Abstract
Generalized and partial lipodystrophy are rare and complex diseases with progressive clinical and humanistic burdens stemming from selective absence of subcutaneous adipose tissue, which causes reduced energy storage capacity and a deficiency of adipokines such as leptin. Treatment options were limited before leptin replacement therapy (metreleptin) became available. This retrospective study evaluates both clinical and humanistic consequences of the disease and treatment.
Chart data were abstracted from a cohort of metreleptin-treated patients with generalized and partial lipodystrophy (n = 112) treated at the US National Institutes of Health. To quantify the quality-of-life consequences of the lipodystrophy disease attributes recorded in chart data, a discrete choice experiment was completed in 6 countries (US, n = 250; EU, n = 750). Resulting utility decrements were used to estimate the quality-adjusted life-year consequences of changes in lipodystrophy attribute prevalence before and after metreleptin.
In addition to metabolic impairment, patients with generalized and partial lipodystrophy experienced a range of lipodystrophy consequences, including liver abnormality (94%), hyperphagia (79%), impaired physical appearance (77%), kidney abnormality (63%), reproductive dysfunction (80% of females of reproductive age), and pancreatitis (39%). Improvement was observed in these attributes following initiation of metreleptin. Quality-adjusted life-year gains associated with 12 months of treatment with metreleptin were estimated at 0.313 for generalized and 0.117 for partial lipodystrophy, reducing the gap in quality of life between untreated lipodystrophy and perfect health by approximately 59% and 31%, respectively.
This study demonstrates that metreleptin is associated with meaningful clinical and quality-of-life improvements.
Keywords: lipodystrophy, leptin, metreleptin, metabolic syndrome, patient outcomes, quality of life
Lipodystrophy syndromes are a heterogeneous cluster of complex, life-threatening, rare diseases with prevalence generally estimated to be below 10 cases/million [1, 2]. A recent US medical chart review estimated clinical prevalence as high as 1 in 20 000 individuals and genetic prevalence of approximately 1 in 7000 individuals, possibly indicating that diagnosis rates have been low and that prevalence could be higher than previously estimated [1-3]. Lipodystrophy syndromes result in numerous adverse health consequences due to the selective absence of subcutaneous adipose tissue, resulting in reduced energy storage capacity, inappropriate lipid storage in muscle, the liver, and other organs, and low levels of adipokines such as leptin [4-6]. Lipodystrophy syndromes may lead to deficiency of nearly all adipose tissue (generalized lipodystrophy, GL), or selected adipose depots may be missing, with other depots preserved (partial lipodystrophy, PL). Patients are at significant risk of developing metabolic problems, including elevated triglycerides, severe insulin resistance, and impaired glycemic control, and abnormalities in multiple organs, including the liver, kidneys, and heart, as well as increased risk of pancreatitis [2, 7-11]. Additional impairments may arise that affect patient quality of life and well-being such as hyperphagia, and female reproductive issues (eg, infertility, polycystic ovarian syndrome), and changes to physical appearance [2, 9]. Lipodystrophy syndromes can affect patients from a very early age, progressively worsening throughout life due to complications of primary disease symptoms and possibly leading to premature mortality [2, 4, 12]. Given the burden of disease, there is an urgent need for safe and effective treatments for lipodystrophy syndromes.
Lipodystrophy syndromes are associated with reduced levels of leptin, a hormone produced in adipose tissue that is involved in the regulation of energy homeostasis, neuroendocrine function, metabolism, and immune function through its effects on the central nervous system and peripheral tissues [13-15]. Because leptin is secreted in proportion to adipose tissue mass, patients with GL and PL often have low leptin levels, which have been implicated as a contributor to lipodystrophy-associated metabolic abnormalities [2, 9]. Recombinant human methionyl leptin (metreleptin) is approved as an adjunct to diet to treat the complications of leptin deficiency in patients with GL (US, EU, Japan) and PL (EU and Japan) [16, 17]. Single-arm open-label studies have indicated that metreleptin can reduce the severity of multiple metabolic abnormalities in patients with lipodystrophy, including hyperglycemia, hypertriglyceridemia, and hepatic steatosis [6, 8, 18-28].
While the attributes of lipodystrophy syndromes with potential to impair quality of life have been documented previously, changes in these attributes following initiation of metreleptin therapy have not been as extensively described [25, 29]. Further, information on the quality of life of patients with lipodystrophies is limited. Dhankar et al evaluated the health-related quality of life in a registry of patients who self-identified as having lipodystrophies, primarily PL [30]. This analysis, however, did not report controlling for metreleptin treatment status.
Given the lack of published evidence on the quality of life of patients with lipodystrophy and the potential quality-of-life benefits associated with metreleptin, this retrospective study assessed patients with GL and PL before and after metreleptin therapy at the National Institutes of Health (NIH). Data on changes in prevalence and severity of specific disease attributes (eg, hyperphagia, pancreatitis, inability to work) were extracted from medical records maintained at NIH. In order to quantify the quality-of-life effect of metreleptin therapy in the NIH-treated patients, this study derived utility values through a discrete choice experiment in which respondents assessed the quality-of-life impact of known lipodystrophy attributes.
Methods
Study Design
This study estimated the quality-of-life impact of metreleptin on patients with lipodystrophy by utilizing (1) medical chart data on changes in lipodystrophy disease attributes from patients treated at NIH; and (2) data on the quality-of-life impact of those attributes from a discrete choice experiment conducted in healthy subjects without lipodystrophy. Data on changes in the prevalence and severity of each complication of lipodystrophy were extracted from medical records collected during ongoing clinical studies of metreleptin in patients with lipodystrophy at NIH [29]. The prevalence of specific disease attributes was assessed before the patients started metreleptin and after 1 year of treatment. Improvement after 1 year in each attribute was evaluated among patients in whom the attribute was present before metreleptin treatment. A discrete choice experiment, in which respondents chose between 2 hypothetical health profiles that differed in levels of impairment and life expectancy, was conducted to determine the utility decrements associated with various characteristics of lipodystrophy syndromes. The utility decrements from the discrete choice experiment were combined with data on prevalence of attributes before and after 1 year of treatment with metreleptin to assess overall quality-of-life consequences of GL and PL and the impact of metreleptin on quality-adjusted life years (QALYs). The QALY is a measure of the value of health outcomes where the change in utility induced by the treatment is multiplied by the duration of the treatment effect [31]. Utilities of health states are generally expressed on a scale ranging from 0 to 1, in which 0 represents the utility of the state “death” and 1 the utility of a state lived in “perfect health”.
Data Sources
NIH chart review
Medical chart data were extracted for a total of 112 patients with GL or PL who received treatment with metreleptin; 105 of these patients were prospectively enrolled in clinical trial NCT00005905 and follow-up study NCT00025883 from 2000 to 2014, which were both single-arm, interventional, phase 2 studies conducted at NIH to evaluate the safety and effectiveness of leptin replacement therapy with metreleptin in patients with lipodystrophy [8, 25]. The other 7 patients were enrolled in trial NCT01778556, a nonrandomized, parallel group study that started in 2013 and assessed the short-term effects of leptin initiation or withdrawal in patients with lipodystrophy [26]. Subjects in all 3 studies were aged ≥6 months, had clinically significant lipodystrophy, circulating leptin levels <12.0 ng/mL in females and <8.0 ng/mL in males, and one or more metabolic abnormalities, including diabetes mellitus defined per American Diabetes Association criteria, insulin resistance (fasting insulin > 30 μU/mL [215 pmol/L]), or hypertriglyceridemia (fasting triglyceride > 200 mg/dL) [32, 33]. Vital signs, laboratory assessments, anthropometric and metabolic measures, measures of vital organ function, metreleptin use, and medical history were recorded at baseline and at scheduled post-baseline visits. Clinical values were collected at baseline and during follow-up visits at 1, 2, 4, 6, 8, and 12 months after therapy start [8, 25]. A chart review was conducted to capture the prevalence of disease attributes before and after treatment with metreleptin, extracting the ongoing health data that were collected systematically over the course of these patients’ study participation at NIH. Data were collected from the start of metreleptin treatment and at each of the follow-up visits, whether or not the patients continued or discontinued metreleptin.
Data from all metreleptin-treated patients were available from the date of study enrollment until death or censoring.
Patient data were extracted by trained abstractors in 2017 and were confirmed by NIH study investigators. Patient demographics and clinical characteristics obtained from medical records included age at start of observation, age at first symptoms, gender, lipodystrophy diagnosis (GL or PL), the number of organs with abnormalities (among the heart, liver, and kidney), and the presence or absence of liver abnormalities, kidney abnormalities, heart abnormalities, episodes of pancreatitis, elevated hemoglobin A1c (HbA1c) (defined as ≥6.5%), and hypertriglyceridemia (defined as ≥200 mg/dL) [34, 35]. The dates of diagnosis for each organ abnormality, episode of pancreatitis, elevated HbA1c, and date of death (if applicable), were also collected.
Discrete choice experiment
Because chart data from patients seen at NIH did not include quality-of-life measures, a separate study was conducted to evaluate the quality-of-life impact of disease attributes associated with lipodystrophy syndromes.
An online discrete choice experiment was implemented through a market research firm, Survey Sampling International (SSI). Data gathered in the discrete choice experiment covered attributes determined to be potential consequences of lipodystrophy syndromes in consultation with clinical experts. Standardized information on each attribute was described in a tutorial reviewed by participants.
Screening criteria for study participants included: age ≥ 18 years and education ≥ elementary/primary school. One thousand survey responses were collected: 250 respondents from the United States and 150 respondents each from France, Germany, Italy, Spain, and the United Kingdom. Quotas for the final sample were set to match census data (US) or Eurostat data on gender, age, and education [36-43]. For participant responses to be considered usable, time spent reading through the tutorial slides of ≥ 4 minutes and correct answers for tutorial comprehension questions were required. Information on participant characteristics, including demographics and health conditions, was collected.
A total of 146 distinct choice cards were constructed with 2 hypothetical health scenarios on each choice card; these choice cards were randomly divided into 12 groups. Each group included 14 choice cards, 12 of which were unique to the group, and 2 of which were included in all groups to assess the consistency of responses. Each choice card included 12 of 20 attributes with varying levels of severity. Each respondent was randomly assigned to 1 of the 12 groups and shown the 14 choice cards within the group in a random order. A fractional factorial design was chosen to reduce study complexity. Respondents were then asked to choose a preferred scenario between the 2 options presented on each choice card, given the attributes and levels presented.
The 2 alternative hypothetical health profiles presented on each choice card were characterized by attribute impairments and life expectancy. Respondents were informed of the attributes and levels used to define the 2 health profiles, including age (at which impairment is experienced), organ damage and its speed of progression, triglyceride control, lymphoma, uncontrollable constant hunger (hyperphagia), depression, and chronic pain, among others, each with 2 to 4 levels (Table 1). The list of attributes included “life expectancy (expected age at death)” to articulate that the health status scenarios were for a life-threatening condition, as well as to facilitate estimation of QALY values. Targeted literature review and consultations with clinical experts at Addenbrooke’s Hospital in the United Kingdom and NIH in the United States were conducted to construct the hypothetical scenarios relevant to lipodystrophy health states.
Table 1.
Outcome measures
| Collected from NIH patient charts | Discrete choice experiment |
|---|---|
| Cardiovascular profile | Heart damage: Present / Absent |
| Vital signs (eg, blood pressurea) | |
| Clinical signs, symptoms, or diagnoses of heart abnormality, including hypertrophy and dilation of ventricle/atrium | |
| Chronic or acute pancreatitis | Pancreas damage: Present / Absent |
| HbA1c laboratory values | Impaired blood sugar control: Partial control / No controlb,c |
| History of work / school attendance | Able/Unable to attend work/school |
| Hyperphagia assessed by clinician | Hyperphagia: Present / Absent |
| Impaired physical appearance | Impaired physical appearance: Present / Absent |
| Irregular menstruation, PCOS | Disruption to female reproductive system: |
| No damage / Polycystic ovary syndrome / Infertility | |
| Kidney profile | Kidney damage: Present / Absent |
| Laboratory values (eg, 24-hour protein excretiond) | |
| Clinical signs, symptoms, or diagnoses of kidney abnormality including proteinuria, enlarged kidneys, nephropathy, hydronephrosis, renal disease, nephromegaly, renal failure, renal calculus, and glomerulosclerosis | |
| Liver profile | Liver damage: Present / Absent |
| Laboratory values (eg, alanine aminotransferase [ALT], aspartate transaminase [AST])e | |
| Clinical signs, symptoms, or diagnoses of liver abnormality, including hepatic steatosis and hepatomegaly | |
| Signs of worsening heart, liver, or kidney profile | Progression of organ damage: No change / Slow / Fast |
| Fasting plasma triglyceride | Triglyceride (blood fat) control: Partial control / No controla,f |
| No analogous attribute | Amputation: Present / Absent |
| Chronic pain: Present / Absent | |
| Depression: Present / Absent | |
| Neuropathy: Present / Absent | |
| Retinopathy: Present / Absent | |
| Loss of response to treatment: Standard risk / Increased risk | |
| Lymphoma: Standard risk / Increased risk |
aA patient is considered to be hypertensive at baseline if the patient is categorized as Stage 2 hypertension (systolic ≥160 mm Hg or diastolic ≥100 mm Hg), Stage 1 hypertension (systolic ≥140 mm Hg and <160 mm Hg; or diastolic ≥90 mm Hg and <100 mm Hg), or prehypertensive (systolic ≥120 mm Hg and <140 mm Hg; or diastolic ≥80 mm Hg and <90 mm Hg). Normal blood pressure is systolic <120 mm Hg and diastolic <100 mm Hg.
bDisease attributes were presented in layman’s terms for ease of comprehension.
cPatients with HbA1c values > 6.5% and ≤8% were considered as patients with “partial control” of blood sugar and patients with HbA1c values >8% were considered as patients with “no control” of blood sugar.
dElevated 24-hour protein excretion is considered patients with a baseline laboratory value of >150 mg.
eElevated ALT (females, >50 U/L; males > 66 U/L) and AST (>40 U/L) are patients with a baseline laboratory value of >2× normal levels.
f Patients with triglyceride values > 200 mg/dL and ≤500 mg/dL were considered as patients with “partial control” of triglyceride levels and patients with triglyceride values of >500 mg/dL were considered as patients with “no control” of triglyceride levels.
Statistical Analyses
Analyses were conducted to examine changes in outcomes/disease progression before and after the start of metreleptin treatment and at each of the follow-up visits whether or not the patients continued metreleptin. Definitions of prevalence at baseline and after treatment are described in Table 2.
Table 2.
Definition of disease attribute prevalence at baseline and post-metreleptin treatment and definition of improvement
| Attribute | Baseline definition | Outcome definition | Definition of improvement |
|---|---|---|---|
| Disruption to female reproductive function | Irregular menstruation or PCOSa | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | More regular menstruation or decreased signs/symptoms of PCOS by last NIH visit based on patient chart notes |
| Heart abnormality | Hypertrophy, any dilation, any regurgitation, cardiomyopathy, and tachycardia (excludes hypertension) | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | N/A—Clinical improvement could not be determined with available data |
| Hypertensionb | Stage 2 hypertension: Systolic ≥ 160 mm Hg or diastolic ≥ 100 mm Hg | Complete control: Systolic <120 mm Hg and diastolic <100 mm Hg | Early improvement: 1-stage improvement in baseline hypertension categorization by year 1 |
|
Stage 1 hypertension: Systolic ≥ 140 mm Hg or <160 mm Hg; or diastolic <100 mm Hg or ≥90 mm Hg Prehypertensive: Systolic ≥120 mm Hg and <140 mm Hg; or diastolic ≥80 mm Hg and <90 mm Hg |
Partial control: ≥1-stage improvement from baseline No control/worsening: Same as baseline definition |
Late improvement: 1-stage improvement in baseline hypertension categorization after year 1 | |
| Hyperphagia | Clinician-documented excessive hunger | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | Clinician-documented reduction in excessive hunger |
| Hyperglycemia | No blood sugar control: HbA1c > 8% | Complete control: HbA1c <6.5% | Early improvement: ≥20% reduction in HbA1c % by year 1 |
| Partial blood sugar control: HbA1c > 6.5% to ≤8% | Partial control: HbA1c ≥ 6.5% to ≤8% after 1 year | ||
| No control/worsening: HbA1c > 8% after 1 year | Late improvement: ≥20% reduction in HbA1c % after year 1 | ||
| Complete blood sugar control: Hba1C <6.5% | Same as baseline definition | N/A | |
| Hypertriglyceridemia | No triglyceride control: >500 mg/dL |
Complete triglyceride control: <200 mg/dL Partial triglyceride control: >200 to ≤500 mg/dL No triglyceride control / worsening: >500 mg/dL after 1 year |
Early improvement: ≥20% reduction in triglyceride levels by year 1 Late improvement: ≥20% reduction in triglyceride levels after year 1 |
| Partial triglyceride control: >200 to <500 mg/dL | |||
| Complete triglycerides control: <200 mg/dL | Same as baseline definition | N/A | |
| Impaired physical appearance | Acanthosis nigricans, hyperkeratosis, or hirsutism | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | Diminished acanthosis nigricans, hyperkeratosis, or hirsutism based on patient chart notes |
| Kidney abnormality | Proteinuria, enlarged kidneys, nephropathy, hydronephrosis, renal disease, nephromegaly, renal failure, renal calculus, and glomerulosclerosis | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | ≥20% reduction in 24-hour protein excretion at year 1 if elevated (>150 mg/24 hrs) at baseline; if additional kidney condition emerged between metreleptin initiation and 1.5 years post-metreleptin (exclusive), then patient was not considered to improve; improvement was not measured for patients without elevated protein excretion at baseline |
| Liver abnormality | Any form of fatty liver or steatosis, fibrosis, cirrhosis, hepatitis, >2x normal levels of ALT (females, >50 U/L; males >66 U/L) and AST (>40 U/L), and hepatomegalyc | Same as baseline definition; continued or newly emergent symptoms after initiation of treatment | ≥20% reduction in ALT or AST at year 1 if elevated at baseline |
| If both ALT and AST were elevated at baseline, both ALT and AST must have 20% reduction at year 1 to be considered improved | |||
| If only one of the laboratory values was considered elevated at baseline, that value must show a ≥20% reduction and the other laboratory values must remain nonelevated at year 1 to be considered improved. Improvement was not measured for patients without elevated ALT or AST at baseline | |||
| Pancreatitis | ≥1 recorded episode of pancreatitis | ≥1 recorded episode of pancreatitis after treatment initiation that did not occur in the context of noncompliance with metreleptin treatment | No recorded episodes of pancreatitis after initiation of metreleptin among patients with a history of pancreatitisd |
| Progression of organ damage |
Fast progression: Organ progression rate ≤9 years/organe Slow progression: Organ progression rate >9 years/organe |
Fast progression: Development of first organ abnormality in the first 3 years of treatment Slow progression: Development of first organ abnormality after the first 3 years of treatment |
N/A—Improvement was not measured |
| Inability to work/attend school | Incomplete school attendance due to disease symptoms (school-age) or not working/working part-time due to disease symptoms (adults) | Same as baseline definition; continued or newly emergent after initiation of treatment | Full school attendance (school-age) or the ability for a patient to work, even if the patient has chosen not to work (adults) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, glycated hemoglobin; NIH, National Institutes of Health; PCOS, polycystic ovary syndrome.
aExcluded subjects in menopause, prepubescent, or status after surgical removal of reproductive organs.
bPatients’ baseline and year 1 hypertension data needed to be available to be included in the count and percent for hypertensive (n = 105).
cHepatomegaly values were not consistently reported.
dExcludes pancreatitis that occurred in the context of noncompliance with metreleptin treatment.
eDefined as the patients’ age at the beginning of treatment divided by the patients’ initial number of organ impairments (kidney, heart, liver, or pancreatitis).
Measuring improvement
To capture the effect of metreleptin in improving clinical signs and symptoms of lipodystrophy over time, definitions of improvement were specified based on expert opinion and published evidence. Improvement was only evaluated among patients reporting the abnormality or attribute before treatment with metreleptin.
For organ abnormalities (heart, liver, kidney), improvement from baseline was analyzed at 1 year after metreleptin initiation. For pancreatitis, the presence of pancreatitis events pre- vs post-metreleptin was analyzed. For non-organ-based signs and symptoms of lipodystrophy, pre- and post-metreleptin clinical status was compared. For laboratory values, “baseline” values are the average of all measurements captured within 0.5 years prior to metreleptin initiation up to 1 week post-metreleptin initiation, except for triglycerides and HbA1c, which were manually adjudicated as the closest laboratory value prior to metreleptin initiation. The long-term efficacy and safety studies of metreleptin in patients with GL and PL by Brown et al and Oral et al, respectively, have previously reported significant reductions in triglyceride levels after metreleptin treatment [25, 44]. Both baseline and follow-up data were required to be available to include a patient in the assessment of each abnormality, with total N for each noted below. For organ abnormalities (heart, kidney, liver), if a new abnormality arose in that organ during the first 1.5 years of metreleptin treatment, that organ was not considered to be improved, even if the patient otherwise met criteria for organ improvement.
Definitions of improvement are presented in Table 2.
Estimation of utility decrements
Data from the discrete choice experiment were used to estimate a conditional logit model, which assumes that individuals derive utility from spending time in particular health states [45-48]. Under the framework used, respondents are assumed to maximize their utility with their choices. The utility function subtracts the sum of the utility impairment associated with each disease attribute from the utility of a year spent in perfect health and then applies this value to the individual’s remaining life. A binary variable indicating the choice card scenario with the most life remaining and a binary variable indicating the choice card scenario with the most impairments were introduced into the estimation equation to reduce the bias stemming from respondents possibly using simple heuristic rules (such as always choose the scenario with the least impairments, or always choose scenarios with more life remaining). An error term following the Type 1 Extreme Value distribution was also included. Standard errors were clustered at the respondent level.
Utility decrements associated with each attribute were estimated using an approach similar to Bansback et al [46]. The utility decrements were computed as follows:
where is the regression coefficient for attribute and is the coefficient on years of life remaining. These decrements were further rescaled in a proportional manner such that the QALY value of the worst possible health state in the study (when all 18 dimensions of utility are maximally impaired) equals the estimated QALY value of the worst possible health state in the UK’s EQ-5D-3L tariff: −0.594 QALYs [49].
The utility decrements from the discrete choice experiment data analysis were combined with data on prevalence of attributes before and after 1 year of metreleptin, to assess overall quality-of-life consequences of GL and PL, and the impact of metreleptin on QALYs using the following formula:
Because the utility survey evaluated a limited number of discrete states in each disease attribute (eg, absent or present), it is possible that clinically meaningful improvement in certain attributes could have been experienced by a patient but would not be reflected in the estimation of that patient’s post-metreleptin utility. Changes in utility are only assigned to those patients in whom the full state change tested in the utility study was observed.
Results
Baseline Characteristics
A total of 112 patients were included in this study. Baseline characteristics are presented in Table 3. The mean age of first lipodystrophy symptom and at metreleptin initiation was 13.4 years and 24.3 years, respectively. Most patients were female (83%) and Caucasian (63.8%) and had congenital GL (42.9%) or familial partial lipodystrophy (FPL) (33.9%). Mean baseline HbA1c was 8.4%, geometric mean triglycerides 531.9 mg/dL, and leptin 3.3 ng/mL. A minority of patients had baseline HbA1c <6.5% and triglyceride levels <200 mg/dL, 25.2% and 19.1% respectively. Most patients were being treated with antidiabetic (89.3%) and/or triglyceride-lowering (51.8%) medications, while 31.3% of patients were being treated with antihypertensive medications at baseline. Patients in this study presented with an array of lipodystrophy disease attributes (Tables 4 and 5), including liver abnormality (94%), hyperphagia (79%), impaired physical appearance (77%), elevated urine protein excretion (41%), kidney abnormality (63%), hypertension (54%), and elevated alanine aminotransferase (ALT) (51%) and aspartate aminotransferase (AST) (43%). Additionally, 80% of females of reproductive age (n = 56) experienced reproductive dysfunction. Patients with GL more frequently experienced elevated protein excretion (50% vs 27% of patients with PL), inability to work/attend school (57% vs 20%), hyperphagia (82% vs 72%), and elevated ALT (66% vs 27%), kidney abnormalities (68% vs 57%), and AST (56% vs 23%). A larger proportion of patients with PL experienced pancreatitis (52% vs 31%), disruption of female reproductive function (55% vs 31%), and hypertension (59% vs 51%). A comparable proportion of patients with GL and PL experienced liver abnormalities (93% vs 95%).
Table 3.
Baseline characteristics
| All Patients N = 112 | GL Patients N = 68 | PL Patients N = 44 | |
|---|---|---|---|
| Baseline information, n (%) | |||
| Age at first GL/PL symptomsa | |||
| Years, mean (SD) | 13.4 (11.2) | 8.8 (7.1) | 20.1 (12.6) |
| Years, [median] {Q1–Q3} | [12] {7–17} | [9] {2–13} | [17] {13–24} |
| Age at metreleptin initiation | |||
| Years, mean (SD) | 24.3 (15.4) | 17.5 (11.4) | 34.6 (15.2) |
| Years, [median] {Q1–Q3} | [18] {14–35} | [15] {12–20} | [35] {19–46} |
| Type of lipodystrophy, n (%)b | |||
| Acquired generalized lipodystrophy | 20 (17.9) | 20 (29.4) | – |
| Acquired partial lipodystrophy | 6 (5.4) | – | 6 (13.6) |
| Congenital generalized lipodystrophy | 48 (42.9) | 48 (70.6) | – |
| Familial partial lipodystrophy | 38 (33.9) | – | 38 (86.4) |
| Laboratory values, mean (SD) | |||
| HbA1c, %c | 8.4 (2.3) | 8.7 (2.3) | 8 (2.2) |
| Triglycerides, mg/dLd,e | 531.9 (228–1219) | 545.2 (220–1251) | 512.5 (244–841) |
| Leptin, ng/mLf | 3.3 (3.4) | 1.3 (1) | 6.4 (3.5) |
| Any baseline antidiabetic medication, n (%)g | 100 (89.3) | 57 (83.8) | 43 (97.7) |
| Any triglyceride-lowering medication, n (%)g | 58 (51.8) | 28 (41.2) | 30 (68.2) |
| Any antihypertensive medication, n (%)g | 35 (31.3) | 18 (26.5) | 17 (38.6) |
Abbreviations: GL, generalized lipodystrophy; HbA1c, glycated hemoglobin; PL, partial lipodystrophy.
aAge at first GL/PL symptoms is the earliest age at which there was evidence of a GL/PL issue (N = 108; 64 GL and 44 PL patients).
bPatients had the following genotypes: AGPAT-2 (n = 26), BSCL2 (n = 15), LMNA (n = 27), PPAR-G (n = 9), or Not Available (n = 9). Additional data on genotype-specific results can be found in Chong et al. and Sekizkardes et al [67, 68].
cBaseline HbA1c laboratory values are sourced for 103 patients. For 8 patients, baseline values were manually adjudicated as the closest laboratory value prior to metreleptin initiation. One GL patient had a missing baseline HbA1c value; percentages reported are of the patients with available baseline HbA1c values (N = 111 patients; 67 GL and 44 PL patients).
dBaseline triglyceride laboratory values are sourced for 102 patients. For 8 patients, baseline values were manually adjudicated as the closest laboratory value prior to metreleptin initiation. Two GL patients had missing baseline triglyceride values; percentages reported are of the patients with available baseline triglycerides values (N = 110 patients; 66 GL and 44 PL patients).
eTriglycerides are reported as geometric mean (25th - 75th percentiles).
fBaseline leptin laboratory values are sourced for 104 patients. For the remaining 8 patients, no leptin data were available.
gMedications filled within 1 year prior to metreleptin initiation were considered baseline medications.
Table 4.
Improvement of disease attributes after treatment with metreleptin
| All patients N = 112 (93F, 19M) | GL patients N = 68 (51F, 17M) | PL patients N = 44 (42F, 2M) | ||||
|---|---|---|---|---|---|---|
| Patients with evidence of issue pre-MET, n (%)a | Patients with evidence of issue who improved post-MET, n (%) | Patients with evidence of issue pre-MET, n (%)a | Patients with evidence of issue who improved post-MET, n (%) | Patients with evidence of issue pre-MET, n (%)a | Patients with evidence of issue who improved post-MET, n (%) | |
| Disruption to female reproductive functionb | 45 (40) | 20 (44) | 21 (31) | 12 (57) | 24 (55) | 8 (33) |
| HbA1c (>6.5%)c | 83 (75) | 66 (80) | 52 (78) | 46 (88) | 31 (70) | 20 (65) |
| Heart | ||||||
| Heart abnormalityd | 50 (45) | - | 36 (53) | - | 14 (32) | - |
| Hypertensione,f | 61 (54) | 36 (59) | 35 (51) | 27 (77) | 26 (59) | 9 (35) |
| Hyperphagiag | 74 (79) | 73 (99) | 51 (82) | 51 (100) | 23 (72) | 22 (96) |
| Impaired physical appearance | 86 (77) | 52 (60) | 56 (82) | 38 (68) | 30 (68) | 14 (47) |
| Kidney | ||||||
| Kidney abnormalitye | 71 (63) | 19 (27) | 46 (68) | 16 (35) | 25 (57) | 3 (12) |
| Elevated 24-hour protein excretionde | 46 (41) | 27 (59) | 34 (50) | 23 (68) | 12 (27) | 4 (33) |
| Liver | ||||||
| Liver abnormalitye | 105 (94) | 38 (36) | 63 (93) | 32 (51) | 42 (95) | 6 (14) |
| Elevated ALTe | 57 (51) | 48 (84) | 45 (66) | 41 (91) | 12 (27) | 7 (58) |
| Elevated ASTe | 48 (43) | 35 (73) | 38 (56) | 30 (79) | 10 (23) | 5 (50) |
| Pancreatitis | 44 (39) | 43 (98) | 21 (31) | 20 (95) | 23 (52) | 23 (100) |
| Triglycerides (>200 mg/dL)h | 89 (81) | 79 (89) | 52 (79) | 47 (90) | 37 (84) | 32 (86) |
| Inability to work/attend school | 48 (43) | 36 (75) | 39 (57) | 31 (79) | 9 (20) | 5 (56) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; F, female; GL, generalized lipodystrophy; HbA1c, glycated hemoglobin; M, male; MET, metreleptin; PL, partial lipodystrophy.
aPatients with evidence of lipodystrophy characteristics at baseline as noted in Table 3.
bOnly female patients of reproductive capacity (GL: 27; PL: 29) were eligible for impairment; all other patients were considered unimpaired. Reproductive capacity was defined as female patients who were postpubescent and premenopausal who had not undergone surgical removal of reproductive organs at the time of starting metreleptin. Prevalence of disruption to reproductive function prior to treatment with metreleptin among female patients of reproductive capacity is 80%. Of 56 female patients of reproductive capacity (GL: 27; PL: 29), 45 presented with evidence of disruption to reproductive function prior to treatment with metreleptin.
cPercentages reported are of the patients with available baseline HbA1c values (GL: 67; PL: 44).
dBaseline data only available as the clinical resolution of heart abnormalities could not be determined with available data post-metreleptin.
eYear 1 post-metreleptin laboratory values are defined as the average of the specific laboratory measurements captured from 0.5 years post-metreleptin (inclusive) to 1.5 years post-metreleptin (exclusive).
fClinical improvement was defined as a 1-stage improvement in baseline hypertension categorization (as defined in Table 2) at year 1 post-metreleptin initiation (eg, moved from Stage 2 hypertension to Stage 1 hypertension, or prehypertensive to normal).
gData on hyperphagia were collected for first 94 patients (GL: 62, PL: 32).
hPercentages reported are of the patients with available baseline triglycerides values (GL: 66; PL: 44).
Table 5.
Prevalence of disease attributes
| Rescaled utility decrement | GL Patients N = 68 | PL Patients N = 44 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence at baselinea (%) | Post-MET prevalencea (%) | Change in prevalence (%) | Change in QALYs | Prevalence at baselinea (%) | Post-MET prevalencea (%) | Change in prevalence (%) | Change in QALYs | ||
| Disruption to female reproductive functionb | −0.026 | 31 | 18 | −13 | 0.005 | 55 | 45 | −10 | 0.003 |
| HbA1c | |||||||||
| >6.5% and ≤8% | −0.038 | 12 | 15 | +3 | −0.001 | 23 | 30 | +7 | −0.003 |
| >8% | −0.082 | 66 | 20 | −46 | 0.038 | 45 | 28 | −17 | 0.015 |
| Heart abnormality c | −0.096 | 53 | 57 | +4 | −0.004 | 32 | 39 | +7 | −0.007 |
| Hyperphagiad | −0.071 | 82 | 11 | −71 | 0.050 | 72 | 9 | −63 | 0.044 |
| Impaired physical appearance | −0.056 | 82 | 29 | −53 | 0.030 | 68 | 41 | −27 | 0.015 |
| Kidney abnormality | −0.066 | 68 | 47 | −21 | 0.014 | 57 | 61 | +4 | −0.003 |
| Liver abnormality | −0.082 | 93 | 47 | −46 | 0.038 | 95 | 84 | −11 | 0.009 |
| Pancreatitise | −0.060 | 31 | 2 | −29 | 0.018 | 52 | 2 | −50 | 0.030 |
| Progression of organ damage | |||||||||
| Slow | 0.030 | 21 | 24 | +3 | 0.001 | 82 | 26 | −56 | −0.017 |
| Fast | −0.080 | 75 | 26 | −49 | 0.039 | 18 | 11 | −7 | 0.006 |
| Triglycerides | |||||||||
| >200 mg/dL and ≤500 mg/dL | −0.012 | 35 | 26 | −9 | 0.001 | 45 | 45 | 0 | <0.001 |
| >500 mg/dL | −0.032 | 44 | 15 | −29 | 0.009 | 39 | 28 | −11 | 0.004 |
| Inability to work/attend school | −0.167 | 57 | 12 | −45 | 0.076 | 20 | 9 | −11 | 0.019 |
Abbreviations: GL, generalized lipodystrophy; HbA1c, glycated hemoglobin; MET, metreleptin; PL, partial lipodystrophy; QALYs, quality-adjusted life years.
aWhen calculating prevalence, it was assumed that a disease attribute is not present when data are not recorded.
bOnly female patients of reproductive capacity (GL: 27; PL: 29) were eligible for impairment; all other patients were considered unimpaired. Reproductive capacity was defined as female patients who were postpubescent and premenopausal who had not undergone surgical removal of reproductive organs at the time of starting metreleptin.
cPrevalence of heart abnormality can only increase as clinical resolution of abnormalities could not be determined with available data.
dData on hyperphagia were collected for first 94 patients (GL: 62, PL: 32).
eOne patient with GL and one patient with PL each experienced a single episode of pancreatitis while on treatment with metreleptin.
Improvement and Change in Prevalence of Disease Attributes
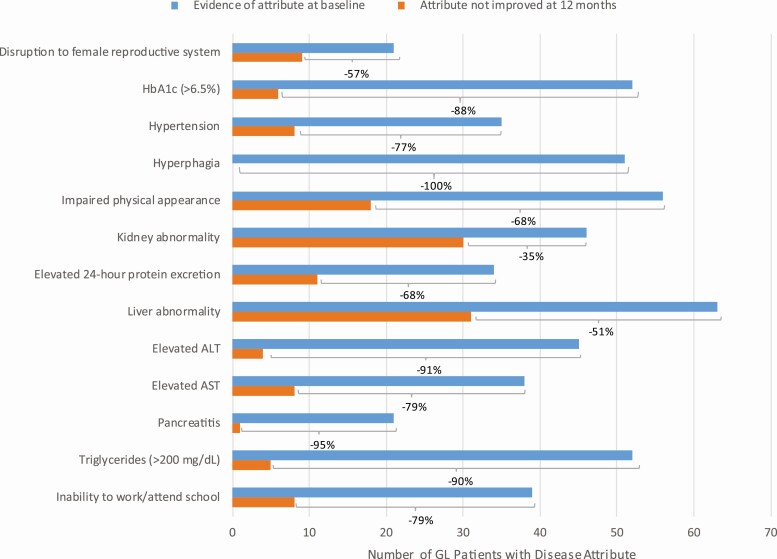
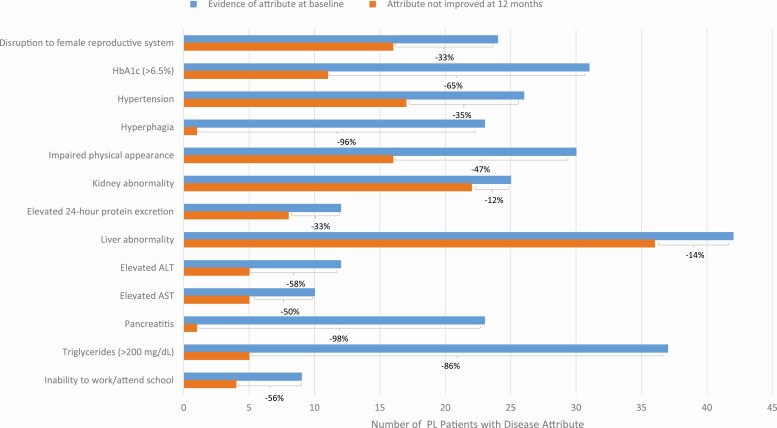
The effect of treatment with metreleptin in improving baseline lipodystrophy complications is presented in Table 4. Percent improved was defined as the proportion of patients with evidence of lipodystrophy characteristics at baseline (as noted in Table 3) who showed improvement after treatment with metreleptin (as defined in Table 2). Among patients with GL, over 50% of patients with baseline complications of hyperphagia, pancreatitis, elevated HbA1c, elevated triglyceride levels, elevated ALT and AST, inability to work/attend school, hypertension, disruption of female reproductive system, impaired physical appearance, liver abnormality, and elevated 24-hour protein excretion at baseline improved after treatment with metreleptin (Fig. 1). Among patients with PL, over 50% of patients with elevated HbA1c, hyperphagia, elevated ALT and AST, pancreatitis, inability to work/attend school, and elevated triglyceride levels improved after treatment with metreleptin (Fig. 2).
Figure 1.
Prevalence of disease attributes at baseline and improvement following treatment in patients with generalized lipodystrophy. This figure presents the prevalence of each disease attribute among patients with generalized lipodystrophy before treatment with metreleptin and the number of patients who did not improve after treatment with metreleptin. The frequency of improvement is shown as a percentage for each disease attribute. Abbreviations: GL, generalized lipodystrophy.
Figure 2.
Prevalence of disease attributes at baseline and improvement following treatment in patients with partial lipodystrophy. This figure presents the prevalence of each disease attribute among patients with partial lipodystrophy before treatment with metreleptin and the number of patients who did not improve after treatment with metreleptin. The frequency of improvement is shown as a percentage for each disease attribute. Abbreviations: PL, partial lipodystrophy.
Attribute prevalence data from patients with GL and PL before and after metreleptin were available for 14 of 18 attributes (Table 5). Attribute prevalence data were collected at baseline and during follow-up visits. Post-metreleptin prevalence includes patients who newly developed the disease attribute after treatment with metreleptin. The largest decreases in attribute prevalence following treatment with metreleptin occurred for hyperphagia (GL prevalence from 82% to 11% and PL prevalence from 72% to 9%), inability to work/attend school (GL prevalence from 57% to 12% and PL prevalence from 20% to 9%), pancreatitis (GL prevalence from 31% to 2% and PL prevalence from 52% to 2%), impaired physical appearance (GL prevalence from 82% to 29% and PL prevalence 68% to 41%), and hyperglycemia (no control/worsening; GL prevalence from 66% to 20% and PL prevalence from 45% to 28%).
Disease Attributes and Utility Decrements
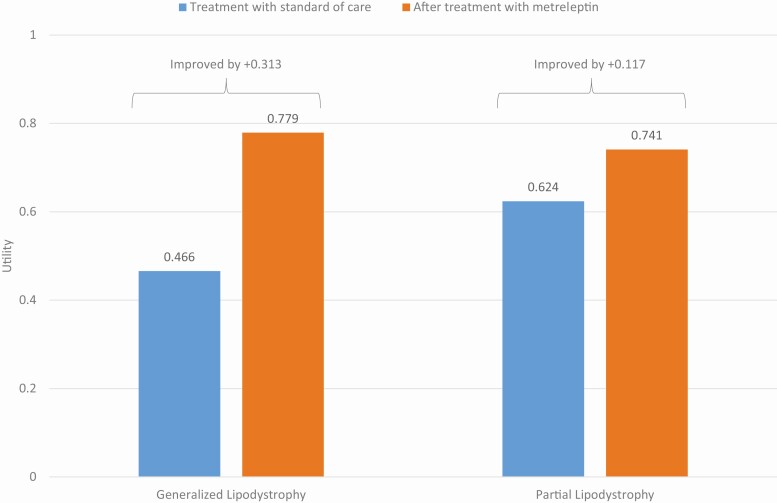
Estimated utility decrements due to lipodystrophy-associated disease attributes, and the calculated changes in QALYs associated with changes in these disease attributes after metreleptin, are shown in Table 5. Overall, QALY gains associated with treatment with metreleptin were estimated at 0.313 across patients with GL (from 0.466 to 0.779; Fig. 3). Changes in “Inability to work/attend school” accounted for 24.3% of the gain. Among patients with PL, QALY gains associated with treatment with metreleptin were estimated at 0.117 (from 0.624 to 0.741; Fig. 3). Changes in “Hyperphagia” accounted for 37.8% of the gain.
Figure 3.
Utility at baseline and following treatment with metreleptin in patients with generalized and partial lipodystrophy. This figure presents the quality-adjusted life year gains associated with 12 months of treatment with metreleptin, estimated at 0.313 for generalized lipodystrophy and 0.117 for partial lipodystrophy, reducing the gap in quality of life between untreated lipodystrophy and perfect health by approximately 59% and 31%, respectively.
Discussion
The rarity of lipodystrophies and the limited treatment options available have constrained study of the conditions and of the effects of metreleptin. This study has combined data from the largest and best-studied group of lipodystrophy patients initiating metreleptin with a de novo study of the quality-of-life effects of lipodystrophy attributes in order to assess the overall quality-of-life impact of lipodystrophy and the benefit associated with metreleptin.
More than half of all patients in this study with pancreatitis, impaired physical appearance, hypertension, hyperphagia, hyperglycemia, elevated triglyceride levels, elevated protein excretion, elevated ALT or AST, or inability to work/attend school experienced clinically meaningful improvement on these attributes following treatment with metreleptin (Table 4). The largest decreases in attribute prevalence following treatment with metreleptin occurred for hyperphagia, inability to work/attend school, pancreatitis, elevated ALT and AST, hyperglycemia, and elevated triglyceride levels.
Especially for female patients, the changed physical appearance is one of the most relevant and debilitating symptoms and there is supportive evidence from Miehle et al investigating 8 lipodystrophy patients after 12 months of treatment with metreleptin [50]. The median fat mass was significantly reduced during metreleptin treatment from 22.3 kg at baseline to 20.0 kg at 1 year (P = 0.031). Five of the 6 patients with FPL lost between 4 and 114 cm3 of facial soft tissue volume in the pre-auricular, buccal, and submandibular area during metreleptin treatment whereas a slight volume gain was seen in 1 FPL patient. The 2 patients with GL developed a volume loss of 20 and 8 cm3 in the buccal region between baseline and 1 year of metreleptin therapy. Another publication by Vatier et al indicates that metreleptin therapy can lead to reduction in facial soft tissue volume in lipodystrophy patients [51]. This study assessed patients’ adherence and satisfaction with metreleptin therapy, as well as self-perception of physical appearance and social interactions, in the 20 patients with PL and GL included in the French metreleptin compassionate program and treated for more than 1 year at the time of the study. Morphological appearance was reported improved under metreleptin therapy in 13 of 17 patients.
Acute pancreatitis (AP), with an incidence between 0.005% and 0.08% per year, is a severe disease associated with a high mortality risk [52]. According to the current guideline on management of severe AP, approximately 20% of patients with AP will develop moderate or severe AP with a mortality rate of 13% to 35% [53-55]. However, hypertriglyceridemia-associated AP leads to a worse clinical outcome than AP associated with other causes, as shown in a meta-analysis comparing 1564 patients with hypertriglyceridemia-associated AP with 5721 patients who had AP of other etiology. Patients with hypertriglyceridemia-associated AP showed a significant, progressive increase in the incidence of persistent organ failure, pancreatic necrosis, and mortality with increasing severity of hypertriglyceridemia [56]. In our investigation, 31% of patients with GL and 52% of patients with PL had experienced pancreatitis. After treatment with metreleptin, only 1 patient with GL and 1 patient with PL had a pancreatitis event.
Overall, patients with GL and PL experience reduced quality of life prior to metreleptin treatment (0.466 and 0.624 QALY, respectively, compared with 1 for a person in perfect health). QALY gains associated with treatment with metreleptin were estimated at 0.313 among patients with GL; in other words, metreleptin treatment reduces the gap in quality of life between untreated GL and perfect health by approximately 59%. QALY gains associated with treatment with metreleptin were estimated at 0.117 among patients with PL, reducing the gap in quality of life between untreated PL and perfect health by approximately 31%. QALY changes of this magnitude in a population with chronic disease are rarely observed. A review of 333 cost-utility analyses estimated that the median incremental QALY gains associated with medical interventions to treat chronic diseases (including cancer, cardiovascular diseases, respiratory diseases, and mental health disorders) range between 0.03 and 0.1 [57].
While some attributes of lipodystrophy syndromes with potential to impair quality of life have been documented previously, changes in many of these attributes following initiation of metreleptin have not been extensively described [25, 29]. The present study addresses this gap but is limited to retrospective evaluation of patient chart data recorded during the course of clinical trials. The efficacy data were reported in phase 2, single-arm, open-label clinical trials (open-label exploratory study and subsequent long-term study) and therefore lack a comparator arm to help evaluate the incremental effect of treatment [8, 25]. Additionally, utility gains were not estimated for all clinically meaningful improvement in disease symptoms. Where the definitions for clinical improvement differed from those for attribute resolution leading to improvement in quality of life, the observed improvement in attributes were not included in quality-of-life calculations.
Because data on quality-of-life effects of lipodystrophy attributes had not previously been collected, it was necessary to develop additional data to quantify the impact of these disease attributes. The discrete choice experiment design used built on an extensive history of such studies [46, 48, 58-62]. At the same time, discrete choice experiments are acknowledged to be imperfect tools to evaluate the quality-of-life impact of disease attributes. The hypothetical nature of these stated preference experiments can create bias [63]. Hypothetical bias can be attributed to choice tasks that do not fully reflect reality, when respondents have incomplete preferences, or if respondents perceive a benefit to over- or understating the importance of certain attributes [64]. Anchoring is another potential limitation. An explicit anchor was not included in the discrete choice experiment, which may have also introduced bias [65]. However, Norman et al demonstrates that while different methods of anchoring utility values on the full health to death QALY scale lead to differences in the scale of utility decrements, the ranking of the health states remains consistent [66].
In conclusion, previous studies have shown metreleptin-mediated improvements in patients with lipodystrophies, including improvement in peripheral insulin sensitivity, hypertriglyceridemia, and biomarkers associated with nonalcoholic fatty liver disease, as well as reduction in hepatic and circulating triglycerides [8, 23-26, 44]. This study finds benefits in these outcomes as well as suggesting benefit in more indirect disease attributes, such as ability to attend school or work among both patients with GL and PL. Regulatory authorities have approved the usage of metreleptin in patients with PL on the basis of the trial primary endpoints (HbA1c and triglyceride levels). However, the results from this study suggest there are other beneficial outcomes that regulators may want to consider when evaluating metreleptin. While additional research is warranted, the results of this study suggest that metreleptin treatment dramatically reduces the gap in quality of life between lipodystrophy treated with standard of care prior to metreleptin and perfect health by 59% in patients with GL and 31% in patients with PL (Fig. 3).
Acknowledgments
We thank Omer Ali, Kristina Shampanier, and Riddhima Sharma for supporting the design and development of the discrete choice experiment and analyzing the resulting data.
Financial Support: This study was funded by Aegerion Pharmaceuticals Inc. (a wholly owned subsidiary of Amryt Pharmaceuticals) and Amryt Pharmaceuticals.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- AP
acute pancreatitis
- AST
aspartate aminotransferase
- GL
generalized lipodystrophy
- HbA1c
glycated hemoglobin A1c
- NIH
National Institutes of Health
- PL
partial lipodystrophy
- QALY
quality-adjusted life-year
Additional Information
Disclosures: K.C., K.A., A.G., and E.T. are employees of Analysis Group, Inc., which has received consultancy fees. H.K. is an employee of Amryt Pharmaceuticals. R.B. and E.C. are clinical researchers at the National Institutes of Health.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Chiquette E, Oral EA, Garg A, Araújo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzaga-Jauregui C, Ge W, Staples J, et al. ; Geisinger-Regeneron DiscovEHR collaboration . Clinical and molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes. 2020;69(2):249-258. [DOI] [PubMed] [Google Scholar]
- 4. Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207(3):245-255. [DOI] [PubMed] [Google Scholar]
- 5. Deeks E. Metreleptin in lipodystrophy: a profile of its use. Drugs Ther Perspect. 2019;35(5):201-208. [Google Scholar]
- 6. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570-578. [DOI] [PubMed] [Google Scholar]
- 7. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220-1234. [DOI] [PubMed] [Google Scholar]
- 8. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am. 2004;33(2):305-331. [DOI] [PubMed] [Google Scholar]
- 11. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298(3):299-308. [DOI] [PubMed] [Google Scholar]
- 12. Gupta N, Asi N, Farah W, et al. Clinical features and management of non-HIV-related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab. 2017;102(2):363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blüher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89(3):991S-997S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34(3):377-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18(1):29-42. [DOI] [PubMed] [Google Scholar]
- 16. Myalept [Summary of Product Characteristics]. 2020. Accessed July 6, 2020.https://www.ema.europa.eu/en/documents/product-information/myalepta-epar-product-information_en.pdf [Google Scholar]
- 17. Myalept (metreleptin) for injection for subcutaneous use [prescribing information]. 2020. Accessed July 6, 2020. http://www.myalept.com/prescribing-information [Google Scholar]
- 18. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994-2002. [DOI] [PubMed] [Google Scholar]
- 19. Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753-760. [DOI] [PubMed] [Google Scholar]
- 20. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebastchi J, Ajluni N, Neidert A, Oral EA. A report of three cases with acquired generalized lipodystrophy with distinct autoimmune conditions treated with metreleptin. J Clin Endocrinol Metab. 2015;100(11):3967-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ajluni N, Dar M, Xu J, Neidert AH, Oral EA. Efficacy and safety of metreleptin in patients with partial lipodystrophy: lessons from an expanded access program. J Diabetes Metab. 2016;7(3):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown RJ, Meehan CA, Cochran E, et al. Effects of metreleptin in pediatric patients with lipodystrophy. J Clin Endocrinol Metab. 2017;102(5):1511-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oral EA, Ruiz E, Andewelt A, et al. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab. 2002;87(7):3110-3117. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez AJ, Mastronardi CA, Paz-Filho GJ. New advances in the treatment of generalized lipodystrophy: role of metreleptin. Ther Clin Risk Manag. 2015;11:1391-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ali OA, Cook K, Gupta D, et al. Effect of leptin replacement therapy (LRT) on survival and disease progression in generalized and partial Lipodystrophy (GL, PL). Diabetes. 2018;67(Supplement 1):106-LB. [Google Scholar]
- 30. Dhankhar P, Isupov T, Araujo-Vilar D, et al. Estimating quality of life of patients with lipodystrophy. Value Health. 2015;18(3):A292. [Google Scholar]
- 31. Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183-1197. [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association. Standards of medical care in diabetes, 2007. Diabetes Care. 2007;30(Suppl 1):S4-S41. [DOI] [PubMed] [Google Scholar]
- 34. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26(1):107-139. [DOI] [PubMed] [Google Scholar]
- 36. Office for National Statistics (ONS). Population Estimates for UK, England and Wales, Scotland and Northern Ireland 2001-2016. 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Accessed October 4, 2017. [Google Scholar]
- 37. Eurostat: Statistics Explained. Education Attainment Statistics. 2017. https://ec.europa.eu/eurostat/statistics-explained/index.php/Educational_attainment_statistics. Accessed October 4, 2017. [Google Scholar]
- 38. Instituto Nacional de Estadistica (INE). Population Figures and Demographic Censuses: Population and Housing Census 2011. 2013. https://www.ine.es/en/censos2011_datos/cen11_datos_inicio_en.htm. Accessed October 4, 2017. [Google Scholar]
- 39. Instituto Nazionale di Statistica. Population and Household: Demographic Indicators 2016. 2017. http://dati.istat.it/Index.aspx?QueryId=18462&lang=en. Accessed October 1, 2017. [Google Scholar]
- 40. Institut National de la Statistique et des Etudes Economoques (INSEE). Demographic Balance Sheet 2016. 2017. https://www.insee.fr/en/statistiques/2382597?sommaire=2382613. Accessed October 1, 2017. [Google Scholar]
- 41. Destatis Statistisches Bundesamt. Current Population: Population by age groups. 2017. https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/Tables/lrbev01.html. Accessed October 1, 2017. [Google Scholar]
- 42. United States Census Bureau. Table 1. Population by Age and Sex: 2014. 2017. https://www.census.gov/data/tables/2016/demo/age-and-sex/2016-age-sex-composition.html. Accessed October 1, 2017. [Google Scholar]
- 43. United States Census Bureau. Table 1. Educational Attainment of Population 18 Years and Over, by Age, Sex, Race, and Hispanic Origin: 2016-All Races. 2017. https://www.census.gov/data/tables/2017/demo/education-attainment/cps-detailed-tables.html. Accessed October 20, 2017. [Google Scholar]
- 44. Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine. 2019;64(3):500-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ali OA, Cook K, Shampanier KS, Tuttle E, Gerrits C, Brown R. Patient quality of life and benefits of leptin replacement therapy (LRT) in generalized and partial lipodystrophy (GL, PL). Diabetes. 2018;67(Supplement 1):1331-P. [Google Scholar]
- 46. Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31(1):306-318. [DOI] [PubMed] [Google Scholar]
- 47. King MT, Viney R, Simon Pickard A, et al. ; MAUCa Consortium . Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics. 2018;36(2):225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Viney R, Norman R, Brazier J, et al. An Australian discrete choice experiment to value eq-5d health states. Health Econ. 2014;23(6):729-742. [DOI] [PubMed] [Google Scholar]
- 49. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095-1108. [DOI] [PubMed] [Google Scholar]
- 50. Miehle K, Stumvoll M, Fasshauer M, Hierl T. Facial soft tissue volume decreases during metreleptin treatment in patients with partial and generalized lipodystrophy. Endocrine. 2017;58(2):262-266. [DOI] [PubMed] [Google Scholar]
- 51. Vatier C, Kalbasi D, Vantyghem MC, et al. Adherence with metreleptin therapy and health self-perception in patients with lipodystrophic syndromes. Orphanet J Rare Dis. 2019;14(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002;56(6 Suppl):S226-S230. [DOI] [PubMed] [Google Scholar]
- 53. Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400. [DOI] [PubMed] [Google Scholar]
- 55. van Dijk SM, Hallensleben NDL, van Santvoort HC, et al. ; Dutch Pancreatitis Study Group . Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024-2032. [DOI] [PubMed] [Google Scholar]
- 56. Zhang R, Deng L, Jin T, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford). 2019;21(9):1240-1249. [DOI] [PubMed] [Google Scholar]
- 57. Wisløff T, Hagen G, Hamidi V, Movik E, Klemp M, Olsen JA. Estimating QALY gains in applied studies: a review of cost-utility analyses published in 2010. Pharmacoeconomics. 2014;32(4):367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Norman R, Viney R, Brazier J, et al. Valuing SF-6D health states using a discrete choice experiment. Med Decis Making. 2014;34(6):773-786. [DOI] [PubMed] [Google Scholar]
- 59. Robinson A, Spencer A, Moffatt P. A framework for estimating health state utility values within a discrete choice experiment: modeling risky choices. Med Decis Making. 2015;35(3):341-350. [DOI] [PubMed] [Google Scholar]
- 60. Rowen D, Labeit A, Stevens K, et al. Estimating a preference-based single index measuring the quality-of-life impact of self-management for diabetes. Med Decis Making. 2018;38(6):699-707. [DOI] [PubMed] [Google Scholar]
- 61. Stein EM, Yang M, Guerin A, et al. Assessing utility values for treatment-related health states of acute myeloid leukemia in the United States. Health Qual Life Outcomes. 2018;16(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pickard AS, Huynh L, Ivanova JI, et al. Value of transfusion independence in severe aplastic anemia from patients’ perspectives - a discrete choice experiment. J Patient Rep Outcomes. 2017;2(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beck MJ, Fifer S, Rose JM. Can you ever be certain? Reducing hypothetical bias in stated choice experiments via respondent reported choice certainty. Transp Res Part B: Methodological. 2016;89:149-167. [Google Scholar]
- 64. Quaife M, Terris-Prestholt F, Di Tanna GL, Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur J Health Econ. 2018;19(8):1053-1066. [DOI] [PubMed] [Google Scholar]
- 65. Lim S, Jonker MF, Oppe M, Donkers B, Stolk E. Severity-stratified discrete choice experiment designs for health state evaluations. Pharmacoeconomics. 2018;36(11):1377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Norman R, Mulhern B, Viney R. The impact of different DCE-based approaches when anchoring utility scores. Pharmacoeconomics. 2016;34(8):805-814. [DOI] [PubMed] [Google Scholar]
- 67. Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27-35. [DOI] [PubMed] [Google Scholar]
- 68. Sekizkardes H, Cochran E, Malandrino N, Garg A, Brown RJ. Efficacy of metreleptin treatment in familial partial lipodystrophy due to PPARG vs LMNA pathogenic variants. J Clin Endocrinol Metab. 2019;104(8):3068-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.