Abstract
Background
Low-density lipoprotein to high density lipoprotein (LDL/HDL) cholesterol ratio has been reported to predict the risk of many metabolic diseases. However, the association between the LDL/HDL cholesterol ratio and nonalcoholic fatty liver disease (NAFLD) has not been established.
Methods
A longitudinal cohort design was adopted in this study; 9767 non-obese subjects without NAFLD were included and analyzed. The subjects were grouped according to the quintile of LDL/HDL cholesterol ratio. The cumulative incidence of NAFLD and the independent effect of the LDL/HDL cholesterol ratio on NAFLD during 5 years of follow-up were calculated using the Kaplan-Meier method and Cox proportional-hazards regression model.
Results
During the 5-year follow-up period, 841 subjects were diagnosed with new-onset NAFLD, and the 1-, 2-, 3-, 4-, and 5-year cumulative incidence rates of NAFLD were 1.16, 4.65, 8.33, 12.43, and 25.14%, respectively. In the multivariable-adjusted Cox proportional-hazards regression model, the LDL/HDL cholesterol ratio was significantly associated with the risk for NAFLD (HR: 1.66, 95% CI: 1.38–1.99, P trend< 0.001), especially among young people (HR: 3.96, 95% CI: 1.50–10.46, P interaction< 0.05). Additionally, receiver operating characteristic curve analysis showed that the LDL/HDL cholesterol ratio was better than HDL cholesterol and LDL cholesterol in predicting new-onset NAFLD.
Conclusions
LDL/HDL cholesterol ratio is an independent predictor of NAFLD in Chinese non-obese people with normal lipids, and its predictive value is higher than that of other lipoproteins. In clinical practice, the LDL/HDL cholesterol ratio can be used to identify people at high risk of NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-021-01457-1.
Keywords: Non-obese, Longitudinal study, Lipoprotein ratios, LDL/HDL cholesterol ratio, Nonalcoholic fatty liver disease
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and the main cause of various liver diseases [1–3]. It is also a risk factor for a series of metabolism-related diseases, such as metabolic syndrome, diabetes, and cardio-cerebrovascular disease [3–5]. It has become an important issue of global public health in the twenty-first century [3]. As reported, the global prevalence of NAFLD is about 25% in adults [6, 7], while in Asia, nearly 30% of adults are affected by NAFLD, and this number is still increasing with the prevalence of obesity [6, 8]. The potential huge disease burden of NAFLD will place great pressure on the security of the medical system and socioeconomic system [3, 6, 9]. At present, there is no effective drug approved by regulatory authorities for the treatment of NAFLD. The prevention and treatment of NAFLD are mainly through lifestyle improvement and weight loss [1, 10]. Therefore, the prevention strategy for identifying and adjusting the potential risk factors is ideal for reducing the risk of NAFLD in the population.
It is well known that obesity is closely associated with the prevalence and severity of NAFLD [3, 6, 11]. However, in recent years, many studies have pointed out that non-obese people are also prone to NAFLD [12, 13]. In previous reports, the prevalence of NAFLD among non-obese people in China was 8.61% [14], and similar results (7.4%) were reported in the United States [15]. Additionally, several recent large cohort studies have shown that compared to obese NAFLD patients, non-obese NAFLD patients are at significantly increased risk for metabolic syndrome and hypertension [16, 17], making early detection and intervention of risk factors in non-obese NAFLD patients extremely important.
Lipid metabolism is also closely related to NAFLD. Previous studies have shown that higher HDL cholesterol is an independent protective factor for NAFLD, while triglycerides (TG), LDL cholesterol, and total cholesterol (TC) are associated with an increased risk of NAFLD [1, 18]. These lipoproteins have been recommended for screening people with a high risk of NAFLD in clinical practice [18]. Recently, some studies have pointed out that the LDL/HDL cholesterol ratio can simultaneously evaluate LDL cholesterol and HDL cholesterol, and its performance in predicting the risk of cardiovascular and cerebrovascular metabolic-related diseases is better than that of a single lipoprotein [19–21]. At present, there are no research data on the association between NAFLD and the LDL/HDL cholesterol ratio. Considering the prevalence of non-obese NAFLD, which has not received widespread attention, the relevant data are still immature. Therefore, this study is based on a large sample of longitudinal cohorts to evaluate the association between the LDL/HDL cholesterol ratio and the risk of new-onset NAFLD in non-obese people with normal lipids.
Methods
Study design and subjects
This research conducted a post hoc analysis of a longitudinal cohort from Wenzhou People’s Hospital [22]. The data were from the Dryad database, which was uploaded and shared by Sun et al. [23]. The longitudinal cohort recruited 33,153 subjects who underwent physical examination at Wenzhou People’s Hospital from the beginning of 2010 to the end of 2014. According to the purpose of the secondary analysis, this study excluded subjects who met the following criteria at baseline: i) subjects who were still taking oral antihypertensive, hypoglycemic and lipid-lowering drugs at baseline; ii) subjects with liver disease; iii) subjects who lacked follow-up data; iv) subjects with BMI ≥ 25 kg/m2 [24]; v) subjects with excessive alcohol consumption (weekly alcohol consumption: male> 140 g, female> 70 g); and vi) subjects with dyslipidemia (fasting sample: HDL cholesterol < 1.04 mmol/l, TG > 1.7 mmol/l, LDL cholesterol > 3.12 mmol/l and TC > 5.2 mmol/l). Finally, this study analyzed 9767 subjects who met the criteria. Because of the previous study has been approved by the institutional review boards of Wenzhou people’s Hospital and the written informed consent of the participants [22], there was no need to apply for ethical approval again for this study.
Data collection and follow-up
Use standardized self-filling spreadsheets to obtain detailed information on basic clinical data, including height, age, weight, gender, and diastolic and systolic blood pressure (D/SBP). Venous blood samples were taken by experienced medical staff after overnight fasting and analyzed by an AbbottAxSYM analyzer. The laboratory parameters included in this study were DBIL: direct bilirubin; ALP: alkaline phosphatase; Cr: creatinine; TP: total protein; FPG: fasting plasma glucose; UA: uric acid; TC; ALB: albumin; TG; AST: aspartate aminotransferase; GLB: globulin; TB: total bilirubin; HDL cholesterol; BUN: blood urea nitrogen; ALT: alanine aminotransferase; LDL cholesterol; and GGT: gamma glutamyl transferase.
The beginning of the follow-up was from the clinician’s first NAFLD evaluation to the subjects, and then the NAFLD evaluation was performed annually by abdominal color ultrasound, while other baseline changes and medication status were not recorded. The follow-up period was 5 years.
Diagnosis of NAFLD
Diagnosis of NAFLD was performed using the abdominal color ultrasound diagnostic procedure published by the Chinese Society of Liver Disease [25], and the main contents included the following five items: i) diffuse hyperechoic liver (compared to spleen and kidney); ii) enlarged liver with blunt, rounded edges; iii) visibility of detailed structures in the liver is reduced; iv) the envelope of the right liver lobe and diaphragm with unclear or nonintact display; and v) hepatic blood flow signal weakening. The diagnosis of NAFLD met the first of the above five items and any other items.
Missing data processing
Missing data are a problem in almost every observational study, while in the data set analyzed in this study, missing data accounted for 8.85% of all variables (Additional file 1, Supplementary Table 1). To reduce the bias caused by missing covariables, which cannot reflect the statistical efficiency of the target sample in the modeling process, the missing data in this study were imputed using multiple imputation [26], and five imputations were created. In this study, all the analysis steps were calculated and compared in the original dataset and imputed dataset, and the results showed that the original data were consistent with the core results of the imputed data. Therefore, the results of multiple regression analysis in this study adopted the values of imputed data, in which the estimates from each imputation were combined according to Rubin’s rules [27]. Additional file 1 provides a detailed statistical analysis process of the original data and estimated data.
Statistical analysis
The relationship between the LDL/HDL cholesterol ratio and NAFLD was evaluated with the LDL/HDL cholesterol ratio as a categorical variable (divided into five groups according to quintiles) and continuous variable; Kaplan-Meier method was used to calculate the cumulative incidence of NAFLD over time. The hazard ratios (HR) and 95% confidence intervals (CI) of the LDL/HDL cholesterol ratio to NAFLD risk were calculated by constructing the Cox proportional hazards regression model. To reflect the direction and size of the association between the ratio of LDL/HDL cholesterol and NAFLD between different models [28], five models were used, in which the crude model being unadjusted. Adjusted model I: only the most basic demographic variable; whereas model II was adjusted for variables that contributed more than 10% to the risk of LDL/HDL cholesterol ratio matching with NAFLD [29]. Adjusted model III was model II plus the variables with P < 0.1 in the univariate analysis, and adjusted model IV comprised all the noncollinear variables (Additional file 1: Supplementary Table 2). Simultaneously, the same steps were used as above to explore some subsidiary analysis of the relationship between HDL cholesterol, LDL cholesterol and NAFLD and to construct a receiver operating characteristic curve (ROC) to estimate the ability of the HDL cholesterol, LDL cholesterol and LDL/HDL cholesterol ratio to predict new-onset NAFLD. Additionally, to further verify the reliability of the positive relationship between the LDL/HDL cholesterol ratio as a continuous variable and new-onset NAFLD, the researchers continue to visualize the association between them through a generalized additive model (GAM, cubic spline smoothing) [30]. Finally, considering that the association between the ratio of LDL/HDL cholesterol and NAFLD may vary in some populations, the researchers conducted exploratory stratified analysis (gender, age and FPG) in some subgroups through the Cox model and used the likelihood ratio test to check the hierarchical differences to determine whether there was interaction.
All analyses in this study were carried out using Empower Stats Software (version 2.2) and R language (version 3.4.3). Judging the distribution of continuous variables by a quantile-quantile plot, the baseline variables were reported as the medians (interquartile ranges), frequencies (percentages) or means (standard deviations). The differences between groups were compared by the Kruskal-Wallis H test or chi-square test or one-way analysis of variance, and the linear trend between groups was tested by linear regression or logistic regression. P < 0.05 (two-tailed) indicates statistical significance.
Results
Subject characteristics
In this study, 9767 non-obese subjects with normal lipids were analyzed. The study population’s median age was 39 (31–51) years old; males accounted for 51.42% and females accounted for 48.58%. During the 5-year follow-up period, 841 subjects were diagnosed with new-onset NAFLD, and the 1-, 2-, 3-, 4-, and 5-year cumulative incidence rates of NAFLD were 1.16, 4.65, 8.33, 12.43, and 25.14%, respectively.
Table 1 summarizes the basic characteristics of the subjects grouped according to the quintiles by the ratio of LDL/HDL cholesterol as a continuous variable. In the group with a higher LDL/HDL cholesterol ratio, the NAFLD prevalence rate and LDL cholesterol level were higher, and the HDL cholesterol level was lower. Additionally, in the five groups from quintile 1 to quintile 5, it can be observed that most of the clinical baseline indexes increased gradually with the increase in the LDL/HDL cholesterol ratio; in contrast, HDL cholesterol showed a gradually decreasing trend (P trend< 0.001).
Table 1.
Baseline Characteristics of participants
| LDL/HDL cholesterol ratio | Q1 (≥0.2, ≤1.1) | Q2 (> 1.1, ≤1.3) | Q3 (> 1.3, ≤1.5) | Q4 (> 1.5, ≤1.8) | Q5 (> 1.8, ≤3.0) | P-value | P-trend |
|---|---|---|---|---|---|---|---|
| N (%) | 1953 | 1952 | 1955 | 1952 | 1955 | ||
| Age, years | 38.00 (31.00–50.00) | 39.50 (31.00–50.00) | 38.00 (31.00–51.00) | 40.00 (32.00–51.00) | 40.00 (31.00–50.00) | 0.443 | 0.214 |
| Gender | 0.003 | 0.0004 | |||||
| Female (%) | 978 (50.08%) | 993 (50.87%) | 950 (48.59%) | 945 (48.41%) | 879 (44.96%) | ||
| Male (%) | 975 (49.92%) | 959 (49.13%) | 1005 (51.41%) | 1007 (51.59%) | 1076 (55.04%) | ||
| NAFLD (%) | 58 (2.97%) | 113 (5.79%) | 145 (7.42%) | 188 (9.63%) | 337 (17.24%) | < 0.001 | < 0.001 |
| BMI, kg/m2 | 20.27 (18.95–21.72) | 20.61 (19.26–22.19) | 20.91 (19.47–22.37) | 21.30 (19.84–22.83) | 21.96 (20.44–23.38) | < 0.001 | < 0.001 |
| Weight, kg | 53.95 (7.35) | 55.22 (7.77) | 56.61 (8.12) | 57.93 (8.33) | 60.38 (8.34) | < 0.001 | < 0.001 |
| Height, m | 1.63 (0.07) | 1.63 (0.07) | 1.64 (0.08) | 1.65 (0.08) | 1.66 (0.08) | < 0.001 | < 0.001 |
| ALP, U/L | 62.00 (52.00–76.00) | 65.00 (54.00–79.00) | 66.00 (56.00–81.00) | 69.00 (57.00–81.00) | 72.00 (59.00–86.00) | < 0.001 | < 0.001 |
| GGT, U/L | 18.00 (14.00–24.00) | 18.00 (15.00–24.00) | 19.00 (15.00–26.00) | 20.00 (16.00–27.00) | 22.00 (17.00–30.00) | < 0.001 | < 0.001 |
| ALT, U/L | 14.00 (11.00–20.00) | 14.00 (11.00–19.00) | 15.00 (11.00–21.00) | 16.00 (12.00–21.00) | 17.00 (13.00–24.00) | < 0.001 | < 0.001 |
| AST, U/L | 20.00 (17.50–24.00) | 20.00 (18.00–24.00) | 20.00 (18.00–24.00) | 21.00 (18.00–24.00) | 21.00 (18.00–25.00) | < 0.001 | 0.942 |
| TP, g/L | 73.48 (4.21) | 73.46 (4.06) | 73.70 (4.14) | 73.90 (3.96) | 74.15 (4.07) | < 0.001 | < 0.001 |
| ALB, g/L | 44.25 (2.87) | 44.34 (2.75) | 44.29 (2.69) | 44.31 (2.61) | 44.28 (2.77) | 0.879 | 0.883 |
| GLB, g/L | 29.24 (3.99) | 29.12 (3.78) | 29.41 (3.83) | 29.59 (3.75) | 29.87 (3.93) | < 0.001 | < 0.001 |
| TB, μmol/l | 11.30 (8.80–14.60) | 11.10 (8.70–14.10) | 11.20 (8.80–14.30) | 11.30 (8.93–14.50) | 11.50 (9.00–14.50) | 0.422 | 0.579 |
| DBIL, μmol/l | 2.20 (1.70–2.90) | 2.10 (1.60–2.80) | 2.10 (1.50–2.80) | 2.10 (1.50–2.80) | 2.10 (1.50–2.90) | 0.004 | 0.960 |
| SBP, mmHg | 115.52 (15.69) | 116.69 (15.68) | 118.11 (16.43) | 118.79 (16.25) | 121.19 (15.82) | < 0.001 | < 0.001 |
| DBP, mmHg | 68.00 (62.00–75.00) | 69.00 (63.00–76.00) | 70.00 (64.00–78.00) | 71.00 (64.00–79.00) | 72.00 (66.00–80.00) | < 0.001 | < 0.001 |
| BUN, mmol/l | 4.20 (3.48–5.10) | 4.20 (3.50–5.10) | 4.30 (3.51–5.10) | 4.40 (3.60–5.26) | 4.40 (3.70–5.30) | < 0.001 | < 0.001 |
| CR, μmol/l | 73.86 (22.90) | 74.98 (26.15) | 77.47 (29.89) | 78.37 (23.72) | 80.46 (22.96) | < 0.001 | < 0.001 |
| UA, mmol/l | 241.46 (77.19) | 251.44 (75.83) | 261.97 (79.06) | 275.10 (79.84) | 288.05 (78.70) | < 0.001 | < 0.001 |
| FPG, mmol/l | 4.90 (4.65–5.20) | 4.93 (4.68–5.21) | 4.98 (4.74–5.29) | 4.99 (4.73–5.29) | 5.06 (4.79–5.35) | < 0.001 | < 0.001 |
| TC, mmol/l | 4.04 (3.64–4.45) | 4.31 (3.89–4.68) | 4.38 (3.99–4.73) | 4.46 (4.10–4.80) | 4.69 (4.38–4.94) | < 0.001 | < 0.001 |
| TG, mmol/l | 0.76 (0.63–0.96) | 0.85 (0.69–1.05) | 0.93 (0.74–1.15) | 1.01 (0.81–1.26) | 1.14 (0.92–1.37) | < 0.001 | < 0.001 |
| HDL cholesterol, mmol/l | 1.84 (1.66–2.02) | 1.66 (1.49–1.80) | 1.51 (1.36–1.63) | 1.36 (1.25–1.49) | 1.21 (1.12–1.31) | < 0.001 | < 0.001 |
| LDL cholesterol, mmol/l | 1.63 (0.32) | 1.97 (0.27) | 2.13 (0.27) | 2.29 (0.27) | 2.55 (0.26) | < 0.001 | < 0.001 |
Values are n(%) or mean (standard deviation) or median (interquartile range)
Abbreviations: NAFLD nonalcoholic fatty liver disease, BMI body mass index, BUN blood urea nitrogen, Cr creatinine, UA uric acid, FPG fasting plasma glucose, TC total cholesterol, TG triglyceride, HDL high-density lipoprotein, LDL low-density lipoprotein, ALP Alkaline phosphatase, GGT gamma-glutamyl transferase, ALT alanine aminotransferase, AST aspartate aminotransferase, TP Total Protein, ALB albumin, GLB globulin, TB Total bilirubin, DBIL Direct bilirubin, DBP diastolic blood pressure, SBP systolic blood pressure
Association between the LDL/HDL cholesterol ratio and new-onset NAFLD
Table 2 summarizes the association of the LDL/HDL cholesterol ratio as a categorical variable and continuous variable with new-onset NAFLD. In the five models, the core direction of the relation between the ratio of LDL/HDL cholesterol and NAFLD did not change significantly. In adjustment model IV, for every 1 unit increase in the ratio of LDL/HDL cholesterol, the NAFLD risk increased by 66% (HR: 1.66, 95% CI: 1.38–1.99, P trend< 0.001). In the subsidiary analysis, the same steps were used to analyze HDL cholesterol and LDL cholesterol. The results show a positive correlation between LDL cholesterol and new-onset NAFLD, while HDL cholesterol was negatively correlated with new-onset NAFLD risk. In the quintile grouping, it can be observed that the NAFLD risk increases linearly with the increase in LDL cholesterol (P trend< 0.001), while with the gradual increase in HDL cholesterol, the NAFLD risk shows a linear downward trend (P trend< 0.001) (Additional file 1: Supplementary Tables 3, 4 and 5). In addition, researchers have also drawn an ROC curve to measure the ability of HDL cholesterol, LDL cholesterol and the LDL/HDL cholesterol ratio to predict the risk of new-onset NAFLD (Fig. 1). The areas under the curve of each lipoprotein were as follows: HDL cholesterol: 0.6335 < LDL cholesterol: 0.6367 < LDL/HDL cholesterol ratio: 0.6713 (Table 3). The predictive ability of the ratio of LDL/HDL cholesterol to new-onset NAFLD was better than that of other lipoproteins.
Table 2.
Hazard ratios for NAFLD events by quintiles of serum lipoproteins and LDL/HDL cholesterol ratio
| HR (95%CI) | |||||||
|---|---|---|---|---|---|---|---|
| Multivariable Analysis | Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
| HDL cholesterol (mmol/l) | |||||||
| Crude Model | 0.25 (0.19, 0.33) | Ref | 0.92 (0.77, 1.10) | 0.71 (0.58, 0.86) | 0.51 (0.41, 0.63) | 0.32 (0.25, 0.41) | < 0.001 |
| Model I | 0.26 (0.20, 0.33) | Ref | 0.92 (0.77, 1.10) | 0.71 (0.58, 0.87) | 0.51 (0.41, 0.64) | 0.32 (0.25, 0.41) | < 0.001 |
| Model II | 0.59 (0.45, 0.77) | Ref | 1.05 (0.88, 1.26) | 0.95 (0.78, 1.17) | 0.85 (0.68, 1.06) | 0.60 (0.46, 0.79) | < 0.001 |
| Model III | 0.59 (0.44, 0.78) | Ref | 1.04 (0.86, 1.25) | 0.91 (0.74, 1.11) | 0.83 (0.66, 1.04) | 0.61 (0.47, 0.80) | < 0.001 |
| Model IV | 0.57 (0.42, 0.75) | Ref | 0.98 (0.82, 1.18) | 0.84 (0.69, 1.04) | 0.80 (0.64, 1.01) | 0.61 (0.46, 0.79) | < 0.001 |
| LDL cholesterol (mmol/l) | |||||||
| Crude Model | 3.24 (2.70, 3.88) | Ref | 1.12 (0.85, 1.49) | 1.45 (1.12, 1.89) | 1.97 (1.53, 2.52) | 3.37 (2.67, 4.26) | < 0.001 |
| Model I | 3.21 (2.68, 3.85) | Ref | 1.12 (0.85, 1.49) | 1.45 (1.11, 1.88) | 1.96 (1.53, 2.51) | 3.34 (2.64, 4.22) | < 0.001 |
| Model II | 3.18 (2.40, 4.21) | Ref | 1.26 (0.93, 1.69) | 1.48 (1.09, 2.02) | 2.00 (1.45, 2.76) | 3.10 (2.17, 4.41) | < 0.001 |
| Model III | 2.72 (2.03, 3.63) | Ref | 1.25 (0.92, 1.70) | 1.40 (1.02, 1.92) | 1.80 (1.29, 2.50) | 2.65 (1.84, 3.82) | < 0.001 |
| Model IV | 2.60 (1.95, 3.48) | Ref | 1.22 (0.90, 3.48) | 1.37 (0.99, 1.88) | 1.70 (1.22, 2.37) | 2.50 (1.74, 3.60) | < 0.001 |
| LDL/HDL cholesterol ratio | |||||||
| Crude Model | 3.42 (2.93, 3.99) | Ref | 1.92 (1.40, 2.63) | 2.47 (1.82, 3.35) | 3.05 (2.27, 4.10) | 5.35 (4.05, 7.07) | < 0.001 |
| Model I | 3.38 (2.90, 3.95) | Ref | 1.92 (1.40, 2.64) | 2.47 (1.82, 3.35) | 3.05 (2.27, 4.10) | 5.32 (4.02, 7.03) | < 0.001 |
| Model II | 1.72 (1.44, 2.06) | Ref | 1.63 (1.18, 2.24) | 1.89 (1.39, 2.58) | 1.87 (1.38, 2.53) | 2.43 (1.80, 3.28) | < 0.001 |
| Model III | 1.64 (1.37, 1.97) | Ref | 1.70 (1.23, 2.35) | 1.83 (1.34, 2.51) | 1.89 (1.39, 2.56) | 2.41 (1.77, 3.27) | < 0.001 |
| Model IV | 1.66 (1.38, 1.99) | Ref | 1.68 (1.21, 2.33) | 1.78 (1.30, 2.44) | 1.86 (1.36, 2.55) | 2.40 (1.76, 3.27) | < 0.001 |
Crude model adjusted for none; Model I adjusted for gender and age; Model II adjusted for TC, TG and BMI; Model III adjusted for gender, age, ALP, GGT, ALT, AST, ALB, GLB, DBIL, CR, UA, FPG, TC, TG, height, BMI, SBP and DBP; Model IV adjusted for Model III and TP, TB, BUN
Abbreviations: NAFLD nonalcoholic fatty liver disease, CI confidence interval, HR hazard ratios; other abbreviations as in Table 1
Fig. 1.
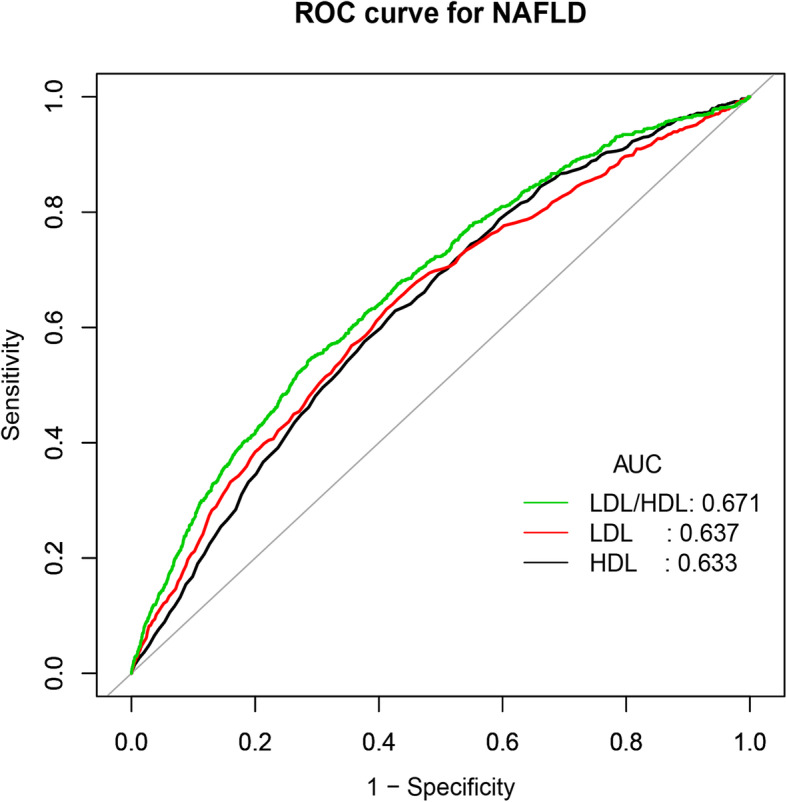
Receiver operating characteristic curve (ROC) of 5-year NAFLD events in non-obese population. AUC: area under the curve; LDL: low density lipoprotein; HDL: high density lipoprotein
Table 3.
AUC with the 95% CI of LDL cholesterol, HDL cholesterol and LDL/HDL cholesterol ratio for predicting NAFLD
| Variables | AUC | 95% CI lower bound | 95% CI upper bound | Best threshold | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| HDL cholesterol | 0.6335 | 0.6147 | 0.6522 | 1.4350 | 0.5734 | 0.6290 |
| LDL cholesterol | 0.6367 | 0.6168 | 0.6566 | 2.2050 | 0.5879 | 0.6314 |
| LDL/HDL cholesterol ratio | 0.6713 | 0.6523 | 0.6904 | 1.6623 | 0.7143 | 0.5422 |
Abbreviations: AUC area under the curve, CI confidence interval, HDL high-density lipoprotein, LDL low-density lipoprotein, NAFLD nonalcoholic fatty liver disease
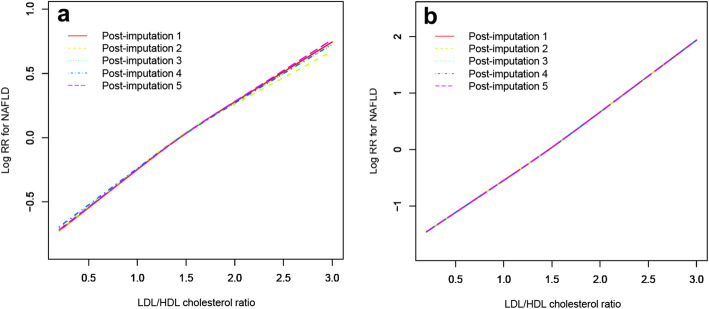
To verify the linear trend between the ratio of LDL/HDL cholesterol and new-onset NAFLD, the researchers established GAM to fit the association between the ratio of LDL/HDL cholesterol as a continuous variable and NAFLD by the cubic spline smoothing technique (Fig. 2). It is obvious from the figure that the linear positive correlation between the ratio of LDL/HDL cholesterol and new-onset NAFLD was stable before and after adjustment.
Fig. 2.
Association between the LDL/HDL cholesterol ratio and the risk of new-onset NAFLD in the unadjusted model (a) and adjusted model (b). Model adjusted for gender, age, ALP, GGT, ALT, AST, ALB, GLB, DBIL, CR, UA, FPG, TC, TG, height, BMI, SBP and DBP. Different line patterns indicated different data sources
Hierarchical analysis and test for interaction
Table 4 evaluates the interaction between the ratio of LDL/HDL cholesterol and age, gender, and glucose metabolism and performs a stratified analysis. Considering the research effect of stratification analysis, stratification factors FPG and age in this study were stratified according to the clinical cutoff point. The significant interaction between the LDL/HDL cholesterol ratio and age was related to NAFLD risk (P interaction< 0.05). In the case of a high LDL/HDL cholesterol ratio, the NAFLD risk decreased with increasing age. Additionally, the LDL/HDL cholesterol ratio and NAFLD risk were linearly positively associated in people < 60 years old, while the ratio of LDL/HDL cholesterol and NAFLD risk seemed to be an inverted U-shaped curve in people ≥60 years old (Additional file 2: Supplementary Fig. 1). However, no significant interaction was observed in gender and FPG stratification (P interaction> 0.05) (Supplementary Table 6, Additional file 1).
Table 4.
Stratified associations between LDL/HDL cholesterol ratio and NAFLD by gender, age, and FPG
| LDL/HDL cholesterol ratio (Quintile), HR (95%CI) | |||||||
|---|---|---|---|---|---|---|---|
| No. of cases | Q1 | Q2 | Q3 | Q4 | Q5 | P-interaction | |
| Gender | > 0.05 | ||||||
| Female | 393 (46.73%) | Ref | 1.84 (1.13, 3.01) | 2.17 (1.36, 3.47) | 2.17 (1.36, 3.45) | 2.58 (1.64, 4.05) | |
| Male | 448 (53.27%) | 1.08 (0.64, 1.85) | 1.73 (1.07, 2.82) | 1.72 (1.07, 2.77) | 1.84 (1.16, 2.91) | 2.48 (1.59, 3.88) | |
| Age, years | < 0.05 | ||||||
| ≥ 60 | 127 (15.10%) | Ref | 3.31 (1.66, 6.62) | 1.57 (0.73, 3.39) | 1.42 (0.67, 3.01) | 2.23 (1.14, 3.34) | |
| ≥ 45, < 60 | 247 (29.37%) | 1.74 (0.78, 3.87) | 2.05 (0.93, 4.48) | 2.26 (1.07, 4.75) | 2.46 (1.17, 5.16) | 3.50 (1.70, 7.20) | |
| ≥ 30, < 45 | 325 (38.64%) | 1.06 (0.41, 2.72) | 2.26 (0.94, 5.42) | 3.01 (1.27, 7.08) | 3.10 (1.33, 7.20) | 3.30 (1.43, 7.59) | |
| < 30 | 142 (16.88%) | 1.78 (0.57, 5.56) | 1.67 (0.58, 4.80) | 2.66 (0.94, 7.54) | 2.81 (1.04, 7.56) | 3.96 (1.50, 10.46) | |
| FPG, mmol/l | > 0.05 | ||||||
| ≤ 6.1 | 745 (88.59%) | Ref | 1.70 (1.20, 2.41) | 1.97 (1.40, 2.76) | 1.93 (1.38, 2.70) | 2.50 (1.80, 3.47) | |
| > 6.1 | 96 (11.41%) | 2.08 (1.04, 4.19) | 4.16 (2.20, 7.89) | 3.24 (1.86, 5.64) | 3.74 (2.18, 6.39) | 3.96 (2.48, 6.32) | |
Adjusted for TC, TG and BMI; Model III adjusted for gender, age, ALP, GGT, ALT, AST, ALB, GLB, DBIL, CR, UA, FPG, TC, TG, height, BMI, SBP and DBP
Note: the model was not adjusted for the stratification variable
Abbreviations: CI confidence interval, HR hazard ratios, FPG fasting plasma glucose, NAFLD nonalcoholic fatty liver disease
Discussion
This study reports the first longitudinal cohort study of the association between the ratio of LDL/HDL cholesterol and new-onset NAFLD risk. During a 5-year prospective follow-up, the cumulative incidence of NAFLD was 25.14%. The study found that in Chinese non-obese people with normal lipids, the LDL/HDL cholesterol ratio was significantly associated with new-onset NAFLD events, which was independent of other risk factors.
Much evidence has shown that HDL cholesterol and LDL cholesterol are closely related to NAFLD [1, 18]. This association was also verified by different models in this study, and the results were the same as the previous conclusion. These conventional lipoproteins have been widely used in the clinic to assess the risk of disease and to judge prognosis. In recent years, many studies have found that the combined indexes of blood lipids, such as the LDL/HDL cholesterol ratio, TG/HDL-cholesterol ratio, and TC/HDL-cholesterol ratio, have better predictive value than single lipoprotein in cardiocerebrovascular diseases, diabetes, and many metabolic diseases [19–21, 31, 32]. However, no studies have been reported on the association between the ratio of LDL/HDL cholesterol and NAFLD, so it is difficult to compare the findings of this study through previous studies. This study was the first to report that there was a positive correlation between the ratio of LDL/HDL cholesterol and new-onset NAFLD risk. This association was linear; even after adjusting for all noncollinear variables, this association remained unchanged. The ROC analysis further evaluated the predictive value of HDL cholesterol, LDL cholesterol and the LDL/HDL cholesterol ratio for new-onset NAFLD. The results showed that the LDL/HDL cholesterol ratio was better than LDL cholesterol and HDL cholesterol in predicting new-onset NAFLD.
This study confirms that a high LDL/HDL cholesterol ratio significantly increases NAFLD risk. Therefore, it may bring beneficial results by effectively reducing the ratio of LDL/HDL cholesterol. A recent nested case-control study of more than 10 million people from South Korea showed that statins could effectively reduce the risk of NAFLD by improving blood lipid metabolism [33]. In other studies of NAFLD treatment, it has also been observed that NAFLD patients benefit from statin treatment [34, 35]. Therefore, the research team suggests that for people with a high LDL/HDL cholesterol ratio, low-dose statins can be used for primary prevention under the guidance of physicians.
This study also investigated the relationship between the ratio of LDL/HDL cholesterol and NAFLD in other subgroups. Studies have shown that there was an interaction between age and LDL/HDL cholesterol ratio-related NAFLD risk. In the group with a higher LDL/HDL cholesterol ratio, the risk of NAFLD decreases with age, which may be related to the unhealthy lifestyle of young people [36]. In the population ≥ 60 years old, the association between them seems to be an inverted U-shaped curve.
This study demonstrates that a high LDL/HDL cholesterol ratio was an independent risk factor for new-onset NAFLD. However, the underlying mechanism is still unclear. In a previous study, Kawamoto et al. found that in non-obese people, the LDL/HDL cholesterol ratio was the best substitute index of insulin resistance (IR) compared to HDL cholesterol, non-HDL cholesterol, TG, LDL cholesterol and the TG/HDL cholesterol ratio [37]. Therefore, researchers speculate that IR may be a potential mediator of NAFLD risk caused by a high LDL/HDL cholesterol ratio. It is well known that the incidence of NAFLD is mainly related to IR and lipid metabolism disorders [1, 2]. In this study, people with abnormal lipid metabolism were excluded. Therefore, a high LDL/HDL cholesterol ratio can be mainly considered a high LDL cholesterol level or low HDL cholesterol level within the normal reference range. In a recent study, Ampuero’s team found that the levels of oxidized LDL cholesterol antibodies/HDL cholesterol were significantly higher in lean NAFLD patients [38]. When the body has elevated levels of oxidized LDL cholesterol antibodies, oxidized LDL cholesterol reduces insulin sensitivity by inhibiting signaling kinase and/or activating the nuclear factor-κB subunit 1 complex responsible for the cellular response to insulin, resulting in IR [39, 40]. High oxidized LDL cholesterol levels will also increase the secretion of the proinflammatory adipocytokines TNF-α and IL6 [40], and the inflammatory response will further lead to IR, which will lead to lipid accumulation and form a vicious cycle [41]. In addition, previous studies have shown that in hepatic steatosis, an alteration in the activity of the transcription factors SREBP-1c and PPAR-alpha is observed. These changes will directly affect the hepatic synthesis of fatty acids, cholesterol and lipoproteins and cause inflammation and antioxidation in the liver [42]. On the other hand, when the HDL cholesterol level is low, the body’s ability to reverse transport cholesterol will decrease, so that the increase in LDL cholesterol level is more prone to LDL cholesterol oxidation [43].
Study strengths and limitations
The advantage of this study lies in adopting a longitudinal cohort design, large sample size and relatively strict adjustment of statistical variables, which can explain the causal association between the ratio of LDL/HDL cholesterol and new-onset NAFLD. This finding provides a new monitoring tool for preventing and intervening in non-obese healthy people and non-obese NAFLD patients in China.
Although this study has some unique advantages, some limitations are still worth discussing. First, the related indicators of IR were not measured in this study, so it was impossible to evaluate the association between the ratio of LDL/HDL cholesterol and IR. Although it has been found by reviewing the literature that IR may be the intermediate factor of the high LDL/HDL cholesterol ratio causing NAFLD, the complex association still needs to be explored in further studies. Second, although the possible risk factors were widely adjusted in this study, there were still some risk factors that could not be measured or obtained, which may lead to unavoidable residual confusion. Third, previous studies have shown that in non-obese patients, the correlation between metabolic syndrome and NAFLD is significantly stronger than that of obese people [16], which may be the main reason why non-obese people are susceptible to NAFLD. However, due to the particularity of the subjects in this study, metabolic syndrome cannot be diagnosed in the subjects according to the current diagnostic criteria of metabolic syndrome [44], so the correlation cannot be further verified. Finally, the definition of non-obesity in this study was BMI < 25 kg/m2, but there were differences in the definition of non-obesity among different ethnic groups [45]; therefore, the results of this study are only for reference for other ethnic groups.
Conclusion
The LDL/HDL cholesterol ratio is an independent predictor of NAFLD in Chinese non-obese people with normal lipids, and its predictive value is higher than that of other lipoproteins. The best cutoff value of the LDL/HDL cholesterol ratio for predicting NAFLD provided in this study was 1.6623. This longitudinal cohort study’s findings provide an objective and simple marker for clinical workers to assess the risk of NAFLD, which helps identify high-risk groups of NAFLD early for early active intervention.
Supplementary Information
Additional file 1: Table S1. The description of missing data. Table S2. Collinearity diagnostics steps. Table S3. Results of multivariate linear regression among original data and post-imputation data. Table S4. Results of multivariate linear regression among original data and post-imputation data. Table S5. Results of multivariate linear regression among original data and post-imputation data. Table S6. Stratified associations between LDL/HDL cholesterol ratio and NAFLD by sex, age, and FPG.
Additional file 2: Figure S1. Association between the LDL/HDL cholesterol ratio and the risk of new-onset NAFLD in people ≥60 years old. Adjusted for gender, ALP, GGT, ALT, AST, ALB, GLB, DBIL, CR, UA, FPG, TC, TG, height, BMI, SBP and DBP. Different line patterns indicated different data sources.
Acknowledgements
Special thanks to Professor Sun’s team for sharing the longitudinal cohort study data and Dr. Yuliang Zhan for supervising the whole study.
Abbreviations
- TC
Total cholesterol
- NAFLD
Nonalcoholic fatty liver disease
- AST
Aspartate aminotransferase
- TG
Triglycerides
- BMI
Body mass index
- LDL/HDL
Low density lipoprotein to high density lipoprotein
- UA
Uric acid
- HR
Hazard ratios
- S/DBP
Systolic/diastolic blood pressure
- DBIL
Direct bilirubin
- BUN
Blood urea nitrogen
- ALP
Alkaline phosphatase
- CI
Confidence interval
- FPG
Fasting plasma glucose
- TP
Total Protein
- ALB
Albumin
- ROC
Receiver operating characteristic curve
- GLB
Globulin
- TB
Total bilirubin
- GGT
Gamma glutamyl transferase
- ALT
Alanine aminotransferase
- GAM
Generalized additive model
- IR
Insulin resistance; Cr: creatinine
Authors’ contributions
Conceptualization and research design; GT-S and YZ. Supervision: GT-S, LZ and CH; Project administration: NP and MC-Z; Writing-Original draft preparation: YZ; Writing- Reviewing and Editing: YZ, GT-S, LZ, CH, NP and MC-Z. The author(s) read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Datasets that support the conclusions of this article were available in the [DRYAD] repository. [https://datadryad.org].
Declarations
Ethics approval and consent to participate
Because of the previous study has been approved by the institutional review boards of Wenzhou people’s Hospital and the written informed consent of the participants, there was no need to apply for ethical approval again for this study.
Consent for publication
Not applicable.
Competing interests
No.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169:ITC65–ITC80. doi: 10.7326/AITC201811060. [DOI] [PubMed] [Google Scholar]
- 2.Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res. 2020;126:679–704. doi: 10.1161/CIRCRESAHA.119.316337. [DOI] [PubMed] [Google Scholar]
- 5.Wattacheril J. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gastroenterol Clin N Am. 2020;49:141–149. doi: 10.1016/j.gtc.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: what we need in the future. Liver Int. 2018;38(Suppl 1):47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 9.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Rodas MC, Valenzuela R, Videla LA. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci. 2015;16:25168–25198. doi: 10.3390/ijms161025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Wang AY, Dhaliwal J, Mouzaki M. Lean non-alcoholic fatty liver disease. Clin Nutr. 2019;38:975–981. doi: 10.1016/j.clnu.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y, Yu M, Sheng G. Association between fasting plasma glucose and nonalcoholic fatty liver disease in a nonobese Chinese population with normal blood lipid levels: a prospective cohort study. Lipids Health Dis. 2020;19:145. doi: 10.1186/s12944-020-01326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 16.Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 17.Lee SW, Lee TY, Yang SS, Tung CF, Yeh HZ, Chang CS. Risk factors and metabolic abnormality of patients with non-alcoholic fatty liver disease: either non-obese or obese Chinese population. Hepatobiliary Pancreat Dis Int. 2018;17:45–48. doi: 10.1016/j.hbpd.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Amor AJ, Perea V. Dyslipidemia in nonalcoholic fatty liver disease. Curr Opin Endocrinol Diabetes Obes. 2019;26:103–108. doi: 10.1097/MED.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XX, Wei M, Shang LX, Lu YM, Zhang L, Li YD, et al. LDL-C/HDL-C is associated with ischaemic stroke in patients with non-valvular atrial fibrillation: a case-control study. Lipids Health Dis. 2020;19:217. doi: 10.1186/s12944-020-01392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Yin P, Lu C, Li J, Zang Z, Liu Y, et al. Association of LDL-C/HDL-C ratio with stroke outcomes within 1 year after onset: a hospital-based follow-up study. Front Neurol. 2020;11:408. doi: 10.3389/fneur.2020.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunutsor SK, Zaccardi F, Karppi J, Kurl S, Laukkanen JA. Is high serum LDL-C/HDL-C cholesterol ratio an emerging risk factor for sudden cardiac death? Findings from the KIHD study. J Atheroscler Thromb. 2017;24:600–608. doi: 10.5551/jat.37184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun DQ, Wu SJ, Liu WY, Wang LR, Chen YR, Zhang DC, et al. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6:e013781. doi: 10.1136/bmjopen-2016-013781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D-Q, et al. Data from: association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study, Dryad, dataset. 2016. 10.5061/dryad.1n6c4. [DOI] [PMC free article] [PubMed]
- 24.World Health Organization. Definition . Diagnosis and classification of diabetes mellitus and its complications—part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. pp. 20–21. [Google Scholar]
- 25.Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, et al. Chinese National Consensus Workshop on nonalcoholic fatty liver disease. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 26.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 28.Fitchett EJA, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, et al. Strengthening the reporting of observational studies in epidemiology for newborn infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16:e202–e213. doi: 10.1016/S1473-3099(16)30082-2. [DOI] [PubMed] [Google Scholar]
- 29.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, et al. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. 2020;19:33. doi: 10.1186/s12944-020-01213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arsenault BJ, Rana JS, Stroes ES, Després JP, Shah PK, Kastelein JJ, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55:35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 33.Lee JI, Lee HW, Lee KS, Lee HS, Park JY. Effects of statin use on the development and progression of nonalcoholic fatty liver disease: a Nationwide nested case-control study. Am J Gastroenterol. 2021;116:116–124. doi: 10.14309/ajg.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 34.Athyros VG, Alexandrides TK, Bilianou H, Cholongitas E, Doumas M, Ganotakis ES, et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement Metabolism. 2017;71:17–32. doi: 10.1016/j.metabol.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg EH, Wolters AAB, Dullaart RPF, Moshage H, Zurakowski D, de Meijer VE, et al. Prescription of statins in suspected non-alcoholic fatty liver disease and high cardiovascular risk, a population-based study. Liver Int. 2019;39:1343–1354. doi: 10.1111/liv.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65:2100–2109. doi: 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 37.Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, et al. Low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Lipids Health Dis. 2010;9:138. doi: 10.1186/1476-511X-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ampuero J, Ranchal I, Gallego-Durán R, Pareja MJ, Del Campo JA, Pastor-Ramírez H, et al. Oxidized low-density lipoprotein antibodies/high-density lipoprotein cholesterol ratio is linked to advanced non-alcoholic fatty liver disease lean patients. J Gastroenterol Hepatol. 2016;31:1611–1618. doi: 10.1111/jgh.13335. [DOI] [PubMed] [Google Scholar]
- 39.Ishigaki Y, Oka Y, Katagiri H. Circulating oxidized LDL: a biomarker and a pathogenic factor. Curr Opin Lipidol. 2009;20:363–369. doi: 10.1097/MOL.0b013e32832fa58d. [DOI] [PubMed] [Google Scholar]
- 40.Santiago-Fernández C, Martin-Reyes F, Tome M, Ocaña-Wilhelmi L, Rivas-Becerra J, Tatzber F, et al. Oxidized LDL modify the human adipocyte phenotype to an insulin resistant, Proinflamatory and Proapoptotic profile. Biomolecules. 2020;10:534. doi: 10.3390/biom10040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. doi: 10.1186/s12944-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenzuela R, Videla LA. Impact of the co-administration of N-3 fatty acids and olive oil components in preclinical nonalcoholic fatty liver disease models: a mechanistic view. Nutrients. 2020;12:499. doi: 10.3390/nu12020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen DE, Fisher EA. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis. 2013;33:380–388. doi: 10.1055/s-0033-1358519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 45.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The description of missing data. Table S2. Collinearity diagnostics steps. Table S3. Results of multivariate linear regression among original data and post-imputation data. Table S4. Results of multivariate linear regression among original data and post-imputation data. Table S5. Results of multivariate linear regression among original data and post-imputation data. Table S6. Stratified associations between LDL/HDL cholesterol ratio and NAFLD by sex, age, and FPG.
Additional file 2: Figure S1. Association between the LDL/HDL cholesterol ratio and the risk of new-onset NAFLD in people ≥60 years old. Adjusted for gender, ALP, GGT, ALT, AST, ALB, GLB, DBIL, CR, UA, FPG, TC, TG, height, BMI, SBP and DBP. Different line patterns indicated different data sources.
Data Availability Statement
Datasets that support the conclusions of this article were available in the [DRYAD] repository. [https://datadryad.org].