Abstract
Context
Loss-of-function mutations of makorin RING finger protein 3 (MKRN3) are the most common monogenic cause of familial central precocious puberty (CPP).
Objective
To describe the clinical and hormonal features of a large cohort of patients with CPP due to MKRN3 mutations and compare the characteristics of different types of genetic defects.
Methods
Multiethnic cohort of 716 patients with familial or idiopathic CPP screened for MKRN3 mutations using Sanger sequencing. A group of 156 Brazilian girls with idiopathic CPP (ICPP) was used as control group.
Results
Seventy-one patients (45 girls and 26 boys from 36 families) had 18 different loss-of-function MKRN3 mutations. Eight mutations were classified as severe (70% of patients). Among the 71 patients, first pubertal signs occurred at 6.2 ± 1.2 years in girls and 7.1 ± 1.5 years in boys. Girls with MKRN3 mutations had a shorter delay between puberty onset and first evaluation and higher follicle-stimulating hormone levels than ICPP. Patients with severe MKRN3 mutations had a greater bone age advancement than patients with missense mutations (2.3 ± 1.6 vs 1.6 ± 1.4 years, P = .048), and had higher basal luteinizing hormone levels (2.2 ± 1.8 vs 1.1 ± 1.1 UI/L, P = .018) at the time of presentation. Computational protein modeling revealed that 60% of the missense mutations were predicted to cause protein destabilization.
Conclusion
Inherited premature activation of the reproductive axis caused by loss-of-function mutations of MKRN3 is clinically indistinct from ICPP. However, the type of genetic defect may affect bone age maturation and gonadotropin levels.
Keywords: precocious puberty, MKRN3, genetic of puberty, MKRN3 phenotype
Makorin RING finger 3 (MKRN3) is an intronless gene located inside a region containing an imprinted gene cluster at chromosome 15q11-q13. It undergoes maternal imprinting; therefore, only the paternal allele is expressed, while the maternal allele is silenced through methylation of CpG islands (1, 2). MKRN3 function is associated with gene transcription and E3 ubiquitin ligase activity (3, 4).
MKRN3 inactivating mutations were first described in 15 patients of both sexes (8 girls and 7 boys) from 5 different families with central precocious puberty (CPP) using whole-exome sequencing analysis in 2013 (5). Different types of mutations (frameshift, stop gain, missense) affecting the MKRN3 protein or the gene promoter region have been subsequently described after this work (4) and MKRN3 defects have been recognized as the most common monogenic cause of familial CPP. Notably, patients with CPP due to MKRN3 mutations have typical clinical and hormonal features of premature activation of the reproductive axis, including early pubertal signs, such as breast and pubic hair development, accelerated linear growth, advanced bone age, and elevated basal and/or gonadotropin-releasing hormone (GnRH)–stimulated luteinizing hormone (LH) levels. Macedo et al. (6) described that the age of pubertal onset ranged from 3.0 to 6.4 years (mean 5.3 years and median 6.0 years) in a small group of Brazilian girls with MKRN3 mutations. Except for the significantly higher levels of basal follicle-stimulating hormone (FSH) in patients with MKRN3 mutations than in those without MKRN3 mutations, no other clinical or hormonal differences were identified between these 2 groups.
A recent systematic review that gathered cohorts from 22 different studies (>800 patients analyzed) revealed a high prevalence of loss-of-function mutations in MKRN3 in patients with familial CPP from different parts of the world, with higher frequency in Occidental countries (4). In the current study, we describe detailed phenotypic characteristics of the largest multiethnic cohort of patients with CPP harboring loss-of-function mutations of MKRN3 evaluated by one medical center. Furthermore, we compare clinical data between patients with different types of mutations and perform in silico analysis of the stability of the protein for missense variants.
Patients and Methods
Study design and participants
Clinical data were extracted from a large cohort of patients with familial or idiopathic CPP (716 cases, 64% females) referenced to a Brazilian Medical Center for molecular analysis (Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil). All index cases had CPP, defined by the development of progressive pubertal signs before 8 years in girls and before 9 years in boys (7). All index patients had CPP confirmed either by pubertal basal (>0.2 UI/L) or GnRH-stimulated LH levels (>5 UI/L by electrochemiluminescence: ECLIA), and they had organic causes ruled out by magnetic resonance imaging of the central nervous system (7, 8).
In order to identify clinical or laboratory predictors of MKRN3 mutations, we assessed data from another cohort of 156 girls with idiopathic CPP followed in the Brazilian Center. All of them were negative for MKRN3 mutations, as well as negative for other established monogenic causes of CPP. Their data were compared with data from the group of individuals with MKRN3 mutations. Because of the low number of boys with CPP (idiopathic and associated with MKRN3 mutations) the same comparison could not be performed.
Clinical and laboratory data collection
The Endocrinology Division of the Hospital das Clínicas in Sao Paulo, Brazil, is a tertiary center; patients were referred from other Brazilian states to investigate genetic causes of CPP. Additionally, we received DNA samples and clinical data from other countries through international collaborations and from a multiethnic cohort encompassing 716 subjects, including 517 index patients and their close relatives. The largest group of subjects were Brazilian (371), whereas the remaining 345 were from other countries (the largest cohort being 228 Spanish cases). Sanger sequencing for MKRN3 was performed in all index cases (517 of the 716 subjects) and 71 individuals had MKRN3 pathogenic variants (36 index cases and 35 relatives). Medical records were systematically reviewed from all confirmed MKRN3 mutated patients, and all available data were collected, including follow-up and treatment information. Relevant clinical data was gathered as follows: (1) age at first pubertal signs (breast buds in girls and testicular enlargement in boys); (2) age at pubarche; (3) age at first evaluation – chronological age (CA); (4) bone age at first evaluation (evaluated according to Greulich and Pyle method); (5) bone age advancement (bone age advance = bone age – chronological age ); (6) height (standard deviation score [SDS]); (7) weight; (8) body mass index and body mass index-SDS (9). The laboratory data included basal and GnRH-stimulated LH and FSH levels, measured either by immunochemiluminometric or ECLIA assays; and basal estradiol or testosterone levels, measured by immunoassays or liquid chromatography-tandem mass spectrometry. Pubertal LH basal values were considered higher than 0.2 UI/L (ECLIA) and the GnRH-stimulated LH levels cutoff was 5 UI/L using ECLIA (7). Whenever available, data regarding follow-up, treatment, and final height were also collected and analyzed in comparison with the predicted target height.
Genetic and molecular analysis
MKRN3 sequencing analysis
Genomic DNA was extracted from peripheral blood leukocytes. We analyzed the coding region of MKRN3 for allelic variants by performing PCR amplification followed by sequencing of the products with the use of the conventional Sanger method, as previously described (5). The local ethics committees (Comissão de Ética para Análise de Projetos de Pesquisa—CAPPesq) approved this study and all individuals and/or their legal guardians gave their written informed consent. DLK1 mutations were excluded in all patients described in this study. A subset of patients with CPP had KISS1 and KISS1R mutations also excluded (5).
All pathogenic variants were evaluated according to the American College of Medical Genetics (ACMG) criteria (10). In order to establish genotype–phenotype correlations, pathogenic variants were classified and grouped either as severe (frameshift, stop gain, and promoter region mutations) or missense mutations. The rationale behind this categorization was that in the first group the mutations led to truncated and possibly more dysfunctional proteins, which could lead to a more pronounced phenotype.
MKRN3 protein stability analysis
Computational protein modelling analysis was performed using Yasara software (Vienna, Austria) with FoldX toolsuite, which provided an analysis of the effect of mutations on the stability, folding and dynamics of proteins (11). This type of analysis first calculates the free energy of unfolding (ΔG) of a target protein. Subsequently, it calculates the energy difference between the wild type and a variant of the protein (ΔΔG) (11).
Statistical analysis
Data were described by simple descriptive statistics or frequencies and percentages. Whenever appropriate, bootstrapping analysis of smaller number of observations was performed to reduce possible bias due to sampling. Data are presented as mean and standard deviation unless otherwise stated. Comparisons between the different groups of mutations and between the genetic and idiopathic cases included Student’s t-test or Wilcoxon signed-rank test for numerical continuous variables as appropriate. Categorical variables were compared between groups using the chi-squared test or Fisher’s exact test as appropriate. Paired t-tests were conducted in the subgroup of treated patients. Statistical analysis was performed in R Studio (version 1.2.1335) and P < .05 was considered statistically significant.
Results
MKRN3 pathogenic variants
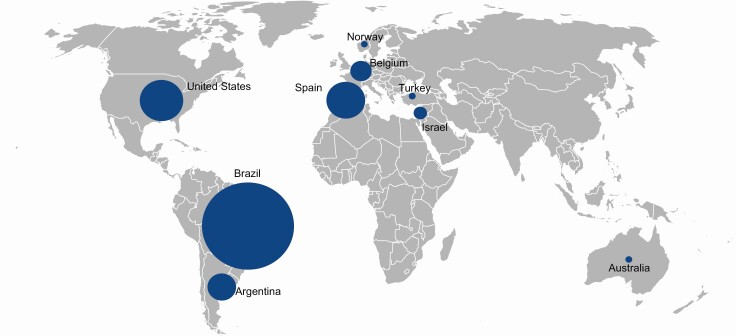
Among 716 cases screened for MKRN3 sequence variants, we identified 71 patients (45 girls and 26 boys) from 36 unrelated families who had pathogenic variants according to ACMG criteria. This cohort comprised 9 different ethnic backgrounds: 40 Brazilian, 9 American, 8 Spanish, 5 Argentinean, 4 Belgian, 2 Israelis, 1 Australian, 1 Norwegian, and 1 Turkish (Fig. 1). Thirty-seven patients were newly reported cases, and the remaining 34 patients were previously described and included for statistical analysis (4–6, 12). Twenty-one families were available for segregation analysis.
Figure 1.
Geographic distribution of 71 patients with CPP due to MKRN3 mutations. The larger circles represent higher numbers of cases: 40 in Brazil, 9 in USA, 8 in Spain, 5 in Argentina, 4 in Belgium, 2 in Israel, 1 in Norway, 1 in Australia, and 1 in Turkey.
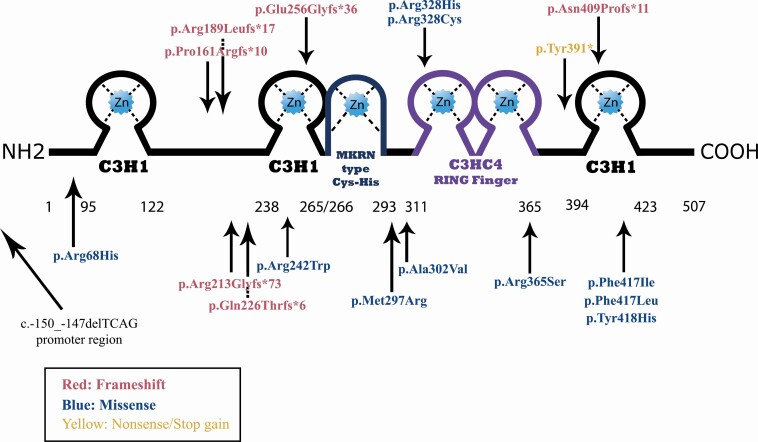
Eighteen different MKRN3 mutations were identified. Eight of them were classified as severe: 6 different frameshift variants, 1 stop gain variant, and 1 promoter region deletion. The promoter region deletion was previously reported and leads to the loss of a transcription factor binding site and was thus considered severe as well (13). The severe mutations of MKRN3 occurred in the great majority of the cohort with CPP: 53 out of 71 patients (75%); or 26 out of 36 index cases (72%). The remaining 10 mutations were missense pathogenic variants (18 out of 71 patients—25%). All identified MKRN3 mutations are represented in Fig. 2.
Figure 2.
Schematic representation of MKRN3 protein and location of severe (red and yellow) and missense (blue) variants. The MKRN3 protein is composed of 3 C3H1 zinc fingers, a MKRN type Cys-His region, and a C3HC4 RING finger. The arrows point to the corresponding region of the mutation in the protein. The promoter region deletion is represented in this image without a corresponding region in the protein because it leads to loss of transcription due to the loss of a DREAM binding site (13).
It is worth noting that the most prevalent MKRN3 mutations, occurring in 34 (46%) patients, were indel variants affecting a poly-C region in cDNA positions 475 to 481 (7 cytosines), which caused frameshift mutations and premature stop codons. These variants have been previously named in a few different ways, generally in the amino acid positions 161 and 162. Here, we adopted a single nomenclature for this hotspot region (p.Pro161Argfs*), because Sanger sequencing reading might differ due to the poly-C region.
Missense variants pathogenicity assessment
According to ACMG criteria, 10 missense MKRN3 variants were categorized either as pathogenic or likely pathogenic (Table 1). In addition, according to Foldx and Yasara software analysis, 6 of them were identified as destabilizing or highly destabilizing, while 4 were identified as neutral or stabilizing. However, missense variants identified as stabilizing or neutral were located in critical regions of the protein structure. The 2 most common regions affected by missense variants were the third C3H1 ring finger and the C3HC4 ring finger of the protein (Table 2).
Table 1.
MKRN3 missense variants identified in patients with CPP and pathogenicity assessment through ACMG criteria
| Position | cDNA | Protein | rs | Populationdata(AF) | ACMG criteria | ||
|---|---|---|---|---|---|---|---|
| gnomAD | ABraOM | Detailed staging | Classification | ||||
| 15:23812024 | c.1095G>T | p.Arg365Ser | rs879255240 | 0.000003976 | Not reported | PS4, PM2, PP1, PP3, PP4, PP5 | Pathogenic |
| 15:23812178 | c.1249T>A | p.Phe417Ile | NA | Not reported | Not reported | PS4, PM2, PP1, PP3, PP4, PP5 | Pathogenic |
| 15:23811132 | c.203G>A | p.Arg68His | rs149274884 | 0.00001201 | Not reported | PM2, PP1, PP2, PP4, PP5, BP4 | Likely pathogenic |
| 15:23811653 | c.724C>T | p.Arg242Trp | rs1277371835 | 0.00001193 | Not reported | PM2, PP1, PP2, PP4, BP4 | Likely pathogenic |
| 15:23811834 | c.905C>T | p.Ala302Val | NA | Not reported | Not reported | PS4, PM2, PP1, PP3, PP4 | Likely pathogenic |
| 15:23811912 | c.983G>A | p.Arg328His | rs1355723562 | 0.00006371 | Not reported | PS4, PM2, PP1, PP3, PP4 | Likely pathogenic |
| 15:23811911 | c.982C>T | p.Arg328Cys | rs1355723562 | 0.00006371 | Not reported | PS4, PM2, PP1, PP3, PP4 | Likely pathogenic |
| 15:23812178 | c.1249T>C | p.Phe417Leu | rs745560329 | 0.000003976 | Not reported | PM2, PM5, PP1, PP2, PP4, BP4 | Likely pathogenic |
| 15:23812181 | c.1252T>C | p.Tyr418His | rs1470111765 | 0.000003976 | Not reported | PM2, PP2, PP1, PP2, PP3, PP4 | Likely pathogenic |
| 15:23811819 | c.890T>C | p.Met297Arg | rs147605349 | 0.00001193 | Not reported | PM2, PP1, PP2, PP3, PP4 | Likely pathogenic |
Abbreviations: AF, allele frequency; ACMG, American College of Medical Genetics and Genomics; gnomAD, Genome Aggregation Database; ABraOM, Online Archive of Brazilian Mutations; ACMG criteria are given according to reference number 10; PS4, pathogenic strong 4; PM2, pathogenic moderate 2; PM5, pathogenic moderate 5; PP1, pathogenic supporting 1; PP2, pathogenic supporting 2; PP3, pathogenic supporting 3; PP4, pathogenic supporting 4; PP5, pathogenic supporting 5; BP4, benign supporting 4.
Table 2.
Protein stability analysis of MKRN3 with missense variants
| Mutation (missense) | ΔΔG | Protein stability | Location within the protein |
|---|---|---|---|
| p.Arg68His | 0.915 | Slightly destabilizing | Before C3H1 zinc finger 1 |
| p.Arg242Trp | –1.090 | Stabilizing | Second C3H1 zinc finger |
| p.Met297Arg | –0.240 | Neutral | Between MRKN type Cys-His domain and C3HC4 ring finger |
| p.Ala302Val | 1.000 | Destabilizing | Between MRKN type Cys-His domain and C3HC4 ring finger |
| p.Arg328His | 1.409 | Destabilizing | C3HC4 ring finger |
| p.Arg328Cys | 1.661 | Destabilizing | C3HC4 ring finger |
| p.Arg365Ser | 1.617 | Destabilizing | C3HC4 ring finger |
| p.Phe417Ile | –1.010 | Stabilizing | Third C3H1 zinc finger |
| p.Phe417Leu | –1.392 | Stabilizing | Third C3H1 zinc finger |
| p.Tyr418His | 2.882 | Highly destabilizing | Third C3H1 zinc finger |
ΔΔG is the energy difference between the wild type and a variant of MKRN3. This analysis can provide a classification of the mutation into 7 categories: (1) highly stabilizing (ΔΔG < −1.84 kcal/mol); (2) stabilizing (−1.84 kcal/mol ≤ ΔΔG < −0.92 kcal/mol); (3) slightly stabilizing (−0.92 kcal/mol ≤ ΔΔG < −0.46 kcal/mol); (4) neutral (−0.46 kcal/mol < ΔΔG ≤ +0.46 kcal/mol); (5) slightly destabilizing (+0.46 kcal/mol < ΔΔG ≤ +0.92 kcal/mol); (6) destabilizing (+0.92 kcal/mol < ΔΔG ≤ +1.84 kcal/mol); and (7) highly destabilizing (ΔΔG > +1.84 kcal/mol) (11).
Clinical and laboratory features
Among the cohort with MKRN3 mutations (71 total cases), first pubertal signs occurred at 6.2 ± 1.2 years in girls and 7.1 ± 1.5 years in boys, and they had a bone age advancement of 2.0 ± 1.6 and 1.8 ± 1.3 years, respectively. Hormonal data showed basal LH levels of 1.9 ± 1.8 IU/L in girls and 1.6 ± 1.2 IU/L in boys. More detailed clinical and hormonal data is provided in Table 3.
Table 3.
Clinical and hormonal features of 71 patients with CPP caused by MKRN3 mutations
| Girls (n = 45) Mean ± SD | Boys (n = 26) Mean ± SD | |
|---|---|---|
| First pubertal signs (years) | 6.22 ± 1.19 | 7.13 ± 1.46 |
| Pubarche (years) | 6.96 ± 1.14 | 7.3 ± 1.58 |
| At first evaluation | ||
| Chronological age (years) | 7.13 ± 1.05 | 8.00 ± 1.53 |
| BA (years) | 9.17 ± 1.98 | 9.51 ± 2.56 |
| BAA (years) | 2.04 ± 1.57 | 1.81 ± 1.30 |
| Height SDS | 1.25 ± 1.06 | 0.43 ± 1.10 |
| BMI SDS | 0.92 ± 0.99 | 1.50 ± 1.26 |
| Basal LH (IU/L) | 1.86 ± 1.78 | 1.59 ± 1.22 |
| Basal FSH (IU/L) | 4.94 ± 2.33 | 2.64 ± 1.87 |
| Estradiol (ng/dL) | 30.0 ± 20.9 | |
| Testosterone (ng/dL) | . | 186 ± 185 |
| LH peak (IU/L) | 20.16 ± 14.93 | 10.87 ± 4.98 |
| FSH peak (IU/L) | 16.94 ± 4.86 | |
| Treatment duration (years) | 2.89 ± 0.91 (n = 20) | 2.72 ± 0.57 (n = 5) |
| Age at menarche in treated subjects (years) | 11.3 ± 1.2 | |
| Age at menarche in untreated subjects (years) | 8.2 ± 1.0 | |
| Final height SDS in treated patients | –0.46 ± 0.61a (n = 16) | –0.9 ± 1.51 (n = 3) |
| TH SDS for treated patients | –0.69 ± 0.86a | –0.1 ± 0.88 (n = 3) |
Data are shown as mean ± standard deviation.
Abbreviations: BA, bone age; BAA, bone age advance; SDS, standard deviation score; LH, luteinizing hormone; FSH, follicle stimulating hormone; TH, target height; NA, not applicable.
a Final height in treated girls did not differ from predicted target height (P = .37).
Genotype–phenotype correlations
The 53 patients who harbored severe MKRN3 mutations had a greater bone age advancement than those with missense mutations (2.3 ± 1.6 vs 1.6 ± 1.4 years, respectively, P = .048), and also had higher basal LH levels (2.2 ± 1.8 vs 1.1 ± 1.1 UI/L, P = .018). Other clinical and laboratory features were not significantly different between the 2 groups (severe vs missense) and are depicted in Table 4.
Table 4.
Phenotype comparison between severe and missense variants of MKRN3
| Severe pathogenica | Missense pathogenicb | P | |
|---|---|---|---|
| Number of patients (%) number of families | 53 (75%) 26 | 18 (25%) 10 | NA |
| Male/Female | 18/35 | 8/10 | NA |
| First pubertal signs (years) | 6.2 ± 1.3 | 6.8 ± 1.2 | .23 |
| Pubarche (years) | 6.8 ± 1.1 | 7.6 ± 1.3 | .19 |
| First evaluation | |||
| CA (years) | 7.3 ± 1.1 | 7.5 ± 1.5 | .62 |
| BAA (years) | 2.3 ± 1.6 | 1.6 ± 1.4 | .047 |
| Height SDS | 1.2 ± 1.1 | 0.9 ± 1.2 | .35 |
| BMI SDS | 1.1 ± 0.9 | 0.8 ± 1.2 | .34 |
| Basal LH (IU/L) | 2.2 ± 1.8 | 1.1 ± 1.1 | .018 |
| GnRH stimulated LH (IU/L) | 20.4 ± 15.8 | 14.7 ± 10.1 | .26 |
| Basal FSH (IU/L) | 4.9 ± 2.7 | 3.7 ± 1.7 | .08 |
| Menarche (years) | 10.6 ± 1.7 | 11.4 ± 1.1 | .22 |
| Final height SDS in treated patients | –0.8 ± 1.1 | –1.3 ± 0.9 | .60 |
| Target height SDS | –0.7 ± 1.0 | –0.1 ± 0.9 | NA |
Data are shown as mean ± standard deviation. The clinical data in this comparison are of the female patients only because male subjects had fewer data available to perform this comparison.
Abbreviations: CA, chronological age; SDS, standard deviation score; FSH, follicle-stimulating hormone; LH, luteinizing hormone; GnRH, gonadotropin-releasing hormone; BMI, body mass index; BAA, bone age advancement; NA, not applicable.
a Severe MKRN3 mutations: p.Pro161Argfs*10; p.Tyr391*; p.Arg213Glyfs*73; p.Asn409Profs*11; p.Gln226Thr fs*6; p.Glu256Gly fs*36; p.Arg189Leufs*17; c.-150_-147delTCAG.
b Missense MKRN3 mutations: p.Arg365Ser; p.Arg328Cys; p.Arg328His; p.Phe417Ile; p.Ala302Val; p.Tyr418His; p.Arg242Trp; p.Phe417Leu; p.Met297Arg; p.Arg68His.
Phenotype comparison between girls with CPP with and without MKRN3 mutations
The 45 girls with CPP caused by MKRN3 loss-of-function mutations were compared with a cohort of 156 Brazilian girls with idiopathic CPP. Girls with MKRN3 mutations had a shorter delay between puberty onset and first evaluation (0.8 ± 0.8 vs 2.4 ± 2.1 years, respectively, P < .001), which occurred at a younger age (7.2 ± 1.1 years vs 8.4 ± 2.0 years; P < .001). Among the MKRN3 subjects, those who had a positive family history of precocious puberty sought medical assistance earlier than those MKRN3 patients without family history (0.42 ± 0.43 vs 1.1 ± 1 years; P < .01). Interestingly, though, even when we compared only the MKRN3 patients without family history of CPP to the cohort of idiopathic CPP the interval remained shorter in the MKRN3 group (1.1 ± 1.0 vs 2.4 ± 2.0; P < .001).
Furthermore, girls with CPP caused by MKRN3 mutations presented with less pronounced height SDS increase at first evaluation (1.2 ± 1.2 vs 1.7 ± 1.1; P = .04). They also presented with higher basal FSH levels than girls with idiopathic CPP (4.9 ± 2.3 IU/L vs 3.8 ± 2.7IU/L; P = .03). However, no cut-off level was established, as the values overlapped. All data are shown in Table 5.
Table 5.
Phenotype comparison between girls with CPP with and without MKRN3 mutations
| MKRN3 CPP (n = 45) | Idiopathic CPP (n = 156) | P | |
|---|---|---|---|
| Thelarche (years) | 6.3 ± 1.2 | 6.0 ± 1.7 | .38 |
| Pubarche (years) | 7.1 ± 1.2 | 6.8 ± 1.9 | .24 |
| First evaluation (years) | 7.2 ± 1.1 | 8.4 ± 2.0 | .001 |
| Diagnostic delay (years) | 0.8 ± 0.8 | 2.4 ± 2.1 | <.001 |
| BAA (years) | 2.1 ± 1.6 | 2.6 ± 1.3 | .08 |
| Height SDS | 1.2 ± 1.2 | 1.7 ± 1.1 | .04 |
| BMI SDS | 0.9 ± 0.9 | 0.8 ± 0.9 | .90 |
| Basal LH (IU/L) | 1.7 ± 1.8 | 1.3 ± 1.4 | .22 |
| Basal FSH (IU/L) | 4.9 ± 2.3 | 3.8 ± 2.7 | .03 |
| Estradiol (ng/dL) | 29.8 ± 20.8 | 30.1 ± 30.3 | .95 |
| LH peak (IU/L) | 20.2 ± 14.4 | 17.3 ± 16.6 | .45 |
| FSH peak (IU/L) | 16.9 ± 4.6 | 14.2 ± 10.2 | .18 |
Data are shown as mean ± standard deviation. Diagnostic delay (years) was defined as the difference between the age at first evaluation and the age at fist pubertal signs.
Abbreviations: BAA, bone age advance; CPP, central precocious puberty; SDS, standard deviation score; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Follow-up and response to GnRH analogue treatment
Among the 71 patients with CPP due to MKRN3 mutations, final height was available for 24 patients treated with GnRH depot analogues and for 8 untreated adults. The untreated adults were diagnosed lately (adult phase) through family screening. Treated patients had a mean time of treatment of 2.9 ± 0.9 years, and mean final height SDS was –0.46 ± 0.61, which was indistinguishable from their mean target height of –0.69 ± 0.86 (P = .37).
Discussion
MKRN3 loss-of-function mutations represent the most common genetic cause of familial CPP (8). They have been described in children from multiple countries and may account for a substantial proportion of inherited CPP cases (33-46%) (12, 14). In the present study, we investigated the genotype and phenotype features of a multiethnic cohort (Latin American, North American, European, Israeli, and Turkish subjects) with deleterious defects of MKRN3. The cohort was composed of 71 patients (from 36 unrelated families) with CPP caused by 18 MKRN3 inactivating mutations. Both female and male patients carrying MKRN3 mutations exhibited classical clinical and biochemical features of premature reactivation of the reproductive axis. Girls started pubertal development at a mean age of 6.2 ± 1.2 years, whereas in boys it was at 7.1 ± 1.5 years. Higher levels of basal FSH and an earlier age at diagnosis were identified in female patients with CPP associated with MKRN3 when compared with an idiopathic CPP group from Brazil, in agreement with the previous study of Macedo et al. (6). The higher FSH levels might be attributed to different frequencies of pulsatile GnRH release and its impact on gonadotropin secretion. While low GnRH pulse frequencies stimulate FSH secretion preferably, higher GnRH pulse frequencies favor LH secretion (15). Therefore, it is reasonable to hypothesize that MKRN3 loss of function could lead to a lower frequency pattern of GnRH pulsatility, which in turn could lead to higher FSH secretion.
The shorter interval between initial manifestations and diagnosis of CPP in patients with MKRN3 mutations was probably related to the fact that 51% of them had a familial history of precocious puberty, increasing the awareness of parents and doctors for premature sexual development in a second case in the same family.
The determination of age at pubertal onset in boys is usually a challenge, because testicular enlargement (first male pubertal sign) is not as obvious as thelarche and menarche in girls. In addition, male patients usually remember only late events of puberty, such as the age at initiation of full facial shaving and the age at voice change. Bessa et al. (12) demonstrated a high frequency of MKRN3 mutations in a series of 20 boys with CPP, previously classified as idiopathic, suggesting the importance of genetic analysis in this group. Notably, boys with CPP due to MKRN3 mutations had puberty initiation at a borderline age according to several studies (7.9-8.5 years) (12, 16, 17). This fact can compromise the precise identification of puberty, leading to an underestimate of the incidence of CPP in the male group. Indeed, in the current study, the clinical data of boys with MKRN3 CPP was also more scarce than for girls, mainly because 87.5% of all male subjects were diagnosed through familial screening; only 5 boys were index cases (out of 36 index cases). Some of the rest of them were underdiagnosed in childhood and the CPP history was only recognized retrospectively in adult life, while others were siblings of index patients and had an earlier diagnosis that might have been undetected otherwise. Therefore, we believe that male CPP caused by MKRN3 mutations can be clinically subtle.
To date, all patients with MKRN3 loss-of-function mutations have a paternal origin when familial segregation analysis was possible. A documented de novo MKRN3 mutation has not been described to date, indicating that true sporadic cases are very uncommon. In fact, a history of premature sexual development on the paternal side can often be difficult to demonstrate or confirm, leading to the misclassification as a sporadic CPP case.
Here, we identified 18 rare inactivating mutations in the MKRN3, including 1 nonsense, 6 frameshifts, and 10 missense mutations, along with a promoter region deletion; some of these variants were previously reported (5, 13, 18). Seven are novel variants, including 2 frameshift mutations (p.Arg189Leufs*17 and p.Asn409Profs*11) and 5 missense variants (p.Ala302Val, p.Tyr418His, p.Arg242Trp, p.Met297Arg, and p.Arg68His). Notably, a recurrent frameshift mutation (p.Pro161Argfs*) was identified in 46% of the patients with CPP. This frameshift affects a cytosine-rich region, confirming this site as a hotspot region.
It is well known that estradiol is the most important hormone in bone maturation, although its serum levels are not good markers of initial pubertal development in girls. While low levels of estradiol in the beginning of puberty enhance longitudinal growth, the gradually higher estradiol levels towards the end of puberty promote growth plate fusion (19). Curiously, severe mutations were associated with greater bone age advancement and higher basal LH levels, suggesting that these mutations could lead to either prolonged or a greater impact of estradiol levels on bone maturation or more rapid advancement of puberty.
The 10 identified rare missense variants were predicted to be pathogenic by in silico analysis and protein modelling. In addition, 60% of these variants are predicted to lead to loss of stability of the protein. Moreover, 3 variants were located within the C3HC4 ring zinc finger domain of the protein and 4 variants within the third C3H1 zinc finger motif. These domains are related to the E3 ubiquitin ligase activity and RNA -binding, respectively, and appear to be essential for protein function. Recently, Abreu et al. (20) described that MKRN3 acts by repressing the promoter activity of KISS1 and TAC3, which are 2 stimulators of GnRH secretion. Additionally, they found that mutations affecting the RING finger domain led to reduced ubiquitin ligase activity and reduced repression of KISS1 and TAC3. Few studies have explored MKRN3 regulation so far. Recently, a study demonstrated that leptin, which is a known permissive factor for puberty, does not seem to influence Mkrn3 expression in mice (21). In addition, the decline of Mkrn3 expression prior to pubertal onset was also shown to be independent of estradiol (20). At the molecular level, microRNAs appear to have an important role in regulation of MKRN3 expression. The microRNA miR-30 has been demonstrated to act on 3 binding sites in a highly conserved region of the Mkrn3 3′-untranslated region (UTR) as a repressor of Mkrn3 to control pubertal onset (22). Taken together, our findings might suggest that missense variants could lead to precocious puberty through at least 2 hypothetical mechanisms: (1) destabilizing the protein and generating reduced inhibition of genes that promote puberty, and (2) affecting critical regions (ie, RING fingers) that are relevant to ubiquitination and overall MKRN3 repressor activity.
Another rarer mechanism has been described in children with CPP who harbored deletions in the 5′-UTR regulatory region of the MKRN3 gene (13). Subsequently, novel heterozygous mutations (–166, –865, –886 nt upstream to the transcription start site) located in the promoter and 5′-UTR regulatory regions of the MKRN3 gene were identified in girls with CPP from Turkey (23). In silico analysis and gene reporter assay for the mutated 5′-UTR predicted a significant change of the mRNA secondary structure and a significant reduction of MKRN3 promoter activity in transfected GN11 cells, respectively.
Recently, Ramos et al. (24) described anthropometric, metabolic and reproductive outcomes of 11 patients with CPP caused by MKRN3 mutations who were submitted to GnRH analog treatment. No deleterious effect was evident in young female adults in this specific group of CPP. Interestingly, a high prevalence of overweight and obesity were observed in CPP patients with or without MKRN3 mutations (47.3% and 50%, respectively), followed by a significant reduction after GnRH analog treatment. Mean final height was similar in CPP groups with or without MKRN3 mutations, indicating adequate response to GnRH analog treatment in both groups. Here we have further expanded this cohort and the results were consistent with the efficacy of GnRH analog treatment in CPP caused by MKRN3 mutations.
Our findings demonstrate that the premature sexual development phenotype caused by MKRN3 loss-of-function mutations is indistinct from idiopathic CPP. Collectively, a shorter time to presentation and higher FSH levels were found in the MKRN3 patients. Some missense variants lead to destabilization of the protein or affected critical regions within the MKRN3 protein structure. Notably, frameshift mutations and other severe defects have a greater impact on phenotype when compared to pathogenic missense variants. Severe mutations result in greater bone age advancement and higher basal LH levels in patients with CPP.
Acknowledgments
J.A. thanks the Spanish PUBERE Registry from the Spanish Society for Pediatric Endocrinology (SEEP).
Financial Support: C.E.S. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #142362/2019-0). Fondo de Investigación del Instituto de Salud Carlos III (fondos FEDER); Grant PI019/0166 (to J.A.) and CIBER de fisiopatología y nutrición (CIBEROBN) (to J.A.), Madrid, Spain. A.P.M.C. was supported by the São Paulo Research Foundation (FAPESP) (#2018/03198-0). D.B.M. was supported by Coordenação de Aperfeiçoamento de Ensino Superior (CAPES) (#88881.170070/2018-01). A.C.L. was supported by Universidade de São Paulo (USP) (# 2008.1.1677.5.4); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #13/03236-5); and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #403525/2016-0 and #302849/2017-7). A.P.A. and U.B.K. were supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R00 HD091381 to A.P.A. and R01 HD082314 to U.B.K.).
Glossary
Abbreviations
- ACMG
American College of Medical Genetics
- CPP
central precocious puberty
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- ICPP
idiopathic central precocious puberty
- LH
luteinizing hormone
- MKRN3
makorin RING finger protein 3
- UTR
untranslated region
Additional Information
Disclosures: V.N.B. has received lecture fees from AbbVie. All other authors declare no competing interests.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Jong MT, Gray TA, Ji Y, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783-793. [DOI] [PubMed] [Google Scholar]
- 2. Nicholls RD, Saitoh S, Horsthemke B. Imprinting in Prader-Willi and Angelman syndromes. Trends Genet. 1998;14(5):194-200. [DOI] [PubMed] [Google Scholar]
- 3. Yellapragada V, Liu X, Lund C, et al. MKRN3 interacts with several proteins implicated in puberty timing but does not influence GNRH1 expression. Front Endocrinol (Lausanne). 2019;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valadares LP, Meireles CG, De Toledo IP, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc. 2019;3(5):979-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macedo DB, Abreu AP, Reis AC, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99(6):E1097-E1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265-274. [DOI] [PubMed] [Google Scholar]
- 8. Soriano-Guillén L, Argente J. Central precocious puberty, functional and tumor-related. Best Pract Res Clin Endocrinol Metab. 2019;33(3):101262. [DOI] [PubMed] [Google Scholar]
- 9. Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. Stanford: Stanford University Press; 1959:272. [Google Scholar]
- 10. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerois R, Nielsen JE, Serrano L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J Mol Biol. 2002;320(2):369-387. [DOI] [PubMed] [Google Scholar]
- 12. Bessa DS, Macedo DB, Brito VN, et al. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology. 2017;105(1):17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macedo DB, França MM, Montenegro LR, et al. Central precocious puberty caused by a heterozygous deletion in the MKRN3 promoter region. Neuroendocrinology. 2018;107(2):127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simon D, Ba I, Mekhail N, et al. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol. 2016;174(1):1-8. [DOI] [PubMed] [Google Scholar]
- 15. Stamatiades GA, Carroll RS, Kaiser UB. GnRH-A key regulator of FSH. Endocrinology. 2019;160(1):57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aycan Z, Savaş-Erdeve Ş, Çetinkaya S, et al. Investigation of MKRN3 mutation in patients with familial central precocious puberty. J Clin Res Pediatr Endocrinol. 2018;10(3):223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortiz-Cabrera NV, Riveiro-Álvarez R, López-Martínez MÁ, et al. Clinical exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Horm Res Paediatr. 2017;87(2):88-94. [DOI] [PubMed] [Google Scholar]
- 18. Valadares LP, Meireles CG, De Toledo IP, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc. 2019;3(5):979-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70(21):4023-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts SA, Abreu AP, Navarro VM, et al. The peripubertal decline in makorin ring finger protein 3 expression is independent of leptin action. J Endocr Soc. 2020;4(7):bvaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019;17(11):e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fanis P, Skordis N, Toumba M, et al. Central precocious puberty caused by novel mutations in the promoter and 5’-UTR region of the imprinted MKRN3 gene. Front Endocrinol (Lausanne). 2019;10:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramos CO, Macedo DB, Canton APM, et al. Outcomes of patients with central precocious puberty due to loss-of-function mutations in the MKRN3 gene after treatment with gonadotropin-releasing hormone analog. Neuroendocrinology. 2020;110(7-8):705-713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.