Abstract
Background
Emerging evidence has implicated that inflammation contributes to the pathogenesis of atrial fibrillation (AF). GlycA is a novel marker of systemic inflammation with low intra-individual variability and high analytic precision. GlycA has been associated with incident cardiovascular disease (CVD) independent of other inflammatory markers. However, whether GlycA is associated with AF, specifically, has yet to be established. We examined the association between GlycA and AF in a multi-ethnic cohort.
Methods
We studied 6,602 MESA participants aged 45–85, with no clinical CVD at baseline, with data on GlycA and incident AF. We used multivariable-adjusted Cox models to evaluate the association between GlycA and incident AF. We also examined other inflammatory markers [high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL6) and fibrinogen] and incident AF for comparison.
Results
The mean (SD) age was 62 (10) years, 53% women. The mean plasma GlycA was 381 (62) μmol/L. Over median follow-up of 12.9 years, 869 participants experienced AF. There was no statistically significant association between GlycA and incident AF after adjusting for sociodemographics, CVD risk factors, and other inflammatory markers [Hazard Ratio (95% CI) per 1 SD increment in GlycA: 0.97 (0.88–1.06)]. Neither hsCRP nor fibrinogen was associated with incident AF in same model. In contrast, IL-6 was independently associated with incident AF [HR 1.12 per 1 SD increment (1.05–1.19)].
Conclusions
Although GlycA has been associated with other CVD types, we found that GlycA was not associated with AF. More research will be required to understand why IL-6 was associated with AF but not GlycA.
Clinical trial registration
MESA is not a clinical trial. However, the cohort is registered at: URL: https://clinicaltrials.gov/ct2/show/NCT00005487 Unique identifier: NCT00005487.
Background
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, characterized by chaotic atrial electrical signals leading to irregular heart rhythm [1, 2]. AF, which has affected more than 30 million people worldwide since 2010, occurs in 1–2% of the general population <65 years of age, but ~9% of those over the age of 65 [3, 4]. Regarded as a chronic illness, AF is associated with compromised quality of life and deleterious consequences including dementia, heart failure, and cardiogenic stroke from embolic events [1]. Therefore, prevention of AF and identification of risk are paramount. Many traditional risk factors such as obesity, hypertension, diabetes, smoking, and coronary artery disease (CAD) are well-known risk factors for AF. However, the methods available for predicting incident AF risk and establishing the underlying pathophysiology are still limited.
Many of the same chronic systemic diseases that are linked to AF (i.e. CAD, hypertension, and obesity) are also associated with persistent low-grade inflammation and increased levels of proinflammatory cytokines [5, 6]. There has been increased recognition of inflammation as a central mediator of AF in hearts that are metabolically stressed [7–9]. The prevalence and prognosis of AF are both associated with serum levels of circulating inflammatory biomarkers and cardiac tissue expression of inflammatory markers [2, 10, 11]. Inflammation exacerbates electrical and structural remodeling of the atrium leading to the formation of an adverse substrate that facilitates onset and maintenance of AF [12]. Moreover, AF itself can induce inflammation during atrial remodeling, thereby perpetuating the arrhythmia [2]. Inflammatory markers, such as interleukin 6 (IL-6), high-sensitivity C-reactive protein (hsCRP), and fibrinogen have been demonstrated to be associated with AF [13–16].
GlycA, an emerging biomarker of systemic inflammation, which is a nuclear magnetic resonance (NMR) signal derived from the N-acetyl methyl group protons of circulating glycosylated proteins: α1-acid glycoprotein, α1-antitrypsin, haptoglobin, α1-antichymotrypsin and transferrin [17]. GlycA is moderately correlated with other known markers for inflammation, such as hsCRP, IL-6, and fibrinogen [18]. Yet, compared to these other common inflammatory markers, GlycA has low intra-individual variability and greater analytic precision, which makes it a more reliable inflammatory biomarker [17]. Moreover, elevated GlycA has been associated with incident cardiovascular events, such as myocardial infarction, ischemic stroke, coronary revascularization, peripheral artery disease, heart failure, and CVD death, independent of other traditional inflammatory markers [19–23]. However, whether GlycA is associated with AF, specifically, has not been well established. This is important to evaluate because GlycA may be a prognostic marker for AF or a target of therapy in the future.
To address this knowledge gap, we sought to determine the association of baseline GlycA and AF in a large multi-ethnic cohort free of overt clinical CVD at baseline. We hypothesized that higher plasma levels of baseline GlycA are associated with increased incidence of AF, independent of other traditional risk factors and other common inflammatory markers.
Material and methods
Transparency and Openness Policy (TOP)
The Multi-Ethnic Study of Atherosclerosis (MESA) participates in the NIH BioLincc Open program, and requests for access to MESA data can be submitted to: https://biolincc.nhlbi.nih.gov/studies/mesa/. Additionally, other researchers can apply to the MESA Coordinating Center to become a new investigator after signing a Data Use Agreement (DUA); more details are available at https://www.mesa-nhlbi.org/. Upon receipt of a DUA with the MESA Coordinating Center, access to the de-identified MESA databases can be made available to other researchers with approved proposals. Our findings should be easily reproducible through the methods described in this paper.
Study sample
MESA is an ongoing prospective cohort study that recruited ethnically diverse individuals free of clinically recognized CVD, including AF, and apparently healthy at baseline from 6 field centers in the United States to assess the characteristics of subclinical CVD, and risk factors for its progression. According to the MESA study protocol, individuals with clinically recognized CVD were excluded from the study. This refers to people who had physician-diagnosed heart attack, angina, stroke, transient ischemic attack, heart failure, or atrial fibrillation, those taking nitroglycerin and those who underwent procedures related to CVD (coronary artery bypass grafting, angioplasty, valve replacement, pacemaker or defibrillator implantation, any surgery on the heart or arteries).
The study initially enrolled a total of 6,814 individuals aged 45 to 84 years, 38% White, 28% African, 22% Hispanic, and 12% Asian between 2000 and 2002. Since the initial visit, 5 subsequent visits have taken place. At each visit, participant demographics, medical history, and physical examination results were collected. Further details about the study design have been reported [24].
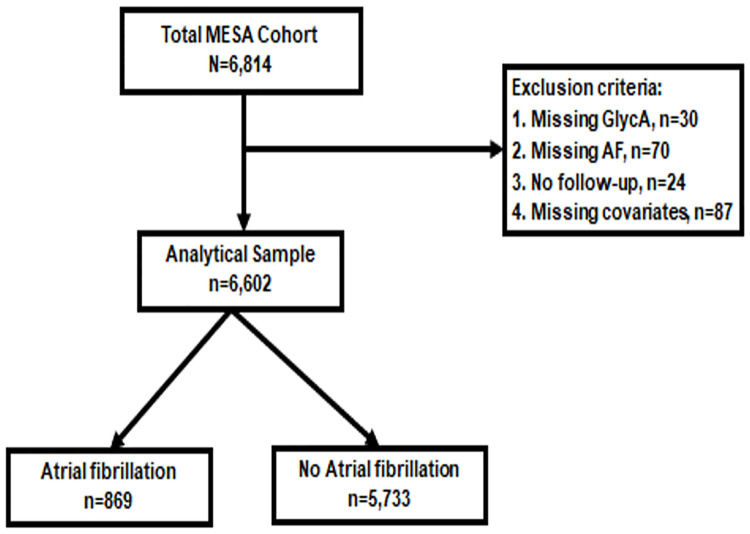
In our analysis, individuals missing either GlycA measurements at baseline or incident AF follow-up data were excluded, leaving a total of 6,602 participants for inclusion (Fig 1).
Fig 1. Flow diagram illustrating study sample inclusion and exclusion criteria as well as the number of participants with and without AF during follow-up.
Ethical approval statement
The MESA study was approved by the institutional review boards (IRB) at each participating center and each study participant provided informed consent. At Johns Hopkins, our study was approved by the Johns Hopkins School of Medicine IRB applicant number NA_00030361.
Exposure assessment
GlycA was analyzed from EDTA plasma samples, which were obtained from MESA baseline visit (2000–2002) and stored at -70°C until measured using NMR LipoProfile® as previously described [21, 23, 25]. The NMR assay detects the level of glycan residues of acute phase proteins and quantifies GlycA levels, which are expected to be elevated during inflammation [17]. The intra-assay and inter-assay coefficients of variance (CV) for NMR LipoProfile® were 1.9% and 2.6%, respectively. GlycA levels haven been shown to be similar when measured after short-term vs. long-term storage, in plasma vs. serum samples, and fasting vs. non-fasting state [17].
Covariates
Using data obtained from the baseline MESA visit, we considered demographics (age, sex, race/ethnicity, and MESA site), behavioral factors (smoking status, pack-years of smoking, and physical activity), socioeconomic factors (education, health insurance), adiposity [Body Mass Index (BMI)], traditional CVD risk factors [systolic blood pressure (SBP), use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and estimated glomerular filtration rate (eGFR)], and other inflammatory markers (hsCRP, IL-6, fibrinogen) for covariate adjustment. Height, weight, and blood pressure were measured following the protocol. BMI was calculated as weight divided by squared height (kg/m2). Physical activity was estimated in metabolic equivalent minutes per week using a 28-item Typical Week Physical Activity Survey [26]. Baseline blood pressure was measured 3 times in the seated position with the Dinamap automated blood pressure device, and the average of the last 2 measurements were used. Glomerular filtration rate was estimated using the formula from the Chronic Kidney Disease Epidemiology Collaboration [27]. Diabetes was defined as fasting blood glucose level ≥126 mg/dl, self-reported diagnosis of diabetes, or use of diabetes medication. Inflammatory biomarkers, including hsCRP, IL-6, and fibrinogen were measured from stored serum samples obtained at the baseline examination [18, 28].
Outcomes assessment
Prevalent AF identified by study visit electrocardiogram (ECG) or self-report at baseline was an exclusion criterion for enrollment in the study. After enrollment, trained staff called study participants or their proxy every 9 to 12 months to identify all new hospitalizations and obtained discharge diagnostic codes. Participants were followed until July 15, 2015. Incident AF was ascertained by 1) study ECGs at a follow-up visit consistent with AF; 2) hospital discharge diagnoses [International Classification of Diseases, Ninth Revision, Clinical Modification code 427.31 (AF) or 427.43 (atrial flutter)] for AF; or 3) Medicare inpatient and outpatient claims data for individuals enrolled in fee-for-service Medicare. Previous work has shown the validity (up to 89%) of discharge diagnoses for identifying AF [29].
Statistical analysis
Baseline GlycA, hsCRP, IL-6, and fibrinogen levels were modeled in quartiles and also continuously per one standard deviation (SD) increment. Baseline characteristics of study population were presented by incident AF status. Kaplan-Meier curves were plotted of AF-free survival by GlycA quartiles. Multivariable-adjusted Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the association of incident AF by GlycA levels.
We evaluated 4 progressively adjusted models. In model 1, we adjusted for demographics and study site (age, sex, race/ethnicity, MESA site). Model 2 adjusted for model 1 covariates with the addition of socioeconomic, behavioral, and adiposity measures (education, health insurance, smoking status, pack-years of smoking, physical activity, and BMI). Model 3 additionally adjusted for CVD risk factors that may be intermediate variables between inflammation/adiposity and AF risk (SBP, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and eGFR). In model 4, we further adjusted for other commonly studied inflammatory markers, specifically log-transformed hsCRP, IL-6, and fibrinogen. In the same fully adjusted model (model 4), we also evaluated the association of the other inflammatory markers with incident AF, to compare with GlycA. In a supplemental analysis, we further adjusted for incident heart failure and incident coronary heart disease (CHD) in addition to all covariates adjusted for in model 3.
In addition, we used restricted cubic spline adjusted for the variables in Model 4 with knots placed at the 5th, 35th, 66.5th, 95th percentiles to characterize the nonlinear association between baseline GlycA (continuous) levels with incident AF. We also examined multiplicative interactions of baseline GlycA (and the other inflammatory markers) with AF by age, sex, and race/ethnicity categories. The analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX). P values were two-sided, with significance level set at 0.05.
Results
Baseline characteristics
The baseline characteristics of the 6,602 participants included in the analyses are shown in Table 1 by incident AF status and S1 Table by baseline GlycA level quartiles. Among the sample, the mean (SD) for age was 62 (10) years with 53% being women, 38% White, 27% Black, 22% Hispanic, and 12% Chinese. The mean (SD) for baseline plasma GlycA level was 381 (62) μmol/L. Those with higher GlycA levels tended to be women, have a lower educational background, and have a higher BMI, SBP, and prevalence of diabetes mellitus. In addition, those with higher GlycA levels tended to have a higher median level of inflammatory biomarkers, such as hsCRP, IL-6, and fibrinogen (S1 Table).
Table 1. Baseline characteristics of participants by presence or absence of incident atrial fibrillation, multi-ethnic study of atherosclerosis (2000–2015).
| Total | AF | No AF | P value | |
|---|---|---|---|---|
| N | 6,602 | 869 (13%) | 5,733 (87%) | |
| a Age, years | 62 (10) | 69 (8) | 61 (10) | < 0.001 |
| < 65 years | 3,739 (57%) | 219 (25%) | 3,520 (61%) | < 0.001 |
| ≥ 65 years | 2,863 (43%) | 650 (75%) | 2,213 (39%) | |
| Sex | ||||
| Women | 3,484 (53%) | 391 (45%) | 3,093 (54%) | |
| Race/ethnicity | ||||
| White | 2,541 (38%) | 411 (47%) | 2,130 (37%) | < 0.001 |
| Chinese American | 791 (12%) | 107 (12%) | 684 (12%) | |
| Black | 1,804 (27%) | 190 (22%) | 1,614 (28%) | |
| Hispanic | 1,466 (22%) | 161 (19%) | 1,305 (23%) | |
| Education | 0.92 | |||
| < bachelor’s degree | 4,267 (65%) | 563 (65%) | 3,704 (65%) | |
| a BMI, kg/m2 | 28 (5) | 28 (5) | 28 (5) | 0.68 |
| Current smoker | 855 (13%) | 94 (11%) | 761 (13%) | < 0.001 |
| Former smoker | 2,423 (37%) | 374 (43%) | 2,049 (36%) | |
| Never smoker | 3,324 (50%) | 401 (46%) | 2,923 (51%) | |
| b Pack-years of smoking if >0, median (IQR) | 16 (6–33) | 23 (9–42) | 16 (6–31) | < 0.001 |
| b Physical activity, MET-minutes/week | 4,035 | 3,435 | 4,170 | < 0.001 |
| (1,995–7,545) | (1,650–6,413) | (2,040–7,740) | ||
| a Systolic blood pressure, mmHg | 126 (22) | 134 (22) | 125 (21) | < 0.001 |
| a eGFR, ml/min per 1.73m2 | 74 (16) | 71 (18) | 75 (16) | < 0.001 |
| a Total cholesterol, mg/dL | 194 (35) | 191 (35) | 195 (35) | 0.005 |
| a HDL-C, mg/dL | 51 (15) | 51 (15) | 51 (15) | 0.53 |
| Diabetes mellitus | 820 (12%) | 140 (16%) | 680 (12%) | < 0.001 |
| Antihypertensive medication | 2,435 (37%) | 443 (51%) | 1,992 (35%) | < 0.001 |
| Lipid-lowering medication | 1,076 (16%) | 187 (22%) | 889 (16%) | < 0.001 |
| b GlycA, μmol/L | 376 (337–420) | 376 (338–417) | 376 (337–421) | 0.94 |
| b hsCRP, mg/L | 1.91 (0.84–4.23) | 1.92 (0.89–4.18) | 1.91 (0.82–4.24) | 0.67 |
| b IL-6 pg/mL | 1.20 (0.77–1.88) | 1.43 (0.92–2.17) | 1.17 (0.75–1.84) | < 0.001 |
| b Fibrinogen, mg/dL | 338 (295–388) | 341 (304–392) | 337 (294–388) | 0.003 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; MET, metabolic equivalent of task; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6.
a Data are presented as mean (standard deviation) for continuous variables and as count (percentages) for categorical variables, unless otherwise specified.
b Data are presented as median (IQR).
Over a median (IQR) follow-up time of 12.9 (10.0–13.6) years, a total of 869 participants (13%) experienced AF. Those who developed AF tended to be men, White, and have a higher age, median pack-years of smoking, SBP, and a greater prevalence of diabetes mellitus, and were more likely to take medications for hypertension and hyperlipidemia. They had lower average physical activity levels and eGFR, although BMI was similar to those who did not develop AF. Participants with AF had similar levels of baseline GlycA, hsCRP, and fibrinogen; however, baseline IL-6 levels were higher among those who developed incident AF (Table 1).
Associations of GlycA with incident AF
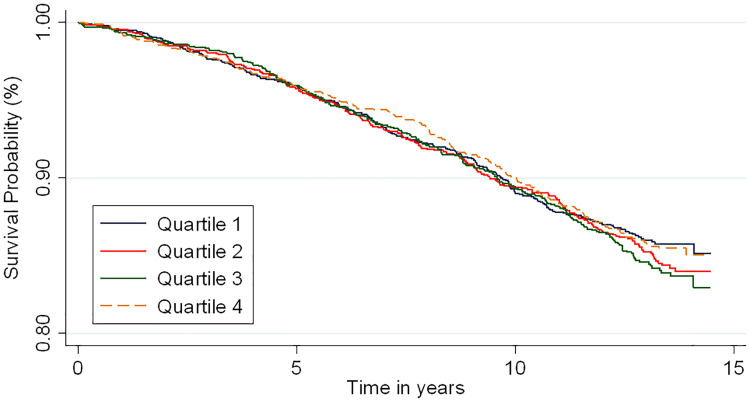
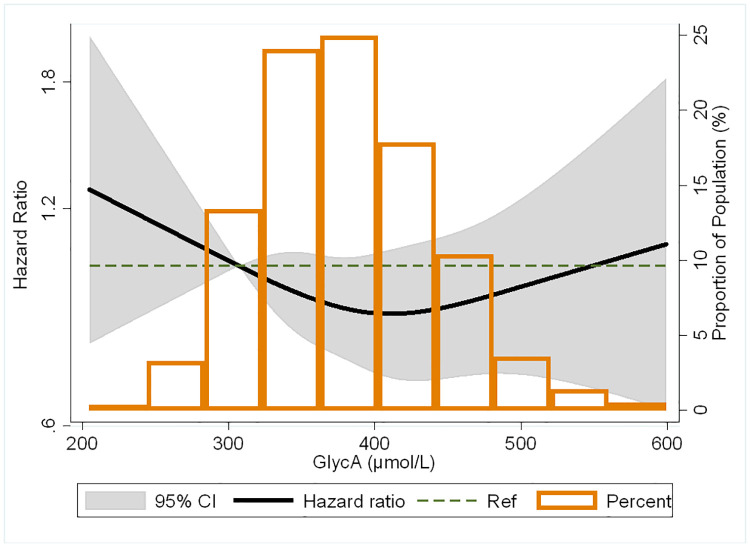
The incidence rates and HRs for the association of baseline GlycA with incident AF are shown in Table 2. In unadjusted analysis, there was no significant difference in survival free of AF by baseline GlycA quartiles (Fig 2, log-rank test: P = 0.64). There remained no significant association of GlycA with incident AF in multivariable adjusted models. After adjusting for demographics, such as age, sex, race/ethnicity, and MESA site (model 1), compared to the lowest GlycA quartile, the adjusted HRs (95% CI) for AF were 1.03 (0.85–1.24), 1.11 (0.92–1.34) and 1.13 (0.92–1.38) for the 2nd, 3rd and 4th quartiles respectively. There also was no statistically significant association after progressively adjusting for behavioral, socioeconomic and adiposity factors (model 2), CVD risk factors that may be intermediate variables between inflammation/adiposity and AF risk (model 3), and other biomarkers for inflammation (model 4). Findings were similar with modeled continuously per 1 SD increment in baseline GlycA. There was no significant interactions by sex (p = 0.05), age (p = 0.65) or race/ethnicity (p = 0.23) for the association of baseline GlycA and incident AF. In the restricted cubic spline model, the association of baseline GlycA with risk of AF appeared U-shaped; however, confidence intervals were wide and associations not statistically significant (Fig 3).
Table 2. Incidence rates (95% CI) and hazard ratios (95% CI) for the association of GlycA with incident atrial fibrillation: The multi-ethnic study of atherosclerosis (2000–2015).
| GlycA in μmol/L | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per 1 SD (62 μmol/L) increment |
|---|---|---|---|---|---|
| [Median (IQR)] | [314 (294–327)] | [359 (348–368)] | [397 (386–407)] | [452 (435–480)] | |
| N | 1,670 | 1,661 | 1,630 | 1,641 | 6,602 |
| Cases | 213 | 226 | 228 | 202 | 869 |
| Incidence rates† | 11.2 (9.8–12.8) | 12.0 (10.5–13.7) | 12.4 (10.9–14.1) | 11.2 (9.8–12.9) | 11.7 (11.0–12.5) |
| Hazard Ratios* | |||||
| Model 1 | 1 (reference) | 1.03 (0.85–1.24) | 1.11 (0.92–1.34) | 1.13 (0.92–1.38) | 1.07 (0.99–1.15) |
| Model 2 | 1 (reference) | 0.98 (0.81–1.19) | 1.00 (0.83–1.22) | 0.98 (0.79–1.21) | 1.02 (0.94–1.10) |
| Model 3 | 1 (reference) | 0.96 (0.79–1.17) | 0.98 (0.81–1.20) | 0.95 (0.77–1.18) | 1.01 (0.93–1.09) |
| Model 4 | 1 (reference) | 0.95 (0.78–1.16) | 0.95 (0.77–1.17) | 0.86 (0.67–1.10) | 0.97 (0.88–1.06) |
Abbreviation: CI, confidence interval; SD, standard deviation.
† Crude incidence rates reported are per 1000 person-years.
*Model 1: Adjusted for age, sex, and race/ethnicity and MESA site.
*Model 2: Model 1 plus education, health insurance, BMI, smoking status, pack-years of smoking, and physical activity.
*Model 3: Model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and eGFR.
*Model 4: Model 3 plus ln(CRP), ln(IL-6) and ln(Fibrinogen).
‡ P value = 0.65, 0.05 and 0.23 for interaction by age, sex and race/ethnicity using quartiles of GlycA as the exposure variable. Results remained similar and not statistically significant when stratified by sex.
Fig 2. Kaplan-Meier survival estimates for the association between GlycA and atrial fibrillation.
Log-rank test: P = 0.64.
Fig 3. Restricted cubic spline of the association between GlycA and atrial fibrillation adjusted for age, sex, race/ethnicity, MESA field site, education, health insurance, BMI, smoking status, pack-years of smoking, physical activity, systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes, eGFR, ln(CRP), ln(IL-6) and ln(fibrinogen).
The black curve represents hazard ratios for atrial fibrillation by proportion of population with the respective GlycA concentration. Grey boundaries represent the 95% CI of the hazard ratios. Knots were at the 5th, 35th, 66.5th, and 95th percentiles which correlates to GlycA levels of 289.8, 353.1, 400.2, and 488.8 μmol/L, respectively.
Given that GlycA has already been demonstrated to be associated with incident heart failure and CHD, we performed a supplemental analysis to determine whether GlycA was still associated with AF after adjusting for interim heart failure event or CHD in addition to all covariates adjusted for in model 3 (S2 Table). In this supplemental analysis, the adjusted HR (95% CI) for AF per 1 SD higher GlycA was 0.96 (0.89–1.04), still showing no statistically significant association between GlycA and AF.
Associations of hsCRP, IL-6, and fibrinogen with incident AF
Incidence rates and HRs for the association of other inflammatory biomarkers at baseline, such as hsCRP, IL-6, and fibrinogen with incident AF were also evaluated among the 6,446 individuals with complete biomarker assessment (Table 3). There was no significant association of hsCRP with incident AF with adjusted HRs (95% CI) of 1.00 (0.82–1.22), 0.94 (0.76–1.16) and 0.98 (0.76–1.26) for the 2nd, 3rd and 4th quartiles, respectively, compared to the first hsCRP quartile, in our fully adjusted model (model 4). Similarly, there was no association of fibrinogen with incident AF. Compared to the lowest fibrinogen quartile, the adjusted HRs (95% CI) for AF were 1.20 (0.98–1.47), 0.96 (0.78–1.19) and 0.94 (0.74–1.20) for the 2nd, 3rd and 4th quartiles respectively. On the other hand, higher IL-6 was independently associated with AF. Compared to the lowest IL-6 quartile, the adjusted HRs (95% CI) for AF were 1.05 (0.84–1.32), 1.33 (1.06–1.67) and 1.42 (1.10–1.82) for the 2nd, 3rd and 4th quartiles respectively, and was 1.12 (1.05–1.19) per 1 SD increment in IL-6. Interactions by age, race/ethnicity and sex were not significant for hsCRP, IL-6 and fibrinogen.
Table 3. Incidence rates (95% CI) and hazard ratios (95% CI) for the association of CRP, IL-6 and fibrinogen with incident atrial fibrillation: The multi-ethnic study of atherosclerosis (2000–2015).
| CRP in mg/L | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per 1 SD |
| [Median (IQR)] | [0.5 (0.3–0.7)] | [1.3 (1.0–1.5)] | [2.8 (2.3–3.4)] | [7.3 (5.4–11.5)] | (5.2 mg/L) increment |
| N | 1,617 | 1,614 | 1,604 | 1,611 | 6,446 |
| Cases | 195 | 230 | 216 | 207 | 848 |
| Incidence rates† | 10.5 (9.1–12.1) | 12.6 (11.1–14.4) | 12.1 (10.6–13.8) | 11.5 (10.1–13.2) | 11.7 (11.0–12.5) |
| Hazard Ratios* | 1 (reference) | 1.00 (0.82–1.22) | 0.94 (0.76–1.16) | 0.98 (0.76–1.26) | 0.99 (0.91–1.08) |
| IL-6 in pg/mL | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per 1 SD |
| [Median (IQR)] | [0.6 (0.5–0.7)] | [1.0 (0.9–1.1)] | [1.5 (1.3–1.7)] | [2.7 (2.2–3.6)] | (1.2 pg/mL) increment |
| N | 1,612 | 1,611 | 1,612 | 1,611 | 6,446 |
| Cases | 140 | 188 | 256 | 264 | 848 |
| Incidence rates† | 7.2 (6.1–8.5) | 10.1 (8.7–11.6) | 14.5 (12.8–16.4) | 15.7 (13.9–17.7) | 11.7 (11.0–12.5) |
| Hazard Ratios* | 1 (reference) | 1.05 (0.84–1.32) | 1.33 (1.06–1.67) | 1.42 (1.10–1.82) | 1.12 (1.05–1.19) |
| Fibrinogen in mg/dL | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per 1 SD |
| [Median (IQR)] | [269 (250–284)] | [317 (307–327)] | [362 (349–373)] | [428 (405–463)] | (72 mg/dL) increment |
| N | 1,643 | 1,583 | 1,638 | 1,582 | 6,446 |
| Cases | 177 | 230 | 223 | 227 | 868 |
| Incidence rates† | 9.3 (8.0–10.8) | 12.9 (11.3–14.7) | 12.1 (10.6–13.8) | 12.6 (11.0–14.4) | 11.7 (11.0–12.5) |
| Hazard Ratios* | 1 (reference) | 1.20 (0.98–1.47) | 0.96 (0.78–1.19) | 0.94 (0.74–1.20) | 0.99 (0.91–1.08) |
Abbreviation: CI, confidence interval; SD, standard deviation.
Sample size = 6,446 after excluding participants with missing data on IL-6 and Fibrinogen.
† Crude incidence rates reported are per 1000 person-years.
*Model: Adjusted for age, sex, race/ethnicity, MESA site, education, health insurance, BMI, smoking status, pack-years of smoking, physical activity, systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and eGFR and log transformed CRP, IL-6, fibrinogen, and including GlycA. Interactions by age, race and sex were not significant for CRP, IL-6 and Fibrinogen using quartiles of each inflammatory biomarker as the exposure variable.
Discussion
Contrary to our hypothesis, in this analysis from a diverse community cohort free of CVD at baseline, we found that higher concentrations of a novel composite inflammatory biomarker GlycA at baseline was not associated with greater incidence of AF over a 12.9-year median follow-up. Given that GlycA was a composite marker reflective of multiple acute phase proteins, we had hypothesized that it would be a superior marker of predicting AF risk compared to the other inflammatory markers. Rather, we only found IL-6 to be independently associated with incident AF.
We investigated this analysis because previous research has found GlycA to be associated with other diseases with an inflammatory etiology, including auto-immune diseases [30–32] and other types of CVD. In a prior MESA study, higher levels of GlycA were associated with having a greater prevalence of an unfavorable cardiovascular health profile, and with poorer status of many of the individual components of the American Heart Association Life Simple 7 score (i.e. BMI, physical activity, smoking, blood pressure, glucose, cholesterol)–health metrics which are also risk factors for AF [18]. Higher GlycA levels have been linked with subclinical atherosclerosis [30, 33, 34], incident CVD [19–22], and with heart failure with preserved ejection fraction [23]. In a prior MESA study comparing the predictive value of GlycA and other inflammatory biomarkers, GlycA had a significantly predictive value comparable to hsCRP, IL-6, and D-dimer, if not superior, for total death, CVD, chronic inflammatory-related severe hospitalization and death, and total cancer [21]. Thus, we had hypothesized that GlycA might be a superior marker for AF too, although our findings came to a different conclusion. Of note, prior work in MESA has found that GlycA is only modestly to moderately correlated with other markers of inflammation [with Pearson correlation coefficient for GlycA with d-dimer (0.09), IL-6 (0.29), hsCRP (0.47), and fibrinogen (0.49) [18], and thus we had anticipated associations with AF independent of other inflammatory markers.
There is extensive evidence that inflammation plays a key role in the development of AF. The association of some inflammatory biomarkers with AF had been studied, but the relationship between GlycA, specifically, and AF had not been explored before our report. Consistent with our results, in a recent Chronic Renal Insufficiency Cohort (CRIC) study that assessed the association between common inflammatory biomarkers (hsCRP, fibrinogen, TNF- α, and IL-6) and AF, only plasma IL-6 level was significantly associated with both the presence of AF at baseline and new-onset of AF in chronic kidney disease patients [35]. A prior MESA study that evaluated the prognostic value of IL-6 for various CVD outcomes found the association of IL-6 with AF appeared greater among statin users compared to non-users [36]. In contrast to our findings, in that analysis there was no longer a statistically significant association of IL-6 with AF in multivariable-adjusted models, but differences between that study and ours may be their stratification by statin use at baseline which reduced statistical power, and we additionally adjusted for multiple inflammatory markers including GlycA which was not included in their models [36].
Other inflammatory markers have been linked to AF in prior work. One study examined the relationship between collagen biomarkers and AF and demonstrated that high levels of plasma collagen biomarkers were also associated with excess risk for AF [37]. Alegret et al showed that CRP and C-C Motif Chemokine Ligand 2 (CCL2) concentrations were significantly increased in AF patient compared to healthy subjects [38]. However, when patients with paroxysmal AF and permanent AF were separately analyzed, while increase in CCL2 was observed in both subgroups, CRP was only elevated in those with the permanent condition. Further analysis of the genetic variants of CRP and CCL2 did not support strong association of the inflammatory biomarkers and AF, leading to the conclusion that the duration of the episode is important to consider in assessing the role of inflammation, and that elevation of CCL2 is the consequence, not the cause of AF.
Together, these collective studies suggest the relationship between AF and the process of inflammation is multifactorial and complex. High levels of inflammatory biomarkers and inflammatory cells such as neutrophils and lymphocytes have been observed in patients with AF. When local or systemic inflammation occurs, it induces atrial electrical and structural remodeling, which triggers AF. In turn, AF promotes inflammation by mechanisms yet to be understood, and perpetuates the arrhythmia [2]. Markers reflective of acute inflammation vs. chronic inflammation may have differing associations with AF. While inflammation has been implicated in the pathophysiology of AF, and several inflammatory biomarkers have been proposed to be associated with AF, the role of inflammation in guiding AF management has not been well established.
Strengths and limitations
Our study must be interpreted in the context of several limitations. First, due to our ascertainment methods, paroxysmal cases of AF that were not present at study visits or did not result in hospitalization may have been missed. Second, incident AF cases were ascertained from hospitalization discharge codes, which could have been misclassified. However, these codes have been demonstrated to have adequate positive predictive value for the ascertainment of AF events [39]. Third, while we included several covariates that likely are to influence the development of AF in the statistical models, residual confounding remains a possibility. Fourth, GlycA levels were measured only once at baseline, and thus we were unable to assess change in GlycA levels over time. Previous work has shown that a single baseline measure could accurately capture the short-term inflammatory status over four to 6 months [40]. However, we do not know if the same accuracy holds for longer periods of time.
Despite these limitations, our study had a number of strengths. We used data from a large multiethnic, multiracial cohort of patients without clinical CVD at baseline who were followed longitudinally with a median follow up of 12.9 years to determine development of AF. We were also able to examine multiple biomarkers for inflammation other than our primary biomarker of interest, GlycA, for comparison in the same population. To our knowledge, our study was the first to examine the association of GlycA with AF.
Conclusions
In summary, we found that elevated GlycA levels at baseline were not associated with higher risk of incident AF, but IL-6 levels were. This suggests that GlycA is not a prediction marker for AF. Further research is required to understand why the inflammatory marker IL-6, but not GlycA, was associated with AF. Several inflammatory biomarkers have been proposed to be associated with AF, but due to the complex pathophysiology of AF each biomarker’s predictive value in guiding AF management has not been well established. Nevertheless, it is undeniable that inflammation contributes to the pathological process of AF. A better understanding of inflammatory markers related to AF improves understanding of the pathophysiology of the disease as well as its risk prediction.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Data Availability
There are restrictions on sharing the actual MESA database because it has patient identifiers and protected health information, and thus is subject to IRB approval. We have signed a data use agreement (DUA) with MESA Coordinating Center that we cannot directly share data and that anyone with access to the data has to follow MESA policies and procedures for data use. We are not allowed to personally share the datasets and have to keep them stored securely in a Cloud using encrypted software for protection. However, other researchers may obtain the minimal dataset required to replicate our study’s findings by submitting a proposal through the NIH BioLINCC (https://biolincc.nhlbi.nih.gov/studies/mesa/). Additionally, one can apply to the MESA Coordinating Center to become a new investigator after signing a Data Use Agreement. Please direct inquires about MESA data access to Craig Johnson (wcraigj@uw.edu) at the MESA Coordinating Center or at https://www.mesa-nhlbi.org/. Our findings should be easily reproducible through the methods described in this paper.
Funding Statement
The MESA study was supported by contracts HHSN268201500003I, N01-HC- 95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC- 95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC- 95169, and R01 HL127659 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. EDM and DZ are additionally funded by the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins University. SJ was supported by the Dean’s Research Fund at Johns Hopkins University School of Medicine. The aforementioned NHBLI funding agency provided financial support of the study for participant recruitment and data collection, as well as support in the form of salaries for authors OO, SRH, MS, and EDM (paid to their respective institution). The aforementioned Johns Hopkins funding source provided support in form of salaries for authors EDM, DZ, and SJ. The commercial affiliation Laboratory Corporation of America Holdings (LabCorp) provided support in the form salary for JDO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation. 2019:CIR0000000000000665. Epub 2019/01/29. 10.1161/CIR.0000000000000665 . [DOI] [PubMed] [Google Scholar]
- 2.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–43. Epub 2015/01/28. 10.1038/nrcardio.2015.2 . [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47. Epub 2013/12/19. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. The American journal of cardiology. 2009;104(11):1534–9. Epub 2009/11/26. 10.1016/j.amjcard.2009.07.022 . [DOI] [PubMed] [Google Scholar]
- 5.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Archives of medical science: AMS. 2017;13(4):851–63. Epub 2017/07/20. 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension (Dallas, Tex: 1979). 2011;57(2):132–40. Epub 2010/12/15. 10.1161/HYPERTENSIONAHA.110.163576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva RM. Influence of Inflammation and Atherosclerosis in Atrial Fibrillation. Current atherosclerosis reports. 2017;19(1):2. Epub 2017/01/20. 10.1007/s11883-017-0639-0 . [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez A, Van Wagoner DR. Oxidant and Inflammatory Mechanisms and Targeted Therapy in Atrial Fibrillation: An Update. J Cardiovasc Pharmacol. 2015;66(6):523–9. Epub 2015/09/04. 10.1097/FJC.0000000000000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52(4):306–13. Epub 2008/09/16. 10.1097/FJC.0b013e31817f9398 . [DOI] [PubMed] [Google Scholar]
- 10.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502. Epub 2015/03/10. 10.1253/circj.CJ-15-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pena JM, MacFadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. European heart journal. 2012;33(4):531–7. Epub 2011/12/22. 10.1093/eurheartj/ehr460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121(8):2955–68. Epub 2011/08/02. 10.1172/JCI46315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7(4):438–44. Epub 2010/02/16. 10.1016/j.hrthm.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155(2):303–9. Epub 2008/01/25. 10.1016/j.ahj.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56(10):789–95. Epub 2010/08/28. 10.1016/j.jacc.2010.02.066 . [DOI] [PubMed] [Google Scholar]
- 16.Mukamal KJ, Tolstrup JS, Friberg J, Gronbaek M, Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study). Am J Cardiol. 2006;98(1):75–81. Epub 2006/06/21. 10.1016/j.amjcard.2006.01.067 . [DOI] [PubMed] [Google Scholar]
- 17.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clinical chemistry. 2015;61(5):714–23. Epub 2015/03/18. 10.1373/clinchem.2014.232918 . [DOI] [PubMed] [Google Scholar]
- 18.Benson EA, Tibuakuu M, Zhao D, Akinkuolie AO, Otvos JD, Duprez DA, et al. Associations of ideal cardiovascular health with GlycA, a novel inflammatory marker: The Multi-Ethnic Study of Atherosclerosis. Clinical cardiology. 2018;41(11):1439–45. Epub 2018/11/20. 10.1002/clc.23069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3(5):e001221. Epub 2014/09/25. 10.1161/JAHA.114.001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis From the JUPITER Trial. J Am Heart Assoc. 2016;5(7). Epub 2016/07/15. 10.1161/JAHA.116.003822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR, Jr. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin Chem. 2016;62(7):1020–31. Epub 2016/05/14. 10.1373/clinchem.2016.255828 . [DOI] [PubMed] [Google Scholar]
- 22.Fashanu OE, Oyenuga AO, Zhao D, Tibuakuu M, Mora S, Otvos JD, et al. GlycA, a Novel Inflammatory Marker and Its Association With Peripheral Arterial Disease and Carotid Plaque: The Multi-Ethnic Study of Atherosclerosis. Angiology. 2019;70(8):737–46. Epub 2019/04/30. 10.1177/0003319719845185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang S, Ogunmoroti O, Ndumele CE, Zhao D, Rao VN, Fashanu OE, et al. Association of the Novel Inflammatory Marker GlycA and Incident Heart Failure and Its Subtypes of Preserved and Reduced Ejection Fraction: The Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2020;13(8):e007067. Epub 2020/08/09. 10.1161/CIRCHEARTFAILURE.120.007067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156(9):871–81. Epub 2002/10/25. 10.1093/aje/kwf113 . [DOI] [PubMed] [Google Scholar]
- 25.Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, et al. High-Density Lipoprotein Subclasses and Noncardiovascular, Noncancer Chronic Inflammatory-Related Events Versus Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4(9):e002295. Epub 2015/09/16. 10.1161/JAHA.115.002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vella CA, Allison MA, Cushman M, Jenny NS, Miles MP, Larsen B, et al. Physical Activity and Adiposity-related Inflammation: The MESA. Medicine and science in sports and exercise. 2017;49(5):915–21. Epub 2016/12/16. 10.1249/MSS.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–7. Epub 2010/03/27. 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;113(4):644–9. Epub 2014/01/08. 10.1016/j.amjcard.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:141–7. Epub 2012/01/25. 10.1002/pds.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119(11):1242–53. Epub 2016/09/23. 10.1161/CIRCRESAHA.116.309637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ormseth MJ, Chung CP, Oeser AM, Connelly MA, Sokka T, Raggi P, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther. 2015;17:117. Epub 2015/05/10. 10.1186/s13075-015-0646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung CP, Ormseth MJ, Connelly MA, Oeser A, Solus JF, Otvos JD, et al. GlycA, a novel marker of inflammation, is elevated in systemic lupus erythematosus. Lupus. 2016;25(3):296–300. Epub 2015/12/08. 10.1177/0961203315617842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tibuakuu M, Fashanu OE, Zhao D, Otvos JD, Brown TT, Haberlen SA, et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS. 2019;33(3):547–57. Epub 2018/11/27. 10.1097/QAD.0000000000002079 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezeigwe A, Fashanu OE, Zhao D, Budoff MJ, Otvos JD, Thomas IC, et al. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–9. Epub 2019/02/05. 10.1016/j.atherosclerosis.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amdur RL, Mukherjee M, Go A, Barrows IR, Ramezani A, Shoji J, et al. Interleukin-6 Is a Risk Factor for Atrial Fibrillation in Chronic Kidney Disease: Findings from the CRIC Study. PLoS One. 2016;11(2):e0148189. Epub 2016/02/04. 10.1371/journal.pone.0148189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cainzos-Achirica M, Enjuanes C, Greenland P, McEvoy JW, Cushman M, Dardari Z, et al. The prognostic value of interleukin 6 in multiple chronic diseases and all-cause death: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2018;278:217–25. Epub 2018/10/13. 10.1016/j.atherosclerosis.2018.09.034 . [DOI] [PubMed] [Google Scholar]
- 37.Duprez DA, Heckbert SR, Alonso A, Gross MD, Ix JH, Kizer JR, et al. Collagen Biomarkers and Incidence of New Onset of Atrial Fibrillation in Subjects With No Overt Cardiovascular Disease at Baseline. Circ Arrhythm Electrophysiol. 2018;11(10):e006557. Epub 2018/10/26. 10.1161/CIRCEP.118.006557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alegret JM, Aragones G, Elosua R, Beltran-Debon R, Hernandez-Aguilera A, Romero-Menor C, et al. The relevance of the association between inflammation and atrial fibrillation. Eur J Clin Invest. 2013;43(4):324–31. Epub 2013/02/13. 10.1111/eci.12047 . [DOI] [PubMed] [Google Scholar]
- 39.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–7. Epub 2009/06/23. 10.1016/j.ahj.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, et al. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1167–70. Epub 2012/05/09. 10.1158/1055-9965.EPI-12-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
There are restrictions on sharing the actual MESA database because it has patient identifiers and protected health information, and thus is subject to IRB approval. We have signed a data use agreement (DUA) with MESA Coordinating Center that we cannot directly share data and that anyone with access to the data has to follow MESA policies and procedures for data use. We are not allowed to personally share the datasets and have to keep them stored securely in a Cloud using encrypted software for protection. However, other researchers may obtain the minimal dataset required to replicate our study’s findings by submitting a proposal through the NIH BioLINCC (https://biolincc.nhlbi.nih.gov/studies/mesa/). Additionally, one can apply to the MESA Coordinating Center to become a new investigator after signing a Data Use Agreement. Please direct inquires about MESA data access to Craig Johnson (wcraigj@uw.edu) at the MESA Coordinating Center or at https://www.mesa-nhlbi.org/. Our findings should be easily reproducible through the methods described in this paper.