Abstract
Objectives
Antiretroviral therapy (ART) use is associated with disrupted lipid and glucose metabolism in people with HIV infection. We aimed to identify plasma lipid species associated with risk of diabetes in the context of HIV infection.
Research Design and Methods
We profiled 211 plasma lipid species in 491 HIV-infected and 203 HIV-uninfected participants aged 35 to 55 years from the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. Cox proportional hazards model was used to examine associations between baseline lipid species and incident diabetes (166 diabetes cases were identified during a median follow-up of 12.6 years).
Results
We identified 11 lipid species, representing independent signals for 8 lipid classes/subclasses, associated with risk of diabetes (P < 0.05 after FDR correction). After adjustment for multiple covariates, cholesteryl ester (CE) (22:4), lysophosphatidylcholine (LPC) (18:2), phosphatidylcholine (PC) (36:4), phosphatidylcholine plasmalogen (34:3), and phosphatidylethanolamine (PE) (38:2) were associated with decreased risk of diabetes (HRs = 0.70 to 0.82 per SD increment), while diacylglycerol (32:0), LPC (14:0), PC (38:3), PE (36:1), and triacylglycerol (50:1) were associated with increased risk of diabetes (HRs = 1.26 to 1.56 per SD increment). HIV serostatus did not modify any lipid-diabetes associations; however, most of these lipid species were positively associated with HIV and/or ART use, including 3 diabetes-decreased ( CE [22:4], LPC [18:2], PE [38:2]) and all 5 diabetes-increased lipid species.
Conclusions
This study identified multiple plasma lipid species associated with incident diabetes. Regardless of the directions of their associations with diabetes, most diabetes-associated lipid species were elevated in ART-treated people with HIV infection. This suggests a complex role of lipids in the link between ART and diabetes in HIV infection.
Keywords: diabetes, HIV infection, lipidomics, prospective cohort
Observed associations of antiretroviral therapy (ART) use, especially protease inhibitors (PI), with hyperglycemia, impaired glucose tolerance, and insulin resistance have raised concerns about diabetes in people with HIV infection (1-3). A higher incidence of diabetes has been reported in HIV-infected people compared with those without HIV infection in several (4, 5), but not all (6), prospective HIV cohort studies. The pathogenesis of diabetes in HIV-infected people, which is not fully understood, may result from a complex interaction of chronic HIV infection, ART use, and factors unrelated to HIV such as diet, lifestyle, and genetic predisposition (7, 8).
Blood lipid disorders, such as high triglycerides and low high-density lipoprotein (HDL) cholesterol, are commonly present in both HIV infection (9) and diabetes (10). Earlier studies have focused on these conventional blood lipid measures, which may ultimately encompass a large number of individual lipid species. Recent metabolomic and lipidomic studies in populations without HIV have identified multiple lipid species associated with diabetes risk and suggested an important role of the structural features of acyl chains in the relationship between lipid species and diabetes (11-16). For example, short and saturated triacylglycerols, but not those with long and unsaturated acyl chains, were associated with increased diabetes risk (11, 14, 16). Our recent lipidomic profiling in 2 long-running HIV cohorts, the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS), identified multiple plasma lipid species elevated in HIV-infected people; and many of these elevated lipid species (eg, ceramides, long and unsaturated triacylglycerols) were associated with increased risk of carotid artery atherosclerosis (17, 18). Thus, lipidomics profiling may help us to better understand the biological link between HIV infection, disrupted lipid metabolism, and cardiometabolic disease. However, to date, the relationship between plasma lipidomic profiles and diabetes risk has not been well-studied in the context of HIV infection.
In the present study, we conducted a lipidomic analysis to examine associations between plasma lipidomic profiles, including 211 lipid species and diabetes risk among 694 participants (491 HIV-infected, 203 HIV-uninfected) in the WIHS and MACS with a median follow-up of 12.6 years. We further examined the potential influences of HIV infection and related factors (eg, ART use) on levels of lipid species and their associations with diabetes risk.
Research Design and Methods
Study Participants
Our current study comprises women from the WIHS and men from the MACS, 2 prospective multicenter cohort studies of individuals with or at high risk of HIV infection. The WIHS was initiated in 1994 and has enrolled more than 4000 women at 6 sites in the United States. Recruitment in the WIHS occurred in 3 waves (1994-1995, 2001-2002, and 2010-2012) from HIV primary care clinics, hospital-based programs, community outreach, support groups, and other locations. The MACS was initiated in 1984 and has enrolled approximately 7000 gay or bisexual men at 4 sites in the United States. MACS recruitment also occurred in 3 waves (1984-1985, 1987, and 2002-2003). To ensure comparability with the HIV-positive participants, site recruitment targeted individuals who engaged in high-risk behaviors for enrollment in the HIV-negative group. WIHS and MACS participants undergo semi-annual visits with a comprehensive physical examination, interviewer-administered questionnaire, and biological specimen collection at each visit. Additional details on the study design and methods have been described previously (19, 20).
Beginning in 2004, the WIHS and MACS initiated a carotid artery imaging study scanning participants without prevalent CVD at 2- to 4-year intervals to ascertain progression of subclinical carotid artery atherosclerosis (21). In 2015, a metabolomics study was introduced to measure metabolomic profiles in plasma samples of participants collected during the baseline carotid artery imaging study (2004-2006) (22). Our current analysis includes 694 participants (391 women and 303 men), aged 35 to 55 years, who were free of CVD, carotid artery plaque, and diabetes at the baseline visit of the carotid artery imaging study. All participants provided informed consent, and the study protocol and consent forms have been approved by the Institutional Review Board at each study site.
Plasma Lipidomic Profiling
Lipidomic profiling was performed on stored frozen plasma specimens using liquid chromatography-tandem mass spectrometry (LC-MS) at the Broad Institute. Details on sample extraction, separation, and MS settings have been described elsewhere (23). Briefly, LC-MS data were acquired using a Nexera X2 U-HPLC (Shimadzu Corp.; Marlborough, MA) coupled to an Exactive Plus mass spectrometer (Thermo Fisher Scientific; Waltham, MA). Lipid identities were denoted by total acyl carbon number and total number of double bonds. Raw data were processed using TraceFinder software (Thermo Fisher Scientific; Waltham, MA) and Progenesis QI (Nonlinear Dynamics; Newcastle upon Tyne, UK). A total of 218 lipid species were quantified, and the median coefficient of variation (CV) was 4.7% (range, 2.8%-31.4%), and 211 lipid species from 11 classes (16 classes/subclasses) were included in the analysis, after excluding 4 lipid species with CV ≥20%, and 3 lipid species with percentage of missing values ≥10%. Missing values due to below minimum detection level were imputed with one-half minimum values for a given lipid species. Detailed information on lipid classes/subclasses has been described elsewhere (18) and is also shown in Supplementary Table 1 (24).
Assessments of HIV Infection, Diabetes, and Other Variables
Data on demographic, behavioral, clinical, and laboratory variables were collected using standardized protocols at semi-annual visits (21). HIV infection was ascertained by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. HIV-specific parameters including CD4+ T-cell counts, HIV-1 viral load, and detailed information on classes of ART drugs (protease inhibitors [PI], nonnucleoside reverse transcriptase inhibitors [NNRTI] and nucleoside reverse transcriptase inhibitors [NRTI] have been described previously (25).
Incident diabetes was defined as the first time a study participant was observed with fasting glucose ≥126 mg/dL, glycated hemoglobin (HbA1c) ≥ 6.5%, or self-report of antidiabetic medication during follow-up. Fasting glucose, fasting insulin and HbA1c were measured using standard assays at central laboratories of the WIHS (Quest Diagnostics, Baltimore, MD) (5, 26) and MACS (Heinz Laboratory, Pittsburgh, PA) (27, 28). Insulin resistance was estimated using the homeostasis model assessment (HOMA-IR) equation, defined as (insulin [μU/mL] × glucose [mg/dL])/405 (29). Measurements of other cardiometabolic traits including body mass index (BMI), systolic blood pressure, high-density lipoprotein (HDL)-cholesterols and triglycerides have been described previously (30).
Statistical Analysis
Baseline characteristics of those with incident diabetes were compared to those without incident diabetes using the Mann–Whitney U test (continuous variables) and χ 2 test (categorical variables) overall, and separately in men and women. Sex-specific inverse normal transformations were performed on each lipid species before analyses.
Associations of plasma lipidomic profiles with risk of diabetes were examined in the following steps. First, multivariate Cox proportional hazards models (referred to as Cox model afterwards) stratified by sex were used to examine the association between each of the 211 lipid species and risk of diabetes, adjusting for age, race/ethnicity, education, and current smoking status (basic model). We used the Cox model stratified by sex to allow each sex to have its own baseline risk for diabetes. False discovery rate (FDR) was applied to control for multiple testing (31). Second, a lipid class/subclass–specific analysis was conducted. This focused on lipid class/subclasses for which at least one lipid species in this class/subclass was significantly associated with risk of diabetes after FDR correction. Lipid species which showed the strongest association (smallest P value) with risk of diabetes within each lipid class/subclass was selected as a representative lipid species (top lipid species) of correlated lipid species in the same class/subclass. If there were additional statistically significant lipid species not highly correlated with the top lipid (Spearman correlation <0.8) within the same class/subclass, we further conducted a joint analysis which included the top and each of the other lipids in the Cox model simultaneously. This lipid class/subclass–specific analysis allowed us to identify additional signals represented by secondary lipid species associated with risk of diabetes. Finally, for the featured lipid species (both top and secondary lipid species), the multivariate Cox model was further adjusted for HIV serostatus and ART use (HIV-uninfected, HIV-infected on ART, HIV-infected ART-naïve), CD4+ T-cell counts, and cardiometabolic risk factors, including BMI, systolic blood pressure, HDL cholesterols, triglycerides, and use of antihypertensive and lipid-lowering medications. Analyses were also conducted separately within HIV-infected and HIV-uninfected participants as well as within men and women. Possible effect modifications of lipid species by HIV infection or by sex were examined by including an interaction term in the Cox model. In addition, we also combined results from men and women by fixed-effect meta-analysis.
Partial Spearman correlation analysis was used to examine correlation coefficients of lipids species with fasting glucose, fasting insulin, HOMA-IR, and other cardiometabolic traits, and HIV parameters (CD4+ T-cell counts and viral load), after adjusting for age and sex. Linear regression was used to compare lipid species levels between HIV-infected and HIV-uninfected participants; and across 3 groups according to HIV status and current ART use (HIV-uninfected, HIV-infected on ART, HIV-infected ART-naïve), adjusting for age and sex. In addition, the associations between lipid species and 3 different classes of ART medications, including PI, NNRTI, and NRTI use, were also examined. All analyses were conducted in R 3.4.0 (The R Foundation for Statistical Computing, 2017).
Results
Participant Characteristics
In 694 participants (303 men and 391 women), we identified 166 incident diabetes cases (67 men and 99 women) during a median follow-up of 12.6 years (interquartile range [IQR], 11.8, 13.2) years. Table 1 shows baseline characteristics of these participants. As compared with women, men were older, better educated, more likely to be white, more likely to have lower BMI, and less likely to smoke. The contrast between incident diabetes cases and non-cases appeared to share a similar pattern for men and women: that is, participants who developed diabetes were more likely to be African Americans and smokers, were less educated, had higher levels of BMI, systolic blood pressure and triglycerides, and lower levels of HDL cholesterols. Baseline demographic, socioeconomic, and behavioral variables between the HIV-infected (n = 491) and HIV-uninfected (n = 203) groups were generally similar and comparable (Supplementary Table 2 (24)), which is in line with the design of WIHS and MACS (19, 20).
Table 1.
Participant Characteristics at Baseline
| All | Men (MACS) | Women (WIHS) | ||||
|---|---|---|---|---|---|---|
| Non-cases | Incident Diabetes | Non-cases | Incident Diabetes | Non-cases | Incident Diabetes | |
| No. of participants | 528 | 166 | 236 | 67 | 292 | 99 |
| Follow-up, years | 12.6 (11.8, 13.2) | - | 12.5 (12.0, 12.7) | - | 13.0 (8.6, 13.5) | - |
| Age, years | 44 (40-49) | 44 (40-49) | 47 (43-51) | 45 (41-50)a | 41 (38-46) | 43 (39-48) |
| Race/ethnicity | ||||||
| White/Other | 179 (33.9) | 31 (18.7)a | 152 (64.4) | 28 (41.8)a | 27 (9.2) | 3 (3.0) |
| Hispanic | 114 (21.6) | 38 (22.9) | 25 (10.6) | 9 (13.4) | 89 (30.5) | 29 (29.3) |
| African American | 235 (44.5) | 97 (58.4) | 59 (25.0) | 30 (44.8) | 176 (60.3) | 67 (67.7) |
| Education | ||||||
| Less than High School | 122 (23.1) | 54 (32.5)a | 15 (6.4) | 9 (13.4)a | 107 (36.6) | 45 (45.4) |
| High School | 117 (22.2) | 41 (24.7) | 27 (11.4) | 14 (20.9) | 90 (30.8) | 27 (27.3) |
| College and above | 289 (54.7) | 71 (42.8) | 194 (82.2) | 44 (65.7) | 95 (32.5) | 27 (27.3) |
| Current smoking | 212 (40.2) | 86 (51.8)a | 68 (28.8) | 31 (46.3) | 144 (49.3) | 55 (55.6) |
| BMI, kg/m2 | 25.9 (23.0-29.7) | 28.4 (25.0-32.1)a | 25.1 (22.6-27.7) | 26.5 (23.7-29.5)a | 26.9 (23.7-31.5) | 29.9 (26.5-34.5)a |
| Fasting glucose, mg/dL | 89 (82-96) | 93 (85-103)a | 96 (89-103) | 99 (92-107) | 85 (80-90) | 89 (83-97)a |
| Fasting insulin, μU/mL | 11 (8-15) | 14 (10-20)a | 12 (9-16) | 15 (12-20)a | 10 (6-15) | 12 (8-19)a |
| HOMA-IR | 2.4 (1.6-3.6) | 3.2 (2.2-4.6)a | 2.8(2.1-3.9) | 3.8(2.7-5.1)a | 2.1(1.3-3.2) | 2.9 (1.8-4.4)a |
| Systolic blood pressure, mmHg | 118 (109-127) | 121 (114-132)a | 121 (114-129) | 128 (120-135)a | 114 (106-123) | 118 (110-127)a |
| Triglyceride, mg/dL | 112 (75-170) | 128 (87-202)a | 125 (81-190) | 172 (115-227)a | 99 (72-145) | 108 (83-160) |
| HDL-cholesterol, mg/dL | 48 (40-58) | 43 (35-54)a | 47 (40-55) | 39 (34-45)a | 50 (40-6) | 48 (38-57)a |
| Antihypertensive medication use | 69 (13.1) | 40 (24.1) | 32 (13.6) | 17 (25.4)a | 37 (12.7) | 23 (23.2)a |
| Lipid-lowering medication use | 52 (9.9) | 11 (6.6) | 41 (17.4) | 10 (14.9) | 11 (3.8) | 1 (1.0) |
| HIV-specific characteristics b | ||||||
| HIV-infected participants | 370 (70.1) | 121 (72.9) | 156 (66.1) | 46 (68.7) | 214 (73.3) | 75 (75.8) |
| CD4+ T-cell count, cells/mm3 | 469 (315-654) | 501 (291-658) | 623 (429-911) | 657 (444-860) | 436 (296-616) | 465 (285-622) |
| Undetectable viral load, ≤80 copies/mL | 213 (57.6) | 55 (45.5)a | 109 (69.9) | 25 (54.3) | 104 (48.6) | 30 (40.0) |
| HIV-1 viral load, copies/mL c | 4413 (910-17 000) | 3350 (548-31 575) | 4413 (1190-19 100) | 20 045(2179-59 192) | 4550 (782-16 750) | 2800 (500-15 000) |
| ART use in past 6 month | 293 (79.2) | 89 (73.5) | 132 (84.6) | 37 (80.4) | 161 (75.2) | 52 (69.3) |
| PI use | 166 (44.9) | 45 (37.2) | 65 (41.7) | 17 (36.9) | 101 (47.2) | 28 (37.3) |
| NNRTI use | 135 (36.5) | 42 (34.7) | 74 (47.4) | 20 (43.5) | 61 (28.5) | 22 (29.3) |
| NRTI use | 290 (78.4) | 86 (71.1) | 131 (84.0) | 35 (76.1) | 159 (74.3) | 51 (68.0) |
Data are median (IQR) or n (%), assessed at baseline unless otherwise noted.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; IQR, interquartile range; MACS, Multicenter AIDS Cohort Study; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WIHS, Women’s Interagency HIV Study.
a P < 0.05 between incident diabetes cases and non-cases.
b HIV-specific characteristics were based on the HIV-infected participants only.
c Participants with undetectable viral load were excluded.
Lipidomic Profiles and Incident Diabetes
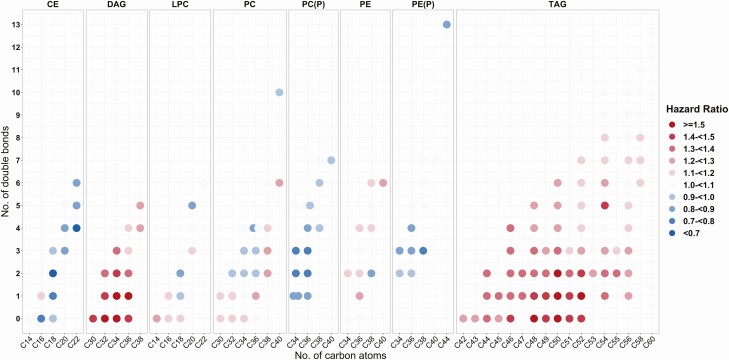
Among 211 individual lipid species examined in this analysis, 80 lipid species from 6 lipid classes (8 classes/subclasses), including cholesteryl esters (CEs), diacylglycerols (DAGs), lysophosphatidylcholines (LPCs), phosphatidylcholines (PCs) (including PC and PC plasmalogen [PC-P] subclasses), phosphatidylethanolamines (PEs) (including PE and PE plasmalogen [PE-P] subclasses), and triacylglycerol (TAGs), showed significant associations with risk of diabetes after FDR adjustment (Supplementary Fig. 1 and Supplementary Table 3 (24)). The top lipid species were CE (22:4), DAG (32:0), LPC (18:2), PC (P-34:3), PC (38:3), PE (36:1), PE (P-38:3) and TAG (50:1). We further plotted the association results of all lipid species in these 8 classes/subclasses with risk of diabetes by numbers of carbons and double bonds of the acyl chains (Fig. 1). Almost all the CEs, PCs-P, and PEs-P showed inverse associations with risk of diabetes, while all DAGs and TAGs showed positive associations with risk of diabetes. DAGs with short and saturated or mono-unsaturated (0 to 1 double bonds) acyl chains and TAGs with medium and saturated or mono-unsaturated acyl chains showed the strongest associations with risk of diabetes. In addition to individual lipid species analyses, we also examined associations of lipid classes/subclasses with risk of diabetes. DAG and TAG lipid classes, especially those with saturated or mono-unsaturated acyl chains, were significantly associated with risk of diabetes (Supplementary Table 4 (24)).
Figure 1.
Associations of individual lipid species from 8 lipid classes/subclasses with risk of diabetes. Individual lipid species are depicted by filled circles and arranged by lipid classes/subclasses according to the number of total carbon atoms (x-axis) and number of double bonds (y-axis). Data are hazard ratios for risk of diabetes per 1-SD increment of lipid species levels, stratified by sex and adjusted for age, race/ethnicity, education, study site, and current smoking. Abbreviations: CE, cholesteryl ester; DAG, diacylglycerol; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PC(P), phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE (P), phosphatidylethanolamine plasmalogen; TAG, triacylglycerol.
Through the joint analyses, we identified 3 secondary lipid species, LPC (14:0), PC (36:4), and PE (38:2), associated with risk of diabetes, independent of the top lipid species in the respective lipid classes/subclasses (Supplementary Fig. 2 (24)). All 3 secondary lipid species had opposite associations with risk of diabetes compared with the top lipid species in the same classes/subclasses, although a moderate level of positive correlations (r = 0.2 to 0.4) were observed between the top and secondary lipid species (Supplementary Table 5 (24)). For example, the top lipid species LPC (18:2) was inversely associated with risk of diabetes (hazard ratio [HR] = 0.72) while the secondary lipid LPC (14:0) had a positive association with risk of diabetes (HR = 1.40).
The multivariate adjusted HRs of the 11 featured (8 top and 3 secondary) lipid species with risk of diabetes are shown in Table 2. Six lipid species, CE (22:4), LPC (18:2), PC (P-34:3), PC (36:4), PE (38:2), and PE (P-38:3) were associated with decreased risk of diabetes (HRs = 0.63 to 0.79 per SD increment), while 5 lipid species, DAG (32:0), LPC (14:0), PC (38:3), PE (36:1) and TAG (50:1) were associated with increased risk of diabetes (HRs = 1.41 to 1.61), after adjustment for demographic, socioeconomic, behavioral, and HIV infection and related factors. After additional adjustment for traditional cardiometabolic risk factors, the strength of the associations between lipid species and risk of diabetes was somewhat attenuated, but all except PE (P-38:3) remained statistically significant.
Table 2.
Associations of 11 Featured Lipid Species With Risk of Diabetes
| Lipid species | All (N = 694) | HIV+ (N = 491) | HIV− (N = 203) | P for interaction | |||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| CE (22:4) | |||||||
| Model 1 | 0.63 (0.54, 0.75) | <0.001 | 0.65 (0.54, 0.78) | <0.001 | 0.54 (0.37, 0.79) | <0.001 | 0.69 |
| Model 2 | 0.70 (0.57,0.85) | <0.001 | 0.68 (0.54, 0.84) | <0.001 | 0.85 (0.49, 1.46) | 0.55 | 0.59 |
| DAG (32:0) | |||||||
| Model 1 | 1.61 (1.37, 1.90) | <0.001 | 1.61 (1.34, 1.95) | <0.001 | 1.59 (1.15, 2.20) | <0.001 | 0.73 |
| Model 2 | 1.56 (1.27, 1.93) | <0.001 | 1.65 (1.29, 2.10) | <0.001 | 1.16 (0.75, 1.80) | 0.49 | 0.38 |
| LPC (18:2) a | |||||||
| Model 1 | 0.72 (0.60, 0.86) | <0.001 | 0.76 (0.62, 0.93) | <0.001 | 0.62 (0.43, 0.88) | <0.001 | 0.44 |
| Model 2 | 0.79 (0.65, 0.96) | 0.02 | 0.84 (0.67, 1.05) | 0.14 | 0.74 (0.47, 1.16) | 0.19 | 0.54 |
| LPC (14:0) a | |||||||
| Model 1 | 1.41 (1.18, 1.69) | <0.001 | 1.30 (1.04, 1.62) | 0.01 | 1.71 (1.21, 2.41) | <0.001 | 0.31 |
| Model 2 | 1.28 (1.06, 1.55) | 0.01 | 1.20 (0.96, 1.51) | 0.1 | 1.35 (0.93, 1.96) | 0.11 | 0.5 |
| PC (P-34:3) | |||||||
| Model 1 | 0.74 (0.63, 0.87) | <0.001 | 0.74 (0.61, 0.90) | <0.001 | 0.74 (0.54, 1.00) | 0.05 | 0.81 |
| Model 2 | 0.79 (0.65, 0.96) | 0.02 | 0.76 (0.60, 0.97) | 0.02 | 0.89 (0.60, 1.34) | 0.59 | 0.59 |
| PC (38:3) a | |||||||
| Model 1 | 1.44 (1.19, 1.73) | <0.001 | 1.30 (1.05, 1.60) | 0.01 | 2.00 (1.34, 2.98) | <0.001 | 0.12 |
| Model 2 | 1.26 (1.02, 1.56) | 0.02 | 1.15 (0.90, 1.48) | 0.23 | 1.60 (1.00, 2.56) | 0.04 | 0.15 |
| PC (36:4) a | |||||||
| Model 1 | 0.76 (0.64, 0.90) | <0.001 | 0.85 (0.70, 1.03) | 0.11 | 0.56 (0.40, 0.78) | <0.001 | 0.04 |
| Model 2 | 0.82 (0.68, 0.98) | 0.03 | 0.90 (0.72, 1.13) | 0.39 | 0.60 (0.39, 0.91) | 0.01 | 0.09 |
| PE (36:1) a | |||||||
| Model 1 | 1.46 (1.22, 1.76) | <0.001 | 1.48 (1.18, 1.84) | <0.001 | 1.42 (1.03, 1.96) | 0.03 | 0.83 |
| Model 2 | 1.37 (1.11, 1.69) | <0.001 | 1.42 (1.09, 1.86) | <0.001 | 1.17 (0.78, 1.74) | 0.43 | 0.95 |
| PE (38:2) a | |||||||
| Model 1 | 0.70 (0.58, 0.83) | <0.001 | 0.74 (0.60, 0.91) | <0.001 | 0.61 (0.44, 0.86) | <0.001 | 0.27 |
| Model 2 | 0.76 (0.63, 0.93) | <0.001 | 0.83 (0.66, 1.04) | 0.12 | 0.66 (0.46, 0.95) | 0.02 | 0.23 |
| PE (P-38:3) | |||||||
| Model 1 | 0.79 (0.67, 0.93) | <0.001 | 0.80 (0.66, 0.97) | 0.02 | 0.76 (0.54, 1.07) | 0.12 | 0.92 |
| Model 2 | 0.89 (0.74, 1.06) | 0.21 | 0.89 (0.72, 1.10) | 0.30 | 0.92 (0.64, 1.34) | 0.69 | 0.91 |
| TAG (50:1) | |||||||
| Model 1 | 1.56 (1.32, 1.83) | <0.001 | 1.57 (1.30, 1.89) | <0.001 | 1.51 (1.08, 2.12) | 0.01 | 0.65 |
| Model 2 | 1.48 (1.20, 1.83) | <0.001 | 1.59 (1.25, 2.02) | <0.001 | 0.94 (0.58, 1.53) | 0.82 | 0.28 |
Data are hazard ratios (HR) and 95% CI of incident diabetes per SD increment of lipid species levels, stratified by sex and adjusted for age, race/ethnicity, education, study site, current smoking, HIV serostatus and treatment status (HIV-uninfected, HIV-infected ART-using, HIV-infected ART-naïve), and CD4 cell counts (Model 1); and further adjusted for systolic blood pressure, HDL-cholesterol, triglycerides, BMI, antihypertensive medication use, and lipid-lowering medication use (Model 2).
Abbreviations: CE, cholesteryl ester; DAG, diacylglycerol; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PC(P), phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE (P), phosphatidylethanolamine plasmalogen; TAG, triacylglycerol.
a Top and Secondary lipid species within the same lipid classes/subclasses were included in the Cox models simultaneously to estimate HRs (95% CI) and P values.
There was little evidence for effect modification by HIV on associations of these 11 lipid species and risk of diabetes. Only PC (36:4) showed a stronger association with risk of diabetes in HIV-uninfected individuals compared with those with HIV infection (raw P for interaction = 0.04) (Table 2). Associations between these lipid species and risk of diabetes were generally consistent between men and women, except for PE (P-38:3) which showed a stronger association with risk of diabetes in men compared with women (raw P for interaction = 0.02) (Supplementary Table 6 (24)). However, none of these interaction terms remained significant after FDR adjustment. Similar results were obtained when using a meta-analysis approach to combine results from men and women and test potential sex-differences in associations between lipid species and risk of diabetes (Supplementary Table 6 (24)).
Considering potential inaccuracy of using the HbA1c test among HIV-infected people (27), we performed a sensitivity analysis by removing HbA1c criteria from the definition of diabetes. This new definition led to a reduction of 14 incident diabetes cases, but the overall patterns of associations between lipid species and risk of diabetes remained the same (data not shown).
Diabetes-Associated Lipid Species and Cardiometabolic Risk Factors
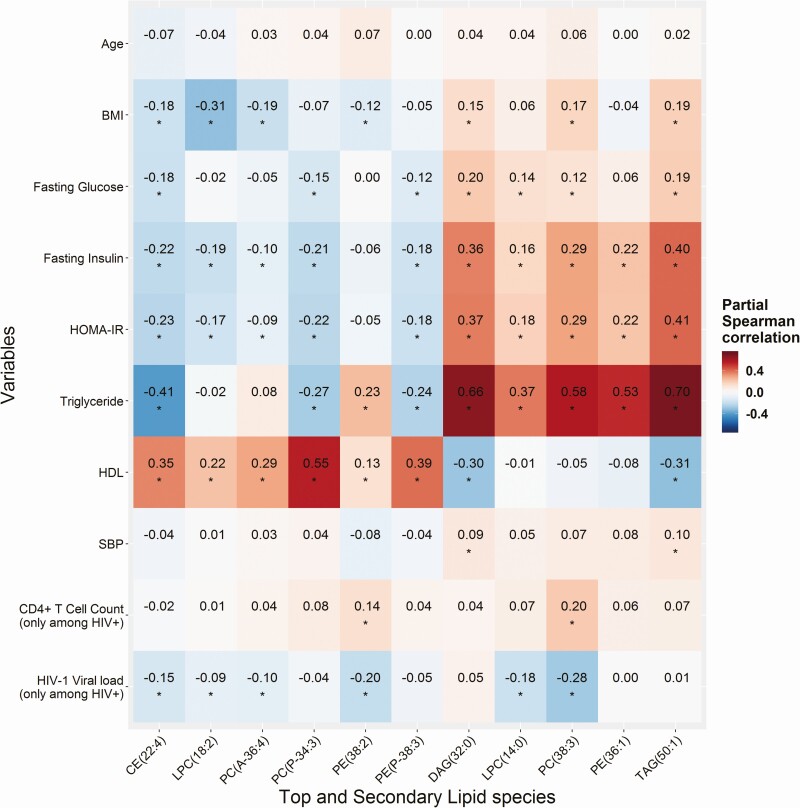
Figure 2 shows correlations between 11 diabetes-associated lipid species and traditional cardiometabolic risk factors. The 6 lipid species that were associated with decreased risk of diabetes generally showed favorable correlations with adiposity (low BMI), glycemic traits (low levels of fasting glucose, fasting insulin, and HOMA-IR), and blood lipids (low triglycerides, high HDL-cholesterol), though there was a weak-to-moderate positive correlation between PE (P-38-3) and triglycerides. The 5 lipid species that were associated with increased risk of diabetes showed unfavorable correlations with these traditional cardiometabolic risk factors, with the strongest correlations observed for high triglycerides.
Figure 2.
Partial Spearman correlation of lipid species with diabetes and HIV related factors. Data are partial Spearman correlation coefficients (r) of 11 diabetes-associated lipid species levels with age, BMI, fasting glucose, fasting insulin, HOMA-IR, HDL-cholesterol and triglycerides (excluding participants taking lipid-lowering medication); systolic blood pressure (excluding participants taking antihypertensive medication); and baseline CD4+ T-cell count and HIV-1 viral load (among people with HIV infection only), adjusting for age and sex. *P value < 0.05. Abbreviations: BMI, body mass index; CE, cholesteryl ester; DAG, diacylglycerol; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PC(P), phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE (P), phosphatidylethanolamine plasmalogen; TAG, triacylglycerol.
Correlation patterns between these lipid species and cardiometabolic risk factors were generally similar between HIV-infected and HIV-uninfected individuals, except for PC (36:4) and PE (38:2), which showed significant inverse correlations with fasting insulin and HOMA-IR only in HIV-uninfected but not in HIV-infected individuals (Supplementary Fig. 3 (24)). Among HIV-infected individuals, a few weak-to-moderate correlations were observed between some of these diabetes-associated lipid species and CD4 T-cell counts and/or HIV-1 viral load (Fig. 2).
HIV Infection, ART Use, and Diabetes-Associated Lipid Species
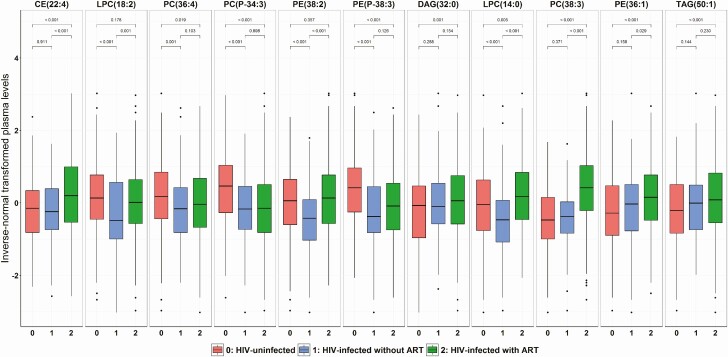
We then examined the relationships of these diabetes-associated lipid species with HIV infection and ART use (Fig. 3 and Supplementary Table 7 (24)). Among 6 lipid species associated with decreased risk of diabetes, PC (36:4), PC (P-34:3), and PE (P-38:3) were significantly lower in HIV-infected individuals (using or not using ART combined) compared with HIV-uninfected individuals, and there were no significant differences between HIV-infected individuals using or not using ART. Plasma LPC (18:2) was significantly lower in HIV-infected compared with HIV-uninfected individuals, but the levels were elevated in HIV-infected individuals using ART compared with those not using ART. Plasma CE (22:4) was significantly higher in HIV-infected compared with HIV-uninfected individuals, which was primarily driven by elevated levels in HIV-infected individuals using ART. PE (38:2) showed no significant differences between HIV-infected and HIV-uninfected individuals, but the levels were significantly higher in HIV-infected using ART compared with those not using ART.
Figure 3.
Plasma levels of diabetes-associated lipid species according to HIV infection and ART use. Data are inverse normal transformed levels of 11 diabetes-associated lipid species in HIV-uninfected individuals (red), HIV-infected ART-naïve (blue), and HIV-infected using ART (green). P values for comparisons between groups were calculated using Wilcoxon rank sum test. Abbreviations: ART, antiretroviral therapy; CE, cholesteryl ester; DAG, diacylglycerol; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PC(P), phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE (P), phosphatidylethanolamine plasmalogen; TAG, triacylglycerol.
Among the 5 lipid species associated with increased risk of diabetes, DAG(32:0), PC(38:3), PE(36:1), and TAG(50:1) were significantly higher in HIV-infected compared with HIV-infected individuals, with the highest levels in HIV-infected individuals on ART (Fig. 3). Although the overall comparison between HIV-infected and HIV-uninfected individuals did not show significant difference in LPC (14:0), the levels were significantly higher in HIV-infected individuals using ART compared with HIV-infected individuals not using ART and HIV-uninfected individuals.
Further analyses by different classes of ART drugs showed that PI-based ART had the most significant influences on plasma levels of these lipid species. PI use was associated with elevated levels of 8 lipid species, NRTI use was associated with elevated levels of 6 lipid species, and NNRTI use was associated with elevated levels of 3 lipid species (Supplemental Fig. 4 (24)).
Discussion
In the combined 2 cohorts of 694 men and women, including HIV-infected and HIV-uninfected individuals, 80 out of 211 lipid species examined from 16 lipid classes were identified to be individually associated with risk of diabetes. Because most lipid species in the same lipid class/subclass were correlated, a lipid class/subclass–specific analysis was conducted to further examine the independent effects of lipid species on risk of diabetes, revealing 11 featured lipid species (8 top and 3 secondary) from 8 lipid classes/subclasses. The observed associations between these lipid species and diabetes risk were supported by their correlations with traditional risk factors. However, their associations with risk of diabetes, except for PE (P-38:3), were only slightly attenuated after controlling for traditional risk factors, suggesting potentially independent effects of these lipid species on risk of diabetes.
Consistent with previous studies in non-HIV cohorts (11-16), our individual lipid species analyses indicated that almost all the CEs, PC(P)s and PE(P)s were inversely associated with risk of diabetes, while many PCs and PEs, and all DAGs and TAGs were positively associated with risk of diabetes. Notably, after controlling for the top lipid species within each class/subclass, we identified 3 secondary lipid species (LPC [14:0], PC [36:4] and PE [38:02]) which showed opposite associations with risk of diabetes, compared with the top lipid species (LPC [18:2], PC [38:3], and PE [36:1]. Before adjusting for the top lipid species, associations of these secondary lipid species with risk of diabetes could have been masked because of their positive correlations with the top lipid species. Consistent with this idea, 2 previous studies in non-HIV cohorts also found all TAGs to be associated with a higher risk of diabetes, while after controlling for total TAG content, several TAGs showed inverse associations with risk of diabetes (14, 16). We found similar results for the TAG-specific analyses, although the emerging inverse associations did not reach significance. These data suggested that correlations among lipid species need to be taken into account in the analyses.
In line with our results on PC(P)s and PE(P)s, plasmalogens have been suggested to have antioxidant, anti-apoptotic and anti-inflammatory functions which may lower risk of diabetes (32, 33). LPCs may improve glucose metabolism through enhanced insulin sensitivity and anti-inflammation (34-36). Alteration in skeletal muscle phospholipid metabolism (increased PCs and decreased PEs) due to CEPT1 deficiency has been related to improved insulin sensitivity in high-fat diet–fed mice (37). However, the biological mechanisms underlying the associations between individual lipid species and risk of diabetes remains unclear. Our data suggest that lipid species within the same class/subclass, even though they are correlated with each other, may have different biological links with diabetes due to structural features of the acyl chains.
This study included both HIV-infected and HIV-uninfected individuals, but we did not find strong evidence for HIV serostatus modification on the associations between lipid species and risk of diabetes. Only one lipid species PC (36:4) showed a suggestive interaction with HIV infection (although it did not pass FDR correction), with a significant inverse association with risk of diabetes in HIV-uninfected individuals but not in HIV-infected. Consistently, PC (36:4) showed inverse correlations with insulin resistance markers only in HIV-uninfected but not in HIV-infected individuals. Previous studies in non-HIV cohorts also showed the inverse associations of PC (36:4) with insulin resistance (38) and type 2 diabetes (15). We observed a significantly lower level of PC (36:4) in HIV-infected compared with HIV-uninfected individuals, but it is unknown whether this might influence its association with risk of diabetes in HIV-infected people.
A unique contribution of this study is that we investigated the relationships of HIV infection and ART use with diabetes-associated lipid species and found some intriguing results. For the 5 lipid species associated with increased risk of diabetes, their plasma levels were all elevated in HIV-infected individuals on ART, especially in those with PI use. This is consistent with reports that PI-based ART may have more detrimental effects on lipid metabolism and glucose metabolism compared with other ART regimens (1-3, 7, 8). The observed relationships of these lipid species with ART use and risk of diabetes are in the expected directions (ie, elevated lipid species in ART use were associated with higher risk of diabetes), which supports one of the pathophysiological hypotheses that ART use may disrupt lipid metabolism, and thus contribute to insulin resistance and increased risk of diabetes (7, 8). However, interestingly, we found that several lipid species (ie, CE [22:4], LPC [18:2] and PE [38:2]) which were associated with lower risk of diabetes, were also elevated in HIV-infected individuals using ART. Although further validation is needed, these unexpected results indicates that ART use might increase levels of some lipid species which could be beneficial for diabetes, suggesting a complex role of ART use in the development of diabetes among HIV-infected individuals (8).
To the best of our knowledge, this study is the first prospective study of lipidomics and risk of diabetes in the context of HIV infection. Major strengths of the study include well-characterized HIV cohorts with both HIV-infected and HIV-uninfected participants, high-throughput and high-quality lipidomic profiling, a long-term follow-up on diabetes status, and multiple cardiometabolic measures which may provide some information on plausible mechanisms. However, this study has several limitations. Due to the nature of observational study design, this study cannot make causal inferences. Our untargeted lipidomics had a broad coverage of lipid species but did not measure absolute values of lipid species levels. Although our cohort allowed sex-specific analyses, sex differences could not be easily identified due to differences in sociodemographic characteristics between women and men. Because of a relatively small number of HIV-uninfected participants, this study has limited statistical power to detect potential effect modification by HIV infection. Future studies with larger number of both HIV-infected and HIV-uninfected individuals are needed to validate and expand our findings.
In summary, this lipidomic study in 2 HIV cohorts identified multiple lipid species in plasma associated with development of diabetes, independent of traditional risk factors. Many of these diabetes-associated lipid species were altered in HIV-infected individuals, especially in those using ART. These findings suggest that ART-related plasma lipidomic alterations may precede the development of diabetes among HIV-infected people, which provides new evidence at a lipid species level on the relationships among HIV infection, ART use, dyslipidemia, and risk of diabetes.
Acknowledgments
We thank all investigators, staffs and participants of the MACS/WIHS Combined Cohort Study (MWCCS) for their contributions to this study. Data in this manuscript were collected by the MWCCS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241-01; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201-01; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204-01; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202-01; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193-01; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245-01; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240-01; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01-HL146242-01; Los Angeles CRS (Roger Detels), U01-HL146333-01; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205-01; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203-01; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208-01; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192-01; and UNC CRS (Adaora Adimora), U01-HL146194-01. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Glossary
Abbreviations
- ART
antiretroviral therapy
- BMI
body mass index
- CE
cholesteryl ester
- DAG
diacylglycerol
- FDR
false discovery rate
- HbA1c
glycated hemoglobin
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment for insulin resistance
- HR
hazard ratio
- IQR
interquartile range
- LPC
lysophosphatidylcholine
- MACS
Multicenter AIDS Cohort Study
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PC
phosphatidylcholine
- PC-P
phosphatidylcholine plasmalogen
- PE
phosphatidylethanolamine
- PE-P
phosphatidylethanolamine plasmalogen
- PI
protease inhibitor
- TAG
triacylglycerol
- WIHS
Women’s Interagency HIV Study
Financial Support: The metabolomics profiling was supported by the National Heart, Lung, and Blood Institute (NHLBI) K01HL129892, and other funding sources for this study include NHLBI R01HL140976 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK119268 to Q.Q., NHLBI R01 HL126543, R01 HL132794, R01HL083760, and R01HL095140 to R.C.K., NHLBI R01HL095129 to W.S.P., and NHLBI K01HL137557 to D.B.H.
Additional Information
Disclosures: None of the authors reported a conflict of interest related to the study. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13(10):F63-F70. [DOI] [PubMed] [Google Scholar]
- 2. Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51-F58. [DOI] [PubMed] [Google Scholar]
- 3. Dubé MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350(9079):713-714. [DOI] [PubMed] [Google Scholar]
- 4. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179-1184. [DOI] [PubMed] [Google Scholar]
- 5. Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61(3):334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ledergerber B, Furrer H, Rickenbach M, et al. ; Swiss HIV Cohort Study . Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45(1):111-119. [DOI] [PubMed] [Google Scholar]
- 7. Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2011;8(1):11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. [DOI] [PubMed] [Google Scholar]
- 9. Feeney ER, Mallon PW. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haffner SM; American Diabetes Association . Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S68-S71. [DOI] [PubMed] [Google Scholar]
- 11. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121(4):1402-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drogan D, Dunn WB, Lin W, et al. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem. 2015;61(3):487-497. [DOI] [PubMed] [Google Scholar]
- 14. Razquin C, Toledo E, Clish CB, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED Trial. Diabetes Care. 2018;41(12):2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merino J, Leong A, Liu CT, et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia. 2018;61(6):1315-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu J, Lam SM, Wan Q, et al. High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care. 2019;42(11):2117-2126. [DOI] [PubMed] [Google Scholar]
- 17. Zhao W, Wang X, Deik AA, et al. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection. Circulation. 2019;139(17):2003-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chai JC, Deik AA, Hua S, et al. Association of lipidomic profiles with progression of carotid artery atherosclerosis in HIV infection. JAMA Cardiol. 2019;4(12):1239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Detels R, Jacobson L, Margolick J, et al. The multicenter AIDS Cohort Study, 1983 to …. Public Health. 2012;126(3):196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61(4):640-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qi Q, Hua S, Clish CB, et al. Plasma Tryptophan-Kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. 2018;67(2):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137(8):841-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang E, Chai J, Deik A, et al. Supplemental data from: Plasma lipidomic profiles and risk of diabetes: two prospective cohorts of HIV-infected and HIV-uninfected individuals. medRxiv. Posted November 13, 2020. Doi: 10.1101/2020.11.11.20229351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008;22(13):1615-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and insulin resistance in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49(4):369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slama L, Palella FJ Jr, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother. 2014;69(12):3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19(13):1375-1383. [DOI] [PubMed] [Google Scholar]
- 29. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 30. Hanna DB, Lin J, Post WS, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis. 2017;215(9):1352-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y. Controlling the false discovery rate–a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. [Google Scholar]
- 32. Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9):1442-1452. [DOI] [PubMed] [Google Scholar]
- 33. Huynh K, Martins RN, Meikle PJ. Lipidomic profiles in diabetes and dementia. J Alzheimers Dis. 2017;59(2):433-444. [DOI] [PubMed] [Google Scholar]
- 34. Lehmann R, Franken H, Dammeier S, et al. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care. 2013;36(8):2331-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yea K, Kim J, Yoon JH, et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem. 2009;284(49):33833-33840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasegawa H, Lei J, Matsumoto T, Onishi S, Suemori K, Yasukawa M. Lysophosphatidylcholine enhances the suppressive function of human naturally occurring regulatory T cells through TGF-β production. Biochem Biophys Res Commun. 2011;415(3):526-531. [DOI] [PubMed] [Google Scholar]
- 37. Funai K, Lodhi IJ, Spears LD, et al. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes. 2016;65(2):358-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nowak C, Salihovic S, Ganna A, et al. Effect of insulin resistance on monounsaturated fatty acid levels: a multi-cohort non-targeted metabolomics and mendelian randomization study. Plos Genet. 2016;12(10):e1006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.