas constant (8.314 J/molK).
By reading the DSC curves and calculating the kinetic parameters of each system (Table 4), the fitted curves of ln (β/Tp2) ~1/Tp and lnβ~1/Tp for each system are plotted in (Figure 6).
Table 4.
Curing reaction kinetics of the o-TTB/E51 system
| heating rate (K/min) | ln β | Tp (K) | ln (β/Tp2) | 1/Tp (K−1) |
|---|---|---|---|---|
| 5 | 1.61 | 417.93 | −10.46 | 2.39 × 10−3 |
| 10 | 2.30 | 430.64 | −9.83 | 2.32 × 10−3 |
| 15 | 2.71 | 434.39 | −9.44 | 2.30 × 10−3 |
| 20 | 3.00 | 439.40 | −9.18 | 2.28 × 10−3 |
Figure 6.
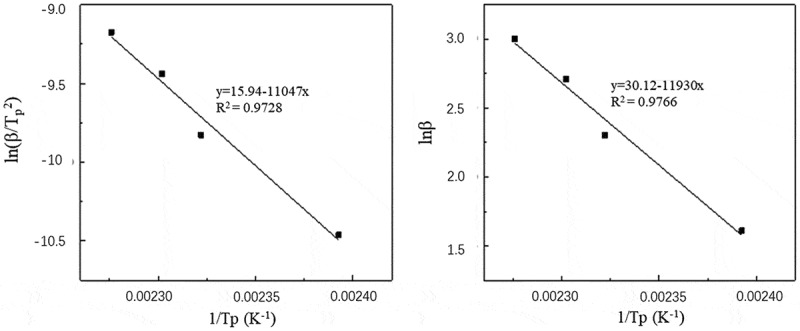
The fitted curve of ln (β/Tp2) ~1/Tp (left) and the fitted curve of lnβ~1/Tp (right) for o-TTB/E51
The apparent activation energy of the o-TTB/E51 system obtained by linear fitting is E = 91.84 kJ/mol, the pre-exponential factor A = 9.24 × 1010 s−1, and the reaction order of the o-TTB/E51 system is 0.92.
Curing kinetics equation
The curing kinetics equation can express the relationship between curing temperature, curing time and curing degree. From the following two formulas (3), (4), the curing reaction kinetics equation of (5) can be obtained, and (5) can be obtained after integration.
| (3) |
| (4) |
| (5) |
| (6) |
where α is the degree of reaction, K is the reaction rate constant (s−1), and t is the reaction time (s).
Eq. (6) shows the relationship between cure, reaction time, and reaction temperature, i.e., the longer the reaction time, the higher the reaction temperature and higher the degree of reaction. Substituting the kinetic parameters previously obtained into Eq. (6), the reaction kinetic equation for each system can be obtained:
TGA of the materials
The thermal stability of o-TTB and cured o-TTB/E51 were investigated by TGA in air atmosphere (Figure 7). According to the TGA curve of o-TTB, a slight weight loss at about 100 oC is caused by the evaporation of trace water, while o-TTB starts to decompose at about 185°C; and the cured 0-TTB/E51 starts to decompose from about 275°C. Therefore, in order to avoid oxidative decomposition of o-TTB and the resin during the curing process, we cured the resin at 120 oC for 2 h and then at 180°C for 1 h, which were lower than the optimized curing temperature.
Figure 7.
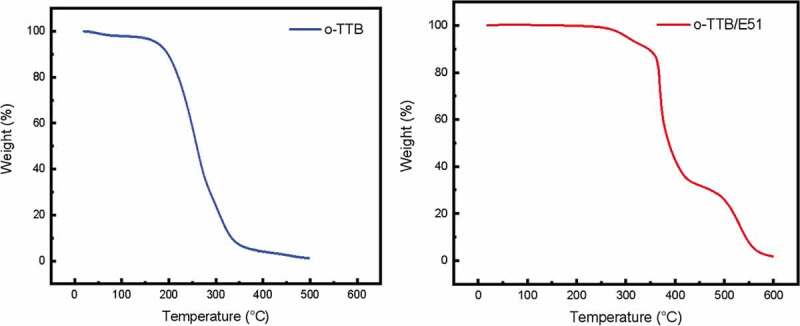
TGA of o-TTB (left) and o-TTB cured E51 (right) in the air. (Heating rate 5°C/min)
Inhibitory properties of o-TTB/E51 cures
The epoxy resin E51 was cured using o-TTB as the curing agent to produce o-TTB/E51 resin with antibacterial performance. The inhibition properties of cured o-TTB/E51 on bacterial growth were investigated by co-culturing the cured o-TTB/E51 resin with S. aureus. The growth curve of bacteria in the presence of o-TTB/E51 was measured by the absorbance method, and the growth curve was compared with that of the control sample. As shown in (Figure 8), the o-TTB/E51 exhibited a significant inhibitory effect on the growth of S. aureus compared to the control sample indicating the excellent antibacterial performance of o-TTB/E51 resin. The results showed that o-TTB/E51 epoxy resin with antibacterial properties was successfully prepared using o-TTB as the curing agent. The good antibacterial performance of the o-TTB/E51 system may be due to the ability of the biguanide groups to carry a positive charge and the high density of the functional group afforded by the branched structure of TTB. When negatively charged bacteria are in contact with the resin surface, the positively charged biguanide groups can kill the bacteria and thus inhibit their growth.
Figure 8.
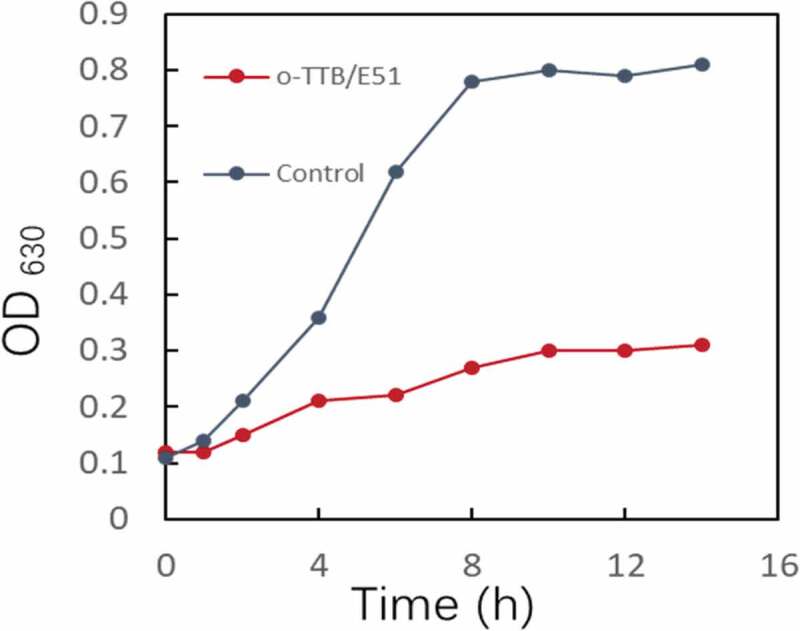
Bacterial growth curves treated with o-TTB/E51 against S. aureus
Conclusions
Three branched polyether biguanides (o-TTB, m-TTB and p-TTB) with broad-spectrum and high antibacterial properties were successfully synthesized by the reaction of branched polyetheramine T403 with o-tolyl biguanide, m-tolyl biguanide and p-tolyl biguanide, respectively. The antibacterial properties of TTB against E. coli and S. aureus were investigated, and the results showed that TTB had high-efficiency and broad-spectrum antibacterial properties. o-TTB had the strongest antibacterial effect against E. coli and S. aureus, and its minimum inhibitory concentration was 20 and 15 µg/mL, respectively. The curing kinetics of the o-TTB/epoxy resin E51 system were investigated by DSC to determine the optimized curing reaction temperature of the systems and determine the curing kinetic parameters and equations. Meanwhile, o-TTB was used as a curing agent to cure epoxy resin E51, and o-TTB/E51 epoxy resin with antibacterial properties was prepared. The antibacterial properties of the cured o-TTB/E51 material were tested by the co-culture method, and the results showed that o-TTB/E51 epoxy resin had significant inhibitory effect on the growth of S. aureus. This is considered to be an antibacterial material with broad application prospects.
Acknowledgments
This work is financially supported by the National Natural Science Foundations of Heilongjiang Province, China (B2018003). The authors are grateful for the support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Doetsch R, Cook T.. Introduction to bacteria and their eco-biology. 1st ed. Baltimore: University Park Press; 1973. [Google Scholar]
- [2].Harada L, Silva E, Campos W, et al. Biotechnological applications of bacteriophages: state of the art. Microbiol Res. 2018;212:38–58. [DOI] [PubMed] [Google Scholar]
- [3].Gabriel G, Som A, Madkour A, et al. Infectious disease: connecting innate immunity to biocidal polymers. Mater Sci Eng R Rep. 2007;57(1):28–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gupta P, Sarkar S, Das B, et al. Pathogenesis and prevention- a journey to break the wall: a review. Arch Microbiol. 2016;198(1):1–15. [DOI] [PubMed] [Google Scholar]
- [5].Hall-Stoodley L, Costerton J, Stoodley P.. Bacterial biofilms: from the Natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. [DOI] [PubMed] [Google Scholar]
- [6].Li P, Sun S, Dong A, et al. Developing of a novel antibacterial agent by functionalization of graphene oxide with guanidine polymer with enhanced antibacterial activity. App Surf Sci. 2015;355:446–452. [Google Scholar]
- [7].Han H, Zhu J, Wu D, et al. Inherent guanidine nanogels with durable antibacterial and bacterially antiadhesive properties. Adv Funct Mater. 2019;29(12):1–17. [Google Scholar]
- [8].Du H, Wang Y, Yao X, et al. Injectable cationic hydrogels with high antibacterial activity and low toxicity. Polym Chem. 2016;7(36):5620–5624. [Google Scholar]
- [9].Coqueiro A, Regasini LO, Stapleton P. In vitro antibacterial activity of prenylated guanidine alkaloids from Pterogyne nitens and synthetic analogues. J Nat Prod. 2014;77(8):1972–1975. [DOI] [PubMed] [Google Scholar]
- [10].Cao Y, Gu J, Wang S. Guanidine-functionalized cotton fabrics for achieving permanent antibacterial activity without compromising their physicochemical properties and cytocompatibility. Cellul. 2020;27(10):6027–6036. [Google Scholar]
- [11].Chen S, Li C, Hou T, et al. Polyhexamethylene guanidine functionalized chitosan nanofiber membrane with superior adsorption and antibacterial performances. React Funct Polym. 2019;145:104379. [Google Scholar]
- [12].Budhathoki J, Peng L, Melander C, et al. Synthesis of guanidinium functionalized polycarbodiimides and their antibacterial activities. ACS Macro Lett. 2012;1:370–374. [DOI] [PubMed] [Google Scholar]
- [13].Liu L, Li Q, Dai J, et al. A facile strategy for the synthesis of guanidinium-functionalized polymer as alkaline anion exchange membrane with improved alkaline stability. J Membr Sci. 2014;453:52–60. [Google Scholar]
- [14].Zhu L, Wang L, Zhang X, et al. Structure-activity relationships of oligoguanidines-influence of counter ion, diamine, and average molecular weight on biocidal activities. Biomacromol. 2003;4(6):1811–1817. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Jiang J, Chen Y. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polym. 1999;40(22):6189–6198. [Google Scholar]
- [16].Feiertag P, Albert M, Ecker-Eckhofen E-M, et al. Structural characterization of biocidal oligoguanidines. Macromol Rapid Commun. 2003;24(9):567–570. [Google Scholar]
- [17].Wang B, Liu B, Peng G, et al. Synthesis and antimicrobial properties of a guanidine-based oligomer grafted with a reactive cationic surfactant through Michael addition. J Appl Polym Sci. 2013;130(5):3489–3497. [Google Scholar]
- [18].Pasero C, D’Agostino I, De Luca F, et al. Alkyl-guanidine compounds as potent broad-spectrum antibacterial agents: chemical library extension and biological characterization. J Med Chem. 2018;61(20):9162–9176. [DOI] [PubMed] [Google Scholar]
- [19].Amjed N, Bhatti I, Zia KM, et al. Synthesis and characterization of stable and biological active chitin-based polyurethane elastomers. Int J Biol Macromol. 2020;154:1149–1157. [DOI] [PubMed] [Google Scholar]
- [20].Wu Z, Xu H, Xie W, et al. Study on a novel antibacterial light-cured resin composite containing Nano-MgO. Colloids Surf B Biointerfaces. 2020;188:110774. [DOI] [PubMed] [Google Scholar]
- [21].Lin Z, Yun L, Bin C, et al. Synthesis of antibacterial polyurethane film and its properties. Pol J Chem Technol. 2020;22(2):56–66. [Google Scholar]
- [22].Tominaga C, Shitomi K, Miyaji H, et al. Antibacterial photocurable acrylic resin coating using a conjugate between silver nanoclusters and alkyl quaternary ammonium. ACS Appl Nano Mater. 2018;1(9):4809–4818. [Google Scholar]
- [23].Zhao B, Jia R, Zhang Y, et al. Design and synthesis of antibacterial waterborne fluorinated polyurethane. J Appl Polym Sci. 2019;136(2):46923. [Google Scholar]
- [24].Zhang Y, Huang C, Chang J. Ca-doped mesoporous SiO2/dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. J Mater Chem B. 2018;6(3):477–486. [DOI] [PubMed] [Google Scholar]
- [25].Kucinska-Lipka J, Gubanska I, Lewandowska A, et al. Antibacterial polyurethanes, modified with cinnamaldehyde, as potential materials for fabrication of wound dressings. Polym Bull. 2019;76(6):2725–2742. [Google Scholar]
- [26].Villani M, Bertoglio F, Restivo E, et al. Polyurethane-based coatings with promising antibacterial properties. Mater. 2020;13(19):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guan Y, Zhang H, Zheng A, et al. Permanent antimicrobial silicone rubber based on bonding guanidine polymers. Polym Adv Technol. 2019;30(7):1555–1563. [Google Scholar]
- [28].Cheng L, Ren S, Lu X. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polym. 2020;12(2):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ahamad T, Nishat N. New Antimicrobial Epoxy-Resin-Bearing Schiff-Base Metal Complexes. J Appl Polym Sci. 2008;107(4):2280–2288. [Google Scholar]
- [30].Nishat N, Ahmad S, Ahamad T. Synthesis, Characterization, and Antimicrobial Studies of Newly Developed Metal-Chelated Epoxy Resins. J Appl Polym Sci. 2006;101(3):1347–1355. [Google Scholar]
- [31].Ahangaran F, Hayaty M, Navarchian AH, et al. Development of self-healing epoxy composites via incorporation of microencapsulated epoxy and mercaptan in poly(methyl methacrylate) shell, Polym. Test. 2018;101(73):395–403. [Google Scholar]
- [32].Qi Z, Tan Y, Gao L, et al. Effects of hyperbranched polyamide functionalized graphene oxide on curing behaviour and mechanical properties of epoxy composites, Polym. Test. 2018;71:145–155. [Google Scholar]
- [33].Qiao H, Liang Y, Xu G, et al. Preparation of a novel phosphorus-nitrogen containing novolac curing agent for epoxy resin and flame-retardancy of its cured epoxy resin. Des Monomers Polym. 2019;22(1):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu X, Kimura M, Sudo A, et al. Accelerating effects of N-Aryl-N′,N′-dialkyl ureas on epoxy-dicyandiamide curing system. J Polym Sci Part A Polym Chem. 2010;48(23):5298–5305. [Google Scholar]
- [35].Jamshidi H, Akbari R, Beheshty M. Toughening of dicyandiamide-cured DGEBA-based epoxy resins using flexible diamine. Iran Polym J (Engl Ed). 2015;24(5):399–410. [Google Scholar]
- [36].Mąka H, Spychaj T, Sikorski W. Deep eutectic ionic liquids as epoxy resin curing agents. Int J Polym Anal Charact. 2014;19(8):682–692. [Google Scholar]
- [37].Wu F, Zhou X, Reaction Mechanism YX, et al. Properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018;8(15):8248–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ge Z, Zhang W, Huang C, et al. Study on Epoxy Resin Toughened by Epoxidized Hydroxy-Terminated Polybutadiene. Mater. 2018;11(6):932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng L, Wang Y, Wang Y, et al. Study on reaction kinetics of epoxy resin cured by a modified dicyandiamide. J Appl Polym Sci. 2013;127(15):1895–1900. [Google Scholar]
- [40].Muirhead K, Earnshaw S, Easton C, et al. incorporation of guanidine and ethylguanidine into thermosetting resins. J Appl Polym Sci. 2012;125(3):372–377. [Google Scholar]
- [41].Zhang X, Liu F, Chen S, et al. Novel flame retardant thermosets from nitrogen-containing and phosphorus-containing epoxy resins cured with dicyandiamide. J Appl Polym Sci. 2007;106(4):2391–2397. [Google Scholar]


