Abstract
Context
Distant metastases (DM) from childhood differentiated thyroid carcinoma (DTC) are uncommon and published studies are limited.
Objective
This work aimed to describe the outcomes of patients with DM from childhood DTC and to evaluate the molecular landscape of these tumors.
Methods
A retrospective study was conducted at a tertiary cancer center including patients with pediatric DTC (diagnosed at age ≤ 18 years from 1946 to 2019) and DM.
Results
We identified 148 patients; 144 (97%) had papillary thyroid carcinoma (PTC) and 104 (70%) were female. Median age at DTC diagnosis was 13.4 years (interquartile range [IQR], 9.9-15.9 years). Evaluable individuals received a median of 2 (IQR, 1-3) radioactive iodine (RAI) treatments at a median cumulative administered activity of 238.0 mCi (IQR, 147.5-351.0 mCi). The oncogenic driver was determined in 64 of 69 PTC samples: RET fusion (38/64; 59%), NTRK1/3 fusions (18/64; 28%), and the BRAF V600E mutation (8/64; 13%). At last evaluation, 93% had persistent disease. The median overall and disease-specific survival after DTC diagnosis were 50.7 and 52.8 years, respectively. Eight (5%) PTC patients died of disease after a median of 30.7 years (IQR, 20.6-37.6 years).
Conclusion
Childhood DTC with DM persists in most patients despite multiple courses of RAI, but disease-specific death is uncommon, typically occurring decades after diagnosis. Fusion genes are highly prevalent in PTC, and all identified molecular alterations have appropriate targeted therapies. Future studies should focus on expanding genotype-phenotype correlations, determining how to integrate molecularly targeted therapy into treatment paradigms, and relying less on repeated courses of RAI to achieve cure in patients with DM from childhood DTC.
Keywords: pediatric thyroid cancer, stage II, somatic mutation, fusion gene, prognosis, lung metastasis
The most common endocrine malignancy, differentiated thyroid carcinoma (DTC) is rarely diagnosed during childhood and accounts for about 4% of all pediatric cancers (1). Approximately 90% of children with DTC have papillary thyroid carcinoma (PTC) whereas less than 10% are diagnosed with follicular thyroid carcinoma (FTC) (2, 3). From 2012 to 2016, the age-adjusted incidence rates of thyroid cancer in the United States ranged from 1.6 cases per million per year in children ages 5 to 9 years to 34 cases per million per year in adolescents (ages 15-19 years), the most commonly affected pediatric age group (4). Rates of pediatric DTC have been increasing over the decades, a phenomenon that cannot solely be explained by an increased diagnosis of small or incidental tumors (1, 2, 4, 5).
At diagnosis, children present with more advanced disease compared with adults. Children with PTC have larger primary tumor sizes with frequent extrathyroidal extension and are more often diagnosed with lymph node and distant metastases (DM) (6-17). The lungs are the primary site of DM, which are identified in up to approximately 25% of childhood DTC patients (7, 13, 15-34), depending on the series. The risk of DM correlates with the presence and extent of lymph node metastases (7, 9, 20, 34). Despite more extensive disease at presentation, children have an extremely low disease-specific mortality regardless of the fact that most patients with DM from pediatric DTC do not reach remission (10, 13, 15, 16, 23, 25, 26, 30, 34-36).
The molecular pathogenesis of DTC, especially PTC, has become better elucidated over the years. Point mutations and fusion genes that activate the mitogen-activated protein kinase pathway play a major role in thyroid cancer development and propagation (37, 38). In pediatric PTC, chromosomal rearrangements involving the REarranged during Transfection (RET) proto-oncogene and the neurotrophic tyrosine receptor kinase (NTRK) genes (NTRK1 and NTRK3) are the most common oncogenic drivers (14, 32, 39-47). The BRAF V600E point mutation is also prevalent in childhood PTC (32, 40-43, 45-50), but at a much lower rate than adult PTC. These differences in molecular pathogenesis most likely explain the differences in clinical behavior observed between older and younger patients with PTC. Knowledge regarding tumor genotype may inform the expected clinical course, response to radioactive iodine (RAI), and potential for gene-targeted therapy for progressive DM. However, there has been very limited research in this area as relates to pediatric DTC.
The management of pediatric thyroid cancer with DM is challenging, given the excellent prognosis and protracted clinical course of childhood DTC, coupled with unique concerns regarding the potential long-term sequelae of overzealous treatment during childhood. To date, there have been few large-scale studies with comprehensive clinical data and extended follow-up periods in patients with DM from childhood DTC, and none of these incorporated testing for somatic molecular alterations. Therefore, data on clinical outcomes and tumor genotype are lacking in these patients. In the present study, we sought to describe the clinical characteristics and long-term outcomes of patients with DM and a diagnosis of pediatric DTC. The second aim was to evaluate the molecular genotype of pediatric PTC in a large subset of study participants and to assess possible relationships between mutational status and clinical characteristics.
Materials and Methods
This study was performed at The University of Texas MD Anderson Cancer Center, a tertiary referral center for thyroid cancer located in Houston, Texas. Using institutional databases, we identified individuals diagnosed with pediatric DTC from 1946 to 2019 and retrospectively reviewed their medical records for eligibility. Patients were considered eligible for study inclusion if they had been diagnosed with DTC during childhood (defined as age 18 years or younger) and were found to have DM at any time point of their follow-up, including adulthood. Patients were excluded if they did not have at least one in-person clinic visit or if there was insufficient clinical information to determine DM status. Some individuals included in the present study have been reported in prior published studies and case reports (20, 21, 36, 51, 52). This study was approved by the MD Anderson Institutional Review Board. A waiver of informed consent was requested and granted by the institutional review board for the data collection and retrospective review.
Data retrieval
Data regarding diagnosis, pathology, molecular test results, surgical treatment, administration of RAI, additional therapies (surgery, systemic therapy, external beam radiation therapy [EBRT], or other interventions), and clinical follow-up (imaging, levels of thyrotropin, thyroglobulin [Tg], and thyroglobulin antibodies [TgAbs]) were extracted from the electronic or paper medical records. If medical records were incomplete, patients were contacted for written consent, and additional records were obtained from outside institutions to supplement the MD Anderson data.
Study definitions
Date of diagnosis was defined as the date of initial histologic confirmation of DTC resulting from primary thyroid surgery or core thyroid biopsy, excisional or core lymph node biopsy, or biopsy of DM. For staging, we used the eighth edition of the tumor node metastases classification of the American Joint Committee on Cancer (AJCC) (53). TNM stage was scored based on the maximal known disease extent during clinical follow-up. For example, if lateral neck lymph node or DM were identified at any moment after initial therapy, the node stage would be scored as N1b or M1, respectively, with the assumption that this disease was present (but unrecognized) at the time of diagnosis. By definition, all patients included in this study were stage group II, even though DM may not have been identified within the first 4 months of diagnosis (as is used for AJCC staging). This was done because, owing to the nature of pediatric DTC and its indolent clinical course, exact staging at diagnosis was not available or inaccurate in some cases because of the delay in recognition of other sites of disease, especially decades ago when sensitive diagnostic imaging and tumor markers were unavailable. For TNM staging, if surgical and pathological data were incomplete or unavailable, or if the patient received systemic therapy prior to surgery, clinical data such as physical exam findings and imaging studies were used to complete staging. If the pathology report at MD Anderson was incongruent with the outside report, we used the MD Anderson interpretation for histopathologic characterization. Patients with PTC were categorized according to the American Thyroid Association (ATA) pediatric risk level classification (low-, intermediate-, or high-risk level) (11). ATA risk level was determined only by the initial clinical and histopathologic findings before RAI.
The date of DM diagnosis was the date of first confirmation of distant disease. If the date of the identification of the DM preceded the histological confirmation of DTC, the date of DTC diagnosis was considered to be the date of DM diagnosis. DM were defined as the presence of at least one of the following: i) RAI uptake consistent with iodine-avid thyroid cancer metastases on the diagnostic and/or therapeutic whole-body scan outside the thyroid bed or cervical/mediastinal lymph nodes; ii) pathologically proven thyroid cancer tissue in the lung, bone, brain, or any other organ; iii) enlarging, discrete pulmonary nodules of any number consistent with metastatic disease, coupled with a detectable Tg or persistently detectable or rising TgAb; iv) multiple (> 10) noncalcified solid pulmonary nodules, in the setting of a detectable Tg or persistently detectable or rising TgAb, that were stable on imaging, located predominantly in a lower lung distribution, and determined by the collaborating radiologist (S.Y.) to be consistent with pulmonary metastases. When pulmonary nodules were predominantly pleural based, they were not considered to be metastases. If imaging or RAI scans were ambiguous regarding the diagnosis of DM, a radiologist (S.Y.) reviewed the chest computed tomography images to classify the patient as having DM or not.
Testing for the molecular oncogenic driver, when obtained, was performed as part of routine patient care in Clinical Laboratory Improvement Amendments–certified laboratories. This analysis was performed across a variety of testing platforms and included immunohistochemistry (IHC; primarily for the BRAF V600E mutation but in one case was also conducted to look for an NTRK fusion gene) as well as DNA and/or RNA sequencing. If a tumor tested positive for a known oncogenic mutation or fusion gene, this was considered to be a true positive result. A negative result was considered as a true negative result only when the tumor was “comprehensively” tested for all relevant oncogenic drivers, defined for this study as testing for BRAF and (N/K/H)RAS mutations and fusion genes involving the RET, NTRK1, NTRK3, and anaplastic lymphoma kinase (ALK) genes. When patients’ tumors tested negative and were not “comprehensively” tested, we considered them as not evaluated.
We scored disease status at last clinical evaluation as shown in Table 1. These categories were adapted from previously published dynamic staging definitions (54, 55). Evaluable patients were considered to have both tumor markers and at least one type of imaging available for review; if data were missing, the disease status of these individuals was considered undeterminable. We did not incorporate an “indeterminate” category because we felt it highly unlikely that any durable detectable Tg or nondeclining TgAb after long-term follow-up in a patient with known structural DM would represent anything other than persistent disease.
Table 1.
Categories of disease status at last known clinical evaluation of patients with childhood differentiated thyroid carcinoma and distant metastases
| Category | Imaging | Thyroglobulin | Thyroglobulin antibodies |
|---|---|---|---|
| No evidence of disease | Negative | Below LLNa,b | Below ULN |
| Persistent disease | |||
| Biochemical disease | Negative | Above LLNa,b | Any level |
| Negative | Below LLN | Positive (stable or rising) | |
| Structural disease | Positive | Above LLNa,b | Any level |
| Positive | Any level | Positive (stable or rising) | |
| Unable to determine | Known | Unknown | Unknown |
| Unknown | Known | Known |
LLN and ULN were determined by the laboratory-provided normal reference ranges for the particular assay used.
Abbreviations: LLN, lower limit of normal; ULN, upper limit of normal.
a Using the athyrotic range for athyrotic patients, if available.
b Includes both suppressed and stimulated thyroglobulin.
Deaths were classified as death from DTC, death from a cause other than DTC, or death from an unknown cause. Disease-specific survival and overall survival were defined as the length of time in years from the date of diagnosis to the date of death or the date of last contact date with the patient (last written or verbal contact with patient/parent[s] or a completed clinic visit). For disease-specific survival, patients with an unknown cause of death were excluded from the analysis. The length of follow-up for disease status was defined as the time between the date of diagnosis and the date of the last known clinical evaluation. Follow-up to last known disease status and follow-up to last known vital status could therefore differ.
Statistical analyses
Study data were collected and managed using REDCap electronic data capture tools (56, 57) hosted at MD Anderson. Descriptive statistics were used to describe diagnostic, pathological, treatment, and outcome variables. Continuous variables are summarized with the median and interquartile range (IQR), unless otherwise specified. Mann-Whitney U tests were performed for continuous variables. We considered differences to be statistically significant at P less than .05 (2-sided). R software (version 3.6.1) and Microsoft Office Professional Plus Excel 2016 (Microsoft Corp) were used for statistical analyses.
Results
Study participants
From 1946 to 2019, a total of 602 patients with a new diagnosis or history of pediatric DTC (PTC = 568; FTC = 34) were registered and seen at least once at MD Anderson. Of these, 148 individuals (24.6%) had been diagnosed with DM at some point during their follow-up. Demographic and clinical characteristics are shown in Table 2. The majority of patients were female (n = 104, 70.3%). Median age at DTC diagnosis was 13.4 years (IQR, 9.9-15.9 years); the youngest patient was 2.8 years at diagnosis. Median age at DTC diagnosis did not significantly differ between females and males (13.6 years with IQR 10.2-16.4 years vs 12.5 years with IQR 9.3-15.9 years, respectively, P = .197). Regarding age of diagnosis, 39 patients (26.4%) were diagnosed before age 10 years, 55 patients (37.2%) were diagnosed from age 10 to 15 years, and 54 participants (36.5%) were diagnosed at age 15 years or older. White (60.1%), Hispanic/Latino (27.4%), Black (4.1%), and Asian (4.1%) were the most prevalent races/ethnicities. Information about clinical presentation was present for 139 of 148 patients. Most of these 139 patients (87.1%) presented with only a palpable nodule or neck mass that led to the diagnosis of DTC.
Table 2.
Characteristics of patients with childhood differentiated thyroid carcinoma and distant metastases
| Characteristic | All patients | PTC | FTC |
|---|---|---|---|
| n = 148 | n = 144 | n = 4 | |
| Sex, No. (%) | |||
| Female | 104 (70.3) | 100 (69.4) | 4 (100) |
| Male | 44 (29.7) | 44 (30.6) | 0 |
| Race/Ethnicity, No. (%) | |||
| White | 89 (60.1) | 85 (59.0) | 4 (100) |
| Hispanic or Latino | 41 (27.7) | 41 (28.5) | 0 |
| Black | 6 (4.1) | 6 (4.2) | 0 |
| Asian | 6 (4.1) | 6 (4.2) | 0 |
| Half Black, half White | 2 (1.4) | 2 (1.4) | 0 |
| Other | 4 (2.7)a | 4 (2.8)a | 0 |
| Age at DTC diagnosis, y | 13.4 (9.9-15.9) | 13.4 (9.8-15.9) | 14.3 (12.2-16.8) |
| Range | 2.8-18.9 | 2.8-18.9 | 12.2-18.5 |
| Clinical presentation at diagnosis, No. (%) | |||
| Palpable thyroid nodule or neck mass | 121 (87.1) | 118 (87.4) | 3 (75.0) |
| Incidental finding by imaging | 6 (4.3) | 6 (4.4) | 0 |
| Compressive symptoms | 3 (2.2) | 3 (2.2) | 0 |
| Nodule/neck mass with compressive symptoms | 8 (5.8) | 8 (5.9) | 0 |
| Other | 1 (0.7) | 0 | 1 (25.0)b |
| Unknown | 9 | 9 | 0 |
Age at diagnosis is shown as median (interquartile range).
Abbreviations: DTC, differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
a Pacific Islander (n = 1), half Asian/half White (n = 1), half Hispanic/half Black (n = 1), and half Hispanic/half White (n = 1).
b In one patient, the DTC diagnosis was made after evaluation of overt hyperthyroidism and diagnosis of a functioning thyroid nodule.
Only 13 of 142 (9.2%) patients with available information (all PTC) had a history of EBRT with exposure to the neck prior to their cancer diagnosis (Supplemental Table 1 [58]). For 7 cases, EBRT was part of their cancer therapy (acute myeloid leukemia [n = 2], alveolar rhabdomyosarcoma, B-cell acute lymphocytic leukemia, Hodgkin lymphoma [n = 2], neuroblastoma, and retinoblastoma). An eighth patient diagnosed with Hodgkin lymphoma before PTC diagnosis was not treated with EBRT. In 6 cases, patients received EBRT for benign conditions. A preexisting thyroid diagnosis was reported in 16 of 138 (11.6%) evaluable patients (hypothyroidism/Hashimoto disease [n = 8], hyperthyroidism/Graves disease [n = 4], and goiter not otherwise specified [n = 4]). Eleven of 133 (8.3%) evaluable patients were reported to have family members with thyroid cancer; none of these reported DTC in a first-degree relative and 7 patients had a second-degree family member with thyroid cancer. One patient was known to have familial Brugada syndrome at diagnosis but none of the other patients had a known familial syndrome at diagnosis.
Treatment
Diagnostic surgery.
Definitive thyroid surgery was preceded by another diagnostic surgical procedure in 39 cases (26.4%, fine-needle aspirations not included). For 32 of 39 patients, the first histologic confirmation of DTC was after a lymph node biopsy; 4 of 39 patients had a lung biopsy; 2 of 39 underwent a Sistrunk procedure with removal of an ectopic PTC; and 1 patient with widespread DM had the diagnosis confirmed after adrenalectomy. The median number of days between diagnostic surgery and initial thyroid surgery was 19 days (IQR, 10-32 days; range, 3-449 days) (Table 3).
Table 3.
Treatment of patients with childhood differentiated thyroid carcinoma and distant metastases
| Treatment | All patients | PTC | FTC |
|---|---|---|---|
| n = 148 | n = 144 | n = 4 | |
| Initial thyroid surgery, No. (%) | |||
| Total/subtotal thyroidectomy | 133 (90.5) | 131 (91.0) | 2 (50.0) |
| Thyroid lobectomy | 11 (7.5) | 9 (6.3) | 2 (50.0) |
| Followed by completion thyroidectomy | 11 | 9 | 2 |
| Other | 3 (2.0)a | 3 (2.1)a | 0 |
| Unknown | 1 | 1 | 0 |
| Initial lymph node resection, No. (%) | |||
| Yes | 119 (86.9) | 118 (88.1) | 1 (33.3) |
| Only central compartment | 13 | 13 | – |
| Only lateral neck (unilateral/bilateral) | 29 | 28 | 1 |
| Both central and lateral neck | 64 | 64 | – |
| Unspecified lymph node resection | 13b | 13b | – |
| No | 18 (13.1) | 16 (11.9) | 2 (66.7) |
| Unknown | 11 | 10 | 1 |
| RAI, No. (%) | |||
| Yes | 146 (98.6) | 142 (98.6) | 4 (100) |
| No | 2 (1.4) | 2 (1.4) | 0 |
| Time from DTC diagnosis to first RAI, mo | 2.7 (1.6-4.5)c | 2.7 (1.6-4.4)d | 3.2 (1.4-18.1) |
| Range | 0.4-474.0 | 0.4-474.0 | 1.0-57.9 |
| No. of RAI administrations per patient | 2 (1-3)c | 2 (1-3)d | 1 (1-1.3) |
| Range | 1-9 | 1-9 | 1-2 |
| Activity of RAI per administration per patient, mCi | 143.8 (97.4-158.0)e | 143.6 (97.0-157.0)f | 150.0 (114.8-175.9) |
| Range | 26.4-532.0 | 26.4-532.0 | 104.5-190.0 |
| Total cumulative activity per patient, mCi | 238.0 (147.5-351.0)g | 241.4 (147.9-352.1)h | 143.4 (119.9-206.3) |
| Range | 29.1-1538.7 | 29.1-1538.7 | 104.5-340.0 |
Numbers are shown as median (interquartile range).
Abbreviations: DTC, differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; mCi, megacurie; PTC, papillary thyroid carcinoma; RAI, radioactive iodine.
a In one patient, the intended total thyroidectomy could not be accomplished because of the invasiveness of the disease. In another patient, the intent of the surgery was a total thyroidectomy, but the pathology report revealed no normal thyroid tissue. In a third patient initially treated in 1946, only a nodulectomy was performed.
b In one case, only a Delphian node was removed. For the other 12 cases, an unspecified selective lymph node excision was performed.
c n = 146 because RAI was not administered in 2 cases (see text).
d n = 142, because RAI was not administered in 2 cases (see text).
e Eleven of 307 doses were excluded from analysis because of missing data regarding the administered activity.
f Eleven of 302 doses were excluded from analysis because of missing data regarding the administered activity.
g n = 139, because 7 patients had at least 1 missing administered activity of RAI and RAI was not administered in 2 cases.
h n = 135, because 7 patients had at least 1 missing administered activity of RAI and RAI was not administered in 2 cases.
Initial surgical treatment.
The majority of patients (n = 114/148, 77.0%) had their initial thyroid surgery outside MD Anderson, and 144 of 147 (98.0%) had a total thyroidectomy, including a completion thyroidectomy after lobectomy in 11 patients. Details regarding the initial thyroid surgery were unknown in one case. Three patients (2.0%) with PTC did not have a total thyroidectomy after diagnosis. In one patient, only a neck dissection was performed since the intended total thyroidectomy could not be accomplished because of the invasiveness of the primary disease. In another patient, the intent of surgery was a total thyroidectomy, but the pathology report revealed no normal thyroid tissue and a residual thyroid lobe was appreciated during RAI therapy; it was assumed that the tumor had completely replaced the bulk of the thyroid gland. In a third patient who was initially treated in 1946, only a nodulectomy was performed. Some extent of lymph node resection was performed in 119 of 137 (86.9%) of the study participants at the initial thyroid surgery (Table 3).
Radioactive iodine.
In the 146 of 148 (98.6%) patients treated with RAI, the first therapeutic RAI was administered after a median of 2.6 months from surgery (IQR, 1.6-4.5 months). The median number of therapeutic RAI administrations was 2 (IQR, 1-3; range, 1-9). In evaluable patients, the median administered activity per therapeutic session was 143.8 mCi (IQR, 98.0-157.3 mCi; range, 26.4-532.0 mCi). The median cumulative administered RAI activity of 139 patients with all dosing data known was 238.0 mCi (IQR, 147.5-351.0 mCi; range 29.1-1538.7 mCi).
Two patients with PTC (1.4%) did not receive RAI. One recently diagnosed patient, whose tumor harbored an NTRK1 fusion gene, had variable RAI uptake in the pulmonary metastases on a diagnostic scan; systemic therapy was started, and RAI had not yet been administered. The second patient with a RET fusion gene–positive tumor did not receive RAI because thyroidectomy could not be accomplished; her disease was treated systemically.
Additional treatment.
Additional surgeries besides the initial diagnostic and therapeutic surgeries took place in 84 (56.8%) patients. EBRT was given to 14 (9.5%) PTC patients (unknown in 1 patient). This approach was used as adjuvant therapy in 5 patients who were treated between 1946 and 1960. Nine PTC patients had palliative radiation therapy for metastatic disease between 1976 and 2013. Cytotoxic or targeted therapy was given to 23 of 143 (16.2%) PTC patients with DM (unknown in 1 PTC patient). None of the FTC patients were treated with systemic therapies (outside of RAI) or EBRT.
Pathology
Pathology characteristics are shown in Table 4 and Supplementary Table 2 (58). PTC was diagnosed in 144 (97.3%) patients and FTC in 4 patients (2.7%). The most common subtypes of PTC were the conventional (n = 37, 25.7%) and follicular variants (n = 27, 18.8%). All 4 patients with FTC (100%) had tumors that were encapsulated and angioinvasive, including 1 patient with an insular variant. The median tumor size for 115 evaluable PTC patients was 3.5 cm (IQR, 2.3-5.5 cm) and 3.0 cm (IQR, 2.5-3.5) for the 4 FTC patients. In terms of TNM staging, PTC tumors were mostly staged as T3 (n = 56, 38.9%) and 126 (87.5%) PTC patients had stage N1b disease. All 4 (100%) FTC tumors were staged as T2 and 1 (25.0%) had N1b disease. In 3 (2.1%) PTC patients and 3 (75.0%) FTC patients, no metastases in lymph nodes were diagnosed. Most PTC patients (76.3%) had multifocal disease and all FTC cases were unifocal. ATA Pediatric Risk level was determinable in 126 of 144 (87.5%) PTC patients: A total of 109/126 (86.5%) were high risk, 13 of 126 (10.3%) were intermediate risk, and 4 of 126 (3.2%) patients were low risk.
Table 4.
Pathology characteristics of patients with childhood differentiated thyroid carcinoma and distant metastases
| Characteristic | All patients | PTC | FTC |
|---|---|---|---|
| n = 148 | n = 144 | n = 4 | |
| Primary tumor size, cm | 3.5 (2.3-5.5)a | 3.5 (2.3-5.5)b | 3.0 (2.5-3.5) |
| Tumor stage, No. (%) | |||
| T1, T1a, or T1b | 21 (14.2) | 21 (14.6) | 0 |
| T2 | 40 (27.0) | 36 (25.0) | 4 (100) |
| T3, T3a, or T3b | 56 (37.8) | 56 (38.9) | 0 |
| T4a or T4b | 16 (10.8) | 16 (11.1) | 0 |
| Txc | 15 (10.1) | 15 (10.4) | 0 |
| Node stage, No. (%) | |||
| N0 | 6 (4.1) | 3 (2.1) | 3 (75.0) |
| N1 | 5 (3.4) | 5 (3.5) | 0 |
| N1a | 7 (4.7) | 7 (4.9) | 0 |
| N1b | 127 (85.8) | 126 (87.5) | 1 (25.0) |
| Nx | 3 (2.0) | 3 (2.1) | 0 |
| Focality, No. (%) | |||
| Unifocal | 22 (23.7) | 18 (20.2) | 4 (100) |
| Multifocal, unilateral | 17 (18.3) | 17 (19.1) | 0 |
| Multifocal, bilateral | 54 (58.1) | 54 (60.7) | 0 |
| Unable to determine/unknown | 55 | 55 | 0 |
Primary tumor size is show as median (interquartile range). Tumor and node stages represent maximal scores during follow-up. Patients were scored according to the eighth edition of the American Joint Committee on Cancer staging system. Pathological staging was leading. If surgical and pathological data were incomplete, clinical data were used to complete staging. Diffusely infiltrating tumors were scored as multifocal and bilateral.
Abbreviations: DTC, differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
a Total of 119 cases.
b Total of 115 cases.
c Includes 2 patients with ectopic PTC.
Distant metastases
The median time from initial DTC diagnosis to DM diagnosis was 2.6 months (IQR, 0.6-18.5 months) for all patients; PTC patients had a median time to DM diagnosis of 2.6 months (IQR, 0.5-15.6 months) whereas FTC patients were diagnosed with DM after a median time of 81.5 months (IQR, 38.7-140.3 months). The median age at diagnosis of DM from DTC was 14.0 years (IQR, 11.1-17.2 years; range, 3.1-69.4 years); PTC patients had a median age at diagnosis of DM from DTC of 14.0 years (IQR, 11.0-17.1 years; range, 3.1-69.4 years) whereas FTC patients were diagnosed with DM at a median age of 22.1 years (IQR, 15.4-29.6 years; range, 12.6-34.8 years). The diagnosis of DM was made in 127 of 148 patients (85.8%) before age 19 years. Twenty-six of 148 patients (17.6%) were diagnosed with DM at younger than 10 years, 56 patients (37.8%) between 10 and 15 years, and 66 patients (44.6%) were diagnosed with DM at age 15 years or older. For 22 (15%) DTC patients, DM were identified before the histological confirmation of DTC. In these patients, DM were identified at a median of 0.4 months (IQR, 0.2-1.2 months) before initial thyroid surgery.
All 144 PTC patients were diagnosed with DM to the lung, including 129 of the 144 patients (89.6%) with lung metastases exclusively. Other sites of DM in PTC patients included bone (n = 13; 9.0%), brain (n = 8; 5.6%), liver (n = 3; 2.1%), and adrenal and renal metastases in a single patient (0.7%) (Supplementary Table 3 [58]). Two of the 4 FTC patients solely had lung metastases and the other two had bone metastases alone.
The majority (76.4%) of patients were diagnosed with DM within 2 years after their cancer diagnosis. After 4 months, 1, 5, 10, and 20 years from DTC diagnosis, the proportion of patients diagnosed with DM was 60.1%, 68.2%, 90.5%, 95.3% and 96.6%, respectively. Details regarding the patients diagnosed with DM beyond 10 years after diagnosis (n = 10, 6.8%) are shown in Supplementary Table 4 (58). Almost all patients with a delayed diagnosis of DM were diagnosed in an earlier era when less sensitive diagnostic testing was available, and the delay did not apparently lead to worse outcomes as 5 of these 7 patients were alive at last contact, with their follow-up ranging from 19.2 to 66.1 years.
Mutational analysis
The tumors from most patients (95/148; 64.2%; PTC = 94; FTC = 1) underwent any testing to identify the oncogenic driver, and of these, 64 specimens (all PTC) were found to have a mutation or fusion gene (Table 5). Fusion genes were identified in 87.5% (56/64), whereas 8/64 (12.5%) tumors had the BRAF V600E mutation. Fusions involved RET in 38 of 64 (59.4%) cases (the most common partner being NCOA4) followed by NTRK1 (11/64; 17.2%) and NTRK3 (7/64; 10.9%). One tumor with a BRAF V600E mutation also had a second point mutation (p.E17K) in the v-akt murine thymoma viral oncogene homolog 1 (AKT1) gene. A RET fusion gene was identified in 3 of 13 tumors (not tested [n = 8], not comprehensively tested [n = 2]) from patients who had received EBRT before their DTC diagnosis. Of the 64 patients with a true positive result, the tissue tested included the primary tumor (n = 17), lymph node metastasis (n = 29), either primary tumor or lymph node (n = 12; exact site unknown), or distant metastasis (n = 6). The methodology that identified the oncogenic drivers was RNA sequencing in 46 cases, solely DNA sequencing in 16 cases, and BRAF V600E IHC in 2 cases (Supplementary Table 5 [58]).
Table 5.
Somatic molecular analysis in patients with childhood papillary thyroid carcinoma and distant metastases
| Molecular analysis | n = 144 | ||
|---|---|---|---|
| Not tested, No. (%) | 50 (34.7) | ||
| Tested with oncogenic driver identified, No. (%) | 64 (45.8) | ||
| RET fusion gene | 38 | ||
| NCOA4/RET | 21 | ||
| CCDC6/RET | 11 | ||
| TRIM24/RET | 2 | ||
| ERC1/RET | 2 | ||
| PRKAR1A/RET | 1 | ||
| EML4/RET | 1 | ||
| NTRK1 fusion gene | 11 | ||
| TPR/NTRK1 | 4 | ||
| TPM3/NTRK1 | 3 | ||
| IRF2BP2/NTRK1 | 2 | ||
| TFG/NTRK1 | 1 | ||
| SQSTM1/NTRK1 | 1 | ||
| NTRK3 fusion gene | 7 | ||
| ETV6/NTRK3 | 5 | ||
| SQSTM1/NTRK3 | 2 | ||
| BRAF V600E mutation | 8a | ||
| Tested without oncogenic driver identified, No. (%) | 30 (20.8) | ||
| Not comprehensively testedb | 25 | ||
| Comprehensively testedc | 5 |
Abbreviations: BRAF, v-Raf murine sarcoma viral oncogene homolog B; NTRK, neurotrophic tyrosine kinase receptor; RET, rearranged during transfection.
aIn one patient, a BRAF V600E and a v-akt murine thymoma viral oncogene homolog 1 (AKT1) mutation were found.
b Twenty of 25 patients were tested only for the BRAF V600E mutation and were negative.
c Tested for BRAF and RAS mutations and RET, NTRK1/3, and ALK fusions.
Of the 31 tumors (PTC = 30) that tested negatively for oncogenic drivers, only 5 PTC cases were comprehensively tested (ie, tested for BRAF/RAS mutations and RET, NTRK1/3, and ALK fusion genes). All 31 of these tumors were tested for the BRAF V600E mutation and were found to be BRAF wild type. Of the 95 tumors with any testing performed, 62 samples (65.3%; all PTC) were tested for (N/K/H)RAS mutations and all were found to be negative (53 samples with another oncogenic driver identified, 5 tumors that were comprehensively tested with no molecular alterations identified, and 4 specimens not comprehensively tested in which RAS was wild type and no other oncogenic driver was identified).
Supplementary Table 5 (58) shows the disease characteristics of the various oncogenic drivers. The youngest patient to have a tumor with an identified oncogenic driver (NTRK3 fusion gene) was diagnosed with PTC and widely metastatic DM at age 4 years. The smallest median tumor size (2.9 cm) was found in patients with a BRAF mutation (RET fusion 4.1 cm; NTRK1 fusion 5.2 cm; and NTRK3 fusion 4.5 cm). The median time to diagnosis of DM was also longest in patients with a somatic BRAF mutation (11.4 months vs 3.1 months, 1.6 months, and 1.2 months in patients whose tumors harbored a RET, NTRK1, or NTRK3 fusion gene, respectively). Given the small numbers of patients, we did not test whether these characteristics differed statistically.
Outcome
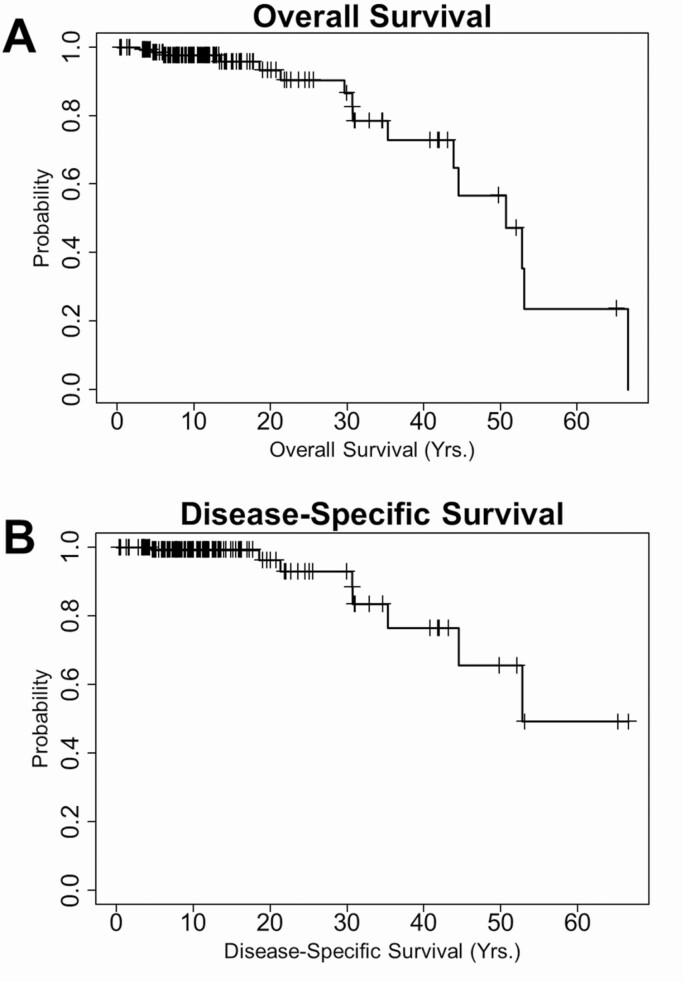
For all 148 patients, the median time from diagnosis to last known vital status was 11.8 years (IQR, 7.2-18.3 years; range, 0.8-66.6 years). Sixteen patients (10.8%) died during follow-up, including 8 (5.4%) patients who died of PTC directly and 2 patients (1.4%) who died of complications of therapy: one at age 10 years of respiratory failure caused by RAI-induced pulmonary fibrosis and the other at age 70 years due to massive hemorrhage as a side effect from an antiangiogenic tyrosine kinase inhibitor. Three deaths were not DTC related, and the cause of death was unknown for 3 patients. None of the FTC patients died. The median overall survival after DTC diagnosis was 50.7 years, whereas the median disease-specific survival (excluding the three subjects 3 patients for whom the cause of death was unknown) was 52.8 years (Fig. 1). The 5-, 10-, 15-, 20-, 25-, and 30-year overall survival rates were 98.5%, 97.7%, 96.1%, 93.5%, 90.6%, and 86.8%, respectively. For disease-specific deaths, these rates were 99.2%, 99.2%, 99.2%, 96.3%, 93.0%, and 93.0%, respectively. Overall and disease-specific survival did not differ by sex or age group (< 10, 10-15, and ≥ 15 years) at DTC or DM diagnosis (Supplementary Figure 1 [58]). For the 132 survivors, the median follow-up to last known vital status was 11.1 years (IQR, 7.0-16.1 years; range, 0.8-65.3 years).
Figure 1.
Kaplan-Meier survival curves of patients diagnosed with distant metastases from childhood differentiated thyroid carcinoma. A, Overall survival (OS; n = 148). Sixteen patients died during follow-up. The median OS was 50.7 years. The 5-, 10-, 15-, 20-, 25-, and 30-year OS rates were 98.5%, 97.7%, 96.1%, 93.5%, 90.6%, and 86.8%, respectively. B, Disease-specific survival (DSS; n = 145). Eight patients died of papillary thyroid carcinoma (PTC); no follicular thyroid carcinoma–related deaths occurred. The median DSS was 52.8 years. The 5-, 10-, 15-, 20-, 25-, and 30-year DSS rates were 99.2%, 99.2%, 99.2%, 96.3%, 93.0%, and 93.0%, respectively. Patients with an unknown cause of death (n = 3) were excluded from the DSS analysis.
The patients who died of PTC (Supplementary Table 6 [58]) were diagnosed between 1956 and 2004. Median follow-up from diagnosis to death from PTC was 30.7 years (IQR, 20.6-37.6 years; range, 4.5-52.8 years) at a median age of 44.5 years (IQR, 36.1-49.9 years; range, 20.6-68.2 years). The youngest patient to die was age 20.6 years and time from diagnosis to death was only 4.5 years; his was an extraordinary case of RAI-avid disease in a patient who had been heavily treated for stage IV neuroblastoma. All PTC patients who died of their disease had pulmonary and extrapulmonary DM at the time of death. Patients who died of PTC received a median cumulative administered RAI activity of 473.5 mCi (IQR, 250.0-805.5 mCi) compared with a median of 226.7 mCi (range, 141.8-331.0 mCi) in patients who were alive at last contact (reported on the 7 and 125 patients with a known cumulative RAI activity, respectively) (Supplementary Table 7 [58]). Molecular testing was conducted in tumors from only 2 of 8 patients who died of DTC, but testing was not comprehensive as defined by this study and no oncogenic driver was identified. Palliative EBRT was administered to 6 of 8 patients, and 5 of 8 of these patients were treated with systemic therapy.
Median follow-up from diagnosis of DTC to last clinical evaluation was 10.5 years (IQR, 6.3-16.2 years; range, 0.8-66.1 years). We were unable to score disease status in 22 of 148 (14.9%) patients because insufficient data were available. At last clinical evaluation, 117 of 126 (92.9%) had persistent disease, of whom 110 had structural evidence of disease and 7 had biochemical evidence of disease. Only 9 of 126 evaluable patients (7.1%) had no evidence of disease as strictly defined by the study (Supplementary Table 8 [58]). These 9 patients were diagnosed from 1986 to 2014 at a median age of 15.5 years and, of the 8 patients with complete records, the median cumulative administered activity of RAI was 125.2 mCi (range, 66.5-175.6 mCi; Supplementary Table 7 [58]). Only spread of DTC to the lungs was identified in these patients. Median follow-up to last known vital status was 15.5 years for these 9 patients having no evidence of disease.
Discussion
Childhood DTC resulting in DM is rare, and previous studies on this topic have been limited by small numbers of patients, inconsistent testing for molecular alterations, short follow-up periods in many pediatric series, and/or a lack of detailed clinical data (especially when derived from large cancer registries). To our knowledge, this single-center study, which focused on the clinical course and the mutational landscape of DTC in 148 patients diagnosed with DM from childhood DTC at any time point, is the largest of its kind with extended follow-up as long as 6 decades in some cases.
The prevalence of DM from childhood DTC diagnosed at any time during follow-up has been reported to be as low as 5% (59) and as high as 42% (18), depending on the population studied and the treating center. In the present study, DM were identified in 24.6% of the individuals diagnosed with DTC of pediatric onset, and more than two-thirds of these patients had their DM recognized within the first year after diagnosis. In some cases, the DM will not be recognized until years into clinical follow-up, especially in patients diagnosed at a time when less sensitive diagnostic testing was available. We believe our study overestimates the true prevalence of DM in pediatric thyroid cancer patients, which is likely to be lower in the larger cohort of all children diagnosed with DTC. This overestimation is most certainly due to a referral bias, in addition to not knowing the exact denominator of all patients ever seen at our institution with a history of DTC diagnosis before age 19 years. Furthermore, we included patients with DM diagnosed during adulthood, which in turn will artificially elevate the proportion of patients who are likely to have more advanced disease (leading to their referral to a tertiary cancer center). Similar to other series, pulmonary metastases were nearly universal, especially in PTC where all patients in our study had lung metastases, and the identification of extrapulmonary sites of disease (most commonly bone followed by brain; 11.5% in our series and 10.4% of PTC cases) was less common (6, 17, 23, 24, 28, 31, 59-62).
FTC represents a minority of DTC cases during childhood and has been reported even less frequently to be associated with DM, occurring in only 2 of 20 cases in a large Japanese series (63). In our study, only 4 FTC patients (2.7% of the entire DM group; 11.8% of all the pediatric FTC patients known in our center) were confirmed to have DM, and the sites of DM were equally split between the lungs and the skeleton. As would be anticipated, and similar to the study by Enomoto et al (63) and others (6), our 4 cases of FTC were unifocal tumors with evidence for vascular invasion in all cases and a low prevalence of cervical lymph node disease. Although death due to pediatric FTC has been reported (23, 29, 59), we had no deaths from FTC in our series. Therefore, if deaths from FTC do occur, they appear to be extraordinarily rare. Given the small number of FTC cases, comparisons with PTC could not be made but it was noted that the latency period from FTC diagnosis to DM diagnosis was longer in the FTC patients, which underscores the importance of long-term follow-up of children with FTC, especially in the presence of angioinvasive tumors.
The present study was not designed to compare the clinical characteristics of patients with stage II DTC (DM+) with those of stage I (DM–) disease, but similar to prior studies (7, 14, 21, 23, 25, 28, 34, 64), patients with DM had larger tumors and a very high rate of lymph node metastases to the lateral neck. The vast majority (86.5%) of evaluable patients were considered to be ATA high risk at diagnosis. Patients with DM treated at our center were more likely to be female, in keeping with the known female predilection in DTC (4). Most of the distantly metastatic tumors comprised conventional PTC followed by the follicular and diffuse sclerosing variants of PTC.
Outcomes from pediatric DTC are consistently reported to be excellent, even when DM is present at diagnosis (3, 10, 13, 30, 34, 35). However, most patients are not cured of their disease and, similar to other recently published studies (14-16, 23, 25, 26, 34), we identified a high prevalence of persistent disease (93%) in this population. In addition to a referral bias, this high rate of persistent disease may reflect the strict definitions used for the study and the better diagnostic studies available in the modern era of DTC care such as more sensitive Tg assays and computed tomography. There is also less reliance on diagnostic and posttreatment RAI scans for the identification of pulmonary metastatic disease, recognizing that RAI nonavid disease does indeed occur in children (18, 19, 34, 52). The DTC was indolent in the vast majority of cases with a notable long-term survival that exceeds any other distantly metastatic pediatric solid tumor.
Death from pediatric DTC was uncommon; the median overall survival was 50.7 years and the median disease-specific survival was 52.8 years after diagnosis. Survival did not differ based on the age at DTC or DM diagnosis and sex of the patient. When death from disease occurred, it was attributed to PTC in all cases after a median of 3 decades after initial diagnosis. In our series, 5.5% of evaluable patients with DM from pediatric-onset DTC died of disease. This is consistent with some studies (6, 15, 25), higher than many others (14, 17, 19, 24, 26, 28, 29, 61), and lower than some series in which death from childhood DTC occurred in 8% to 22% of cases with DM (10, 13, 18, 27, 32-34). An interesting observation in our study was that those who died of their disease all developed extrapulmonary metastatic disease. Most of the patients in the present study who died of PTC were diagnosed in an earlier era when less sensitive clinical tools were available for disease monitoring, when higher cumulative RAI activities were given (thus theoretically increasing the risk of secondary tumor mutations and more aggressive disease), and before there were advances in the understanding of the molecular alterations that drive PTC and the availability of molecularly targeted therapy. Moving forward, it is likely that the death rate will be lower as knowledge regarding pediatric DTC expands and treatment approaches evolve. Notably, 2 patients died of complications related to therapy, 1 of hemorrhage due to a tyrosine kinase inhibitor prescribed for progressive disease and the other of pulmonary fibrosis caused by overaggressive therapy with RAI. In the Chernobyl pediatric cohort, pulmonary fibrosis was identified in 7.2% of 69 patients and resulted in the death of 1 (1.5% of the 69 patients) (65). Therefore, in children with diffuse pulmonary disease that is RAI avid, additional careful consideration must be given to RAI dosing and frequency and monitoring for pulmonary complications of treatment.
Our study is one of the first to report the underlying oncogenic driver in a large number of patients with DM from childhood DTC. Not unexpectedly, the most frequently found oncogenic driver was a fusion gene (RET fusion genes being predominant, representing 59% of PTC samples with a known driver). NTRK fusion genes were also common (28% of known drivers; NTRK1 > NTRK3) but the BRAF V600E mutation was surprisingly seen in approximately 13% of these tumors. As understood for PTC in general (37, 45), oncogenic mutations are usually mutually exclusive. We found the same to hold true in this study since only 1 tumor in a patient with an aggressive presentation of metastatic PTC had 2 concurrent somatic mutations (BRAF V600E and AKT1). Of 69 PTC samples considered to be evaluable for molecular alteration status, the oncogenic driver was identified in 93% of cases. In another recent study with comprehensive molecular testing (32), all 10 patients with DM were found to have a molecular alteration, suggesting that the molecular driver can be identified in almost all cases of pediatric stage II PTC after comprehensive testing. Importantly, this has treatment implications because the most commonly identified fusion genes and point mutations all have commercially available, molecularly targeted therapies that can be used for systemic therapy if clinically indicated.
The medical literature is sparse as relates to the molecular profiles of DM tumors and most prior studies have been limited by the lack of comprehensive molecular testing, especially for fusion genes involving NTRK. In line with previous studies (25, 32), RET fusion genes were the most commonly found oncogenic drivers in patients with DM from childhood DTC. A RAS mutation was not found in any of our cases, and we are aware of only 2 reported cases of a RAS mutation (1 NRAS and 1 HRAS) in young patients with DM (25, 32). ALK fusion genes were also not identified in our patient population. Although fusion genes involving ALK have been described in pediatric PTC (45), unequivocal cases of ALK fusion gene–positive DM childhood PTC have not yet been published. Therefore, it can be concluded that point mutations in the RAS gene and ALK fusion genes are rare in this population. Furthermore, there have been cases of DM tumors with MET (32) and AGK-BRAF fusion genes (50, 66). In the 5 PTC patients comprehensively tested for somatic molecular alterations with negative results, we did not identify a fusion gene involving BRAF (n = 4) or MET (n = 3). In 3 of these cases, the tumor was tested in a laboratory (using DNA and RNA sequencing) in which we have seen false-negative results, in one the testing was primarily conducted via analysis of cell-free DNA (liquid biopsy), and the fifth case had DNA-based testing alone. Therefore, we cannot rule out a false-negative result in these cases. In the setting of an uninformative test result when systemic therapy is warranted and knowledge of the oncogenic driver paramount, repeat testing in a different laboratory using RNA-based next-generation sequencing should be considered, understanding that this approach provides optimal screening for fusion genes involving genes such as NTRK1 and NTRK3 (67).
Our sample size was too small to statistically analyze genotype-phenotype correlations. However, it appears that patients with BRAF-mutated tumors had smaller tumor sizes and were diagnosed with DM later than patients with other identified oncogenic drivers. It is hypothesized that, owing to the increased risk of RAI-refractory disease in BRAF-mutated PTC (68, 69), it takes longer to recognize DM in these cases because of the poor sensitivity of RAI scans. There were no obvious differences in the clinical characteristics between RET and NTRK fusion genes.
This study was not designed to evaluate the prevalence of RAI-refractory disease since the images of diagnostic and posttherapy RAI scans were not available in all cases and for all episodes of therapy. Although the criteria for RAI-refractory disease have been defined in adults (68), this has not been studied in children. Some of the adult criteria, such as lack of RAI uptake in known structural disease, would certainly be applicable to pediatric DTC but others such as the 600 mCi threshold is not translatable to children, understanding that the cumulative mCi activity that defines RAI refractory disease in an 8-year-old would be quite different from an 18-year-old. Given the high rate of persistent disease in our study, despite a median of 2 RAI treatments and a median cumulative activity of 248.9 mCi, it would appear that RAI-refractory disease is more common than previously recognized. However, unlike older patients with DTC, RAI-refractory disease in children, even if fluorodeoxyglucose avid, can remain indolent for decades (70) and can be associated with declining Tg levels despite no further RAI therapy (71). Understanding that the long-term prognosis in patients with DM from DTC diagnosed during childhood is excellent and recognizing the high rates of persistent disease despite aggressive RAI therapy, one could strongly argue that repeated RAI therapy is unlikely to benefit these patients and may result in more harm than good over the course of their lives. Ultimately, further studies of the genotype-phenotype correlations in pediatric DTC and a better understanding of what distinguishes those cured of their disease from those with persistent disease will help to inform decisions regarding RAI during childhood.
Strengths and limitations
The main strengths of the present study are the size of the cohort, the length of follow-up, and the large group of patients whose tumors were tested molecularly. We were able to describe the clinical outcomes of 148 patients with DM from childhood DTC through decades of follow-up in many cases. We also identified the oncogenic drivers in the largest number of childhood DTC patients with DM. Limitations of the study are as would be expected for a retrospective study, wherein data were not prospectively collected or documented in a systematic fashion. We also had several patients in whom we could not determine disease status because of incomplete staging and, in exceptional cases, structural disease may have been overcalled (for example, an abnormal cervical ultrasound without confirmatory biopsy; persistent but stable lung nodules that may or may not be active sites of cancer); in these cases the Tg or TgAb was still abnormal and therefore, at a minimum, would still be categorized as persistent disease. Furthermore, our patient population is likely to be sicker with more advanced disease compared with other academic centers because of our practice in a comprehensive cancer center with known expertise in thyroid cancer management. Thus, our reported outcomes may be worse.
In conclusion, at a tertiary cancer center, DM occurs in up to 25% of pediatric DTC cases. These cases are more enriched for PTC compared with the expected distribution in DTC as a whole, and the lungs are universally affected in PTC. Although most cases are diagnosed within 1 year after DTC diagnosis, the recognition of DM can be delayed by years, especially in FTC, which highlights the importance of long-term follow-up in children with DTC. Fortunately, despite a high prevalence of persistent biochemical and structural disease, the prognosis remains excellent, with death from DTC occurring in few patients and typically decades after diagnosis. Given the excellent long-term survival outcomes and high proportion of persistent disease as seen in our study and others, it behooves us to reconsider the aggressiveness of treatment at a young age when the patient may be more at risk for the life-long sequelae of surgical therapy and RAI. Gene rearrangements causing RET and NTRK1/3 fusion genes occurred in 88% of PTC cases with a known oncogenic driver, and all identified alterations currently have targeted systemic therapies available. Future studies should focus on expanding the genotype-phenotype correlations in stage II pediatric DTC, determining the best way to integrate molecularly targeted therapy into treatment paradigms, and relying less on repeated courses of RAI to achieve cure in patients with DM from childhood DTC.
Acknowledgments
We are forever grateful to the patients and families who contributed to the present study; your courage in the face of adversity inspires us. This study could not have been completed without the help of Pamela Waddilove, Danielle Litofsky, Jennifer Ellefson, and Damaris Cruz-Goldberg. We also acknowledge our colleagues in Pathology and Endocrine Neoplasia who helped to answer questions and cared for patients on the study. We thank the Prince Bernhard Culture Fund and the Academy Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences for their financial support. We thank the Junior Scientific Masterclass Groningen for extending the MD/PhD program to allow Dr Nies to perform this research. Finally, we are very grateful to Ms Sandra Montez and Dr David Hong in Investigational Cancer Therapeutics for their assistance with molecular testing.
Financial Support: Marloes Nies received funding from the Junior Scientific Master Class Groningen, the Prince Bernhard Culture Fund, and the Academy Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences for her research at The University of Texas MD Anderson Cancer Center. This work was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (award No. P30CA016672) and used the Biostatistics Resource Group.
Glossary
Abbreviations
- AJCC
American Joint Committee on Cancer
- ALK
anaplastic lymphoma kinase
- ATA
American Thyroid Association
- DM
distant metastases
- DTC
differentiated thyroid carcinoma
- EBRT
external beam radiation therapy
- FTC
follicular thyroid carcinoma
- IHC
immunohistochemistry
- IQR
interquartile range
- NTRK
neurotrophic tyrosine receptor kinase
- PTC
papillary thyroid carcinoma
- RAI
radioactive iodine
- RET
rearranged during transfection
- Tg
thyroglobulin
- TgAb
thyroglobulin antibody
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References (58).”
References
- 1. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4): e945-e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernier MO, Withrow DR, Berrington de Gonzalez A, et al. Trends in pediatric thyroid cancer incidence in the United States, 1998-2013. Cancer. 2019;125(14):2497-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156(1):167-172. [DOI] [PubMed] [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016, Based on November 2018 SEER Data Submission, Posted to the SEER Web Site, April 2019. Vol. 2020. National Cancer Institute; 2019. [Google Scholar]
- 5. Steliarova-Foucher E, Stiller CA, Pukkala E, Lacour B, Plesko I, Parkin DM. Thyroid cancer incidence and survival among European children and adolescents (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2150-2169. [DOI] [PubMed] [Google Scholar]
- 6. Welch Dinauer CA, Tuttle RM, Robie DK, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf). 1998;49(5):619-628. [DOI] [PubMed] [Google Scholar]
- 7. Wada N, Sugino K, Mimura T, et al. Treatment strategy of papillary thyroid carcinoma in children and adolescents: clinical significance of the initial nodal manifestation. Ann Surg Oncol. 2009;16(12):3442-3449. [DOI] [PubMed] [Google Scholar]
- 8. Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens). 2007;6(3):200-209. [PubMed] [Google Scholar]
- 9. Machens A, Lorenz K, Nguyen Thanh P, Brauckhoff M, Dralle H. Papillary thyroid cancer in children and adolescents does not differ in growth pattern and metastatic behavior. J Pediatr. 2010;157(4):648-652. [DOI] [PubMed] [Google Scholar]
- 10. Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34(6): 1192-1202. [DOI] [PubMed] [Google Scholar]
- 11. Francis GL, Waguespack SG, Bauer AJ, et al. ; American Thyroid Association Guidelines Task Force . Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinelli C, Strambi S, Rossi L, et al. Surgical management of papillary thyroid carcinoma in childhood and adolescence: an Italian multicenter study on 250 patients. J Endocrinol Invest. 2016;39(9):1055-1059. [DOI] [PubMed] [Google Scholar]
- 13. Hay ID, Johnson TR, Kaggal S, et al. Papillary thyroid carcinoma (PTC) in children and adults: comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the Mayo Clinic during eight decades (1936-2015). World J Surg. 2018;42(2):329-342. [DOI] [PubMed] [Google Scholar]
- 14. Galuppini F, Vianello F, Censi S, et al. Differentiated thyroid carcinoma in pediatric age: genetic and clinical scenario. Front Endocrinol (Lausanne). 2019;10:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alzahrani AS, Alswailem M, Moria Y, et al. Lung metastasis in pediatric thyroid cancer: radiological pattern, molecular genetics, response to therapy, and outcome. J Clin Endocrinol Metab. 2019;104(1):103-110. [DOI] [PubMed] [Google Scholar]
- 16. Zhang XY, Song HJ, Qiu ZL, et al. Pulmonary metastases in children and adolescents with papillary thyroid cancer in China: prognostic factors and outcomes from treatment with 131I. Endocrine. 2018;62(1):149-158. [DOI] [PubMed] [Google Scholar]
- 17. Bal CS, Garg A, Chopra S, Ballal S, Soundararajan R. Prognostic factors in pediatric differentiated thyroid cancer patients with pulmonary metastases. J Pediatr Endocrinol Metab. 2015;28(7-8):745-745. [DOI] [PubMed] [Google Scholar]
- 18. Schlumberger M, De Vathaire F, Travagli JP, et al. Differentiated thyroid carcinoma in childhood: long term follow-up of 72 patients. J Clin Endocrinol Metab. 1987;65(6):1088-1094. [DOI] [PubMed] [Google Scholar]
- 19. Dottorini ME, Vignati A, Mazzucchelli L, Lomuscio G, Colombo L. Differentiated thyroid carcinoma in children and adolescents: a 37-year experience in 85 patients. J Nucl Med. 1997;38(5):669-675. [PubMed] [Google Scholar]
- 20. Vassilopoulou-Sellin R, Klein MJ, Smith TH, et al. Pulmonary metastases in children and young adults with differentiated thyroid cancer. Cancer. 1993;71(4):1348-1352. [DOI] [PubMed] [Google Scholar]
- 21. La Quaglia MP, Black T, Holcomb GW III, et al. Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children’s Cancer Group. J Pediatr Surg. 2000;35(6):955-959; discussion 960. [DOI] [PubMed] [Google Scholar]
- 22. Bal CS, Kumar A, Chandra P, Dwivedi SN, Mukhopadhyaya S. Is chest x-ray or high-resolution computed tomography scan of the chest sufficient investigation to detect pulmonary metastasis in pediatric differentiated thyroid cancer? Thyroid. 2004;14(3):217-225. [DOI] [PubMed] [Google Scholar]
- 23. Demidchik YE, Demidchik EP, Reiners C, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243(4): 525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein Hesselink MS, Nies M, Bocca G, et al. Pediatric differentiated thyroid carcinoma in the Netherlands: a nationwide follow-up study. J Clin Endocrinol Metab. 2016;101(5):2031-2039. [DOI] [PubMed] [Google Scholar]
- 25. Chesover AD, Vali R, Hemmati SH, Wasserman JD. Lung metastasis in children with differentiated thyroid cancer: factors associated with diagnosis and outcomes of therapy. Thyroid. Published online July 16, 2020. doi: 10.1089/thy.2020.0002 [DOI] [PubMed] [Google Scholar]
- 26. Vaisman F, Bulzico DA, Pessoa CH, et al. Prognostic factors of a good response to initial therapy in children and adolescents with differentiated thyroid cancer. Clinics (Sao Paulo). 2011;66(2):281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chow SM, Law SC, Mendenhall WM, et al. Differentiated thyroid carcinoma in childhood and adolescence-clinical course and role of radioiodine. Pediatr Blood Cancer. 2004;42(2): 176-183. [DOI] [PubMed] [Google Scholar]
- 28. Prpić M, Franceschi M, Jukić T, et al. Differentiated thyroid cancer in pediatric population (≤ 18 years): postoperative treatment with radioactive iodine (I-131). Acta Clin Croat. 2019;58(1):119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamam M, Uyanik E, Edís N, Mulazimoglu M, Ozpacaci T. Differentiated thyroid carcinoma in children: clinical characteristics and long-term follow-up. World J Nucl Med. 2020;19(1):28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golpanian S, Perez EA, Tashiro J, Lew JI, Sola JE, Hogan AR. Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int. 2016;32(3):201-208. [DOI] [PubMed] [Google Scholar]
- 31. Handkiewicz-Junak D, Wloch J, Roskosz J, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007;48(6):879-888. [DOI] [PubMed] [Google Scholar]
- 32. Pekova B, Sykorova V, Dvorakova S, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30(12):1771-1780. [DOI] [PubMed] [Google Scholar]
- 33. Pekova B, Dvorakova S, Sykorova V, et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr Connect. 2019;8(6):796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sugino K, Nagahama M, Kitagawa W, et al. Distant metastasis in pediatric and adolescent differentiated thyroid cancer: clinical outcomes and risk factor analyses. J Clin Endocrinol Metab. 2020;105(11):dgaa545. [DOI] [PubMed] [Google Scholar]
- 35. Pawelczak M, David R, Franklin B, Kessler M, Lam L, Shah B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following ¹³¹I treatment: a systematic review. Thyroid. 2010;20(10): 1095-1101. [DOI] [PubMed] [Google Scholar]
- 36. Vassilopoulou-Sellin R, Goepfert H, Raney B, Schultz PN. Differentiated thyroid cancer in children and adolescents: clinical outcome and mortality after long-term follow-up. Head Neck. 1998;20(6):549-555. [DOI] [PubMed] [Google Scholar]
- 37. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoo SK, Lee S, Kim SJ, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PloS Genet. 2016;12(8):e1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hardee S, Prasad ML, Hui P, Dinauer CA, Morotti RA. Pathologic characteristics, natural history, and prognostic implications of BRAFV600E mutation in pediatric papillary thyroid carcinoma. Pediatr Dev Pathol. 2017;20(3):206-212. [DOI] [PubMed] [Google Scholar]
- 40. Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (Basel). 2019;10(9):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin North Am. 2017;46(2):389-403. [DOI] [PubMed] [Google Scholar]
- 42. Picarsic JL, Buryk MA, Ozolek J, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol. 2016;19(2):115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nikita ME, Jiang W, Cheng SM, et al. Mutational analysis in pediatric thyroid cancer and correlations with age, ethnicity, and clinical presentation. Thyroid. 2016;26(2):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cordioli MICV, Moraes L, Bastos AU, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2017;27(2):182-188. [DOI] [PubMed] [Google Scholar]
- 45. Vanden Borre P, Schrock AB, Anderson PM, et al. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations, including kinase fusions. Oncologist. 2017;22(3):255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mostoufi-Moab S, Labourier E, Sullivan L, et al. Molecular testing for oncogenic gene alterations in pediatric thyroid lesions. Thyroid. 2018;28(1):60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alzahrani AS, Alswailem M, Alswailem AA, et al. Genetic alterations in pediatric thyroid cancer using a comprehensive childhood cancer gene panel. J Clin Endocrinol Metab. 2020;105(10):dgaa389. [DOI] [PubMed] [Google Scholar]
- 48. Cordioli MI, Moraes L, Cury AN, Cerutti JM. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr Relat Cancer. 2015;22(6):R311-R324. [DOI] [PubMed] [Google Scholar]
- 49. Alzahrani AS, Murugan AK, Qasem E, Alswailem M, Al-Hindi H, Shi Y. Single point mutations in pediatric differentiated thyroid cancer. Thyroid. 2017;27(2):189-196. [DOI] [PubMed] [Google Scholar]
- 50. Sisdelli L, Cordioli MICV, Vaisman F, et al. AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatr Blood Cancer. 2019;66(7):e27707. [DOI] [PubMed] [Google Scholar]
- 51. Vassilopoulou-Sellin R, Libshitz HI, Haynie TP. Papillary thyroid cancer with pulmonary metastases beginning in childhood: clinical course over three decades. Med Pediatr Oncol. 1995;24(2):119-122. [DOI] [PubMed] [Google Scholar]
- 52. Waguespack SG, Sherman SI, Williams MD, Clayman GL, Herzog CE. The successful use of sorafenib to treat pediatric papillary thyroid carcinoma. Thyroid. 2009;19(4):407-412. [DOI] [PubMed] [Google Scholar]
- 53. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing/American College of Surgeons ;2017. [Google Scholar]
- 54. Tuttle RM, Leboeuf R. Follow up approaches in thyroid cancer: a risk adapted paradigm. Endocrinol Metab Clin North Am. 2008;37(2):419-435, ix-x. [DOI] [PubMed] [Google Scholar]
- 55. Sung TY, Jeon MJ, Lee YH, et al. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2017;102(3):793-800. [DOI] [PubMed] [Google Scholar]
- 56. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nies M, Vassilopoulou-Sellin R, Bassett RL, et al. Data from “Distant metastases from childhood DTC—Supplemental Material.” Deposited September 12, 2020. 10.6084/m9.figshare.12948386.v2 [DOI]
- 59. Popovtzer A, Shpitzer T, Bahar G, Feinmesser R, Segal K. Thyroid cancer in children: management and outcome experience of a referral center. Otolaryngol Head Neck Surg. 2006;135(4):581-584. [DOI] [PubMed] [Google Scholar]
- 60. Park S, Jeong JS, Ryu HR, et al. Differentiated thyroid carcinoma of children and adolescents: 27-year experience in the Yonsei University Health System. J Korean Med Sci. 2013;28(5):693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reiners C, Biko J, Haenscheid H, et al. Twenty-five years after Chernobyl: outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2013;98(7):3039-3048. [DOI] [PubMed] [Google Scholar]
- 62. Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2(5): 356-358. [DOI] [PubMed] [Google Scholar]
- 63. Enomoto K, Enomoto Y, Uchino S, Yamashita H, Noguchi S. Follicular thyroid cancer in children and adolescents: clinicopathologic features, long-term survival, and risk factors for recurrence. Endocr J. 2013;60(5):629-635. [DOI] [PubMed] [Google Scholar]
- 64. Livhits MJ, Pasternak JD, Xiong M, et al. Pre-ablation thyroglobulin and thyroglobulin to thyroid-stimulating hormone ratio may be associated with pulmonary metastases in children with differentiated thyroid cancer. Endocr Pract. 2016;22(11):1259-1266. [DOI] [PubMed] [Google Scholar]
- 65. Hebestreit H, Biko J, Drozd V, et al. Pulmonary fibrosis in youth treated with radioiodine for juvenile thyroid cancer and lung metastases after Chernobyl. Eur J Nucl Med Mol Imaging. 2011;38(9):1683-1690. [DOI] [PubMed] [Google Scholar]
- 66. Chu YH, Wirth LJ, Farahani AA, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol. 2020;33(12):2458-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Penault-Llorca F, Rudzinski ER, Sepulveda AR. Testing algorithm for identification of patients with TRK fusion cancer. J Clin Pathol. 2019;72(7):460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aashiq M, Silverman DA, Na’ara S, Takahashi H, Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel). 2019;11(9):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sabra MM, Dominguez JM, Grewal RK, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. 2013;98(5):E829-E836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kwong N, Marqusee E, Gordon MS, et al. Long-term, treatment-free survival in select patients with distant metastatic papillary thyroid cancer. Endocr Connect. 2014;3(4):207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on 131I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging. 2011;38(4):651-655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References (58).”