Abstract
Context
The origin of Graves disease (GD) remains elusive. However, evidence of an association between GD and viral infections is emerging. Human leukocyte antigen (HLA) class I presents viral antigens to circulating immune cells and plays a crucial role in the defense against viral infections.
Objective
This work aimed to investigate HLA class I expression, enterovirus presence, and the viral immune response proteins signal transducer and activation of transcription 1 (STAT1) and protein kinase R (PKR) in thyroid tissue from GD patients.
Methods
We collected thyroid tissue from core needle biopsies or surgical specimens from 48 GD patients and 24 controls. Standard immunohistochemistry was used to detect HLA class I and enteroviral capsid protein 1 (VP1) on formalin-fixed and paraffin-embedded tissue. STAT1 and PKR were examined by combined immunofluorescence staining. HLA class I expression score was the main outcome measure.
Results
The HLA class I expression score, which takes both proportion and intensity of immunostaining into account, was significantly higher in GD patients (3.1 ± 3.3) than in controls (0.5 ± 0.9) (P < .001). Significantly more VP1 positive thyroid cells were found GD samples (50.1 ± 30.5%) than in controls (14.9 ± 10.5%) (P < .001). STAT1 and HLA class I were found within the same thyroid cells and PKR and VP1 were also colocalized within thyroid cells.
Conclusion
HLA class I is upregulated in GD and enterovirus protein is prevalent in thyroid tissue. The colocalization of HLA class I with STAT1 and VP1 with PKR indicates an antiviral tissue response. These findings support the concept of a link between viral infections and GD.
Keywords: Graves disease, autoimmune thyroid disease, HLA class I, STAT1, enterovirus, viral infections
Graves disease (GD) is a prevalent, autoimmune endocrine disease, caused by an interplay between genetic and environmental factors (1, 2). The incidence of GD shows seasonal trends and variable geographical distribution, indicating possible epidemic triggering factors (3). Evidence of an association between viral infections and autoimmune thyroid disease (AITD) is emerging (4-11). Enterovirus, in particular, has been linked to numerous autoimmune diseases, and the association with type 1 diabetes is especially strong (12-15).
The class I major histocompatibility complex, usually known as human leukocyte antigen (HLA) class I in humans, is a complex of molecules expressed on nucleated cells. HLA class I normally presents peptides from intracellular host proteins. During viral infections, HLA class I presents viral antigens on the cell surface, which is vital for recognition and destruction of infected cells by CD8⁺ T cells (cytotoxic T cells). By contrast, HLA class II presents with extracellular pathogens, such as bacteria.
Antigen presentation is possibly a key process in inducing thyroid autoimmunity. An association between aberrant HLA class II expression and thyroid autoimmunity is recognized (16), and the HLA class II gene variant HLA-DR3is a major AITD susceptibility gene (17-19). HLA class I and its role in thyroid autoimmunity, on the other hand, is less explored. However, HLA class I alleles have been linked to AITD (20, 21), and our group recently reported that HLA class I is upregulated in Hashimoto thyroiditis (22).
HLA class I is upregulated in response to interferon-activated signal transducer and activator of transcription 1 (STAT1) (23). Moreover, STAT1 promotes transcription of several genes involved in antiviral defense mechanisms, one of which is protein kinase R (PKR) (24-30), an enzyme that inhibits viral protein synthesis (31, 32).
To further investigate the association between viral infection and thyroid autoimmunity, we aimed to i) examine the expression of HLA class I in thyroid cells in GD, ii) examine the presence of the antiviral immune response proteins STAT1 and PKR in GD, and iii) confirm the presence of enterovirus in GD. Using immunohistochemistry (IHC) and combined immunofluorescence (IF), we evaluated the expression of HLA class I, STAT1, PKR, and enteroviral capsid protein 1 (VP1) in thyroid tissue from a cohort of both recent-onset and chronic GD.
Material and Methods
Study participants and thyroid tissue collection
For this study, we used previously collected thyroid tissue samples from 48 patients with GD and 24 controls at Oslo University Hospital (11). Age, sex, smoking habits, thyroid function, disease duration, and treatment at the time of tissue sampling were recorded (Table 1). The duration of disease was defined as the time elapsed between clinical diagnosis and the collection of thyroid tissue. The patients were divided into 2 subgroups according to disease duration: newly diagnosed GD (within the last 3 months) or chronic GD (median disease duration of 24 months). Thyroid tissue samples were collected by core needle biopsy in patients with newly diagnosed disease, and from surgical specimens during thyroidectomy in patients with chronic disease. Thyroid tissue samples from 24 patients undergoing neck surgery for reasons other than AITD, such as thyroid tumors or parathyroid adenomas, served as controls. Control samples were taken from normal thyroid tissue adjacent to the pathological lesion. To insure no preexisting or unrecognized thyroid autoimmunity, we measured serum antibodies against thyroid peroxidase (TPO-Ab), thyroglobulin, and thyrotropin receptor (TRAb) in all control individuals.
Table 1.
Characteristics of Patients and Controls at Study Inclusion
| Control group | Graves disease, all | P a | New Graves disease | Chronic Graves disease | P b | |
|---|---|---|---|---|---|---|
| (n = 24) | (n = 48) | (n = 22) | (n = 26) | |||
| Duration, mo | – | 18.0 ± 28.0 | – | 0.0 (2.4) | 24.0 (114) | – |
| Age, y | 52.0 ± 14.4 | 44.0 ± 13.1 | .021 | 40.1 ± 11.9 | 47.2 ± 13.4 | .062 |
| Female, No., % | 22 (91.7) | 42 (87.5) | .596 | 19 (86.4) | 23 (88.5) | .828 |
| TSH, mIU/L | 1.4 (0.7-2.5) | 0.0 (0.0-0.0) | < .001 | 0.0 (0.0-0.0) | 0.0 (0.0-0.3) | .003 |
| FT4, pmol/L | 13.1 (12.1-14.5) | 24.2 (16.7-45.8) | < .001 | 40.0 (27.0-55.2) | 18.6 (15.6-21.4) | < .001 |
| TRAb, IU/L | < .09 | 8.6 (3.4-16.6) | < .001 | 8.6 (4.7-12.1) | 8.9 (2.7-18.0) | .796 |
| Smoker, No., % | 5 (21.7) | 18 (40.9) | .119 | 5 (25.0) | 13 (54.2) | .053 |
| Ophthalmopathy, No., % | 0 (0.0) | 23 (50.0) | < .001 | 3 (15.0) | 20 (76.9) | < .001 |
| Antithyroid drugs, No., % | 0 (0.0) | 23 (47.9) | < .001 | 0 (0.0) | 23 (88.5) | < .001 |
| Steroids before inclusion, No., % | 0 (0.0) | 10 (20.8) | .017 | 0 (0.0) | 10 (38.5) | .001 |
Values presented as median and range, median and interquartile ranges, mean±SD, numbers and percentages. Reference ranges: TSH equal to 0.5 to 3.6 mIU/L, FT4 equal to 8 to 20 pmol/L, TRAb less than 1.5 IU/L. P values compared to controls and tested with t test, Mann-Whitney U test, or Pearson chi-square test. Smoking and ophthalmopathy data were missing for 7 patients.
Abbreviations: FT4, free thyroxine; TRAb, thyrotropin receptor antibody; TSH, thyrotropin.
a P controls vs all Graves patients. bP newly diagnosed vs chronic disease.
Formalin-fixed, paraffin-embedded (FFPE) tissue samples were cut into 3-μm slices and mounted on slides for further analysis. The regional ethics committee approved the study and written informed consent was obtained from all participants.
Immunohistochemistry
HLA class I and VP1 immunostaining was performed with a standard IHC protocol in all samples. Antigens were unmasked by heating in 10-mM citrate buffer of pH 6.0, in a pressure cooker in a microwave oven (800 W) for 20 minutes, followed by 20 minutes of cooling at room temperature. Sections were blocked with 10% goat serum and primary antibodies diluted in Dako REAL antibody diluent (Agilent S202230). The Dako antienteroviral VP1 (5D8/1 clone) was used at a dilution of 1:1400 (55 ng/mL) for 30 minutes. The HLA class I primary antibody (Abcam class I HLA [EMR8/5] Ab70328) was used at a dilution of 1:1500 for 1 hour. Primary antibodies were visualized using the Dako REAL EnVision Detection system (Agilent K5007).
Combined immunofluorescence
To examine the presence of STAT1 and PKR, and to test the localization of these proteins in relation to HLA class I and VP1 within the same FFPE sections, we performed combined IF of STAT1/HLA class I and PKR/VP1. Owing to limited resources and a restricted supply of the STAT1 antibody, we performed IF staining in a subset of the samples only. Seventeen samples of especially good quality and size were analyzed. Antigen retrieval and blocking were performed as described in the IHC protocol described earlier. The sections were immunostained sequentially with STAT1 (Ab109320, dilution 1:500, overnight primary incubation at 4 °C), followed by HLA class I (Abcam class I HLA [EMR8/5] Ab70328, dilution 1:1000, 1 hour at room temperature). The same subset of cases were stained for PKR (Abcam Ab32052 at a dilution of 1:700, overnight at 4 °C) and VP1 (Dako antienteroviral VP1 [5D8/1 clone], 1:1000 dilution, overnight at 4 °C). Primary antibodies were detected using species-specific secondary antibodies (antirabbit or antimouse immunoglobulin G [H + L]) conjugated to either Alexa Fluor 488 or Alexa Fluor 555 (Invitrogen) at a dilution of 1:400 for 1 hour at room temperature, together with DAPI 1:1000.
Image acquisition and analysis
Bright-field image acquisition and analysis were performed on a Nikon 50i microscope fitted with a DS-Fi camera and a DSL2 camera control unit. Images were captured and analyzed using the ImageJ platform. IF image collection and processing were achieved using a Leica DM4000 B LED upright fluorescence microscope and Leica Image analysis software (LAS X).
Immunohistochemical analysis
The slides were analyzed by light microscopy at 400× magnification by 2 independent scientists (T.W. and S.J.R.). Tissue samples were interpreted in a blinded fashion. First, samples were classified as positive or negative for HLA class I. Only immunostaining of thyroid follicular cells (thyrocytes) was evaluated. Next, HLA class I immunoreactivity was graded according to the semiquantitative Allred scoring system. The Allred scoring system takes both intensity (ranging from 0 to 3) and proportion (ranging from 0 to 5) into account, with 8 being the maximum score possible and 0 being the lowest score. This system is commonly used in clinical settings to assess the immunostaining of pathological specimens (33).
We assessed VP1 staining by counting all positively stained thyrocytes alongside the total number of thyrocytes in 10 consecutive counting grids (0.058 mm2) at 400× magnification, thus yielding a percentage of positively stained thyrocytes.
Statistics
If normally distributed, continuous variables were summarized with means and SD (mean ± SD) and, if skewed, with medians and interquartile ranges (median [25th percentile to 75th percentile]). Categorical variables were summarized as frequencies and percentages. Statistical significance of differences between groups was calculated with the independent-samples t test if fulfilling criteria of normality, and with the Mann-Whitney U test if not. The Pearson chi-square test was used to determine the statistical significance of differences in proportions. Associations between clinical features and results were explored using binary logistic regression, Pearson correlation for continuous data, and the Spearman correlation for ordinal data. All analyses were performed using IBM SPSS Statistics version 25 and GraphPad Prism 7.02. We considered P values less than .05 significant.
Results
Few studies have looked at the immunological tissue responses in GD thyroid tissue. In the present study, we found HLA class I upregulation and antiviral proteins both in recent-onset GD and chronic GD.
Study population
Thyroid samples from 26 patients with chronic GD, 22 patients with newly diagnosed GD, and 24 controls were analyzed. Patient characteristics and demographic data are presented in Table 1. All new GD samples were collected within 3 months after diagnosis, while the median disease duration before sampling was 24 months for chronic GD. None of the recent-onset GD patients received antithyroid treatment prior to inclusion, whereas 23 out of 26 chronic GD patients were on antithyroid treatment (22 on carbimazole and 1 on propylthiouracil) at inclusion. The GD group was significantly younger (44.0 ± 13.1 years) than the control group (52.0 ± 14.4 years). Eighteen out of 44 (40.9%) GD patients and 5 out of 23 (21.7%) controls were current cigarette smokers (P = .119). Smoking status data was missing from four GD patients and one control. The sex distribution was equal in each group (87.5% female in GD and 91.7% female in the control group). Three patients in the GD group (6.3%) had one additional autoimmune disease (ankylosing spondylitis, Addison disease and Sjögren syndrome). Two patients in the control group (8.3%) had an autoimmune disease (ulcerous colitis and psoriasis). Ten chronic GD patients received steroids (methylprednisolone intravenously n = 6, prednisolone orally n = 4) due to Graves ophthalmopathy (GO).
Human leukocyte antigen class I is upregulated in Graves disease
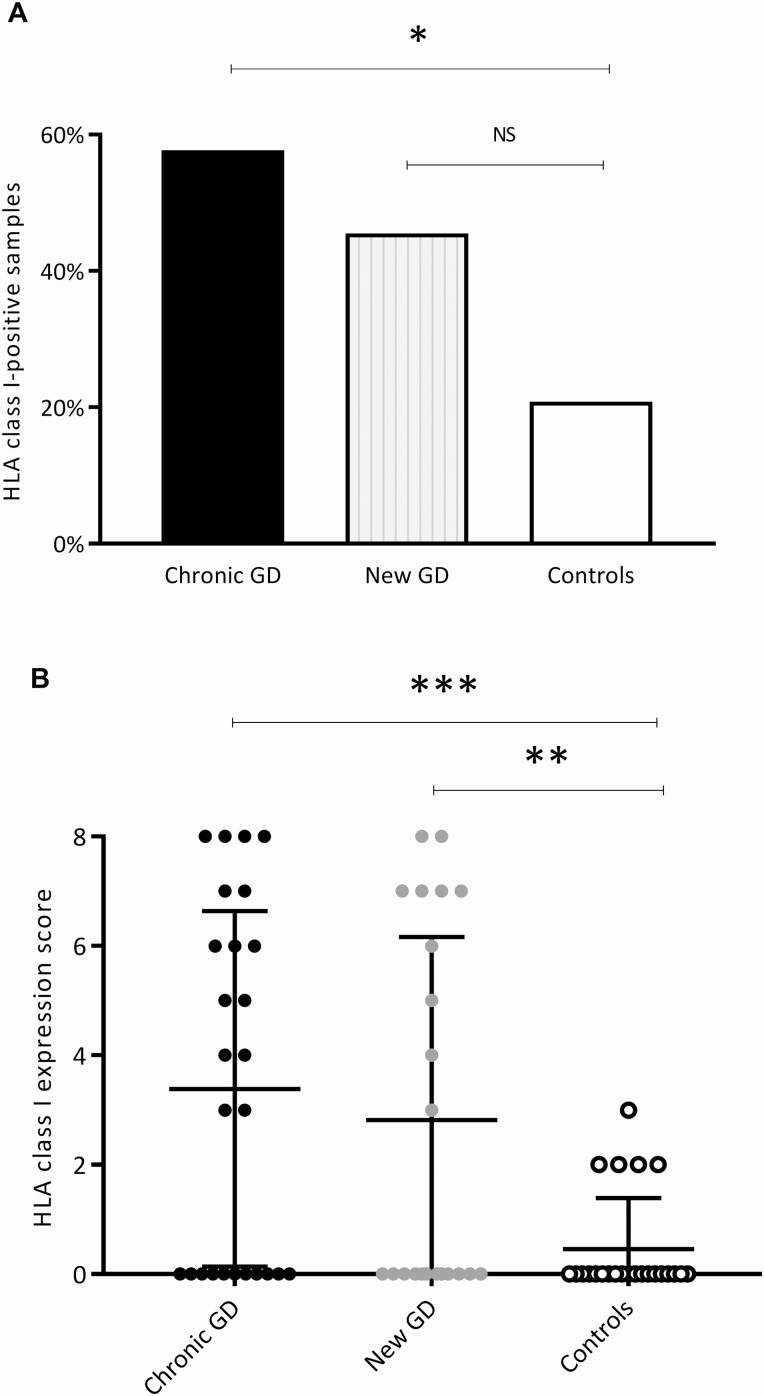
HLA class I presents viral antigens to CD8+ T cells of the immune system and plays a vital role in the body’s defense against virus. We herein demonstrate that HLA class I expression is significantly higher in GD compared to controls (Fig. 1A). First, we examined the number of positive samples in GD and controls. We found increased HLA class I expression in thyroid tissue in 25 of 48 (52.1%) samples in the GD group, but in only 5 of 24 (20.8%) samples in the control group (P = .011). The chronic GD group had a significantly higher number of HLA class I–positive samples than controls, with 15 of 26 (57.7%) being positive (P = .012) (Fig. 2A). The proportion of HLA class I–positive samples in the new GD group was also higher than in the control group (45.5% vs 20.8% respectively); however, the difference did not reach statistical significance. The proportion of HLA class I–positive samples did not differ significantly between the recent-onset and chronic GD group.
Figure 1.
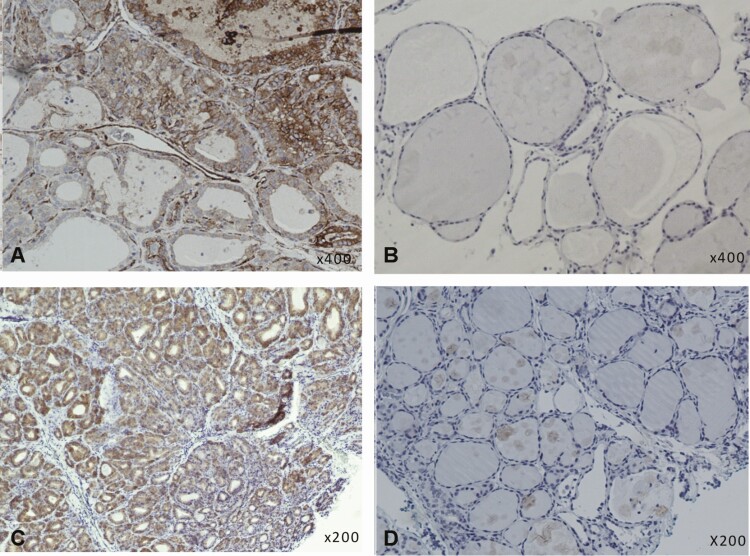
Immunohistochemical staining for HLA class I and VP1 in thyroid tissue samples from GD patients and controls. HLA class I and VP1 was found in more GD samples than controls. A, Positive HLA class I staining (brown color) in thyroid follicular cells in a new GD sample. B, Negative HLA class I staining in a control sample. C, Globally positive VP1 staining (brown color) in thyroid follicular cells in a chronic GD sample. D, Negative VP1 staining in a control sample. All samples stained with a standard horseradish peroxidase immunohistochemistry protocol. GD, Graves disease; HLA, human leukocyte antigen; VP1, enteroviral capsid protein 1.
Figure 2.
HLA class I immunodetection and HLA class I expression score. Significantly more HLA class I–positive samples were found in the GD group than in the control group. A, Proportion of samples with HLA class I positivity in the 2 GD subgroups and in the controls. B, HLA class I expression score (Allred score), taking both immunostaining intensity and proportion of stained tissue into account (maximum score 8, and lowest score 0). The HLA class I expression score was significantly higher in the chronic GD group and in the newly diagnosed GD group than in controls. Bars represent mean and SD. *P less than or equal to .05; **P less than or equal to .01; *** P less than or equal to .001. GD, Graves disease; HLA, human leukocyte antigen; NS, not significant.
Next, we assessed HLA class I expression using an IHC scoring tool that takes both intensity and proportion of immunostaining into account (ranging from 0 to 8) (33). The mean HLA class I expression score was 3.1 ± 3.3 in GD patients and 0.5 ± 0.9 in controls (P < .001). Each subgroup had a significantly higher HLA class I expression score compared to the control group (3.4 ± 3.3 in chronic GD and 2.8 ± 3.3 in new GD) (Fig. 2B). Additionally, when considering HLA class I–positive samples only (score ≥ 1), the HLA class I expression score differed significantly between GD patients (6.0 ± 1.7) and controls (2.2 ± 0.4) (P < .001). Moreover, no samples in the control group had an HLA class I expression score higher than 3. The HLA class I expression score did not significantly differ between the two GD subgroups.
The proportion of HLA class I–positive samples was slightly higher in the 10 patients who received steroids before study inclusion (60.0%) compared to the group that did not receive steroids (50.0%) (P = .578). Moreover, the HLA class I score was higher (4.0 ± 3.6) in the steroid group compared to the GD patients who did not receive steroids (2.9 ± 3.2) (P = 0.347).
Thyroid function (thyrotropin [TSH] and free thyroxine), TRAb level, age, smoking status, and duration of disease did not significantly influence or correlate with the proportion of HLA class I–positive samples or HLA class I expression score. Although the mean TRAb was higher in the HLA class I–positive samples (17.7 ± 5.8 IU/L) than in the HLA class I–negative sample (9.3 ± 1.5 IU/L), the difference did not reach statistical significance.
Smoking increases the risk of GD, and especially GO, but several studies indicate that smoking protects against the development of TPO-Ab and thyroglobulin antibodies (34-37). In the present study, the proportion of GD patients with positive TPO-Ab was not different between smokers and nonsmokers (68.4% vs 66.7%, respectively) (P = .907). The median TPO-Ab titer, however, was nonsignificantly lower in smokers compared to nonsmokers (124 vs 180 kIU/L) (P = .511). Smokers had a higher median thyroglobulin antibody titer than nonsmokers (742 vs 116 kIU/l) (P = .356).
Signal transducer and activation of transcription 1 is colocalized with human leukocyte antigen class I within thyroid cells
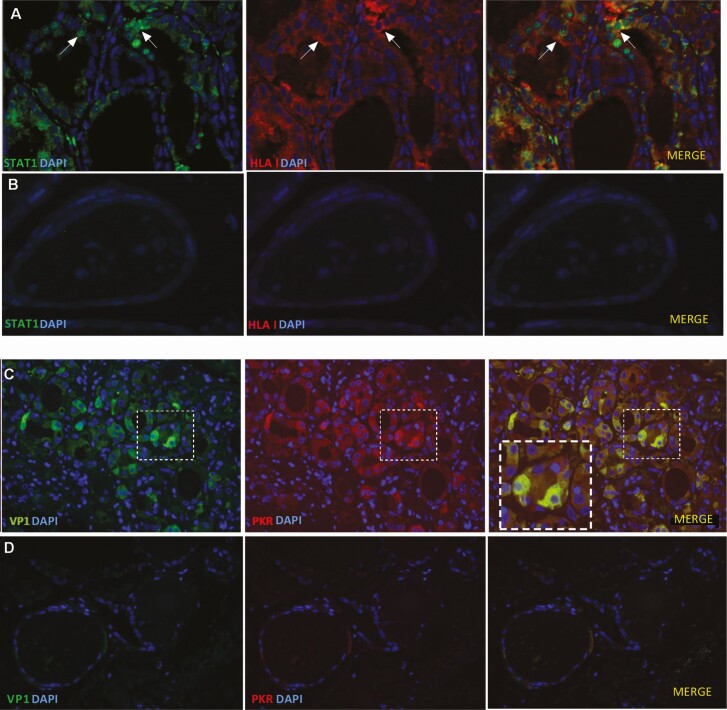
STAT1 is a cytoplasmic protein, which on activation by interferons translocates to the nucleus and initiates transcription of antiviral response proteins. We analyzed STAT1 in relation to HLA class I expression in a subset of samples (4 with newly diagnosed GD, 9 chronic GD, and 4 controls). Interestingly, we found elevated STAT1 in all 4 GD samples with newly diagnosed disease and in only 3 of 9 samples in the chronic GD group. Two of the 4 control samples were also positive (Table 2). STAT1 expression was colocalized with HLA class I, and we observed both cytosolic and nuclear STAT1 (Fig. 3A).
Table 2.
Sequential immunofluorescence in a subset of samples
| HLA class I | STAT 1 | PKR | VP1 | |
|---|---|---|---|---|
| Chronic GD 1 | Positive | Negative | Negative | Negative |
| Chronic GD 2 | Positive | Positive | Positive | Positive |
| Chronic GD 3 | Positive | Negative | Positive | Positive |
| Chronic GD 4 | Negative | Negative | Positive | Positive |
| Chronic GD 5 | Positive | Negative | Positive | Positive |
| Chronic GD 6 | Positive | Positive | Positive | Positive |
| Chronic GD 7 | Negative | Negative | Negative | Negative |
| Chronic GD 8 | Negative | Negative | Positive | Positive |
| Chronic GD 9 | Positive | Positive | Positive | Positive |
| New GD 1 | Positive | Positive | Positive | Positive |
| New GD 2 | Positive | Positive | Positive | Positive |
| New GD 3 | Positive | Positive | Positive | Positive |
| New GD 4 | Positive | Positive | Positive | Positive |
| Control 1 | Positive | Positive | Positive | Positive |
| Control 2 | Positive | Positive | Negative | Negative |
| Control 3 | Negative | Negative | Negative | Negative |
| Control 4 | Negative | Negative | Negative | Negative |
Seventeen samples were stained sequentially with immunofluorescence for HLA class I, STAT1, PKR, and VP1. The table shows that the majority of the GD samples were both HLA class I and STAT1 positive (shown in bold). All new GD samples stained positively for all proteins studied.
Abbreviations: GD, Graves disease; HLA, human leukocyte antigen; PKR, protein kinase R; STAT1, signal transducer and activator of transcription 1; VP1, enteroviral capsid protein 1.
Figure 3.
Combined immunofluorescence of HLA class I/STAT1 and PKR/VP1 in GD samples and controls. Nuclear and cytosolic STAT1 was found in thyroid cells and was colocalized with HLA class I. PKR and VP1 were also colocalized within thyroid cells. A, An example of STAT1 (green) and HLA class I (red) immunofluorescence staining in GD thyroid tissue. Arrows indicate thyroid cells with nuclear STAT1 and intracellular HLA class I. B, Control sample with negative STAT1 and HLA class I staining. C, An example of VP1 (green) and PKR (red) immunofluorescence staining in GD thyroid tissue. The insets represent higher magnification (of the area outlined by the white boxes) and shows colocalized VP1 and PKR within thyrocytes. D, Control sample with negative VP1 and PKR staining. Merged images with blue nuclear DAPI staining. All scale bars at 25 μm. DAPI, 4’,6-diamidino-2-phenylindole; GD, Graves disease; HLA, human leukocyte antigen; PKR, protein kinase R; STAT1, signal transducer and activator of transcription 1; VP1, enteroviral capsid protein 1.
Enteroviral capsid protein 1 was detected in thyroid tissue
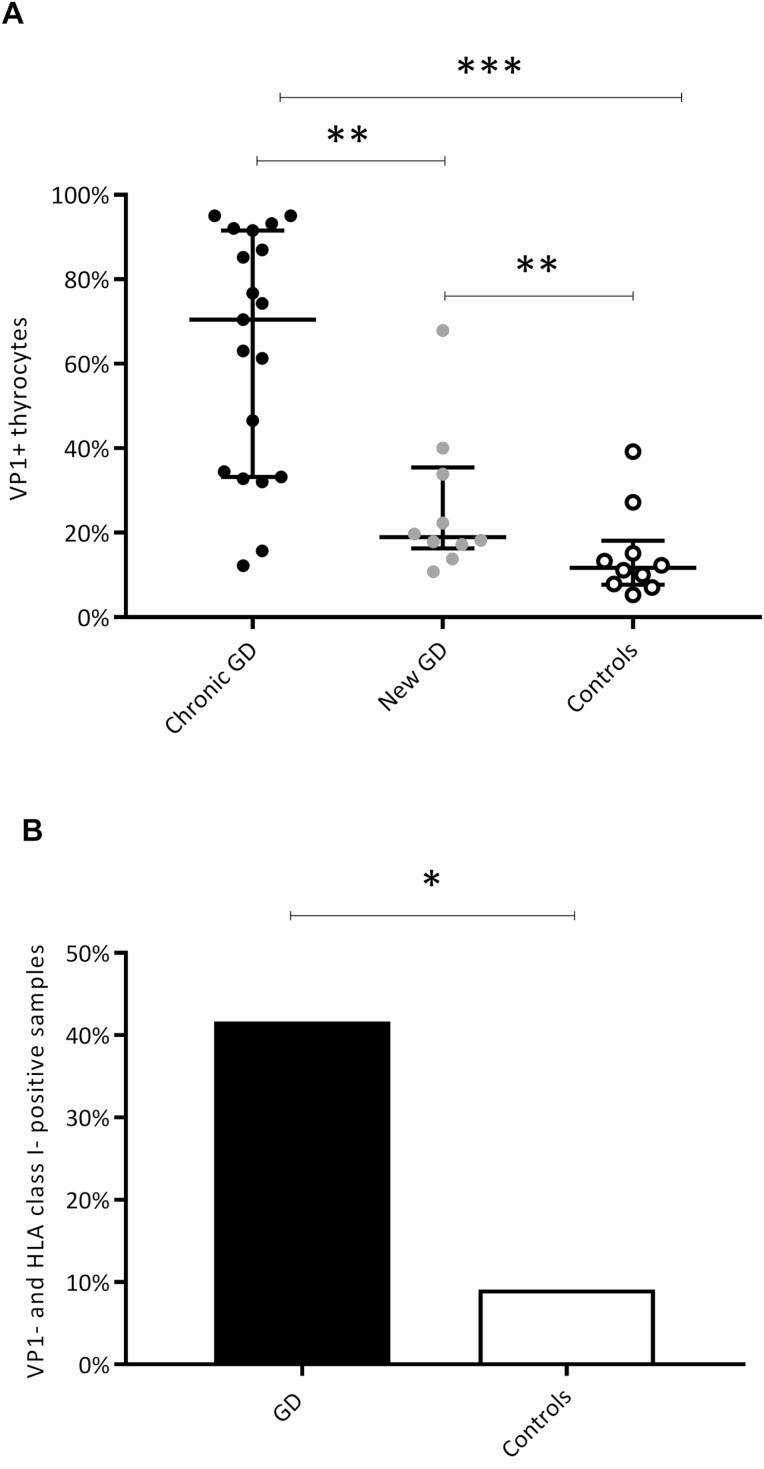
The presence of enterovirus can be explored by staining for the enteroviral capsid protein VP1 (38) (Figs. 1C and 3C). We previously reported both increased VP1 and the presence of enterovirus RNA (in situ hybridization) in the same GD cohort (11). VP1 was found in more GD samples (29 of 48 [60.4%]) than in controls (10 of 24 [41.7%]). This difference was significant only when comparing chronic GD patients (19 of 26 [73.1%]) to control individuals (10 of 24 [41.7%]) (P = .025). When analyzing the VP1-positive samples, there were also significantly more VP1+ thyrocytes in the GD samples (median 40.0% [19.0%-80.9]) than in the controls (median 11.7% [7.7%-18.1%]) (P < .001) (Fig. 4A). The 2 disease stages differed in the numbers of VP1+ thyrocytes, with the chronic GD patients having a significantly higher number (median 70.4% [33.2%-91.5%]) than the newly diagnosed GD group (median 19.0% [16.3-35.4%]) (P = .003) (Fig. 4A). Finally, significantly more GD samples (20 out of 48 [41.7%]) than control samples (2 out of 24 [8.3%]) (P = .004) were double positive (both VP1+ and HLA class I+ thyrocytes) (Fig. 4B).
Figure 4.
VP1 assessment in GD samples and controls. We confirmed the presence of VP1 in GD samples. A, Number of VP1+ thyrocytes in the GD subgroups and controls. There were significantly more VP1+ thyrocytes both in the chronic GD group and the new GD group compared to controls. The chronic GD group had significantly more VP1+ thyrocytes than the new GD group. Bars represent median and interquartile range. B, Proportion of double-positive samples (VP1+/HLA I+) in the GD group and the controls. We found significantly more double-positive samples in the GD group than in the control group. *P less than or equal to .05; **P less than or equal to .01; ***P less than or equal to .001. GD, Graves disease; HLA, human leukocyte antigen; VP1, enteroviral capsid protein 1.
VP1 and HLA class I expression score were positively correlated (Pearson correlation = 0.48, P < .001). Moreover, the odds of being HLA class I positive were 4.0 times higher in the VP1-positive group compared to the VP1-negative group (P = .007).
Protein kinase R was colocalized with enteroviral capsid protein 1
The antiviral enzyme PKR inhibits viral messenger RNA translation, thus preventing viral replication. The transcription of PKR is positively regulated by STAT1. We detected PKR in 11 out of 13 GD samples and in 1 out of 4 control samples (see Table 2). PKR was found in all new GD samples. Interestingly, PKR was colocalized with VP1 within the same thyroid cells (see Fig. 3C).
Plasmacytoid dendritic cells correlate with human leukocyte antigen class I
We previously reported an increased density of plasmacytoid dendritic cells (PDCs) and upregulated expression of the type 1 interferon downstream response protein myxovirus resistance protein A (MxA) in newly diagnosed GD (39). CD8+ T cells, on the other hand, were mainly found in chronic GD in this cohort (39). CD8+ T cells recognize the HLA class I–viral antigen complex, and induce destruction of virally infected cells. When combining previous results with current HLA class I findings, we found a significantly positive correlation between HLA class I score and density of number of PDCs in thyroid tissue (Pearson correlation 0.332, P = .029). The number of VP1+ thyrocytes correlated with CD8+ T cells (Pearson correlation = 0.338, P = .012). Ten of 25 (40.0%) VP1-positive GD samples had CD8+ T cells, whereas only one of the 16 (6.3%) VP1-negative GD samples had CD8+ T cells (P = .017).
Discussion
In this study consisting of thyroid tissue samples both from newly diagnosed and chronic GD patients, we show that HLA class I upregulation is a prominent feature of GD. Moreover, HLA class I is colocalized with the antiviral immune response protein STAT1, whereas the enterovirus capsid protein VP1 is colocalized with PKR, suggesting an active antiviral thyroid tissue immune response in GD. Additionally, we confirm earlier findings of VP1 in the same cohort, which was particularly prevalent in chronic GD tissue samples.
HLA class I was upregulated in thyroid cells both in chronic and new GD tissue samples. These findings are in line with the seminal work of Hanafusa and Bottazzo, who found upregulated HLA class I in 16 out of 26 surgical thyroid samples from GD patients (16). We found increased HLA class I expression in 5 out of 24 controls, but the intensity and proportion of HLA class I staining were significantly lower in the control samples than in the GD samples.
Amplified HLA class I has been found in several autoimmune diseases, with type 1 diabetes being the most studied (40). The role of the amplified HLA class I expression is, however, unknown. We hypothesize that the amplified HLA class I response is due to an active immune response following viral infection. Numerous theories on how viral infections can induce autoimmunity exist (reviewed in [41]). Infection leads to the production of inflammatory cytokines and expression of costimulators on antigen-presenting cells, thus attracting immune cells to the infected area and decreasing the threshold for self-attack. Interferon α, which is produced in large quantities by PDCs in viral infections, stimulates HLA class I upregulation (42) and induces thyroglobulin degradation (43), which is a potential mechanism for pathological thyroglobulin presentation. Moreover, infectious microbes may produce antigens similar to self-antigens, thus evoking an immune attack against the self. Proteins derived from infectious agents, such as Borrelia burgdorferi and Yersinia enterocolitica, show structural homology with the TSH receptor (44). Furthermore, tissue injury might lead to the release of otherwise hidden antigens that can prompt an immune response. Finally, HLA class I upregulation might be a virus-evading strategy. HLA class I acts as a self-tolerance checkpoint for natural killer cells. Some viruses, including hepatitis C virus, herpesvirus, and flavivirus, induce upregulation of HLA class I to avoid natural killer cell–mediated attacks (45-47).
Antithyroid drugs and steroids both are immunosuppressive, and a suppressive effect on HLA class I cannot be ruled out. However, we could not find any significant differences in HLA expression in participants treated with steroids or antithyroid drugs compared to those without any treatment.
Interestingly, Mack et al showed that orbital fibroblasts from GD patients expressed HLA class II in response to cigarette toxins in combination with interferon γ stimulation (48). The increased capacity of antigen presentation due to smoking and interferon γ, which is a viral response cytokine, might be one of the reasons that smokers are more prone to develop GO. However, no such association has been shown for HLA class I expression and GO. We found more HLA class I–positive samples and a higher HLA class I score in smoking GD patients, but the difference did not reach statistical significance.
Viral infections have been linked to several autoimmune diseases, and there is evidence of persistent enterovirus infection of the β cells of the pancreas in patients with type 1 diabetes (12, 13). Enteroviruses, among other viruses, have also been associated with AITD (4-11). In patients genetically predisposed for AITD, an additional AITD-susceptibility gene was expressed when exposed to products of microbial infection (49). This interplay of genetic predisposition and infectious insults could explain why common infections give rise to autoimmunity in some but are negligible in others. To perform double staining for VP1 and antiviral tissue markers, we first repeated VP1 staining and found similar results as previously reported (11). Immunoreactive VP1 was present in thyroid samples from GD patients and controls, but significantly more VP1 was found in GD patients than in control individuals. Notably, the chronic GD tissue samples had remarkably high VP1 numbers. Moreover, the positive correlation between VP1 and HLA class I might indicate that the HLA class I upregulation is a response to enterovirus infection. Interestingly, we previously confirmed the presence of the SIV isoform of the coxsackie and adenovirus receptor in thyroid tissue, thus proving that thyroid cells are susceptible to enteroviral infection (22).
STAT1 was detected with an antiserum that does not discriminate between phosphorylated and nonphosphorylated STAT1. However, the active, phosphorylated form of STAT1 relocates from the cytosol to the nucleus. We identified both cytosolic and nuclear STAT1, suggesting the presence of both inactive and active forms of STAT1 (see Fig. 3A). We found that STAT1 colocalized with HLA class I, thus supporting the previously proven link between activated STAT1 and upregulated HLA class I (23). The antiviral protein PKR, which is enhanced by STAT1 activation, was also found in the GD samples. PKR was colocalized within the same thyroid cells as VP1, thus demonstrating that enterovirus infection may lead to PKR expression (see Fig. 3C). This response resembles that of studies in which infection of thyrocytes or stimulation of cells with viral products caused upregulation of HLA class I, PKR, STAT1, and toll-like receptor 3, among other immune proteins (4, 10, 50).
All recent-onset GD analyzed for STAT1 and PKR were positive, suggesting an active, interferon-driven immune response. Moreover, we confirmed that the chronic GD participants had more VP1+ thyroid cells. These findings are consistent with our previous results, which also showed an interferon-driven response in recent-onset GD. PDCs, which secrete type 1 interferon in the presence of virus, were prevalent in recent-onset GD tissue. Likewise, the type 1 interferon surrogate marker MxA was also prominent in recent-onset GD (39). CD8+ T cells, on the other hand, were characteristic of chronic GD (39). When combining results from these previous analyses performed in the same cohort, our findings point to an acute antiviral response in recent-onset GD and a persistent viral infection in chronic GD.
We show some important differences between chronic and new GD, hypothesizing that these 2 patient groups might differ in their ability to resolve viral infection. However, our method of detecting enterovirus is not suitable to answer whether the VP1 represents persistent or acute infection. As well as applying other methods, this hypothesis should be addressed with a different study design, allowing thyroid tissue biopsies in a study population at several time points. Moreover, new technologies that allow us to target the peptide-binding capacities of the HLA molecules could provide answers as to what thyroid cells are presenting (51). Furthermore, novel methods could be applied to characterize the specific T-cell response in adaptive immunity (52). Finally, more sensitive methods than IHC should be applied to confirm the presence of virus in thyroid tissue in autoimmune thyroid diseases. Yet, the timing in break of tolerance and debut of clinical manifestations in autoimmune diseases are difficult to establish.
In conclusion, we report HLA class I hyperexpression in thyroid tissue from a large GD cohort consisting both of recent-onset and chronic GD. Additionally, we found the immune response protein STAT1 colocalized with HLA class I and VP1 with PKR, which is indicative of an active, antiviral host response. These results are similar to our recently reported findings in Hashimoto thyroiditis, demonstrating common features in AITD. Moreover, we confirm the presence of VP1 both in chronic and new GD, with VP1 being especially evident in chronic GD. Taken together with earlier reports of MxA, PDCs, and CD8+ T cells in the same tissue samples, we believe our study adds evidence to the theory of a link between enterovirus and thyroid autoimmunity.
Strengths and Limitations
The thyroid tissue cohort used in this study is unique because of its considerable proportion of newly diagnosed GD patients. Moreover, the cohort is large when taking into consideration the limited access to thyroid tissue, especially at the onset of disease. However, this study has several limitations. The size of the control group was limited. We did not analyze PKR and STAT1 in all samples. Moreover, the tissue samples from the chronic GD patients were larger in size because they were taken from surgical specimens. However, when counting the VP1+ cells, a fraction based on the total number of cells in the tissue sample was used and the proportion of positively stained thyrocytes was used in the HLA class I expression score.
Acknowledgments
We would like to thank the study participants. A special thanks to Nina Gjerlaugsen at the Hormone Laboratory at Oslo University Hospital in Oslo, and Christine Flaxman and Marie Louise Zeissler at the Islet Biology laboratory at the University of Exeter for technical support and advice. Additionally, we are thankful to Prof Frode Jahnsen at the Department of Pathology and Centre for Immune Regulation at Oslo University Hospital for technical microscopy support.
Financial Support: This work was supported by the South-Eastern Norway Regional Health Authority (Helse Sør-Øst grants, no. 2017024, to S.S.H and K.D.J.); the faculty of Medicine at the University of Oslo, which provided a traveling grant; the European Union’s Seventh Framework Programme PEVNET (FP7/2007-2013) (grant no. 261441) to S.J.R. and N.G.M. Participants of the PEVNET consortium are described at http://www.uta.fi/med/pevnet/publications.html); and a Juvenile Diabetes Research Foundation (JDRF) Career Development Award (5-CDA-2014-221-A-N to S.J.R.).
Author Contributions: T.W. contributed to IHC examinations, data collection, analysis, and interpretation; and drafting of the manuscript. S.S.H. contributed to all parts of the study; study design, clinical coordination and patient recruitment; data collection, analysis, and interpretation; and drafting of the manuscript. T.P. contributed to the surgery and writing of the manuscript. S.J.R. and N.G.M., contributed to the IHC analysis, data analysis and interpretation, and writing the manuscript. K.D.-J., as the principal investigator of the study, had the initial study idea and contributed to the study design; funding; regulatory issues; international collaboration; data collection, analysis, and interpretation; and writing of the manuscript. T.W., S.S.H., and K.D.-J. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- AITD
autoimmune thyroid disease
- FFPE
formalin-fixed, paraffin-embedded
- GD
Graves disease
- GO
Graves ophthalmopathy
- HLA
human leukocyte antigen
- IF
immunofluorescence
- IHC
immunohistochemistry
- MxA
myxovirus resistance protein A
- PDC
plasmacytoid dendritic cell
- PKR
protein kinase R
- STAT1
signal transducer and activator of transcription 1
- TPO
thyroid peroxidase
- TRAb
thyrotropin receptor antibodies
- TSH
thyrotropin
- VP1
enteroviral capsid protein 1
Additional Information
Disclosures: The authors have nothing to disclose, and no competing financial interests exist.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015;64:82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen QY, Huang W, She JX, Baxter F, Volpe R, Maclaren NK. HLA-DRB1*08, DRB1*03/DRB3*0101, and DRB3*0202 are susceptibility genes for Graves’ disease in North American Caucasians, whereas DRB1*07 is protective. J Clin Endocrinol Metab. 1999;84(9):3182-3186. [DOI] [PubMed] [Google Scholar]
- 3. Misaki T, Iida Y, Kasagi K, Konishi J. Seasonal variation in relapse rate of Graves’ disease after thionamide drug treatment. Endocr J. 2003;50(6):669-672. [DOI] [PubMed] [Google Scholar]
- 4. Hammerstad SS, Stefan M, Blackard J, et al. Hepatitis C virus E2 protein induces upregulation of IL-8 pathways and production of heat shock proteins in human thyroid cells. J Clin Endocrinol Metab. 2017;102(2):689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desailloud R, Goffard A, Page C, et al. Detection of enterovirus RNA in postoperative thyroid tissue specimens. Clin Endocrinol (Oxf). 2009;70(2):331-334. [DOI] [PubMed] [Google Scholar]
- 6. Desailloud R, Sané F, Caloone D, Hober D. Persistent infection of a carcinoma thyroid cell line with coxsackievirus B. Thyroid. 2009;19(4):369-374. [DOI] [PubMed] [Google Scholar]
- 7. Nagata K, Hara S, Nakayama Y, et al. Epstein-Barr virus lytic reactivation induces IgG4 production by host B lymphocytes in Graves’ disease patients and controls: a subset of Graves’ disease is an IgG4-related disease-like condition. Viral Immunol. 2018;31(8):540-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Zhang WP, Yao L, et al. PRDM1 expression via human parvovirus B19 infection plays a role in the pathogenesis of Hashimoto thyroiditis. Hum Pathol. 2015;46(12):1913-1921. [DOI] [PubMed] [Google Scholar]
- 9. Caselli E, D’Accolti M, Soffritti I, et al. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol J. 2017;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammerstad SS, Blackard JT, Lombardi A, et al. Hepatitis C virus infection of human thyrocytes: metabolic, hormonal, and immunological implications. J Clin Endocrinol Metabol. 2019;105(4):1157-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammerstad SS, Tauriainen S, Hyöty H, Paulsen T, Norheim I, Dahl-Jørgensen K. Detection of enterovirus in the thyroid tissue of patients with Graves’ disease. J Med Virol. 2013;85(3):512-518. [DOI] [PubMed] [Google Scholar]
- 12. Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64(5):1682-1687. [DOI] [PubMed] [Google Scholar]
- 13. Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56(1):185-193. [DOI] [PubMed] [Google Scholar]
- 14. Kahrs CR, Chuda K, Tapia G, et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ. 2019;364:l231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Bourlet T, Andreoletti L, et al. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation. 2000;101(3):231-234. [DOI] [PubMed] [Google Scholar]
- 16. Hanafusa T, Pujol-Borrell R, Chiovato L, Russell RC, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigen on thyrocytes in Graves’ disease: relevance for autoimmunity. Lancet. 1983;2(8359):1111-1115. [DOI] [PubMed] [Google Scholar]
- 17. Farid NR, Stone E, Johnson G. Graves’ disease and hla: clinical and epidemiologic associations. Clin Endocrinol (Oxf). 1980;13(6):535-544. [DOI] [PubMed] [Google Scholar]
- 18. Heward JM, Allahabadia A, Daykin J, et al. Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J Clin Endocrinol Metabol. 1998;83(10):3394-3397. [DOI] [PubMed] [Google Scholar]
- 19. Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet. 2000;95(5):432-437. [DOI] [PubMed] [Google Scholar]
- 20. Dong RP, Kimura A, Okubo R, et al. HLA-A and DPB1 loci confer susceptibility to Graves’ disease. Hum Immunol. 1992;35(3):165-172. [DOI] [PubMed] [Google Scholar]
- 21. Simmonds MJ, Howson JM, Heward JM, et al. A novel and major association of HLA-C in Graves’ disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet. 2007;16(18):2149-2153. [DOI] [PubMed] [Google Scholar]
- 22. Weider T, Richardson SJ, Morgan NG, Paulsen TH, Dahl-Jørgensen K, Hammerstad SS. Upregulation of HLA class I and antiviral tissue responses in Hashimoto’s thyroiditis. Thyroid. 2020;30(3):432-442. [DOI] [PubMed] [Google Scholar]
- 23. Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84(3): 431-442. [DOI] [PubMed] [Google Scholar]
- 24. Fu XY, Kessler DS, Veals SA, Levy DE, Darnell JE Jr. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci U S A. 1990;87(21):8555-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czerkies M, Korwek Z, Prus W, et al. Cell fate in antiviral response arises in the crosstalk of IRF, NF-κB and JAK/STAT pathways. Nat Commun. 2018;9(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamauchi S, Takeuchi K, Chihara K, et al. STAT1 is essential for the inhibition of hepatitis C virus replication by interferon-λ but not by interferon-α. Sci Rep. 2016;6:38336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84(3):443-450. [DOI] [PubMed] [Google Scholar]
- 28. Larner AC, Chaudhuri A, Darnell JE Jr. Transcriptional induction by interferon. New protein(s) determine the extent and length of the induction. J Biol Chem. 1986;261(1):453-459. [PubMed] [Google Scholar]
- 29. Kessler DS, Veals SA, Fu XY, Levy DE. Interferon-α regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4(10):1753-1765. [DOI] [PubMed] [Google Scholar]
- 30. Bluyssen HA, Muzaffar R, Vlieststra RJ, et al. Combinatorial association and abundance of components of interferon-stimulated gene factor 3 dictate the selectivity of interferon responses. Proc Natl Acad Sci U S A. 1995;92(12):5645-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meurs EF, Watanabe Y, Kadereit S, et al. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66(10):5805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balachandran S, Roberts PC, Brown LE, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13(1):129-141. [DOI] [PubMed] [Google Scholar]
- 33. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474-1481. [DOI] [PubMed] [Google Scholar]
- 34. Effraimidis G, Tijssen JG, Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009;94(4):1324-1328. [DOI] [PubMed] [Google Scholar]
- 35. Strieder TG, Prummel MF, Tijssen JG, Endert E, Wiersinga WM. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf). 2003;59(3):396-401. [DOI] [PubMed] [Google Scholar]
- 36. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2004;89(12):6077-6086. [DOI] [PubMed] [Google Scholar]
- 37. Pedersen IB, Laurberg P, Knudsen N, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158(3): 367-373. [DOI] [PubMed] [Google Scholar]
- 38. Richardson SJ, Leete P, Dhayal S, et al. Evaluation of the fidelity of immunolabelling obtained with clone 5D8/1, a monoclonal antibody directed against the enteroviral capsid protein, VP1, in human pancreas. Diabetologia. 2014;57(2):392-401. [DOI] [PubMed] [Google Scholar]
- 39. Hammerstad SS, Jahnsen FL, Tauriainen S, et al. Immunological changes and increased expression of myxovirus resistance protein A in thyroid tissue of patients with recent onset and untreated Graves’ disease. Thyroid. 2014;24(3):537-544. [DOI] [PubMed] [Google Scholar]
- 40. Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59(11):2448-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19(1):80-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akeno N, Smith EP, Stefan M, et al. IFN-α mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J Immunol. 2011;186(8):4693-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faustino LC, Lombardi A, Madrigal-Matute J, Owen RP, Libutti SK, Tomer Y. Interferon-α triggers autoimmune thyroid diseases via lysosomal-dependent degradation of thyroglobulin. J Clin Endocrinol Metab. 2018;103(10):3678-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benvenga S, Guarneri F, Vaccaro M, Santarpia L, Trimarchi F. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid. 2004;14(11):964-966. [DOI] [PubMed] [Google Scholar]
- 45. Herzer K, Falk CS, Encke J, et al. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77(15):8299-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glasner A, Oiknine-Djian E, Weisblum Y, et al. Zika virus escapes NK cell detection by upregulating major histocompatibility complex class I molecules. J Virol. 2017;91(22):e00785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gustafsson RK, Engdahl EE, Hammarfjord O, et al. Human herpesvirus 6A partially suppresses functional properties of DC without viral replication. PloS One. 2013;8(3):e58122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mack WP, Stasior GO, Cao HJ, Stasior OG, Smith TJ. The effect of cigarette smoke constituents on the expression of HLA-DR in orbital fibroblasts derived from patients with Graves ophthalmopathy. Ophthalmic Plast Reconstr Surg. 1999;15(4):260-271. [DOI] [PubMed] [Google Scholar]
- 49. Ye XP, Yuan FF, Zhang LL, et al. ; China Consortium for the Genetics of Autoimmune Thyroid Disease . ITM2A expands evidence for genetic and environmental interaction in Graves disease pathogenesis. J Clin Endocrinol Metab. 2017;102(2):652-660. [DOI] [PubMed] [Google Scholar]
- 50. Harii N, Lewis CJ, Vasko V, et al. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto’s autoimmune thyroiditis. Mol Endocrinol. 2005;19(5):1231-1250. [DOI] [PubMed] [Google Scholar]
- 51. Høydahl LS, Frick R, Sandlie I, Løset GÅ. Targeting the MHC ligandome by use of TCR-like antibodies. Antibodies (Basel). 2019;8(2):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52..Newell EW, Davis MM. Beyond model antigens: high-dimensional methods for the analysis of antigen-specific T cells. Nat Biotechnol. 2014;32(2):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.