Abstract
Context
Aggressive pituitary tumors (APTs) are characterized by unusually rapid growth and lack of response to standard treatment. About 1% to 2% develop metastases being classified as pituitary carcinomas (PCs). For unknown reasons, the corticotroph tumors are overrepresented among APTs and PCs. Mutations in the alpha thalassemia/mental retardation syndrome X-linked (ATRX) gene, regulating chromatin remodeling and telomere maintenance, have been implicated in the development of several cancer types, including neuroendocrine tumors.
Objective
To study ATRX protein expression and mutational status of the ATRX gene in APTs and PCs.
Design
We investigated ATRX protein expression by using immunohistochemistry in 30 APTs and 18 PCs, mostly of Pit-1 and T-Pit cell lineage. In tumors lacking ATRX immunolabeling, mutational status of the ATRX gene was explored.
Results
Nine of the 48 tumors (19%) demonstrated lack of ATRX immunolabelling with a higher proportion in patients with PCs (5/18; 28%) than in those with APTs (4/30;13%). Lack of ATRX was most common in the corticotroph tumors, 7/22 (32%), versus tumors of the Pit-1 lineage, 2/24 (8%). Loss-of-function ATRX mutations were found in all 9 ATRX immunonegative cases: nonsense mutations (n = 4), frameshift deletions (n = 4), and large deletions affecting 22-28 of the 36 exons (n = 3). More than 1 ATRX gene defect was identified in 2 PCs.
Conclusion
ATRX mutations occur in a subset of APTs and are more common in corticotroph tumors. The findings provide a rationale for performing ATRX immunohistochemistry to identify patients at risk of developing aggressive and potentially metastatic pituitary tumors.
Keywords: ATRX (alpha thalassemia/mental retardation syndrome X-linked), aggressive PitNETs, pituitary carcinoma, pituitary adenoma, Cushing’s disease
Pituitary neuroendocrine tumors (PitNETs) (1), traditionally designated as pituitary adenomas, are usually benign tumors with indolent, nonaggressive course. Recently, the European Society of Endocrinology published criteria that define aggressive PitNETs as tumors demonstrating an unusually fast growth and/or lack of response to all standard treatment modalities including surgery, and radio- and pharmacological therapies (2). Pituitary carcinomas (PCs) are defined by the presence of noncontiguous craniospinal or distant metastases (3). While PCs are rare and constitute only 0.1% to 0.2% of all pituitary neoplasms (4), the prevalence of aggressive pituitary tumors (APTs) without metastases is less well known. An estimate of 3% has been suggested based on indices of increased proliferation and extensive p53 staining in tumor specimens from 451 patients reported to the German Pituitary Tumor Registry (5). Little is known about genetic abnormalities driving invasive and metastatic pituitary tumors. Whether they develop through malignant progression of benign pituitary tumors or occur as de novo malignant tumors caused by early, single, or multiple genetic changes predisposing for distant dissemination is unknown.
The functioning corticotroph tumors causing Cushing’s disease represent less than 5% of the benign, slow-growing PitNETs (6, 7). However, they are overrepresented among APTs and PCs, where they constitute approximately 30% to 40% (8, 9). One suggested explanation for this was a lower expression of the cell cycle inhibitor p27 in normal corticotroph cells and corticotroph tumors (10); however, the mechanisms are still unclear. Silent corticotroph tumors are also considered potentially more aggressive according to the current World Health Organization classification of the pituitary tumors (3), although a recent meta-analysis could not identify an increased recurrence rate in this subtype (11).
In patients with APTs, genetic abnormalities have previously only been reported in single sporadic cases, none has consistently been found in larger groups of patients (12). In a case of clinically nonfunctioning gonadotroph carcinoma, a low level of HER2/neu gene amplification was demonstrated by using fluorescence in situ hybridization and chromogenic in situ hybridization analysis (13). The presence of mi-RNAs probably targeting PTEN (phosphatase and tensin homolog) and TIMP2 (tissue inhibitor of metalloproteinases 2) was reported as potential drivers of metastatic growth in a case with a nonfunctioning PC (14). A single case of PC was reported in a patient with succinate dehydrogenase subunit B gene mutation and history of paraganglioma (15). Finally, tumor protein p53 mutations in 2 PCs have been described (16).
Alpha thalassemia/mental retardation syndrome X-linked (ATRX) interacts with death domain-associated protein (DAXX) and the histone H3.3 variant in heterochromatin remodeling and maintenance of telomere structure and function (17, 18). Inactivation of ATRX or, less frequently, DAXX in ATRX/DAXX mutated tumors, leads to telomere destabilization and facilitates the process of alternative lengthening of telomeres (ALTs), which results in cancer cell immortality (19, 20). Somatic ATRX gene mutations are associated with several different tumor types, including astrocytomas in adults (21) and neuroendocrine tumors (NETs) such as pancreatic NETs (22, 23), neuroblastomas (24), and paragangliomas/pheochromocytomas (25, 26). Interestingly, in neuroendocrine tumors, ATRX abnormalities seem to predict malignant tumor phenotype, being present in high-grade malignant tumors such as neuroblastoma (24), or associated with poor prognosis and/or metastatic potential, such as in pancreatic NET (27), and pheochromocytomas/paraganglioma (26).
We have previously demonstrated normal immunohistochemical expression of ATRX protein in a large cohort of 246 well-characterized PitNETs localized to the sellar region, including 37 corticotroph tumors. However, 1 of 2 studied pituitary carcinomas (a corticotroph carcinoma in a patient with Cushing’s disease) did not express the protein due to a large deletion of the ATRX gene (28).
In the present study, we aimed to further explore ATRX protein expression and mutational status of the ATRX gene in a large cohort of aggressive PitNETs and pituitary carcinomas.
Material and Methods
Patient cohort
Pituitary tumor specimens were obtained from a multicenter cohort of 48 patients (15 female, 33 male), with a median age 45 (range 16-73 years) at diagnosis. Inclusion criteria were at least 1 pituitary surgery and tumor progression despite radiotherapy, and/or while on treatment with dopamine agonists or somatostatin analogues, or metastatic disease. Thirty patients had APTs and 18 had PCs with cerebrospinal and/or systemic metastases. The median time from diagnosis of the pituitary tumor to metastases was 8.5 (range 1.2-36) years (Table 1). The patients were treated at specialized centers in 11 European countries (Belgium, Denmark, Finland, France, Hungary, Italy, Norway, Poland, Serbia, Sweden, and UK). Patients’ data and tumor characteristics at the first presentation, treatments given, and outcome were collected in anonymized standardized questionnaires filled in by all participating centers.
Table 1.
Patient and tumor characteristics in the study population
| Total | APT | PC | |
|---|---|---|---|
| Total n | 48 | 30 | 18 |
| Age at diagnosis, year (median, range) | 45 (16-73) | 46.5 (18-73) | 42 (16-69) |
| Male n (%) | 33 (69) | 23 (77) | 10 (56) |
| Macroadenomasa | 44/45 | 28/29 | 16/16 |
| Invasive growtha | 39/42 | 24/27 | 15/15 |
| No of surgeries (median, range) | 3 (1-10) | 3 (1-10) | 3.5 (1-8) |
| No of radiotherapies (median, range) | 1 (0-4) | 1 (0-2) | 2 (1-4) |
| Resistance to DA/ somatostatin analogsb | 27/27 | 18/18 | 10/10 |
| Time to metastases from first surgery, year (median, range) | 8.5 (1.2-36) | ||
| Treatment with cytotoxic drugsb | 35/37 | 21/23 | 14/14 |
| ATRX negative, n (%) | 9 (19) | 4 (13) | 5 (28) |
| Tumor subtypes (IHC) | |||
| Corticotrophc | 22 | 10 | 12 |
| Lactotroph | 15 | 12 | 3 |
| Somatotroph | 4 | 2 | 2 |
| Somato/lactotroph | 2 | 1 | 1 |
| TSH/FSH | 1 | 1 | 0 |
| Silent Pit 1 positive PitNET | 3 | 3 | 0 |
| Null cell | 1 | 1 | 0 |
Abbreviations: APT, aggressive pituitary tumor; PC, pituitary carcinoma; DA, dopamine agonist; IHC, immunohistochemistry.
a MRI at first tumor presentation in patients with available information.
b In patents with available information.
c Six clinically silent (2 PCs, 4 APTs).
Information on pituitary tumor size and local extension at the first magnetic resonance imaging (MRI) was available in 45 and 43 patients, respectively. All but 1 lactotroph tumor were macroadenomas at the time of diagnosis. By the time of pituitary surgery, invasion of the cavernous sinuses, bone and/or brain was evident on MRI in the 39 cases, including the single patient who had a microadenoma. Of the 48 patients, 39 had more than 1 pituitary surgery, and 33 more than 2. Forty-six out of the 48 patients had received at least 1 radiotherapy. In 1 case, tumor size and extension were considered too large for radiotherapy, and in the second case the reason for not performing radiotherapy was not available. No tumor treated with dopamine agonists and/or somatostatin analogues (octreotide, lanreotide, pasireotide) was controlled by these medications (Table 1). In addition to standardized medical therapy, 34 patients had received treatment with chemotherapy, temozolomide in 33 including 1 patient with additional bevacizumab, and another 1 with an mTOR inhibitor and 2 immune checkpoint inhibitors.
Tumors were classified based on the laboratory and clinical signs of pituitary hormone hypersecretion, expression of anterior pituitary hormones in the tumor cells, and, in the cases of hormone-negative nonfunctioning tumors, by their expression of pituitary-specific transcription factors. Corticotroph tumors were the most common: 22/48, of which 16 were functioning tumors causing Cushing’s disease. Lactotroph tumors were the second most common, n = 15 (Table 1).
The index patient with ATRX mutation has been previously reported (28) and is also included in the present study. Of the 48 patients, 3 had syndromes predisposing for pituitary tumors, 1 had MEN1 (29), 1 had Lynch syndrome (30), and 1 patient belonged to a kindred with familial predisposition for pituitary tumors, but without MEN1 or AIP mutation. In addition, pituitary tumor tissue from a corticotroph nonaggressive macroadenoma in a patient with Lynch syndrome was investigated. This case was not included in the statistical analyses as it did not fulfil the criteria for aggressive tumors.
In 45 patients, at least 1 specimen from pituitary surgery was available for analyses. In the remaining 3 patients, there was only specimen from the metastasis. For 7 patients with carcinoma, material from both pituitary surgery and from metastatic tumor was available. The presence of representative tumor tissue was confirmed in hematoxylin and eosin stained slides from all specimens.
Immunohistochemical analyses
Immunohistochemistry (IHC), with antibodies towards growth hormone (GH), prolactin (PRL), thyrotroph hormone (TSH), adrenocorticotroph hormone (ACTH), gonadotroph hormones, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), was performed at the local IHC laboratories according to the routine protocols. Immunohistochemical analysis with antibodies towards pituitary-specific transcription factors was performed at Uppsala University Hospital by using anti-SF1 antibody (Abcam, ab217317), anti-Pit-1 antibody (Novus Biologicals, NBP1-92273), and anti-T-Pit antibody (Atlas Antibodies, AMAb91409), according to the standard protocols.
ATRX protein expression was studied on whole sections from formalin-fixed paraffin-embedded tissue blocks. For the patients operated on more than once, available tissue specimens from multiple surgeries were examined. In the majority of cases, IHC was performed at Uppsala University Hospital in a DAKO-Autostainer Link 48 with heat-induced epitope retrieval at high pH. Purified polyclonal anti-ATRX antibody (HPA001906, Atlas Antibodies; dilution 1:100; incubation time 20 minutes) was used. Specimens from 2 adult astrocytomas, 1 with ATRX mutation and 1 without ATRX mutation, both confirmed by using molecular genetic analysis, were used as negative and positive controls. In addition, immunolabelled endothelial cells served as an internal positive control. Four cases from Foch Hospital (Suresnes, France) and a case from University Hospital in Copenhagen, Denmark, were stained in Ventana Benchmark by using the same antibody and according to the locally optimized protocols.
Molecular genetic analysis
Molecular genetic analysis was performed on tumor tissue from the pituitary specimen in all nine cases demonstrating lack of ATRX immunolabelling. In 2 patients, specimens from metastases were also analyzed. If there was more than 1 specimen from the pituitary surgery, the specimen with the most representative tumor tissue was used. In 1 patient, a partial lack of ATRX protein labelling was observed in the pituitary specimen and a total lack in metastatic tumor tissue. In this patient, an attempt was made to microdissect tissue and extract DNA separately from ATRX negative and positive area of the pituitary tumor. In addition, the specimen from metastasis with negative ATRX staining was analyzed. All but 1 specimen were examined by a next-generation sequencing (NGS) panel targeting 20 genes (31) related to cancers of the central nervous system as in the initial study (28). The proportion of tumor cells exceeded 70% in all the specimens. One specimen was analyzed using an exome-wide sequencing approach.
Next-generation sequencing
DNA was purified from 10-µm paraffin slides using GeneRead DNA FFPE Kit (Qiagen, Germany) according to the manufacturer’s instructions. NGS was performed with a custom designed central nervous system panel covering the entire coding sequence or hotspot regions of 20 genes frequently mutated in brain tumors (32). DNA was quantified using an RNase P TaqMan Copy Number Reference Assay performed on a QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems, Foster City, CA). Libraries were prepared in 2 primer pools using the Ion AmpliSeq Library Kit Plus and Ion Xpress Barcode Adapters 1–96 Kit in 10 µL of reaction volume with 5 ng of template DNA. Library quantitation was performed using the Ion Library Quantitation Kit. Sample preparation, chip loading, and sequencing were performed using Ion Chef and Ion Torrent S5 System with Ion S5 Chef solutions, Ion S5 sequencing reagents and Ion 530/540 Chip Kits. All Ion products were supplied by Ion Torrent/ThermoFisher Scientific, Carlsbad, CA, USA. Data analysis, including base calling, quality scoring, trimming, demultiplexing, and alignment, was performed using standard Ion Torrent Suite v5.10 workflows. BAM alignment files were manually analyzed for alterations in the coding sequences of the 20 genes using Golden Helix GenomeBrowse 3.0 (Golden Helix, Bozeman, MT, USA). The sequencing experiments included ATRX wild-type control samples from healthy donors.
One specimen was analyzed using hybridization capture-based high-throughput NGS platform from Illumina (33).
Ethics approval
The study has been approved by Regional Ethics Committee in Uppsala (Dnr 2018/327).
Results
Lack of ATRX protein expression is frequent in corticotroph tumors
Nine of the 48 tumors (19%) demonstrated lack of ATRX immunolabelling in the tumor cells. Five were carcinomas and 7 were corticotroph tumors, representing 32% of all corticotroph tumors (7 out of 22). Lack of protein expression was more common in patients with functioning corticotroph tumors (6/16, 38%) than in those with silent corticotroph tumors (1/6, 17%). Of the remaining 2 ATRX-immunonegative tumors, 1 was a lactotroph APT with a fatal outcome, and 1 was a somato-lactotroph carcinoma that initially presented as a prolactinoma and subsequently evolved into acromegaly (Table 2).
Table 2.
Patient and tumor characteristics in ATRX mutated vs intact cases
| ATRX mutated | ATRX intact | |
|---|---|---|
| Total n | 9 | 39 |
| Age at diagnosis, year (median, range) | 45 (23-72) | 45 (16-73) |
| Male, n (%) | 6 (67) | 27 (69) |
| Aggressive pituitary tumors, n (%) | 4 (44) | 26 (67) |
| Pituitary carcinomas, n (%) | 5 (56) | 13 (33) |
| Tumor subtypes (IHC) | ||
| Corticotroph (n = 22) | 7 | 15 |
| PC (n = 12) | 4 | 8 |
| APT (n = 10) | 3 | 7 |
| Lactotroph (n = 15) | 1 | 14 |
| PC (n = 3) | 0 | 3 |
| APT (n = 12) | 1 | 11 |
| Somato/lactotroph (n = 2) | 1 | 1 |
| PC (n = 1) | 1 | 0 |
| APT (n = 1) | 0 | 1 |
| Other subtypesa (n = 9) | 0 | 9 |
| PC (n = 2) | 0 | 2 |
| APT (n = 7) | 0 | 7 |
Abbreviations: APT, aggressive pituitary tumor, IHC, immunohistochemistry; PC, pituitary carcinoma.
a Somatotrophs (4); silent Pit 1 positive (3); double TSH/FSH (1); null cell PitNET (1).
More than 1 pituitary specimen was available for analysis in 6 of 7 patients who underwent multiple surgeries. In 5 of the 6 patients, all specimens demonstrated lack of ATRX in all tumor cells. In 1 patient, the specimen from the first surgery could not be assessed, and there was partial lack of ATRX expression in pituitary tumor from the second surgery and a total lack in the metastasis. In 5 patients with PC, specimens from metastases were available in 4 and demonstrated negative ATRX staining in the tumor cells. The remaining 39 pituitary tumors demonstrated intact nuclear ATRX expression.
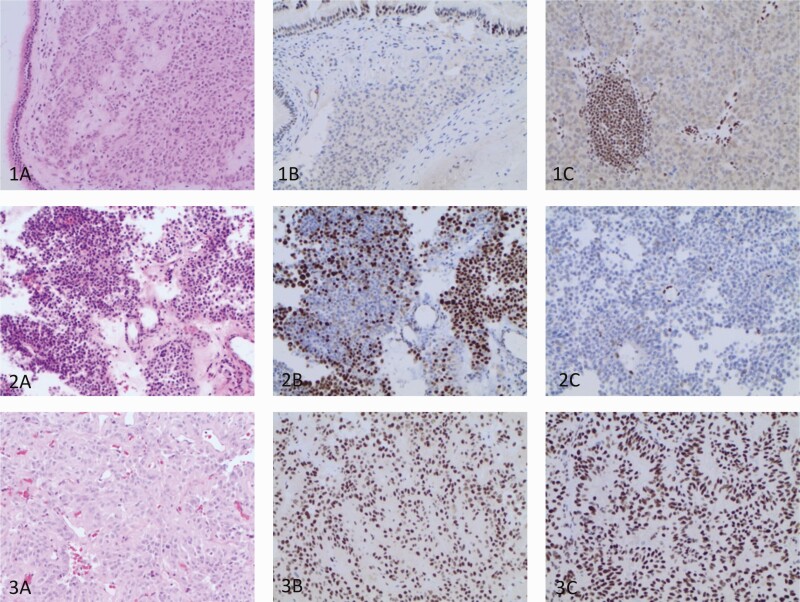
Examples of PitNETs with normal ATRX staining, total lack of immunolabelling and partial negative ATRX staining in primary and metastatic tumors are illustrated in Fig. 1.
Figure 1.
Histopathological and immunohistochemical features of PitNETs. Row 1: Hematoxylin eosin staining of a primary pituitary tumor invading into the respiratory mucosa (1A) with a total lack of ATRX in tumor cells nuclei both the primary pituitary tumor (1B) and a lymph gland metastasis (1C) in a patient with a functioning somato-lactotroph carcinoma. ATRX expression is intact in respiratory epithelium, endothelial cells and lymphocytes. Row 2: Hematoxylin eosin staining from the primary pituitary tumor in a patient with a silent corticotroph carcinoma (2A). A partial nuclear ATRX-loss in a proportion of cells in the specimen from the second pituitary surgery (2B) and a total ATRX-loss in the metastasis (2C) ATRX expression is preserved in the nuclei of the endothelial cells. Row 3: Hematoxylin eosin staining of the specimen from the first surgery (3A) and normal ATRX expression in the nuclei of the tumor cells in the specimens from 2 pituitary surgeries (3B, 3C) in a patient with a silent Pit-1 positive PitNET.
All ATRX-immunonegative tumors harbor loss-of-function ATRX gene abnormalities
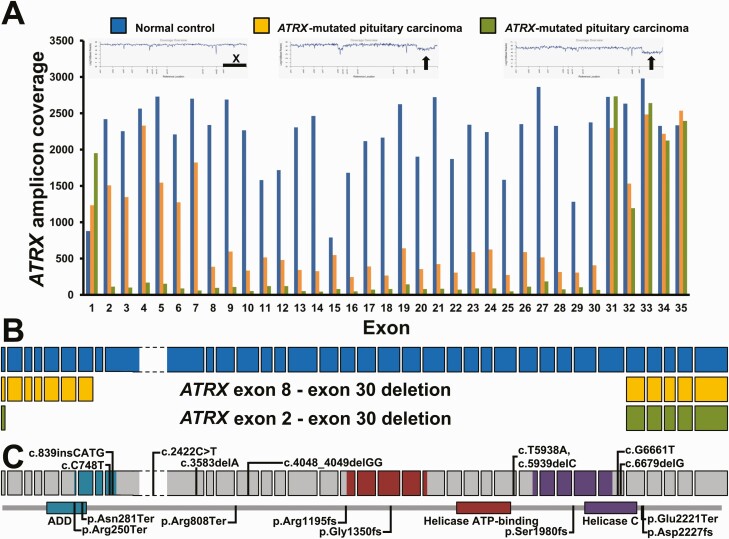
ATRX loss-of-function gene abnormalities were found in all 9 ATRX-immunonegative tumors (Table 3) (31). Two different damaging ATRX mutations with large differences in mutation frequencies were identified in the same primary tumor in 2 carcinomas from male patients. One of these 2 tumors demonstrated a partial lack of ATRX at IHC. An attempt to extract separately DNA from ATRX-immunopositive and negative fraction was, however, unsuccessful, as the same mutational status was confirmed in both fractions. Interestingly, only the predominant mutation from this pituitary tumor was present in the metastasis (6 years later) with a frequency of 98%, suggesting clonal heterogeneity and evolution of the primary tumor (Table 3) (31). Three tumors did not show any ATRX single nucleotide variants or small indels, but had large, intragenic deletions corresponding to most of the coding sequences (22-28 of 36 exons) (Fig. 2A and 2B). One of these tumors was the corticotroph tumor previously reported, whereas the other 2 were lactotroph and somato-lactotroph, respectively. All identified ATRX single nucleotide variants and small indels were positioned throughout the coding sequence of the ATRX gene (Fig. 2C). In addition to the ATRX mutations, 8 out of 9 ATRX-immunonegative tumors had other genetic abnormalities: inactivating somatic mutations in tumor suppressor genes TP53 (6), PTEN (2), RB1 (1), NF2 (1), and a homozygous deletion of CDKN2A/B in both primary tumor and metastasis in 1 patient (Table 3). Recurrent copy number variants (CNVs) that were estimated from the sequencing data were all gains, and involved chromosomes 5, 7, 9p21.3 encompassing CDKN2A/B loci as well as the CIC locus on 19q.
Table 3.
Genetic alterations in ATRX-negative APT and PC by panel NGS
| Pt. | Specimen | Local. | ATRX expression | Genes | Coding | Amino Acid | Freq. (%)# |
|---|---|---|---|---|---|---|---|
| 1 | Cushing/PC | Pituitary | loss | ATRX | c.134_6217del | p.D45-K2027del | Nu |
| 2 | Cushing/PC | Pituitary | loss | ATRX | c.748C>T | p.Arg250Ter | 89 |
| Lynch sy | TP53 | c.524G>A | p.Arg175His | 84 | |||
| PTEN | c.697C>T | p.Arg233Ter | 10 | ||||
| 3 | Lactotroph/APT | Pituitary | loss | ATRX | c.21_6699del | p.E8-K2233del | Nu |
| TP53 | c.584T>A | p.Ile195Asn | 92 | ||||
| RB1 | c.1725_1726 insAACAA | p.Ser576fs | 13 | ||||
| RB1 | c.1218_1697del | p.N406-S565del | He | ||||
| 4 | Cushing/PC | Pituitary | loss | ATRX | c.6679delG | p.Asp2227fs | 81 |
| ATRX | c.3583delA | p.Arg1195fs | 12 | ||||
| 5* | Silent ACTH/PC | Pituitary | retained (major)/loss (minor) | ATRX | c.4048_4049delGG | p.Gly1350fs | 28 |
| ATRX | c.6661G>T | p.Glu2221Ter | 31 | ||||
| TP53 | c.644G>A | p.Ser215Asn | 30 | ||||
| 5a | Silent ACTH/PC | Pituitary | loss (major)/retained (minor) | ATRX | c.4048_4049delGG | p.Gly1350fs | 67 |
| ATRX | c.6661G>T | p.Glu2221Ter | 10 | ||||
| TP53 | c.644G>A | p.Ser215Asn | 8 | ||||
| 5 | Silent ACTH/PC | Metastasis | loss | ATRX | c.4048_4049delGG | p.Gly1350fs | 98 |
| 6 | Cushing/APT | Pituitary | loss | ATRX | c.2422C>T | p.Arg808Ter | 72 |
| TP53 | c.1024C>T | p.Arg342Ter | 51 | ||||
| PTEN | c.697C>T | p.Arg233Ter | 55 | ||||
| 7 | Cushing/APT | Pituitary | loss | ATRX | c.839_840insCATG | p.Asn281Ter | 44 |
| TP53 | c.818G>A | p.Arg273His | 85 | ||||
| NF2 | c.1052G>A | p.Arg351His | 20 | ||||
| 8 | Cushing/APT | Pituitary | loss | ATRX | c.5938T>A, c.5939delC | p.Ser1980fs | 88 |
| TP53 | c.375G>A | p.( = ) | 81 | ||||
| 9 | Mixed GH-PRL/PC | Pituitary | loss | ATRX | c.595_6699del | p.N199-K2233del | He |
| CDKN2A | c.1_501del | p.M1-A167del | Ho | ||||
| CDKN2B | c.1_414del | p.M1-D138del | Ho | ||||
| 9 | Mixed GH-PRL/PC | Metastasis | loss | ATRX | c.595_6699del | p.N199-K2233del | He |
| CDKN2A | c.1_501del | p.M1-A167del | Ho | ||||
| CDKN2B | c.1_414del | p.M1-D138del | Ho |
Abbreviations: APT, aggressive pituitary tumors; PC, pituitary carcinoma; NGS, next-generation sequencing; ACTH, adrenocorticotropic hormone, GH, growth hormone; PRL, prolactin; #, estimated ploidy level of larger gene deletions: Nu: nullizygous, He: hemizygous deletion, Ho: homozygous deletion.
a The same mutations were detected in ATRX immunopositive and immunonegative tissue fractions indicating that they could not be successfully separated.
Figure 2.
ATRX deletions in 2 patients with corticotroph carcinoma. (A) Profile of the average amplicon coverage of ATRX coding sequence from exon 1 through exon 35. A healthy donor of female origin was included in all NGS experiments (blue). Low amplicon coverage corresponding to deletion of ATRX sequence was observed for 2 patients, highlighted with orange and green, respectively. Coverage overviews are shown as inserts. The black horizontal bar indicates the position of chromosome X. Arrows mark the deletions in ATRX. (B) Schematic illustration of the 2 large, intragenic ATRX deletions spanning exon 2/exon 8 through exon 30. The large exon 9 of ATRX, depicted with stippled lines, is compressed for clarity. (C) Diagram of ATRX variants at coding and protein levels, respectively. Definition of ATRX domains were from UniProt. ADD, ATRX-DNMT3-DNMT3L; Helicase C, Helicase C-terminal.
Discussion
Little is known about genetic abnormalities driving invasive and metastatic growth of PitNETs. Here, we demonstrate a loss of ATRX protein expression caused by severe loss-of-function ATRX gene alterations in almost a fifth of highly APTs, with a higher prevalence in PC than in APT, and in corticotroph tumors than in other lineage subtypes. This indicates that corticotroph tumors are prone to develop ATRX gene abnormalities.
We reported previously normal ATRX expression in 246 PitNETs localized to the sellar region. However, in 1 female patient diagnosed with Cushing’s disease and a pituitary macroadenoma at an age of 36 years, we found negative ATRX immunolabelling caused by a large deletion of the ATRX gene (28). This tumor had progressed over time and had become metastatic despite multiple transsphenoidal surgeries, pharmacological therapy, and 3 different modalities of radiation therapy. ATRX staining was absent in all the tumor specimens including the 1 from the first surgery.
In the present extended study, we demonstrate ATRX gene defects in 8 additional patients. Thus, 9 out of 48 patients (19%) with APTs or carcinomas harbored loss-of-function ATRX gene alterations, more frequently in patients with PC than with APT (28% vs 13%). Five out of the total 9 patients with ATRX gene defects had carcinomas. Of the 4 APT patients, 2 died due to progressive tumor growth, in another there was a short time from the tumor diagnosis to the study end, and in the last patient search of metastases was not performed due to advanced dementia. Further studies with longer follow-up are needed to assess to what extent an initial ATRX defect leads to a metastatic disease.
In addition to our previously reported case of ATRX mutated corticotroph carcinoma (28), a corticotroph carcinoma with an ATRX mutation in combination with PTEN and TP53 mutations has been described; however, without detailed presentation of genetic data (34).
In a recent study (35), whole exome sequencing of 18 corticotroph tumors lacking mutations in the USP8 (ubiquitine specific peptidase 8) gene, mutations that drive corticotroph tumors in approximately 50% of patients with Cushing’s disease, demonstrated ATRX mutations concomitantly with TP53 mutations in 2. Although detailed clinical data regarding aggressiveness of the 2 ATRX mutated tumors were not presented, both were recurrent and required surgery on 2 and >3 occasions, respectively, and Ki67 proliferative index was increased in 1 of the cases (35). Lack of ATRX immunolabelling was recently found in 3 lactotroph macroadenomas from a cohort of 42 pediatric PitNETs, but molecular genetic confirmation of the ATRX mutations was not provided (36). Recently, ALT phenotype has been reported in 3 of 106 PitNETs, 2 were recurrent nonfunctioning PitNETs without specification of cell linage differentiation, and 1 was a somatotroph tumor (37). Two of the 3 ALT-positive PitNETs demonstrated loss of ATRX or DAXX at protein level, indicating a homozygous loss of the gene or alternative mechanism of gene silencing. However, no ATRX or DAXX mutations were identified by sequencing (37).
In patients who had repeated pituitary surgeries in the present cohort, an ATRX defect was already present in the first removed tumor, though in 1 patient tumor tissue from the first surgery was not evaluable. This indicates that ATRX abnormalities represent an early genetic event contributing to aggressive behavior and, at least in a subset of patients, to metastatic spread. Where material from both the pituitary tumor and metastasis was available (n = 4), identical patterns of a complete loss of ATRX were seen in 3, whereas 1 one case, partial loss of ATRX was identified in the pituitary tumor and a complete loss in the metastasis. A similar case of a PitNET with ALT-negative phenotype in the original tumor, and ALT-positive phenotype and a partial loss of ATRX in a recurrent tumor, was recently reported (37). These findings suggest that an ATRX mutation may occur, though rarely, in pituitary tumors with primarily intact ATRX, contributing to malignant tumor progression.
In the ATRX-mutated cases in our cohort, we demonstrated different loss-of-function ATRX defects including nonsense mutations, frameshift indels, and, in 3 cases, large, intragenic deletions of almost the whole gene (22-28 of the 36 exons). Interestingly, large deletions of almost the whole ATRX gene have only rarely been reported in other tumor types, such as astrocytomas (21, 32), pancreatic NETs (22), and pheochromocytomas and paragangliomas (25). Yet, a recent study on ATRX alterations in neuroblastoma demonstrated a strong tendency for large, intragenic deletions of exons 1-9, encoding the first half portion of the ATRX protein (38). In our cohort, there was no predominance of a particular type of mutation in carcinomas compared with APTs, or in corticotroph compared with Pit-1-lineage tumors. However, the number of mutated cases may be too low to make conclusions on a potential genotype–phenotype association.
Blood samples or normal tissues from patients were not included in the sequencing experiments to test for germline mutations. The variant allele frequencies of mutations in ATRX reported in this study are in favor of somatic rather than germline origin. Furthermore, IHC revealed normal ATRX expression in non-neoplastic cells in all the mutated specimens, arguing for the somatic origin of the ATRX gene defects.
In the present study, we had the opportunity to investigate ATRX in 2 patients with corticotroph tumors, 1 nonaggressive macroadenoma and 1 carcinoma, and Lynch syndrome, a cancer predisposing syndrome with mutations in genes involved in DNA mismatch repair (MLH1, MSH2, MSH6, PMS2, EPCAM). Both tumors harbored an MSH2 mutation, but only the severe case, a carcinoma, in addition exhibited an ATRX mutation.
Additional cancer-related mutations were identified and associated with ATRX alterations in 8 of 9 cases, TP53 mutations in 6 (3 aggressive corticotroph tumor, 2 corticotroph carcinomas, and 1 aggressive lactotroph tumor), PTEN mutations in 2, and RB1, NF2, and CDKN2A/B in single cases. TP53 mutations have rarely been previously reported in pituitary tumors (16). However, recently, TP53 mutations were demonstrated in 6 out of 18 of corticotroph USP8 wild-type tumors and correlated with larger tumors and higher Ki67 index (35). Our findings, together with previous report, may suggest an association of the TP53 mutations with corticotroph tumors with more aggressive phenotype. Findings of multiple mutations in the ATRX mutated tumors may indicate genetic instability leading to multiple cancer-related genetic events. However, more extensive molecular genetic analyses are needed to get full insight into genetic landscape of aggressive PitNETs.
The strength of the present study is the well characterized cohort of APT and PC and a relatively large number of patients, having in mind the rarity of the condition. A limitation is a short follow-up of some of the patients with ATRX defects, which limits conclusions on the metastatic potential of this mutation.
Although many APT/carcinomas exhibit histological features consistent with increased proliferation (Ki-67 index > 3%, increased mitotic count, and p53 expression) (4), and coexistence of 2 of the 3 markers is associated with increased risk of tumor progression and recurrence (39), the presence of these features does not fully predict future aggressive behavior (40, 41). To our knowledge, the present findings is the first time that a gene mutation with well-known oncogenic potential has been consistently reported in a proportion of aggressive PitNETs.
Currently, temozolomide is the first-line chemotherapy for APT and PC (29). The drug induces an initial response rate of 40%, but subsequently most tumors relapse and long-term effective alternative therapies are still lacking (42). Mutated ATRX is an attractive therapeutic target for the subgroup of ATRX negative pituitary tumors. There is ongoing intensive research aiming to develop pharmacological therapies targeting ATRX and ALT (43, 44).
In summary, the results of this study provide a rationale for performing ATRX immunohistochemistry as a simple, inexpensive, and widely available laboratory test to identify patients at increased risk for development of highly aggressive and potentially metastatic PitNETs, especially in macroadenomas causing Cushing’s disease or in clinically silent corticotroph tumors. Patients with pituitary tumors harboring an ATRX mutation should be offered closer follow-up, including work-up for metastatic dissemination, and invasive treatment at the early stages of the disease.
Acknowledgments
We thank Svetlana Popova, Uppsala University Hospital, for her help in performing immunohistochemical analyses; Ansgar Heck, Kristin Astrid B. Øystese and Jens Bollerslev (University of Oslo, Norway), Bjarne Winther Kristensen and Janne Christiansen (Department of Pathology, University of Southern Denmark, Odense), Olli Tynninen (Department of Pathology, University of Helsinki and HUSLAB, Helsinki, Finland), Angeliki Papagiannopoulou (Department of Pathology, Linköping University Hospital, Sweden), Eva C. Lindberg (Department of Pathology, Lund University Hospital, Sweden), Alia Shamikh (Karolinska University Hospital, Sweden), Lilla Reiniger and Hajnalka Rajnai (Department of Pathology and Experimental Cancer Research, Medical Faculty, Semmelweis University, Budapest, Hungary), Gavin Baker, Brian Herron (Royal Victoria Hospital, Belfast) and Anastasia M. Lapshina (Department of Pathology, University Hospital Moscow, Russia) for their contribution in the selection of the patients and collection of the surgical specimens; Christine Gaasdal Kassentoft and Britt Elmedal Laursen, Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark, for molecular analysis performed at one of the ATRX mutated cases, and Evelina Sjöstedt, Uppsala University, for formatting Fig. 1.
Financial Support: Olivera Casar-Borota was supported by the Swedish Cancer Society (grant number 190157 Fk) and by a grant from the Swedish state under the agreement between the Swedish government and the county councils (ALF-agreement). Henning B. Boldt was supported by the Region of Southern Denmark. Camilla Schalin-Jäntti received funding from the Helsinki University Hospital Research Funds (TYH2018223, TYH20191254). Pia Burman received a research grant from Skåne University Hospital, Region of Skåne, Sweden.
Glossary
Abbreviations
- ACTH
adrenocorticotroph hormone
- ALT
alternative lengthening of telomere
- APT
aggressive pituitary tumor
- ATRX
alpha thalassemia/mental retardation syndrome X-linked
- DAXX
death domain-associated protein
- FSH
follicle-stimulating hormone
- GH
growth hormone
- IHC
immunohistochemistry
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- NET
neuroendocrine tumor
- NGS
next-generation sequencing
- PC
pituitary carcinoma
- PitNET
pituitary neuroendocrine tumors
- PRL
prolactin
- PTEN, PTEN
phosphatase and tensin homolog
- TSH
thyrotroph hormone
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Asa SL, Casar-Borota O, Chanson P, et al. ; attendees of 14th Meeting of the International Pituitary Pathology Club, Annecy, France, November 2016 . From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocr Relat Cancer. 2017;24(4):C5-C8. [DOI] [PubMed] [Google Scholar]
- 2. Raverot G, Burman P, McCormack A, et al. ; European Society of Endocrinology . European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1-G24. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd RV, Osamura RY, Kloppel G, Rosai J.. World Health Organization Classification of Tumours of Endocrine Organs. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 4. Miermeister CP, Petersenn S, Buchfelder M, et al. . Histological criteria for atypical pituitary adenomas – data from the German pituitary adenoma registry suggests modifications [published correction appears in Acta Neuropathol Commun. 2016;4(1):21]. Acta Neuropathol Commun. 2015;3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156(2):203-216. [DOI] [PubMed] [Google Scholar]
- 6. Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95(9):4268-4275. [DOI] [PubMed] [Google Scholar]
- 7. Tjörnstrand A, Gunnarsson K, Evert M, et al. . The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur J Endocrinol. 2014;171(4):519–526. [DOI] [PubMed] [Google Scholar]
- 8. Pernicone PJ, Scheithauer BW, Sebo TJ, et al. . Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804-812. [DOI] [PubMed] [Google Scholar]
- 9. Dudziak K, Honegger J, Bornemann A, Horger M, Müssig K. Pituitary carcinoma with malignant growth from first presentation and fulminant clinical course–case report and review of the literature. J Clin Endocrinol Metab. 2011;96(9):2665-2669. [DOI] [PubMed] [Google Scholar]
- 10. Lidhar K, Korbonits M, Jordan S, et al. . Low expression of the cell cycle inhibitor p27Kip1 in normal corticotroph cells, corticotroph tumors, and malignant pituitary tumors. J Clin Endocrinol Metab. 1999;84(10):3823-3830. [DOI] [PubMed] [Google Scholar]
- 11. Fountas A, Lavrentaki A, Subramanian A, Toulis KA, Nirantharakumar K, Karavitaki N. Recurrence in silent corticotroph adenomas after primary treatment: a systematic review and meta-analysis. [Published online ahead of print, December 21, 2018]. J Clin Endocrinol Metab. Doi: 10.1210/jc.2018-01956. [DOI] [PubMed] [Google Scholar]
- 12. Srirangam Nadhamuni V, Korbonits M. Novel insights into pituitary tumorigenesis: genetic and epigenetic mechanisms. [Published online ahead of print March 23, 2020]. Endocr Rev. 2020;bnaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roncaroli F, Nosé V, Scheithauer BW, et al. . Gonadotropic pituitary carcinoma: HER-2/neu expression and gene amplification. Report of two cases. J Neurosurg. 2003;99(2):402-408. [DOI] [PubMed] [Google Scholar]
- 14. Wei Z, Zhou C, Liu M, et al. . MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary. 2015;18(5):710-721. [DOI] [PubMed] [Google Scholar]
- 15. Tufton N, Roncaroli F, Hadjidemetriou I, et al. . Pituitary carcinoma in a patient with an SDHB mutation. Endocr Pathol. 2017;28(4):320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV. P53 gene mutations in pituitary carcinomas. Endocr Pathol. 2007;18(4):217-222. [DOI] [PubMed] [Google Scholar]
- 17. Xue Y, Gibbons R, Yan Z, et al. . The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci U S A. 2003;100(19):10635-10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong LH, McGhie JD, Sim M, et al. . ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20(3):351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heaphy CM, de Wilde RF, Jiao Y, et al. . Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clynes D, Jelinska C, Xella B, et al. . Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun. 2015;6:7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiao Y, Killela PJ, Reitman ZJ, et al. . Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiao Y, Shi C, Edil BH, et al. . DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haase S, Garcia-Fabiani MB, Carney S, et al. . Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets. 2018;22(7):599-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molenaar JJ, Koster J, Zwijnenburg DA, et al. . Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483(7391):589-593. [DOI] [PubMed] [Google Scholar]
- 25. Fishbein L, Khare S, Wubbenhorst B, et al. . Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Job S, Draskovic I, Burnichon N, et al. . Telomerase activation and ATRX mutations are independent risk factors for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2019;25(2):760-770. [DOI] [PubMed] [Google Scholar]
- 27. Singhi AD, Liu TC, Roncaioli JL, et al. . Alternative lengthening of telomeres and loss of DAXX/ATRX expression predicts metastatic disease and poor survival in patients with pancreatic neuroendocrine tumors. Clin Cancer Res. 2017;23(2):600-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casar-Borota O, Botling J, Granberg D, et al. . Serotonin, ATRX, and DAXX expression in pituitary adenomas: markers in the differential diagnosis of neuroendocrine tumors of the sellar region. Am J Surg Pathol. 2017;41(9):1238-1246. [DOI] [PubMed] [Google Scholar]
- 29. Bengtsson D, Schrøder HD, Andersen M, et al. . Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689-1698. [DOI] [PubMed] [Google Scholar]
- 30. Bengtsson D, Joost P, Aravidis C, et al. . Corticotroph pituitary carcinoma in a patient with lynch syndrome (LS) and pituitary tumors in a nationwide LS cohort. J Clin Endocrinol Metab. 2017;102(11):3928-3932. [DOI] [PubMed] [Google Scholar]
- 31. Casar-Borota O, Boldt HB, Engström BE, et al. . Data from: corticotroph aggressive pituitary tumors and carcinomas frequently harbor ATRX mutations. Figshare. Deposited 20 July 2020. https://figshare.com/s/f04f8753859badbd154a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zacher A, Kaulich K, Stepanow S, et al. . Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. 2017;27(2):146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng DT, Mitchell TN, Zehir A, et al. . Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo F, Wang G, Wang F, Xu D, Liu X. Identification of novel genes involved in the pathogenesis of an ACTH-secreting pituitary carcinoma: a case report and literature review. Front Oncol. 2018;8:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sbiera S, Perez-Rivas LG, Taranets L, et al. . Driver mutations in USP8 wild-type Cushing’s disease. Neuro Oncol. 2019;21(10):1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Schmidt RE, Dahiya S. Pituitary adenoma in pediatric and adolescent populations. J Neuropathol Exp Neurol. 2019;78(7):626-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heaphy CM, Bi WL, Coy S, et al. . Telomere length alterations and ATRX/DAXX loss in pituitary adenomas. Mod Pathol. 2020;33(8):1475-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeineldin M, Federico S, Chen X, et al. . MYCN amplification and ATRX mutations are incompatible in neuroblastoma. Nat Commun. 2020;11(1):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trouillas J, Roy P, Sturm N, et al. ; members of HYPOPRONOS . A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(1):123-135. [DOI] [PubMed] [Google Scholar]
- 40. McCormack A, Dekkers OM, Petersenn S, et al. ; ESE survey collaborators . Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265-276. [DOI] [PubMed] [Google Scholar]
- 41. Trouillas J, Burman P, McCormack A, et al. . Aggressive pituitary tumours and carcinomas: two sides of the same coin? Eur J Endocrinol. 2018;178(6):C7-C9. [DOI] [PubMed] [Google Scholar]
- 42. Burman P, Lamb L, McCormack A. Temozolomide therapy for aggressive pituitary tumours – current understanding and future perspectives. Rev Endocr Metab Disord. 2020;21(2):263-276. [DOI] [PubMed] [Google Scholar]
- 43. Kent T, Gracias D, Shepherd S, Clynes D. Alternative lengthening of telomeres in pediatric cancer: mechanisms to therapies. Front Oncol. 2019;9:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liang J, Zhao H, Diplas BH, et al. . Genome-wide CRISPR-Cas9 screen reveals selective vulnerability of ATRX-mutant cancers to WEE1 inhibition. Cancer Res. 2020;80(3):510-523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.