Abstract
Aims
The association of glycemic variability with microvascular disease complications in type 2 diabetes (T2D) has been under-studied and remains unclear. We investigated this relationship using both Action to Control Cardiovascular Risk in Diabetes (ACCORD) and the Veteran Affairs Diabetes Trial (VADT).
Methods
In ACCORD, fasting plasma glucose (FPG) was measured 1 to 3 times/year for up to 84 months in 10 251 individuals. In the VADT, FPG was measured every 3 months for up to 87 months in 1791 individuals. Variability measures included coefficient of variation (CV) and average real variability (ARV) for fasting glucose. The primary composite outcome was time to either severe nephropathy or retinopathy event and secondary outcomes included each outcome individually. To assess the association, we considered variability measures as time-dependent covariates in Cox proportional hazard models. We conducted a meta-analysis across the 2 trials to estimate the risk of fasting glucose variability as well as to assess the heterogenous effects of FPG variability across treatment arms.
Results
In both ACCORD and the VADT, the CV and ARV of FPG were associated with development of future microvascular outcomes even after adjusting for other risk factors, including measures of average glycemic control (ie, cumulative average of HbA1c). Meta-analyses of these 2 trials confirmed these findings and indicated FPG variation may be more harmful in those with less intensive glucose control.
Conclusions
This post hoc analysis indicates that variability of FPG plays a role in, and/or is an independent and readily available marker of, development of microvascular complications in T2D.
Keywords: microvascular complications, glycemic control, long-term glycemic variability, type 2 diabetes, interaction
Microvascular complications (retinopathy, nephropathy, and neuropathy) are common pathologic consequences of type 2 diabetes (T2D). It is well recognized that chronic hyperglycemia is an important risk factor for the development of microvascular disease in patients with T2D (1). As a result of this strong link between hyperglycemia and diabetes complications, the role of glycemic control (eg, intensive vs standard control of glucose or glycated hemoglobin A1c [HbA1c] levels) has been extensively studied (2-4). Several clinical trials (eg, the UK Prospective Diabetes Study [UKPDS], Action to Control Cardiovascular Risk in Diabetes [ACCORD]) and meta-analyses of clinical trials show that more intensive glucose lowering in T2D improves microvascular outcomes (especially kidney and eye events) compared with standard glycemic control group (5). However, the effects are often relatively modest, and are largely driven by improvements in proteinuria or background retinopathy, rather than in more clinically relevant outcomes such as end-stage renal disease or photocoagulation.
Glycemic control evaluated in previous trials examined only average glycemic exposure. It has been estimated that total glycemic exposure (derived from average HbA1c and duration of diabetes) predicted only 11% of the risk of developing retinopathy in the type 1 diabetic cohort of the Diabetes Control and Complications Trial (DCCT) cohort (6, 7). On the other hand, increasing evidence implicates glycemic variability as an important contributor to the development of microvascular diabetes complications. Using a newly diagnosed diabetes cohort extracted from the Tayside and Fife in the Scottish Care Information–Diabetes Collaboration (SCI-DC) (8), Li et al recently showed that patients with a higher HbA1c variability score had an increased risk of developing microvascular complications. Although their risk factors and outcomes were extracted from medical records based on ICD9/ICD10 codes or changes of lab results, ie, estimated glomerular filtration rate (eGFR), this work suggested that renal, eye, and nerve complications may be linked to visit-to-visit variation in HbA1c in new-onset T2D. This appears to be supported by findings in diabetes patients with a longer duration of diabetes, including those from the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (9) and several observational studies. For example, HbA1c variability was an independent risk factor (after adjustment for average HbA1c) for albuminuria but was not associated with diabetic retinopathy in the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study (10). The Rio de Janeiro Study reported that glucose variability was associated with severe renal outcomes (eg, renal failure), but not retinopathy, after accounting for overall glucose control (11). However, none of the studies have examined the relationship of glucose variability with microvascular complications during intensive vs standard glucose lowering—a clinical setting in which variability may be most relevant.
Therefore, the goal of the present study was to use 2 large comprehensive trials of glucose lowering (ACCORD and the Veteran Affairs Diabetes Trial [VADT]) to study the effects of fasting plasma glucose (FPG) variability on microvascular outcomes (including both nephropathy and retinopathy). As both ACCORD and VADT were designed to compare glucose-lowering strategies, we were able to assess the differential effects of FPG variability between intensive and standard treatment arms. By using consistent microvascular outcomes across the 2 studies, we were able to perform a meta-analysis to pool the information from the 2 trials to provide a more precise estimate of the risk of FPG variability. Our results show that FPG variability increased risk of microvascular events well beyond that accounted for by average glycemic levels in both trials. Interestingly, the effect of long-term visit-to-visit FPG variability was found to be stronger in the standard treatment group than in the intensive treatment group in both the ACCORD and VADT trials.
Methods
Study Design and Participants
The Action to Control Cardiovascular Risk in Diabetes trial (ACCORD) was a double-blinded, 2-by-2 factorial, randomized controlled, parallel treatment trial in which 10 251 participants were assigned to receive intensive treatment targeting HbA1c concentration of less than 6.0% (42.1 mmol/mol) or standard treatment targeting HbA1c of 7.0% to 7.9% (53-62.8 mmol/mol)—as well as to distinct blood pressure and lipid interventions arms (3). The ACCORD study included participants with T2D, HbA1c concentrations of 7.5% (58.5mmol/mol) or more, and who were aged 40 to 79 years with a history of cardiovascular disease or 55 to 79 years with evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least 2 risk factors for cardiovascular disease (dyslipidemia, hypertension, smoking, or obesity). During the study, FPG concentrations were measured every 4 months in the initial year, then annually up to a maximum of 84 months. Within 4 months after randomization, the median glycated hemoglobin level had fallen from 8.1% (65 mmol/mol) at baseline to 6.5% (47.5 mmol/mol) in the intensive therapy group and to 7.5% (58.5 mmol/mol) in the standard therapy group. Details of the design and principal results of ACCORD trial were reported previously (3, 12). Our analysis used all in-study FPG measures through the full ACCORD study (Supplementary Fig. 1 (13)).
The Veteran Affairs Diabetes Trial (VADT) was a randomized trial that enrolled 1791 military veterans (mean age, 60.4 years) who had a suboptimal response to therapy for T2D (HbA1c > 7.5% or 58.5mmol/mol) to receive either intensive or standard glucose control. The design and the principal results have been described previously (2). Following an established algorithm, the 2 groups were treated with similar medications (but different doses) with a goal of the intensive treatment group of achieving near normal glucose control and an absolute difference in HbA1c of >1.5% between treatment groups. HbA1c and fasting glucose were measured every 3 months up to a maximum of 84 months. At 3 months into the trial median HbA1c levels had decreased in both groups and had stabilized by 6 months, with a level of 8.4% (68 mmol/mol) in the standard-therapy group and 6.9% (52 mmol/mol) in the intensive-therapy group. Similar temporal patterns were seen with FPG.
Primary and Secondary Outcomes
Our primary microvascular outcome for both studies reflects a composite endpoint of advanced kidney and eye disease. In ACCORD, this composite microvascular outcome was its primary outcome and was defined as the development of end-stage renal disease (ESRD, ie, initiation of dialysis or a rise of serum creatinine to 3.3 mg/dL [292 μmol/L]), or retinal photocoagulation or vitrectomy to treat retinopathy. These serious outcomes were previously defined and described as the key microvascular outcomes by ACCORD investigators and were referred to as Neph-3 and Eye-1, respectively (3). As the VADT did not collect the same renal clinical outcomes as in ACCORD, we defined nephropathy in VADT in a similar, but not identical, fashion as 2 consecutive values of serum creatinine more than 3.3 mg per deciliter or with consecutive values of glomerular filtration rate (GFR) of less than 30 mL per minute.(2) Time to event reflected the first of the 2 consecutive lab values meeting the criteria for either outcome. Retinopathy in the VADT was defined by retinal photocoagulation or vitrectomy, as within ACCORD. The GFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation as described previously (14). Secondary outcomes for each study included the individual components of the primary composite outcome, ie, time to renal failure (nephropathy) and retinal photocoagulation or vitrectomy (retinopathy) (3). To conduct a meta-analysis of the 2 studies, we redefined the nephropathy outcome in ACCORD by the definition used in VADT in order to make the outcome consistent and further reduce the study heterogeneity.
In addition to the 3 above key microvascular outcomes (eye, renal, or combined events) in ACCORD, there were several other prespecified microvascular outcomes in ACCORD for kidney function and diabetes eye complications. The additional predefined ACCORD microvascular outcomes were also explored for a more comprehensive assessment of less advanced or broader combinations of microvascular outcomes and included:
Neph-1: Doubling of baseline serum creatinine or more than 20 mL/min per 1.73 m2 decrease in estimated GFR.
Neph-2: Development of macroalbuminuria (urine albumin:creatinine ratio ≥33.9 mg/mmol)
[Neph-3: Defined above, as part of the primary composite outcome]
Neph-4: Development of Neph-1, Neph-2, or Neph-3
Neph-5: Development of microalbuminuria (urine albumin:creatinine ratio ≥3.4 mg/mmol)
[Eye-1: Defined above as part of primary composite outcome]
Eye-2: Eye surgery for cataract extraction
Eye-3: Three-line change in visual acuity
Eye-4: Severe vision loss (Snellen fraction <20/200)
A detailed description of the prespecification of outcomes in ACCORD was reported previously (3).
Fasting Plasma Glucose Variability
Commonly used measures of visit-to-visit glucose variability include SD, coefficient of variation (CV), variability independent of mean (VIM), and average real variability (ARV) (9, 15, 16). We selected CV and ARV for this analysis as they appear to be complementary measures of variability as previously published (17). CV measures the spread of the data over time vs while ARV measures smoothness of data over time. Definitions of these 2 variability measures have been described previously (17) and are provided in the Supplementary Table 1 (13). Mean FPG levels revealed substantial treatment group separation was achieved over the initial 4 months of ACCORD and the initial 6 months of VADT. This pattern persisted during the remaining duration of the trials. Therefore, observations from (including) the fourth month and beyond in ACCORD and from (including) the sixth month and beyond in the VADT were used for FPG variability calculation to exclude the rapid change of FPG resulting from the glucose-lowering trial designs (Supplementary Fig. 1 (13)).
Statistical Analysis
Data are expressed as means (SD) for continuous variables or as numbers and percentages for categorical variables. Differences between patients who did and did not develop an event were analyzed using the Wilcoxon test for continuous variables and the χ 2 test or Fisher exact test, as appropriate, for categorical variables shown in Supplementary Table 2 and Supplementary Table 3 (13).
Multivariable analyses were performed by Cox proportional hazard models. We evaluated risk of fasting glucose variability while controlling for average glycemic control (defined as cumulative average of HbA1c). Both were included as continuous and time-dependent covariates (18) in the Cox proportional hazard models. This process dynamically matched the risk variables and time of outcome event, so that we do not use any measures after the event has happened (19). The proportionality of all model predictors was confirmed in plots of Schoenfeld residuals over time. To ease interpretation of statistical models, hazard ratios (HR) for all variables of glycemic control were standardized to a change of one SD. Nonnormally distributed variables, such as CV, were log-transformed to approach normal distribution. Analyses were performed after adjusting for (i) Model-1: age only; (ii) Model-2: age and covariates reflecting significant baseline differences in characteristics (including blood pressure and lipids treatment arms in ACCORD study) between those who did and did not develop microvascular events during the study (seeTable 1 legend and Supplementary Tables 2 and 3 (13)); and (iii) Model-3: covariates in Model-2 plus cumulative mean of HbA1c reflecting average glycemic control to clarify whether variability measures provided information beyond standard glycemic measures.
Table 1.
Hazard Ratios for Glycemic Exposure Variables Estimated by Cox Proportional Hazards Model in ACCORD and VADT
| Model-1 | Model-2 | Model-3 | ||||
|---|---|---|---|---|---|---|
| Age adjustment | Multivariate adjustment | Model 2 + cumulative HbA1c | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| ACCORD | ||||||
| Primary Composite (n = 1106) a | ||||||
| Log(CV)-glucose | 1.35 (1.25-1.44) | <0.001 | 1.19 (1.10-1.29) | <0.001 | 1.18 (1.09-1.28) | <0.001 |
| ARV-glucose | 1.27 (1.20-1.35) | <0.001 | 1.15 (1.07-1.23) | <0.001 | 1.14 (1.06-1.22) | <0.001 |
| Nephropathy (n = 269) b | ||||||
| Log(CV)-glucose | 1.34 (1.18-1.53) | <0.001 | 1.29 (1.12-1.47) | <0.001 | 1.28 (1.12-1.47) | <0.001 |
| ARV-glucose | 1.30 (1.17-1.45) | <0.001 | 1.25 (1.11-1.40) | <0.001 | 1.24 (1.10-1.39) | <0.001 |
| Retinopathy (n = 880) C | ||||||
| Log(CV)-glucose | 1.35 (1.25-1.46) | <0.001 | 1.13 (1.03-1.23) | 0.005 | 1.12 (1.03-1.22) | 0.010 |
| ARV-glucose | 1.26 (1.18-1.35) | <0.001 | 1.08 (1.00-1.18) | 0.052 | 1.07 (0.99-1.17) | 0.091 |
| VADT | ||||||
| Primary Composite (n = 186) d | ||||||
| Log(CV)-glucose | 1.58(1.32-1.89) | <0.001 | 1.43(1.18-1.73) | <0.001 | 1.39(1.14-1.69) | <0.001 |
| ARV-glucose | 1.31(1.16-1.48) | <0.001 | 1.20(1.04-1.38) | <0.001 | 1.17(1.01-1.35) | <0.001 |
| Nephropathy (n = 42) e | ||||||
| Log(CV)-glucose | 2.31(1.59-3.35) | <0.001 | 1.92(1.30-2.83) | 0.001 | 1.91(1.27-2.86) | 0.002 |
| ARV-glucose | 1.53(1.22-1.91) | <0.001 | 1.36(1.06-1.75) | 0.016 | 1.34(1.03-1.75) | 0.027 |
| Retinopathy (n = 154) f | ||||||
| Log(CV)-glucose | 1.43 (1.18-1.73) | <0.001 | 1.29(1.05-1.59) | 0.004 | 1.31(1.06-1.63) | 0.005 |
| ARV-glucose | 1.26 (1.10-1.45) | <0.001 | 1.18(1.01-1.38) | 0.031 | 1.19(1.01-1.40) | 0.043 |
In Model 2, glycemic control variables that were significant in age-adjusted models (Model-1) were further adjusted for significantly different baseline factors (see Supplementary Tables 2 and 3 (13)). For the primary outcome, in Model 2, we additionally adjusted race, diabetes duration, CVD history, history of heart failure, history of eye disease, smoker status, baseline DBP, baseline SBP, baseline HDL, baseline triglycerides, baseline HbA1c, baseline albumin to creatinine ratio (ACR), and baseline eGFR for ACCORD and duration of diabetes, pack-years of cigarette smoked, baseline SBP, and baseline ACR. In Model-3, models were additionally adjusted for cumulative mean of HbA1c as a reflection of average glycemic control.
P values < 0.05 (bold font) are considered significant.
n = event number.
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; ARV, average real variability; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HR, hazard ratio; VADT, Veteran Affairs Diabetes Trial.
a Primary composite: development of end-stage renal disease (ESRD, ie, initiation of dialysis or a rise of serum creatinine to 3.3 mg/dL [291.72 μmol/L]) or photocoagulation or vitrectomy.
b Nephropathy: renal failure or ESRD or serum creatine >3.3 mg/dL (291.72 μmol/L).
c Retinopathy: photocoagulation or vitrectomy.
d Primary composite: Nephropathy or Retinopathy;
e Nephropathy: 2 consecutive values of serum creatinine ≥3.3mg per deciliter (291.72 μmol/L or eGFR < 30 mL/min/1.73 m2).
f Retinopathy: Photocoagulation or Vitrectomy.
Five sensitivity analyses were conducted. We first excluded patients with advanced baseline eye or kidney disease. Baseline eye disease was defined as “cataract removal,” “retinal laser photocoagulation for diabetic retinopathy,” “laser for cataract capsule,” or “vitrectomy for diabetic retinopathy” for either eye. Baseline kidney disease was defined as eGFR <45 mL per minute or macroalbuminuria, ie, urine albumin:creatinine ratio ≥33.9 mg/mmol. We also adjusted for “insulin use” in the model to assess whether the risk of glucose variability is independent of insulin use. Non-insulin users were those who reported “No” at all time points; all remaining participants were considered insulin users. We increased the minimum number of glucose measures per individual for inclusion to 5 which preserved 80% of sample. As the intensive-therapy arm was aborted prematurely because of increased mortality in ACCORD (4), we also tested glucose measures only up to cessation of intensive treatment in the ACCORD study. Finally, we assessed the contribution of adverse lifestyle behaviors on the effect of glucose variability, within a subset sample (n = 2034) in ACCORD, where smoking, dietary patterns, and activity data were available. A summary score of unhealthy behaviors, from 0 to 3, was generated from these factors. A similar score was also generated in VADT, as previously described (20). As these additional adverse behavior adjustments did not modify the results, they were not included in the presented models. We also used study visit HbA1c measures to capture glycemic variability. However, HbA1c showed a generally weaker association with primary and second microvascular outcomes than did variability in fasting glucose (results not shown).
We also pooled the results from the 2 studies to provide a more precise and generalizable overall effect. As random-effects meta-analysis (21) relies on the estimates of between study variance (which is ideally conducted in more than 2 studies) to apply it correctly (22), we instead used fixed-effects meta-analysis to integrate the results from the 2 studies for all 3 outcomes (composite, renal, and eye) using the same outcome definitions. Statistical heterogeneity of the 2 studies was assessed with the I2 statistic (23), where values of 30% to 60% represent a moderate level of heterogeneity. Meta-analyses were used to pool the risk of glucose variability from the 2 cohorts as well as to assess the pooled differential risks between the 2 treatment arms (intensive vs standard glucose control). We tested the null hypothesis (that the difference of glucose variability risks between treatment groups is zero) using the following approach: first, for each trial, we calculated a trial-specific interaction HR by adding an interaction term between treatment group and glucose variability measures in the Cox proportional hazard models; second, we combined these trial-specific interaction HRs across trials using a fixed-effects model. For the stratified analysis and stratified meta-analysis, we derived the HRs of glucose variability for all 3 microvascular outcomes, and their 95% CIs, in each study separately in the intensive and standard treatment group. We then calculated the pooled HR of glucose variability using fixed-effect meta-analysis models.
All statistical analyses were performed using R version 3.5.3 (https://www.r-project.org). Meta-analyses were conducted by a R package, “meta” (24). A 2-sided P < 0.05 was considered statistically significant.
Results
There were 10 026 participants in ACCORD who had at least 2 measurements of fasting glucose after baseline and before a microvascular event occurred. Of these, 1106 developed a primary composite microvascular event. In the VADT, 1658 individuals had at least 2 measurements of fasting glucose after the first 6 months and before a microvascular event occurred, and 186 developed a primary composite event. The mean and median follow-up times were 59.5 and 59.8 months for the ACCORD, and 67.1 and 68.8 months for the VADT cohort. This provided on average 9 (median 6) and 18 (median 20) measures of fasting glucose in ACCORD and VADT, respectively. In ACCORD, the number of missing glucose measures ranged from 2.3% at the second visit (month 4) to 12.5% at the last time point (month 84). In the VADT, missing glucose measures at each visit were 4% or less. As noted in the ACCORD and VADT trial original publications for microvascular complications (2, 3), the proportion of participants lost to follow-up was very low (0.5%) (12). There were 3% of eye outcomes missing for the ACCORD cohort, while the rates of missing data for other outcomes were negligible for both studies. Baseline characteristics for both cohorts are shown by incident event status during the studies for both primary and secondary outcomes (Supplementary Tables 2 and 3 (13)).
The Pearson correlation coefficient between CV-glucose and ARV-glucose in ACCORD was 0.86, while correlation coefficients between glucose variability measures and the cumulative mean of HbA1c were relatively low, at 0.25 and 0.26 for CV and ARV, respectively. In the VADT, a similar degree of correlation was observed between CV and ARV and the cumulative mean of HbA1c. Plots of cumulative CV and ARV in ACCORD and VADT across all visits separated by treatment arms show that variability in intensive groups was slightly higher than in standard treatment group throughout the study (Supplementary Fig. 2 and Supplementary Fig. 3, upper panel (13)). Similar plots show that glucose variability was higher among insulin users than in nonusers (Supplementary Fig. 2 and Supplementary Fig. 3, lower panel (13)).
Glucose Variability and Risk of Microvascular Outcome in ACCORD and VADT
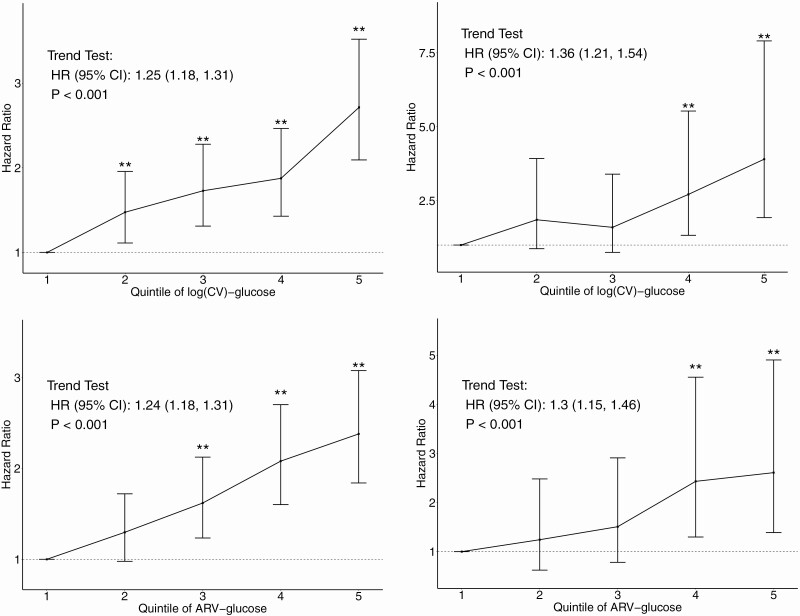
In an age-adjusted model (Model 1), the risk for microvascular outcomes with increasing quintiles of glucose variability demonstrated a striking linear trend (illustrated in Fig. 1, panel A).Table 1(top panel) shows estimated hazard ratios (HR) for glucose variability measures in 3 models for the primary and 2 secondary outcomes in ACCORD. Both CV and ARV were significant risk factors (P < 0.05) for the primary composite and secondary microvascular outcomes in Model 1 (age-adjusted only) and remained significant predictors for both primary and secondary microvascular outcomes after the adjustment for differences in risk factors for microvascular outcomes (Model 2). All these associations, except for the risk of ARV with retinopathy, remained significant after adjustment for the cumulative mean of HbA1c (Model 3). The estimated risks (ie, HRs) associated with glucose variability was in general higher for nephropathy than retinopathy.
Figure 1.
Hazard ratio (HR) estimates for quintiles of Log(CV)-glucose and ARV-glucose for the primary composite outcome in ACCORD and VADT in age-adjusted models. Vertical bars shown are the 95% CI associated with HR estimates. ** indicates estimated HR in the indicated variability quintile is significantly higher than the HR of lowest variability quintile (quintile 1). Trend test results are presented as the text annotation in the figures.
As ACCORD also defined a broad range of other renal and eye outcomes, we also examined risk of glucose variability measures for these secondary outcomes, using the fully adjusted Model 3 (Supplementary Fig. 4 (13)). Thus, there were a total of 5 nephropathy-related microvascular outcomes with event numbers ranging from 486 (Neph-2) to 2751 (Neph-4). The strongest association of glycemic variability with nephropathy was with the primary renal outcome Neph-3 (HR 1.27; 95% CI, 1.11-1.46). However, glycemic variability appeared to be a significant risk factor for all nephropathy outcomes except Neph-5 (development of microalbuminuria). While Eye-3 (3-line change in visual acuity) had more than 3000 events, the number of events for the secondary eye disease outcomes were generally smaller (ranging from 490 to 551). Although glycemic variability increased the risk for all eye outcomes, only the Eye-1 risk (photocoagulation or vitrectomy) was significant. As with the primary outcomes, the associations of glycemic variability with the development of other eye outcomes appeared weaker compared with the risk for development of secondary nephropathy outcomes. In the VADT, risk for microvascular outcomes with increasing glucose variability also demonstrated a generally linear trend (illustrated in Fig. 1, panel B).Table 1(bottom panel) shows HRs of glucose measures for the primary and secondary outcomes. CV and ARV of fasting glucose were significant risk factors for the primary composite outcome in Model 1 (age-adjusted only) and remained significant risk factors of the primary microvascular outcome after all adjustments, including the cumulative mean of HbA1c (Model 3).
In sensitivity analyses, glucose variability generally remained a significant predictor of incident microvascular disease events after removing participants with baseline eye disease and renal disease or adjusting for insulin use. Risk of glucose variability was even slightly higher after restricting analyses to those with ≥5 glucose measures, and the primary results in the ACCORD did not change after using glucose data collected only during the shorter active glucose-lowering treatment phase of the study (all shown in Supplementary Table 5 (13)).
Meta-analysis of ACCORD and VADT
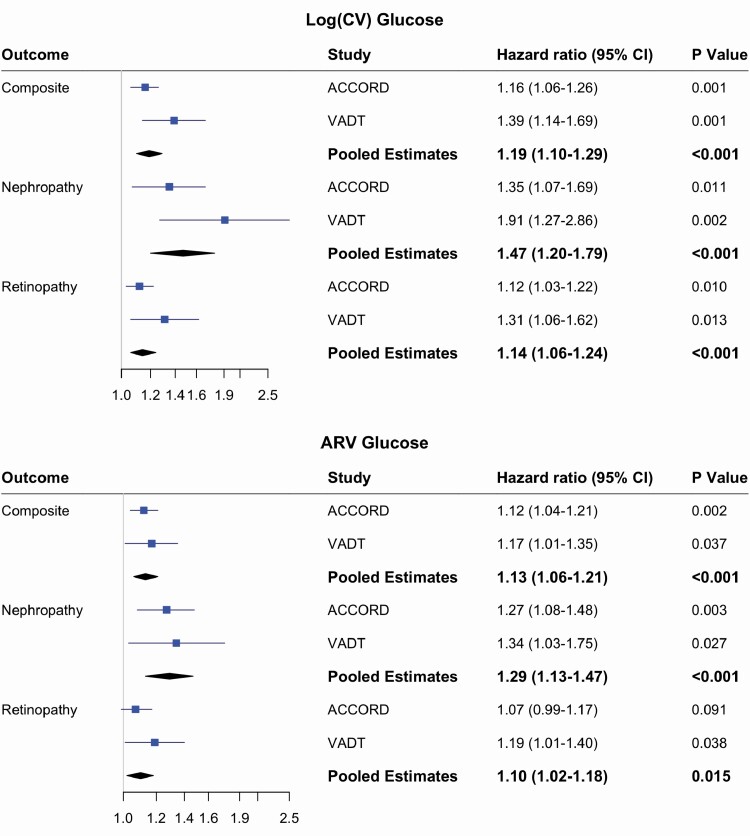
In order to perform a meta-analysis of the effect of measures of glucose variability, we modified the ACCORD definition of nephropathy to be consistent with that used in the VADT (as described above) so that all outcomes were similar between studies. This definition reduced event numbers within ACCORD to 950 composite events and 99 nephropathy events, while eye events remained unchanged (baseline covariates and event numbers shown in Supplementary Table 4) (13). We then reassessed the risk of glucose variability for composite and renal outcomes in ACCORD using the fully adjusted model (Fig. 2). The HRs for all outcomes in ACCORD remained similar and significant, and as when using the original ACCORD definition, were slightly lower compared with HRs estimates based on the VADT. For example, the HR for CV for the composite outcome was estimated to be 1.16 (1.06-1.26) in ACCORD vs 1.39 (1.14-1.69) in the VADT.
Figure 2.
Meta-analysis of the risk of glucose variability for microvascular complications in ACCORD and VADT. Hazard ratios for each individual study were estimated using fully adjusted models (Model-3 in text). Fixed-effect meta-analysis and inverse variance weighted method was used. Composite outcome: Nephropathy or Retinopathy; Nephropathy: 2 consecutive values of serum creatinine ≥3.3 or eGFR < 30; Retinopathy: Photocoagulation or Vitrectomy.
The meta-analysis of the ACCORD and VADT demonstrated that glucose variability (measured by both CV and ARV) is a significant risk factor for the composite microvascular outcome as well as for both nephropathy and retinopathy individually, with estimated I2 statistics of 63%, 55%, and 42%, respectively. The pooled risk estimate of glucose variability was greatest for nephropathy (HR of 1.29 for ARV and 1.38 for CV, Fig. 2).
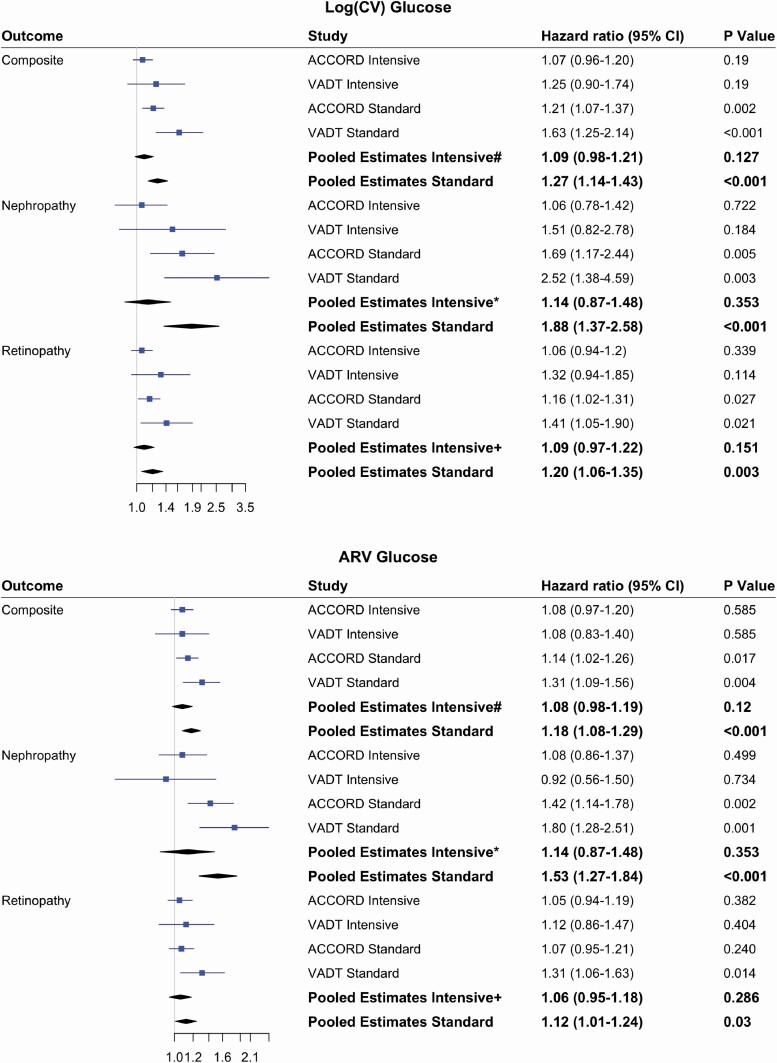
To explore a differential effect between the treatment arms, we added a glucose variability by treatment interaction and assessed the pooled interaction effect. For the composite outcome, the P value for the CV-glucose interaction was 0.07. In stratified-metanalysis, the HR for CV-glucose in the standard treatment group was significant for the composite outcome, as well as for nephropathy and retinopathy individually (Fig. 3). In contrast, it was not a significant predictor for any outcome in the intensive treatment group. This difference between treatment groups was most apparent for development of renal outcomes, with a HR of 1.88 (1.37-2.58, P < 0.001) in the standard group vs 1.14 (0.87-1.48, P = 0.35) in the intensive group. This treatment related modification of the relationship of glucose variability with each outcome was also apparent in each study, with the HRs for CV-glucose achieving statistical significance only in the standard treatment group (Fig. 3). This pattern of differential effects of glucose variability by treatment group was also present for ARV-glucose.
Figure 3.
Meta-analysis of the risk of glucose variability for microvascular complications stratified by treatment arms of ACCORD and VADT. Hazard ratios (95% CI) in each study were estimated based on fully adjusted Model-3. Fixed-effect meta-analysis and inverse variance weighted method was used. Differential risks of glucose variability were assessed by assessing an interaction between glucose variability and treatment groups. For composite outcome#, P value of interaction is 0.07 and 0.24 for CV and ARV respectively. For nephropathy*, P values for interactions were 0.06 and 0.08 for CV and ARV respectively. For retinopathy+, P values for interactions were 0.06 and 0.30 for CV and ARV, respectively.
Discussion
We show that among patients with advanced T2D, fasting glucose visit-to-visit variability was associated with the development of microvascular complications (both for the composite of nephropathy and retinopathy as well as each outcome category individually) even after adjusting for other risk factors, including overall level of glucose control. A 20% to 30% increase in the risk of developing composite events with 1 SD increase in glucose variability was present in both in ACCORD and the VADT study cohorts. Even greater increases in risk for nephropathy were seen in both studies. Pooled analysis of these 2 separate studies confirmed these findings and showed a nearly 20% increase in risk for the primary microvascular composite outcome, and a 50% increase in risk for nephropathy, for the same 1 SD increase in CV-glucose variability. A unique finding in this pooled analysis was that effects of glucose variability appeared particularly robust in those randomized to the standard treatment group, with an approximate 65% increase in risk for nephropathy in the pooled analysis. Although P values for testing the treatment modification on the risk of glucose variability (0.07 for primary composite outcome and 0.06 for both nephropathy and retinopathy) support stratified analyses, the results should be considered exploratory.
Results from several previous investigations of long-term glycemic variability and microvascular outcomes are generally supportive of our findings (10, 25-29). However, our analysis based on 2 independent glucose-lowering trials, ACCORD (n = 10 026) and VADT (n = 1658), exceeds the sample sizes of all previous reports using trial design (8, 25, 27-29) and was able to focus on clinically important and carefully adjudicated nephropathy and retinopathy outcomes. Aside from the ADVANCE trial (9), most other studies were observational (retrospective and prospective cohort studies based on medical records or registries) and were not assessing glucose variation in the context of glucose lowering. Importantly, nephropathy outcomes in these studies were typically not clinically relevant events but were based either on mild changes in urine albumin to creatinine ratio (25) or eGFR (8, 29). However, these studies had also noted a stronger relationship between glucose variability and renal disease than with eye disease, a pattern that is more definitively confirmed with our results. Both ACCORD and VADT also included extensive assessments of demographic, medical history, and laboratory data, permitting comprehensive adjustment for covariates in models. Taking advantage of multiple secondary microvascular outcomes captured in ACCORD also allowed us to investigate the contribution of glucose variability over a spectrum of microvascular events for T2D patients.
As both ACCORD and VADT randomized participants to intensive and standard glucose-lowering arms, this offered a unique opportunity to investigate the differential effects of glucose variability across treatment groups. As was apparent within each cohort and confirmed in the meta-analyses of the 2 trials, glycemic variation appeared most harmful in those with less intensive glucose control. As the unit of comparison, 1 SD of glucose variability, was calculated based on variability in the whole group, the comparison between groups was based on a similar degree of variability and allows us to directly compare the risk between the 2 treatment arms. Of note, despite finding that glucose variability was higher in “insulin users” than in “non-insulin users,” the risk of glucose variability remained present after adjusting for insulin use. This indicates that the risk of glucose variability for microvascular outcomes is not simply a function of greater insulin use. For both ACCORD and VADT, when both CV and ARV were included in Model 3, risk of CV for the primary outcome and retinopathy remained the same while the risk of ARV diminished (data not shown). These results suggest that for the primary outcome and retinopathy the spread of glucose as measured by CV better identifies risks. Finally, as the ACCORD study recorded adherence to glycemic medications, we were able to explore whether this behavior explained the relationship between glucose variability and outcomes. As illustrated in Supplementary Table 6, accounting for adherence to diabetes medications in several different ways did not notably alter the risk of glucose variability (13).
These results have several important implications. They strongly corroborate the additional risk that glucose variation may have in development of microvascular complications, even when accounting for overall glucose control. From a clinical perspective, understanding the risk of glucose variability during glucose lowering is particularly relevant as this may inform healthcare providers when they make decisions to target lower HbA1c goals. Although fasting glucose is inexpensive, routinely measured, and readily extractable for potential use in automated algorithms calculating variability, these results are also valuable as proof-of-principle as more continuous glucose monitoring devices will undoubtedly further refine long-term assessments of glucose variability in the near future. Our data also suggest that additional attention to glucose variability may be needed to prevent microvascular complications in those with relatively poor glycemic control. A potential explanation, consistent with our multivariate analyses, is that glucose variability is an independent contributor to microvascular disease. Indeed, many pathophysiologic mechanisms have been proposed to explain how glucose fluctuations may cause vascular injury (15, 30). Thus, the combination of marked hyperglycemia and increased glucose variability may further increase overall risk for these events, and in particular, for renal outcomes. However, these results do not imply that glucose variation is only important in the setting of very poor glucose control. Moreover, we have previously reported that glucose variation was associated with increased risk for cardiovascular disease (17) and was particularly prominent in those undergoing more aggressive glucose lowering. Thus, the effects of glucose variation on T2D complications may not be relevant solely to those in poor glucose control. These intriguing findings clearly need to be validated.
Our study has several limitations. Despite our efforts to account for key covariates in models predicting microvascular disease, residual confounding remains a possibility. In addition, participants in ACCORD and VADT reflect more advanced diabetes, and caution must be taken when applying our findings to younger and healthier T2D patients. Finally, our study is not able to tease out whether glycemic variability is a key mediator of microvascular disease progression or simply a marker.
In summary, our study indicates higher visit-to-visit fasting glucose variability is related to increased risk for microvascular complications, including nephropathy, and retinopathy. This relationship appears strongest in those individuals who were assigned to the standard treatment arms and who on average had worse overall glucose control. Our results suggest that visit-to-visit glucose variability may be another relevant component of overall glycemic management in T2D. Further efforts are needed to determine how best to clinically track glucose variability and whether therapies reducing variability can improve outcomes.
Acknowledgments
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors also acknowledge the contributions of the Hines VA Cooperative Studies Program Coordinating Center.
VADT Study Co-Chairs: Carlos Abraira, M.D., Miami VA Medical Center; William C. Duckworth, M.D., Carl T. Hayden VA Medical; Center Co-Chairs Miami Office: Christina Paul, Danielle Arca, L.M.H.C.* Lottie Cason*, Rebecca Martinez Zolotor, M.S.*, Louisa Williams*; Carl T. Hayden VA Medical Center Office: Susan L. Collier, H.T., M.M.T., C.R.C., Nafis Ahmed, B.S.*, Angela Boyd, R.T., C.R.C., C.C.R.C.* CSP Coordinating Center Edward Hines Jr. VA Hospital: Domenic Reda, Ph.D, Director, Hines CSPCC Thomas Moritz, M.S., Study Biostatistician: Robert Anderson, Ph.D., Sub-Protocol Biostatistician; Mary Ellen Vitek, Quality Assurance Specialist, Tamara Paine, National Study Coordinator, Lizy Thottapurathu, M.S., Statistical Programmer, Ping Luo, Ph.D., M.S., Sub-Protocol Statistical Programmer, Ken Bukowski, B.S., Database Programmer, Danuta Motyka, Database Programmer, Victoria Barillas, Statistical Assistant, Rodney Brown, A.A., Statistical Assistant, Barbara Christine, Statistical Assistant, Laurel Anfinsen, M.S.*, Statistical Programmer; Mary Biondic, B.A.* Database Programmer; Rita Havlicek*, Statistical Assistant; Joe Kubal, M.A.* National Study Coordinator, Statistical Assistant; Maureen McAuliffe*, Statistical Assistant; Madeline McCarren, Ph.D., M.P.H.*, Study Biostatistician; Maria Rachelle*, Statistical Assistant; Linda Rose, M.P.A.*, National Study Coordinator; Jerome Sacks, Ph.D.*, Subprotocol Biostatistician; Tom Sindowski, B.A.*, Statistical Assistant; Jonathan Thomas, M.A.*, National Study Coordinator; Candice Zahora, B.S.*, National Study Coordinator; CSPCRPCC Albuquerque, NM: Mike R. Sather, Ph.D., F.A.S.H.P., Center Director; Stuart Warren, J.D., Pharm.D., Study Pharmacist, Jolene Day, Pharmaceutical Project Manager; Jeff Haroldson, Pharm.D., B.C.P.S.*, Study Pharmacist; Executive Committee: Carlos Abraira, M.D., William Duckworth, M.D., Stephen N. Davis, M.D., Nicholas Emanuele, M.D., Steven Goldman, M.D., Rodney Hayward, M.D., Jennifer Marks, M.D., Thomas Moritz, M.S., Peter Reaven, M.D., Domenic Reda, Ph.D., Stuart Warren, J.D., Pharm.D., Franklin Zieve, M.D., Ph.D., Wendy Wendell, R.N., B.S.N., Jeff Haroldson, Pharm.D., B.C.P.S.*, Paula Harper, R.N., C.D.E.*, William G. Henderson, Ph.D.*, Robert R. Henry, M.D.*, M. Sue Kirkman, M.D.* Madeline McCarren, Ph.D., M.P.H.*, Jerome Sacks, Ph.D.*; Data and Safety Monitoring Board James Gavin, III, M.D., Ph.D., Emily Chew, M.D., Barbara Howard, Ph.D. Ted Karrison, Ph.D., Ivan V. Pacold, M.D., Daniel Seigel, Sc.D., Frank Vinicor, M.D., Barry Massie, M.D. (consultant)* Steven Goldman, M.D. Steven Rapcsak, M.D., Gulshan Sethi, M.D., Mark Sharon, M.D., Hoang Thai, M.D., Karen Zadina, R.N., M.A., Janice Christensen, M.D.*, Douglass Morrison, M.D., Ph.D.*, Peter Spooner, M.D.*, Alex Westerband, M.D.*; Consultants: Barry Materson, M.D., M.B.A., Eliot Brinton, M.D.; Ronald Klein, M.D., M.P.H., John A. Colwell, M.D., Ph.D, Ernst J. Schaefer, M.D., Carlton S. Gass, Ph.D.; Central Laboratories: David A. Ehrmann, M.D., Paul Rue; Central Biochemistry Lab: Ernst J. Schaefer, M.D., Judith R. McNamara, M.T. (retired); MAVERIC Core Laboratory: Mary Brophy, M.D., Donald Humphries, Deborah Govan, Laurie McDonnell, Laura Carlton, Yugia Weng; Cost-Effectiveness Lab: Rodney A. Hayward, M.D., Sarah Krein, Ph.D., R.N.; ECG Laboratory: Steven Goldman, M.D, Karen Zadina, R.N., M.A. Study Sites: Charleston: Jeremy Soule, M.D., Susan Caulder, R.N., M.S.N, C.D.E., Clare Pittman, R.N., M.S., C.D.E., Omayra Alston, Ronald K. Mayfield, M.D.*, Greg Moffitt, M.D.*, Julius Sagel, M.D.*, Frank Sanacor, Pharm.D.* Elizabeth Ganaway, R.N.*; Miami: Jennifer Marks, M.D., Lorraine Okur, R.N., Lucille Jones, R.N., Hermes Florez, M.D., Donna Pfeifer, Ph.D, ARNP, APRN, BC, Luis Samos, M.D., Andrew L. Taylor, M.D.* Lyons/East Orange Mark B. Zimering, M.D., Adilia Sama, R.N., Frances Rosenberg, R.N., Heidi Garcia, R.N., Norman Ertel, M.D., Leonard Pogach, M.D., John J. Shin, M.D., Felice Caldarella, M.D.*, Constantino Carseli, M.D.* Mamta Shah, M.D.* Fresno: Paulette Ginier, M.D., F.A.C.P., George Arakel, M.D., Yangheng Fu, M.D. Don Tayloe, M.D., Jack E. Allen, R.N.*, Elizabeth Fox, M.S.N, C.N.S, NP-C, C.D.E*, Paula G. Hensley, R.N.*; Hines: Nicholas Emanuele, M.D., Kathleen Kahsen, R.N., C.D.E., Patricia Linnerud, R.N., M.S., Lily Agrawal, M.D., Nasrin Azad, M.D.; Houston: Marco Marcelli, M.D., Glenn R. Cunningham, M.D., Natalie M. Nichols, L.V.N., Emilia Cordero, R.N., Rabih Hijazi, M.D.* Farid Roman, M.D.* Paromita Datta*, Mariana Garcia Touza*; Indianapolis: Amale Lteif, M.D., Karen L. Moore, R.N., B.S.N, Christina Lazar-Robinson, M.D., Sanjay Gupta, M.D.*, M. Sue Kirkman, M.D.*, Martha Mendez, R.N.*, Zehra Haider, M.D.*, Lora Risley, R.N.*; Lexington: Dennis Karounos, M.D., Linda Barber, R.N., C.D.E., Janet Hibbard, B.S., James W. Anderson, M.D., L. Raymond Reynolds, M.D., Jeff Carlsen, M.D.*, Robert W. Collins, M.D.*, As’ad Ehtisham, M.D.*; Long Beach: Moti L. Kashyap, M.D., Barbara Matheus, RNP,MSN,CDE,BC-ADM, Tina Rahbarnia, B.S., Anthony N. Vo, M.D., Nancy Downey, M.S.N., N.P.*, Lynette Fox, M.S.N., N.P.-C*, Richard M. Gonzales, M.D.*, C. Daniel Meyers, M.D.*, Subramaniam Tavintharan, M.D.*; Minneapolis: Frank Q. Nuttall, M.D., Ph.D., Lisa Cupersmith, R.N., Kathy Dardick, R.N., Linda Kollman, R.N., Angeliki Georgopoulos, M.D., Catherine Niewoehner, M.D.; Nashville Stephen N. Davis, M.D., Paula Harper, R.N., C.D.E., Diana Davis, R.N., B.S.N., C.D.E., Jessica Devin, M.D., Annis Marney, M.D., Julia Passyn-Dunn, M.D., Jennifer Perkins, M.D., John Stafford, M.D. Al Powers, M.D.*, Linda Balch, R.N., C.D.E.*, Patricia Harris, R.N.*, Omaha: Robert J. Anderson, M.D., Diana Dunning, B.S.N., M.A., C.D.E. Steve Ludwig, R.N., Marlene Vogel, R.N., Cyrus DeSouza, M.D., Robert Ecklund, M.D.*, Sarah Doran, B.S.N.*, Claire Korolchuk, R.N.*, Mary McElmeel, B.S.N., M.S.*, Sarah Wagstaff, B.S.N.*; Phoenix: Peter Reaven, M.D., Bradley Solie, R.N., C.D.E., John Matchette, P.A.-C, Christian Meyer, M.D., Sylvia Vela, M.D., Nadeem Aslam, M.D.*, Eliot Brinton, M.D.*, Joy Clark, M.S.N, F.N.P-C, C.C.R.C., C.D.E.*, Alisa Domb, R.N.*, Linda McDonald, R.N.*, Lynae Shurtz, R.N., B.S.N.*; Pittsburgh: R. Harsha Rao, M.D., Janice N. Beattie, B.S.N., C.D.E., Carol Franko, C.R.N.P., Frederick R. DeRubertis, M.D., David Kelly, M.D.*, Melisse Maser, C.R.N.P.*, Juleen Paul, CRNP, C.D.E.*, Richmond: Franklin Zieve, M.D., Ph.D., Susan J. Clark, R.N., M.S., C.C.R.C., Ann Grimsdale, R.N., Sonja Fredrickson, M.D., James Levy, M.D., Dianne Schroeder, M.D.; Salem: Ali Iranmanesh, M.D., Barbara Dunn, P.A.-C, Donna Arsura, R.N., Csaba Kovesdy, M.D., Suzanne Hanna, M.D.* Ashraf Iranmanesh, Pharm.D.*, Christy Florow*, Fe Remandaban, R.N.*, Erica Smith, L.P.N.*; San Diego: Robert R. Henry, M.D., Miriam Keller, R.N., B.S., Vanita Aroda, M.D., Charles Choe, M.D., Steven Edelman, M.D., Andrea Gasper, PA-C, Dereck MaFong, M.D., Sunder Mudaliar, M.D., Deborah Oh, M.D., Rahil Bandukwala, M.D.*, Anna Chang, M.D.*, Sandeep Chaudhary, M.D.*, Sithophol Chinnapongse, M.D.*, Louie Christiansen, M.D.*, Neelima Chu, M.D.*, Dennis Kim, M.D.*, Mark Lupo, M.D.*, Chandran, Manju, M.D.*, Ray Plodkowski, M.D.*, Roopa Sathyaprakash, M.D.*, Janet Wilson, M.D.*, Joseph Yu, M.D.*, Gina Macaraeg, R.N.*, Shelley Townes, R.N.*; San Antonio: Ralph DeFronzo, M.D., Lisa Johnson, M.S., RD/LD, Ken Cusi, M.D., Devjit Tripathy, M.D. Mandeep Bajaj, M.D.*, Janet Blodgett, M.D.*, Sangeeta Kayshup, M.D.*, Mary Helen Vasquez, R.N., C.D.E.*, Barbara Walz, R.N., B.S.N., C.D.E.*, Tess Weaver, M.S.N., APRN., B.C., F.N.P.*; San Juan. Julio Benabe, M.D., Zuleika Mercado, M.P.H., Brunilda Padilla, B.S.N., Jocelyn Serrano-Rodriguez, R.N., Carlos Rosado, M.D., Edwin Mejias, M.D.*, Tania Tejera, M.D.*, Clorinda Geldrez*, Elda Gonzalez-Melendez, B.S.N.*, Maria Natal, R.N.* Maribel Rios Jimenez, R.N.*; Tucson: Jayendra H. Shah, M.D., Wendy S. Wendel, R.N., B.S.N., Lynnette Scott, R.N., Lynne A. Gurnsey, R.N, Fabia A. Kwiecinski, M.D., Thomas Boyden, M.D.*, Merilyn G. Goldschmid, M.D., Virginia Easton*.
* Participants who left the VADT before its conclusion.
Glossary
Abbreviations:
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ARV
average real variability
- CV
, coefficient of variation
- eGFR
estimated glomerular filtration rate
- FPG
fasting plasma glucose
- GFR
glomerular filtration rate
- HbA1c
glycated hemoglobin A1c
- HR
hazard ratio
- T2D
type 2 diabetes
- VADT
Veteran Affairs Diabetes Trial
Financial Support: This work was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development. Additional support was received from the National Institute of Health R01-067690 and 5R01-094775 to P.D.R., and the American Diabetes Association to P.D.R. J.J.Z is supported by National Institute of Health grant K01DK106116, R21HL150374, R01HG006139, and Arizona Biomedical Research Centre (ABRC) new investigator award.
Clinical Trial Information: Glycemic Control and Complications in Diabetes Mellitus Type 2 (VADT) is registered at ClinicalTrials.gov with identifier: NCT00032487. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is registered at ClinicalTrials.gov number, NCT00000620. The study protocols are available on ClinicalTrials.gov.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Individual deidentified participant data of ACCORD was requested through biolincc (https://biolincc.nhlbi.nih.gov/home/). The independent scientific review board reviewed and approved the request. VADT deidentified participant data will not be available online but upon request, as permitted by VA policy.
References
- 1. Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168(22):2440-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. [DOI] [PubMed] [Google Scholar]
- 3. Ismail-Beigi F, Craven T, Banerji MA, et al. ; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572. [DOI] [PubMed] [Google Scholar]
- 5. Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431-437. [DOI] [PubMed] [Google Scholar]
- 6. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial–revisited. Diabetes. 2008;57(4):995-1001. [DOI] [PubMed] [Google Scholar]
- 7. The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44(8):968-983. [PubMed] [Google Scholar]
- 8. Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-to-visit HbA1c variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2020;43(2):426-432. [DOI] [PubMed] [Google Scholar]
- 9. Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359-2365. [DOI] [PubMed] [Google Scholar]
- 10. Penno G, Solini A, Bonora E, et al. ; Renal Insufficiency And Cardiovascular Events Study Group . HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013;36(8):2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerstein HC, Miller ME, Byington RP, et al. ; The Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou J, Koska J, Bahn G, Reaven P. Supplementary materials for Fasting Glucose Variation Predicts Microvascular Risk in ACCORD and VADT. GitHub.Deposited November 13, 2020. https://github.com/jinjinzhou/GluVarMicroCVD/tree/main/manuscripts
- 14. Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. [DOI] [PubMed] [Google Scholar]
- 15. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707-1708. [DOI] [PubMed] [Google Scholar]
- 16. Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with Type 2 diabetes. Diabet Med. 2016;33(12):1640-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou JJ, Schwenke DC, Bahn G, Reaven P, Investigators V. Glycemic variation and cardiovascular risk in the veteran’s affairs diabetes trial. Diabetes Care. 2018;41(10):2187-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lachin JM, Bebu I, Bergenstal RM, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the Diabetes Control and Complications Trial. Diabetes Care. 2017;40(6):777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145-157. [DOI] [PubMed] [Google Scholar]
- 20. Zhou JJ, Koska J, Bahn G, Reaven P. Glycaemic variation is a predictor of all-cause mortality in the Veteran Affairs Diabetes Trial. Diab Vasc Dis Res. 2019;16(2):178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 22. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. [DOI] [PubMed] [Google Scholar]
- 23. Loke YK, Price D, Herxheimer A; Cochrane Adverse Effects Methods Group . Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol. 2007;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarzer G. meta: An R package for meta-analysis. R News. 2007;7(3):40-45. [Google Scholar]
- 25. Hsu CC, Chang HY, Huang MC, et al. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55(12):3163-3172. [DOI] [PubMed] [Google Scholar]
- 26. Takao T, Ide T, Yanagisawa H, Kikuchi M, Kawazu S, Matsuyama Y. The effects of fasting plasma glucose variability and time-dependent glycemic control on the long-term risk of retinopathy in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91(2):e40-e42. [DOI] [PubMed] [Google Scholar]
- 27. Sugawara A, Kawai K, Motohashi S, et al. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55(8):2128-2131. [DOI] [PubMed] [Google Scholar]
- 28. Takao T, Suka M, Yanagisawa H, Matsuyama Y, Iwamoto Y. Predictive ability of visit-to-visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract. 2017;128:15-23. [DOI] [PubMed] [Google Scholar]
- 29. Lin CC, Chen CC, Chen FN, et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med. 2013;126(11):1017.e1-1017.10. [DOI] [PubMed] [Google Scholar]
- 30. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Individual deidentified participant data of ACCORD was requested through biolincc (https://biolincc.nhlbi.nih.gov/home/). The independent scientific review board reviewed and approved the request. VADT deidentified participant data will not be available online but upon request, as permitted by VA policy.