Abstract
Considering advice from others is a pervasive element of human social life. We used the judge-advisor paradigm to investigate the neural correlates of advice evaluation and advice integration by means of functional magnetic resonance imaging. Our results demonstrate that evaluating advice recruits the “mentalizing network,” brain regions activated when people think about others’ mental states. Important activation differences exist, however, depending upon the perceived competence of the advisor. Consistently, additional analyses demonstrate that integrating others’ advice, i.e., how much participants actually adjust their initial estimate, correlates with neural activity in the centromedial amygdala in the case of a competent and with activity in visual cortex in the case of an incompetent advisor. Taken together, our findings, therefore, demonstrate that advice evaluation and integration rely on dissociable neural mechanisms and that significant differences exist depending upon the advisor’s reputation, which suggests different modes of processing advice depending upon the perceived competence of the advisor.
Keywords: Advice evaluation, Advice integration, Judge-advisor paradigm, Functional magnetic resonance imaging (fMRI)
Few things are as central to our everyday lives as judgment and decision-making. Often, however, we do not make these judgments and decisions alone but receive advice from others, sometimes because we actively consult experts, sometimes because others spontaneously offer advice. While integrating the advice of others into our judgments and decisions is, thus, an essential process of everyday life, relatively little is known about the processes that underlie advice-taking. Behaviorally, advice-taking has been investigated extensively in social psychology using the so called “judge-advisor system” (JAS: Sniezek & Buckley, 1995). In this paradigm, a person (the “judge”) first makes a decision or judgment, then receives advice from another person (the “advisor”) and, subsequently, has the opportunity to revise her initial judgment. Comparing the judge’s final and initial estimates allows calculating the “weight (assigned to a given piece) of advice” (WOA; Yaniv, 2004a).
Research using the JAS has shown that heeding advice is usually beneficial as it leads to better judgments and decisions (Sniezek, Schrah, & Dalal, 2004; Yaniv, 2004b). It has also provided us with a good understanding of the situational and personal factors that influence the degree to which advice is taken into account (for a review, see Bonaccio & Dalal, 2006). However, little is known about the cognitive processes that are involved in advice-taking. The only existing models of advice-taking (Jungermann & Fischer, 2005; Soll & Larrick, 2009) are prescriptive in that they are more interested in predicting under which circumstances and to what degree judges utilize advice, but do not elaborate on the underlying neural mechanisms. More general theories in judgment and decision-making research suggest that belief updating includes two consecutive processes, namely information evaluation and information integration (Anderson, 1991; Hogarth & Einhorn, 1992). Applying this idea to the JAS suggests that advice is first evaluated with regard to its quality based on different information such as the advisors’ expertise or reputation and then integrated. This second step, advice integration, can be thought of as the process of computing a weighted mean of the advice and the initial judgment. Plausibly, the weights assigned to the advice on one hand and the initial judgment on the other depend on the perceived quality of advice, derived during advice evaluation, as well as the judge’s confidence in the initial estimate.
Although advice evaluation and advice integration are conceptually different processes, they are difficult—if not impossible—to differentiate using a purely behavioral approach. In light of the important conceptual differences, however, we hypothesize advice evaluation and advice integration to rely on different neural mechanisms. To test this hypothesis, we employed event-related functional magnetic resonance imaging (fMRI) to investigate and differentiate the neural correlates of advice evaluation and advice integration. Furthermore, we were interested to explore the impact of the advisor’s expertise on the neural mechanisms of advice-taking, as the advisor’s reputation has been shown to strongly influence advice-taking in previous research (Harvey & Fischer, 1997; Yaniv & Kleinberger, 2000). Here, an intriguing possibility is that differences in (perceived) advisor competence lead to differences in neural activations (cf. Klucharev, Smidts, & Fernandez, 2008) implicating different modes of processing for competent and less competent advisors.
To these ends, we developed an fMRI-compatible version of the JAS, in which participants had to estimate distances between pairs of European capitals and were subsequently given (1) the possibility to reflect upon their own initial judgment and revise it (no advice) or (2) were given advice from an allegedly (2a) incompetent or (2b) competent advisor (reputation of advisor: COM vs. INCOM) and the subsequent possibility to revise their own initial judgment (Figure 1). In order to investigate the neural correlates of advice evaluation, we employed a general linear model (GLM)-based subtraction analysis, which allows contrasting regional brain activation during the evaluation of one’s own initial judgment with those during the evaluation of advice provided by either a competent or incompetent advisor. In order to also explore the neural correlates of advice integration, we used a second set of statistical analyses employing a model-based approach: here, the weight assigned by participants to a given piece of advice (WOA) was calculated for each trial and used to construct a parametric regressor to search for brain regions, whose hemodynamic response profile co-varies with the extent to which participants actually adjust their initial judgment to the advice.
Figure 1.
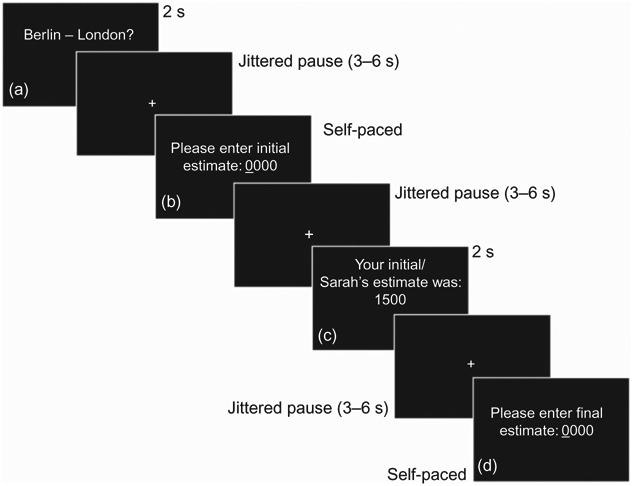
Individual trial structure. (a) Presentation of pair of capital cities. (b) Input screen. Judgments were made via button press. (c) Evaluation screen. (d) Input screen allowing participants to confirm or change their initial judgment via button press.
METHODS
Participants
Twenty-seven right-handed, healthy volunteers (aged 21–50, mean age: 28.26; 16 females) with no record of neurologic or psychiatric illness participated in this fMRI study. All volunteers were naive with respect to the experimental task as well as to the purpose of the study. Handedness was confirmed using the Edinburgh Handedness Questionnaire (Oldfield, 1971). All subjects gave informed written consent to the study protocol, which had been approved by the local ethics committee of the Medical Faculty of the University of Cologne, Germany.
Experimental protocol
Before participation, all participants received standardized instructions and were familiarized with the task on a laptop computer. Participants were instructed that they would be asked to estimate the distance between pairs of European capitals in kilometres (Figure 1a) and to enter their initial estimate by pressing buttons on an MRI-compatible response pad (LumiTouch, Burnaby, Canada) to generate a four-digit number (Figure 1b). In order to ensure that four buttons had to be pressed during each trial, numbers smaller than four-digit numbers had to be started by pressing the zero-button an adequate number of times. Before starting the scanning sessions participants were given time to familiarize themselves with the MRI-compatible response pad. After having provided the initial estimate, participants were either given the opportunity to re-evaluate their own initial judgment, which was shown to them on the stimulus screen, or would be given advice also presented on the stimulus screen, i.e., they would be informed about the estimate of one of two other participants concerning the given pair of European capitals (Figure 1c). In order to assign two advisors to each participant before the scanning session, we implemented a computerized mock random generator, which was said to determine the advisors’ names, whose estimates would later be shown to the study participant inside the scanner. Unbeknownst to the subjects, the random generator always assigned two same-sex advisors, who were described as either being the 7th or the 73rd best of an alleged group of 100 participants, who, as the participants of the fMRI study were told, had taken part in a pilot study leading up to the neuroimaging experiment. In spite of this suggestion, the advice provided during scanning was, in fact, algorithmically varied as a function of the initial judgment provided by the participant and could deviate by either 20 or 60% from the initial judgment. After being presented with the advice or the possibility to rethink their own initial judgment, participants were given the opportunity on each trial to change or confirm their initial judgment by again pressing the buttons on the response pad (Figure 1d). No feedback about the actual distance between the two cities was given to the participant on any given trial. The interstimulus interval (ISI) was jittered between 2 s and 6 s. During the experiment, 60 trials were presented in a fully randomized order. All visual stimuli were presented during scanning using the software package Presentation (Version 11.3; Neurobehavioral Systems, Albany, CA, USA) and were displayed on a custombuilt, shielded TFT screen at the rear end of the scanner room visible via a mirror mounted to the head coil (~12° × 8° viewing angle, 245 mm distance from the subject’s eyes).
Behavioral data analysis
The dependent variable was the WOA calculated as the absolute value of the change between initial and final judgment in relation to the absolute distance between the initial judgment and the advice given for each trial:
The WOA assumes a value of 0 when final and initial judgments are identical, i.e., when the judge completely ignores the advice given. In cases where the judge adjusts completely to the advice given, the WOA value becomes 1, whereas values >1 indicate that the final judgment has been adjusted so strongly that it surpasses the given advice. The WOA is used as a measure of advice utilization in almost all previous behavioral studies on advice-taking (e.g., Gino, 2008; Gino & Moore, 2007; Gino, Shang, & Croson, 2009; Harvey & Fischer, 1997; See, Morrison, Rothman & Soll, 2011; Yaniv, 2004b). The behavioral measurements, i.e., the initial and final distance judgments, obtained during the fMRI experiment were analyzed off-line using MATLAB (MathWorks, Natick, MA, USA). The effect of the experimental factor (reputation of advisor: COM vs. INCOM) on WOA values was compared by means of a repeated measures analysis of variance (repeated measures ANOVA).
Functional magnetic resonance imaging
Images were acquired on a Siemens Trio 3T whole-body scanner (Erlangen, Germany) using blood-oxygen-level-dependent (BOLD) contrast (Gradient-echo planar imaging (EPI) pulse sequence, TR = 2200 ms, in plane resolution = 3.1 × 3.1 mm, 36 axial slices, 3.1 mm thickness) covering the whole brain. Image acquisition was preceded by four dummy images allowing for magnetic field saturation. These were discarded prior to further processing. Images were analyzed using Statistical Parametric Mapping (SPM8) (www.fil.ion.ucl.ac.uk/spm). First, the EPI images were corrected for head movements by affine registration using a two-pass procedure, by which images were initially realigned to the first image and subsequently to the mean of the realigned images. After realignment, the mean EPI image for each subject was spatially normalized to the Montreal Neurological Institute (MNI) single subject template using the “unified segmentation” approach (Ashburner & Friston, 2003). The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subjects’ data into the space of the MNI tissue probability maps, were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes that were hereby transformed into the MNI single subject space and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8 mm full-width half-maximum (FWHM) Gaussian kernel to meet the statistical requirements of the GLM and to compensate for residual macro-anatomical variations across subjects.
The fMRI data were analyzed using a GLM as implemented in SPM8. Each experimental condition was modelled using a series of stick-functions denoting the individual events. These were convolved with a canonical hemodynamic response function (HRF) and its first-order temporal derivative. Low-frequency signal drifts were filtered using a cut-off period of 128 s. Parameter estimates were subsequently calculated for each voxel using weighted least squares to provide maximum likelihood estimators based on the temporal autocorrelation of the data (Kiebel & Holmes, 2003). No global scaling was applied. For each subject, simple main effects for each experimental condition were computed by applying appropriate baseline contrasts. These individual first-level contrasts were then fed into a second-level group-analysis using an ANOVA (factor: condition, blocking factor: subject) employing a random-effects model. In the modelling of variance components, we allowed for violations of sphericity by modelling non-independence across images from the same subject and allowing unequal variances between conditions and subjects using the standard implementation in SPM8. The resulting SPM(T) maps were interpreted by referring to the probabilistic behavior of Gaussian random fields (Worsley et al., 1996) and thresholded at p < .05 (cluster-level corrected for multiple comparisons). The cluster-forming threshold at the voxel-level was set to puc < .001.
On the group level, two sets of specific statistical analyses were conducted: the first set of analyses investigated categorical differences between the experimental conditions in order to assess the neural signature of advice evaluation. In order to do so, our analysis was tailored to the event during each trial, in which participants were presented with their own initial judgment (SELF) or the advice given by either a competent (COM) or incompetent advisor (INCOM) (Figure 1c). Consequently, the main effect of SELF, COM, and INCOM were calculated. To assess activation differences between the three conditions appropriate contrasts were used (SELF > COM, SELF > INCOM; COM > SELF, COM > INCOM; INCOM > SELF, INCOM > COM; (INCOM + COM) > SELF; SELF > (INCOM + COM)). Furthermore, we analyzed the commonality across all three conditions (SELF ∩ INCOM ∩ COM) by means of a conjunction null analysis thresholded at p < .05 (family-wise error (FWE) corrected for multiple comparisons).
The second set of analyses used a model-based approach to investigate the neural correlates of advice integration. Here, we used the WOA value calculated for each trial to also construct a regressor, which investigates the parametric modulation of general advice processing by WOA and, thus, allows to search for brain regions, whose hemodynamic response in relation to advice presentation co-varies with the extent to which participants actually adjust their initial judgment to that of either the competent (WOA_COM) or incompetent (WOA_INCOM) advisor.
Functional activations were anatomically localized by using the SPM anatomy toolbox (Eickhoff et al., 2007) employing a maximum probability map (MPM). This map (Eickhoff, Heim, Zilles, & Amunts, 2006) denotes the most likely anatomical area at each voxel of the MNI single subject template based on probabilistic cytoarchitectonic maps derived from the analysis of cortical areas in a sample of 10 human post-mortem brains, which were subsequently normalized to the MNI reference space. If no cytoarchitectonic maps were available, the macro-anatomical labels are provided by the Automated Anatomic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002).
RESULTS
Behavioral data
Mean WOA values calculated from the behavioral data obtained during scanning are shown in Figure 2. Repeated measures ANOVA of WOA values showed a significant main effect of the reputation of the advisor (COM vs. INCOM; F(1, 26) = 41.527, p < .001), indicating a stronger adjustment of the initial judgment towards advice given by the competent advisor.
Figure 2.
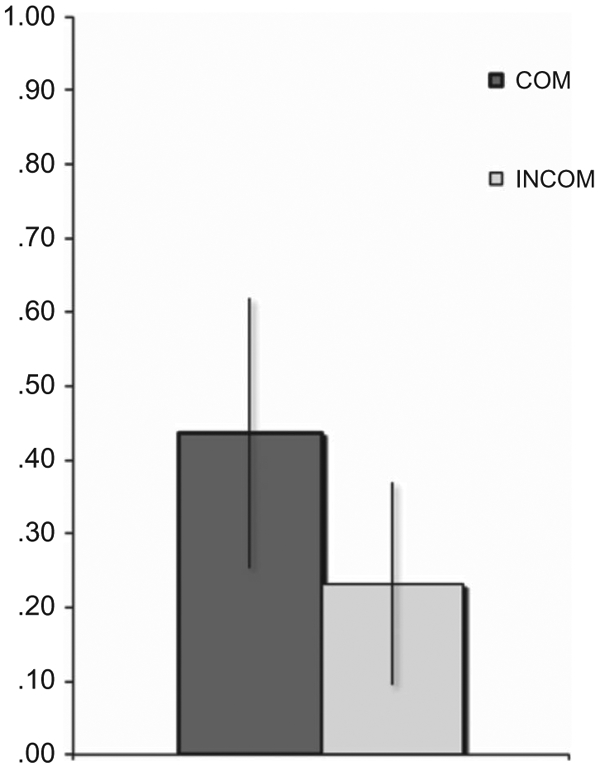
Mean WOA values for experimental conditions, in which advice was provided. Error bars depict standard deviations. COM: competent advisor, INCOM: incompetent advisor.
Neural correlates of advice evaluation
Conjunction analysis
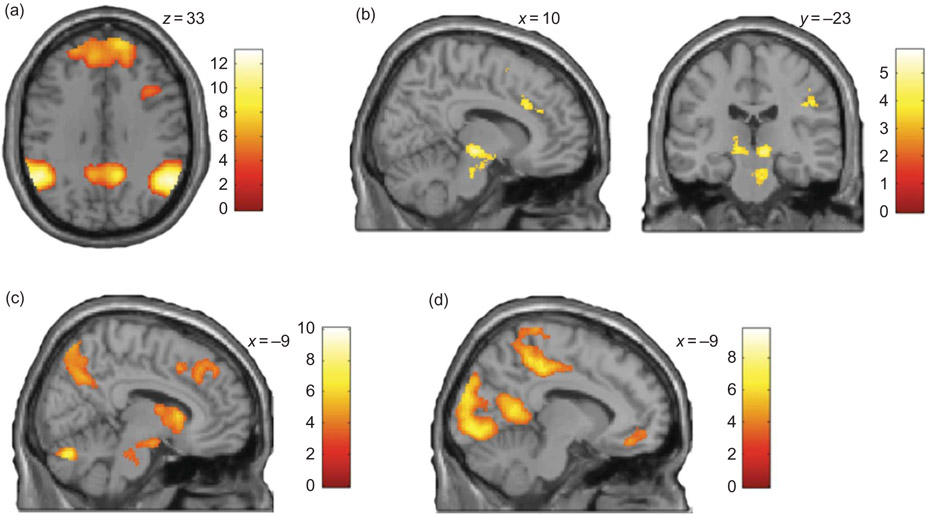
By applying a conjunction analysis across all three experimental conditions focusing on the event during which either advice was provided or the participant was shown her own initial judgment on the stimulus screen (SELF ∩ INCOM ∩ COM), i.e., the moment of advice evaluation, the conjunction analysis demonstrates involvement of a neural network including dorso-medial prefrontal, inferior parietal and medial temporal cortices bilaterally, inferior and middle frontal gyrus bilaterally, and the precuneus (Figure 3a, Table 1).
Figure 3.
Neural correlates of advice evaluation. (a) Results of conjunction analysis (SELF ∩ COM ∩ INCOM). (b) Differential effects of the evaluation of competent vs. incompetent advice (COM > INCOM). (c) Neural correlates of being presented with advice irrespective of the advisor’s reputation as compared to re-assessing one’s own initial judgment (COM + INCOM) > SELF and (d) neural correlates of being presented with one’s own initial judgment as compared to being given advice (SELF > (COM + INCOM)).
TABLE 1.
Neural correlates (main effects at p < .05 cluster-level corr. for multiple comparisons; conjunction analysis at p < .05 voxel-level FWE corr. MNI coordinates of principally activated voxels for each cluster are given)
| Brain region | x | y | z | k | T |
|---|---|---|---|---|---|
| SELF ∩ COM ∩ INCOM (Figure 3a) | |||||
| Right uperior frontal fyrus | 18 | 58 | 24 | 9190 | 10.511 |
| Right angular gyrus | 54 | −54 | 28 | 3504 | 12.791 |
| Right middle temporal gyrus | 64 | −28 | −9 | 2768 | 11.141 |
| Left supramarginal gyrus | −58 | −52 | 24 | 2650 | 13.101 |
| Left middle temporal gyrus | −62 | −32 | −10 | 1610 | 9.501 |
| Right precuneus | 8 | −56 | 36 | 1478 | 10.391 |
| Right inferior frontal gyrus (p. orbitalis) | 51 | 26 | −4 | 1321 | 8.481 |
| Right middle frontal gyrus | 44 | 16 | 45 | 1296 | 8.951 |
| Left middle frontal gyrus | −42 | 15 | 48 | 607 | 10.471 |
| Left inferior frontal gyrus (p. orbitalis) | −54 | 30 | −10 | 342 | 8.591 |
| Left inferior frontal gyrus (p. triangularis) | −56 | 20 | 14 | 272 | 8.391 |
| Left inferior temporal gyrus | −52 | −3 | −36 | 146 | 7.471 |
| Left middle orbital gyrus | −30 | 56 | −9 | 124 | 6.941 |
| Right middle cingulate cortex | 2 | −22 | 39 | 33 | 5.711 |
| Right middle occipital gyrus | 34 | −93 | 2 | 18 | 5.41 |
| Left inferior temporal gyrus | −58 | −16 | −30 | 16 | 5.48 |
| Left cerebellum | −20 | −70 | −28 | 3 | 5.26 |
| Right inferior occipital gyrus | 45 | −84 | −9 | 1 | 5.20 |
| Left cerebellum | −22 | −74 | −30 | 1 | 5.41 |
| Right inferior temporal gyrus | 46 | 2 | −34 | 1 | 5.13 |
| COM > INCOM (Figure 3b) | |||||
| Left inferior parietal lobule | 6 | −57 | −18 | 369 | 4.14 |
| Substantia nigra | 9 | −20 | −8 | 2517 | 5.511 |
| Left SMA | −3 | 4 | 56 | 1882 | 5.211 |
| Right rolandic operculum | 56 | −18 | 21 | 803 | 4.22 |
| Left precentral gyrus | −50 | 2 | 34 | 397 | 4.00 |
| Right cerebellum | 30 | −58 | −32 | 322 | 4.39 |
| Left inferior frontal gyrus (p. triangularis) | −36 | 28 | 3 | 172 | 4.70 |
| Left precentral gyrus | −33 | −3 | 62 | 153 | 3.84 |
| Left rolandic operculum | −52 | 9 | 2 | 142 | 3.89 |
| Left cerebellum | −9 | −51 | −14 | 137 | 4.27 |
| Right rolandic operculum | 58 | 4 | 14 | 116 | 4.30 |
| Left inferior frontal gyrus (p. triangularis) | −50 | 42 | 10 | 94 | 4.41 |
| INCOM > COM (not illustrated) | |||||
| Left insula lobe | −26 | 20 | 16 | 231 | 4.81 |
| Right cuneus | 15 | −93 | 8 | 138 | 4.11 |
| (COM + INCOM) > SELF (Figure 3c) | |||||
| Right angular gyrus | 28 | −60 | 40 | 10,643 | 10.071 |
| Right middle frontal gyrus | 46 | 39 | 21 | 10,165 | 7.891 |
| Left precentral gyrus | −50 | 6 | 34 | 8588 | 8.501 |
| Left inferior parietal lobule | −34 | −50 | 40 | 8509 | 9.621 |
| Left cerebellum | −32 | −64 | −32 | 7555 | 8.491 |
| Left superior medial gyrus | 2 | 26 | 40 | 4450 | 7.641 |
| Right caudate nucleus | 14 | 0 | 12 | 1452 | 5.901 |
| Left middle frontal gyrus | −26 | −4 | 50 | 1018 | 6.711 |
| Right amygdala | 10 | −8 | −18 | 690 | 4.79 |
| Right middle temporal gyrus | 57 | 0 | −30 | 421 | 4.61 |
| SELF > (COM + INCOM) (Figure 3d) | |||||
| Right cuneus | 12 | −93 | 15 | 14,311 | 9.841 |
| Right middle cingulate cortex | 9 | −26 | 45 | 6748 | 6.781 |
| Right superior temporal gyrus | 54 | −33 | 21 | 6500 | 6.541 |
| Left postcentral gyrus | −52 | −10 | 15 | 2762 | 5.671 |
| Left precuneus | −9 | −54 | 10 | 1846 | 6.331 |
| Left postcentral gyrus | −36 | −26 | 52 | 1081 | 5.09 |
| Left middle temporal gyrus | −42 | −45 | 8 | 1029 | 5.661 |
| Right precentral gyrus | 50 | −14 | 54 | 992 | 6.791 |
| Right calcarine gyrus | 14 | −60 | 16 | 834 | 4.79 |
| Left superior temporal gyrus | −57 | 3 | −12 | 619 | 5.601 |
| Left rolandic operculum | −44 | 2 | 10 | 317 | 5.491 |
| Left rectal gyrus | −10 | 44 | −15 | 287 | 4.73 |
| Right mid orbital gyrus | 9 | 44 | −14 | 265 | 4.75 |
| Right fusiform gyrus | 32 | −42 | −14 | 242 | 4.61 |
| Left inferior frontal gyrus (p. orbitalis) | −32 | 36 | −16 | 208 | 5.411 |
| Right insula lobe | 40 | −12 | −4 | 181 | 4.68 |
| Anterior cingulate cortex | −2 | 34 | 8 | 162 | 4.33 |
| Left superior temporal gyrus | −40 | −3 | −9 | 119 | 4.67 |
Note:
also significant at p < .05 FWE voxel-level corr.
Competent vs. incompetent advisor
Significant activation differences are also observed when contrasting advice evaluation in the case of a competent to the case of an incompetent advisor (COM > INCOM): here, a differential increase of neural activity is observed in the left inferior parietal lobe (IPL) predominantly in the left hemisphere, in the supplementary motor area (SMA), and anterior cingulate cortex (ACC). Also, a differential effect is seen in the substantia nigra (SN), the ventral tegmental area (VTA), and the ventral striatum (VS) (Figure 3b, Table 1). The inverse contrast (INCOM > COM) demonstrated a differential increase of neural activity in the left insula and right cuneus (not illustrated, Table 1).
Advice vs. no advice
Our first set of analyses further explored differences between being presented with one’s own initial judgment as compared to being presented with advice (regardless of whether this was provided by a competent or an incompetent advisor): here, our results demonstrate that being presented with advice ((COM + INCOM) > SELF) leads to a differential increase in a bilateral fronto-parietal network including right and left middle frontal gyri and inferior parietal cortex, the ACC, the VTA, and the caudate nucleus bilaterally (Figure 3c; see Table 1). Using the inverse contrast, i.e., investigating brain regions which show a differential increase of neural activity when presented with one’s own initial judgment as compared to being presented with advice (SELF > (COM + INCOM)), demonstrates involvement of visual and motor cortices, the precuneus and the right temporo-parietal junction (TPJ) (Figure 3d; see Table 1).
Neural correlates of advice integration
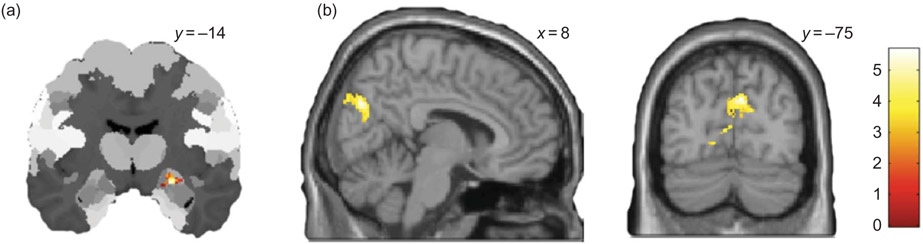
The second set of analyses used a parametric modulation to investigate the neural correlates of advice integration by taking into consideration the WOA values that were calculated for each trial, during which advice was provided to the participant. This model-based analysis demonstrates that adjusting to the competent advisor’s opinion (WOA_COM) correlates with neural activity in the right centromedial amygdala (CMA) and the left inferior frontal gyrus (Figure 4a; see Table 2) while adjustment to the incompetent advisor’s opinion (WOA_INCOM) correlates with neural activity in primary visual and inferotemporal cortices (Figure 4b; see Table 2).
Figure 4.
Neural correlates of advice integration. (a) Parametric effect of integrating competent advice (WOA_COM). (b) Parametric effect of integrating incompetent advice (WOA_INCOM).
TABLE 2.
Neural correlates of parametric modulation at p < .05 cluster-level corr. for multiple comparisons; MNI coordinates of principally activated voxels for each cluster are given
| Brain region | x | y | z | k | T |
|---|---|---|---|---|---|
| WOA_COM (Figure 4a) | |||||
| Left superior frontal gyrus | −26 | −2 | 66 | 337 | 4.76 |
| Left cuneus | −12 | −63 | 28 | 189 | 4.29 |
| Right centromedial amygdala (CMA) | 24 | −14 | −9 | 174 | 5.411 |
| Left superior parietal lobule | −28 | −75 | 46 | 173 | 4.35 |
| Left inferior frontal gyrus (p. orbitalis) | −36 | 24 | −14 | 172 | 4.60 |
| WOA_INCOM (Figure 4b) | |||||
| Right cuneus | 8 | −75 | 32 | 999 | 5.691 |
| Left calcarine gyrus | −20 | −52 | 4 | 274 | 5.351 |
| Left calcarine gyrus | −4 | −76 | 9 | 225 | 4.97 |
| Left lingual gyrus | −12 | −70 | −4 | 151 | 4.90 |
Note:
also significant at p < .05 FWE voxel-level corr.
It should be noted that the localization of neural activity to the centromedial portion of the amygdala needs to be interpreted with caution given the current resolution of fMRI and the susceptibility of the medial temporal lobe to imaging artefacts. Nevertheless, the applied histological atlas allows anatomical allocation in a probabilistic fashion, hereby accommodating some of the uncertainty in establishment of structure-function relationships (cf. Amunts et al., 2005; Eickhoff et al., 2005). Moreover, this approach has already been repeatedly shown to allow the attribution of functional activation to specific parts of the amygdala (Müller et al., 2011; Mutschler et al., 2010; Roy et al., 2009).
DISCUSSION
We have used fMRI in conjunction with the well-established judge-advisor paradigm to investigate the neural correlates of advice-taking. Specifically, we aimed at differentiating the neural substrate of advice evaluation, i.e., assessing the quality of advice, from the neural substrate of advice integration, i.e., combining advice and the initial judgment into a final judgment, and at investigating differences in advice evaluation and integration depending upon the perceived competence of the advisor. While the evaluation of advice—irrespective of the advisor’s reputation—recruits dorso-medial prefrontal and temporo-parietal cortices, evaluation of advice coming from an allegedly competent as compared to an incompetent advisor leads to recruitment of IPL, nigrostriatal areas, SMA, and the anterior insula. Similarly, differences in the underlying neural correlates also exist for advice integration: here, our results demonstrate that adjustment of one’s own initial judgment correlates with activity change in the right CMA in the case of a competent advisor and with activity change in visual and inferotemporal cortex in the case of an incompetent advisor.
Neural correlates of advice evaluation
As the neural correlate of advice evaluation our study implicates the involvement of a neural network, which bears strong resemblance with what has been described as the “mentalizing network,” a set of brain regions known to become active when people think about other people’s mental states (e.g., Frith & Frith, 2008; Spreng & Grady, 2010; Van Overwalle, 2009). Indeed, the evaluation of the quality of advice given to us might be closely linked to our assessment of the advisor’s putative mental states, most notably her reasoning behind the advice (Yaniv, 2004a) or her intentions, i.e., whether she might be inclined to offer helpful rather than misleading pieces of information (van Swol, 2009). Consistent with previous research suggesting that similar processes may underlie thinking about oneself and others (e.g., Benoit, Gilbert, Volle, & Burgess, 2010), the neural network observed to underlie advice evaluation (regardless of the nature of the advisor) is also (at least in part) recruited during situations when participants are presented with their own initial judgment as evidenced by the triple conjunction analysis (e.g., Lombardo et al., 2010; Spreng, Mar, & Kim, 2009).
When contrasting whether evaluation was concerned with advice provided by others as compared to those situations when participants were faced with their own initial judgment, our analysis shows a differential recruitment of a fronto-parietal network commonly described as the neural correlate of top–down control of attention in the former case (e.g., Corbetta, Patel, & Shulman, 2008), suggesting that assessment of advice coming from others might be an attentionally more demanding exercise. The fronto-parietal network, however, has also been implicated in a wide range of other tasks (e.g., mental calculation, control of eye-movements, action monitoring, working memory) and has been described as the “task-positive” or “extrinsic” network to denote its recruitment during goal-directed tasks, which require processing of externally presented rather than endogenously generated stimuli (e.g., Boly et al., 2007; Fox et al., 2005).
Furthermore, the contrast comparing advice evaluation to the contemplation of one’s own initial estimate demonstrates involvement of the caudate nuclei, which have previously been implicated in reward-based learning (e.g., Delgado, Frank, & Phelps, 2005; Ding & Gold, 2010; Sharot, Shiner, & Dolan, 2010) and the acquisition and control of goal-directed actions (Brovelli, Nazarian, Meunier, & Boussaoud, 2011). Interestingly, the caudate nuclei have also recently been shown to be relevant for learning and decision-making in a social context, namely when people learn from other’s experience: in an fMRI study by Canessa, Motterlini, Alemanno, Perani, and Cappa (2011) it was shown that the caudate nucleus tracked the outcomes of previously made decisions both in the case of observing others making such decisions or making them oneself. Consistent with this, findings from a recent study by Cooper, Dunne, Furey, and O’Doherty (2012) suggest a dissociation between the involvement of the dorsal and ventral striatum during reward-based decision-making depending upon the role of the participant, i.e., being an observer or an active participant of the decision-making process.
The inverse contrast, which targets a differential increase of neural activity related to thinking about one’s own initial judgment as compared to thinking about advice (regardless of whether this comes from a competent or incompetent advisor), resulted in activations of visual cortex, the precuneus and right TPJ. Consistent with our findings, the latter regions have, indeed, been highlighted as contributing to self-referential processing and the differentiation between self and other (e.g., Bahnemann, Dziobek, Prehn, Wolf, & Heekeren, 2010; Brass, Ruby, & Spengler, 2009; Giardina, Caltagirone, & Oliveri, 2011; Lenggenhager, Smith, & Blanke, 2006; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008).
Furthermore, our analysis of advice evaluation focused on differences between evaluating advice coming from an allegedly competent as compared to an incompetent advisor (and vice versa): here, our results, indeed, show striking activation differences for the perception of a piece of advice coming from a competent or an incompetent advisor. Given that visual stimulation was identical in both conditions, these differences are likely to be the result of a top–down modulation. Our findings provide evidence for a differential increase of neural activity in IPL during the perception of competent as compared to incompetent advice, predominantly in the left hemisphere. In the context of decision-making, this area is known to play an important role in integrating prior with current sensory information (e.g., Preuschhof, Schubert, Villringer, & Heekeren, 2010). As IPL is known to act as a crucial interface between action observation and preparation (Kockler et al., 2010; Tunik, Rice, Hamilton, & Grafton, 2007), even during the performance of an unrelated task (Sinke, Sorger, Goebel, & de Gelder, 2010), it is tempting to interpret this finding in terms of a higher computational load being put on regions relevant for the integration and usage of sensory information for action control and preparation in the presence of advice from a competent rather than an incompetent person.
Additionally, a differential effect for advice evaluation in the case of a competent as compared to an incompetent advisor was found in the nigrostriatal system. More specifically we observed a differential increase of neural activity in the midbrain (SN & VTA) and the ventral striatum, areas, which are thought be relevant for reinforcement learning by computing a reward prediction error (e.g., Murray et al., 2008; Schultz, Dayan, & Montague, 1997). Consistent with this suggestion, striatal-midbrain connectivity has actually been shown to predict how reinforcements are used to guide decisions (e.g., Kahnt et al., 2009). Interestingly, a recent study by Biele and colleagues (2011) invokes the notion that evaluations of the outcomes from recommended options will be more positive than non-recommended options and that the underlying neural correlate of this “outcome-bonus” consists in activity change in the nigrostriatal system. What this indicates is that advice provided by others may provide an additional (possibly “intrinsic”) reward (cf. Campbell-Meiklejohn, Bach, Roepstorff, Dolan, & Frith, 2010; Schilbach et al., 2010), which can influence decision-making processes. Other research demonstrates that the opinions of others can lead to social conformity—adjusting our judgments in line with group opinion—which might also be based on mechanisms of reinforcement learning and engagement of the VS (Burke, Tobler, Schultz, & Baddeley, 2010; Klucharev, Hytonen, Rijpkema, Smidts, & Fernandez, 2009). On a more abstract level, these findings are consistent with a proposition made by Behrens and colleagues (2008), who argue and empirically demonstrate that social information is acquired using the same associative processes, which are assumed to underlie reward-based learning.
Our findings add to this most interesting body of evidence by demonstrating that the relevant midbrainstriatal system is more strongly activated in the presence of an allegedly competent as compared to an incompetent advisor. This finding is in line with evidence, which suggests that social reputation has a powerful impact on humans’ behavior and was shown to activate reward-related neurocircuitry, particularly when participants are asked to act in front of socially reputable observers (Izuma, Saito, & Sadato, 2008, 2010).
Neural correlates of advice integration
In a second set of analyses, we took a model-based approach using the WOA value for each trial, in which advice was given, to construct a parametric regressor, which allows searching for brain regions, whose hemodynamic responses scale with the magnitude of the WOA values. This analysis demonstrates that adjusting to the competent advisor’s opinion correlates with neural activity in the right CMA and left inferior frontal gyrus (Figure 3c). While the amygdaloid complex is known to play a crucial role in social cognition, emotion, value representation, and decision-making (e.g., Bzdok et al., 2011; Gospic et al., 2011; Pessoa, 2010; Seymour & Dolan, 2008), the CMA, in particular, plays a crucial role in modulating various other brain regions relevant for the occurrence of behavioral responses: e.g., the CMA projects to the midbrain periaqueductal gray (PAG), the hypothalamus and the brainstem, which are known to coordinate various defensive responses and autonomic arousal (Misslin, 2003; Mosher, Zimmerman, & Gothard, 2010). Unfortunately, we did not have any psychophysiological measures (such as skin conductance or heart rate) available during our study to investigate this further. Also, projections from the CMA exist to the basal forebrain and, here, provide an important modulatory input to fronto-striatal dopaminergic neurotransmission relevant for reward-based decision-making (Fudge & Haber, 2000). Via this mechanism the amygdala has been suggested to directly influence behavioral responding with the centromedial complex possibly being more relevant for avoidance-related behaviors and learning (Fudge & Haber, 2000; Gozzi et al., 2010; Prevost, McCabe, Jessup, Bossaerts, & O’Doherty, 2011). Also, classical conditioning-like learning processes, which have been closely related to amygdala function, could thereby guide more sophisticated action-selection processes that underlie judgment and decision-making processes (Seymour & Dolan, 2008).
In line with the suggestion of an influence of CMA on fronto-striatal circuitry relevant for decision-making, our analysis demonstrates that activity change in CMA correlates with the extent to which participants actually adjusted to the competent advisor’s opinion. This finding is also largely consistent with findings by Biele et al. (2011): in their study it was found that the amygdala also implemented the “outcome-bonus” observed in the nigrostriatal system, i.e., a response pattern specific to the added “social” value a decision might produce. Both results indicate that the amygdala—and in particular its centromedial complex—is involved in realizing the influence a given advice may have on decision-making.
Future investigations using the judge-advisor paradigm could include changes of the design to allow for the investigation of the effective connectivity between CMA and the nigrostriatal system. In light of the known modulatory effects of oxytocin (OXC) on the amygdala (e.g., Gamer & Büchel, 2012; Viviani et al., 2011) and a possible relationship of amygdala activity and the perception of trustworthiness in an advisor (Bzdok et al., 2011), future studies could also explore OXC’s impact on advice-taking. Similarly, the known effects of testosterone on amygdalar function (e.g., van Wingen, Ossewaarde, Bäckström, Hermans, & Fernández, 2011) could be taken to suggest that single-shot testosterone administration might be effective in influencing advice-taking, possibly by increasing egocentric biases (e.g., Wright et al., 2012).
Concerning the neural correlate of advice integration in the inferior frontal gyrus, it appears relevant that numerous studies have demonstrated recruitment of inferior frontal cortex during task switching, response inhibition and cognitive control (Christakou et al., 2009; Derrfuss, Brass, Neumann, & von Cramon, 2005; Goghari & MacDonald, 2009; Jakobs et al., 2009; Sridharan, Levitin, & Menon, 2008; Swick, Ashley, & Turken, 2008). More specifically, evidence suggests that the inferior frontal gyrus can be differentially engaged when cognitive and motivational signals interact during inhibitory control (Padmala & Pessoa, 2010; Savine & Braver, 2010; Schulz et al., 2009).
When using the WOA values from trials in which an incompetent advisor’s opinion was presented to the participants, to perform a parametric analysis, we observe a correlation with neural activity in visual and inferotemporal cortices. It is noteworthy that activations in these areas are also found for self-referential processing. This, one might argue, could be taken to suggest that adjustment to an incompetent person’s advice may rely in part on similar mechanisms as contemplating one’s own initial judgment. Apart from this speculation, activity change in visual and inferotemporal cortices has been related to visual imagery (e.g., Cichy, Heinzle, & Haynes, 2012) and the acquisition of perceptual expertise (e.g., DeGutis & D’Esposito, 2007; Suzuki & Tanaka, 2011).
Clearly, our study has limitations: in our task the revealing of the advisor and the revealing of the advice amount takes place at the same time, which makes it difficult to make strong claims about the processes that underlie the evaluation of the advisor as compared to the evaluation of advice per se to be dissociable. While it is debatable whether in everyday life situations source and content of advice are commonly dissociated, future research could help to provide evidence in this respect. Another aspect that may be seen to complicate the interpretation of the fMRI data is uncertainty about when exactly participants compute their second estimate. It is conceivable that participants computed this already at the time point when they received the advice not at the second entry screen, thereby requiring the second estimate to be kept in working memory. However, a similar argument could also be made for the initial estimate. As we do not have any evidence suggesting that first and second estimate differ systematically, we treat both estimation periods similarly. Future research could help to investigate this matter further and could also include assessments of participants’ subjective confidence in their initial estimates in order to investigate its neural basis.
CONCLUSIONS
Taken together, our study provides empirical evidence for the involvement of different neural networks during advice evaluation and advice integration. Furthermore, our findings suggest differences depending upon the perceived competence of the advisor: evaluation of advice coming from an allegedly competent as compared to an allegedly incompetent advisor differentially engages brain regions that have previously been related to reinforcement learning and action control. Similarly, significant differences exist in the neural correlates of advice integration depending upon the advisor’s reputation: in the case of an incompetent advisor, the neural correlate hints towards a relation to visual imagery suggesting that participants may resort to perceptually based strategies similar to when they re-assess their own initial judgments. By contrast, a more pronounced behavioral adjustment towards the advice given by a competent advisor correlates with activity changes in the amygdala, a brain region crucially important for emotional (and reinforcement-based) learning processes. Overall, our study, thus, highlights how inherently social aspects of the environment, such as the reputation of conspecifics, provide important constraints for judgment and decision-making processes reflected on the neural level.
Acknowledgments
The authors gratefully acknowledge the help with data collection provided by members of the Institute of Neuroscience and Medicine at the Research Centre Juelich, in particular Barbara Elghahwagi and Dorothe Krug. L.S. would also like to thank Anja Kassecker for her assistance during the recruitment of study participants, data collection, and analysis.
L.S. was funded by the Koeln Fortune Program/Medical Faculty, University of Cologne and by the Volkswagen Foundation. S.B.E. was funded by the Human Brain Project (R01-MH074457-01A1), the DFG (IRTG 1328) and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model). A.M. was funded by the Volkswagen Foundation. K.V. was funded by the German Ministery of Research and Education (BMBF) and the Volkswagen Foundation.
REFERENCES
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, … Zilles K (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anatomy and Embryology (Berlin), 210(5–6), 343–352. [DOI] [PubMed] [Google Scholar]
- Anderson NH (1991). Contributions to information integration theory (1, 2, 3). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Ashburner J, & Friston KJ (2003). Rigid body registration. In Frackowiak RS, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, …Penny WD (Eds.), Human brain function (2nd ed., pp. 635–655). London: Academic Press. [Google Scholar]
- Bahnemann M, Dziobek I, Prehn K, Wolf I, & Heekeren HR (2010). Sociotopy in the temporoparietal cortex: Common versus distinct processes. Socical Cognitive and Affective Neuroscience, 5(1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Hunt LT, Woolrich MW, & Rushworth MF (2008). Associative learning of social value. Nature, 456(7219), 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, & Burgess PW (2010). When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. NeuroImage, 50(3), 1340–1349. [DOI] [PubMed] [Google Scholar]
- Biele G, Rieskamp J, Krugel LK, & Heekeren HR (2011). The neural basis of following advice. PLOS Biology, 9(6), e1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, … Laureys S (2007). Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(29), 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccio S, & Dalal RS (2006). Advice taking and decision-making: An integrative literature review, and implications for the organizational sciences. Organizational Behavior and Human Decision Processes, 101, 127–151. [Google Scholar]
- Brass M, Ruby P, & Spengler S (2009). Inhibition of imitative behavior and social cognition. Philosophical Transactions of the Royal Society London B: Biological Sciences, 364(1528), 2359–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Nazarian B, Meunier M, & Boussaoud D (2011). Differential roles of caudate nucleus and putamen during instrumental learning. NeuroImage, 57(4), 1580–1590. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Tobler PN, Schultz W, & Baddeley M (2010). Striatal BOLD Response Reflects the Impact of Herd Information on Financial Decisions. Frontiers in Human Neuroscience, 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, … Eickhoff SB (2011). ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Structure and Function, 215(3–4), 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, & Frith CD (2010). How the opinion of others affects our valuation of objects. Current Biology, 20(13), 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa N, Motterlini M, Alemanno F, Perani D, & Cappa SF (2011). Learning from other people’s experience: A neuroimaging study of decisional interactive-learning. NeuroImage, 55(1), 353–362. [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, & Rubia K (2009). Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage, 48(1), 223–236. [DOI] [PubMed] [Google Scholar]
- Cichy RM, Heinzle J, & Haynes JD (2012). Imagery and Perception Share Cortical Representations of Content and Location. Cerebral Cortex, 22(2), 372–380. doi: 10.1093/cercor/bhr106 [DOI] [PubMed] [Google Scholar]
- Cooper JC, Dunne S, Furey T, & O’Doherty JP (2012). Human dorsal striatum encodes prediction errors during observational learning of instrumental actions. Journal of Cognitive Neuroscience, 24(1), 106–118. doi: 10.1162/jocn_a_00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGutis J, & D’Esposito M (2007). Distinct mechanisms in visual category learning. Cognitive, Affective, and Behavioral Neuroscience, 7(3), 251–259. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Frank RH, & Phelps EA (2005). Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience, 8(11), 1611–1618. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, & von Cramon DY (2005). Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Human Brain Mapping, 25(1), 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, & Gold JI (2010). Caudate encodes multiple computations for perceptual decisions. Journal of Neuroscience, 30(47), 15747–15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, & Amunts K (2006). Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage, 32(2), 570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, & Amunts K (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage, 36(3), 511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, & Zilles K (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, & Frith U (2008). Implicit and explicit processes in social cognition. Neuron, 60(3), 503–510. [DOI] [PubMed] [Google Scholar]
- Fudge JL, & Haber SN (2000). The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience, 97(3), 479–494. [DOI] [PubMed] [Google Scholar]
- Gamer M, & Büchel C (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37(1), 87–93. doi: 10.1016/j.psyneuen.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Giardina A, Caltagirone C, & Oliveri M (2011). Temporo-parietal junction is involved in attribution of hostile intentionality in social interactions: An rTMS study. Neuroscience letters, 495(2), 150–154. [DOI] [PubMed] [Google Scholar]
- Gino F (2008). Do we listen to advice just because we paid for it? The impact of advice cost on its use. Organizational Behavior and Human Decision Processes, 107, 234–245. [Google Scholar]
- Gino F, & Moore DA (2007). Effects of task difficulty on use of advice. Journal of Behavioral Decision Making, 20(1), 21–35. [Google Scholar]
- Gino F, Shang J, & Croson R (2009). The impact of information from similar or different advisors on judgment. Organizational Behavior and Human Decision Processes, 108, 287–302. [Google Scholar]
- Goghari VM, & MacDonald AW 3rd. (2009). The neural basis of cognitive control: Response selection and inhibition. Brain and Cognition, 71(2), 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, & Ingvar M (2011). Limbic justice—Amygdala involvement in immediate rejection in the Ultimatum Game. PLOS Biology, 9(5), e1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovanelli A, Bertollini C, Crestan V, Schwarz AJ,… Bifone A (2010). A neural switch for active and passive fear. Neuron, 67(4), 656–666. [DOI] [PubMed] [Google Scholar]
- Harvey N, & Fischer I (1997). Taking advice: Accepting help, improving judgment, and sharing responsibility. Organizational Behavior and Human Decision Processes, 70, 117–133. [Google Scholar]
- Harvey N, Harries C, & Fischer I (2000). Using advice and assessing its quality. Organizational Behavior and Human Decision Processes, 81, 252–273. [DOI] [PubMed] [Google Scholar]
- Hogarth RM, & Einhorn HJ (1992). Order effects in belief updating: The belief-adjustment model. Cognitive Psychology, 24, 1–55. [Google Scholar]
- Izuma K, Saito DN, & Sadato N (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–294. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, & Sadato N (2010). The roles of the medial prefrontal cortex and striatum in reputation processing. Society for Neuroscience, 5(2), 133–147. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, & Eickhoff SB (2009). Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. NeuroImage, 47(2), 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann H, & Fischer K (2005). Using expertise and experience for giving and taking advice. In Betsch T & Haberstroh S (Eds.), The routines of decision making (pp. 157–173). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, & Wrase J (2009). Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. Journal of Cognitive Neuroscience, 21(7), 1332–1345. [DOI] [PubMed] [Google Scholar]
- Kiebel S, & Holmes AP (2003). The general linear model. In Frackowiak RS, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, …Penny WD (Eds.), Human brain function (2nd ed., pp. 725–769). London: Academic Press. [Google Scholar]
- Klucharev V, Hytonen K, Rijpkema M, Smidts A, & Fernandez G (2009). Reinforcement learning signal predicts social conformity. Neuron, 61(1), 140–151. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Smidts A, & Fernandez G (2008). Brain mechanisms of persuasion: How “expert power” modulates memory and attitudes. Socical Cognitive and Affective Neuroscience, 3(4), 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockler H, Scheef L, Tepest R, David N, Bewernick BH, Newen A, … Vogeley K (2010). Visuospatial perspective taking in a dynamic environment: Perceiving moving objects from a first-person-perspective induces a disposition to act. Consciousness and Cognition, 19(3), 690–701. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Smith ST, & Blanke O (2006). Functional and neural mechanisms of embodiment: Importance of the vestibular system and the temporal parietal junction. Reviews in the Neurosciences, 17(6), 643–657. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, … Baron-Cohen S (2010). Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, 22(7), 1623–1635. [DOI] [PubMed] [Google Scholar]
- Misslin R (2003). The defense system of fear: Behavior and neurocircuitry. Neurophysiologie Clinique, 33(2), 55–66. [DOI] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, & Gothard KM (2010). Response characteristics of basolateral and centromedial neurons in the primate amygdala. Journal of Neuroscience, 30(48), 16197–16207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Habel U, Derntl B, Schneider F, Zilles K, Turetsky BI, & Eickhoff SB (2011). Incongruence effects in crossmodal emotional integration. NeuroImage, 54(3), 2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, … Fletcher PC (2008). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry, 13(3), 239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Speck O, Schulze-Bonhage A, Hennig J, Seifritz E, & Ball T (2010). Time scales of auditory habituation in the amygdala and cerebral cortex. Cerebral Cortex, 20(11), 2531–2539. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Padmala S, & Pessoa L (2010). Interactions between cognition and motivation during response inhibition. Neuropsychologia, 48(2), 558. doi: 10.1016/j.neuropsychologia.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2010). Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?”. Neuropsychologia, 48(12), 3416–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschhof C, Schubert T, Villringer A, & Heekeren HR (2010). Prior Information biases stimulus representations during vibrotactile decision making. Journal of Cognitive Neuroscience, 22(5), 875–887. [DOI] [PubMed] [Google Scholar]
- Prevost C, McCabe JA, Jessup RK, Bossaerts P, & O’Doherty JP (2011). Differentiable contributions of human amygdalar subregions in the computations underlying reward and avoidance learning. European Journal of Neuroscience, 34(1), 134–145. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, … Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, & Braver TS (2010). Motivated cognitive control: Reward incentives modulate preparatory neural activity during task-switching. Journal of Neuroscience, 30(31), 10294–10305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, & Vogeley K (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17(2), 457–467. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, … Vogeley K (2010). Minds made for sharing: Initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience, 22(12), 2702–2715. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, & Fan J (2009). Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human Brain Mapping, 30(9), 2821–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See KE, Morrison EW, Rothman NB,, & Soll JB (2011). The detrimental effects of power on confidence, advice taking, and accuracy. Organizational Behavior and Human Decision Processes, 116(2), 272–285. [Google Scholar]
- Seymour B, & Dolan R (2008). Emotion, decision making, and the amygdala. Neuron, 58(5), 662–671. [DOI] [PubMed] [Google Scholar]
- Sharot T, Shiner T, & Dolan RJ (2010). Experience and choice shape expected aversive outcomes. Journal of Neuroscience, 30(27), 9209–9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinke CB, Sorger B, Goebel R, & de Gelder B (2010). Tease or threat? Judging social interactions from bodily expressions. NeuroImage, 49(2), 1717–1727. [DOI] [PubMed] [Google Scholar]
- Sniezek JA, & Buckley T (1995). Cueing and cognitive conflict in judge-advisor decision making. Organizational Behavior and Human Decision Processes, 62, 159–174. [Google Scholar]
- Sniezek JA, Schrah GE, & Dalal RS (2004). Improving judgment with prepaid expert advice. Journal of Behavioral Decision Making, 17, 173–190. [Google Scholar]
- Soll JB, & Larrick R (2009). Strategies for revising judgment: How (and how well) people use others’ opinions. Journal of Experimental Psychology: Learning, Memory and Cognition, 35, 780–805. [DOI] [PubMed] [Google Scholar]
- Spreng RN, & Grady CL (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–1123. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, & Kim AS (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki W, & Tanaka K (2011). Development of monotonic neuronal tuning in the monkey inferotemporal cortex through long-term learning of fine shape discrimination. European Journal of Neuroscience, 33(4), 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, & Turken AU (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, & Grafton ST (2007). Beyond grasping: Representation of action in human anterior intraparietal sulcus. NeuroImage, 36(Suppl 2), T77–T86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- van Overwalle F (2009). Social cognition and the brain: A meta-analysis. Human Brain Mapping, 30(3), 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swol LM (2009). The effects of suspicion and advisor motives on confidence and advice utilization. Communication Research, 36, 857–873. [Google Scholar]
- van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ,& Fernández G (2011). Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience, 191, 38–45. doi: 10.1016/j.neuroscience.2011.04.042 [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, … Stoop R (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science, 333(6038), 104–107. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, & Evans AC (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4, 58–74. [DOI] [PubMed] [Google Scholar]
- Wright ND, Bahrami B, Johnson E, Di Malta G, Rees G, Frith CD, & Dolan RJ (2012). Testosterone disrupts human collaboration by increasing egocentric choices. Proceedings of Biological Sciences, 279(1736), 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv I (2004a). Receiving other people’s advice: Influence and benefit. Organizational Behavior and Human Decision Processes, 93, 1–13. [Google Scholar]
- Yaniv I (2004b). The benefit of additional opinions. Current Directions in Psychological Science, 13, 75–78. [Google Scholar]
- Yaniv I, & Kleinberger E (2000). Advice taking in decision making: Egocentric discounting and reputation formation. Organizational Behavior and Human Decision Processes, 83, 260–281. [DOI] [PubMed] [Google Scholar]