Abstract
29Si NMR spectroscopy, the method of continuous variations, and density functional theory computations show that sodium hexamethyldisilazide (NaHMDS) is a disolvated dimer in toluene, a mixture of disolvated dimer and tetrasolvated monomer in THF/toluene, and exclusively monomer in neat THF. The dioxane-solvated NaHMDS only partially deaggregates to monomer even in neat dioxane. 15N–29Si coupling constants and 29Si chemical shifts show a high and dependable correlation with the aggregation state. Monitoring either chemical shift or coupling constant versus THF concentration even in the high-temperature, rapid-exchange limit affords the solvation numbers consistent with DFT computations. The preparation of 15N-labelled NaHMDS has been improved.
A select few groups have been hailing the organic chemistry community to pay more attention to organosodium chemistry. We joined in only recently and possibly may not be the last to do so. To this end, we submit that sodium hexamethyldisilazide (NaHMDS) is arguably the preeminent organosodium reagent in both academic and industrial laboratories.1 Despite its prominence, studies of its properties in solution are restricted to a couple of NMR spectra2,3 and a handful of computations.4
We have embarked on an extensive study using a combination of methods to determine the structure of NaHMDS in over 30 commonly employed organic solvents to begin to study how aggregation and solvation influence reactivity and selectivity.5 An unexpectedly important protocol revolves around the interrogation of structure using 15N–29Si coupling observed in 15N-labelled NaHMDS. Lukevics and coworkers used natural abundance to examine 1JN–Si coupling in a series of disilazanes including NaHMDS in benzene.3 Unaided by additional data, their suggestion that NaHMDS is tetrameric in benzene was suspect,2a,b but the tactic had merit.
We find a highly predictable correlation of 15N–29Si coupling with aggregation state. The high sensitivity, resolution, and quantitation offered by 29Si NMR spectroscopy and the low cost of the 15N label (7% the cost of an NMR tube) render this of potential interest to those studying M-N(SiR3)2 and M-N(SiR3)(R) derivatives. We present herein preliminary studies that focus on three prominent solvents: toluene, THF, and dioxane.
A 29Si{1H} INEPT experiment circumvented the problems posed by background signal from glass NMR tubes and NOE effects,6 allowing the relative integrations to be ascertained. [15N]NaHMDS was prepared in 45% overall yield from 15NH4Cl by an optimized protocol.7 1H and 13C NMR spectra are archived Supporting Information. Density functional theory (DFT) computations were carried out at the M06–2X level of theory8,9 for geometry optimizations and single-point calculations.10 The standard Def2-SVP basis set was used for geometry optimizations and the expanded basis set Def2-TZVP for single point calculations.11,12
The high solubility of NaHMDS in toluene and insolubility in hexane implicated an explicit π complexation as observed crystallographically for many metals, including sodium.13 The 29Si NMR spectrum shows a doublet with 1JN–Si = 7.9 Hz akin to that for the benzene solvate noted by Lukevics, which proves to be characteristic of dimeric NaHMDS (Figure 1).
Figure 1.
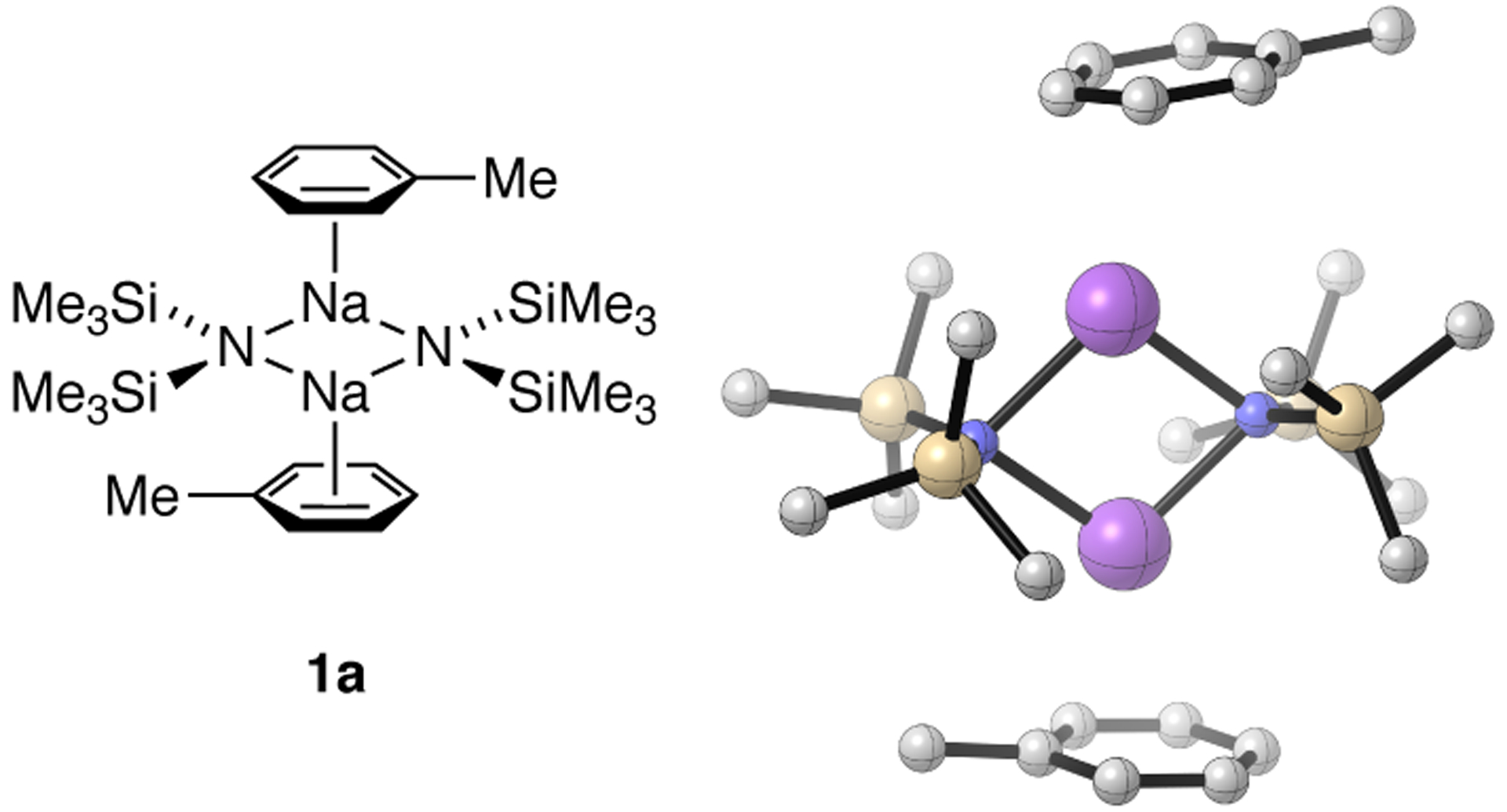
DFT-computed toluene-complexed dimer 1a.
The dimer assignment was secured using the method of continuous variations (MCV).14 Mixtures of NaHMDS and the structurally similar sodium tetramethyldisilazide [NaTMDS; (HMe2Si)2NNa]15 in toluene shows two homodimers along with a heterodimer manifesting a 29Si singlet corresponding to the TMDS fragment and a doublet with coupling characteristic of dimers (1JN–Si = 8.9 Hz; Figure 2c). Plotting the proportions of homo- and heteroaggregates versus measured16 mole fraction of NaHMDS (XNaHMDS) affords the Job plot in Figure 3. Quantitative heterodimerization is supported computationally (eq 1) and presumably derives from steric relief in the NaHMDS homodimer 1. An analogous Job plot is obtained in toluene with 5.0 equiv of THF, conditions in which THF quantitatively displaces toluene to form 1b as shown by titrations.13 Dimer 1b has been characterized crystallographically.2c
Figure 2.

29Si NMR spectra of (a) [15N]NaHMDS (0.20 M) in toluene at −80 °C showing homodimer 1a, (b) NaTMDS (0.20 M) in toluene at −80 °C showing homodimer 2a, (c) a 1:1 mixture of [15N]NaHMDS and NaTMDS (0.20 M total titer) in toluene recorded at −80 °C showing homo- and heterodimers 1a and 3a, and (d) 29Si NMR spectrum of 0.15 M [15N]NaHMDS in 0.75 M THF with Me2NEt (DMEA) as cosolvent recorded at −120 °C shows dimer 1b and monomer 4.
Figure 3.

Job plot showing relative integrations of the 29Si resonances of NaHMDS homodimer 1a (red), NaTMDS-derived homodimer 2a (blue), and heterodimer 3a (green; eq 1) versus the measured16 mole fraction of NaHMDS (XNaHMDS) at 0.20 total molarity17 in neat toluene-d8 at −80 °C.
Titration of solutions of [15N]NaHMDS (0.15 M) in DMEA to record 29Si NMR spectra with added THF at −120 °C reveals a markedly upfield-shifted resonance displaying a large coupling (1JN–Si = 13.4 Hz, Figure 2d) characteristic of NaHMDS monomers (Figure 4). The monomer becomes the sole observable form by 10 equiv (1.50 M).
Figure 4.

DFT-computed, THF-complexed dimer 1a and monomer 4.
Exchange of free and sodium-bound THF is rapid even at −120 °C. Although computations are supportive of both di-and tetrasolvated dimer, a host of other monodentate solvents show disolvation to be the norm.5 Couplings and chemical shifts are proxies for aggregation even in the high-temperature, rapid-exchange limit as illustrated in Figure 5. Figure 6 shows the temperature dependence of the deaggregation, revealing the anticipated preference for monomer at lower temperatures. Moreover, the curves in Figures 5 and 6 result from fits according to the equilibrium in Figure 4 with tetrasolvated monomer 4. Analogous fits assuming a trisolvated monomer are decidedly inferior.
Figure 5.

The 29Si chemical shift (green) and 15N–29Si coupling constants (black) plotted versus [THF] in 2:1 pentane/toluene as cosolvent measured at −20 °C. The curves are fits to a model based on an A2S2–AS4 equilibrium (Supporting Information).
Figure 6.

The 15N–29Si coupling constants plotted versus [THF] in 2:1 pentane/toluene as cosolvent measured at −20 °C (black), 20 °C (green), and 50 °C (blue). The functions are fits to a model based on an A2S2–AS4 equilibrium (Supporting Information).
The correlation of coupling constant to aggregation state can also be used to assign dimer-monomer ratios in solvents that eluded assignment at lower temperatures. The prominent ethereal solvent 1,4-dioxane produced a highly insoluble white crystalline material at low temperature probably owing to a polymeric network of monomers characterized crystallographically,18 precluding solution structural studies. 29Si spectra in the rapid exchange limit at 20 °C show dioxane-concentration-dependent coupling consistent with partial deaggregation of dimer 5 to monomer 6 at 20 °C. In neat dioxane, the 10.7 Hz coupling indicates that approximately 50% of the titer derives from monomer 6. DFT computations indicate monomer 6 is only trisolvated.
We have shown that chemical shift and 15N–29Si coupling for the dimers (7.5–8.5 Hz) and monomers (13.0–13.5 Hz), in conjunction with results from a much more broadly based study,5 are highly diagnostic of aggregation state. From a single spectrum, even at ambient temperatures, one can assess the relative proportions of monomers and dimers. Ironically, in over a dozen papers describing the structure of lithium hexamethyldisilazide using 15N-labelled substrate in >100 solvents, we did not record a single 29Si NMR spectrum: we didn’t need them. Belatedly, we find the analogous [15N]LiHMDS/THF dimer and monomer 15N–29Si couplings are 7.0 and 11.7 Hz, respectively. We suspect that other organometallic complexes with silazide-based ligands are likely to show diagnostic trends as well.
Supplementary Material
Figure 7.

The 15N–29Si coupling constants plotted versus [THF] (black) and [1,4-dioxane] (green) in 2:1 pentane/toluene as cosolvent measured at 20 °C. The functions are to fit to a model based on an A2S2–AS4 equilibrium (Supporting Information).
Funding Sources
We thank the National Institutes of Health (GM131713) for support.
Footnotes
Supporting Information: Spectroscopic, computational, and MCV data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.“Sodium hexamethyldisilazide”.; Watson BT; Lebel H In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, New York; 2005, p 1–10. [Google Scholar]
- 2.(a) Knizek J; Krossing I; Noth H; Schwenk H; Seifert T Synthesis and Structures of Sodium Phenylhydrazides. Chem. Berichte/Recueil 1997, 130, 1053. [Google Scholar]; (b) Driess M; Pritzkow H; Skipinski M; Winkler U Synthesis and Solid State Structures of Sterically Congested Sodium and Cesium Silyl(Fluorosilyl)Phosphanide Aggregates and Structural Characterization of the Trimeric Sodium Bis(Trimethylsilyl)Amide. Organometallics 1997, 16, 5108. [Google Scholar]; (c) Karl M; Seybert G; Massa W; Harms K; Agarwal S; Maleika R; Stelter W; Greiner A; Neumüller WH; Dehnicke K Amidometallate von Seltenerdelementen. Synthese Und Kristallstrukturen von [Na(12-Krone-4)2][M{N(SiMe3)2}3(OSiMe3)] (M = Sm, Yb), [Na(THF)3Sm{N(SiMe3)2}3(C≡C-Ph)], [Na(THF)6][Lu2(μ-NH2)(μ-NSiMe3){N(SiMe3)2}4] Sowie von [NaN(SiMe3)2(THF)]2. Anwen-dungen Der. Zeitschrift für Anorg. und Allg. Chemie 1999, 625, 1301. [Google Scholar]; (d) Ojeda-Amador AI; Martínez-Martínez AJ; Kennedy AR; Armstrong DR; O’Hara CT Monodentate Coordination of the Normally Chelating Chiral Diamine (R,R)-TMCDA. Chem. Commun 2017, 53, 324. [DOI] [PubMed] [Google Scholar]; (e) Forbes GC; Kennedy AR; Mulvey RE; Rodger PJA TEMPO: A Novel Chameleonic Ligand for s-Block Metal Amide Chemistry. Chem. Commun 2001, 1, 1400. [Google Scholar]; (f) Neufeld R; Michel R; Herbst-Irmer R; Schöne R; Stalke D Introducing a Hydrogen-Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexamethyldisilazides (MHMDS, M=Li, Na, K, Rb and Cs) with Ammonia. Chem. - A Eur. J 2016, 22, 12340. [DOI] [PubMed] [Google Scholar]; (g) Goodwin CAP; Smith A; Ortu F; Vitorica-Yrezabal IJ; Mills DP Salt Metathesis versus Protonolysis Routes for the Synthesis of Silylamide Hauser Base (R2NMgX; X = Halogen) and Amido-Grignard (R2NMgR) Complexes. Dalt. Trans 2016, 45, 6004. [DOI] [PubMed] [Google Scholar]; (h) Schüler P; Görls H; Westerhausen M; Kriec S Bis(trimethylsilyl)amide Complexes of s-Block Metals with Bidentate Ether and Amine Ligands. Dalton Trans 2019, 48, 8966. [DOI] [PubMed] [Google Scholar]; (i) A molecular weight measurement of NaHMDS in Et2O implicated dimer: Wannagat, U. N-Metallated Silicon-Nitrogen Derivatives: Preparation, Structure, and Reactions. Pure Appl. Chem 1969, 19, 329. [Google Scholar]
- 3.Kupce E; Lukevics E Silicon-29-Nitrogen-15 Spin-Spin Coupling Constants in Silazanes. Organometallics 1988, 7, 1649. [Google Scholar]
- 4.Luo G; Luo Y; Qu J Direct Nucleophilic Trifluoromethylation Using Fluoroform: A Theoretical Mechanistic Investigation and Insight into the Effect of Alkali Metal Cations. New. J. Chem 2013, 37, 3274. [Google Scholar]
- 5.Woltornist RA; Collum DB, unpublished.
- 6.Uhlig FD; Marsmann HC Silicon-29 NMR Some Practical Aspects in Gelest Catalog Silicon Compounds: Silanes & Silicones Aufl. (2003) 208. Morrisville, PA. USA: self-published. [Google Scholar]
- 7. [15N]Hexamethyldisilazane was prepared from [15N]NH319 by mixing [15N]ammonium chloride (3.0 g, 55.0 mmol, >99% 15N isotopic purity) with 6.00 g (0.15 mol) of granular NaOH in a 25 mL in a one-neck, round-bottom flask equipped cooled to −78 °C. The mixture was warmed with a heat gun for approximately 20 min. After the transfer of ammonia was complete, 1-(trimethylsilyl)imidazole (14.7 g, 15.3 mL, 105 mmol, 98% purity) was added to ammonia at −78 °C and stirred. HCl begins to off-gas and imidazole precipitates immediately. Anhydrous diethyl ether (20 mL) is then added to the flask, and the mixture is held at 0 °C for 40 min. Cholesterol (3.0 g) was added to the [15N]hexamethyldisilazane with stirring for 45 min to remove excess 1-(trimethylsilyl)imidazole. Short-path distillation at atmospheric pressure removed the diethyl ether. Vacuum distillation (40 mm Hg, 20 °C) afforded 4.75 mL (47% yield) of (Me3Si)215NH.; (b) [15N]-Sodium hexamethyldisilazide (1) was prepared by a known procedure as follows.20 Sliced sodium metal (1.20 g, 52.4 mmol) was added to a flame-dried, fine-mesh swivel frit setup (Supporting Information) in the glovebox. The apparatus was moved to a Schlenk line for the remainder of the procedure. Under an argon atmosphere, [15N]HMDS (7.31 g, 9.45 mL, 45.0 mmol) and 40 mL DMEA were added to the reaction flask at room temperature. Isoprene (2.62 mL, 26.2 mmol) dissolved in 8 mL of dry DMEA was then added over 1 hr via syringe pump to the mixture. After addition of isoprene, the reaction was stirred at rt for an additional 2 hr. The solution was then filtered through the frit, canula transferred to a second coarse swivel frit setup, and the solution evaporated to dryness under vacuum for at least 10 h to yield a white powder. The powder was then suspended in dry pentane (~20 mL), stirred for 1 h, and filtered. Washing with an additional 20 mL of pentane yielded 6.70 g (91% yield) of [15N]NaHMDS, which was transferred to the glovebox and stored at room temperature. It should be noted that NaHMDS can be recrystallized as described previously20 but with no detectable improvement. 1H NMR spectrum (500 MHz, toluene-d8) δ 0.160 ppm; 13C NMR spectrum (125.72 MHz, toluene-d8) δ 7.28 ppm; 29Si NMR spectrum (99.36 MHz, toluene-d8) −14.6 ppm.; NaTMDS (2) was prepared using the same method described for NaHMDS. 13C NMR spectrum (125.72 MHz, toluene-d8) δ 5.93 ppm; 29Si NMR spectrum (99.36 MHz, toluene-d8) −25.57 ppm.
- 8.Zhao Y; Truhlar DG The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Non-covalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc 2008, 120, 215. [Google Scholar]
- 9.In a number of organosodium studies we have noted that computations at the MP2 level of theory can fail to afford minima or single-point energies on what seem like pedestrian structures. This has not been a problem using the M06 functionals.
- 10.Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Zakrzewski VG; Montgomery JA Jr.; Stratmann RE; Burant JC; Dapprich S; Millam JM; Daniels AD; Kudin KN; Strain MC; Farkas O; Tomasi J; Barone V; Cossi M; Cammi R; Mennucci B; Pomelli C; Adamo C; Clifford S; Ochterski J; Petersson GA; Ayala PY; Cui Q; Morokuma K; Malick DK; Rabuck AD; Raghavachari K; Foresman JB; Cioslowski J; Ortiz JV; Baboul AG; Stefanov BB; Liu G; Liashenko A; Piskorz P; Komaromi I; Gomperts R; Martin RL; Fox DJ; Keith T; Al-Laham MA; Peng CY; Gill A; Nanayakkara C; Gonzalez M; Challacombe PMW; Johnson B; Chen W; Wong MW; Andres JL; Gonzalez C; Head-Gordon M; Replogle ES; Pople JA Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- 11.Weigend F; Ahlrichs R Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys 2005, 7, 3297. [DOI] [PubMed] [Google Scholar]
- 12.CYLview, 1.0b; Legault CY, Université de Sherbrooke, 2009. (http://www.cylview.org).
- 13.Yamada S Cation-π Interactions in Organic Synthesis. Chem. Rev 2018, 118, 11353. [DOI] [PubMed] [Google Scholar]
- 14.Renny JS; Tomasevich LL; Tallmadge EH; Collum DB Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem., Int. Ed 2013, 52, 11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Eppinger J; Herdtweck E; Anwander R Synthesis and Characterisation of Alkali Metal Bis(Dimethylsilyl) Amides: Infinite All-Planar Laddering in the Unsolvated Sodium Derivative. Polyhedron 1998, 17, 1195 [Google Scholar]; (b) Schneider J; Popowski E; Reinke H Darstellung, Charakterisierung Und Reaktionsverhalten von NatriumUnd Kaliumhydridosilylamiden R2(H)Si-N(M)R’ (M = Na, K) - Kristallstruktur von [(ME3C)2(H)Si-N(K)SiMe3]2 ·THF. Zeit. Anorg. Allg. Chem 2003, 629, 55. [Google Scholar]
- 16.The measured mole fraction—the mole fraction within only the ensemble of interest—eliminates the distorting effects of impurities. This problem has been highlighted:; Hibberta DB; Thordarson P The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun 2016, 52, 12792. [DOI] [PubMed] [Google Scholar]
- 17.The concentration of NaHMDS, although expressed in units of molarity, refers to the concentration of the monomer subunit (normality).
- 18.(a) Edelmann FT; Pauer F; Wedler M; Stalke D Preparation and Structural Characterization of Dioxane Coordinated Alkali Metal Bis(Trimethylsilyl)Amides. Inorg. Chem 1992, 31, 4143. [Google Scholar]; For an extensive review on the chemistry of the alkali metal amides, see:; Mulvey RE; Robertson SD Synthetically Important Alkali-Metal Utility Amides: Lithium, Sodium, and Potassium Hexamethyl-disilazides, Diisopropylamides, and Tetramethylpiperidides. Angew. Chem., Int. Ed 2013, 52, 11470. [DOI] [PubMed] [Google Scholar]
- 19.Mailyan AK; Chen JL; Li W; Keller AA; Sternisha SM; Miller BG; Zakarian A Short Total Synthesis of [15N5]-Cylindrospermopsins from 15NH4Cl Enables Precise Quantification of Freshwater Cyanobacterial Contamination. J. Am. Chem. Soc 2018, 140, 6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasevich LL; Collum DB Method of Continuous Variation: Characterization of Alkali Metal Enolates Using 1H and 19F-NMR Spectroscopies. J. Am. Chem. Soc 2014, 136, 9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


