Abstract
Context
The long-term effects of dipeptidyl peptidase-4 inhibitors on β-cell function and insulin sensitivity in latent autoimmune diabetes in adults (LADA) are unclear.
Objective
To investigate the effects of sitagliptin on β-cell function and insulin sensitivity in LADA patients receiving insulin.
Design and Setting
A randomized controlled trial at the Second Xiangya Hospital.
Methods
Fifty-one patients with LADA were randomized to sitagliptin + insulin (SITA) group or insulin alone (CONT) group for 24 months.
Main Outcome Measures
Fasting C-peptide (FCP), 2-hour postprandial C-peptide (2hCP) during mixed-meal tolerance test, △CP (2hCP – FCP), and updated homeostatic model assessment of β-cell function (HOMA2-B) were determined every 6 months. In 12 subjects, hyperglycemic clamp and hyperinsulinemic euglycemic clamp (HEC) tests were further conducted at 12-month intervals.
Results
During the 24-month follow-up, there were no significant changes in β-cell function in the SITA group, whereas the levels of 2hCP and △CP in the CONT group were reduced at 24 months. Meanwhile, the changes in HOMA2-B from baseline were larger in the SITA group than in the CONT group. At 24 months, first-phase insulin secretion was improved in the SITA group by hyperglycemia clamp, which was higher than in the CONT group (P < .001), while glucose metabolized (M), insulin sensitivity index, and M over logarithmical insulin ratio in HEC were increased in the SITA group (all P < .01 vs baseline), which were higher than in the CONT group.
Conclusion
Compared with insulin intervention alone, sitagliptin plus insulin treatment appeared to maintain β-cell function and improve insulin sensitivity in LADA to some extent.
Keywords: sitagliptin, islet β-cell function, insulin sensitivity, latent autoimmune diabetes in adults
Latent autoimmune diabetes in adults (LADA) is a subtype of autoimmune type 1 diabetes, accounting for approximately 6% to 10% of patients with phenotypic type 2 diabetes (1, 2). Importantly, the decreasing rate of islet β-cell function in LADA is 3 times that in type 2 diabetes, as we have reported (3). Thus, the therapeutic goal for LADA is to suppress autoimmune islet destruction, preserve β-cell function, and prevent complications. However, the optimal intervention strategy for LADA is currently not yet clear. Since insulin therapy had been shown to have a beneficial effect in LADA (4, 5), we tried to explore promising agents plus insulin as a therapeutic strategy for LADA.
Dipeptidyl peptidase-4 (DPP4) inhibitors, a class of oral antidiabetic agents, seem to have the potential to preserve β-cell function in LADA in our pilot study (6). However, due to the small sample size, relatively short follow-up period, and the rough assessment of β-cell function, there are few clinical trials to date that have evaluated the potential long-term effects of DPP-4 inhibitors on islet β-cell function accurately in patients with LADA.
Here, we carried out a 2-year randomized controlled parallel study to investigate the potential long-term effects of the DPP-4 inhibitor sitagliptin on islet β-cell function in patients with LADA receiving insulin. The hyperglycemic clamp test was used to quantify islet β-cell function in some participants.
In addition to islet β-cell function, insulin sensitivity is another important target. Some data regarding DPP-4 inhibitors have implied potential benefits to improve insulin sensitivity in patients with type 2 diabetes, but are still inconclusive at present (7, 8). In this study, we further explored the possible effects of sitagliptin on insulin sensitivity in patients with LADA by hyperinsulinemic euglycemic clamp (HEC) technique.
Materials and Methods
Participants
This was an open-label randomized controlled clinical trial conducted in the Department of Metabolism and Endocrinology at the Second Xiangya Hospital of Central South University. Recruitment occurred between December 2014 and December 2017. The inclusion criteria were as follows: (1) diagnosis of diabetes (World Health Organization 1999 criteria); (2) age 25-70 years; (3) duration of diabetes ≤3 years; (4) insulin independence for at least 6 months postdiagnosis; (5) glutamic acid decarboxylase autoantibody (GADA) positivity; and (6) fasting C-peptide (FCP) ≥0.2 nmol/L. The exclusion criteria included (1) insulin requirements >0.8 U/kg/day; (2) chronic or acute infection within 4 weeks prior to visit 1; (3) history of any malignancy; (4) pregnancy, lactation, or planned pregnancy within 2 years; (5) specific types of diabetes; (6) congestive heart failure; (7) serum creatinine ≥1.5 mg/dL for males and ≥1.4 mg/dL for females; (8) history of pancreatitis; or (9) other severe diseases.
All procedures followed were in accordance with the ethical standards of the Ethics Committee of the Second Xiangya Hospital of Central South University and with the Helsinki Declaration. The study protocol was available at www.clinicaltrials.gov (identifier: NCT01159847). Informed consent was obtained from all participants included in the study.
Study Protocol
Briefly, patients who met the inclusion and exclusion criteria entered a screening period for at least 4 weeks. During this period, the subjects used single insulin therapy to achieve the glycemic target of glycosylated hemoglobin A1c (HbA1c) ≤7.5%. After the screening period, 51 eligible patients entered a 24-month treatment period and were randomized 1:1 to receive insulin therapy with sitagliptin 100 mg daily (SITA group, n = 25) or without sitagliptin (CONT group, n = 26) according to simple randomization procedures (computer-generated random numbers) and were followed at baseline, 6, 12, 18, and 24 months. The allocation sequence was concealed from the researchers and assessing patients in sequentially numbered envelopes. Only after the enrolled participants completed all baseline assessments, the corresponding envelopes were opened and the patients were allocated to the intervention. In our initial protocol, every participant in the study was suggested to receive hyperglycemic clamp and HEC tests at baseline, 12 months, and 24 months. Due to the large volume of blood requirement, frequent blood collection, and time-consuming procedures of the clamp technique, research patients who refused the glucose clamp tests were allowed to participate in the study for better feasibility. Eventually, 12 subjects (6 patients in each group) with excellent compliance finished all clamp tests.
During the study, other antidiabetic agents were not allowed except insulin and sitagliptin. Basal insulin analogues (glargine), premixed insulin (aspart 30 or lispro 25), or basal (glargine) + bolus insulin (aspart) regimens were assigned to patients based on their glycemic profile (9). The insulin doses were adjusted by physicians to reach the glycemic goal without hypoglycemia based on the American Association of Clinical Endocrinologists Comprehensive Diabetes Management Algorithm 2013 (9).
Two prespecified primary outcomes were chosen: (1) the change in β-cell function by the standardized mixed-meal tolerance test (MMTT) at 6, 12, 18, and 24 months of the study; and (2) the change in β-cell function with hyperglycemic clamp and insulin sensitivity with HEC in 12 LADA patients at 12 and 24 months. Secondary parameters included glycemic control measured by HbA1c and possible immunomodulatory effects on T-cell subsets and transcription factors, which have been published elsewhere (10).
To detect a 2-hour postprandial C-peptide (2hCP) difference between 2 groups of 0.56 nmol/L (SD, 0.62 nmol/L) according to our previous data for 12 months, with a 2-sided 5% significance level and a power of 90%, a sample size of 25 patients per group was necessary given an anticipated dropout rate of 10% per year.
GADA Assay
GADA was analyzed by a radioligand assay in duplicate as previously described (11). GADA positivity was defined as a GADA titer ≥18 U/mL, and high-titer GADA was defined as ≥180 U/mL (12). In the Islet Autoantibody Standardization Program 2012, the sensitivity and specificity for the GADA assay were 78.0% and 96.7%, respectively.
Mixed-meal Tolerance Test
Serum glucose and C-peptide levels were measured before and 120 minutes after a standard 543.6-kcal MMTT (44.4% of calories as carbohydrate, 47.7% as fat, and 7.9% as protein). Serum C-peptide levels were detected by a chemiluminescence method using the Adiva Centaur XP immunoassay system (Siemens, Germany). The inter- and intra-assay variation coefficients were 3.7% to 4.1% and 1.0% to 3.3%, respectively. Sitagliptin was held for 7 days (>10 half-lives). Long-acting insulin was withheld the night before visits, and morning doses of insulin were withheld on the day of MMTT, hyperglycemic clamp test (the second day), and HEC test (the third day). The updated homeostasis model assessment (HOMA2) (13) was used to measure β-cell function (HOMA2-B) and insulin resistance (HOMA2-IR) based on FCP and fasting blood glucose (FBG) according to the calculator available at https://www.dtu.ox.ac.uk/homacalculator/index.php.
Assessment of Insulin and HbA1c
Serum insulin was measured by a radioimmunoassay (Boehringer Mannheim, Mannheim, Germany), and HbA1c was measured by automated liquid chromatography (VARIANT-II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA).
Hyperglycemic Clamp Test
At baseline, and 12 and 24 months, 12 subjects completed hyperglycemic clamp tests after fasting for at least 12 hours as described before (14). A rapid infusion of glucose solution (20%) was administered intravenously to raise the blood glucose level to reach the plateau of 13.9 mmol/L. Blood was sampled through an intravenous catheter needle inserted into the median cubital vein of the other arm. The venous blood was arterialized by placing the hand in a 45°C thermostat (15). After infusion of a bolus of glucose (150 mg/kg), blood glucose was measured with a glucose analyzer (glucose/lactic acid analyzer, Biotin, Inc., EKF, Germany) at 2-minute intervals. Thereafter, blood glucose was maintained for 150 minutes at the level of 13.9 mmol/L by adjusting the glucose infusion rate (GIR) according to blood glucose level assessed every 5 minutes. Blood samples were collected at 2-minute intervals during the first 10 minutes and at 10-minute intervals for the remaining 140 minutes for measurement of serum insulin.
To estimate acute β-cell function, the first-phase insulin secretion (1PH) was analyzed as the area under the curve of the insulin levels for the first 10 minutes of the clamp, using the trapezoidal rule. Moreover, the second-phase insulin secretion (2PH) was regarded as the mean insulin concentration for the remaining 140 minutes. In addition, the maximum insulin secretion (MIS) was calculated as the average serum insulin levels between 120 and 150 minutes of the clamp.
Hyperinsulinemic Euglycemic Clamp Test
The HEC test was performed according to the protocol based on DeFronzo et al. (16) and slightly adjusted according to Parvanova et al. (17). Briefly, after an overnight fast, a catheter was inserted into 1 antecubital vein for the insulin and glucose infusion. Regular human insulin (Humulin R, Eli Lilly, Indianapolis, Ind) was infused. A priming insulin dose was administered at a rate of 4 mU/(kg body weight·min) for the first 10 minutes, followed by a constant insulin infusion at a rate of 2 mU/(kg body weight·min) for the remaining 140 minutes of clamping to achieve insulin concentration ~200 mU/L with total suppression of hepatic glucose production (17, 18). Another catheter connected to a T-branch pipe for blood sampling was inserted into the median cubital vein of the other arm. The blood glucose level was maintained with a target of 5 mmol/L for 150 minutes by adjusting the GIR of 20% glucose solution according to blood glucose measured every 5 minutes. Blood samples were drawn for measurement of serum insulin every 10 minutes during 120 to 150 minutes of the clamp test.
The insulin sensitivity, expressed in terms of glucose metabolized (M), was calculated as average value of GIR in steady state of clamp (120-150 minutes) minus space correction as there was no need to correct for urinary loss of glucose in HEC. The insulin sensitivity index (ISI, M/I × 100) was determined as the ratio of the M value in steady state divided by the average serum insulin concentration (I) in steady state multiplied by 100. In addition, M/log I ratio (ie, the ratio of M divided by the natural logarithmical insulin concentration in steady state) was also included to assess insulin sensitivity since the effects of insulin on glucose uptake increase in a logarithmical manner (19, 20).
Safety Assessments
Safety and tolerability were evaluated based on adverse events, vital signs, and physical and laboratory examinations throughout the study. Adverse events were assessed by physicians for the frequency, intensity, and relationship with drugs. Hypoglycemia was confirmed as a blood glucose level ≤3.9 mmol/L with related symptoms.
Statistics
Statistical analysis was performed with IBM SPSS Statistics 20 (IBM Corporation, USA). The normal distribution of data was tested by the 1-sample Kolmogorov–Smirnov test. Continuous data with a normal distribution are presented as mean ± SD or as indicated. Categorical variables are expressed as the number of cases, percentages or as indicated. Comparisons between groups were performed using independent Student’s t-test for variables with a normal distribution. Covariance analysis was adopted to adjust the effects of FBG and low-density lipoprotein (LDL) cholesterol on islet function. A chi-squared test was used to compare categorical variables between groups. The changes in parameters during follow-up were analyzed by repeated measures analysis of variance (ANOVA), and the degree of freedom for F value was adjusted if spherical symmetry was rejected. Multivariate analysis of variance (MANOVA) was used to compare the variables between groups at each follow-up time point. Due to the exploratory analysis of intragroup and intergroup treatment effects, the P value of multiple comparison was not adjusted. A 2-sided P value of <.05 was considered significant.
Results
Baseline Characteristics and Demographics
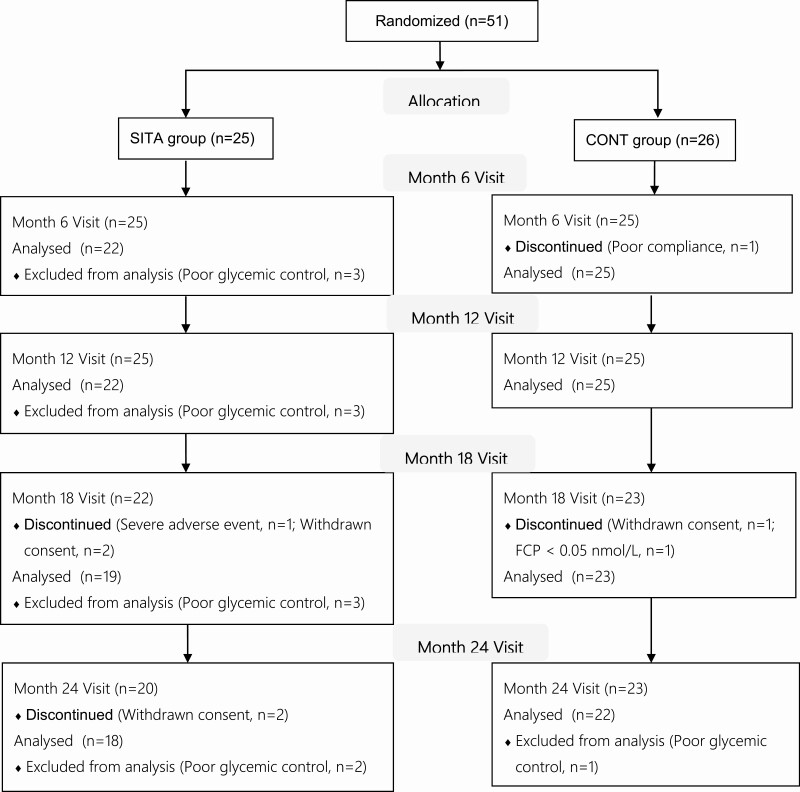
The analysis excluded patients who could not meet the HbA1c control targets in scheduled and extra visits to eliminate the effect of glucose toxicity on islet function. Among 51 randomized cases, 22 subjects in SITA group and 25 patients in CONT group were analyzed at 6 and 12 months, while 18 in SITA group and 22 in CONT group were included for analysis at 24 months (Fig. 1). Twenty subjects (10 cases in each group) received premixed insulin regimens.
Figure 1.
Flow diagram of randomized patients. FCP, fasting C-peptide.
Among the patients who finished 1-year follow-up, the baseline FBG level in SITA group was higher than that in CONT group (Table 1, P = .024). Moreover, the baseline level of LDL cholesterol in SITA group was higher too (P = .036). There were no significant differences in the baseline FCP, 2hCP, and △CP (△CP = 2hCP – FCP) between 2 groups after adjusting for FBG and LDL cholesterol by covariance analysis. No significant differences were observed in other baseline parameters between these 2 groups.
Table 1.
Baseline characteristics and demographics
| SITA group (n=22) | CONT group (n=25) | P value | |
|---|---|---|---|
| Men/women | 13/9 | 15/10 | .985 |
| Age (years) | 48.2 ± 11.5 | 48.2 ± 12.3 | .985 |
| Duration of diabetes (years) | 1.8 ± 1.4 | 2.3 ± 1.8 | .310 |
| Daily insulin does (U) | 10.9 ± 10.6 | 13.4 ± 9.3 | .384 |
| Insulin dose (U/kg/day) | 0.18 ± 0.17 | 0.22 ± 0.15 | .511 |
| BMI (kg/m2) | 23.2 ± 2.8 | 23.8 ± 2.6 | .433 |
| Waist circumference (cm) | 81.8 ± 10.3 | 84.1 ± 6.8 | .361 |
| Waist circumference in men (cm) | 84.0 ± 12.0 | 85.5 ± 6.8 | .682 |
| Waist circumference in women (cm) | 78.6 ± 6.6 | 82.1 ± 6.6 | .273 |
| Hip circumference (cm) | 94.6 ± 5.7 | 95.9 ± 5.2 | .424 |
| Waist-to-hip ratio | 0.86 ± 0.07 | 0.88 ± 0.06 | .435 |
| Waist-to-hip ratio in men | 0.87 ± 0.08 | 0.88 ± 0.07 | .667 |
| Waist-to-hip ratio in women | 0.85 ± 0.06 | 0.87 ± 0.05 | .468 |
| Systolic blood pressure (mmHg) | 120 ± 12 | 119 ± 17 | .697 |
| Diastolic blood pressure (mmHg) | 75 ± 10 | 75 ± 12 | .915 |
| GADA titer (U/mL) | 469.5 ± 374.9 | 435.8 ± 399.8 | .768 |
| High-titer GADA (%) | 63.6 | 56.0 | .595 |
| FBG (mmol/L) | 6.8 ± 1.3a | 6.1 ± 1.0 | .024 |
| 2hBG (mmol/L) | 12.5 ± 3.8 | 12.3 ± 4.2 | .863 |
| HbA1c (%) | 6.3 ± 0.7 | 6.3 ± 0.8 | .907 |
| FCP (nmol/L) | 0.45 ± 0.17 | 0.43 ± 0.19 | .796b |
| 2hCP (nmol/L) | 1.60 ± 0.62 | 1.65 ± 0.70 | .991b |
| △CP (nmol/L) | 1.15 ± 0.55 | 1.22 ± 0.62 | .926b |
| HOMA2-B (%) | 55.0 ± 22.7 | 68.4 ± 36.0 | .139 |
| HOMA2-IR | 1.10 ± 0.42 | 1.00 ± 0.45 | .425 |
| Triglyceride (mmol/L) | 1.38 ± 0.82 | 1.34 ± 0.84 | .848 |
| Total cholesterol (mmol/L) | 4.79 ± 0.74 | 4.57 ± 0.75 | .324 |
| HDL cholesterol (mmol/L) | 1.28 ± 0.27 | 1.39 ± 0.31 | .227 |
| HDL cholesterol in men (mmol/L) | 1.19 ± 0.26 | 1.32 ± 0.35 | .280 |
| HDL cholesterol in women (mmol/L) | 1.42 ± 0.24 | 1.49 ± 0.21 | .500 |
| LDL cholesterol (mmol/L) | 3.00 ± 0.71a | 2.54 ± 0.74 | .036 |
Data are shown as mean ± SD, frequency, and percentage.
Abbreviations: BMI, body mass index; GADA, glutamic acid decarboxylase autoantibody; FBG, fasting blood glucose; 2hBG, 2-hour postprandial blood glucose; HbA1c, glycosylated hemoglobin A1c; FCP, fasting C-peptide; 2hCP, 2-hour postprandial C-peptide; △CP, 2hCP – FCP; HOMA2-B, updated homeostasis model assessment of β-cell function; HOMA2-IR, updated homeostasis model assessment of insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a P < .05 vs CONT group (Student t-test for variables with normal distribution and chi-squared test for categorical variables).
b Adjustment for FBG and LDL cholesterol (covariance analysis).
Glycemic Control
There were no significant differences in HbA1c levels between the 2 groups at each follow-up time point. Compared with the baseline, there were no significant differences in HbA1c levels at different follow-up time points in both groups (Table 2, all P > .05).
Table 2.
Changes of glycemic control, BMI and islet β-cell function evaluated by MMTT
| 6 months | 12 months | 18 months | 24 months | |||||
|---|---|---|---|---|---|---|---|---|
| SITA group | CONT group | SITA group | CONT group | SITA group | CONT group | SITA group | CONT group | |
| n | 22 | 25 | 22 | 25 | 19 | 23 | 18 | 22 |
| HbA1c (%/mmol/mol) | ||||||||
| Baseline | 6.3 ± 0.7/45 ± 8 | 6.3 ± 0.8/45 ± 9 | 6.3 ± 0.7/45 ± 8 | 6.3 ± 0.8/45 ± 9 | 6.2 ± 0.7/44 ± 8 | 6.2 ± 0.8/44 ± 9 | 6.1 ± 0.7/43 ± 8 | 6.2 ± 0.8/44 ± 9 |
| Follow-up | 6.3 ± 0.7/45 ± 8 | 6.5 ± 0.8/48 ± 9 | 6.3 ± 0.9/45 ± 10 | 6.4 ± 0.8/46 ± 9 | 6.1 ± 0.8/43 ± 9 | 6.5 ± 0.8/48 ± 9 | 6.4 ± 0.7/46 ± 8 | 6.4 ± 0.7/46 ± 8 |
| Change from baseline | 0.1 ± 0.7/1 ± 8 | 0.2 ± 1.0/2 ± 11 | 0.0 ± 0.9/0 ± 10 | 0.1 ± 1.0/1 ± 11 | –0.1 ± 0.6/–1 ± 7 | 0.3 ± 0.9/3 ± 10 | 0.2 ± 0.6/2 ± 7 | 0.2 ± 0.8/2 ± 9 |
| BMI (kg/m 2 ) | ||||||||
| Baseline | 23.2 ± 2.8 | 23.8 ± 2.6 | 23.2 ± 2.8 | 23.8 ± 2.6 | 23.4 ± 2.9 | 23.9 ± 2.6 | 23.4 ± 3.0 | 23.8 ± 2.6 |
| Follow-up | 23.0 ± 2.6 | 24.0 ± 2.8 | 22.9 ± 2.7 | 24.1 ± 2.5 | 23.3 ± 2.8 | 24.3 ± 2.7 | 23.2 ± 2.6 | 24.4 ± 2.9 |
| Change from baseline | –0.2 ± 1.1 | 0.2 ± 1.4 | –0.3 ± 1.4 | 0.3 ± 1.3 | –0.2 ± 1.0 | 0.4 ± 1.6 | –0.2 ± 1.1 | 0.6 ± 1.4 |
| FCP (nmol/L) | ||||||||
| Baseline | 0.45 ± 0.17 | 0.43 ± 0.19 | 0.45 ± 0.17 | 0.43 ± 0.19 | 0.45 ± 0.18 | 0.43 ± 0.19 | 0.45 ± 0.18 | 0.42 ± 0.19 |
| Follow-up | 0.39 ± 0.15 | 0.39 ± 0.21 | 0.38 ± 0.19 | 0.37 ± 0.21 | 0.42 ± 0.18 | 0.38 ± 0.20 | 0.45 ± 0.30 | 0.40 ± 0.24 |
| Change from baseline | –0.05 ± 0.16 | –0.04 ± 0.19 | –0.07 ± 0.15 | –0.06 ± 0.21 | 0.02 ± 0.42 | –0.02 ± 0.46 | 0.00 ± 0.25 | –0.02 ± 0.25 |
| HOMA2-B (%) | ||||||||
| Baseline | 55.0 ± 22.7 | 68.4 ± 36.0 | 55.0 ± 22.7 | 68.4 ± 36.0 | 55.9 ± 23.9 | 70.7 ± 36.4 | 57.1 ± 24.0 | 70.8 ± 37.3 |
| Follow-up | 55.7 ± 21.5 | 55.0 ± 28.7 | 59.8 ± 23.2 | 54.2 ± 26.6 | 68.2 ± 30.7 | 51.7 ± 28.9c | 69.7 ± 39.5 | 51.8 ± 29.9 |
| Change from baseline | 0.7 ± 24.6 | –13.4 ± 31.9 | 4.8 ± 23.1a | –14.2 ± 38.5 | 12.3 ± 25.5b | –19.0 ± 39.8 | 12.6 ± 41.7a | –19.0 ± 41.8 |
| 2hCP (nmol/L) | ||||||||
| Baseline | 1.60 ± 0.62 | 1.65 ± 0.70 | 1.60 ± 0.62 | 1.65 ± 0.70 | 1.61 ± 0.66 | 1.70 ± 0.70 | 1.61 ± 0.68 | 1.68 ± 0.71 |
| Follow-up | 1.43 ± 0.57 | 1.37 ± 0.73c | 1.40 ± 0.71 | 1.16 ± 0.66e | 1.55 ± 0.81 | 1.35 ± 0.68c | 1.38 ± 0.64 | 1.39 ± 0.71c |
| Change from baseline | –0.17 ± 0.50 | –0.28 ± 0.66 | –0.21 ± 0.44 | –0.49 ± 0.60 | –0.06 ± 0.55 | –0.35 ± 0.60 | –0.23 ± 0.50 | –0.29 ± 0.53 |
| △CP (nmol/L) | ||||||||
| Baseline | 1.15 ± 0.55 | 1.22 ± 0.62 | 1.15 ± 0.55 | 1.22 ± 0.62 | 1.16 ± 0.58 | 1.27 ± 0.63 | 1.16 ± 0.60 | 1.25 ± 0.64 |
| Follow-up | 1.03 ± 0.49 | 0.97 ± 0.63 | 1.02 ± 0.56 | 0.79 ± 0.56d | 1.12 ± 0.73 | 0.97 ± 0.59c | 0.92 ± 0.42 | 0.98 ± 0.56c |
| Change from baseline | –0.12 ± 0.50 | –0.25 ± 0.62 | –0.13 ± 0.40a | –0.43 ± 0.53 | –0.04 ± 0.53 | –0.31 ± 0.58 | –0.24 ± 0.50 | –0.27 ± 0.52 |
Data are expressed as mean ± SD.
Abbreviations: BMI, body mass index; HbA1c, glycosylated hemoglobin A1c; FCP, fasting C-peptide; HOMA2-B, updated homeostasis model assessment of β-cell function; 2hCP, 2-hour postprandial C-peptide; △CP, 2hCP – FCP.
a P < 0.05, bP < 0.01, vs. CONT group (MANOVA), cP < 0.05, dP < 0.01, and eP < 0.001 vs the baseline in CONT group (repeated measures ANOVA).
Body Mass Index
No significant differences were observed in body mass index (BMI) between the 2 groups at each follow-up time point and there were no significant differences in BMI at each follow-up time point in both groups compared with the baseline (Table 2, all P > .05). Changes in BMI at 24 months from baseline in the 2 groups seemed different, but no statistical significance was found (P = .051).
Daily Insulin Dose
There were no significant differences in the insulin requirements (U/kg/day) between the 2 groups at each follow-up time point. Moreover, compared with those at baseline, no significant differences in insulin requirements were found at different follow-up visits in both groups (Table S1 in (21)).
Evaluation of Islet β-cell Function by MMTT
Among the patients who completed the 1-year follow-up, changes in islet β-cell function in the SITA group (n = 22) were not noted, while the levels of 2hCP in the CONT group (n = 25) significantly decreased at 6 months (Table 2, mean [95% CI] change from baseline: –0.28 [–0.56, –0.01] nmol/L, P = .043) and 12 months (–0.49 [–0.73, –0.24] nmol/L, P < .001). Likewise, the levels of △CP declined at 12 months (–0.43 [–0.65, –0.21] nmol/L, P = .001). Furthermore, changes in △CP as well as HOMA2-B from baseline in the SITA group were significantly higher than those in the CONT group (difference [95% CI] in changes of △CP from baseline: 0.30 [0.01, 0.57] nmol/L, P = .042; difference [95% CI] in changes of HOMA2-B from baseline: 19.0 [0.1, 38.0] %, P = .049)
Based on the results from patients who completed 1.5-year follow-up, there were no notable changes in islet β-cell function in the SITA group (n = 19), while the HOMA2-B, 2hCP and △CP in the CONT group (n = 23) were significantly reduced from baseline at 18 months (mean [95% CI] change from baseline in HOMA2-B: –19 [–36.2, –1.8] %, P = .032; 2hCP: –0.35 [–0.61, –0.09] nmol/L, P = .010; △CP: –0.30 [–0.55, –0.06] nmol/L, P = .019). The changes in HOMA2-B from baseline in the SITA group were significantly higher than those in the CONT group (difference [95% CI]: 31.3 [9.9, 52.6] %, P = .005).
Among the patients who finished the 2-year follow-up, there was still no significant change in islet β-cell function in the SITA group (n = 18) from baseline; in the CONT group (n = 22), 2hCP and △CP decreased gradually at 24 months from baseline (mean [95% CI] change from baseline in 2hCP: –0.29 [–0.53, –0.06] nmol/L, P = .018; △CP: –0.27 [–0.50, –0.04] nmol/L, P = .024). The changes in HOMA2-B from baseline in the SITA group were higher than those in the CONT group at 24 months (difference [95% CI]: 31.6 [4.6, 58.4] %, P = .023).
Evaluation of Islet β-cell Function by Hyperglycemic Clamp
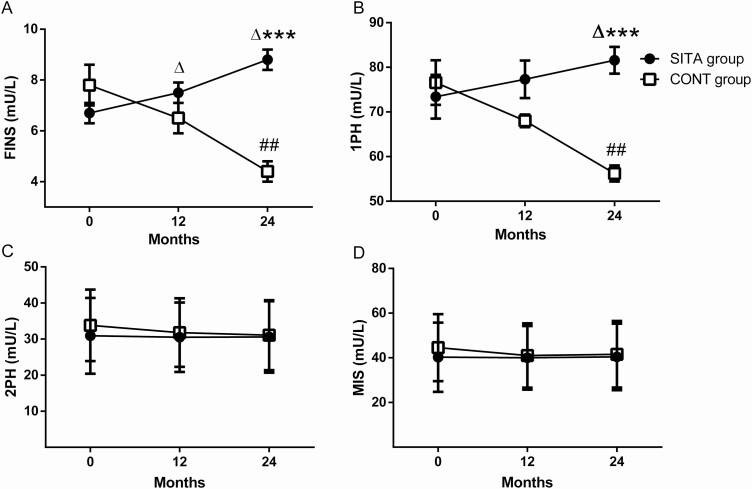
At baseline, no significant difference in demographic and clinical characteristics were observed between the 2 groups who completed the hyperglycemic clamp and HEC tests (6 cases in each group, data not shown). Among these 12 patients, 1 in the SITA group and 2 cases in the CONT group had premixed insulin therapy. The detailed figures of insulin levels during hyperglycemic clamp at baseline, 12 months, and 24 months for each group are presented elsewhere (21). The levels of fasting insulin (FINS) in the SITA group increased continually from 6.7 ± 1.1 mU/L to 7.5 ± 1.0 mU/L at 12 months (Fig. 2A, mean [95% CI] change from baseline: 0.8 [0, 1.6] mU/L, P = .049) and further to 8.8 ± 1.0 mU/L at 24 months (2.1 [0.6, 3.6] mU/L, P = 0.017). In contrast, the FINS levels in CONT group decreased significantly from baseline to 24 months (4.4 ± 1.1 vs 7.8 ± 2.1 mU/L, –3.4 [–5.1, –1.7] mU/L, P = .004). At 24 months, the FINS level in SITA group was significantly higher than that in CONT group (difference [95% CI]: 4.4 [3.0, 5.7] mU/L, P < .001). Similarly, the 1PH insulin response increased from baseline to 24 months in SITA group (Fig. 2B, 73.4 ± 12.1 vs 81.6 ± 7.4 mU/L, 8.2 [0.5, 15.9] mU/L, P = .040), but declined significantly in CONT group (baseline, 76.6 ± 12.2 vs 24 months, 56.2 ± 4.5 mU/L; –20.4 [–29.4, –11.4] mU/L, P = .002). The levels of 1PH in SITA group were higher than those in CONT group at 24 months (difference [95% CI]: 25.4 [17.6, 33.3] mU/L, P < .001). Whereas there were no significant changes in the 2PH and MIS in 2 groups during follow-up (Figs. 2C and 2D, both P > .05).
Figure 2.
Islet β-cell function evaluated by hyperglycemic clamp. Black circles: SITA group (n=6); white squares: CONT group (n=6). Fasting insulin (FINS, A), the first-phase insulin secretion (1PH, B), the second-phase insulin secretion (2PH, C) and the maximum insulin secretion (MIS, D) were calculated at the indicated month. Data were expressed as mean and SEM. *P < .05, **P < .01, ***P < .001 vs CONT group (MANOVA), △P < .05 vs the baseline in SITA group and #P < .05, ##P < 0.01 vs the baseline in CONT group (repeated measures ANOVA).
Evaluation of Insulin Sensitivity by HEC
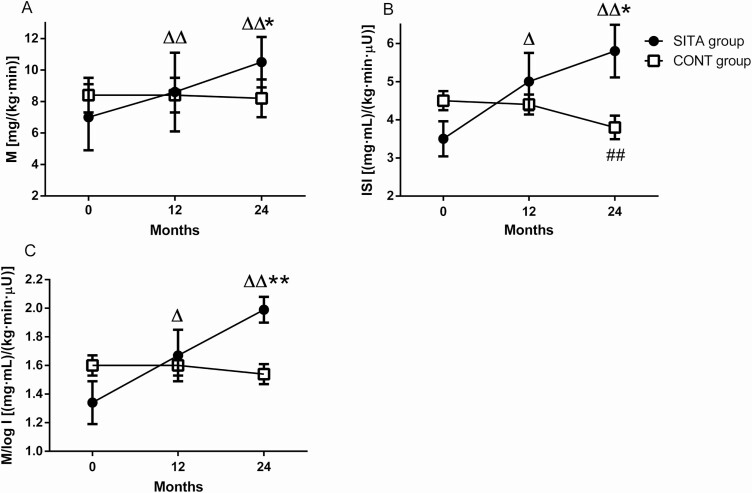
The detail figures of insulin levels and GIR during HEC at baseline, 12 months, and 24 months for each group are shown elsewhere (21). In the SITA group, M increased gradually from 7.0 ± 2.1 mg/(kg·min) to 8.6 ± 2.5 mg/(kg·min) at 12 months (Fig. 3A, mean [95% CI] change from baseline: 1.6 [0.7, 2.6] mg/[kg·min], P = .007) and to 10.4 ± 1.6 mg/(kg·min) at 24 months (3.4 [1.4, 5.5] mg/[kg·min]), P = .007), while M in the CONT group did not change during follow-up. Moreover, M levels in SITA group were much higher than those in the CONT group at 24 months (10.4 ± 1.6 vs 8.2 ± 1.2 mg/[kg·min], difference [95% CI]: 2.2 [0.4, 4.0] mg/[kg·min], P = .019). Likewise, the ISI levels in SITA group increased from baseline to 12 months (Fig. 3B, 5.0 ± 1.8 vs 3.5 ± 1.1 [mg·mL]/[kg·min·μU], mean [95% CI] change from baseline: 1.5 [0.3, 2.7] [mg·mL]/[kg·min·μU], P = .027) and further to 5.8 ± 1.7 (mg·mL)/(kg·min·μU) at 24 months (2.3 [0.9, 3.6] [mg·mL]/[kg·min·μU], P = .008), but decreased significantly in the CONT group from baseline to 24 months (3.8 ± 0.8 vs 4.5 ± 0.6 [mg·mL]/[kg·min·μU], –0.7 [–1.1, –0.4] [mg·mL]/[kg·min·μU], P = .003). Moreover, the ISI levels in SITA group were much higher than those in the CONT group at 24 months (difference [95% CI]: 2.0 [0.3, 3.7] [mg·mL]/[kg·min·μU], P = .024). Similarly, the M/log I ratio in the CONT group did not alter during follow-up while M/log I in SITA group increased from baseline to 12 months (Fig. 3C, 1.7 ± 0.4 vs 1.3 ± 0.4 [mg·mL]/[kg·min·μU], mean [95% CI] change from baseline: 0.3 [0.1, 0.5] [mg·mL]/[kg·min·μU], P = .011) and further to 24 months (0.7 [0.3, 1.0] [mg·mL]/[kg·min·μU], P = .004); it was higher than that in CONT group (2.0 ± 0.2 vs 1.5 ± 0.2 [mg·mL]/[kg·min·μU], difference [95% CI]: 0.5 [0.2, 0.7] [mg·mL]/[kg·min·μU], P=.004).
Figure 3.
Insulin sensitivity evaluated by hyperinsulinemic euglycemic clamp. Black circles: SITA group (n=6); white squares: CONT group (n=6). Glucose metabolized (M, A), insulin sensitivity index (ISI, B) and M/log I ratio were calculated at the indicated month. Data were presented as mean and SEM. *P < .05 vs CONT group (MANOVA), △P < .05, △△P < .01 vs the baseline in SITA group and #P < .05, ##P < .01 vs the baseline in CONT group (repeated measures ANOVA).
Safety and Tolerability
One patient in the SITA group dropped out at 18 months due to severe elevation of transaminase (hepatitis B virus related), which returned to a normal level after entecavir therapy. No other severe adverse event or serious adverse event was reported. During 24-month follow-up, 2 cases in the SITA group (one at 18 months and the other case at 24-month follow-up) and 1 in the CONT group (at 12 months) had detected hypoglycemia (blood glucose level <3.9 mmol/L).
Discussion
This study suggested that sitagliptin combined with insulin therapy seemed to delay the decline of islet β-cell function and improve insulin sensitivity in patients with LADA compared with insulin treatment alone. To our knowledge, this is the first prospective study using the glucose clamp technique to evaluate the effects of DPP-4 inhibitors on insulin secretion and insulin resistance in patients with LADA.
In this study, the baseline islet β-cell function (FCP, HOMA2-B, 2hCP and △CP) as well as insulin resistance (HOMA2-IR) were comparable between 2 groups. During the follow-up period, both groups were in good glycemic control, eliminating the effect of glucose toxicity on islet function and the possibility of islet β-cell resistance to glucagon-like peptide-1 (GLP-1) under poor glycemic control (22). In addition, 1-week sitagliptin washout (>10 half-lives) before the visits eliminated the potential bias in the assessment of islet function by acute stimulation of insulin secretion via incretin in the SITA group (23). Currently, there are several methods to measure islet β-cell function. (1) The hyperglycemic clamp test, which is considered as the standard method to evaluate islet β-cell function, is only suitable for small-sample scientific research due to the labor-consuming, time-costing, high-cost technique. (2) Intravenous glucose tolerance test is not affected by gastrointestinal hormones and individual absorption but with complex procedures, frequent blood collection and poor repeatability. (3) Minimum model has similar disadvantages with hyperglycemic clamp. (4) Oral glucose tolerance test is simple and suitable for epidemiological studies. However, it is affected by glucose absorption, thus is not accurate enough, and it is difficult to reflect the first-phase insulin secretion. (5) Arginine stimulation test and glucagon stimulation test are simple methods but unable to measure the second-phase insulin secretion and to estimate the response of islet β-cells to glucose. (6) MMTT, containing more stimuli than oral glucose tolerance test, simulates insulin secretion under more physiological conditions. However, the load of MMTT is needed to be standardized. (7) HOMA is simple to evaluate β-cell function and insulin sensitivity, thus it is of great value in epidemiological studies. But sometimes it may overestimate the islet β-cell function. In a word, there is not any perfect method to quantify β-cell function to date given that insulin secretion is a dynamic and nonlinear process in addition to its synthesis and storage processes. Herein, we attempted to evaluate β-cell function by the combined application of several methods, such as FCP, HOMA2-B, MMTT 2hCP, and △CP as well as hyperglycemic clamp test. In general, the MMTT is the recommended test and C-peptide, not affected by exogenous insulin, is the appropriate outcome measure for the assessment of β-cell function in intervention trials in type 1 diabetes (24-27). The 2hCP and △CP values during MMTT may reflect the secretory capacity in response to a mixed meal, and our group has utilized 2hCP, △CP as well as FCP to measure the effects of oral antidiabetic agents including rosiglitazone (28, 29), sitagliptin (6) and saxagliptin or combined with vitamin D3 (30) on β-cell function in a series of clinical trials in patients with LADA. FCP is frequently used in clinical practice due to its good correlation with MMTT or glucagon stimulated C-peptide (31-34) and the ability to predict β-cell function failure in autoimmune type 1 diabetes (35, 36). Moreover, FCP is not affected by gastrointestinal status compared with 2hCP and △CP, but it is affected by fasting blood glucose. Matthews et al. updated the homeostasis model assessment in 1998 (13), making it closely related with glucose clamp test, minimum model etc. In our study, although there was no significant difference in FCP between the 2 groups during follow-up, the parameters of HOMA2-B, 2hCP, and △CP consistently demonstrated the protective benefit of sitagliptin on islet β-cell function in patients with LADA. In addition, mathematical model could extract more useful information on overall β-cell function (37-40), but the outcomes varied. The inability to develop a mathematical model of insulin secretion was 1 of our limits. Herein, our results of the hyperglycemic clamp test displayed that sitagliptin combined with insulin therapy could improve the first-phase insulin secretion and the reserve function of islet β-cells. Some studies have revealed that GLP-1 analogue exenatide could improve the acute insulin response in intravenous glucose tolerance test in patients with type 2 diabetes (41). However, it is not clear whether sitagliptin improves 1PH indirectly by increasing active GLP-1. Here, both MMTT and hyperglycemic clamp test showed that sitagliptin plus insulin treatment appeared to halt the decline of islet β-cell function in patients with LADA compared with insulin alone. Similarly, in the few clinical trials of DPP-4 inhibitors in LADA (saxagliptin: 6 months (42), 12 months (30); sitagliptin: 12 months (6), 21 months (43), and 48 months (44); linagliptin (45): 24 months), except the Norway study (43) and our pilot study of saxagliptin (30) (further discussion below), these studies consistently revealed the protective effects of DPP-4 inhibitors on islet function in LADA. However, the above trials may have limited clinical significance because of short follow-up periods (6, 30, 42), small sample sizes (44, 45), historical control (44), and significant different baseline FCP levels between the groups (45), and so on. In our study, the longitudinal follow-up was up to 24 months, and a total of 40 patients with LADA were followed at 24 months.
A randomized controlled clinical trial conducted in Norway and published in 2019 (43) (hereafter referred to as Norway study) did not show an advantage on β-cell function in 32 LADA patients taking sitagliptin compared with 32 LADA patients receiving insulin, which was not completely consistent with our study. The reasons for this inconsistency may include the following: (1) distinct treatment protocols: the Norway study did a direct comparison between insulin and sitagliptin, and insulin was not permitted in the sitagliptin group; (2) different methods for evaluating islet function: previous studies have indicated that the glucagon stimulation test conducted in the Norway study appeared to be less sensitive and less reproducible than the MMTT we carried out (24) in addition to the hyperglycemic clamp test applied in our study; and (3) different study population: the participants in the Norway study seemed to have larger BMIs, representing varying degrees of insulin resistance, which could affect the efficacy of DPP-4 inhibitors (46). Nevertheless, from another point of view, the Norway study showed that the protective effects of single sitagliptin therapy on β-cell function in LADA may be comparable with insulin since the current evidence-based medicine has confirmed the benefits of insulin on β-cell function in LADA (4, 5).
Interestingly, in our recent pilot study, compared with conventional therapy (metformin and/or insulin), vitamin D3 plus saxagliptin and conventional therapy could maintain β-cell function in LADA rather than saxagliptin plus conventional therapy (group B) (30). In addition to different study drugs, relatively higher levels of HbA1c in group B than those in our study implied that influences of glucose toxicity on islet function might account for this disparity and saxagliptin without Vitamin D3 probably could not totally reverse this glucotoxicity to maintain β-cell function.
Although sitagliptin seem to have the potential to preserve β-cell function in LADA, the benefit of sitagliptin may not be great enough to make clinically relevant changes, such as the daily insulin requirements. We supposed, besides β-cell function and insulin sensitivity, daily insulin requirements were affected by many other factors, such as exercises and diets, both of which were difficult to be controlled in the long–term clinical trials. Larger-scale studies with restricting the potential confounding factors (exercises, diets, etc.) are required for confirmation in the future.
In our study, no statistical differences were observed in the proportions of patients receiving premixed insulin between SITA and CONT groups; or between the subgroups having clamp tests. In addition, there were at least 15 hours between the last injection of premixed insulin and the blood sampling. However, it is worth mentioning that our study didn’t eliminate the confounding effect of premixed insulin on the β-cell function related measurements due to their pharmacokinetic properties even though the potential influence may be minimal and similar between the two groups. Future studies with further restrictions of insulin are required, such as premixed insulin analogues are withheld the night before visits.
In addition, our study showed that the M and ISI levels in the SITA group were significantly higher than those in the CONT group at 24 months, suggesting the benefits of sitagliptin for improving insulin sensitivity in LADA. Since the effect of sitagliptin combined with insulin on BMI was neutral (see Table 2), the changes in insulin sensitivity in SITA group should not be interpreted by weight loss. Previous studies have found that DPP-4 inhibitors can ameliorate insulin resistance in diabetic animal models (47, 48) and patients with type 2 diabetes (7), which may be related to its anti-inflammatory action (49-51). However, the small sample size (12 subjects; 6 patients in each group) was 1 of the limitations of our study as a result of the complex procedure of glucose clamp technique and frequent blood collection. Moreover, it is worth mentioning that we followed the uncommon HEC protocol from Parvanova et al. (17), but not from DeFronzo et al. (16), which would also be one of the limitations. Larger-scale studies are needed for confirmation in the future.
DPP-4 inhibitors are known to be multitarget agents. At present, the specific mechanism of islet β-cell preservation and insulin sensitivity improvement in LADA has not been fully elucidated. DPP4 (also known as CD26) is widely distributed on the surface of endothelial cells, epithelial cells, natural killer cells, lymphocytes, and monocytes. DPP4/CD26 not only has peptidase activity, but also plays an important role in immune regulation. As a cell surface antigen, CD26 has a costimulatory function on T-cell activation and proliferation (52). Our group’s recent study implied that sitagliptin could modulate cellular immunity by increasing the proportion of T helper 2 cells, decreasing the proportion of T helper 17 cells in LADA patients and down-regulating the messenger RNA expression of T box expressed in T cells and related orphan receptor C in LADA patients (10).
In conclusion, compared with insulin alone, sitagliptin combined with insulin therapy appeared to delay the decline of islet β-cell function and improve insulin sensitivity in patients with LADA to some extent. Our findings provide clues for the treatment of LADA.
Acknowledgments
We are indebted to the patients with LADA participating in this study. We also extend our sincere thanks to Prof. Zhengqi Liu from University of Virginia Health System, USA, Prof. Jian Zhou, and Prof. Yuqian Bao from Shanghai Jiao Tong University Affiliated Six People’s Hospital, China, and Dr. Yijun Li from the General Hospital of People’s Liberation Army for suggestions in manuscript revision process. The authors thank all other colleagues from the department of Metabolism and Endocrinology, the Second Xiangya Hospital, Central South University who greatly assisted us in the study.
Financial Support: This research was supported by the National Key Research and Development Program of China (grant no. 2018YFC2001005, 2018YFC1315603) and European Foundation for the Study of Diabetes (EFSD/CDS/Lilly Collaborative Grant Programme-2009).
Clinical Trial Information: NCT01159847, clinicaltrials.gov registered at July 12, 2010.
Glossary
Abbreviations
- 1PH
first-phase insulin secretion
- 2hCP
2-hour postprandial C-peptide
- 2PH
second-phase insulin secretion
- ANOVA
analysis of variance
- BMI
body mass index
- DPP4
dipeptidyl peptidase-4
- FBG
fasting blood glucose
- FCP
fasting C-peptide
- FINS
fasting insulin
- GADA
glutamic acid decarboxylase autoantibody
- GIR
glucose infusion rate
- GLP
glucagon-like peptide-1
- HbA1c
glycosylated hemoglobin A1c
- HEC
hyperinsulinemic euglycemic clamp
- HOMA2-B
homeostatic model assessment of β-cell function
- I
serum insulin concentration
- IR
insulin resistance
- ISI
insulin sensitivity index
- LADA
latent autoimmune diabetes in adults
- LDL
low-density lipoprotein
- M
glucose metabolized
- MANOVA
multivariate analysis of variance
- MIS
, maximum insulin secretion
- MMTT
mixed-meal tolerance test
- SITA
sitagliptin + insulin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Xiang Y, Huang G, Zhu Y, et al. ; China National D, Metabolic Disorders Study G. Identification of autoimmune type 1 diabetes and multiple organ-specific autoantibodies in adult-onset non-insulin-requiring diabetes in China: a population-based multicentre nationwide survey. Diabetes Obes Metab. 2019;21(4):893-902. [DOI] [PubMed] [Google Scholar]
- 2. Hawa MI, Kolb H, Schloot N, et al. ; Action LADA consortium . Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care. 2013;36(4):908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang L, Zhou ZG, Huang G, Ouyang LL, Li X, Yan X. Six-year follow-up of pancreatic beta cell function in adults with latent autoimmune diabetes. World J Gastroenterol. 2005;11(19):2900-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive beta-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45(5):622-626. [DOI] [PubMed] [Google Scholar]
- 5. Maruyama T, Tanaka S, Shimada A, et al. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008;93(6):2115-2121. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y, Yang L, Xiang Y, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin maintains β-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab. 2014;99(5):E876-E880. [DOI] [PubMed] [Google Scholar]
- 7. Tian M, Liang Z, Liu R, et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: a randomized trial. Eur J Endocrinol. 2016;174(2):147-155. [DOI] [PubMed] [Google Scholar]
- 8. Lyu X, Zhu X, Zhao B, et al. Effects of dipeptidyl peptidase-4 inhibitors on beta-cell function and insulin resistance in type 2 diabetes: meta-analysis of randomized controlled trials. Sci Rep. 2017;7:44865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garber AJ, Abrahamson MJ, Barzilay JI, et al. ; American Association of Clinical Endocrinologists . AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19(2):327-336. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Yang L, Cheng Y, et al. Altered T-cell subsets and transcription factors in latent autoimmune diabetes in adults taking sitagliptin, a dipeptidyl peptidase-4 inhibitor: A 1-year open-label randomized controlled trial. J Diabetes Investig. 2019;10(2):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen JS, Hejnaes KR, Moody A, et al. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes. 1994;43(3):459-467. [DOI] [PubMed] [Google Scholar]
- 12. Zhou Z, Xiang Y, Ji L, et al. ; LADA China Study Group . Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013;62(2):543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191-2192. [DOI] [PubMed] [Google Scholar]
- 14. Yang L, Liu X, Liang H, Cheng Y, Huang G, Zhou Z. Pathophysiological characteristics in patients with latent autoimmune diabetes in adults using clamp tests: evidence of a continuous disease spectrum of diabetes. Acta Diabetol. 2019;56(11):1217-1224. [DOI] [PubMed] [Google Scholar]
- 15. Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30(9):936-940. [DOI] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-E223. [DOI] [PubMed] [Google Scholar]
- 17. Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456-1462. [DOI] [PubMed] [Google Scholar]
- 18. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165-1174. [DOI] [PubMed] [Google Scholar]
- 19. Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderwald C, Gastaldelli A, Tura A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. 2011;96(2):515-524. [DOI] [PubMed] [Google Scholar]
- 21. Yang L, LiangH, LiuX, et al. Supplementary data (Islet function in sitagliptin-treated LADA). figshare. Online resource. Deposited 9 November 2020. 10.6084/m9.figshare.13207283.v1. [DOI]
- 22. Højberg PV, Zander M, Vilsbøll T, et al. Near normalisation of blood glucose improves the potentiating effect of GLP-1 on glucose-induced insulin secretion in patients with type 2 diabetes. Diabetologia. 2008;51(4):632-640. [DOI] [PubMed] [Google Scholar]
- 23. Gudipaty L, Rosenfeld NK, Fuller CS, Gallop R, Schutta MH, Rickels MR. Effect of exenatide, sitagliptin, or glimepiride on β-cell secretory capacity in early type 2 diabetes. Diabetes Care. 2014;37(9):2451-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. ; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31(10):1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Besser RE, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care. 2013;36(2):195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenbaum CJ, Harrison LC; Immunology of Diabetes Society . Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52(5):1059-1065. [DOI] [PubMed] [Google Scholar]
- 27. Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53(1):250-264. [DOI] [PubMed] [Google Scholar]
- 28. Zhou Z, Li X, Huang G, et al. Rosiglitazone combined with insulin preserves islet beta cell function in adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2005;21(2):203-208. [DOI] [PubMed] [Google Scholar]
- 29. Yang Z, Zhou Z, Li X, Huang G, Lin J. Rosiglitazone preserves islet beta-cell function of adult-onset latent autoimmune diabetes in 3 years follow-up study. Diabetes Res Clin Pract. 2009;83(1):54-60. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Yan X, Wu C, et al. Adding vitamin D3 to the dipeptidyl peptidase-4 inhibitor saxagliptin has the potential to protect β-cell function in LADA patients: A 1-year pilot study. Diabetes Metab Res Rev. 2020;36(5):e3298. [DOI] [PubMed] [Google Scholar]
- 31. Hendriksen C, Faber OK, Drejer J, Binder C. Prevalence of residual B-cell function in insulin-treated diabetics evaluated by the plasma C-etide response to intravenous glucagon. Diabetologia. 1977;13(6):615-619. [DOI] [PubMed] [Google Scholar]
- 32. Garcia-Webb P, Bonser A, Welborn TA. Correlation between fasting serum C-peptide and B cell insulin secretory capacity in diabetes mellitus. Diabetologia. 1982;22(4):296. [DOI] [PubMed] [Google Scholar]
- 33. Gjessing HJ, Matzen LE, Frøland A, Faber OK. Correlations between fasting plasma C-peptide, glucagon-stimulated plasma C-peptide, and urinary C-peptide in insulin-treated diabetics. Diabetes Care. 1987;10(4):487-490. [DOI] [PubMed] [Google Scholar]
- 34. Group TDR. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab. 1987;65(1):30-36. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Cheng J, Huang G, Luo S, Zhou Z. Tapering decay of β-cell function in Chinese patients with autoimmune type 1 diabetes: a four-year prospective study. J Diabetes. 2019;11(10):802-808. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Huang G, Lin J, Yang L, Zhou Z. Variation of C peptide decay rate in diabetic patients with positive glutamic acid decarboxylase antibody: better discrimination with initial fasting C peptide. BMC Endocr Disord. 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51(Suppl 1):S221-S226. [DOI] [PubMed] [Google Scholar]
- 38. Gallenberger M, zu Castell W, Hense BA, Kuttler C. Dynamics of glucose and insulin concentration connected to the β-cell cycle: model development and analysis. Theor Biol Med Model. 2012;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han K, Kang H, Kim J, Choi M. Mathematical models for insulin secretion in pancreatic β-cells. Islets. 2012;4(2): 94-107. [DOI] [PubMed] [Google Scholar]
- 40. Boutayeb W, Lamlili MEN, Boutayeb A, Derouich M. Mathematical modelling and simulation of beta-cell mass, insulin and glucose dynamics: effect of genetic predisposition to diabetes. J Biomed Sci Eng. 2014:330-342. [Google Scholar]
- 41. Xu W, Bi Y, Sun Z, et al. Comparison of the effects on glycaemic control and β-cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel-group trial (the CONFIDENCE study). J Intern Med. 2015;277(1):137-150. [DOI] [PubMed] [Google Scholar]
- 42. Buzzetti R, Pozzilli P, Frederich R, Iqbal N, Hirshberg B. Saxagliptin improves glycaemic control and C-peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Metab Res Rev. 2016;32(3):289-296. [DOI] [PubMed] [Google Scholar]
- 43. Hals IK, Fiskvik Fleiner H, Reimers N, et al. Investigating optimal β-cell-preserving treatment in latent autoimmune diabetes in adults: results from a 21-month randomized trial. Diabetes Obes Metab. 2019;21(10):2219-2227. [DOI] [PubMed] [Google Scholar]
- 44. Awata T, Shimada A, Maruyama T, et al. Possible long-term efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for slowly progressive type 1 diabetes (SPIDDM) in the stage of non-insulin-dependency: an open-label randomized controlled pilot trial (SPAN-S). Diabetes Ther. 2017;8(5):1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansen OE, Boehm BO, Grill V, et al. C-peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2-year double-blind, randomized, controlled study. Diabetes Care. 2014;37(1):e11-e12. [DOI] [PubMed] [Google Scholar]
- 46. Dennis JM, Shields BM, Hill AV, et al. ; MASTERMIND Consortium . Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care. 2018;41(4):705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng W, Zhou J, Song S, et al. Dipeptidyl-peptidase 4 inhibitor sitagliptin ameliorates hepatic insulin resistance by modulating inflammation and autophagy in ob/ob mice. Int J Endocrinol. 2018;2018:8309723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhuge F, Ni Y, Nagashimada M, et al. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65(10):2966-2979. [DOI] [PubMed] [Google Scholar]
- 49. Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300(2):E410-E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97(9):3333-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Satoh-Asahara N, Sasaki Y, Wada H, et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2013;62(3):347-351. [DOI] [PubMed] [Google Scholar]
- 52. Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55-70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.