Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic and complex respiratory disorder associated with airflow limitation and increased inflammatory response of the lungs to harmful particles. The purpose of this original study was to describe the results and profile of the Shahrekord Prospective Epidemiological Research Studies in IrAN (PERSIAN) regarding COPD in southwestern Iran.
Methods
This study of asthma and respiratory diseases is a subcohort of the more extensive cohort study, i.e., Shahrekord PERSIAN cohort, a population-based prospective study on people aged 35–70 years in southwestern Iran (n = 10,075). The sample size of the subcohort was 8500 people. Annual follow-ups (person-year) of the cohort were designed to be conducted up to 2036. The instruments to collect data on various exposures were derived from the questionnaires previously developed in extensive multinational studies (occupational exposures, smoking, housing status, and fuel consumption, history of respiratory and chronic diseases, comorbidity, etc.). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the lower limit of normal (LLN) spirometric criteria were used to confirm COPD diagnosis.
Results
The response rate was 93.85%. The mean age of the participants was 49.48 ± 9.32; 47.9% were male, and 52.9% were female; nearly 16% of the population was current smokers; the fuel used by most of the participants for heating the house and cooking was gas. The most common comorbidity among participants was dyslipidemia; 30% of people have three or more comorbidities. According to GOLD and LLN criteria, the Prevalence of COPD was 3.6% and 8.4%, respectively. 4.3% of the participants had a history of chronic lung disease. The group of subjects with COPD had higher mean age, fewer years of schooling, a higher percentage of smokers with a smoking history of 10 or more pack years. 4.6% of patients had a history of chronic lung disease, 17.6% had a history of asthma in childhood, and 5.2% had a family history of respiratory and pulmonary diseases.
Conclusion
Epidemiological research is necessary to create an appropriate framework to fight COPD. This framework requires a better description of men and women at risk of developing COPD and describing people with early-stage illnesses.
Keywords: Chronic obstructive, Pulmonary disease, Longitudinal, PERSIAN Cohort study, Iran
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex respiratory disorder that is caused by airflow limitation and increased inflammatory response of the lungs to harmful particles and gases, which is usually progressive and irreversible [1]. According to the World Health Organization, COPD is not a single disease but is a so-called umbrella disease that covers a wide range of pulmonary diseases, including emphysema and bronchitis [2]. It affects 6–10% of the world's population [3] and is one of the most important causes of mortality and disability across the globe [4]. The burden of COPD has risen over time [5], and the current costs associated with this disease are remarkable and will increase in the future [6, 7].
In a recently published meta-analysis study, the pooled Prevalence of COPD was 15.70%. Among all WHO regions, the highest prevalence was in the Americas, and the lowest was in the Southeast Asia/West Pacific region [8]. In Iran, the prevalence of COPD in estimating the burden of obstructive pulmonary disease was 8.3% [9]. The Prevalence of COPD in Tehran, the capital of Iran, was 9.2% [10] and in Isfahan, )neighboring Chaharmahal and Bakhtiari Province( was 5.7% [11]. In another study in Iran from 5 different geographical areas in Iran (north, south, east and center), the overall prevalence of COPD was 4.9%, which is the highest province of Kerman (13.9%) and then Tehran 4.4%, Ahvaz was 3.8%, Mazandaran was 3.7%, and Mashhad was 2.8%, respectively [12].
Despite the significant impact of COPD on health and the economy, this chronic disease has not yet drawn enough attention from the public healthcare institutes and is not known among the general population. One possible reason for this significant problem is the lack of epidemiological data on the prevalence and risk factors of COPD in developing countries, especially in Iran. According to previous studies, the diagnosis of COPD is underreported in Iran [10].
Evidence and epidemiological data on the status and progression of COPD in Iran are minimal and contradictory; the methods for examining this disease in various studies are different, and there is a paucity of evidence about the natural history of the disease. On the other hand, inconsistencies in the information on the Prevalence of COPD, chronic bronchitis and asthma in the Iranian population may affect the decision made by health care system officials, policy-makers and insurance organizations. They may prevent them from taking adequate preventive and treatment measures to prevent potential severe effects and stupendous costs [13].
Therefore, this longitudinal study was conducted to investigate the need for longitudinal observational studies on COPD in Chaharmahal and Bakhtiari province, southwestern Iran. The province is geographically located at approximately 2,153 m above sea level and is known as the roof of Iran. The effect of altitude on other medical conditions, such as pulmonary hypertension and heart failure, has previously been reported, but the potential mechanisms proposed for the effect of altitude on the Prevalence of COPD are highly contradictory and controversial. In the PREPOCOL-PLATINO-BOLD-EPI-SCAN study, authors claimed that " known risk factors were less frequent at high altitude and high altitude had no significant influence in COPD prevalence" [14]. In contrast, the PREPOCOL study results in five Colombian cities and four geographically diverse in Peru showed that the Prevalence of COPD increased with increasing altitude [15, 16].
In Iran, especially Chaharmahal and Bakhtiari province, no cohort study has yet been conducted to investigate the problems of lack of diagnosis of COPD and the need for intervention. Therefore, this work is a futuristic cohort study with a 20-year follow-up period in Chaharmahal and Bakhtiari, southwestern Iran, making it possible to do cross-sectional and longitudinal data analysis.
Materials and methods
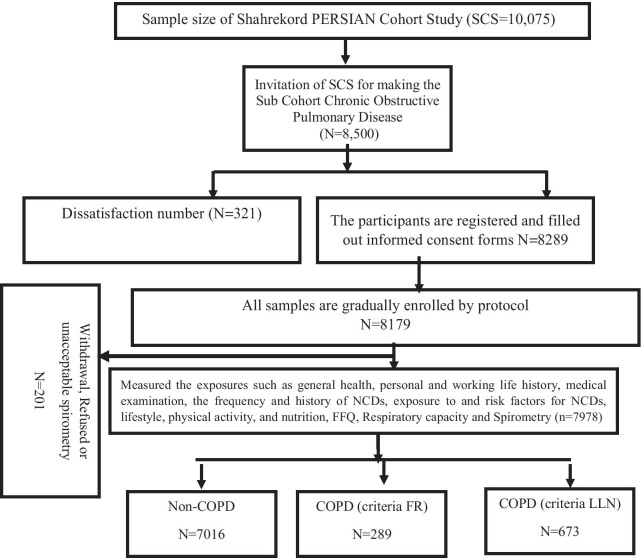
The study of asthma and respiratory diseases is a subcohort of a more extensive cohort study, i.e., Shahrekord Cohort Study (SCS), a population-based prospective study on people aged 35–70 years in southwestern Iran. SCS was designed to serve as one of the centers of the Prospective Epidemiological Research Studies in IrAN (PERSIAN) Cohort (n = 10,075) and is being conducted in the southwest of Iran [17]. The sample size of the subcohort was 8500 people. This study began in November 2015 in Shahrekord and has been scheduled to continue until 2036, with a total follow-up of 200,000 person-years each year (Fig. 1). Details of the protocol and the objectives of the Shahrekord PERSIAN cohort have already been published [18].
Fig. 1.
Summarizing Person's Recruitment and Assessment for Iranian Sub Cohort Chronic Obstructive Pulmonary Disease in Southwest of Iran
Outcome definition
The primary outcome of this study is COPD, and mortality from COPD is an airway inflammatory disease. That is associated with continuous airflow limitation, which is usually progressive and irreversible. The most common symptoms of COPD are coughing, phlegm production and difficulty breathing, which should be considered for the clinical diagnosis of the disease [19]. However, none of the symptoms is sufficient to make a diagnosis, and if there are several additional symptoms and tests to diagnose the disease, the likelihood of a diagnosis of COPD increases. According to ICD-10, COPD includes emphysema and chronic bronchitis. Chronic coughing is usually the first symptom that occurs. Chronic bronchitis is defined as a condition of cough and sputum for at least three months for two consecutive years during the past year. Diagnosis of emphysema is only possible by describing the changes in the anatomy of the lung tissue and cannot be considered a disease per se [1, 20]. The most common and easiest way to confirm the diagnosis of COPD is spirometry. Most studies have only used a questionnaire to diagnose COPD, but in this study, both instruments were used for the diagnosis of COPD (Table1). Data collection methods and exposure variables in a subcohort of Shahrekord PERSIAN Cohort were reported in Table 1.
Table 1.
Data collection methods, exposure variables and tools in a subcohort of Shahrekord PERSIAN cohort
| Data and collection methods | Exposure variables |
|---|---|
| Questionnaires (self-reported) |
History and current occupational exposures, personal history and habits (smoking, alcohol, tobacco smoking and drinking), as well as sedentary time, the age at which smoking began and the stages of change of readiness to quit smoking in current smokers, fuel status for home heating and cooking, Housing status, history of contact with animals, exposure to agricultural toxins, pesticides and detergents Medical history including pulmonary diseases, the history of asthma in childhood, respiratory symptoms, respiratory infections, chronic illnesses, drug use, family history of respiratory and pulmonary diseases, and questions about whether Have you ever had a doctor or other health care professionals diagnose one of the following conditions: Chronic bronchitis, emphysema, pulmonary fibrosis and sleep apnea Comorbidities: Cardiovascular disease (Myocardial Infarction, Cardiac Ischemia, Heart Failure, stroke), Hypertension, Type 2 diabetes, Metabolic syndrome, Dyslipidemia, Anxiety, Depression, Renal failure, Fatty liver, Musculoskeletal disorders, Pulmonary blood pressure, Gastroesophageal reflux disease (GERD), Pulmonary cancer, Pulmonary fibrosis |
| Clinical examination | Blood pressure, Heart rate, Electrocardiogram(EKG), Breathlessness on exertion |
| Anthropometry indexes | Height, Weight, Waist/Hip/Wrist Circumference, Body Components (fat, water, muscle), Bioimpedance |
| Blood samples | Fasting Blood Sugar, Serum Triglyceride, Total cholesterol, Low and High-Density Lipoprotein, Cholesterol |
| Spirometry | Pre- and Post-bronchodilator for diagnosis of COPD |
| COPD assessment test (CAT) | Only COPD and at-risk |
| Routine data and BioBank | Link all variables to Shahrekord PERSIAN cohort Data Base. A biobank that has been designed to store blood, nail, hair and urine samples for future research studies. |
COPD, chronic obstructive pulmonary disease
A pulmonary function test was conducted by using a spirometer (New Spirolab, MIR, Italy, 2015) according to the criteria of the American Thoracic Society/European Respiratory Society (ATS/ERS). All tests were conducted in a quiet room in a sitting position on a comfortable chair. The spirometer was calibrated using a syringe by trained technicians daily before the study began. All participants were informed about all stages in the investigation and the pulmonary function test. All steps of the spirometry maneuver were performed practically by technicians so that the participant could see how to do proper inhaling and exhaling. The person was instructed to take a deep, full breath and then exhale forcefully. In-depth and complete inhalation is no less important than strong and complete exhalation. Inadequate and incomplete inhalation will lead to an insufficient volume of exhalation, resulting in a false decrease in forced vital capacity values and an increase in the likelihood of a restrictive pattern. Pulmonary function tests were conducted in triplicate for each individual with a single and acceptable method. By comparing the curves of the three pulmonary function tests, the maximal values of FEV1 (forced expiratory volume in one second) and FEV6 (forced expiratory volume in 6 second) forced vital capacity (FVC), maximum peak expiratory flow (PEF) in 25%, 50% and 75% of FVC (PEF25-75), Maximum Ventilatory Volume (MVV) were obtained. Spirometry data were interpreted according to the ATS/ERS recommendations by two respiratory medicine specialists. The pulmonary function test parameters values were presented as the percent of predicted values for the respective age, height, and weight [21].
The GOLD criteria (The Global Initiative for Chronic Obstructive Lung disease) uses a fixed ratio of forced expiratory volume in 1 second (FEV1) over forced vital capacity (FVC) < 0.7 for the diagnosis of COPD [22]. The post-bronchodilator spirometry was conducted for patients with a pre-bronchodilator FEV1/FVC ratio < 80% and 15 min after administration of 2 puffs (200 µg) of salbutamol via a spacer standard to evaluate the reversibility of the obstruction. We used a pre-bronchodilator FEV1/FVC ratio < 0·8 or an FVC < 80% as cutoffs for whether or not to do post-bronchodilator spirometry, to avoid underestimating FVC, which could result in a normal FEV1/FVC ratio. COPD was defined as the presence of post-bronchodilator FEV1/FVC of less than 70%. Although using this fixed ratio is easy and common, but the value varies greatly with age and decreases with age, thus leading to underestimation in adults under 45 years and overestimation in older people [23, 24]. For these reasons, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) recommends setting the cut-off to 5% of normal to avoid potential Misclassification [25, 26]. Therefore, in this study, Spirometry data were expressed in predicted percentage according to the lower limit of normal (LLN), FEV1/FVC ratio < LLN, and also according to the GOLD criteria with a constant ratio FEV1/FVC < 0.70 and FEV1 < 80%. In addition, COPD severity was determined for all participants according to the GOLD criteria as follows: Stage 0 (at risk); stage 1 (mild): FEV1/FVC < 70% and FEV1 ≥ 80%; stage 2 (moderate): FEV1/FVC < 70% and 50% ≤ FEV1 < 80%; stage 3 (extreme): FEV1/FVC < 70% and 30% ≤ FEV1 < 50%; and stage 4 (extremely severe): FEV1/FVC < 70% and FEV1 < 30% [1, 27].
Contraindications for the use of spirometry drugs included cardiac infarction, pulmonary embolism, diagnosed aneurysm, uncontrolled blood pressure over 140 mmHg, previous surgery on the eyes, ears, brain, abdomen and chest, liver, heart or kidney failure, cancer, and endocrine disorders).
Definition of exposures
A questionnaire was used to collect information about various exposures. After obtaining informed consent, complete information about various exposures was collected by experienced interviewers through face-to-face interviews. The main Questionnaire used in this study was derived from valid questionnaires that had been used in multinational studies. The main exposures of COPD disease are specifically studied. The Questionnaire also addresses the history and current occupational exposures, individual history and habits (smoking, alcohol, tobacco smoking and drinking), as well as sedentary time, the age at which smoking began and the stages of change of readiness to quit smoking in current smokers, fuel status for home heating and cooking, housing situation, history of contact with animals, exposure to agricultural toxins, pesticides and detergents.
All participants were asked questions about medical history, including pulmonary diseases, the history of asthma in childhood, respiratory symptoms, respiratory infections, chronic illnesses, drug use, family history of respiratory and pulmonary diseases, and questions about whether Have you ever had a doctor or other health care professionals diagnose one of the following conditions: Chronic bronchitis, emphysema, pulmonary fibrosis and sleep apnea.
Comorbidities about which the subjects were asked questions due to their clinical significance in COPD, included cardiovascular disease (myocardial infarction, cardiac ischemia, and stroke), hypertension, type 2 diabetes, syndrome metabolic, dyslipidemia, anxiety, depression, Osteoporosis, fatty liver, Rheumatoid Arthritis, pulmonary fibrosis [21].
In terms of smoking, the participants were divided into three groups as follows: Non-smokers, i,e., the people who never or occasionally smoked (those who have not yet smoked or have smoked less than 100 cigarettes during their lifetime); current smokers, i.e., the people who smoke one or more cigarettes a day; and ex-smokers, i.e., the people who are not currently smokers but smoked regularly in the past). Current exposure to cigarette smoke or passive smoking was also considered to be smoking-positive given the smoking of other family members or colleagues and exposure to parental cigarette smoke in childhood. Other additional variables such as anthropometric measurements and laboratory variables, which have already been published in the SCS protocol, were also collected [18]. The list of exposures and variables of this cohort were reported in Table 1.
Generalizability of cohort (external cohort credibility)
Subcohort COPD was designed for a sample of approximately 8500 people out of 10,075 individuals aged 35–70 years for 20 years in Chaharmahal and Bakhtiari province, southwest of Iran. It seems that since this cohort contains a balanced ratio of men and women and urban and rural populations, it is likely to represent the community.
Results
The response rate was 93.85%. Based on the results, 7978 people participated in the subcohort COPD, 6388 (80.1%) were urban, and 1590 (19.9%) were rural. The mean age of the population enrolled in the study was 49.48 (the standard deviation (SD), 9.32) years. 47.9% were male and 52.9% were female; 94.3% were married; 48.6% were of the Ethnicity Fars, and 39.1% were of the Ethnicity Lur Bakhtiari; 14.3% of the participants were illiterate, and 34.7% of participants were in the 35‐44 age category. 51.6% of the participants were housewives/unemployed/retired, and 18.2% were employees (Table 2).
Table 2.
Baseline characteristics of the subcohort, by area residence urban/rural
| Variables | Total N = 7978 |
Urban N = 6388 |
Rural N = 1590 |
|---|---|---|---|
| Age—mean ± SD (years) | 49.48 ± 9.32 | 49.04 ± 9.20 | 51.25 ± 9.58 |
| 35–44 years | 2772 (34.7%) | 2326 (36.4%) | 446 (28.1%) |
| 45–54 years | 2708 (33.9%) | 2185 (34.2%) | 523 (32.9%) |
| 55–64 years | 1914 (24%) | 1460 (22.9%) | 454 (28.6%) |
| ≥ 65 | 584 (7.3%) | 416 (6.5%) | 167 (10.5%) |
| Sex | |||
| Men | 3824 (47.9%) | 3198 (50.1%) | 625 (39.3%) |
| Female | 4154 (52.1%) | 3189 (49.9%) | 965 (60.7%) |
| Ethnicity | |||
| Fars | 3874 (48.6%) | 3721 (58.3%) | 152 (9.6%) |
| Lur Bakhtiari | 3122 (39.1%) | 1775 (27.8%) | 1347 (84.7%) |
| Turk Qashqai | 649 (8.1%) | 619 (9.7%) | 30 (1.9%) |
| Other | 333 (4.2%) | 272 (4.3%) | 61 (3.8%) |
| Number of family members—mean ± SD | 4.00 ± 1.29 | 3.89 ± 1.14 | 4.45 ± 1.69 |
| Number of family members—n (%) | |||
| 1 | 84 (1.1%) | 59 (0.9%) | 25 (1.6%) |
| 2 | 782 (10%) | 617 (9.9%) | 165 (10.5%) |
| 3 | 1745 (22.3%) | 1477 (23.6%) | 267 (17%) |
| 4 or more | 5205 (66.6%) | 4096 (65.5%) | 1109 (70.8%) |
| Marital status | |||
| Single | 137 (1.7%) | 112 (1.8%) | 25 (1.6%) |
| Married | 7520 (94.3%) | 6074 (95.1%) | 1445 (90.9%) |
| Widow and divorced | 321 (4%) | 201 (3.1%) | 120 (7.5%) |
| Education (N years)—mean ± SD | 8.95 ± 6.01 | 10.16 ± 5.69 | 4.13 ± 2.08 |
| Illiterate | 1142 (14.3%) | 593 (9.3%) | 549 (34.5%) |
| ≤ 5 years | 1689 (21.2%) | 1120 (17.5%) | 569 (35.8%) |
| 6–8 years | 891 (11.2%) | 702 (11%) | 189 (11.9%) |
| 9–12 years | 2059 (25.8%) | 1855 (29%) | 203 (12.8%) |
| > 12 years | 2197 (27.5%) | 2117 (33.1%) | 80 (5%) |
| Occupational status | |||
| Employee | 1450 (18.2) | 1389 (21.7%) | 60 (3.8%) |
| Farmer/rancher/herder | 293 (3.7) | 122 (1.9%) | 171 (10.8%) |
| Carpet weaver/tailor/weaver | 311 (3.9) | 239 (3.7%) | 72 (4.5%) |
| Heavy car driver/mechanic and oil change worker | 507 (6.4%) | 448 (7%) | 59 (3.7%) |
| Building contractor/worker and builder | 470 (5.9%) | 311 (4.9%) | 159 (10%) |
| Housewife/unemployed/retired | 4120 (51.6%) | 3142 (49.2%) | 978 (61.5%) |
| Others | 827 (10.4%) | 736 (11.5%) | 91 (5.7%) |
The mean body mass index of the participants was 27.70% (SD, 4.58); 44.9% of the population had overweight (25–30) and 27.4% had obesity (> 30). Nearly 16% of the population was current smokers, with a higher proportion of men than women (33.3% VS 0.4%). The fuel used by most of the participants for heating the house and cooking was gas (65.2%) and also, the type of kitchen or cooking area for most participants was an open kitchen inside the house (74.9%). The most common comorbidity among participants was dyslipidemia (71.9%) and later hypertension (27.1%). 31.7% of participants had at least one comorbid and 30% of people have three or more comorbidities (Table 3).
Table 3.
Baseline behavioral and clinical characteristics of the subcohort, by gender and area residence
| Characteristics | Total N = 7978 |
Men N = 3824 |
Women N = 4154 |
Urban N = 6388 |
Rural N = 1590 |
|---|---|---|---|---|---|
| Weight status | 74.16 ± 13.27 | 78.15 ± 13.26 | 70.52 ± 12.20 | 75.49 ± 12.95 | 68.89 ± 13.27 |
| Body mass index (BMI)—mean (SD) | 27.70 ± 4.58 | 26.62 ± 4.02 | 28.68 ± 4.83 | 27.87 ± 4.47 | 27.00 ± 4.93 |
| Underweight (BMI < 20)—n (%) | 259 (3.3%) | 165 (4.4%) | 94 (2.3%) | 163 (2.6%) | 96 (6%) |
| Healthy (20–25)—n (%) | 1934 (24.4%) | 1100 (29.1%) | 834 (20.2%) | 1447 (22.9%) | 487 (30.7%) |
| Overweight (25–30)—n (%) | 3550 (44.9%) | 1813 (48%) | 1737 (42%) | 2948 (46.6%) | 601 (37.9%) |
| Obesity (> 30)—n (%) | 2171 (27.4%) | 699 (18.5%) | 1472 (35.6%) | 1768 (27.9%) | 403 (25.4%) |
| Smoking status | |||||
| Current smoker n (%) yes | 1288 (16.1%) | 1272 (33.3%) | 16 (0.4%) | 1078 (16.9%) | 210 (13.2%) |
| Former smoker n (%) yes | 612 (7.7%) | 596 (15.6%) | 16 (0.4%) | 511 (8%) | 101 (6.4%) |
| Never smoker n (%) yes | 6078 (76.2%) | 1956 (51.2%) | 4122 (99.2%) | 4798 (75.1%) | 1279 (80.4%) |
| Pack-years | |||||
| 1–10 | 974 (12.2%) | 777 (20.3%) | 32 (0.8%) | 728 (11.4%) | 144 (9.1%) |
| 10–20 | 583 (7.3%) | 712 (18.6%) | 0 (0.0%) | 587 (9.2%) | 100 (6.3%) |
| > 20 | 343 (4.3%) | 379 (9.9%) | 0 (0.0%) | 274 (4.3%) | 67 (4.2%) |
| Fuels used | |||||
| Gas | 5198 (65.2%) | 2519 (65.9%) | 2679 (64.5%) | 4777 (74.8%) | 421 (26.5%) |
| Oil/gasoline | 1801 (22.6%) | 868 (22.7%) | 933 (22.5%) | 1183 (18.5%) | 618 (38.9%) |
| Wood/firewood/animal dung | 979 (12.3%) | 437 (11.4%) | 542 (13%) | 427 (6.7%) | 551 (34.7%) |
| Cooking area (kitchen type) | |||||
| Closed kitchen inside the house | 1905 (23.9%) | 826 (21.6%) | 1079 (26%) | 1371 (21.5%) | 534 (33.6%) |
| Open kitchen inside the house | 5979 (74.9%) | 2951 (77.2%) | 3028 (72.9%) | 4951 (77.5%) | 1028 (64.7%) |
| Outside of the house | 94 (1.2%) | 47 (1.2%) | 47 (1.1%) | 66 (1%) | 28 (1.8%) |
| Kitchen ventilation status | |||||
| Ventilated | 4422 (55.4%) | 2277 (59.5%) | 2145 (51.6%) | 3899 (61%) | 522 (32.8%) |
| Not ventilated | 3556 (44.6%) | 1547 (40.5%) | 2009 (48.4%) | 2488 (39%) | 1068 (67.2%) |
| Comorbidities diseases status | |||||
| Cardiovascular disease | 523 (6.6%) | 306 (8%) | 2.7 (5.2%) | 429 (6.7%) | 94 (5.9%) |
| Chronic lung diseases (asthma, tuberculosis, emphysema and bronchitis) | 346 (4.3%) | 155 (4.1%) | 191 (4.6%) | 302 (4.7%) | 44 (2.8%) |
| Hypertension | 2125 (27.1%) | 1032 (27.6%) | 1093 (26.7%) | 1753 (28%) | 372 (23.6%) |
| Diabetes mellitus | 955 (12%) | 424 (11.5%) | 531 (13.1%) | 815 (13.2%) | 140 (9.2%) |
| Dyslipidemia | 5610 (71.9%) | 2579 (69.3%) | 3031 (74.4%) | 4451 (70.9%) | 1159 (76%) |
| Metabolic syndrome | 2006 (25.2%) | 1660 (30.5%) | 846 (20.4%) | 1798 (28.3%) | 208 (13.1%) |
| Anxiety and depression | 1346 (16.9%) | 353 (9.2%) | 993 (23.9%) | 1147 (18%) | 199 (12.5%) |
| Musculoskeletal disorders | 3929 (56.5%) | 1562 (47.4%) | 2367 (64.7%) | 3316 (59.7%) | 613 (43.8%) |
| Rheumatoid arthritis | 385 (4.8%) | 115 (3%) | 270 (6.5%) | 318 (5%) | 67 (4.2%) |
| Osteoporosis | 733 (9.2%) | 59 (1.5%) | 674 (16.2%) | 615 (9.6%) | 118 (7.4%) |
| Fatty liver | 1280 (16%) | 489 (12.8%) | 791 (19%) | 1134 (17.8%) | 146 (9.2%) |
| Comorbidities—n (%) | |||||
| None | 1211 (15.2%) | 676 (17.7%) | 535 (12.9%) | 972 (15.2%) | 239 (15%) |
| 1 | 2529 (31.7%) | 1265 (33.1%) | 1264 (30.4%) | 1848 (28.9%) | 681 (42.8%) |
| 2 | 1848 (23.2%) | 849 (22.2%) | 999 (24%) | 1515 (23.7%) | 333 (20.9%) |
| 3 or more | 2390 (30%) | 1034 (27%) | 1356 (32.6%) | 2053 (32.1%) | 337 (21.2%) |
According to the GOLD criteria, 289 (3.6%) patients had COPD, and according to the LLN criteria, 673 (8.4%) had COPD. 4.3% of the participants had a history of chronic lung disease (asthma, tuberculosis, emphysema, and bronchitis), 11.9% had a history of asthma in childhood and 3.9% had a family history of respiratory and pulmonary diseases and also 13% of the participants had a history of chronic cough and of those who had a chronic cough, 47.6% had a history of chronic phlegm; 2.7% of the participants had Shortness of breath and wheezing. 47.8% of patients in the mild stage (GOLD I), 40.1% in the moderate stage (GOLD II), 9.8% in the severe stage of the disease (GOLD III) and 2.4% in the very severe stage of the disease (GOLD IV) were located (Table 4).
Table 4.
Baseline airway obstruction in the subcohort of COPD and comparison between sex and residence group
| Variables | All N = 7978 |
Men N = 3824 |
Women N = 4154 |
P value | Urban N = 6388 |
Rural N = 1590 |
P value |
|---|---|---|---|---|---|---|---|
| Airway obstruction—LLN | 673 (8.4%) | 345 (9%) | 328 (7.9%) | 0.039 | 558 (8.7%) | 115 (7.2%) | 0.029 |
| Airways obstruction—FR | 289 (3.6%) | 159 (4.2%) | 130 (3.1%) | 0.008 | 243 (3.8%) | 46 (2.9%) | 0.045 |
| Chronic lung disease (asthma, tuberculosis, emphysema and bronchitis)—n (%) | 346 (4.3%) | 155 (4.1%) | 191 (4.6%) | 0.127 | 302 (4.7%) | 44 (2.8%) | < 0.0001 |
| History of asthma in childhood—n (%) | 949 (11.9%) | 511 (13.4%) | 438 (10.5%) | 0.028 | 756 (11.8%) | 193 (12.1%) | 0.098 |
| family history of respiratory and pulmonary diseases—n (%) | 311 (3.9%) | 138 (3.6%) | 173 (4.2%) | 0.261 | 251 (3.9%) | 60 (3.8%) | 0.879 |
| Symptoms | |||||||
| Chronic cough—n (%) | 1038 (13%) | 383 (10%) | 655 (15.8%) | < 0.0001 | 937 (14.7%) | 101 (6.4%) | < 0.0001 |
| Chronic cough with phlegm—n (%) | 494 (47.6%) | 201 (52.5%) | 293 (44.7%) | 0.009 | 444 (47.4%) | 50 (49.5%) | 0.382 |
| Shortness of breath and Wheezing—n (%) | 217 (2.7%) | 75 (2%) | 142 (3.4%) | < 0.0001 | 188 (2.9%) | 29 (1.8%) | 0.007 |
| Pre-bronchodilator spirometry (n = 7978) | |||||||
| FEV1 (L.)—mean ± SD | 2.84 ± 0.79 | 2.93 ± 0.81 | 2.76 ± 0.76 | < 0.0001 | 2.92 ± 0.78 | 2.55 ± 0.74 | < 0.0001 |
| FEV6 (L.)—mean ± SD | 2.08 ± 0.86 | 3.17 ± 0.87 | 2.99 ± 0.084 | < 0.0001 | 3.16 ± 0.85 | 2.74 ± 0.80 | < 0.0001 |
| FVC (L)—mean ± SD | 3.08 ± 0.86 | 3.18 ± 0.88 | 3.00 ± 0.84 | < 0.0001 | 3.17 ± 0.85 | 2.74 ± 0.80 | < 0.0001 |
| FEV1/FVC—mean ± SD | 92.48 ± 7.47 | 92.43 ± 7.63 | 92.52 ± 7.32 | 0.601 | 92.27 ± 7.45 | 93.33 ± 7.50 | < 0.0001 |
| FEV1/FEV6—mean ± SD | 92.39 ± 7.48 | 92.60 ± 7.45 | 92.62 ± 7.32 | 0.678 | 92.42 ± 7.36 | 93.38 ± 7.41 | < 0.0001 |
| PEF (L)—mean ± SD | 5.07 ± 2.09 | 5.28 ± 2.19 | 4.88 ± 1.96 | < 0.0001 | 5.24 ± 2.12 | 4.37 ± 1.79 | < 0.0001 |
| MVV (L/min)—mean ± SD | 99.67 ± 27.7 | 102.54 ± 28.3 | 97.02 ± 26.96 | < 0.0001 | 102.27 ± 27.55 | 89.37 ± 26.21 | < 0.0001 |
| Post-bronchodilator spirometry (n = 761) | |||||||
| FEV1 (L.)—mean ± SD | 3.23 ± 0.21 | 3.41 ± 0.74 | 2.98 ± 0.88 | < 0.0001 | 3.35 ± 0.42 | 2.78 ± 0.81 | < 0.0001 |
| FEV6 (L.)—mean ± SD | 2.51 ± 0.74 | 3.67 ± 0.84 | 3.12 ± 0.36 | < 0.0001 | 3.41 ± 0.65 | 2.98 ± 0.63 | < 0.0001 |
| FVC (L)—mean ± SD | 3.89 ± 0.48 | 3.91 ± 0.73 | 3.84 ± 0.61 | < 0.0001 | 3.99 ± 0.53 | 2.93 ± 0.61 | < 0.0001 |
| FEV1/FVC—mean ± SD | 91.28 ± 6.94 | 91.40 ± 6.86 | 91.62 ± 6.82 | 0.687 | 91.39 ± 6.87 | 92.64 ± 6.91 | < 0.0001 |
| FEV1/FEV6—mean ± SD | 92.73 ± 7.21 | 92.92 ± 7.69 | 92.89 ± 7.41 | 0.964 | 92.68 ± 7.44 | 93.81 ± 7.48 | < 0.0001 |
| PEF (L)—mean ± SD | 5.51 ± 2.32 | 5.68 ± 2.38 | 5.21 ± 2.01 | < 0.0001 | 5.84 ± 2.39 | 4.98 ± 1.94 | < 0.0001 |
| MVV (L/min)—mean ± SD | 99.87 ± 28.1 | 102.66 ± 28.2 | 98.12 ± 28.91 | < 0.0001 | 102.69 ± 27.97 | 90.21 ± 27.31 | < 0.0001 |
| Severity of COPD | |||||||
| Mild | 138 (47.8%) | 92 (55.1%) | 46 (37.7%) | 0.003 | 111 (46.8%) | 27 (51.9%) | 0.801 |
| Moderate | 116 (40.1%) | 52 (31.1%) | 64 (52.5%) | 98 (41.4%) | 18 (34.6%) | ||
| Severe | 28 (9.8%) | 19 (11.4%) | 9 (7.4%) | 22 (9.3%) | 6 (11.5%) | ||
| Very severe | 7 (2.4%) | 4 (2.4%) | 3 (2.5%) | 6 (2.5%) | 1 (1.9%) |
LLN, low limit normal; FR, fixed ratio; FEV1, forced expiratory volume in 1 second; FEV6, forced expiratory volume in 6 second; FVC, forced vital capacity; PEF, peak expiratory flow
The group of subjects with COPD had higher mean age, fewer years of schooling, a higher percentage of subjects of smokers with a smoking history of 10 or more pack years. The fuel used by most of the participants for heating the house and cooking was gas (50.9%), and also, the type of kitchen or cooking area for most participants was an open kitchen inside the house (68.9%). 4.6% of patients had a history of chronic lung disease, 17.6% had a history of asthma in childhood and 5.2% had a family history of respiratory and pulmonary diseases (Table 5).
Table 5.
Baseline behavioral and clinical characteristics of obstructed patients
| Indicator | Fixed ratio | LLN | ||
|---|---|---|---|---|
| COPD (N = 289) |
Non-COPD (6746) |
COPD (N = 673) |
Non-COPD (6547) |
|
| Weight status, mean (SD) kg | 74.17 ± 14.18 | 74.16 ± 13.24 | 74.26 ± 13.19 | 74.15 ± 13.28 |
| Body mass index (BMI) | ||||
| Mean (SD) | 27.24 ± 4.52 | 27.71 ± 4.58 | 27.57 ± 4.56 | 27.71 ± 4.58 |
| Underweight (BMI < 20)—n (%) | 74 (25.7%) | 246 (3.2%) | 24 (3.6%) | 235 (3.2%) |
| Healthy (20–25)—n (%) | 130 (45.1%) | 1860 (24.4%) | 166 (24.9%) | 1768 (24.4%) |
| Overweight (25–30)—n (%) | 71 (24.7%) | 3420 (44.8%) | 299 (44.8%) | 3251 (44.9%) |
| Obesity (> 30)—n (%) | 75 (26%) | 2100 (27.5%) | 178 (26.7%) | 1993 (27.5%) |
| Smoking status | ||||
| Current smoker | 75 (26%) | 1213 (15.8%) | 159 (23.6%) | 1129 (15.5%) |
| Ex-smoker | 30 (10.4%) | 582 (7.6%) | 56 (8.3%) | 556 (7.6%) |
| Never smoker | 184 (63.7%) | 5894 (76.7%) | 458 (68.1%) | 5620 (76.9%) |
| Pack-years | ||||
| 1–10 | 51 (17.6%) | 1171 (15.2%) | 107 (15.9%) | 1043 (14.3%) |
| 10–20 | 36 (12.4%) | 377 (4.9%) | 71 (10.5%) | 372 (5.1%) |
| > 20 | 18 (6.2%) | 247 (3.2%) | 37 (5.5%) | 270 (3.7%) |
| Occupational status | ||||
| Housewife/unemployed/retired | 133 (46%) | 3972 (51.7%) | 337 (50.1%) | 3768 (51.6%) |
| Employee | 47 (16.3%) | 1403 (18.2%) | 119 (17.7%) | 1331 (18.2%) |
| Farmer/rancher/herder | 21 (7.3%) | 277 (3.6%) | 33 (4.9%) | 265 (3.6%) |
| Carpet weaver/tailor/weaver | 12 (4.2%) | 301 (3.9%) | 25 (3.7%) | 288 (3.9%) |
| Car driver/mechanic and oil change worker | 20 (6.9%) | 493 (6.4%) | 41 (6.1%) | 472 (6.5%) |
| Building contractor/worker and builder | 24 (8.3%) | 448 (5.8%) | 45 (6.7%) | 427 (5.8%) |
| Others | 32 (11.1%) | 795 (10.3%) | 73 (10.8%) | 754 (10.3%) |
| Fuels used | ||||
| Gas | 147 (50.9%) | 5051 (65.7%) | 410 (60.9%) | 4788 (65.5%) |
| Oil/gasoline | 88 (30.4%) | 1713 (22.3%) | 164 (24.4%) | 1637 (22.4%) |
| Wood/firewood/animal dung | 54 (18.7%) | 925 (12%) | 99 (14.7%) | 880 (12%) |
| Cooking area (kitchen Type) | ||||
| Closed kitchen inside the house | 82 (28.4%) | 1823 (23.7%) | 174 (25.9%) | 1731 (23.7%) |
| Open kitchen inside the house | 199 (68.9%) | 5780 (75.2%) | 483 (71.8%) | 5496 (75.2%) |
| Outside of the house | 8 (2.8%) | 86 (1.1%) | 16 (2.4%) | 78 (1.1%) |
| Kitchen ventilation status | ||||
| Ventilated | 149 (51.6%) | 4273 (55.6%) | 360 (53.5%) | 4062 (55.6%) |
| Not ventilated | 140 (48.4%) | 3416 (44.4%) | 313 (46.5%) | 3243 (44.4%) |
| Symptoms | ||||
| Chronic cough | 32 (11.1%) | 1006 (14.9%) | 79 (11.7%) | 958 (14.6%) |
| Chronic cough with phlegm | 14 (45.2%) | 480 (47.7%) | 31 (41.9%) | 463 (48%) |
| Shortness of breath and Wheezing | 15 (5.3%) | 202 (2.7%) | 27 (4.1%) | 190 (2.6%) |
| Chronic lung disease (asthma, tuberculosis, emphysema and bronchitis) | 13 (4.6%) | 333 (4.4%) | 27 (4.1%) | 319 (4.4%) |
| History of asthma in childhood | 51 (17.6%) | 898 (13.3%) | 109 (16.1%) | 840 (12.8%) |
| family history of respiratory and pulmonary diseases | 15 (5.2%) | 296 (4.4%) | 39 (5.7%) | 272 (4.1%) |
COPD, chronic obstructive pulmonary disease; LLN, Low limit normal
Discussion
Results of baseline cohort and profile publication are one of the most important outcomes after the completion of enrollment in cohort studies. This is useful for researchers, helps develop research fields and is helpful for health care system planners.
COPD is a preventable and curable disease [28]. Prevention of this disease should be taken into account as with other non-communicable chronic diseases, such as cardiovascular disease and cancer. Epidemiological studies are necessary to create an appropriate framework for fighting COPD. This framework requires a better description of men and women at risk of developing COPD and a description of people with an early-stage illness. This framework should also provide a better understanding of the risk factors that can be changed through interventions[29]. Subcohort COPD is the first longitudinal prospective study for COPD with population sampling in Iran. This study seeks to provide a broad description of the characteristics of men and women with COPD and to identify other causes of airway obstruction that can lead to an outcome. This study provides a unique opportunity to advance and consult observations on people with mild and unknown illnesses. The inclusion of healthy people and patients in the study provided a new opportunity to describe and follow a subgroup of the COPD patients who had not already been diagnosed, while many of them may at risk or have mild COPD that had not previously been diagnosed by a doctor or other health care professionals [30]. Most national estimates of COPD prevalence rates have been usually based on the data derived from the patients' self-report questionnaires and without an objective measurement of pulmonary function using a spirometer [10]. This study provides a good opportunity to assess the incidence of COPD by mean of both GOLD and LLN criteria from spirometry data so that its results can be compared with multivariate models of people who have not previously been diagnosed with COPD and who have COPD on the basis of known risk factors; in fact, this study examined the problems due to lack of COPD diagnosis and the need for intervention, and provides an opportunity to address the question Will undiagnosed COPD be clinically important? COPD is associated with a high incidence rate of one or more diseases. Comorbidities such as cardiovascular disease, musculoskeletal disorders, and metabolic syndrome are common in patients with COPD and significantly affect the quality of life of the patients, prognosis, and survival [31–34]. Increasing knowledge about the prevalence and effects of comorbidities in COPD is essential to adopt better intervention strategies and revise primary health care guidelines. COPD prognostic indicators currently focus primarily on prediction of mortality risk; creating a large COPD cohort for primary care and evaluating a wide range of outcomes enable us to review existing prognostic indicators and, if necessary, to develop new and appropriate prognostic indicators to predict other outcomes such as exacerbation and recurrence of disease, hospital admissions, and hospitalization due to exacerbation of respiratory diseases in primary care to be used in primary health care [30]. This study is a valuable work concerning increasing the longitudinal information and identifying the prognostic factors for COPD and the contribution of each of these factors to the progression and development of the disease due to its similarity to other cohort studies conducted around the world in terms of methodology, data collection methods and many other distinctive features [29]. Subcohort COPD is a good platform for standard research with Access to a database (such as lifestyle information, records and occupational exposures, smoking status and exposure to cigarette smoke and exposure time, fuel status, medical records, illnesses, and outcomes). Reported by patients, housing status and lung function test information, etc.). Using linear regression models with FEV1 as a dependent variable, we can estimate the progression of COPD over time. The data of this cohort study provide an appropriate infrastructure for the development of mathematical and statistical models to predict COPD and the survival rate of patients, and also the grounds for the analysis of the effects of various exposures, smoking and age using regression models and mortality rate from COPD as a dependent variable. There is a need for research to identify the impact of occupational exposure in COPD, especially among non-smokers by large, prospective and longitudinal studies. Therefore, in addition to the impact of occupational exposure, the potential interaction between occupational exposure and smoking was also addressed in this study. This study also provides a basis for answering an important and challenging research question about disease improvement and management, as well as interdisciplinary collaboration ranging from epidemiology to basic clinical research.
Conclusion
We expect results from this and future research to help improve the health status and determine specific biological pathways or treatments for health care services planning and management decisions. Ultimately, this information will help policy-makers and public health decision-makers develop policies to improve the diagnosis, management and control of COPD. A biobank that has been designed to store blood, nail, hair and urine samples for future research is another strength of this study. Researchers who are interested in using the information can refer to the following web page: http://persiancohort.com.
Acknowledgements
We hereby gratefully thank all people, especially the participants, the field team, the staff of the Research and Technology Deputy of the SKUMS, the Research Councils of the Faculty of Public Health, Modeling in Health Research Center, Shahrekord and Ardal health centers of the SKUMS, the Treatment, Health, and Resource and Management Deputies of the SKUMS. Also, we thank the central quality control team of the PERSIAN Cohort for providing the Central Protocol and the checklists of making arrangements and training the interviewers and monitoring their performance as well as assisting in implementing the pre-pilot phase, especially Sareh Eghtesad, Farzin Rouzafazai, Ameneh Shayan-Raad, Zahra Mahmoudi, Romina Mohammadi, Esmaeil Omidi, Reza Gojani, Kamal Solati, Arsalan Khaledifar, Morteza Hashemzadeh, Mahdi Sheikh, Hossein Poustchi and Reza Malekzadeh.
Authors' contributions
AA in study design and principal investigator, FZK and AA participated in data gathering. FZK, AA, ASB, and HR wrote the first draft, and the statistical analysis was conducted by FZK and AA, ASB and HR contributed to the study design, spirometry data collection and interpreting the results. All authors contributed to the data collection, interpreting the results and commenting on the initial manuscripts. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The SCS was funded by Iran's Ministry of Health and Medical Education (number 700/120), to develop cohort studies across Iran and financially and non-financially supported by the SKUMS (number 2763 and 3366).
Availability of data and materials
The study is ongoing. The general information is available from http://cohort.skums.ac.ir. All researchers across Iran and the world can have free access to the findings of this study, and necessary processes are available at the Cohort website to reproduce the research project, participate in collaborative research projects, and use the data. After requested, under conditions of collaboration and endowment, Access to the data is available for interested researchers from the corresponding author in A.A (aliahmadi2007@gmail.com).
Declarations
Ethics approval and consent to participate
This study was conducted with the observance of the Declaration of Helsinki and the National Ethical Guidelines in Biomedical Research in Iran. As well, the study protocol was approved by the Ethics Committee of the SKUMS (IR.SKUMS.REC 1394.286 and IR. SKUMS.1396.110) at regional and national scales. All participants provided signed and fingerprinted informed written consent. For illiterate participants, the informed consent form was read. After consent, the consent form was taken with a fingerprint. The consent of the participant was also signed and confirmed by his first-degree family member or legally authorized representatives. We provided signed and fingerprinted informed written consent according to the guidelines enforced by the Ethics Committee of the SKUMS. The participants can withdraw from the study whenever they wish. Data are stored in a codified confidential database.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Zeynab Kiani, Email: fatemezeynabkiani@gmail.com.
Ali Ahmadi, Email: aliahmadi2007@gmail.com.
Akbar Soleymani Babadi, Email: dr.akbarsoleymani@yahoo.com.
Hamid Rouhi, Email: hammfer@yahoo.com.
References
- 1.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Gordillo MA, Collado-Mateo D, Olivares PR, Adsuar JC, Merellano-Navarro E. A cross-sectional assessment of health-related quality of life among patients with chronic obstructive pulmonary disease. Iran J Public Health. 2017;46(8):1046. [PMC free article] [PubMed] [Google Scholar]
- 3.Halbert R, Natoli J, Gano A, Badamgarav E, Buist AS, Mannino D. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 4.Melville A, Pless-Mulloli T, Afolabi O, Stenton S. COPD prevalence and its association with occupational exposures in a general population. Eur Respir J. 2010;36(3):488–493. doi: 10.1183/09031936.00038309. [DOI] [PubMed] [Google Scholar]
- 5.Doucet M, Rochette L, Hamel D. Incidence, prevalence, and mortality trends in chronic obstructive pulmonary disease over 2001 to 2011: a public health point of view of the burden. Can Respir J. 2016;2016:7518287. doi: 10.1155/2016/7518287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maleki-Yazdi MR, Kelly SM, Lam SS, Marin M, Barbeau M, Walker V. The burden of illness in patients with moderate to severe chronic obstructive pulmonary disease in Canada. Can Respir J. 2012;19(5):319–324. doi: 10.1155/2012/328460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang-Tan T, Zhang S, Tavares RV, Stutz M, Ismaila AS, Vaillancourt J, et al. The burden of illness related to chronic obstructive pulmonary disease exacerbations in Québec, Canada. Can Respir J. 2017;2017:8184915. doi: 10.1155/2017/8184915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varmaghani M, Dehghani M, Heidari E, Sharifi F, Moghaddam SS, Farzadfar F. Global prevalence of chronic obstructive pulmonary disease: systematic review and meta-analysis. East Mediterr Health J. 2019;25(1):47–57. doi: 10.26719/emhj.18.014. [DOI] [PubMed] [Google Scholar]
- 9.Sharifi H, Ghanei M, Jamaati H, Masjedi MR, Aarabi M, Sharifpour A, et al. Burden of obstructive lung disease in Iran: Prevalence and risk factors for COPD in North of Iran. Int J Prev Med. 2020;11:78. doi: 10.4103/ijpvm.IJPVM_478_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifi H, Masjedi MR, Emami H, Ghanei M, Eslaminejad A, Radmand G, et al. Burden of obstructive lung disease study in Tehran: prevalence and risk factors of chronic obstructive pulmonary disease. Lung India J. 2015;32(6):572. doi: 10.4103/0970-2113.168129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amra B, Golshan M, Fietze I, Penzel T, Welte T. Correlation between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome in a general population in Iran. J Res Med Sci. 2011;16(7):885. [PMC free article] [PubMed] [Google Scholar]
- 12.Sharifi H, Ghanei M, Jamaati H, Masjedi MR, Aarabi M, Sharifpour A, et al. Burden of obstructive lung disease study in Iran: first report of the prevalence and risk factors of COPD in five provinces. Lung India J. 2019;36(1):14. doi: 10.4103/lungindia.lungindia_129_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varmaghani M, Farzadfar F, Sharifi F, Rashidain A, Moin M, Moradi-Lakeh M, et al. prevalence of asthma, COPD, and chronic bronchitis in Iran: A systematic review and meta-analysis. Iran J Allergy Asthma Immunol. 2016;15(2):93. [PubMed] [Google Scholar]
- 14.Horner A, Soriano JB, Puhan MA, Studnicka M, Kaiser B, Vanfleteren LE, et al. Altitude and COPD prevalence: analysis of the PREPOCOL-PLATINO-BOLD-EPI-SCAN study. Respir Res. 2017;18(1):162. doi: 10.1186/s12931-017-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaganath D, Miranda JJ, Gilman RH, Wise RA, Diette GB, Miele CH, et al. prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16(1):40. doi: 10.1186/s12931-015-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest. 2008;133(2):343–349. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 17.Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–655. doi: 10.1093/aje/kwx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaledifar A, Hashemzadeh M, Solati K, Poustchi H, Bollati V, Ahmadi A, et al. The protocol of a population-based prospective cohort study in southwest of Iran to analyze common non-communicable diseases: Shahrekord cohort study. BMC Public Health. 2018;18(1):660. doi: 10.1186/s12889-018-5364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghobadi H, Ahari SS, Kameli A, Lari SM. The relationship between COPD assessment test (CAT) scores and severity of airflow obstruction in stable COPD patients. Tanaffos. 2012;11(2):22–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 21.Kiani FZ, Ahmadi A. Prevalence of different comorbidities in chronic obstructive pulmonary disease among Shahrekord PERSIAN cohort study in southwest Iran. Sci Rep. 2021;11:1548. doi: 10.1038/s41598-020-79707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 23.Hoesein FAM, Zanen P, Lammers J-WJ. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med. 2011;105(6):907–915. doi: 10.1016/j.rmed.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Hardie J, Buist AS, Vollmer W, Ellingsen I, Bakke P, Mørkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JE, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not < 70% Chest. 2007;131(2):349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 27.Quanjer PH, Pretto JJ, Brazzale DJ, Boros PW. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J. 2013;43(2):505–512. doi: 10.1183/09031936.00086313. [DOI] [PubMed] [Google Scholar]
- 28.Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716–723. doi: 10.1164/rccm.201206-0996OC. [DOI] [PubMed] [Google Scholar]
- 29.Bourbeau J, Tan WC, Benedetti A, Aaron SD, Chapman KR, Coxson HO, et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11(2):125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 30.Adab P, Fitzmaurice D, Dickens A, Ayres J, Buni H, Cooper B, et al. Cohort profile: The Birmingham Chronic Obstructive Pulmonary Disease (COPD) cohort study. Int J Epidemiol. 2016;46(1):23–23. doi: 10.1093/ije/dyv350. [DOI] [PubMed] [Google Scholar]
- 31.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9(1):871–888. doi: 10.2147/COPD.S49621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corlateanu A, Covantev S, Mathioudakis AG, Botnaru V, Siafakas N. Prevalence and burden of comorbidities in chronic obstructive pulmonary disease. Respir Investig. 2016;54(6):387–396. doi: 10.1016/j.resinv.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Gebreyes YF, Goshu DY, Geletew TK, Argefa TG, Zemedu TG, Lemu KA, et al. prevalence of high bloodpressure, hyperglycemia, dyslipidemia, metabolic syndrome and their determinants in Ethiopia: evidences from the National NCDs STEPS Survey, 2015. PLoS ONE. 2018;13(5):e0194819. doi: 10.1371/journal.pone.0194819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin H-L, Yin S-Q, Lin Q-Y, Xu Y, Xu H-W, Liu T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine. 2017;96(19):e6836. doi: 10.1097/MD.0000000000006836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study is ongoing. The general information is available from http://cohort.skums.ac.ir. All researchers across Iran and the world can have free access to the findings of this study, and necessary processes are available at the Cohort website to reproduce the research project, participate in collaborative research projects, and use the data. After requested, under conditions of collaboration and endowment, Access to the data is available for interested researchers from the corresponding author in A.A (aliahmadi2007@gmail.com).