Abstract
Several studies over the past 3 decades document a higher prevalence of primary aldosteronism (PA) among hypertensive patients than generally presumed. PA exists as a spectrum from mild to severe aldosterone excess. Although a variety of PA subtypes exist, the 2 most common are aldosterone-producing adenomas (APAs) and bilateral hyperaldosteronism (BHA). The distinction is important, because APA—and other subtypes, with aldosterone production mostly from 1 adrenal—can be cured surgically, and BHA should be treated medically with mineralocorticoid-receptor antagonists (MRAs). The major shortcomings in the tailored management of patients with possible PA are the low rates of screening for case identification and the expensive and technically challenging imaging and interventional procedures required to distinguish APA from BHA, especially adrenal vein sampling (AVS). When AVS identifies an APA and allows the patient to be cured surgically, the procedure is of great value. In contrast, the patient with BHA is treated with MRA whether AVS is performed or not. Consequently, it is prudent to gauge how likely it is to benefit from imaging and AVS in each case prior to embarking on these studies. The explosion of information about PA in the past decade, including predictors of APA and of surgical benefit, are useful in limiting the evaluation for some patients with a positive PA screening test. This article will review our suggestions for approaching these patients in a pragmatic style, recognizing the limitations to even the best resources and facilities.
Keywords: primary aldosteronism, adrenal, aldosterone, hypertension, adrenal vein sampling
Case 1
A 56-year-old African American man with a longstanding history of hypertension and hypokalemia is referred to you after he was found to have elevated serum aldosterone (38 ng/dL or 1050 pmol/L) and a suppressed plasma renin activity (<0.6 ng/mL/h, the lower limit of the assay, aldosterone/renin ratio >63 (ng/dL)/(ng/mL/h) or >1748 (pmol/L)/(ng/mL/h), positive screen >20 or >550, respectively). His creatinine is 1.21 mg/dL (107 µmol/L) and serum potassium 3.5 mmol/L. His current antihypertensive regimen includes: lisinopril 40 mg daily, amlodipine 10 mg daily, metoprolol 50 mg twice daily, and eplerenone 50 mg daily, plus potassium chloride 20 mEq 3 times daily. His primary care physician ordered a computed tomography scan (CT) of the abdomen without contrast, which revealed a 2 cm left adrenal nodule with a density of –5 Hounsfield units, consistent with a lipid-rich cortical adenoma, and an irregular thickening of the medial limb of the right adrenal, which is pressed against the liver and difficult to characterize. His other medical history includes obesity, type 2 diabetes mellitus, and paroxysmal atrial fibrillation. After 1 mg dexamethasone at bedtime, the 8 a.m. serum cortisol was 0.8 µg/dL (22 nmol/L) and the serum aldosterone 36 ng/dL (1001 pmol/L). Adrenal vein sampling (AVS) was performed and revealed aldosterone lateralization to the right (Table 1). Would you refer the patient for surgery?
Table 1.
AVS results in case 1
| Right AV | IVC | Left AV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | A | A/C | C | A | A/C | C | A | A/C | LI | |
| Precosyntropin | 29.1 | 5030 | 173 | 3.8 | 46.2 | 12.2 | 20.4 | 380 | 18.6 | 9.3 |
| Postcosyntropin | 1232 | 24700 | 20.1 | 8.6 | 54.9 | 6.4 | 906 | 2350 | 2.6 | 7.7 |
Abbreviations: A, aldosterone in ng/dL (multiply by 273 for pmol/L); AV, adrenal vein; AVS, adrenal vein sampling; C, cortisol in µg/dL (multiply by 27.6 for nmol/L); IVC, inferior vena cava; LI, lateralization index (to right AV).
Case 2
A 78-year-old woman with hypertension since her 50s is treated with amlodipine 10 mg daily, losartan 100 mg daily, and hydrochlorothiazide 12.5 mg daily. She had stage 2 breast cancer treated with surgery and chemotherapy 10 years ago. A CT scan with and without contrast ordered for acute abdominal pain shows a 0.8-cm mass in the lateral limb of the right adrenal gland with a precontrast density of –10 Hounsfield units. Screening for pheochromocytoma and hypercortisolemia are normal. The plasma renin activity is 0.7 ng/mL/h, the serum potassium is 3.7 mEq/L, serum creatinine 0.9 mg/dL (80 µmol/L), and serum aldosterone is 12 ng/dL (330 pmol/L; aldosterone/renin ratio = 17 (ng/dL)/(ng/mL/h) or 473 (pmol/L)/(ng/mL/h)). She is referred to an endocrinologist (you). Her blood pressure is 132/88 mm Hg, and physical examination shows 1 to 2+ bilateral pitting edema. What would you do next?
Primary Aldosteronism—Definition and Clinical Relevance
Primary aldosteronism (PA) refers to renin-independent, inappropriate aldosterone production from 1 or both adrenal glands. Chronic and excessive aldosterone production and activation of the mineralocorticoid receptor (MR) leads to retention of salt and water, expansion of the intravascular volume, and kaliuresis. Depending on severity, individual genetic makeup, and dietary composition, hypertension and/or hypokalemia might result. MR activation in the brainstem increases outflow from the sympathetic nervous system (1), a second mechanism that raises blood pressure independent of renal function. Long thought to be a rare entity, PA is now recognized as the most common cause of secondary hypertension. PA prevalence reports have been heterogeneous in regard to the populations studied and diagnostic methods (2), but, generally, the frequency of PA is higher among patients with resistant hypertension (3, 4) and increases with age (5). Roughly, 5% to 10% of all hypertensive patients (6-8) and up to 20% of those with resistant hypertension (3, 4) have PA, which ranges from low-renin hypertension to florid disease, often resulting from aldosterone-producing adenomas (APAs).
More than its prevalence, the most compelling argument for recognizing and optimally treating PA derives from its associated deleterious cardiovascular and renal consequences. Compared with patients with primary (essential) hypertension of similar severity, patients with PA are more prone to stroke, atrial fibrillation, myocardial infarction, and even death (9-16). Beyond the cardio-renal morbidity mediated through blood pressure elevations, direct and sustained MR activation in various target organs induces inflammation, fibrosis, and necrosis (17, 18). Via such mechanisms, PA promotes left ventricular hypertrophy, microalbuminuria, endothelial dysfunction, QT interval prolongation, and metabolic syndrome (19-26). Early recognition and targeted treatment of PA is essential to circumvent many of its clinical complications (9, 27, 28).
PA Histopathology and Clinical Implications
Although a few familial monogenic forms of PA have been described, most cases are sporadic. With the advent of highly specific antibodies against human aldosterone synthase (CYP11B2) (29), the spectrum of PA histopathology has been redefined over the past decade. Sporadic PA can occur from a single APA, from distinct multifocal areas, or from zona glomerulosa hyperplasia, which can occur in 1 or both adrenal glands (30-32). Advanced DNA sequencing techniques coupled with precise mapping of aldosterone-producing cortical areas by CYP11B2 immunostaining have led to the identification of aldosterone-driver somatic mutations in the overwhelming majority of APAs (33-35). Such mutations facilitate inappropriate calcium entrance into CYP11B2-expressing cortical cells, stimulating their aldosterone synthesis. Affected genes include KCNJ5 (encoding the inwardly rectifying potassium channel GIRK4), ATP1A1 (encoding the α subunit of Na+/K+-ATPase), ATP2B3 (encoding a Ca2+-ATPase), CACNA1D (encoding a subunit of the L-type voltage-gated calcium channel CaV1.3), CACN1H (encoding a T-type voltage-gated calcium channel CaV2.3), CTNNB1 (encoding β-catenin), and CLCN2 (encoding the voltage-gated chloride channel protein 2) (36-38). Curiously, the prevalence of somatic mutations identified in APAs varies with sex, race, and geographic location (39), pointing toward additional, nongenetic factors as contributors to PA pathogenesis.
A study of adrenal tissue obtained from deceased individuals across a broad age range found that the continuous zona glomerulosa observed at a young age is replaced by discrete microscopic aldosterone-producing cell clusters in older individuals (5). Such aldosterone-producing cell clusters have been found to be even more numerous in the adrenal glands of patients with bilateral hyperaldosteronism (BHA), and more than half of these aldosterone-producing structures harbored CACNA1D mutations (40). Non-nodular hyperplasia was observed in only 4 of 15 cases with BHA, debunking the dogma that such cases are typically caused by bilateral adrenal hyperplasia. Moreover, genetic and histopathological heterogeneity have been identified in patients with presumed unilateral PA based on AVS data (30, 35, 41), suggesting that asynchronous processes could involve both adrenal glands.
Treatment Options for Patients With PA
When PA arises strictly from 1 adrenal gland, it can be cured with unilateral adrenalectomy, whereas BHA requires indefinite medical therapy (8). In the absence of a selective CYP11B2 inhibitor, the current standard of care for PA medical management includes mineralocorticoid receptor antagonists (MRAs) (8). Although MRAs can be efficacious in normalizing blood pressure and serum potassium (11, 42), treatment should also block the MR in target tissues; however, this second component of treatment is difficult to assess clinically or biochemically. A rise of plasma renin to the normal range suggests adequate MR antagonism, but often renin remains suppressed despite doses that increase the risk of hyperkalemia, particularly so in patients with underling renal disease. Data from a large retrospective cohort study indicated that medical treatment often fails to mitigate the excessive cardiovascular risk, incident chronic kidney disease, and mortality observed in PA patients compared with those with essential hypertension, even after adjusting for blood pressures and other covariates, which suggests that MRAs are often not adequately dosed to block all the adverse effects of PA on target organs (10, 15, 16). Conversely, surgical treatment with unilateral adrenalectomy often cures PA (43-45) and simultaneously abrogates its associated cardiovascular risk (10, 15, 16). Limited data also suggest that surgery provides long-term cost (46) and quality-of-life benefits (47).
Individualized treatment for PA, however, relies on numerous factors, not only patient-specific consideration but also practical aspects, such as availability of resources. Ideally, surgical treatment should be theoretically considered in every patient with PA who might have unilateral disease, unless major comorbidities or personal preferences preclude surgery. Except for the most severe cases, medical therapy with MRA normalizes blood pressure and potassium balance irrespective of the PA subtype. Only 2 MRAs are available in the United States and most other countries: spironolactone, which is the most efficacious MRA but prone to side effects because of its off-target effects at the androgen and progesterone receptors; and eplerenone, a selective MRA, but with roughly half the potency of aldosterone (48, 49). Amiloride and triamterene, which antagonize the epithelial sodium channels in the renal distal convoluted tubule and the collecting duct, can be used as alternatives, but these drugs do not offer the protective effects mediated via MR blockade in target tissues. Moreover, compared with patients treated with surgery, PA patients managed medically commonly need intensification of their treatment and have higher rates of hypokalemia (50). Reconsideration for surgery should be given whenever hypertension and/or hypokalemia become recalcitrant, which could be due to disease progression, but also inconsistent adherence to therapeutic regimens that often include several drugs and dietary sodium restriction.
Identifying Surgical Candidates
Adrenalectomy is the treatment of choice for patients with aldosterone production from one adrenal gland, such as an APA in 1 gland as the sole source, but most cases are not so simple. Traditionally, sporadic PA was subtyped into unilateral (presumed APA) and bilateral (initially called “bilateral hyperplasia”) forms; however, the preceding discussion of PA pathophysiology demonstrates why this dichotomy is an oversimplification, both biochemically (AVS results) and histologically. For example, some cases of lateralized PA are cured with surgery, yet no adenoma is identified; furthermore, zona glomerulosa hyperplasia is only rarely observed (40). We suggest interpreting AVS data to show either BHA or significant dominance of 1 side, which we will operationally define as lateralized hyperaldosteronism (LHA) based on consensus criteria (8, 51). These lateralization criteria—although generally useful—are derived from retrospective data and expert opinion and not from prospective trials, which would require a study in which all patients who undergo AVS have the dominant adrenal gland removed, no matter how small the difference. Even for cases of LHA with convincing dominance of 1 adrenal, the other adrenal typically produces some aldosterone, and an imaged tumor in the dominant adrenal might not be the source of aldosterone (45). In reality, many PA patients are more correctly described as bilateral, but with 1 adrenal being dominant, through 1 of several histologic subtypes (Table 2). Given these caveats and ambiguities, the art of PA care includes deciding when that dominance is sufficient to warrant surgical intervention.
Table 2.
Primary aldosteronism subtypes accounting within LHA and BHA based on AVS
| LHA | BHA |
|---|---|
| Single aldosterone-producing adenoma | Bilateral aldosterone-producing adenomas |
| Unilateral micronodule(s) | Bilateral micronodules |
| Asymmetric, lateralized hyperaldosteronism Adenoma + bilateral micronodules Asymmetric bilateral micronodules Bilateral asymmetric adenomas | Weak adenoma + bilateral micronodules |
Zona glomerulosa hyperplasia can be present in both LHA and BHA.
Abbreviations: AVS, adrenal vein sampling; BHA, bilateral hyperaldosteronism; LHA, lateralized hyperaldosteronism.
In most centers with relatively high thresholds for screening, LHA accounts for roughly half of PA cases (50), and the probability of LPA (and APA) increases with disease severity (52, 53). In contrast with other autonomous adrenal hormone excess disorders, such as ACTH-independent hypercortisolemia or pheochromocytoma, cross-sectional imaging is generally unreliable for identifying the source of PA. LHA can often occur from lesions that are below the resolution of conventional cross-sectional imaging, such as micronodules, aldosterone-producing cell clusters, or focal hyperplasia (35, 54). Furthermore, cross-sectional imaging cannot distinguish between aldosterone-producing lesions and nonfunctional adenomas (45), both of which become increasingly common with age (55, 56). The overall agreement between cross-sectional imaging and AVS for PA laterality is only ~50% to 60% (57-60). The accuracy of CT for PA subtyping is highest in patients younger than 35 years of age, although still imperfect in this population (58, 61, 62). Under these considerations, expert consensus guidelines for PA management endorse AVS as the standard of care whenever unilateral adrenalectomy is considered, except for patients younger than 35 years with profound PA and a single adrenal nodule detected by imaging (8, 51). Subsequent to the publication of Endocrine Society clinical practice guidelines, what have we learned about the reliability of AVS for PA subtyping and the likelihood that a patient with PA will be cured of PA after adrenalectomy?
In the absence of a “gold standard” for PA subtyping short of the biochemical and clinical response to adrenalectomy, the Subtyping Primary Aldosteronism: a Randomized Trial comparing Adrenal vein sampling and CompUted tomography Scan (SPARTACUS) study of Dekkers and colleagues challenged the superiority of AVS over CT. In the only randomized controlled trial published to date, 200 PA patients were followed for 1 year after CT- or AVS-guided unilateral adrenalectomy (63). The primary endpoint of the study was the intensity of medical therapy needed to achieve target blood pressure 1 year after adrenalectomy, and no statistical difference was found between the 2 groups. The biochemical failure rate, however, was higher in patients who underwent adrenalectomy based on CT (20% vs. 11% of those who had AVS-directed surgery). Furthermore, although AVS was conducted only after cosyntropin stimulation, the failure rates after adrenalectomy were considerably higher than those reported from high-volume AVS referral centers using similar protocols (58). Given these caveats and other limitations in study design, SPARTACUS has generated considerable controversy (64).
Two major limitations of studies that compare CT and AVS for PA subtyping should be considered. First, clinical pathology reports typically provide only histological, and not functional information, even in major referral centers, and cortical adenomas identified in pathological specimens from PA patients have historically been presumed to be APAs. In 2014, an antibody specific for CYP11B2 without cross-reactivity for CYP11B1 (11-hydroxylase) was developed (29), but CYP11B2 immunostaining has so far been used as a research tool only. Second, hormonal follow-up data, particularly beyond the immediate postoperative period, often have been scarce. Importantly, partial or transient resolution of PA can be achieved by debulking with unilateral adrenalectomy even in patients with BHA (65). In 2017, an international expert consortium formulated a consensus using the Delphi method on measures to evaluate outcomes following unilateral adrenalectomy for PA: The Primary Aldosteronism Surgical Outcome (PASO) study (43). Implementing the standardized PASO criteria, postoperative data were collected from 18 international centers and compared outcomes in 526 patients who had unilateral adrenalectomy directed by AVS with 235 patients whose operations were based on CT findings (66). Compared with patients in the AVS group, patients in the CT group had lower rates of complete biochemical success (80% vs. 93%, P < 0.001) and higher rates of absent biochemical success (12% vs. 2%, P < 0.001). Another large multicenter study also found that the proportion of patients cured of hypertension was higher when surgery was guided by AVS (50). Taken together, existing evidence strongly favors AVS for selecting surgical candidates, not only to ensure that patients do not have futile surgeries—as would happen if imaging points toward a contralateral nonfunctional adenoma—but also to evaluate the source(s) of aldosterone for patients with either bilateral nodularity or no detectable adrenal abnormalities on cross-sectional imaging studies.
Radionuclide imaging of APA has been attempted for decades. The initial single-photon agent NP-59 required dexamethasone suppression of the normal adrenal cortex and was insensitive for identifying tumors <3 cm in diameter (67). Positron-emitting agents such as [11C]-metomidate (68) or [68Ga]-pentixafor (69) are capable of detecting small tumors, but the high cost and limited availability leave little advantage over AVS, at least currently.
Patient Preparation Quandaries
Ideally, medications that could alter the renin-angiotensin-aldosterone system should be avoided at the time of AVS. Medications that can raise renin, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and even more so MRAs are of a particular concern, because renin physiologically drives aldosterone synthesis from normal glomerulosa cells in both adrenal glands, potentially obscuring lateralization in some APA cases. Thus, expert guidelines recommend to avoid such medications for up to 4 weeks before AVS (8). When PA is severe, hypertension and/or hypokalemia are difficult to control with the few antihypertensive agents that do not interfere with the renin-angiotensin-aldosterone system, such as α 1-adrenergic blockers, hydralazine, and verapamil. In addition, hypokalemia impairs aldosterone synthesis, including autonomous production from an APA, which can attenuate lateralization gradients. Nevertheless, several studies have now shown that AVS lateralization remains reliable despite concomitant therapy with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and or even MRAs, particularly when renin is suppressed on the day of AVS (70, 71); however, nonlateralized studies during MRA therapy should be interpreted with caution.
Variations in Protocols for AVS Procedure and Data Interpretation
Because AVS is a technically challenging procedure, its success is highest for the few interventional radiologists who practice in high-volume referral centers (72-74). In centers with low AVS volume or lacking 1 dedicated AVS radiologist, the success rate can be improved when prompt cortisol assays are available to confirm catheter placement and allow resampling the same day (75, 76). The left adrenal vein (AV) is accessed off the superior wall of the left renal vein, but the right AV is a thin, elusive vessel that perpendicularly joins the inferior vena cava (IVC), which makes its engagement challenging and the major source of AVS failure (74). Even with AV access, considerable heterogeneity among procedural protocols and data interpretation limit comparison of results from even the most experienced centers (77). Among the procedural variations, samples from the 2 AVs and IVCs are obtained either simultaneously with catheters in each AV or sequentially, with catheter repositioning between sampling (Table 3). The procedure can be conducted with and/or without cosyntropin administration, either as a bolus, an infusion, or bolus followed by infusion. Some centers employ microcatheters for “super-selective” sampling of AV branches inside the glands (78). Finally, the number of samples taken and the rate of blood collection, ranging from gravity-assisted drip to a slow draw, are additional variables.
Table 3.
Major variables in AVS protocols and interpretation
| Protocol |
| • Catheterization: simultaneous vs. sequential |
| • Cosyntropin: |
| - None |
| - Bolus |
| - Continuous infusion, starting 5-60 minutes before procedure |
| - Bolus + infusion, typically starting at catheterization |
| • Number of samples obtained: 1-6 |
| Interpretation |
| • Selectivity index: >1.1-3 basal; >1.1-5 postcosyntropin |
| • Lateralization index: >2-4 basal; >2-4 postcosyntropin |
| • Contralateral suppression index: |
| - [Aldosterone/cortisol]nondominant AV < [Aldosterone/cortisol]IVC |
| - [Aldosterone]nondominant AV < [Aldosterone]IVC |
Abbreviations: AV, adrenal vein; AVS, adrenal vein sampling; IVC, inferior vena cava.
The current standard of interpretation is to first determine that bilateral AV catheterization is successful by comparing the concentrations of cortisol, a major product of the adrenal cortex, between each AV and IVC or peripheral blood samples (Table 3). The AV/IVC cortisol ratio is called the selectivity index (SI), and values of minimum 2 and 5 are typically used as indicators of adequate AV catheterization in the absence or presence of cosyntropin, respectively (8). The AV samples generally contain a mixture of AV and mixed venous blood, and the higher the SI, the greater the confidence in the resultant interpretation. Next, the “cortisol-corrected aldosterone ratios” from the 2 AV and IVC samples are compared with localize the source(s) of aldosterone; the lateralization index (LI) computes the ratio of aldosterone/cortisol between the dominant vs. nondominant or contralateral adrenal gland. The contralateral suppression index (CSI) assesses if the aldosterone/cortisol ratio for the nondominant adrenal gland is lower than found in the peripheral circulation, which is used to confirm that the nondominant adrenal is not a significant source of aldosterone. The CSI generally uses cortisol-corrected aldosterone values, but comparing raw aldosterone concentrations has also been proposed (79, 80). In principle, the higher the LI and the lower the CSI, the stronger the evidence for unilateral PA. When AVS is performed with cosyntropin stimulation, a minimum LI value of 4 is widely accepted as consistent with LHA; in contrast, some centers where AVS is performed without cosyntropin use a minimum LI value of 2 or even lower to indicate LHA (50). Without prospectively defined LI cutpoints and considering the heterogeneity of PA pathophysiology, the sobering reality is that AVS can only estimate the relative aldosterone contributions from the 2 adrenals, but even high LIs cannot fully exclude asymmetric bilateral disease (Table 2). In rare instances, a study interpreted as BHA could underestimate the dominance of 1 side and the benefit of surgery, but the reasons for such bizarre results are not known.
AVS Caveats and Limitations
Implications of Cosyntropin Use
The use of cosyntropin has two major advantages: (1) by stimulating adrenal blood flow and cortisol production, cosyntropin enhances the SI value and thus the confidence of correct catheter placement (81, 82); (2) cosyntropin minimizes potential ACTH-dependent hormonal fluctuations because of the stress of the procedure. The relative effect of cosyntropin stimulation on physiologic vs. aberrant aldosterone production has been debated and insufficiently studied. High doses of cosyntropin stimulate aldosterone from normal zona glomerulosa cells, which could mask lateralization for an APA with modest autonomy (79, 83-86). At the same time, some APAs can display a disproportionate response to cosyntropin (52, 73, 87, 88), possibly from enhanced expression of ACTH receptors (MC2R) (89). The very few centers that perform AVS both before and after administration of cosyntropin have found discordant results in up to one-quarter of patients (52, 79). Even in patients with consistently lateralized results, cosyntropin was equally likely to enhance, dampen, or preserve the same aldosterone gradient between the 2 adrenal glands (52). Such fluctuations of the LI during AVS further prove that PA is a complex and heterogeneous group of disorders. The optimal management of patients who have inconsistent results during AVS is elusive. Our approach to such cases includes a multidisciplinary assessment and shared decision-making that involves that patient. When patients have difficult-to-control hypertension and/or hypokalemia and are good surgical candidates, we favor unilateral adrenalectomy. Longitudinal follow-up of such cases will be essential to determine which patients might harbor APAs in evolution or have asymmetrical BHA.
Concomitant Cortisol Excess
Mild ACTH-independent cortisol excess (MACE) has been reported in a considerable number of patients with PA (90, 91). All patients with PA who have at least 1 adrenal nodule of minimum 1 cm or bilateral hyperplasia should be evaluated for autonomous cortisol production (92). The most sensitive test for MACE is a 1-mg dexamethasone suppression test (92, 93). Causes of false-positive results include estrogen use, which increases corticosteroid-binding globulin, disrupted circadian rhythm, protocol deviations, and malabsorption or accelerated clearance of dexamethasone (94). An abnormal result should prompt further evaluation to confirm and to determine the magnitude of hypercortisolemia (94). As is our practice, some centers perform dexamethasone suppression testing for cortisol and aldosterone in all PA patients, to screen for both hypercortisolemia and glucocorticoid-remediable aldosteronism (familial hyperaldosteronism type 1) because AVS is of no benefit for these patients, whose aldosterone production is bilateral.
There are 2 clinically important aspects of concomitant autonomous hypercortisolemia in patients with PA. When the cortisol excess is clinically overt, an adenoma is usually present, and unilateral adrenalectomy of the hyperfunctional adrenal gland should be the treatment of choice, irrespective of PA subtype or laterality. This approach reflects both the numerous and serious comorbidities associated with Cushing syndrome and the lack of safe, inexpensive medical treatment options for these patients (92). Second, in a PA patient who also has MACE, AVS interpretation can be obfuscated because cortisol becomes an unreliable normalizing factor for aldosterone. The autonomous hypercortisolemia can lower ACTH and, consequently, cortisol production from the contralateral adrenal. The lower cortisol concentration increases the aldosterone/cortisol ratio on the side opposite to the overactive tumor, which might change the interpretation from lateralization to the side of the tumor (the true result) to BHA or apparent dominance of the contralateral adrenal as an artifact. The right approach to such patients can be challenging; the severity of PA and hypercortisolemia, and the rate of growth of the adrenal mass on follow-up imaging are typically taken into consideration in each individual case. Adrenal adenomas that produce clinically significant hypercortisolemia tend to be 2.4 cm in diameter or larger (95), but the minimum tumor size and serum cortisol value after dexamethasone to interfere with AVS interpretation are not known. One small study found little interference of MACE with AVS in patients with moderately severe PA when cosyntropin was used, but independent adjudication of BHA was not possible (96). Several other adrenal products, including metanephrines (97), 11-deoxycotisol, androstenedione, 11β-hydroxyandrostenedione, and several other steroids (98-100), have been suggested as alternatives to cortisol for normalizing aldosterone during AVS, but these approaches have not been standardized. Metanephrine release in particular fluctuates with catheter occlusion of the adrenal vein, and all cortical hormones are regulated to some extent by ACTH, which limits the utility of these alternatives.
Salvaging Incomplete AVS Data
As discussed previously, the rates of AV catheterization failure are not negligible, particularly on the right side, and when AVS is conducted without cosyntropin stimulation or in low-volume centers. If the cortisol-corrected aldosterone ratio in the successfully catheterized AV sample is less than the value in the IVC blood, unilateral PA originating from the opposite adrenal is highly likely, based on the CSI < 1 (101, 102). Imaging abnormalities limited to the unsuccessfully accessed side, usually the right AV, might further support an APA on that side. Conversely, a high cortisol-corrected aldosterone ratio in the successful AV sample compared with the IVC value cannot distinguish lateralized from bilateral disease (102).
Alternative steroids tend to have much greater AV to peripheral gradients than cortisol, owing to less protein binding and resulting shorter half-lives (98, 100, 103). Because of their high SI values and adrenal-specific origins, 11β-hydroxyandrostenedione and 11-deoxycortisol are promising candidates for salvaging AVS cases with low cortisol SI. Finally, simultaneous measurement of aldosterone precursors and APA-derived steroids, including 18-hydroxycorticosterone (104) or 18-hydroxycortisol and 18-oxo-cortisol (105), might also help to confirm interpretations. Validation data and wider access to assays for these alternative steroids, however, are needed before clinical implementation of these approaches.
Practical Considerations of AVS
Other limitation of AVS include its high cost, invasiveness, risk of complications, higher radiation exposure than that received during a CT scan (106), and limited availability of experienced centers with skilled operators. Low-volume centers have success rates as low as 8% to 10% (107), which is unacceptable for an invasive and costly procedure. Conversely, the rates of success are high and procedure-associated complications are infrequent in centers of expertise (72, 88, 108). Thus, referral to a high-volume center is imperative whenever surgical treatment of PA is considered. Although CT availability is ubiquitous, it should not be used in lieu of AVS, with the rare exceptions discussed previously, and AVS should be used exclusively for subtyping, and not for diagnosis of PA (109).
Thinking Twice Before You Recommend AVS
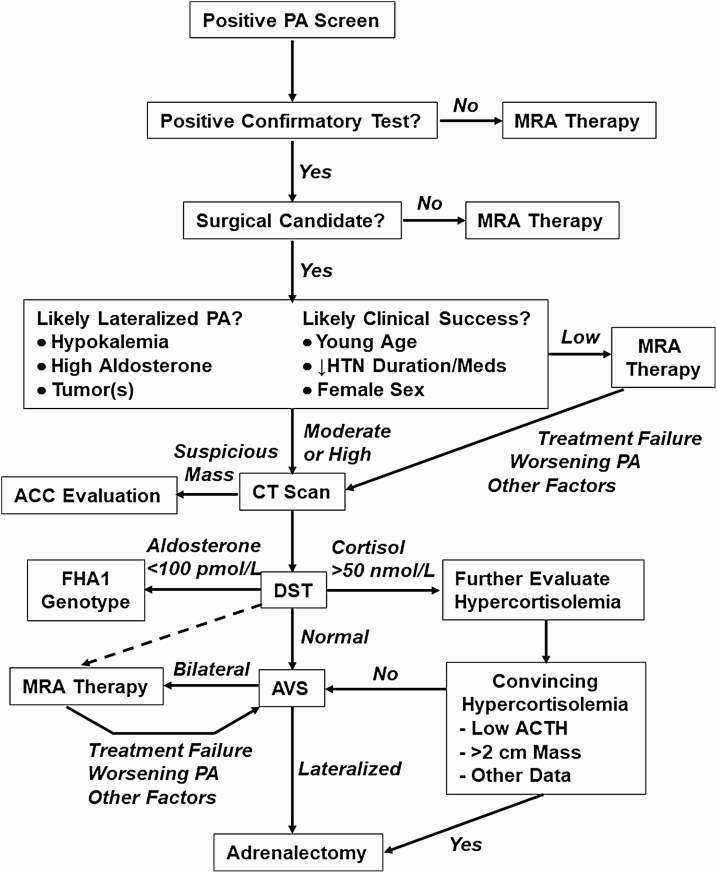
Although we have reviewed all the limitations and caveats associated with AVS, we still find ample evidence that AVS is the most accurate test that we currently have for subtyping PA. We also recognize that with expanded screening, the majority of PA patients will have BHA and ultimately will be treated medically with MRA. For BHA patients, AVS does not provide any additional benefit beyond treatment with MRA, yet these patients undergo AVS because one does not know their subtype a priori. Until a good, minimally invasive test to distinguish APA from BHA is validated, AVS could be offered to most patients with PA, but resource and cost considerations suggest that we should attempt to limit the number of patients who undergo AVS. A practical approach is to first ask yourself 2 questions: (1) “What are the odds that this patient has LHA?” and (2) “If I send this patient to surgery, how much clinical benefit will be gained from adrenalectomy?” Particularly in older patients and those with metabolic syndrome and/or impaired renal function, the PA might not be the sole reason for their hypertension, and these patients will still require antihypertensive therapy even if PA is cured. Many of these considerations are outlined in the algorithm in Fig. 1 and elaborated next.
Figure 1.
Expanded algorithm for triaging patients with positive screen for PA. Importantly, MRA is the treatment of choice in those with positive screens but negative confirmatory testing. For patients who are surgical candidates, CT scan and dexamethasone suppression testing (DST) (order can be inverted) precede the decision to recommend AVS, which incorporates the likelihood of lateralization and of significant clinical benefit from adrenalectomy. The likelihood stratification step is admittedly an imprecise and nuanced exercise, but as more outcomes data are reported and decision tools developed, the confidence in this process will increase. Even after a normal DST result, MRA therapy (dashed arrow) remains an option if AVS expertise is not available or if other factors create reluctance for the patient or endocrinologist to pursue AVS next. Patients with an adrenal cortical adenoma, particularly if >2 cm in diameter plus low ACTH and other convincing evidence of hypercortisolemia are sent to adrenalectomy, and the PA is reassessed postoperatively. For adrenal masses with suspicious features (large size, necrosis, high density, irregular borders), evaluation for adrenocortical carcinoma (ACC) is warranted. Whenever MRA therapy is used, including after a bilateral result from AVS, periodic reassessment is indicated, and AVS should be reconsidered if response to MRA is inadequate or is worsening of PA is identified. A cortisol of 50 nmol/L is 1.8 μg/dL; an aldosterone of 100 pmol/L is 4 ng/dL. Abbreviations: AVS, adrenal vein sampling; CT, computed tomography; MRA, mineralocorticoid-receptor antagonist; PA, primary aldosteronism.
Likelihood of Lateralized PA
A historical view of PA provides some insight to predictors of APA. When PA was first identified, testing was limited to patients with hypertension and hypokalemia or patients with adrenal tumors. In that era, before the development of AVS, roughly two-thirds of patients proved to have APAs, and their aldosterone production was generally very high. Not surprisingly, hypokalemia, high aldosterone, and imaged tumor(s) have been identified as predictors of LHA using AVS (110, 111). Refined models with better predictive values have been developed using additional parameters and machine learning algorithms (112), but none are perfect, particularly for distinguishing truly unilateral PA from asymmetric bilateral PA. Although the imaging of a tumor favors LHA, we emphasize that the imaged tumor is not necessarily the source of aldosterone (45) and that AVS is still recommended to guide adrenalectomy in most patients with high probability of LHA. Conversely, patients with severe PA and bilateral tumors often lateralize with AVS, although some might have asymmetrical bilateral PA. More importantly, patients with mild aldosterone excess and normokalemia are likely to have BHA, particularly when identified from broad screening programs (113). Nevertheless, some PA cases with low probability of lateralization might prove to have LHA after AVS and benefit substantially from surgery given age and other factors discussed in the following section.
Likelihood of Clinical Improvement
Several studies from major centers have attempted to define patient criteria that predict blood pressure reduction or normalization following adrenalectomy for PA. These studies are difficult to compare because criteria for surgery, patient characteristics, length of follow-up, and definitions of “cure” or “improvement” are inconsistent. For this reason, the PASO study group applied the agreed-on criteria for complete/partial/absent biochemical (serum aldosterone) and clinical (blood pressure and potassium) improvement to a series of nearly 700 patients who underwent AVS-guided adrenalectomy (43). When patients are subtyped using AVS, complete biochemical success was observed in 94% of patients after adrenalectomy, much higher than observed in SPARTACUS. Predictably, clinical improvements in blood pressure were not as consistent but still very good, with complete or partial success in 37% and 47%, respectively, leaving absent success in only 16% of patients. The best predictors of blood pressure normalization following adrenalectomy were female sex and younger age, and a greater number of blood pressure medications reduced the likelihood of complete but not partial clinical success. Earlier studies also found that young age and fewer antihypertensive medications predicted the best clinical responses to adrenalectomy, as well as lower body mass index, shorter duration of hypertension, higher aldosterone/renin ratio, and weak family history of hypertension (114, 115)
Based on these data, one can conceptualize a stereotypical patient who is most likely to benefit from AVS: a thin young woman with recent onset of hypertension and hypokalemia and no family history of hypertension, whose blood pressure is marginally controlled with 2 medications plus potassium supplements, a suppressed plasma renin, and a serum aldosterone >30 ng/dL. Unfortunately, most PA patients do not fit this mold, but the converse is also true: the endocrinologist can identify patients who are unlikely to have LHA and who are less likely to benefit from AVS. Older patients with longstanding hypertension, no history of hypokalemia, weakly positive screening and confirmatory tests, and additional factors such as obesity and a strong family history of hypertension are not likely to have LHA and be cured of their hypertension. PA has been associated with severity of sleep apnea (116, 117), but cure of PA will not normalize blood pressure when other secondary causes of hypertension remain. This decision point also illustrates the art of medicine and the judgement that must be exercised in approaching the PA patient.
Rather than draconian guidelines, we suggest that patients are stratified according the likelihood of (LHA + high clinical success) into high, medium, and low groups (Fig. 1). Using this model, high-likelihood patients should be offered AVS at an experienced center if willing and able to undergo adrenalectomy, and AVS should only be offered to low likelihood patients for a compelling reason. We have sent healthy octogenarians with few other medical problems to AVS and surgery, because recent worsening of hypertension and new onset hypokalemia mitigated concerns of futility. For the medium likelihood patient, we have “the talk,” in which we try our best to lay out the options, pros and cons, and provide our best recommendation.
Two points are worth repeating. First, if medical therapy without AVS is chosen, AVS remains an option in the future should medical therapy prove ineffective or poorly tolerated, or should the clinical severity worsen (Fig. 1). Second, the most likely outcome following surgery is partial clinical success, which is in many cases a resounding victory! We typically counsel patients who fit in the high- and medium-probability groups and for whom we recommend AVS that we cannot guarantee that the hypertension will be cured, but the odds are >90% that the potassium loss will be stopped, and very likely we will achieve target blood pressure with a single daily dose of a drug or drug combination—which might include a thiazide diuretic after correction of kaliuresis. Though not a “cure,” such a result is highly desirable compared with uncontrolled hypertension and intermittent hypokalemia despite 4 to 6 drugs taken 2 to 3 times a day plus copious potassium supplements. Furthermore, surgery is likely to afford long-term protection from cardiovascular consequences and end-organ damage beyond blood pressure and potassium control.
Back to the Patients
Case 1
Despite having a 2-cm left adrenal nodule, AVS demonstrated right aldosterone lateralization. Because the nodule had the typical characteristics of a lipid-rich cortical adenoma, malignancy is not a concern. Such a nodule could be either a nonfunctional adenoma or a cortisol-producing adenoma. The possibility of autonomous cortisol production is important both for AVS data interpretation as well as for establishing the goals of clinical management. In this patient, a 1-mg dexamethasone suppression test was conducted before AVS and resulted in a suppressed morning cortisol of 0.8 µg/dL (22 nmol/L). Review of older abdominal imaging showed that the left adrenal nodule had remained stable over the past 3 years. The patients underwent right laparoscopic adrenalectomy, which led to biochemical cure of PA (undetectable serum aldosterone 2 weeks after adrenalectomy) and blood pressure controlled with amlodipine alone.
Case 2
We had “the talk” with this woman and learned that her main concern was that the adrenal tumor represented recurrence of her breast cancer. With the low precontrast density, we could assure her that the mass is a cortical adenoma. We also discussed PA and the various options, and we recommended that we try changing her losartan and hydrochlorothiazide to spironolactone, starting at 12.5 mg per day. Over several months, her regimen was converted to spironolactone monotherapy, 25 mg per day, and her blood pressure is 118/78 mm Hg. Her edema (likely from the amlodipine) has resolved, for which she is elated. Her serum potassium is 4.4 mEq/L, serum creatinine 1.1 mg/dL (97 µmol/L), and plasma renin activity 2.2 ng/mL/h.
Summary
AVS is currently the best available tool for PA subtyping. To ensure the highest likelihood of success, AVS should ideally be performed by interventional radiologist with focused expertise in a high-volume center. In addition to its limited availability and high cost, AVS reliability suffers from heterogeneous procedure protocols and data interpretation criteria. Considering the complex array of pathologies leading to PA, lateralized AVS results might not always indicate strictly unilateral PA, but rather asymmetrical bilateral disease. Ultimately, the goal of PA subtyping is to identify LHA patients who would benefit significantly from unilateral adrenalectomy, and we recommend AVS for those patients. When the likelihood of LHA and/or large clinical benefit is not high, a shared decision-making process should review the options, including a trial of MRA therapy, and AVS can be reconsidered in the future. Until noninvasive subtyping tools such as functional imaging (69, 118) and peripheral biomarkers of APAs (98, 100, 119, 120), that would streamline patients with BHA directly to medical therapy, all patients with PA who are likely to substantially benefit from surgery should be offered AVS. Should screening for PA increase appropriately in the future, randomized trials for optimal subtyping criteria might be possible.
Acknowledgments
Financial Support: A.F.T. was supported by grants K08DK109116 from the National Institute of Diabetes and Digestive and Kidney Diseases, 2019087 from the Doris Duke Charitable Foundation, and U070002 from the Michigan Institute for Clinical & Health Research (MICHR). R.J.A. receives support from grant R01DK115392 (Dr. Anand Vaidya, principal investigator, Brigham and Women’s Hospital) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Glossary
Abbreviations
- APA
aldosterone-producing adenoma
- AV
adrenal vein
- AVS
adrenal vein sampling
- BHA
bilateral hyperaldosteronism
- CSI
contralateral suppression index
- CT
computed tomography
- IVC
inferior vena cava
- LHA
lateralized hyperaldosteronism
- LI
lateralization index
- MACE
mild ACTH-independent cortisol excess
- MR
mineralocorticoid receptor
- MRA
mineralocorticoid-receptor antagonist
- PA
primary aldosteronism
- PASO
Primary Aldosteronism Surgical Outcome study
- SI
SPARTACUS, Subtyping Primary Aldosteronism: a Randomized Trial comparing Adrenal vein sampling and CompUted tomography Scan.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Kontak AC, Wang Z, Arbique D, et al. Reversible sympathetic overactivity in hypertensive patients with primary aldosteronism. J Clin Endocrinol Metab. 2010;95(10):4756-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892-896. [DOI] [PubMed] [Google Scholar]
- 4. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921-1926. [DOI] [PubMed] [Google Scholar]
- 5. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. 2012;44(3):157-162. [DOI] [PubMed] [Google Scholar]
- 7. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 8. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 9. Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911-918. [DOI] [PubMed] [Google Scholar]
- 10. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fourkiotis V, Vonend O, Diederich S, et al. ; Mephisto Study Group . Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2013;168(1):75-81. [DOI] [PubMed] [Google Scholar]
- 12. Iwakura Y, Morimoto R, Kudo M, et al. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99(5): 1593-1598. [DOI] [PubMed] [Google Scholar]
- 13. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243-1248. [DOI] [PubMed] [Google Scholar]
- 14. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331-336. [DOI] [PubMed] [Google Scholar]
- 15. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulatero P, Milan A, Williams TA, Veglio F. Mineralocorticoid receptor blockade in the protection of target organ damage. Cardiovasc Hematol Agents Med Chem. 2006;4(1):75-91. [DOI] [PubMed] [Google Scholar]
- 18. Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maule S, Mulatero P, Milan A, et al. QT interval in patients with primary aldosteronism and low-renin essential hypertension. J Hypertens. 2006;24(12):2459-2464. [DOI] [PubMed] [Google Scholar]
- 20. Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454-459. [DOI] [PubMed] [Google Scholar]
- 21. Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109(23):2857-2861. [DOI] [PubMed] [Google Scholar]
- 22. Rossi GP, Bernini G, Desideri G, et al. ; PAPY Study Participants . Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48(2):232-238. [DOI] [PubMed] [Google Scholar]
- 23. Kozàkovà M, Buralli S, Palombo C, et al. Myocardial ultrasonic backscatter in hypertension: relation to aldosterone and endothelin. Hypertension. 2003;41(2):230-236. [DOI] [PubMed] [Google Scholar]
- 24. Freel EM, Mark PB, Weir RA, et al. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5(6):740-747. [DOI] [PubMed] [Google Scholar]
- 25. Stehr CB, Mellado R, Ocaranza MP, et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28(10):2120-2126. [DOI] [PubMed] [Google Scholar]
- 26. Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826-4833. [DOI] [PubMed] [Google Scholar]
- 27. Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62-69. [DOI] [PubMed] [Google Scholar]
- 28. Lin YH, Lin LY, Chen A, et al. ; TAIPAI Study Group . Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis. 2012;221(1):154-159. [DOI] [PubMed] [Google Scholar]
- 29. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dekkers T, ter Meer M, Lenders JW, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99(7):E1341-E1351. [DOI] [PubMed] [Google Scholar]
- 31. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ono Y, Yamazaki Y, Omata K, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020;105(3):e282-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nanba K, Omata K, Else T, et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J Clin Endocrinol Metab. 2018;103(10):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Sousa K, Boulkroun S, Baron S, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dutta RK, Arnesen T, Heie A, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37-K41. [DOI] [PubMed] [Google Scholar]
- 37. Seidel E, Schewe J, Scholl UI. Genetic causes of primary aldosteronism. Exp Mol Med. 2019;51(11):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nanba K, Blinder AR, Rege J, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75(3):645-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Funder JW. Primary aldosteronism. Hypertension. 2019;74(3):458-466. [DOI] [PubMed] [Google Scholar]
- 40. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nanba K, Omata K, Tomlins SA, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol. 2016;175(2):K1-K6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuda Y, Kawate H, Matsuzaki C, et al. Eplerenone improves carotid intima-media thickness (IMT) in patients with primary aldosteronism. Endocr J. 2016;63(3):249-255. [DOI] [PubMed] [Google Scholar]
- 43. Williams TA, Lenders JWM, Mulatero P, et al. ; Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burrello J, Burrello A, Stowasser M, et al. The primary aldosteronism surgical outcome score for the prediction of clinical outcomes after adrenalectomy for unilateral primary aldosteronism. Ann Surg. 2020;272(6):1125-1132. [DOI] [PubMed] [Google Scholar]
- 45. Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol. 2017;87(6):665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sywak M, Pasieka JL. Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89(12):1587-1593. [DOI] [PubMed] [Google Scholar]
- 47. Velema M, Dekkers T, Hermus A, et al. ; SPARTACUS investigators . Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J Clin Endocrinol Metab. 2018;103(1):16-24. [DOI] [PubMed] [Google Scholar]
- 48. Funder JW. Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control. 2013;6:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15(8):709-716. [DOI] [PubMed] [Google Scholar]
- 50. Rossi GP, Rossitto G, Amar L, et al. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension. 2019;74(4):800-808. [DOI] [PubMed] [Google Scholar]
- 51. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151-160. [DOI] [PubMed] [Google Scholar]
- 52. Wannachalee T, Zhao L, Nanba K, et al. Three discrete patterns of primary aldosteronism lateralization in response to cosyntropin during adrenal vein sampling. J Clin Endocrinol Metab. 2019;104(12):5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Puar TH, Loh WJ, Lim DS, et al. Aldosterone-potassium ratio predicts primary aldosteronism subtype. J Hypertens. 2020;38(7):1375-1383. [DOI] [PubMed] [Google Scholar]
- 54. Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cawood TJ, Hunt PJ, O’Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009;161(4):513-527. [DOI] [PubMed] [Google Scholar]
- 56. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637-644. [DOI] [PubMed] [Google Scholar]
- 57. Zhu L, Zhang Y, Zhang H, et al. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Medicine (Baltimore). 2016;95(39):e4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99(8):2712-2719. [DOI] [PubMed] [Google Scholar]
- 59. Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329-337. [DOI] [PubMed] [Google Scholar]
- 60. Mathur A, Kemp CD, Dutta U, et al. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg. 2010;211(3):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Umakoshi H, Ogasawara T, Takeda Y, et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol. 2018;88(5):645-651. [DOI] [PubMed] [Google Scholar]
- 62. Wannachalee T, Caoili E, Nanba K, Nanba A, Rainey WE, Shields JJ, Turcu AF. The concordance between imaging and adrenal venous sampling varies with aldosterone-driver somatic mutation. J Clin Endocrinol Metab. 2020;105(10):e3628-e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dekkers T, Prejbisz A, Kool LJS, et al. ; SPARTACUS Investigators . Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739-746. [DOI] [PubMed] [Google Scholar]
- 64. Beuschlein F, Mulatero P, Asbach E, et al. The SPARTACUS trial: controversies and unresolved issues. Horm Metab Res. 2017;49(12):936-942. [DOI] [PubMed] [Google Scholar]
- 65. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009;94(7):2437-2445. [DOI] [PubMed] [Google Scholar]
- 66. Williams TA, Burrello J, Sechi LA, et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension. 2018;72(3):641-649. [DOI] [PubMed] [Google Scholar]
- 67. Wong KK, Komissarova M, Avram AM, Fig LM, Gross MD. Adrenal cortical imaging with I-131 NP-59 SPECT-CT. Clin Nucl Med. 2010;35(11):865-869. [DOI] [PubMed] [Google Scholar]
- 68. Burton TJ, Mackenzie IS, Balan K, et al. Evaluation of the sensitivity and specificity of 11C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012;97(1):100-109. [DOI] [PubMed] [Google Scholar]
- 69. Heinze B, Fuss CT, Mulatero P, et al. Targeting CXCR (CXC Chemokine Receptor Type 4) for molecular imaging of aldosterone-producing adenoma. Hypertension. 2018;71(2):317-325. [DOI] [PubMed] [Google Scholar]
- 70. Haase M, Riester A, Kröpil P, et al. Outcome of adrenal vein sampling performed during concurrent mineralocorticoid receptor antagonist therapy. J Clin Endocrinol Metab. 2014;99(12):4397-4402. [DOI] [PubMed] [Google Scholar]
- 71. Nanba AT, Wannachalee T, Shields JJ, et al. Adrenal vein sampling lateralization despite mineralocorticoid receptor antagonists exposure in primary aldosteronism. J Clin Endocrinol Metab. 2019;104(2):487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005;25(Suppl 1):S143-S158. [DOI] [PubMed] [Google Scholar]
- 73. Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol. 2009;70(1):14-17. [DOI] [PubMed] [Google Scholar]
- 74. Jakobsson H, Farmaki K, Sakinis A, Ehn O, Johannsson G, Ragnarsson O. Adrenal venous sampling: the learning curve of a single interventionalist with 282 consecutive procedures. Diagn Interv Radiol. 2018;24(2):89-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Auchus RJ, Michaelis C, Wians FH Jr, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249(2):318-321. [DOI] [PubMed] [Google Scholar]
- 76. Betz MJ, Degenhart C, Fischer E, et al. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur J Endocrinol. 2011;165(2):301-306. [DOI] [PubMed] [Google Scholar]
- 77. Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97(5):1606-1614. [DOI] [PubMed] [Google Scholar]
- 78. Kitamoto T, Kitamoto KK, Omura M, et al. Precise mapping of intra-adrenal aldosterone activities provides a novel surgical strategy for primary aldosteronism. Hypertension. 2020;76(3):976-984. [DOI] [PubMed] [Google Scholar]
- 79. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826-1835. [DOI] [PubMed] [Google Scholar]
- 80. Desrochers MJ, St-Jean M, El Ghorayeb N, et al. Basal contralateral aldosterone suppression is rare in lateralized primary aldosteronism. Eur J Endocrinol. 2020;183(4):399-409. [DOI] [PubMed] [Google Scholar]
- 81. Elliott P, Holmes DT. Adrenal vein sampling: substantial need for technical improvement at regional referral centres. Clin Biochem. 2013;46(15):1399-1404. [DOI] [PubMed] [Google Scholar]
- 82. Monticone S, Satoh F, Giacchetti G, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012;59(4): 840-846. [DOI] [PubMed] [Google Scholar]
- 83. Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008;26(5):989-997. [DOI] [PubMed] [Google Scholar]
- 84. Seccia TM, Miotto D, De Toni R, et al. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: comparison of 3 different protocols. Hypertension. 2009;53(5):761-766. [DOI] [PubMed] [Google Scholar]
- 85. Rossi GP, Ganzaroli C, Miotto D, et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens. 2006;24(2):371-379. [DOI] [PubMed] [Google Scholar]
- 86. Takeda Y, Umakoshi H, Takeda Y, et al. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J Hypertens. 2019;37(5):1077-1082. [DOI] [PubMed] [Google Scholar]
- 87. Wolley MJ, Ahmed AH, Gordon RD, Stowasser M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin Endocrinol (Oxf). 2016;85(5):703-709. [DOI] [PubMed] [Google Scholar]
- 88. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227-1235. [DOI] [PubMed] [Google Scholar]
- 89. El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Front Endocrinol (Lausanne). 2016;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heinrich DA, Adolf C, Holler F, et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab. 2019;104(11):5658-5664. [DOI] [PubMed] [Google Scholar]
- 92. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 93. Galm BP, Qiao N, Klibanski A, Biller BMK, Tritos NA. Accuracy of laboratory tests for the diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2020;105(6):2081-2094. [DOI] [PubMed] [Google Scholar]
- 94. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827-834. [DOI] [PubMed] [Google Scholar]
- 96. O’Toole SM, Sze WC, Chung TT, et al. Low grade cortisol co-secretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J Clin Endocrinol Metab. 2020;105(10):e3776-e3784. [DOI] [PubMed] [Google Scholar]
- 97. Dekkers T, Deinum J, Schultzekool LJ, et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension. 2013;62(6):1152-1157. [DOI] [PubMed] [Google Scholar]
- 98. Eisenhofer G, Dekkers T, Peitzsch M, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62(3):514-524. [DOI] [PubMed] [Google Scholar]
- 99. Li H, Zhang X, Shen S, et al. Adrenal androgen measurement for assessing the selectivity of adrenal venous sampling in primary aldosteronism. Steroids. 2018;134:16-21. [DOI] [PubMed] [Google Scholar]
- 100. Turcu AF, Wannachalee T, Tsodikov A, et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension. 2020;75(1):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pasternak JD, Epelboym I, Seiser N, et al. Diagnostic utility of data from adrenal venous sampling for primary aldosteronism despite failed cannulation of the right adrenal vein. Surgery. 2016;159(1):267-273. [DOI] [PubMed] [Google Scholar]
- 102. Strajina V, Al-Hilli Z, Andrews JC, et al. Primary aldosteronism: making sense of partial data sets from failed adrenal venous sampling-suppression of adrenal aldosterone production can be used in clinical decision making. Surgery. 2018;163(4):801-806. [DOI] [PubMed] [Google Scholar]
- 103. Ceolotto G, Antonelli G, Maiolino G, et al. Androstenedione and 17-α-hydroxyprogesterone are better indicators of adrenal vein sampling selectivity than cortisol. Hypertension. 2017;70(2):342-346. [DOI] [PubMed] [Google Scholar]
- 104. Auchus RJ, Chandler DW, Singeetham S, et al. Measurement of 18-hydroxycorticosterone during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2007;92(7):2648-2651. [DOI] [PubMed] [Google Scholar]
- 105. Nakamura Y, Satoh F, Morimoto R, et al. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96(8):E1272-E1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fuss CT, Treitl M, Rayes N, et al. Radiation exposure of adrenal vein sampling: a German Multicenter Study. Eur J Endocrinol. 2018;179(4):261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vonend O, Ockenfels N, Gao X, et al. ; German Conn’s Registry . Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension. 2011;57(5):990-995. [DOI] [PubMed] [Google Scholar]
- 108. Monticone S, Satoh F, Dietz AS, et al. Clinical management and outcomes of adrenal hemorrhage following adrenal vein sampling in primary aldosteronism. Hypertension. 2016;67(1):146-152. [DOI] [PubMed] [Google Scholar]
- 109. Umakoshi H, Naruse M, Wada N, et al. ; WAVES-J Study Group . Adrenal venous sampling in patients with positive screening but negative confirmatory testing for primary aldosteronism. Hypertension. 2016;67(5):1014-1019. [DOI] [PubMed] [Google Scholar]
- 110. Küpers EM, Amar L, Raynaud A, Plouin PF, Steichen O. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab. 2012;97(10):3530-3537. [DOI] [PubMed] [Google Scholar]
- 111. Kobayashi H, Abe M, Soma M, et al. ; JPAS Study Group . Development and validation of subtype prediction scores for the workup of primary aldosteronism. J Hypertens. 2018;36(11):2269-2276. [DOI] [PubMed] [Google Scholar]
- 112. Burrello J, Burrello A, Pieroni J, et al. Development and validation of prediction models for subtype diagnosis of patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;dgaa974. doi: 10.1210/clinem/dgaa974. [DOI] [PubMed] [Google Scholar]
- 113. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045-1050. [DOI] [PubMed] [Google Scholar]
- 114. Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135(4):258-261. [DOI] [PubMed] [Google Scholar]
- 115. Zarnegar R, Young WF Jr, Lee J, et al. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247(3):511-518. [DOI] [PubMed] [Google Scholar]
- 116. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131(2):453-459. [DOI] [PubMed] [Google Scholar]
- 117. Buffolo F, Li Q, Monticone S, et al. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic study. Hypertension. 2019;74(6):1532-1540. [DOI] [PubMed] [Google Scholar]
- 118. Abe T, Naruse M, Young WF Jr, et al. A novel CYP11B2-specific imaging agent for detection of unilateral subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(3):1008-1015. [DOI] [PubMed] [Google Scholar]
- 119. Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Williams TA, Peitzsch M, Dietz AS, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67(1):139-145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.