Abstract
Context
Postprandial hyperglycemia increases systemic inflammation and is a risk factor for cardiovascular disease. A ketone monoester (KME) drink containing β-hydroxybutyrate (β-OHB) rapidly lowers plasma glucose, which may be a strategy protecting against postprandial hyperglycemia.
Objective
We hypothesized that KME would attenuate 2-hour postprandial glucose, lower systemic inflammation, and improve vascular function in adults with obesity.
Methods
In a randomized crossover design, 14 participants with obesity (age = 56 ± 12 years; body mass index = 32.8 ± 7.7 kg/m2) consumed KME (12 g β-OHB) or placebo 15 minutes prior to each meal for 14 days with all meals provided and matched between conditions. Postprandial glycemia was assessed by continuous glucose monitoring. Vascular function and inflammation were assessed before and after treatment periods.
Results
Postprandial glucose was 8.0% lower in KME versus placebo (g = 0.735; P = 0.011) and 24-hour average glucose reduced by 7.8% (g = 0.686; P = 0.0001). Brachial artery flow-mediated dilation increased from 6.2 ± 1.5% to 8.9 ± 3.3% in KME (g = 1.05; P = 0.0004) with no changes in placebo (condition × time interaction, P = 0.004). There were no changes in plasma cytokines; however, lipopolysaccharide-stimulated monocyte caspase-1 activation was lower following KME supplementation versus placebo (stimulation × condition × time interaction; P = 0.004). The KME supplement was well tolerated by participants and adherence to the supplementation regimen was very high.
Conclusions
In adults with obesity, 14 days of premeal KME supplementation improves glucose control, enhances vascular function, and may reduce cellular inflammation. KME supplementation may be a viable, nonpharmacological approach to improving and protecting vascular health in people with heightened cardiometabolic risk.
Keywords: β-hydroxybutyrate, glucose-lowering, flow-mediated dilation, NLRP3 inflammasome, inflammation, postprandial glucose
Accumulating evidence indicates that the ketone body β-hydroxybutyrate (β-OHB) has many important physiological functions extending beyond its traditional role as an energy source during periods of fasting or low carbohydrate availability (1). β-OHB is now a recognized signaling molecule that can influence metabolic control, inflammation, oxidative stress, and cardiovascular function (2-5). β-OHB has direct anti-inflammatory effects through inhibition of the nucleotide-binding domain, leucine-rich repeat, pyrin domain containing 3 (NLRP3) inflammasome and reduced production of interleukin (IL)-1β and IL-18 (3). In cell culture and rodent models, β-OHB has also been shown to reduce vascular cell senescence and is hypothesized to improve endothelial and smooth muscle cell function (4, 5). These recent discoveries have generated substantial interest in exploring the role of ketogenic diets and fasting as therapies for cardiometabolic disease (6, 7).
Exogenous ketone supplements provide an alternative means to raise circulating β-OHB without the need for restrictive dietary practices. The most potent exogenous ketone supplement is the ketone monoester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (8). Upon ingestion, the ketone monoester is cleaved by gut and liver esterases to produce a rapid elevation in circulating β-OHB to levels of ~1 to 3 mM, equivalent to several days of fasting or following a strict ketogenic diet. Elevations in circulating β-OHB can rapidly shift metabolism; we have recently shown that acute supplementation with a single ketone monoester drink improves glucose tolerance in both normoglycemic adults (9) and adults with obesity (10). These findings suggest that raising circulating β-OHB may be a strategy for attenuating the harmful cardiometabolic effects of elevated postprandial hyperglycemia (11, 12). Chronic supplementation with exogenous ketones provides the potential for targeted and sustained elevation in circulating ketone bodies, in the absence of restrictive dietary practices, that could be used to not only reduce prevailing glucose levels but capitalize on several of the therapeutic signaling properties recently attributed to β-OHB. Although the safety and tolerability of oral supplementation with ketone monoester have been established (13, 14), the potential therapeutic implications have not been explored.
The purpose of this double-blind placebo-controlled crossover study was to investigate the effect of a 14-day premeal β-OHB supplementation intervention on postprandial glycemia, vascular function, and inflammatory markers in adult humans with obesity. We hypothesized that thrice daily ingestion of a β-OHB supplement 15 minutes prior to each meal would reduce postprandial hyperglycemia, improve vascular function, and lower inflammation when compared to placebo.
Methods
Study Approval
The present trial was approved by the University of British Columbia Clinical Research Ethics Board (ID H18-02930) and was registered as “Ketone Supplementation, Glucose Control, and Cardiovascular Function” (ClinicalTrials.gov identifier NCT03817749). The trial conformed with the standards set by the Declaration of Helsinki and subsequent revisions. All participants provided written informed consent prior to commencement of trial activities.
Participants Recruitment and Randomization
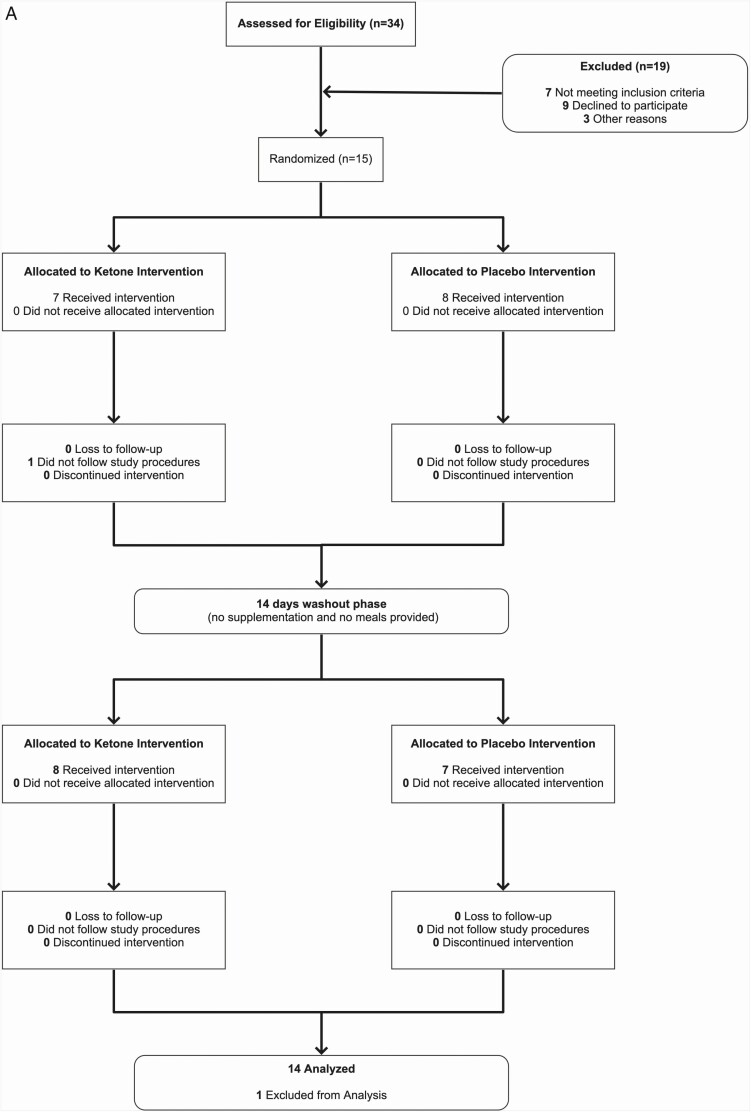
Participant recruitment was performed between March 2019 and January 2020 through advertisements around the University of British Columbia Okanagan Campus and the greater Kelowna area. An initial phone screening was performed to assess participant eligibility. If deemed eligible for the study, participants were invited into the lab for an in-person familiarization visit. Recruitment was closed once 15 participants were enrolled in the study. Overall, 14 participants completed the trial and were included in the final analysis (Figure 1; Table 1). Following the familiarization visit, participants were randomly assigned to either the ketone or placebo condition for 14 days and after a washout period of at least 14 days completed the alternate condition. The randomization schedule was prepared using an online generator using block sizes of 2 and 4 (https://www.sealedenvelope.com/simple-randomiser/v1/lists [accessed 12 Mar 2019]). Sealed opaque envelopes were used to reveal the randomization sequence, which was denoted as A-B or B-A with identity of the ketone and placebo supplement blinded to both researchers and participants (details below).
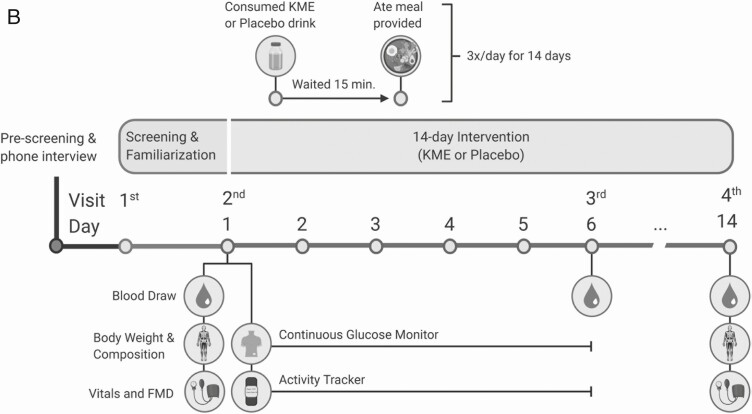
Figure 1.
Trial details. A, flow chart of study recruitment and facilitation. B, trial schematic. All pre- and post-intervention measures were made with participants in a fasting, non-supplemented state.
Table 1.
Anthropometric, Hemodynamic, and Fasting Metabolic Responses to 14-Day Intervention
| Placebo (n = 14) | Ketone (n = 14) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Body Weight (kg) | 90.3 (24.3) | 89.2 (24.4)a | 90.5 (24.5) | 89.5 (24.7)a |
| Body mass index (kg/m2) | 32.7 (7.6) | 32.3 (7.7)a | 32.8 (7.7) | 32.4 (7.7)a |
| Lean body mass (kg) | 52.5 (12.0) | 52.3 (12.7) | 52.4 (11.9) | 51.8 (12.1) |
| Fat mass (kg) | 37.8 (16.9) | 36.9 (16.9) | 38.1 (17.2) | 37.7 (17.6) |
| % Fat mass | 40.4 (9.7) | 39.2 (9.9) | 40.6 (9.7) | 40.5 (10.2) |
| Waist circumference (cm) | 106.2 (18.3) | 106.2 (18.6) | 106.5 (19.2) | 105.3 (19.1) |
| Systolic blood pressure (mmHg) | 131 (17) | 126 (16)a | 132 (17) | 127 (18)a |
| Diastolic blood pressure (mmHg) | 81 (12) | 79 (13) | 81 (12) | 80 (10) |
| Heart rate (bpm) | 65 (10) | 62 (10) | 63 (10) | 62 (9) |
| Glucose (mmol/L) | 4.69 (0.54) | 4.79 (0.53) | 4.79 (0.47) | 5.00 (0.49) |
| Insulin (uIU/mL) | 20.8 (12.) | 21.9 (14.9) | 22.4 (15.1) | 20.0 (11.8) |
| C-peptide (pmol/L) | 2275.0 (896.7) | 2279.8 (903.8) | 2590.5 (1062.9) | 2226.0 (1106.8) |
| NEFA (mmol/L) | 0.65 (0.19) | 0.50 (0.22)a | 0.61 (0.13) | 0.50 (0.12)a |
| HOMA-IR (AU) | 2.11 (1.15) | 2.08 (1.06) | 2.29 (1.58) | 2.04 (1.15) |
Data are means (SD).
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance; NEFA, nonesterified fatty acids.
asignificantly different compared to PRE (main effect of time), P < 0.05
Inclusion Criteria
-
•
Elevated waist circumference (>102 cm for males, >88 cm for females) and/or
-
•
Obesity (body mass index > 30 kg/m2) and/or
-
•
Diagnoses of prediabetes based on A1C (5.7%-6.4%) and/or fasting plasma glucose (5.6-6.9 mmol/L) using American Diabetes Association (ADA) criteria (15)
-
•
Age 30 to 69 years
Exclusion Criteria
-
•
Competitively trained endurance athlete
-
•
Actively attempting to lose weight
-
•
History of mental illness or existing neurological disease(s)
-
•
Previous cardiovascular events (eg, heart attack, stroke)
-
•
Tobacco or drug use
-
•
Diagnoses of diabetes
-
•
History of hypoglycemia
-
•
Irritable bowel syndrome or inflammatory bowel disease
-
•
Taking medication that may interfere with insulin sensitivity
-
•
Currently following a ketogenic diet or taking ketone supplements
-
•
Unable to commit for 2 separate 14-day supplementation and controlled feeding periods
Intervention Design
The present trial was a randomized, placebo-controlled double-blinded crossover trial following the 2010 CONSORT guidelines (16). All experimental visits were conducted in the Exercise Metabolism and Inflammation Laboratory at the University of British Columbia, Okanagan Campus, Canada. Participants attended 3 visits to the lab per condition (Pre-intervention, continuous glucose monitor [CGM] removal, and Post-intervention). Following completion of the first condition, participants resumed free-living for at least 2 weeks before commencing the alternate condition.
The intervention involved 14 days of 3-times daily premeal supplementation with either a ketone monoester or placebo supplement. Both ketone monoester and placebo supplement were provided in single serving opaque bottles (ie, 42 bottles per condition). Participants were instructed to ingest one supplement bottle 15 minutes prior to breakfast, lunch, and dinner in order to allow for β-OHB absorption. Participants were permitted to consume only water during the 15-minute period prior to meals. Compliance was assessed by self-report log and returning of empty supplement bottles. Given the bitter taste of the ketone monoester, participants consumed single dose of the ketone supplement (30 mL, 12 g β-OHB; ie, the same dose used throughout the study) during the familiarization visit to ensure tolerability.
All food was provided to participants for each 14-day intervention period. Breakfast, lunch, and dinner meals were provided by a local meal preparation service and were composed of approximately 50% energy from carbohydrate, 30% fat, and 20% protein. Participants selected meals and prepackaged snacks prior to their first intervention period, in order to meet individual estimated daily energy requirements based on the Harris-Benedict equation with an activity factor of 1.4 (17). An example menu plan is provided in Supplemental Table S1 and Figure S5 (18). A research assistant delivered one day of meals and snacks to participants prior to the first visit of both conditions to ensure the exact same foods were eaten prior to pre-intervention measures. Participants received 5 days’ worth of meals and snacks at the pre-intervention visit with subsequent food being picked up or delivered based on participant preference. In addition to providing all food to be consumed, participants recorded all food and drink consumed during their first trial condition in a detailed food log and any deviations from the meal plan were matched for the second trial condition.
Visit Protocols
Pre- and Post-Intervention Visits
Participants arrived at the lab between 8:00-9:00 am local time, following an overnight fast (>10 hours), having abstained from alcohol and caffeine for at least 12 hours and any exercise other than activities of daily living for 24 hours. During the pre-intervention visit, anthropometric measurements were taken, venous blood was drawn, and a CGM device was inserted into each participant’s subcutaneous abdominal tissue by a trained researcher. Following this, participants lay supine in a bed for 15 minutes, after which endothelial function and blood pressure were assessed, respectively. Upon completion of this visit, participants were given 5 days’ worth of food and supplements and left the lab wearing a wrist-worn activity monitor. The remaining 9 days of supplements were given during the CGM removal visit. Aside from consuming premeal supplements and eating only the food provided to them, participants were instructed to maintain regular activities of daily living. Researchers maintained regular communication with participants via email and text messages to ensure compliance and to address any concerns. The post-intervention visit was identical to the pre-intervention visit, except for the insertion of the CGM and provision of supplements.
Supplement Details
The ketone supplement used in this study was (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester (∆G, HVMN Inc, San Francisco, USA). The ketone supplement contained 0.4 g of β-hydroxybutyrate (β-OHB) per mL, natural flavoring, and <2% stevia leaf extract. A taste-matched placebo drink was formulated based on a recipe provided by HVMN, Inc and included the same natural flavoring and stevia leaf extract with 5 mL Bittrex stock (0.005 g denatonium benzoate powder in 40 mL water) and 1.5 mL arrowroot stock (2 g arrowroot powder in 50 mL water) to match the bitter flavor and viscosity of the ketone monoester. The ketone and placebo supplements were aliquoted into opaque 30 mL bottles (12 g β-OHB per dose; 36 g β-OHB per day) and labeled either A or B by a third-party researcher. The labeled bottles were stored in separate, identical plastic containers. The third-party researcher maintained the blinding code and the researchers involved the trial facilitation were blinded to condition throughout the data collection and analysis phases.
Trial Outcomes
All data analysis was performed by researchers blinded to condition, and whenever possible, measurement time point. Researchers were unblinded once all data analysis was complete.
Anthropometry and Body Composition
Body mass was measured to the nearest 0.1 kg and body composition was obtained via bioelectrical impedance (Tanita TBF-410GS; Tanita Corp, Arlington Heights, USA). Height was measured to the nearest 1 cm using a stadiometer. Waist circumference was measured at the middle distance between the last floating rib and the iliac crest using a tape measure. Waist circumference was measured by the same researcher for the entire study.
Blood Samples
Fasting venous blood samples were obtained from participants during the pre- and post-intervention visits by a trained phlebotomist. Three 4 mL EDTA tubes were obtained per blood draw. Blood was analyzed for C-peptide, insulin, plasma glucose, nonesterified fatty acids (NEFA), circulating cytokines including interleukin (IL)-1ra, IL-6, IL-10, IL-18, and tumor necrosis factor-α (TNF-α).
Blood β-OHB levels were measured via finger prick (FreeStyle Precision Neo, Abbott Laboratories, IL, USA) 15 minutes postconsumption to provide an indication of expected β-OHB levels achieved by premeal supplementation throughout the trial.
Quantification of Activated Caspase-1
Activated caspase-1, a marker of NLRP3 inflammasome activation, was quantified by flow cytometry using the carboxyfluorescein fluorochrome inhibitor of caspases (FAM-FLICA) Caspase-1 Assay Kit containing the fluorescent inhibitor probe FAM-YVAD-FMK) (FAM-FLICA; ImmunoChemistry Technologies). The FAM-YVAD-FMK was reconstituted to 150× stock with dimethyl sulfoxide as per the product specifications and stored at −20 °C for later use. The 150× stock FAM-YVAD-FMK was then diluted to 30× working concentration with phosphate-buffered saline (PBS). The working concentration was used within 10 minutes of preparation. Treatment of Pre and Post samples was identical. EDTA blood was processed within 10 minutes of sample collection by aliquoting whole blood into 2 polypropylene culture tubes: 1 unstimulated and 1 stimulated with lipopolysaccharide (LPS). Both tubes contained media with 5.5 mM glucose (RPMI 1640; Corning). LPS, a known activator of the NLRP3 inflammasome (19) (10 ng/mL in culture) was added to the stimulated tube in order to induce caspase-1 activation in human monocytes. Both sample conditions were then incubated at 37 °C and 5% CO2 in the dark for 1 hour. Following the first incubation, FAM-YVAD-FMK (30× concentration) was added to each sample tube (1× final concentration). Samples were incubated for a further 50 minutes at 37 °C and 5% CO2 in the dark. Conjugated antibody specific for human cluster of differentiation (CD)14-Vioblue (Clone REA599; Miltenyi Biotec) and CD45-APCVio770 (Clone REA747; Miltenyi Biotec) were added to each culture and incubated for 10 minutes. Red blood cell lysis buffer (Miltenyi Biotec; 1× final concentration) was then added to the samples, which were lysed for 15 minutes in the dark at room temperature. Samples were centrifuged at 250g for 5 minutes at 20 °C and then resuspended in apoptosis wash buffer (ImmunoChemistry Technologies; 1× final concentration). Samples were washed twice by centrifuging at 250g for 5 minutes at 20 °C and then resuspended in apoptosis wash buffer. Propidium iodide (PI; Miltenyi Biotec) was added immediately prior to data acquisition for dead cell exclusion.
Samples were run immediately through a MACSQuant Analyzer (Miltenyi Biotec) flow cytometer with a stop gate set for 15 000 CD14+ monocytes (Supplemental Figure S6E) (18). Positive staining for either CD14, CD45, or FAM-YVAD-FMK (representative of activated caspase-1) is indicative of greater presence of the target antigen. CD45+/CD14+/FAM-YVAD-FMK+ monocytes and CD45+/FAM-YVAD-FMK+ granulocytes were identified using a hierarchical gating strategy (Supplemental Figure S6) (18). First, singlets were selected (Figure Supplemental S6A) followed by exclusion of dead cells (PI+; Supplemental Figure S6B) (18). Leukocytes were selected by positive staining for CD45 APC-Vio770 (Supplemental Figure S6C) (18). To select monocytes, CD45+ leukocytes that exhibited positive staining for the monocyte-specific marker CD14 VioBlue (Supplemental Figure S6D) (18) were backgated to confirm membership by selecting based on characteristic scatter profile (Supplemental Figure S6E) (18). Presence of active caspase-1 (FAM-YVAD-FMK+) in CD45+/CD14+ monocytes was then quantified (Supplemental Figure S6F) (18). All gates were set with fluorescence minus-one (FMO) controls. Data were analyzed with MACSQuantify (Version 2.13, Miltenyi Biotec).
To assess a complimentary marker of downstream caspase-1 activation, secretion of IL-1β was measured by collecting supernatants from parallel whole blood cultures that were stimulated with LPS for 2 hours. Cytokine secretion was not measured in unstimulated culture supernatants (which is analogous to fasting plasma) because IL-1β is typically undetectable in such samples.
Continuous Glucose Monitoring
24-hour glucose control and 2-hour postprandial glucose responses were measured via blinded CGM (iPro2 Professional, Medtronic, Dublin, Ireland). CGM devices were inserted during the pre-intervention visits and were worn for at least 4 days during the supplementation period. To calibrate CGM devices, participants measured blood glucose via finger prick (FreeStyle Precision Neo, Abbott Laboratories, IL, USA) 15 minutes prior to each meal (immediately before consuming a supplement dose; 3× daily), and once before bed. Following CGM removal, CGM data was uploaded onto the Medtronic CareLink iPro2 Software and calibration data were entered for each day worn. Raw CGM data files were cleaned and cross-referenced by 2 independent researchers that were blinded to condition allocation after all trials were complete. First, time-course graphs were created for the entire CGM wearing period and were visually inspected for issues related to loss of signal or device malfunction (eg, flat-lining or glucose reading <3.5 mM for an extended period). Inspections revealed that <10% of data was flagged as missing or irregular based on these criteria. Viable data were then organized by day in order to investigate individual meals. Breakfast, lunch, and dinner meals were identified using the premeal, presupplementation calibration timestamps and confirmed by participant logbook entries. The calibration timestamp served as the premeal glucose measurement and 2-hour postprandial glucose was measured starting at the beginning of mealtime, as recorded in participants’ logbooks. Researchers then went through participant logbooks to confirm that meals consumed were matched between conditions. If meals were not matched, the data was flagged and was not used in analysis. A valid day consisted of full, uninterrupted CGM data (no loss of signal or data integrity concerns) for a 24-hour period where meal matching was achieved. Using data from valid condition-matched days, 2-hour glucose area under the curve (AUC) and incremental glucose AUC (iAUC) were calculated for breakfast, lunch, and dinner using Prism GraphPad 8.4.1. AUC and iAUC outcomes are the mean of all 3 meals for each condition. Measures of 24-hour glucose exposure and variability were calculated using an open-access glycemic variability calculator spreadsheet (EasyGV 9.0.R2, University of Oxford). The CGM data for 24-hour glucose measurements were derived from same matching days used for AUC analysis.
Hemodynamic Assessments
Endothelial function was measured by flow-mediated dilation (FMD) of the right brachial artery using a 12.5 MHz linear transducer attached to a high-resolution ultrasound machine (Terason 3200, Teratech, USA) following current guidelines (20, 21). Following 15 minutes of supine rest, simultaneous recordings of brachial artery diameter and blood velocity were obtained for 1 minute from a longitudinal section of the upper arm, 2 to 3 cm proximal to the antecubital fossa. The insonation angle for velocity acquisition was 60º. A pneumatic cuff was positioned 1 to 2 cm distal from the olecranon process of the forearm and was rapidly inflated to >50 mmHg above systolic blood pressure for 5 minutes. The cuff was then rapidly deflated and acquisition of brachial artery diameter and blood velocity continued for 3 minutes postdeflation. All ultrasound images were recorded using screen capture technology and stored as video files for offline analysis, which was performed using edge-detection software (BloodFlow Software Version 5.1, Australia) (22). Arterial blood pressure was measured using an automatic blood pressure monitor with participants in a supine position following completion of the FMD test (Omron 10 Series BP786N, Omron Healthcare Inc., Kyoto, Japan).
Physical Activity Monitoring
In order to track physical activity, participants were given wrist-worn devices (FitBit Charge 3, San Francisco, USA) to measure step count for the duration of the intervention. Participants were instructed to wear the device at all times (waking and sleep). Blinding of daily steps was not possible; however, all data from devices were synced to a computer by a researcher and participants were not able to see their total physical activity trends.
Questionnaires
Participants were asked to report their feelings of hunger and fullness following their meals, and gastrointestinal symptoms following supplement ingestion during the first 4 days of each condition. The hunger and fullness questionnaire was a 4-question, 10-cm visual analog scale (VAS). The questions were (1) “How hungry do you feel?” (2) “How satisfied do you feel?” (3) “How full do you feel?” and (4) “How much do you think you can eat?” The gastrointestinal symptoms questionnaire was a 5-point, 10-cm VAS that asked participant to rate their feelings of nausea, urge to vomit, bloating, belching, and cramping.
Sample Size
Sample size was determined based on our previous observations that ketone monoester ingestion prior to an oral glucose tolerance test attenuates glucose AUC by ~15% (9). Using means and standard deviations from the existing literature for glucose AUC in individuals with prediabetes following an oral glucose tolerance test (OGTT) (1800 ± 360 mmol/L.min) a sample size of 10 was calculated to detect a conservative 15% reduction in the primary outcome of glucose AUC (calculated effect size, d = 1.1), assuming 90% power with an alpha level of 0.05 and a moderate correlation between repeated measures of r = 0.7 (calculated using G*Power v3.1). To preserve power and account for any dropouts or missing CGM data we aimed to recruit 15 participants.
Blood Analysis
For all blood analysis, EDTA blood was collected at each timepoint and centrifuged for 15 minutes at 2000g at 4 °C. Plasma was aliquoted and frozen immediately at −80 °C for batch analysis.
Cytokines
Plasma cytokines (IL-1β, IL-6, TNF-α, IL-10, IL-18 and IL-1RA) and whole blood culture supernatant IL-1β were assessed by U-PLEX Metabolic Group 1 (human) assay kit (Mesoscale Discovery). The U-PLEX assay was performed in duplicate with samples diluted 1:1 as per the specifications of the kit and analyzed on a MESO QuickPlex SQ 120 Imager (Mesoscale Discovery). The dynamic range (lower limit of detection [LLOD]; upper limit of detection [ULOD]) for each cytokine was as follows: IL-1β (LLOD = 0.061 pg/mL; ULOD = 1910 pg/mL); IL-6 (LLOD = 0.175 pg/mL; ULOD = 495 pg/mL); TNF-α (LLOD = 0.411 pg/mL; ULOD = 1830); IL-10 (LLOD = 0.1 pg/mL; ULOD = 929 pg/mL); IL-18 (LLOD = 0.352, ULOD = 21 200 pg/mL); IL-1RA (LLOD = 0.983 pg/mL; ULOD = 2530 pg/mL).
Circulating Metabolic Markers
Circulating free fatty acids were batch-analyzed using the Wako HR Series NEFA-HR kit using the ACS/ACOD method on a Chemwell 2910 Chemistry Analyzer (Awareness Technologies).
Plasma glucose samples were batch-analyzed using the Glucose Hexokinase assay (G7517-120; Pointe Scientific Inc.) on a Chemwell 2910 Chemistry Analyzer (Awareness Technologies). Samples for insulin and C-peptide were analyzed in duplicate as per the specifications of the U-PLEX Metabolic Group1 (human) assay kit (Mesoscale Discovery). The dynamic ranges were as follows: Insulin (LLOD = 0.32 μIU/mL; ULOD = 7360020μIU/mL); C-peptide (LLOD = 14 pg/mL; ULOD = 7610 pg/mL).
Statistical Analysis
Data were analyzed using Prism GraphPad 8.4.1. Data are presented as mean ± SD unless otherwise noted. Given the randomized crossover design, statistical analysis was first performed to assess time period effect (ie, trial 1 vs 2) on outcomes, and then the effect of condition (A vs B—conditions blinded for analyses) was assessed. Q-Q plots and Shapiro-Wilk tests were used to assess normality and skewness within each trial and condition. The criteria of >1.5 × the interquartile range (IQR) was used to assess potential outliers and/or influential data points. If one or more criteria were not met, data were either log-transformed or excluded as appropriate. The 2-hour postprandial AUC and iAUC were calculated using Prism GraphPad 8.4.1 and differences in postprandial glucose AUCs and measures of 24-hour glucose control between conditions were compared using a paired t test. Hunger/fullness and gastrointestinal symptoms VAS differences were compared between conditions using a Wilcoxon’s Signed Rank Test for matched pairs. Pearson Product-Moment correlations were used to test exploratory relationships and simple linear regressions were used to model significant correlations.
All dependent variables collected in the fasted state before (Pre) and after (Post) the 14-day supplementation period were analyzed using a linear mixed-effects model (LMEM) with condition and time as fixed effects and participant as a random factor. Tukey post hoc tests were used to evaluate within-condition preplanned contrasts following significant interactions. Cohen’s d effect sizes were calculated for pairwise comparisons between Pre and Post within each condition with a Hedge’s g correction for small sample sizes.
Within the fasted plasma cytokine data, IL-6 and IL-10 data were log-transformed to meet statistical assumptions. One data set within IL-18 placebo data was excluded from analyses as it was overtly influential, resulting in N = 14 in ketone and N = 13 in placebo. IL-1RA and TNF-α data were log-transformed and analyzed with and without one dataset due to values >1.5 × IQR. However, since neither exclusion nor inclusion of these data affected the outcome, these data were included in the reported analyses. Presence of an order effect for IL-1RA was detected and controlled for statistically.
Caspase-1 activation measured by flow cytometry was analyzed by LMEM with participant as a random factor and 3 fixed factors to examine condition (ketone vs placebo), time (Pre vs Post), LPS-stimulation status (stimulated vs unstimulated). A significant 3-way interaction was further probed with a 2-factor (condition × stimulation) LMEM to explore whether the change in caspase-1 data from Pre to Post was significantly different between condition or stimulation status. All flow cytometry data met statistical assumptions except for 1 potential outlier with data >1.5 × interquartile range for the Pre-Post change in LPS-stimulated caspase-1 in the ketone condition. Inclusion of this data point did not impact the 3-way interaction in the overall LMEM (P = 0.004 vs P = 0.008) or the 2-way interaction (P = 0.014 vs P = 0.007) but did alter the statistical significance of the post hoc test (P = 0.07 vs P = 0.014). Data with or without this potential statistical outlier are reported in the text. Statistical significance was set at P < 0.05.
Results
Participants
A total of 10 females and 4 males completed the intervention (age = 56 ± 12 years) (Figure 1A; Table 1). The supplement compliance rate was 98.7% ± 3.2% for the entire trial and there were no differences in compliance between the ketone and placebo conditions (98.0% ± 4.1% vs 99.3% ± 1.8% respectively; P = 0.25). To assess whether ketone supplementation impacts appetite-related variables compared to placebo in free-living conditions in the current study, participants reported feelings of hunger, fullness, satisfaction, and capacity to eat more using a VAS during the post-supplementation/post-meal period. There were no significant differences in any of these measures between ketone and placebo across the 14 days (Supplemental Figure S1) (18). Similarly, the reported gastrointestinal symptoms were very low and there were no significant differences between conditions (Supplemental Figure S2) (18). There was no difference in daily step count, measured by wrist-worn activity trackers, between ketone (8300 ± 3826 steps/day) and placebo (8889 ± 5564 steps/day) conditions (P = 0.654).
Fasting Metabolic Outcomes
There were no changes in fasting plasma glucose, insulin, C-peptide, or homeostasis model assessment of insulin resistance (HOMA-IR; Table 1). There were small, yet systematic, reductions in measures of body mass (−1.03 kg; main effect of time P < 0.001), systolic blood pressure (−5 mmHg; P = 0.025), and NEFA (−0.037 mmol/L; P = 0.004) following each of the 14-day intervention periods that were independent of ketone or placebo condition (Table 1). A single, 1-g β-OHB dose elevated circulating β-OHB to 1.8 ± 1.3 mM [range, 0.6 to 4.7mM] within 15 minutes of consumption (Supplemental Figure S3) (18).
Continuous Glucose Monitoring
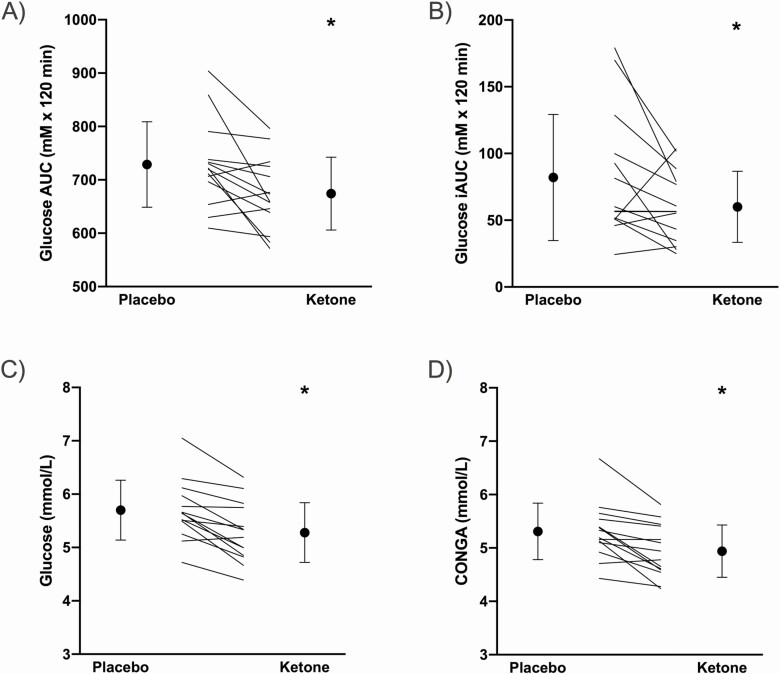
The average of the 2-hour post-meal glucose area under the curve (AUC) following breakfast, lunch, and dinner was measured using continuous glucose monitoring (CGM) during the first 4 days of the intervention. Premeal ketone supplementation reduced 2-hour postprandial glucose AUC by 8% compared with placebo (mean difference [95% CI] = −54.6 mmol/L × 120 minutes [−94.4, −14.8], g = 0.735, P = 0.011; Figure 2A). When controlling for premeal glucose, ketone supplementation attenuated the 2-hour postprandial incremental glucose AUC (iAUC) by 36% compared with placebo (−21.9 mmol/L × 120 minutes [−43.9, −0.1]; g = 0.574, P = 0.049; Figure 2B). Within the same condition, there were no differences between breakfast, lunch, or dinner meals for either postprandial glucose AUC or iAUC. There were significant reductions in 24-hour mean glucose (∆ −0.41 mmol/L [−0.25, −0.58], g = 0.686 P = 0.0001) and continuous overall net glycemic action (CONGA; ∆ −0.37 mmol/L [−0.181, −0.562], g = 0.577, P = 0.001) in the ketone condition compared to placebo (Figure 2).
Figure 2.
Glucose responses to intervention as measured by CGM. A, 2-hour glucose AUC; B, 2-hour glucose iAUC; C, 24-hour average glucose; and D, continuous overall net glycemic action (CONGA). *Significantly different compared to placebo condition, P < 0.05 (paired t test).
Vascular Function
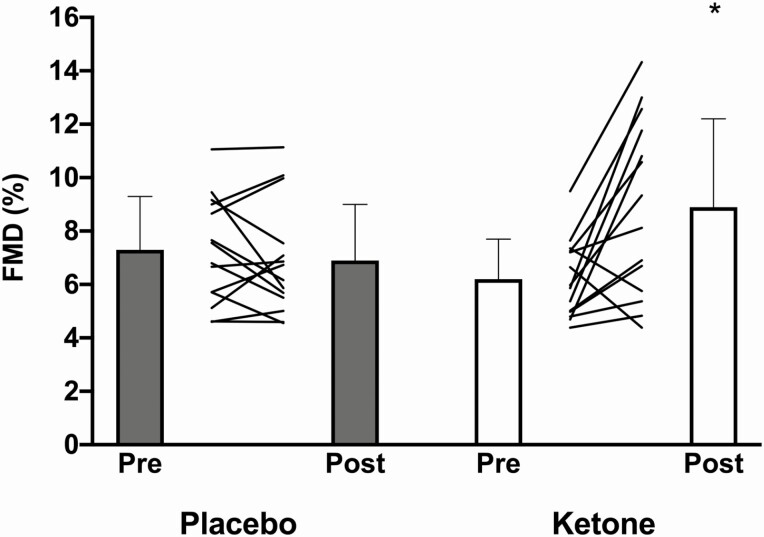
There was a significant time × condition interaction such that %FMD significantly increased from 6.2 ± 1.5% to 8.9 ± 3.3% (∆ +2.7% [0.98, 4.4]; g = 1.05; P = 0.0004) following 14-days of ketone supplementation, whereas there was no change in %FMD in the placebo condition (7.3 ± 2.0% to 6.9 ± 2.1%; ∆ −0.35% [−1.4, 2.1], g = −0.173, P = 0.942) (Figure 3; Table 2). There were no differences in shear-rate AUC or time to peak between conditions. Although there was a significant interaction in baseline diameter (P = 0.018), the absolute FMD in placebo was identical pre- vs post-intervention, and sensitivity analysis further revealed that baseline diameter did not affect absolute FMD across trials (P = 0.394).
Figure 3.
Flow-mediated dilation (FMD) responses before (Pre) and after (Post) 14-day premeal supplementation with either placebo or ketone. Data are not corrected for baseline diameter, as sensitivity analysis revealed no effect of baseline diameter on the FMD response. The time × condition interaction was significant (P < 0.05) in a linear mixed-effect model. *Significantly different compared to Pre within condition, P < 0.05.
Table 2.
FMD Responses to 14-Day Intervention
| Placebo (n = 14) | Ketone (n = 14) | Two-way LMEM | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Condition | Time | Interaction | |
| Baseline diameter (mm) | 3.91 (0.81) | 4.05 (0.90) | 3.98 (0.75) | 3.82 (0.71)a | 0.130 | 0.904 | 0.018 |
| Peak diameter (mm) | 4.19 (0.84) | 4.33 (0.95) | 4.22 (0.77) | 4.15 (0.73) | 0.242 | 0.440 | 0.090 |
| FMD (mm) | 0.28 (0.09) | 0.28 (0.10) | 0.24 (0.06) | 0.33 (0.12)a | 0.676 | 0.017 | 0.011 |
| FMD (%) | 7.3 (2.0) | 6.9 (2.1) | 6.2 (1.5) | 8.9 (3.3)a | 0.335 | 0.021 | 0.004 |
| SRAUC (103 au) | 16.6 (6.1) | 16.9 (5.6) | 17.2 (8.5) | 20.3 (11.2) | 0.458 | 0.474 | 0.675 |
| Time to peak (s) | 59 (26) | 51 (21) | 64 (26) | 66 (19) | 0.084 | 0.668 | 0.350 |
Data are means (SD).
Abbreviations: FMD, flow-mediated dilation; LMEM, linear mixed-effects model; SRAUC, shear rate area under the curve.
aSignificantly different preplanned post hoc contrasts compared with Ketone Pre, P < 0.05
Monocyte NLRP3 Activation Status
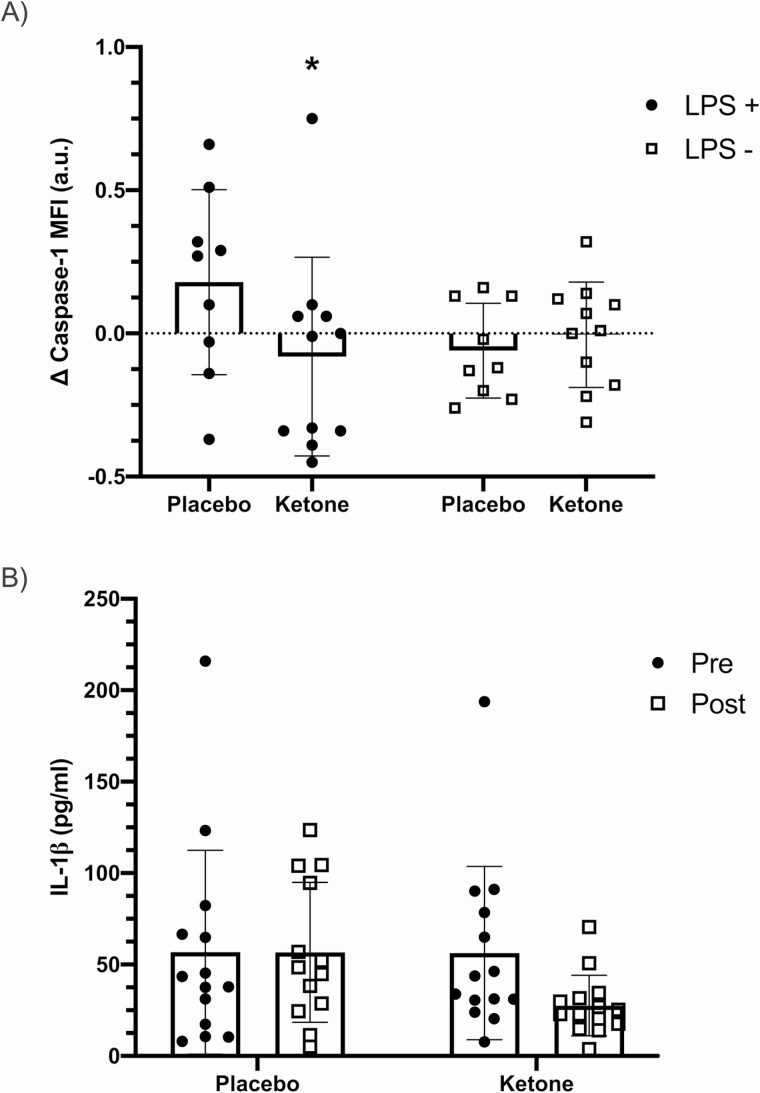
LPS-stimulated caspase-1 activation was significantly different in whole blood cultures following ketone supplementation compared to placebo (stimulation × time × condition interaction, P = 0.004; Figure 4A). As a follow-up exploration of this significant 3-way interaction, a 2-factor linear mixed model on the change scores from Pre to Post supplementation was conducted and a significant condition × stimulation interaction (P = 0.007) was found. The post hoc test for the change in caspase-1 activation in LPS-stimulated cultures comparing ketone with placebo approached statistical significance with inclusion of one statistical outlier (change score >1.5 × interquartile range) (∆ −0.26 [−0.54, 0.02], g = 0.775, P = 0.07). When this potential outlier was removed from the analyses, the reduction in LPS-stimulated caspase-1 activation in the ketone condition was statistically significant compared to placebo (∆ −0.32 [−0.58, −0.067], g = 1.24, P = 0.014).
Figure 4.
A, Change in monocyte caspase-1 activation after the 14-day intervention (Post minus Pre) measured in whole blood, cultured with lipopolysaccharide (LPS+) or without (LPS−). The stimulation × condition interaction was significant (P < 0.05). B, IL-1β expression following 2-hour of LPS-stimulation before (Pre) and after (Post) the 14-day intervention (time × condition interaction P = 0.053). *Significantly different from placebo condition on preplanned contrast (P < 0.05). Abbreviation: MFI, median fluorescence intensity.
To further probe the significant reduction in caspase-1 activation in monocytes following ketone monoester supplementation, we measured IL-1β secretion into cultures after a 2-hour stimulation with LPS (Figure 4B). Results indicated a tendency for a significant time × condition interaction (P = 0.053) with a ~51% reduction in LPS-stimulated IL-1β secretion following ketone monoester supplementation period (g = 0.808).
Circulating Cytokines
Table 3 reports fasting measures of circulating cytokines. Overall, there were no changes in either proinflammatory (IL-18, IL-6, and TNF-α) or anti-inflammatory (IL-10 and IL-1ra) cytokines measured in fasting blood samples before and after the 14-day intervention (all cytokines, P > 0.05; Table 3).
Table 3.
Fasting Plasma Cytokine Responses to 14-Day Intervention
| Placebo (n = 14) | Ketone (n = 14) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| IL-18 (pg/mL)* | 326.6 (174.2) | 311.8 (174.6) | 329.4 (168.2) | 321.9 (149.2) |
| IL-1RA (pg/mL) | 205.1 (117.5) | 197.5 (94.4) | 232.9 (103.8) | 219.8 (125.1) |
| IL-6 (pg/mL) | 1.30 (0.73) | 1.20 (0.46) | 1.23 (0.72) | 1.39 (0.86) |
| IL-10 (pg/mL) | 0.300 (0.158) | 0.270 (0.129) | 0.315 (0.173) | 0.286 (0.142) |
| TNF-α (pg/mL) | 1.83 (0.63) | 1.77 (0.50) | 1.94 (0.66) | 1.82 (0.66) |
Data are means (SD).
Abbreviations: IL, interleukin; IL-1RA, interleukin-1 receptor agonist; TNF-α, tumor necrosis factor alpha.
*n = 13 for IL-18 placebo due to removal of outlier.
Discussion
The purpose of this double-blind placebo-controlled crossover study was to investigate the impact of a 14-day premeal ketone monoester supplementation on postprandial glucose, vascular function, and markers of inflammation in participants with obesity. The main findings from this study are: (1) premeal ketone supplementation significantly lowered postprandial glucose AUC and improved 24-hour glucose control during the supplementation period; (2) brachial artery FMD significantly increased following ketone supplementation; (3) ketone supplementation reduced monocyte NLRP3 activation in ex vivo LPS-stimulated whole blood cultures; and (4) the intervention was well tolerated with high compliance. These findings demonstrate that 14 days of ketone monoester supplementation is a viable strategy for improving glycemic control, which could benefit cardiovascular function and impact immune cell function in adults with obesity.
Ketone Supplementation Lowers 2-hour Glucose AUC and Improves 24-hour Glycemic Control
The primary objective of the current study was to determine if premeal ketone monoester supplementation could attenuate postprandial hyperglycemia. We found that ketone supplementation lowered the average 2-hour post-meal glucose AUC by 8%. Accounting for premeal baseline, ketone monoester supplementation lowered 2-hour glucose iAUC by 36%. We have previously shown that ingestion of a ketone supplement prior to a single OGTT lowers glucose AUC in lean adults (9) and adults with obesity (10). The rapid glucose-lowering effects of a single dose of ketone supplement are supported by several recent studies where ketone supplements were consumed prior to exercise (2, 23-25). The current study convincingly extends these findings by demonstrating that the glucose-lowering effect of consuming a ketone supplement persists across the day in an ecologically relevant setting where meals were controlled but participants were free-living.
Although the exact mechanisms are yet to be fully elucidated, β-OHB appears to lower glucose by causing a reduction in hepatic glucose output and not an increase in peripheral glucose uptake (8, 26-28). Previous work has found that infusion of ketone bodies can reduce plasma glucose without alterations in insulin or glucagon (27, 29, 30). However, an acute rise in β-OHB can increase circulating insulin concentrations to a small extent (8, 31) yet we have demonstrated that prior ingestion of ketone monoester does not augment insulin secretion during an OGTT (9, 10), which suggests the glucose-lowering effects of oral exogenous ketone supplementation are not due to higher insulin. Acute β-OHB ingestion inhibits lipolysis via direct binding to GPR109A (also known as the hydroxycarboxylic acid receptor 2 [HCAR2]; or niacin receptor 1 [NIACR1]) (32), which reduces circulating NEFA (9, 10) and suppresses hepatic glucose output (33). Overall, the ability of premeal ketone monoester supplementation to lower postprandial hyperglycemia across multiple meals and days seen here indicates that such a strategy could help improve overall glucose control.
In the current study, we measured the response of circulating β-OHB levels 15 minutes after ingestion of a single dose of 12 g β-OHB during the familiarization visit. We found that β-OHB levels increased to 1.8 ± 1.3 mmol/L (range, 0.6-4.7 mmol/L) at 15 minutes after supplementation. β-OHB was not measured throughout the 14-day intervention to maintain blinding. Discussions of an optimal β-OHB threshold must be prefaced by the intended therapeutic application of ketone supplementation (6). In the case of glucose-lowering, studies have found that β-OHB levels as low as 0.8 to 1.0 mM elicit glucose-lowering effects (8, 31). Accordingly, although each premeal supplement dose in this study may have led to lower circulating β-OHB than previous acute (8-10) and longer-term ketone supplementation studies (13), it was effective at significantly increasing β-OHB and lowering postprandial glucose.
Although the primary outcome was 2-hour postprandial glucose levels, it is likely that the glucose-lowering effects of β-OHB persisted beyond this observation window. Previous studies have shown that circulating β-OHB levels remain elevated for >3 hours following ingestion of a single ketone dose (8). Using CGM as a research tool allowed us to further explore the impacts of ketone versus placebo supplementation on glucose control. In support of a more global effect on improving glucose control, we observed significant reductions in 24-hour mean glucose and CONGA, a measure of glycemic variability, in the ketone condition compared to placebo. We attribute the collective improvements in glycemic control to the direct presence of elevated circulating β-OHB during CGM monitoring, as 14 days of ketone supplementation did not affect fasting plasma glucose measured during the post-intervention visit, and there was no change in HOMA-IR despite improvements in 24-hour glucose control.
Ketone Supplementation Improves Vascular Function
Acute hyperglycemia negatively impacts vascular function (11, 12, 34), and postprandial hyperglycemia is an independent risk factor for cardiovascular disease (35). Atherogenic changes are preceded by marked reductions in endothelial-dependent vascular function (36). Given the glucose-lowering effects of premeal β-OHB ingestion (9, 10), we hypothesized that 14 days of ketone supplementation would improve endothelial function assessed by brachial artery FMD. In line with our hypothesis, we found that ketone supplementation significantly increased brachial artery FMD. The changes in FMD appear related to improvements in vascular function per se, as there was no difference in the shear stress stimulus between trials.
Mechanistically, acute oscillatory hyperglycemia increases production of reactive oxygen species (ROS) and inflammatory cytokines, which impairs FMD to a greater extent than stable hyperglycemia in people with normal and impaired glucose tolerance (11, 37). Accordingly, we speculate that a systematic reduction in postprandial glucose with ketone ingestion attenuated the pulsatile production of ROS and inflammatory mediators, which mitigated the negative effects on endothelial function throughout the 14-day intervention period. Moreover, β-OHB promotes protection against oxidative stress through upregulation of antioxidant defense genes (38), providing an additional mechanism that protects the endothelium beyond lowered glucose.
Acute hyperglycemia also increases sympathetic nerve activity (39) and chronically elevated sympathetic nerve activity is linked to endothelial dysfunction and cardiovascular disease progression (40, 41). β-OHB inhibits sympathetic nerve activity in a dose-dependent manner through the G protein-coupled receptor 41 (GPR41) in cardiac tissue and sympathetic neurons (42), and GPR41 is also expressed on endothelial cells. Antagonization of endothelial GPR41 by short-chain fatty acids induces vasodilation and lowers blood pressure during phenylephrine infusion in mice (43). Whether β-OHB has a similar effect on endothelial GPR41 has yet to be determined. Preliminary evidence from human trials supports that β-OHB may lower blood pressure following acute (10) and short-term ketone supplementation (44). Interestingly, β-OHB infusion reduces total peripheral vascular resistance without concomitant changes in arterial blood pressure in healthy adults and patients with heart failure (45). However, in the current trial, systolic blood pressure measured in the fasting state was reduced following both the placebo and β-OHB supplementation interventions, which is likely attributable to the tightly controlled and standardized healthy diet provided to the participants.
The observed improvements in FMD may have also been facilitated by the direct action of β-OHB on the vascular endothelium (4, 5). In a rat model of diabetes, the provision of 200 mg/kg/day β-OHB for 10 weeks prevented aortic endothelial cell injury and increased serum NO through the β-hydroxybutyrylation of histone H3K9 (5). This in turn upregulated vascular endothelial growth factor expression, a factor critical for vascular integrity and function. Similarly, Han et al (2018) (4) found that β-OHB protects the vasculature in vivo by preventing replicative- and stress-induced senescence of vascular cells and inducing vascular cell quiescence via the upregulation of Oct4. Evidence from a recent human trial shows that β-OHB infusion (infusion rate = 0.18 g/kg/h; average plasma concentration = 3.78 mM) increases myocardial blood flow by 75% in healthy, middle-to-older age adults (46), which may be driven by subtle alterations in cellular redox potential (47) and/or vasodilatory effects of β-OHB (48). Interestingly, we found a positive correlation between circulating β-OHB levels achieved after 15 minutes of ingestion and the change in FMD in the ketone condition (r = 0.601, P = 0.023; Figure S4) (18). This exploratory finding warrants follow-up to directly assess the relationship between changes in brachial artery FMD and circulating β-OHB with acute ketone ingestion. Whether 14 days of ketone monoester supplementation led to improved FMD through attenuating post-meal glucose spikes, reducing postprandial ROS and inflammation, altered sympathetic nerve activity, or via direct effects on vascular cells cannot be elucidated in a human trial, but our findings are the first to support a vascular benefit of raising β-OHB in vivo in humans.
Ketone Supplementation Lowers Monocyte NLRP3 Activation Status but Does Not Impact Circulating Cytokines
β-OHB has been shown to reduce inflammation by inhibiting the NLRP3 inflammasome pathway (3). The NLRP3 inflammasome activates caspase-1, which subsequently cleaves pro-IL-1β and pro-IL-18 to their mature secreted cytokine products (19, 49). We observed a reduction in LPS-stimulated caspase-1 activation following ketone supplementation but not placebo. These findings suggest that β-OHB effectively suppressed NLRP3 inflammasome activation in the presence of an inflammatory stimulus. Further supporting this, the downstream reduction in IL-1β secretion from LPS-stimulated cultures showed a tendency (P = 0.053) to be reduced following ketone supplementation (Figure 4B). These results are consistent with previous works showing that β-OHB treatment in vitro attenuates caspase-1 activation and consequent IL-1β secretion in culture (3, 50, 51). Youm et al (2015) showed that β-OHB directly attenuates canonical NLRP3 activation by preventing K+ efflux from the cell (3). However, our model likely activated the alternative NLRP3 pathway rather than the canonical pathway as we provided a single (LPS only) rather than dual stimulus, in which case K+ efflux would not be required for NLRP3 activation (49). In further support of this, we did not see compromised cell viability in LPS-stimulated samples compared with unstimulated samples (data not shown), suggesting that our ex vivo model of NLRP3 activation did not induce pyroptosis. Consequently, the suppressive effect of β-OHB may not have been as robust in our model. Our findings demonstrate that 14 days of oral β-OHB supplementation may reduce NLRP3 activation through an additional unknown upstream mechanism.
While β-OHB may directly inhibit NLRP3 activation (3, 50, 51), 14 days of premeal ketone monoester supplementation also induced metabolic changes which may have impacted NLRP3 activation. Hyperglycemic activation of NLRP3 is mediated by thioredoxin-interacting protein (TXNIP) activation in response to ROS (52, 53). The reduction in postprandial hyperglycemia from ketone supplementation in the current study may have been sufficient to attenuate NLRP3 activation in response to an inflammatory stimulus. While the mechanism remains speculative, the lowering of cellular inflammation assessed by NLRP3 following β-OHB supplementation may represent a therapeutic approach to help suppress the vicious cycle of hyperglycemia and inflammation that can drive metabolic disease.
The suppression of stimulated NLRP3 by β-OHB did not translate into changes in proinflammatory (IL-18, IL-6, and TNF-α) or anti-inflammatory (IL-10 and IL-1ra) cytokines measured in fasting blood samples before and after the 14-day intervention. Given the low concentrations of resting circulating cytokines in the participants in the current study, more prolonged exposure to β-OHB or a sample with greater baseline inflammation may be necessary in order to tease out more subtle effects at these low levels. Furthermore, it is likely that local production and concentration of cytokines is more important in mediating inflammation in obesity; measuring cytokines in plasma may lack the sensitivity needed to detect changes at the local cellular level.
Trial Considerations
Participants demonstrated very high compliance to the intervention protocols, which we attribute to the straightforward instructions provided, along with regular dialogue via text/phone/email that was maintained between researchers and participants. We expected greater variation in the compliance rate due to the bitter taste of the supplements; however, the familiarization visit likely helped with this, as participants confirmed on this visit that they felt confident in their ability to consume the ketone monoester (or placebo) 3× per day for each 14-day trial period. Additionally, the reported gastrointestinal symptoms were very low and there were no differences between conditions, which is in agreement with previous reports (13).
There were small, yet systematic, reductions in measures of body mass, systolic blood pressure, and NEFA independent of condition, which we attribute to the provision of a tightly controlled diet consisting primarily of unprocessed, whole foods with balanced macronutrient composition. Interestingly, the addition of 36 g of β-OHB per day, which would theoretically equate to ~175 kcal, to the calorie-matched diet in the ketone condition did not impact changes in body mass compared with calorie-free placebo nor did it cause any weight gain. The small additional energy from the ketone supplement may have altered total or resting energy expenditure, but we did not assess these parameters in the free-living study. Consumption of a ketone monoester has previously been shown to reduce appetite in the fasting state, possibly through the attenuation of the hunger-hormone ghrelin (28); however, we found no differences in measures of hunger and fullness between ketone and placebo across the 14 days.
Limitations and Future Directions
The randomized crossover design and level of control used in this study were very strong; however, this study is not without limitations. This was a short-term intervention and the long-term effects of ketone monoester supplementation on metabolic and vascular health remain to be determined. The ketone supplement was well tolerated and adherence was high over the 14-day period, and we attribute the glucose-lowering effects to the presence of elevated β-OHB. It will be important to determine whether adherence to ketone monoester supplementation can be maintained over a longer-term intervention period.
Although the volunteers recruited for this study had obesity, they did not have overt insulin resistance or impaired glucose tolerance, and therefore the results may not be applicable to clinical populations with dysglycemia (eg, prediabetes or type 2 diabetes). Moreover, whether the potential glucose-lowering effects of β-OHB would be sufficient to reduce cardiovascular risk in people with impaired glucose tolerance or overt type 2 diabetes is yet to be determined. Continuous glucose monitors measure interstitial glucose, which can be impacted by changes in subcutaneous blood flow (eg, in response to aerobic exercise) (54). Importantly, blood flow to subcutaneous fat does not appear to be affected by elevated β-OHB (55), and in the current trial there was no difference in physical activity between conditions
There were no effects of ketone monoester supplementation on circulating cytokines, but this does not preclude conclusions regarding the effect of ketone supplementation on local inflammatory pathways in key tissues impacted by hyperglycemia, including the liver, adipose tissue, and vasculature. Future mechanistic studies in humans and animals are required to elucidate the tissue-specific effects of β-OHB on local inflammatory pathways.
Conclusions
In summary, 14 days of premeal ketone monoester supplementation lowers postprandial and 24-hour mean glucose, improves brachial artery FMD, and reduces LPS-stimulated monocyte NLRP3 activation in adults with obesity. These findings provide important evidence for the therapeutic potential of ketone supplementation for protecting and improving cardiometabolic health. The glucose-lowering effects of ketone supplementation may have clinical implications for mitigating heightened cardiovascular risk due to postprandial hyperglycemia. This trial establishes an efficacious foundation for future, longer-term interventions that are now warranted to fully elucidate potential clinical application of ketone supplementation for improving cardiometabolic health.
Acknowledgments
The authors would like to thank the dedicated community volunteers who participated in this trial, the undergraduate research assistants who aided in data collection, and members of the School of Health and Exercise Sciences at the University of British Columbia—Okanagan Campus who provided meaningful input and discussion throughout the trial. Thank you to Mr. Morgan Decksheimer and the entire team at Meal Prep for You (Kelowna, BC) for preparing all meals, and for the seamless coordination of meal pickups with researchers and participants. We acknowledge the assistance of Mr. Cody Durrer for creating and maintaining blinding throughout the trial and analyses phases. We also thank Dr. Thomas Solomon who provided critical insight on the manuscript.
Financial Support: The trial was funded by a Heart and Stroke Foundation Grant-in-Aid (G-17-0018639). J.L is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award (MSH-141980) and a Michael Smith Foundation for Health Research (MSFHR) Scholar Award (16890). J.W was supported by a MSFHR Fellowship Award (17941).
Clinical Trial Information: ClinicalTrials.gov registration no. NCT03817749.
Author Contributions: J.L. conceived of the study and secured funding for the trial. J.L. and J.W. designed the study and H.N. provided input. J.W. and H.N. were involved in the recruitment of participants and facilitation of the trial in its entirety. J.W. and H.N. analyzed all data and performed all statistical analysis with oversight and consultation with J.L. All authors contributed to creating figures, preparing the manuscript, and revising subsequent versions. All authors approved the final version of this manuscript.
Glossary
Abbreviations
- β-OHB
β-hydroxybutyrate
- AUC
area under the curve
- CGM
continuous glucose monitoring
- CONGA
continuous overall net glycemic action
- FMD
flow-mediated dilation
- GPR
G protein-coupled receptor
- HOMA-IR
homeostasis model assessment of insulin resistance
- iAUC
incremental glucose AUC
- IL
interleukin
- KME
ketone monoester
- LLOD
lower limit of detection
- LMEM
linear mixed-effects model
- LPS
lipopolysaccharide
- NEFA
nonesterified fatty acid
- NLRP3
nucleotide-binding domain, leucine-rich repeat, pyrin domain containing 3 protein
- OGTT
oral glucose tolerance test
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
- ULOD
upper limit of detection
- VAS
visual analog scale
Additional Information
Disclosures: The authors declare no direct competing interests related to the current study. J.L. is the Chief Scientific Officer for the not-for-profit Institute for Personalized Therapeutic Nutrition. J.L. holds shares in Metabolic Insights Inc., a for-profit company developing noninvasive metabolic monitoring devices.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Newman JC, Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu. Rev. Nutr. 2017;37:51-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256-268. [DOI] [PubMed] [Google Scholar]
- 3. Youm YH, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han Y, et al. β-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol. Cell. 2018;71(6):1064-1078.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu X, Miao D, Liu Z, et al. β-hydroxybutyrate antagonizes aortic endothelial injury by promoting generation of VEGF in diabetic rats. Tissue Cell. 2020;64:101345. [DOI] [PubMed] [Google Scholar]
- 6. Walsh JJ, Myette-Côté É, Neudorf H, Little JP. Potential therapeutic effects of exogenous ketone supplementation for type 2 diabetes: a review. Curr Pharm Des. 2020;26(9):958-969. [DOI] [PubMed] [Google Scholar]
- 7. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stubbs BJ, Cox PJ, Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myette-Côté É, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596(8):1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Myette-Côté É, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr. 2019;110:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
- 12. O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249-255. [DOI] [PubMed] [Google Scholar]
- 13. Soto-Mota A, Vansant H, Evans RD, Clarke K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol. 2019;109:104506. [DOI] [PubMed] [Google Scholar]
- 14. Clarke K, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. [DOI] [PubMed] [Google Scholar]
- 16. The Lancet. Consort 2010. Lancet 2010;375(9721):1136. [DOI] [PubMed] [Google Scholar]
- 17. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci. 1918;4(12):370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh JJ, Neudrof H, Little JP. Supplemental Data associated with 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial., Repository: OSF. Deposited November 30, 2020. https://osf.io/cf7ez/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21(7):677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40(30):2534-2547. [DOI] [PubMed] [Google Scholar]
- 22. Woodman RJ, et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 2001;91(2):929-937. [DOI] [PubMed] [Google Scholar]
- 23. Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8(OCT):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans M, Egan B. Intermittent Running and Cognitive Performance after Ketone Ester Ingestion. Med Sci Sports Exerc. 2018;50(11):2330-2338. [DOI] [PubMed] [Google Scholar]
- 25. O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42:1031-1035. [DOI] [PubMed] [Google Scholar]
- 26. Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100(2):636-643. [DOI] [PubMed] [Google Scholar]
- 27. Miles JM, Haymond MW, Gerich JE. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J Clin Endocrinol Metab 1981;52(1):34-37. [DOI] [PubMed] [Google Scholar]
- 28. Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring). 2018;26(2):269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binkiewicz A, Sadeghi-Najad A, Hochman H, Loridan L, Senior B. An effect of ketones on the concentrations of glucose and of free fatty acids in man independent of the release of insulin. J Pediatr. 1974;84(2):226-231. [DOI] [PubMed] [Google Scholar]
- 30. Balasse E, Ooms HA. Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium beta-hydroxybutyrate infusions in man. Diabetologia. 1968;4(3):133-135. [DOI] [PubMed] [Google Scholar]
- 31. Neudorf H, et al. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019;1801171:1–10. [DOI] [PubMed] [Google Scholar]
- 32. Taggart A, Kero J, Gan X, et al. (D)-B-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor. J Biol Chem 2005;280(29):26649-26652. [DOI] [PubMed] [Google Scholar]
- 33. Kehlenbrink S, Koppaka S, Martin M, et al. Elevated NEFA levels impair glucose effectiveness by increasing net hepatic glycogenolysis. Diabetologia. 2012;55(11):3021-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loader J, Montero D, Lorenzen C, et al. Acute hyperglycemia impairs vascular function in healthy and cardiometabolic diseased subjects: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35(9):2060-2072. [DOI] [PubMed] [Google Scholar]
- 35. Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34(10):2237-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Habas K, Shang L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell. 2018;54:139-143. [DOI] [PubMed] [Google Scholar]
- 37. Piconi L, Quagliaro L, Da Ros R, et al. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2(8):1453-1459. [DOI] [PubMed] [Google Scholar]
- 38. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smorschok MP, Sobierajski FM, Purdy GM, et al. Peripheral chemoreceptor deactivation attenuates the sympathetic response to glucose ingestion. Appl Physiol Nutr Metab. 2019;44(4):389-396. [DOI] [PubMed] [Google Scholar]
- 40. Masuo K, Rakugi H, Ogihara T, Esler M, Lambert G. Cardiovascular and renal complications of type 2 diabetes in obesity:role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev. 2010;6(2):58-67. [DOI] [PubMed] [Google Scholar]
- 41. Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108(19):8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Natarajan N, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genomics 2016;48(11):826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holland AM, Qazi AS, Beasley KN, Bennett HR. Blood and cardiovascular health parameters after supplementing with ketone salts for six weeks. J. Insul. Resist. 2019;4(1):1-8. [Google Scholar]
- 45. Nielsen R, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139(18):2129-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gormsen LC, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: A positron emission tomography study. J Am Heart Assoc. 2017;6(3):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xin L, et al. Nutritional ketosis increases NAD+/NADH ratio in healthy human brain: an in vivo study by 31P-MRS. Front. Nutr. 2018;5:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fioretto P, et al. Glomerular filtration rate is increased in man by the infusion of both D, L-3-Hydroxybutyric acid and sodium D, L-3-Hydroxybutyrate. J Clin Endocrinol Metab. 1987;65(2):331-338. [DOI] [PubMed] [Google Scholar]
- 49. Gaidt M, et al. Human monocytes engage an alternative inflammasome pathway2016; 44(4):833-846. [DOI] [PubMed] [Google Scholar]
- 50. Goldberg EL, Asher JL, Molony RD, et al. β-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017;18(9):2077-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bae HR, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. 2016: [DOI] [PMC free article] [PubMed]
- 52. Maedler K, Sergeev P, Ris F, Oberholzer J. Glucose-induced (beta) cell production of IL-1(beta) contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 2002;110(6):851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136-140. [DOI] [PubMed] [Google Scholar]
- 54. Yardley JE, Sigal RJ, Kenny GP, Riddell MC, Lovblom LE, Perkins BA. Point accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol Ther. 2013;15(1):46-49. [DOI] [PubMed] [Google Scholar]
- 55. Häggendal E, Kerstell J, Steen B, Svanborg A. Blood flow and uptake of oxygen and substrates in forearm muscle and subcutaneous fat tissue in man: a study in normal and diabetic subjects. Acta Med Scand. 1968;183(1–6):79–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.