Abstract
Background
To explore population aging and the epidemic trend of pulmonary tuberculosis (PTB) in the elderly, and provide a basis for the prevention and control of pulmonary tuberculosis among the elderly.
Methods
We collected clinical information of 239,707 newly active PTB patients in Shandong Province from 2005 to 2017. We analyzed and compared the clinical characteristics, reported incidence and temporal trend of PTB among the elderly group (≥60 years) and the non-elderly group (< 60 years) through logistic model and Join-point regression model.
Results
Among the total PTB cases, 77,192(32.2%) were elderly. Compared with non-elderly patients, newly active elderly PTB patients account for a greater proportion of male cases (OR 1.688, 95% CI 1.656–1.722), rural population cases (OR 3.411, 95% CI 3.320–3.505) and bacteriologically confirmed PTB cases (OR 1.213, 95%CI 1.193–1.234). The annual reported incidence of total, elderly, pulmonary bacteriologically confirmed cases were 35.21, 68.84, 35.63 (per 100,000), respectively. The annual reported incidence of PTB in the whole population, the elderly group and the non-elderly group has shown a slow downward trend since 2008. The joinpoint regression model showed that the overall reported incidence of PTB in the elderly significantly decreased from 2007 to 2017 (APC = -5.3, P < 0.05). The reported incidence of bacteriologically confirmed PTB among elderly patients declined rapidly from 2005 to 2014(2005–2010 APC = -7.2%, P < 0.05; 2010–2014 APC = -22.6%, P < 0.05; 2014–2017 APC = -9.0%, P = 0.1). The reported incidence of clinically diagnosed PTB among elderly patients from 2005 to 2017 (11.48–38.42/100,000) increased by about 235%. It rose significantly from 2007 to 2014 (APC = 9.4, P<0.05).
Conclusions
Compared with the non-elderly population, the reported incidence of PTB in the elderly population is higher. The main burden of PTB will shift to the elderly, men, rural population, and clinically diagnosed patients. With the intensification of aging, more researches on elderly PTB prevention and treatment will facilitate the realization of the global tuberculosis (TB) control targets.
Keywords: Aging, Pulmonary tuberculosis, Incidence, Join-point regression model
Background
The World Health Organization (WHO) estimated that 10 million people worldwide suffered from tuberculosis in 2018, of which 1.4 million died. Recent years, the global burden of PTB has been relatively stable. PTB accounted for 85% of all notified tuberculosis cases worldwide, and 88% of deaths from tuberculosis [1]. According to the data from the fifth national tuberculosis epidemiological sampling survey in China in 2010, there were still 4.99 million active PTB patients nationwide [2]. Although the number and incidence of TB in China had been declining in recent years, there were still 833,000 newly diagnosed tuberculosis cases in 2019, with an incidence rate of 58/100,000. Among them, PTB formed a sizeable majority (about 95%) [3]. Globally, the number of new cases of tuberculosis in China still ranked second in 2018, accounting for 9% of all new cases worldwide [1].
Elderly people are both susceptible to new TB infection, and at high risk for reactivation of latent TB. So the elderly population represents a large reservoir of TB infection [4]. Elderly PTB patients have a low positive rate of sputum smears, making diagnosis difficult and more likely to have delayed diagnosis. In addition, in the elderly, due to decreased immunity, more chronic comorbidities and more prone to treatment-related adverse drug reactions, the treatment effect is poor and the mortality rate is high [4–7]. Therefore, with the increase of the aging population, PTB is still one of the diseases that cannot be ignored. The prevention and control of PTB in the elderly need more attention.
China’s aging process is accelerating and it entered an aging society in 1999. The Report on the Development of China’s Elderly Career (2013) issued by the Chinese Academy of Social Sciences pointed out that elderly population in china has exceeded 200 million, and will increase by 1 million per year by 2025 [8]. Shandong Province is the most populous province in China. Shandong’s elderly population ranks first in the country by scale. In 2017, Shandong Province had a population of 23.173 million people aged 60 and above, accounting for 21.4% of the total population of the province. Moreover, the aging population of Shandong Province is experiencing a period of fast development [9], and the aging population of Shandong Province is significantly higher than that of the whole country.
This article describes and compares the reported incidence and trends of newly active PTB among elderly (≥60 years) and non-elderly (< 60 years) in seven cities in Shandong Province, China from 2005 to 2017.
Methods
Ethics statement
Ethical approvals of this study were obtained from the Ethics Committee of Shandong Provincial Hospital, affiliated with Shandong University (SPH) and the Ethic Committee of Shandong Provincial Chest Hospital (SPCH), China. Before data analysis and reporting, all personal identifiers of TB patients were removed.
Study population and data collection
In this study, 77,192 elderly and 162,515 non-elderly new PTB cases were collected from the PTB information management system of the Shandong Center for Disease Control and Prevention (CDC). PTB must be reported within 24 h and registered in the CDC system. Failure to report is a crime in China. Because the reporting and registration of PTB are mandatory in China within the law, CDC has a very lower missing error rate of data on PTB incidence, the data can largely reflect the actual incidence. This study investigated the reported PTB cases in 7 cities in Shandong Province (Dezhou, Jinan, Jining, Liaocheng, Linyi, Weifang and Yantai) from 2005 to 2017. It covered 54% of the population, 50% of health institutions and 51% of health stations in Shandong Province. This study collected data on demographics, clinical information, and disease incidence. The Shandong Statistical Yearbook provided population data every year.
Laboratory methods and laboratory quality control
All patients with presumptive PTB (cough or fever for more than 2 weeks, weight loss or dysplasia, history of tuberculosis exposure, abnormal chest radiographs) were required to submit at least 2 sputum specimens and use the Ziehl-Neelsen smear microscope to check for acid-fast bacilli (AFB) before starting treatment. Sputum specimens were collected through expectoration, gastric suction, induced sputum and bronchoscopy. For internal quality control, all positive smears were reconfirmed by another examiner in the same laboratory. For external quality assessment, 10% of the isolates were randomly selected from each laboratory and blindly inspected by the upper-level laboratory.
Data inclusion and definitions
The diagnostic criteria for bacteriologically confirmed PTB was at least 2 smear-positive sputum specimens, or 1 smear-positive sputum specimen plus chest radiograph abnormalities consistent with active PTB, or 1 smear-positive sputum specimens plus 1 culture-positive sputum specimen. The clinically diagnosis of PTB mainly depended on clinical symptoms (cough, fever, hemoptysis, etc.), abnormal chest radiographs, pathology, TST, anti-tuberculosis treatment effects, etc. Except for HIV co-infected patients (in China, HIV-positive people are immediately referred to an HIV specialist hospital), all PTB cases were included in this study. The new case criteria were patients who had never received tuberculosis treatment or had taken anti-tuberculosis drugs for < 1 month. Patients who were diagnosed as active after tuberculosis cure or treatment (whether it is a real relapse or a new episode of tuberculosis caused by reinfection) were relapsed cases.
Statistical analyses
Categorical variables including gender, race, class, geographic location, patient type (bacteriologically confirmed PTB or clinically diagnosed PTB) were summarized with proportions; continuous variables were summarized with average values. We calculated the annual reported incidence rate (100,000) by dividing the annual number of cases by the annual population size. We used the method to calculate the total reported incidence rate and calculate the reported incidence rate classified by gender, age group, patient type and geographic location. Through univariate analysis, odds ratios (ORs) and 95% confidence intervals (CIs) were obtained. Chi-square test was used to compare the specificity of elderly and non-elderly PTB patients in some aspects, and p < 0.05 was considered significant.
The join-point regression model was used to test the trend of reported incidence from 2005 to 2017. In the model, trends were described by annual percentage changes (APC). The estimation of APC was by fitting a simple linear logarithmic regression model. The Z test was used to evaluate whether APC was significantly different from 0. Non-significant (P ≥ 0.05) APC were described as stable, while significant (P < 0.05) positive or negative APC were described as increasing or decreasing.
The analysis was performed using SPSS software (version 20.0) and Joinpoint (version 4.8.0.1).
Results
Characteristics of patients
From 2005 to 2017, a total of 239,707 new active PTB cases were reported in seven selected cities in Shandong Province. The total number of elderly patients was 77,192 (32.2%), and the average age was 69.8 years. Among them, 59,284(76.80%) were male patients, Han nationality accounted for 99.87%, rural patients accounted for 91.06%, 39,958 cases (51.76%) were positive for sputum smears.
Compared with the non-elderly PTB, the proportion of men (OR 1.688, 95% CI 1.656–1.722), rural population (OR 3.411, 95% CI 3.320–3.505) and bacteriologically confirmed PTB (OR 1.213, 95%CI 1.193–1.234) in the elderly PTB cases was higher. (Table 1).
Table 1.
Sociodemographic and clinical characteristics of the elderly and non-elderly PTB patients, Shandong Province, China, 2005–2017
| Characteristics | Age ≥ 60 y, no. (%), n = 77,192 | Age < 60 y, no. (%), n = 162,515 | Total OR (95% CI), n = 239,707 | p value |
|---|---|---|---|---|
| Total | 77,192(32.20) | 162,515(67.80) | 239,707 | <0.001 |
| Sex | ||||
| Male | 59,284(76.80) | 107,624(66.22) | 1.688(1.656–1.722) | <0.001 |
| Female | 17,908(23.20) | 54,891(33.78) | ||
| Stratum | ||||
| Rural | 70,288(91.06) | 121,729(74.90) | 3.411(3.320–3.505) | <0.001 |
| Urban | 6904(8.94) | 40,786(25.10) | ||
| Ethnic group | ||||
| Han | 77,092(99.87) | 161,786(99.55) | 3.474(2.818–4.282) | <0.001 |
| Other | 100(0.13) | 729(0.45) | ||
| Patients type | ||||
| Bacteriologically confirmed PTB | 39,958(51.76) | 76,280(46.94) | 1.213(1.193–1.234) | <0.001 |
| Clinically diagnosed PTB | 37,234(48.24) | 86,235(53.06) | ||
| Geographical location | ||||
| Jinan | 6336(8.21) | 15,354(9.45) | ||
| Yantai | 7213(9.34) | 20,914(12.87) | ||
| Weifang | 8281(10.73) | 23,486(14.45) | ||
| Linyi | 23,078(29.90) | 40,330(24.82) | ||
| Dezhou | 8837(11.45) | 16,771(10.32) | ||
| Liaocheng | 12,352(16.0) | 22,639(13.93) | ||
| Jining | 11,095(14.37) | 23,021(14.17) | <0.001 | |
OR Odds ratio, PTB Pulmonary tuberculosis
Total and annul incidence rate
The overall reported incidence of the elderly was 68.84/100,000, and the reported incidence of PTB in the elderly was significantly higher than that of non-elderly people. From 2005 to 2017, the annual reported incidence of newly active PTB (78.79–51.31/100,000) in the elderly dropped by about 35%, and the reported incidence was the highest in 2007, at 88.75/100,000. The reported incidence of bacteriologically confirmed PTB in elderly patients (67.32–13.13/100,000) decreased by approximately 81%. (Table 2).
Table 2.
Incidence of pulmonary tuberculosis in Shandong, China, 2005–2017
| Incidence per 100,000 population | Changea(%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2005–2017 | |
| Total | 33.56 | 37.43 | 39.74 | 41.83 | 39.78 | 38.41 | 35.59 | 36.28 | 35.47 | 33.57 | 31.16 | 28.48 | 27.50 | −18.06% |
| Total(≥60) | 78.79 | 81.36 | 88.75 | 88.30 | 77.63 | 74.43 | 67.23 | 71.04 | 65.96 | 61.14 | 58.77 | 52.87 | 51.31 | −34.88% |
| Total(<60) | 26.72 | 30.26 | 31.43 | 33.98 | 33.11 | 31.90 | 29.54 | 29.31 | 28.97 | 27.43 | 24.66 | 22.50 | 21.64 | −19.01% |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Sex(≥60) | ||||||||||||||
| Male | 122.05 | 124.83 | 136.02 | 134.83 | 117.17 | 114.12 | 101.50 | 107.40 | 98.76 | 91.51 | 88.82 | 78.25 | 75.41 | −38.21% |
| Female | 34.32 | 36.76 | 40.29 | 40.66 | 37.06 | 33.71 | 31.94 | 33.35 | 32.00 | 29.62 | 27.61 | 26.69 | 26.68 | −22.26% |
| Sex(<60) | ||||||||||||||
| Male | 34.28 | 38.8 | 40.45 | 43.54 | 43.09 | 41.84 | 38.53 | 38.41 | 37.96 | 35.99 | 32.77 | 29.78 | 28.88 | −15.75% |
| Female | 18.94 | 21.5 | 22.16 | 24.17 | 22.85 | 21.67 | 20.28 | 19.9 | 19.68 | 18.55 | 16.24 | 14.95 | 14.14 | −25.34% |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Patients type(≥60) | ||||||||||||||
| Bacteriologically confirmed PTB | 67.32 | 65.67 | 63.5 | 58.84 | 51.44 | 47.88 | 35.73 | 28.42 | 24.75 | 16.83 | 15.43 | 14.57 | 13.13 | −80.5% |
| Clinically diagnosed PTB | 11.48 | 15.71 | 25.29 | 29.5 | 26.21 | 26.6 | 31.51 | 42.54 | 41.16 | 44.28 | 43.34 | 38.41 | 38.42 | 234.67% |
| Patients type(<60) | ||||||||||||||
| Bacteriologically confirmed PTB | 21.24 | 22.9 | 20.42 | 19.75 | 19.15 | 17.89 | 13.51 | 10.22 | 9.14 | 6.11 | 5.05 | 4.66 | 4.12 | −80.6% |
| Clinically diagnosed PTB | 5.48 | 7.36 | 11.01 | 14.23 | 13.95 | 14.01 | 16.03 | 19.09 | 19.83 | 21.32 | 19.61 | 17.85 | 17.52 | 219.71% |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
PTB Pulmonary tuberculosis
aThe % changes were calculated as follows: (incidence in 2017 – incidence in 2005)/incidence in 2005
Temporal trends and join-point regression model
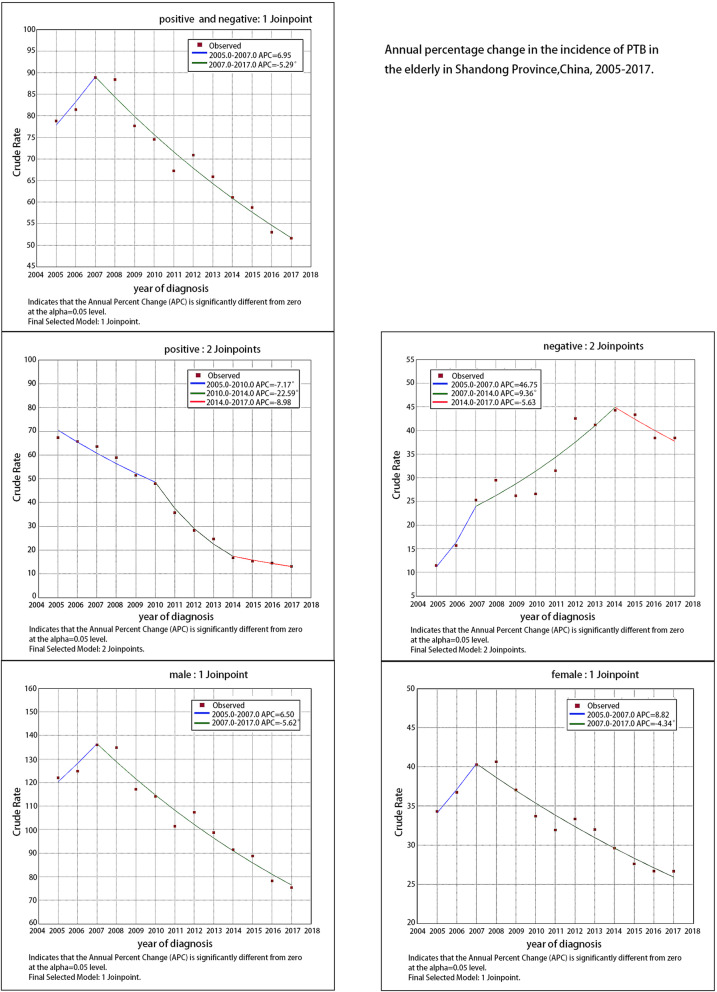
The join-point regression model showed that the overall reported incidence of PTB in the elderly remained at a stable level from 2005 to 2007, with a low annual increase rate (APC = 6.9, P = 0.3); it decreased significantly from 2007 to 2017 (APC = -5.3, P < 0.05). The reported incidence of bacteriologically confirmed PTB among elderly patients declined rapidly from 2005 to 2014. It decreased by 7.2% per year from 2005 to 2010 (P < 0.05), and it decreased by 22.6% from 2010 to 2014 (P < 0.05). It remained stable from 2014 to 2017 and the annual decline rate was low (APC = -9.0, P = 0.1).
The reported incidence of clinically diagnosed PTB among elderly patients from 2005 to 2017 (11.48–38.42/100,000) increased by about 235%. It remained stable from 2005 to 2007, with a low annual rate of increase (APC = 46.8, P = 0.2), it rose significantly from 2007 to 2014 (APC = 9.4, P<0.05), and remained stable from 2014 to 2017 (APC = -5.6, P = 0.4). (Table 2, Table 3, Fig. 1).
Table 3.
Annual percentage change in incidence of pulmonary tuberculosis in Shandong, China, 2005–2017
| Period | Trend | APC (95% CI) | P-value | |
|---|---|---|---|---|
| Total(≥60) | 2005–2007 | Stable | 6.9(−5.7, 21.3) | 0.3 |
| 2007–2017 | Decrease | −5.3(−6.2,-4.4) | <0.05 | |
| Sex | ||||
| Male | 2005–2007 | Stable | 6.5(−7.1,22.1) | 0.3 |
| 2007–2017 | Decrease | −5.6(−6.6,-4.6) | <0.05 | |
| Female | 2005–2007 | Stable | 8.8(−5.0,24.6) | 0.2 |
| 2007–2017 | Decrease | −4.3(− 5.3,-3.4) | <0.05 | |
| Patients type | ||||
| Bacteriologically confirmed PTB | 2005–2010 | Decrease | −7.2(−9.8,-4.4) | <0.05 |
| 2010–2014 | Decrease | −22.6(−28.7,-15.9) | <0.05 | |
| 2014–2017 | Stable | −9.0(−18.4,1.5) | 0.1 | |
| Clinically diagnosed PTB | 2005–2007 | Stable | 46.8(−30.2208.4) | 0.2 |
| 2007–2014 | Increase | 9.4(1.8,17.5) | <0.05 | |
| 2014–2017 | Stable | −5.6(−20.4,11.9) | 0.4 | |
| Total(<60) | 2005–2008 | Increase | 7.8(2.9,13.0) | <0.05 |
| 2008–2014 | Decrease | −3.7(−5.6,-1.7) | <0.05 | |
| 2014–2017 | Decrease | −8.0(−12.7,-3.1) | <0.05 | |
| Sex | ||||
| Male | 2005–2008 | Increase | 8.1(2.9,13.5) | <0.05 |
| 2008–2014 | Decrease | −3.4(−5.4,-1.3) | <0.05 | |
| 2014–2017 | Decrease | −7.7(−12.5,-2.6) | <0.05 | |
| Female | 2005–2008 | Increase | 7.3(2.5,12.3) | <0.05 |
| 2008–2014 | Decrease | −4.4(−6.3,-2.5) | <0.05 | |
| 2014–2017 | Decrease | −8.8(−13.5,-3.9) | <0.05 | |
| Patients type | ||||
| Bacteriologically confirmed PTB | 2005–2010 | Decrease | −4.9(−8.4,-1.3) | <0.05 |
| 2010–2017 | Decrease | −20.7(−23.6,-17.6) | <0.05 | |
| Clinically diagnosed PTB | 2005–2008 | Increase | 33.2(18.4,49.9) | <0.05 |
| 2008–2014 | Increase | 7.8(4.0,11.7) | <0.05 | |
| 2014–2017 | Stable | −6.6(−13.4,0.7) | 0.1 | |
APC Annual percent change, PTB Pulmonary tuberculosis
Fig. 1.
Annual percentage change in the reported incidence of PTB in the elderly in Shandong Province, China, 2005–2017. PTB = pulmonary tuberculosis
The trend of male and female reported incidence among elderly patients was consistent with the overall trend, which was stable from 2005 to 2007 and significantly decreased from 2007 to 2017 (P < 0.05). The proportion of male patients in elderly patients was significantly greater than that of female patients. (Tables 2 and 3).
TB cases by age group and aging trend
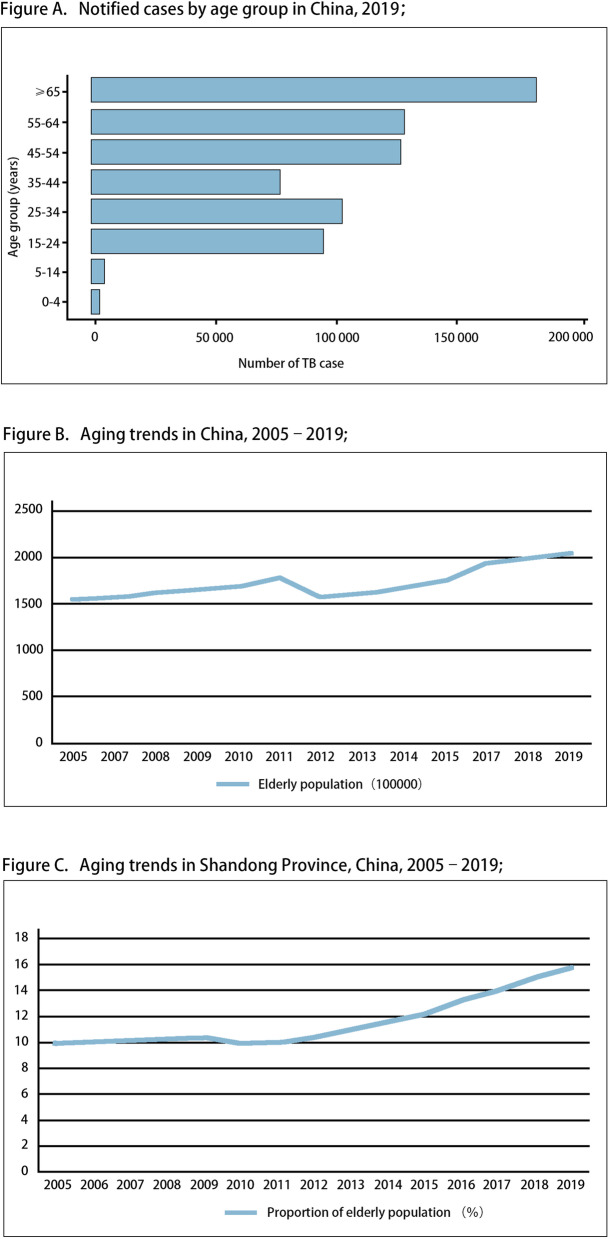
The Global Tuberculosis Report grouped the reported TB cases by age, 2019 [3]. (Fig. 2a).
Fig. 2.
a, Global estimates of TB incidence, 2019; b, Aging trends in China, 2005–2019; c, Aging trends in Shandong Province, China, 2005–2019; TB = tuberculosis
From 2005 to 2019, the aging population of China and China’s Shandong Province had shown an upward trend year by year [10]. (Fig. 2b, c).
Discussion
As the aging of population intensifies, investigating and understanding the prevalence of PTB in the elderly has the important meaning for more effective prevention and control of tuberculosis. This study analyzes the clinical and epidemiological characteristics of PTB in Shandong Province, China. The main findings are: 1) The reported incidence of newly active PTB in the elderly is significantly higher than that of non-elderly people; 2) compared with non-elderly patients, newly active elderly PTB patients account for a greater proportion of male cases, rural population cases and bacteriologically confirmed PTB cases; 3) The reported incidence of newly active PTB in the elderly and the reported incidence of bacteriologically confirmed PTB both decreased significantly, and the reported incidence of clinically diagnosed PTB in elderly cases increased significantly.
In this study, a total of 239,707 cases of newly active PTB were reported in seven cities in Shandong Province from 2005 to 2017. Its annual reported incidence rate was 35.21 per 100,000, which was lower than the global data in 2018 [1], and also lower than many other reported incidence levels in China [11–17]. The reported incidence is at a low level nationwide. This shows that the prevention and treatment of tuberculosis in China’s Shandong Province has achieved good effects.
In this study, the average annual reported incidence of newly active PTB in the elderly was much higher than that of the non-elderly. Similarly, tuberculosis were also more common among the elderly in many countries such as the United States, the United Kingdom, Japan, and other East and Southeast Asian countries [18–20]. The tuberculosis incidence rate in Africa peaked among population aged 25 to 44 [20]. The age distribution of tuberculosis incidence in Africa was different from our study. This was due to its high proportion of tuberculosis cases co-infected with HIV among young people in Africa [1, 18]. It was well-known that HIV-infected people had a 19 times higher risk of tuberculosis than normal people [1, 18]. Actually, most previous studies [18, 20] are consistent with our conclusions, which suggest that the increased incidence of newly active PTB due to aging is a common problem in TB control. After Mycobacterium tuberculosis infection, there are two cases of the onset: the onset of new infection and the activation of latent infection. Changes in immune function among elderly were considered to be an important risk factor for the increased susceptibility to tuberculosis and the reactivation of latent tuberculosis infection [18, 19, 21]. The potential mechanisms of impaired immune system function among aging population included various DNA damage, protein misfolding, and decreased cell function at the cellular and molecular level [22, 23]. Research indicated that the lungs became more inflammatory with age on level of individuals. These all increased the risk of tuberculosis infection in the elderly [21]. The risk of latent tuberculosis infection accumulated throughout life [24], so its incidence was higher among older people [25]. In addition, aging was also a major risk factor for some human diseases, such as cancer, diabetes, cardiovascular disease and neurodegenerative diseases [23], which increased the risk of PTB. In sum, population aging may lead to a high incidence of newly active PTB.
PTB was divided into bacteriologically confirmed and clinically diagnosed PTB [26]. TB patients plays an important role as the infection source. Bacteriologically confirmed PTB was more contagious, and might cause PTB outbreaks in some areas [27–31]. In this study, the proportion of bacteriologically confirmed cases in the elderly was higher than that of the non-elderly people (OR 1.213, P < 0.001). Some research showed that the incidence of tuberculosis in men was higher than that in women [27, 32]. Our study showed that compared with women, aging may have a greater impact on increasing the reported incidence of PTB in men (OR 1.688, P < 0.001). This phenomenon can be explained by the physiological differences between the sexes, social and cultural differences such as smoking, alcohol, drug abuse and other behavioral risk factors, as well as social network patterns that affect the source of infection [18]. Furthermore, this study showed that aging might increase the incidence of newly active PTB in rural population more than urban population (OR 3.411, P < 0.001). Poor economic conditions, urban-rural differences in life and production methods, and low awareness of tuberculosis might be the reasons for the high incidence of tuberculosis in rural areas [33–35]. The aging population may exacerbate the epidemic of PTB. Therefore, the above results suggest that elderly men in rural areas may be the priority of tuberculosis prevention and control work in future.
China’s strict regulations and measures for tuberculosis, including routine infectious disease reporting system, directly-observed treatment strategy (DOTS) [36, 37], and some non-tuberculous specific interventions such as improving living standards and improving the environment [38], were the main reasons for the decline of the overall reported incidence of PTB in Shandong. In this study, the overall reported incidence of PTB and the reported incidence of bacteriologically confirmed PTB decreased from 2007 (P < 0.05). Pharmacological methods alone were not enough to treat tuberculosis, and social determinants of health must also take into account. This could really improve the burden of tuberculosis [39]. Therefore, some measures taken by the Shandong Provincial Government around 2007 should explain why the trend in the reported incidence of PTB began to decline in 2007. These measures included the implementation of Shandong Province’s policy to completely abolish agricultural taxes, the implementation of Shandong’s rural residents’ minimum living security system, and further measures against environmental pollution, such as the province’s pollution source survey conducted in early 2008.
In this study, the reported incidence of clinically diagnosed PTB increased by 234.67%, which increased sharply from 2005 to 2014, similar to the results of other studies [37]. This suggests that the burden of PTB has gradually changed from bacteriologically confirmed cases to clinically diagnosed cases. The sensitivity of TB diagnosis is low depending only on symptoms, chest radiography and AFB sputum smear [40]. Therefore, to improve the diagnosis of clinically diagnosed PTB, many countries and organizations, including China, had carried out specific work, such as the development and use of TB antibody test, interferon-γ release assay, T cell detection, HRCT, bronchoscopy and other diagnostic methods [41–43]. This may be the main reason why the reported incidence of clinically diagnosed PTB increased. In addition, the role of ultrasound in the diagnosis of tuberculosis should also be concerned. Ultrasound was an effective diagnostic tool in detecting signs of extra-pulmonary tuberculosis. For example, ultrasound could be used to detect tuberculosis-related effusion, residual pleural thickening, mediastinal lymphadenopathy, and transthoracic biopsy guidance [44, 45]. Therefore, ultrasound can assist in the diagnosis of PTB with extra-pulmonary tuberculosis. Clinically diagnosed PTB could also cause the spread of tuberculosis [46], and was more difficult to diagnose [47, 48]. Therefore, as the burden of disease shifts, it is very important to diagnose and treat the patients with presumptive clinically diagnosed PTB as soon as possible.
The limitations of this study were as follows: First, studies had shown that tuberculosis recurrence or activation of latent infections would be a major factor of TB patients morbidity and mortality in the future [38]. Aging might increase the recurrence of tuberculosis [49]. However, our study only included newly cases of PTB. Therefore, further researches on recurrent PTB cases could be considered in near future. Second, since only one province in eastern China was examined, regional differences may limit the generalizability of the results.
Conclusion
Population aging was an important risk factor for the increased reported incidence of total newly active PTB. The main burden of PTB in Shandong were shifting to males, rural population, and clinically diagnosed cases. Therefore, elderly men in rural areas are probably going to be the main focus of tuberculosis prevention and control. The application and development of rapid and accurate diagnostic methods for clinically diagnosed PTB is vitally important for further TB control.
Acknowledgments
We thank Shandong Provincial Hospital, Shandong Provincial Chest Hospital, 13 municipal-level and 21 county-level local health departments for clinical, demographic data.
Abbreviations
- PTB
Pulmonary tuberculosis
- TB
Tuberculosis
- OR
Odds ratio
- CI
Confidence interval
- WHO
The World Health Organization
- CDC
Center for Disease Control and Prevention
- AFB
Acid-fast bacilli
- APC
Annual percentage changes
- DOTS
Directly-observed treatment strategy
Authors’ contributions
H.C.L., S.J.L. conceived and designed the study. H.C.L., Q.Q.A., and S.Q.L. directed its implementation including the data analysis and writing of the paper. S.J.L.W.M.S. and Y.F.L. analyzed the data; Q.Y.Z., J.Y.L and T.T.X. contributed materials/analytic tools; S.J.L., H.C.L. wrote and revised the manuscript. All authors reviewed and approved the manuscript.
Funding
This work was supported by Department of Science & Technology of Shandong Province (CN) (No.2007GG30002033; No.2017GSF218052) and Jinan Science and Technology Bureau (CN) (No.201704100). The funding body/bodies did not provide any assistance in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The raw data was managed by the Shandong Provincial Chest Hospital (SPCH), China. SPCH granted its permission to the study. The participants remained anonymous during data analysis, so only verbal informed consent was obtained from the study participants during the data collection phase. No individuals’ identity can be revealed upon publication. The study was approved by the Ethic Committee of Shandong Provincial Hospital, affiliated to Shandong University (SPH) and the Ethic Committee of SPCH, China.
Consent for publication
Not Applicable.
Competing interests
We authors declare that we don’t have any competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Global tuberculosis report 2019. 2019. [Google Scholar]
- 2.LX W, et al. The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberculosis. 2012;34(08):485–508. [Google Scholar]
- 3.World Health Organization . Global tuberculosis report 2020. 2020. [Google Scholar]
- 4.Di Gennaro F, et al. Active pulmonary tuberculosis in elderly patients: a 2016–2019 retrospective analysis from an Italian referral hospital. Antibiotics. 2020;9(8):489. doi: 10.3390/antibiotics9080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velayutham BRV, Nair D, Chandrasekaran V, Raman B, Sekar G, Watson B, Charles N, Malaisamy M, Thomas A, Swaminathan S. Profile and response to anti-tuberculosis treatment among elderly tuberculosis patients treated under the TB control programme in South India. PLoS One. 2014;9(3):e88045. doi: 10.1371/journal.pone.0088045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan R, Kumar K, Ganesh M, Kumar AMV, Krishnan N, Swaminathan S, Edginton M, K A, Gupta D. The profile and treatment outcomes of the older (aged 60 years and above) tuberculosis patients in Tamilnadu, South India. PloS One. 2013;8(7):e67288. doi: 10.1371/journal.pone.0067288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults – time to take notice. Int J Infect Dis. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Ma GC, Hao CQ, Shang GX. The development and enlightenment of American geriatrics and medical model. Soft Science of Health. 2014;28(07):486-8.
- 9.Liu P, Zhou H. Current situation and countermeasures of population aging in Shandong Province. Journal of Shandong Institute of Business and Technology. 2013;27(04):89-94.
- 10.National Bureau of Statistics, China Population and Employment Statistical Yearbook[Internet]. 2019. [cited 16 February 2021]. Available from: https://www.yearbookchina.com/navibooklist-n3020021405-1.html.
- 11.Ming H, et al. Analysis on Preventive Measures of TB in Different Profession and Age in Hunan Province. J Gannan Med Univ. 2019;39(09):914–9.
- 12.Yang QR, Xu L, Zhou QB. Analysis of incidence trend of different age groups of patients with pulmonary tuberculosis in Yunnan province from 2005 to 2015. Soft Sci Health. 2017;31(01):60–4.
- 13.Gao HQ, Niu WK, Chen QF. Epidemiology of pulmonary tuberculosis in people aged ≥60 years in Shaoxing, Zhejiang. Dis Surveillance. 2015;30(01):46–9.
- 14.Liang S, et al. Epidemiological characteristics of pulmonary tuberculosis among elderly population in Liaoning, 2013-2017. China Tropical Medicine. 2019;19(06):552–5.
- 15.Lei RR, et al. Epidemic characteristics of pulmonary tuberculosis in the elderly people aged 65 above in Chongqing, 2009-2018. China Trop Med. 2019;19(07):654–8.
- 16.Pang XW, Li XR, Li JX. Epidemiological features of pulmonary tuberculosis in elderly of Tianjin from 2009 to 2017. Chin J Prev Contr Chron Dis. 2018;26(09):656–9.
- 17.Zhang BB, Li HH. Analysis of epidemic situation of senile pulmonary tuberculosis in Heilongjiang Province from 2011 to 2017. J Tuberc Lung Health. 2018;7(04):293–7.
- 18.Yew WW, Yoshiyama T, Leung CC, Chan DP. Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology. 2018;23(6):567–575. doi: 10.1111/resp.13303. [DOI] [PubMed] [Google Scholar]
- 19.Manabe T, Takasaki J, Kudo K. Seasonality of newly notified pulmonary tuberculosis in Japan, 2007–2015. BMC Infect Dis. 2019;19(1):497. [DOI] [PMC free article] [PubMed]
- 20.Mori T, Leung CC. Tuberculosis in the global aging population. Infect Dis Clin N Am. 2010;24(3):751–768. doi: 10.1016/j.idc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Piergallini TJ, Turner J. Tuberculosis in the elderly: why inflammation matters. Exp Gerontol. 2018;105:32–39. doi: 10.1016/j.exger.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku C, Dodd PJ. Forecasting the impact of population ageing on tuberculosis incidence. PLoS One. 2019;14(9):e0222937. doi: 10.1371/journal.pone.0222937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L, et al. Space-time clustering and associated risk factors of pulmonary tuberculosis in southwest China. Infect Dis Poverty. 2018;7(1):91. [DOI] [PMC free article] [PubMed]
- 27.Mao Q, Zeng C, Zheng D, Yang Y. Analysis on spatial-temporal distribution characteristics of smear positive pulmonary tuberculosis in China, 2004–2015. Int J Infect Dis. 2019;80:S36–S44. doi: 10.1016/j.ijid.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Sanchez M, Brugueras S, de Andrés A, Simon P, Gorrindo P, Ros M, Masdeu E, Millet JP, Caylà JA, Orcau À, the Contact Tracing Group of the Tuberculosis Investigation Unit of Barcelona Tuberculosis incidence among infected contacts detected through contact tracing of smear-positive patients. PLoS One. 2019;14(4):e0215322. doi: 10.1371/journal.pone.0215322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2012;41(1):140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semunigus T, et al. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in Dessie and Debre Birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob. 2016;15(1):50. [DOI] [PMC free article] [PubMed]
- 31.Rutherford ME, Hill PC, Maharani W, Apriani L, Sampurno H, van Crevel R, Ruslami R. Risk factors for mycobacterium tuberculosis infection in Indonesian children living with a sputum smear-positive case. Int J Tuberc Lung Dis. 2012;16(12):1594–1599. doi: 10.5588/ijtld.12.0389. [DOI] [PubMed] [Google Scholar]
- 32.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the end TB era. Lancet Respir Med. 2018;6(4):299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 33.Dye C, Lönnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87(9):683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GUO C, du Y, SHEN SQ, LAO XQ, QIAN J, OU CQ. Spatiotemporal analysis of tuberculosis incidence and its associated factors in mainland China. Epidemiol Infect. 2017;145(12):2510–2519. doi: 10.1017/S0950268817001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen XB, et al. Comparison of differences in the incidence and molecular epidemiological characteristics of tuberculosis between urban and rural areas in northern Guizhou. Pract Prev Med. 2019;26(03):321–3.
- 36.Wang LP, Liu JM, Chin DPD. Progress in tuberculosis control and the evolving public-health system in China. Lancet. 2007;369(9562):691–696. doi: 10.1016/S0140-6736(07)60316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, Chen M, Zhao Y, Jiang S, du X, He G, Li J, Wang S, Chen W, Xu C, Huang F, Liu X, Wang Y. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 38.Huynh GH, et al. Tuberculosis control strategies to reach the 2035 global targets in China: the role of changing demographics and reactivation disease. BMC Med. 2015;13(1):88. [DOI] [PMC free article] [PubMed]
- 39.Pizzol D, Veronese N, Marotta C, di Gennaro F, Moiane J, Chhaganlal K, Monno L, Putoto G, Mazzucco W, Saracino A. Predictors of therapy failure in newly diagnosed pulmonary tuberculosis cases in Beira, Mozambique. BMC Res Notes. 2018;11(1):99. doi: 10.1186/s13104-018-3209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, Kimerling ME, Chheng P, Thai S, Sar B, Phanuphak P, Teeratakulpisarn N, Phanuphak N, Dung NH, Quy HT, Thai LH, Varma JK. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 41.Li XX, Jiang SW, Zhang H, Jing KH, Wang L, Li WB, Liu XQ, Yao HY, Wang LX. Clinical and radiographic predictors in diagnosing sputum smear-negative pulmonary tuberculosis in HIV-negative patients: a cross-sectional study in China. Chin Med J. 2013;126(19):3662–3667. [PubMed] [Google Scholar]
- 42.Nakanishi M, Demura Y, Ameshima S, Kosaka N, Chiba Y, Nishikawa S, Itoh H, Ishizaki T. Utility of high-resolution computed tomography for predicting risk of sputum smear-negative pulmonary tuberculosis. Eur J Radiol. 2010;73(3):545–550. doi: 10.1016/j.ejrad.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad M, Ibrahim WH, Sarafandi SA, Shahzada KS, Ahmed S, Haq IU, Raza T, Hameed MA, Thomas M, Swehli HAI, Sattar HA. Diagnostic value of bronchoalveolar lavage in the subset of patients with negative sputum/smear and mycobacterial culture and a suspicion of pulmonary tuberculosis. Int J Infect Dis. 2019;82:96–101. doi: 10.1016/j.ijid.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Bobbio F, di Gennaro F, Marotta C, Kok J, Akec G, Norbis L, Monno L, Saracino A, Mazzucco W, Lunardi M. Focused ultrasound to diagnose HIV-associated tuberculosis (FASH) in the extremely resource-limited setting of South Sudan: a cross-sectional study. BMJ Open. 2019;9(4):e027179. doi: 10.1136/bmjopen-2018-027179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Gennaro F, et al. Potential diagnostic properties of chest ultrasound in thoracic tuberculosis—a systematic review. Int J Environ Res Public Health. 2018;15(10):2235. doi: 10.3390/ijerph15102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behr MA, Warren SA, Salamon H, Hopewell PC, de Leon AP, Daley CL, Small PM. Transmission of mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353(9151):444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 47.Tiamiyu AB, et al. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2020. Evaluation of GeneXpert MTB/RIF as a diagnostic tool in patients with sputum smear-negative TB in a high HIV burden region in Nigeria. [DOI] [PubMed] [Google Scholar]
- 48.Getahun H, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet (British) 2007;369(9578):2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 49.Guzzetta G, Kirschner D. The roles of immune memory and aging in protective immunity and endogenous reactivation of tuberculosis. PLoS One. 2013;8(4):e60425. doi: 10.1371/journal.pone.0060425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.