Abstract

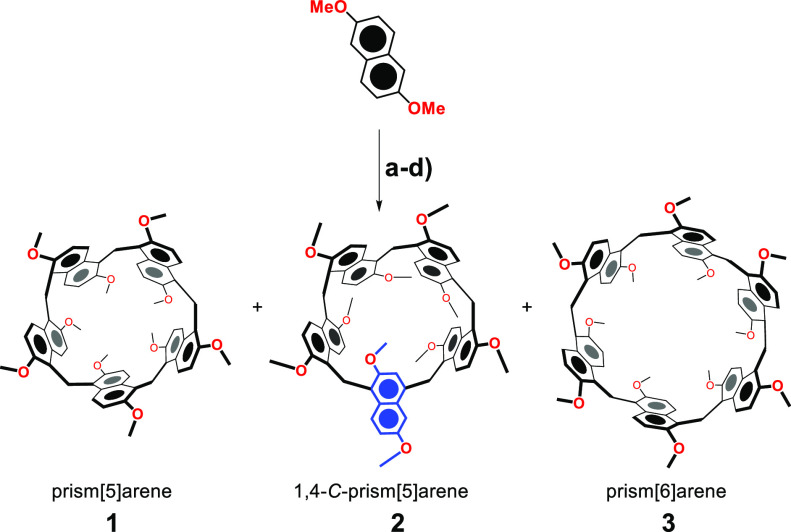
The novel title macrocycles, based on methylene-bridged 1,5-naphthalene units, have been obtained by template effect in a thermodynamically controlled synthesis. In detail, the prism[5]arene 1 or the prism[6]arene 3 was selectively removed from the equilibrium mixture by using the complementary ammonium-templating agent. When only the solvent 1,2-DCE was used, the 1,4-confused derivative 2 was obtained. The prism[5]arene here described shows a deep π-electron-rich aromatic cavity that exhibits a great affinity for the quaternary ammonium guests, originating from favorable cation···π and +NC–H···π interactions. This recognition motif is the basis of the templated synthesis of the prism[n]arenes here reported.
Since 1967, when Charles Pedersen1 reported the first template synthesis of crown-ethers, a plethora of peculiar macrocyclic structures have been designed and obtained by guest-templated2 strategies. Even now, supramolecular chemists have never stopped imagining novel and intriguing macrocyclic structures. Among them, pillararenes3 and oxatubarenes4 have recently shown intriguing supramolecular functions and properties.5 Inspired by the attractive shapes of oxatubarenes and pillararenes and encouraged by their supramolecular performances,5 we have envisioned novel cyclo-structures (e.g., 1 and 3 in Scheme 1), based on methylene-bridged 1,5-naphthalene units.6 We were attracted by their deep π-electron-rich aromatic cavity and by their prism shape (vide infra), which has inspired the name prismarene.7 The prismarenes 1–3, as well as pillararenes, can be classified as cyclophanes.8 The cyclophanes8 are generally obtained by a reversible acid- or base-catalyzed condensation of the respective monomers with an aldehyde. Interestingly, in some cases, under thermodynamically controlled macrocyclization conditions, the selectivity toward a specific cyclo-oligomer can be driven by a template effect.9−12 In this way, it can be possible to isolate a specific macrocycle from an equilibrium mixture by adding an appropriate complementary guest.10−12 In this regard, a well-known example of thermodynamically controlled synthesis concerns the pillararene macrocycles.9,11,12
Scheme 1. Synthesis of Prism[n]arenes 1–3.
Reagents and conditions: (a) 1,2-DCE, TFA, paraformaldehyde, 70 °C, 22 h: 1 (0.3%), 2 (40%); (b) conditions a and 42+·2I–: 1 (47%), 2 (16%); (c) conditions a and 5+·I–: 1 (32%), 2 (8%); (d) 6+·I–, 1,2-DCE, TFA, 70 °C, 72 h: 1 (0.3%), 2 (6%), 3 (20%).
In analogy with other examples of cyclophanes,3,13 we started the synthetic approaches to prismarenes by using the monomer 2,6-dimethoxynaphthalene and formaldehyde in the presence of an acid catalyst. In initial tests, when 2,6-dimethoxynaphthalene (0.5 M)13 was reacted in 1,2-dichloroethane (1,2-DCE) as the solvent, with paraformaldehyde in the presence of BF3·Et2O at 30 °C, only linear oligomers were obtained.14 With the aim of obtaining cyclic structures, we resorted to different conditions, which included the use of trifluoroacetic acid (TFA) as the acid, higher reaction temperature, and dilution. Thus, when 2,6-dimethoxynaphthalene (2.5 mM) and paraformaldehyde (1.2 equiv) were reacted in the presence of TFA (15 equiv) in DCE at 70 °C, the envisioned prism[5]arene macrocycle 1 was obtained in 0.3% yield after 22 h (Scheme 1), while its 1,4-confused15 isomer (1,4-C-prism[5]arene) 2 was obtained in higher yield (40%).
The possible role of the solvent for the formation of prism[5]arene 1 and 1,4-C-prism[5]arene 2 was then explored. Thus, 2 was obtained in lower yield when solvents such as o-dichlorobenzene or chloroform were used (Table S1). Temperature was also found to be crucial for the formation of 2. Indeed, only 11% of 1,4-C-prism[5]arene was collected when the reaction was performed at room temperature. The HR-ESI mass spectrum confirmed the molecular mass of 2 (found: 1000.4218 m/z, calculated for [M]+ 1000.4186). Detailed 1D and 2D NMR studies (SI) at 213 K clearly indicated that 4/5 of the naphthalene rings of 2 were bridged through their 1,5-positions, while the naphthalene group in blue in Scheme 1 showed a 1,4-bridging pattern (confused-naphthalene ring), as confirmed by X-ray crystallographic analysis (Figure 1). Interestingly, when the TFPB– salt16 of dication 42+ (TFPB: tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) was added to a CD2Cl2 solution of 2, its 1H NMR spectrum at 183 K (600 MHz, SI) showed dramatic changes indicative of the formation of a pseudo[2]rotaxane.16 In detail, the formation of the 42+ ⊂ 2 pseudorotaxane was ascertained by the presence of shielded 1H NMR signals at negative value of chemical shifts (from 0 to −2 ppm, SI) attributable to the protons of the guest inside the aromatic cavity of the host.16 An association constant value of 470 M–1 was calculated by direct integration of the slowly exchanging 1H NMR signals17 of the threaded 42+ ⊂ 2 and the free species (SI, Figure 2).
Figure 1.
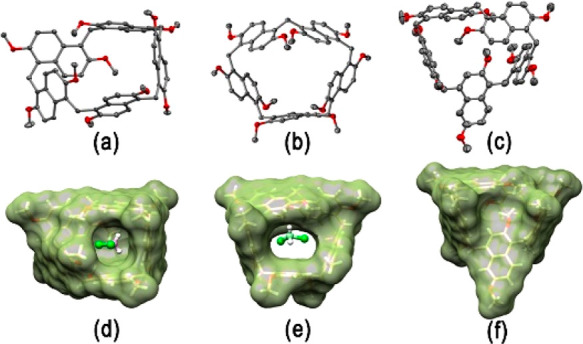
Ellipsoid representation (50% probability) of the (a) α and (b) γ forms of prism[5]arene 1 and (c) β form of 1,4-C-prism[5]arene 2. Solvent molecules, disordered atoms with low occupancy factors, and hydrogen atoms are not included for clarity. For each molecule the α and β forms have similar conformations. The solvent-excluded molecular surfaces (1.4 Å probe) of the (d) α and (e) γ forms of prism[5]arene and (f) β form of 1,4-C-prism[5]arene. The encapsulated CH2Cl2 solvent molecules are represented as a ball-and-stick model.
Figure 2.
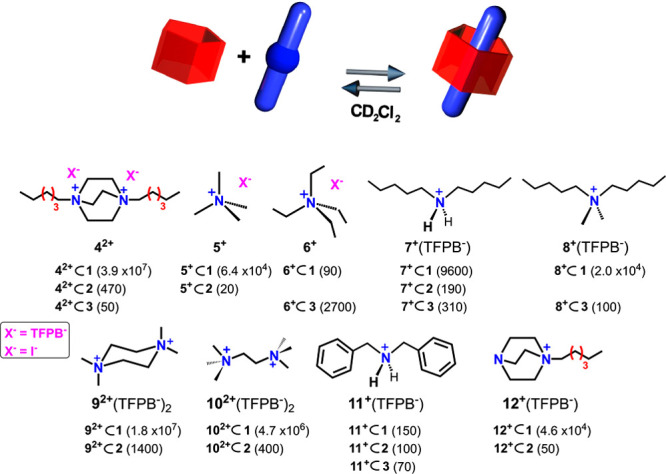
(Top) Schematic complexation equilibrium of the prism[5]arene 1 with guests 42+–12+. Binding constant values of their host–guest complexes with the prism[n]arenes 1–3, determined by 1H NMR experiments in CD2Cl2 (600 MHz) (SI). Errors <15% calculated as mean values of three measures.
With this result in hand, we decided to perform the synthesis of prismarenes in the presence of 42+ as iodide salt (Figure 2), with the aim of investigating its possible template effect over the thermodynamic equilibrium12 distribution of the cyclo-oligomers in Scheme 1. When 2,6-dimethoxynaphthalene (2.5 mM) and paraformaldehyde (1.2 equiv) were reacted in the presence of TFA (15 equiv) in 1,2-DCE at 70 °C, and by adding the 42+ iodide salt, impressively, prism[5]arene 1 was obtained in 47% yield after 22 h. Interestingly, the yield of 1 was decreased to 32% when the tetramethylammonium cation 5+ was instead used as potential templating agent. The HR-ESI mass spectrum of prism[5]arene 1 confirmed its molecular mass. 1D and 2D NMR spectra (CD2Cl2, 298 K, 600 MHz) were in accord with the D5 symmetry of 1.
Small colorless single crystals of three pseudopolymorphic forms of prism[5]arene 1 and two pseudopolymorphic forms of 1,4-C-prism[5]arene 2, suitable for X-ray structure determination, were analyzed using synchrotron radiation and cryo-cooling techniques. The three prism[5]arene pseudopolymorphs, α, β, and γ forms (Figure 1 and SI), are distinguished by a different amount of cocrystallized CH2Cl2 solvent molecules (three, two, or one solvent molecule for each prism[5]arene molecule, respectively). All three forms are composed of a racemic mixture of inherently chiral prism[5]arene molecules, in which all the naphthalene moieties show the same orientation of the 2,6-methoxy substitution pattern (Figure 1a,b). In the α and β forms, there is formation of a molecular cavity sealed on one site by a methoxy group (Figure 1d), while in the more symmetric γ form a central hole is present (Figure 1e) (see SI). In all three pseudopolymorphs, the CH2Cl2 molecules hosted inside each prism[5]arene hole/cavity are sealed by the neighboring molecules (void volume of 85–95 Å3, 67–60% filled by the vdW volume of a CH2Cl2 molecule). The two pseudopolymorphic forms of 1,4-C-prism[5]arene 2 (α = monoclinic form, β = triclinic form) are also composed of a racemic mixture of inherently chiral molecules. The 1,4-naphthalene ring assumes a conformation that completely fills the cavity of the prismarene (Figure 1f).
At this point of our study, we decided to perform a series of experiments in order to investigate a possible interconversion process between the two isomers, the prism[5]arene 1 and the 1,4-C-prism[5]arene 2. When prism[5]arene 1 was heated at 70 °C in 1,2-DCE and in the presence of TFA, the conversion to 1,4-C-prism[5]arene 2 was complete after 16 h. This result clearly indicates that 1,4-C-prism[5]arene 2 is the thermodynamic isomer, while prism[5]arene 1 is the kinetic one. Density functional theory (DFT) calculations (SI) substantially agree with these results. The prism[5]arene 1 is predicted to be less stable than its confused-isomer 2 by 5.1 kcal/mol. Interestingly, this energy difference decreases to 2.6 kcal/mol when considering the equilibrium geometries of 1 and 2 hosting 1,2-DCE inside their cavity. We have also computed the equilibrium geometries of the most stable transition states (TS) for the macrocyclization steps of the intermediate carbocation. The TS (SI) for the 1,4 attack is predicted to lie 3.3 kcal/mol above the one involved in 1,5 attack. This energy difference increases to 4.3 kcal/mol when considering 1,2-DCE inside the cavity, thus confirming that formation of the 1,5 adduct occurs faster.
To add further support to our points, we examined the reaction in Scheme 1 by HPLC (SI). In accordance with the above conclusion, after 270 min of reaction, prism[5]arene 1 is clearly the favored product. In fact, the amount of 1 is larger than that of its C-confused-isomer 2. After 330 min the 1,4-C-isomer 2 prevails over 1 (SI). Differently, when the reaction was conducted in the presence of guest 42+, the HPLC monitoring evidenced that prism[5]arene 1 was the favored product over time.
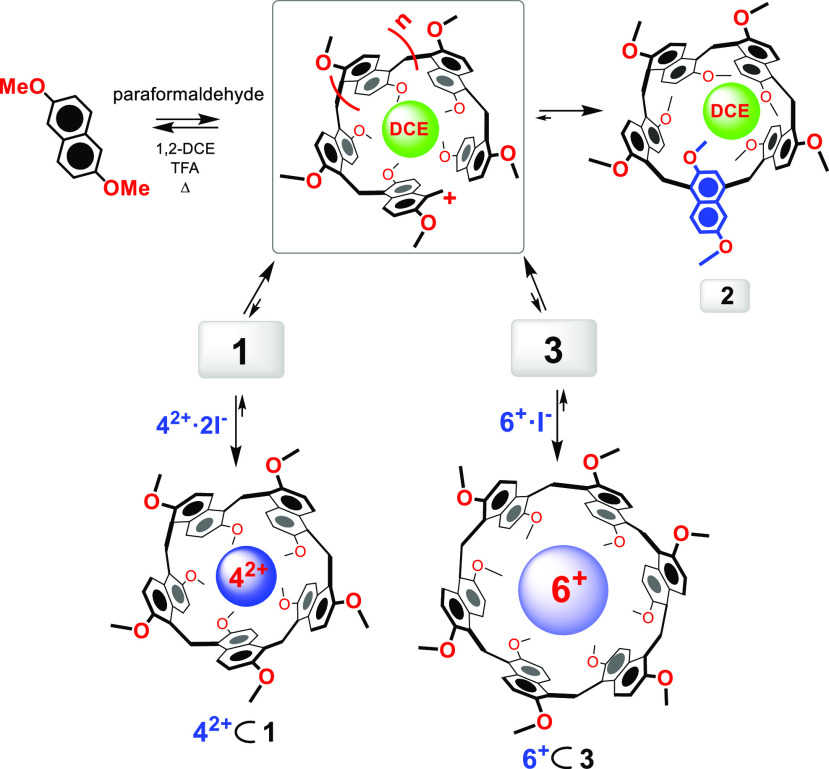
Finally, when 1,4-C-prism[5]arene 2 was treated in the presence of 42+ in 1,2-DCE at 70 °C for 22 h, derivative 1 was obtained in 20% yield. In summary, these results clearly indicate that the formation of 1 and 2 occurs through a thermodynamically controlled templated process (Figure 3) in which the solvent 1,2-DCE and the cationic guest 42+ act as templating agent in the formation of 1,4-C-prism[5]arene 2 and prism[5]arene 1, respectively.12 It is worth mentioning here that this result is also an uncommon example of template control over the regiochemistry of a macrocyclic product.12
Figure 3.
Thermodynamically controlled templated synthesis of prism[n]arenes.
With this results in hand, we investigated the use of a different templating agent. Thus, when the tetraethylammonium cation 6+ was used as iodide salt (Scheme 1 and Figure 3), the prism[6]arene 3 was isolated in 20% yield, in addition to 1,4-C-prism[5]arene 2 (6%) and prism[5]arene 1 (0.3%). This result clearly indicates that the equilibrium distribution of the prism[n]arenes 1–3 can be controlled by using the appropriate complementary templating agent able to remove its host from the equilibrium mixture (Figure 3). Significantly, prism[6]arene 3 was quantitatively converted to 1,4-C-prism[5]arene 2 after treatment with TFA at 70 °C, for 16 h in 1,2-DCE, thus confirming that 2 is the thermodynamic product, while 1 and 3 are the kinetic ones in the equilibrium mixture.
Our attention then turned to the recognition ability of prism[n]arenes 1–3 (Figures 2 and 4 and SI). In detail, when 1,4-dihexyl-DABCO 42+, as TFPB– salt, was added to a CD2Cl2 solution of prism[5]arene 1 (in equimolar ratio), the formation of pseudo[2]rotaxane 42+ ⊂ 1 (Figure 4e,f) was observed, as confirmed by 1D and 2D NMR studies and HR-MS-CID spectrum (SI). An association constant value of 3.9 × 107 M–1 (298 K, CD2Cl2) was determined for the 42+ ⊂ 1 complex by a series of competition experiments18 (Figure 2 and SI). This value is significantly higher than that observed for the formation of 42+ ⊂ 2 pseudorotaxane (470 M–1). This result is in accord with the findings summarized in Figure 3.
Figure 4.
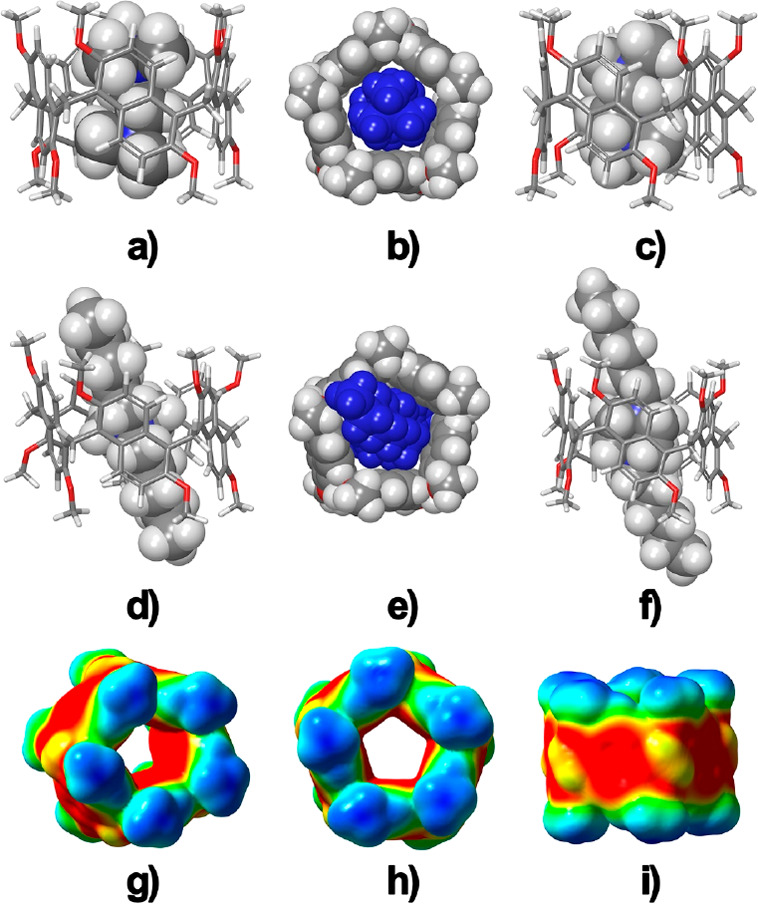
DFT-optimized structures (B97D3/SVP/SVPFIT) of complexes (a, b) 102+ ⊂ 1; (c) 92+ ⊂ 1; (d) 8+ ⊂ 1; (e, f) 42+ ⊂ 1. (g–i) ESPs mapped onto electron density isosurfaces (ρ = 0.004) for the prism[5]arene 1.
In fact, the guest 42+ shows a greater thermodynamic affinity for prism[5]arene 1 with respect to its C-isomer 2, and therefore it plays the templating agent role in its synthesis. Analogous conclusions are inferred by comparing the association constant values of the two endo-complexes of the tetramethylammonium 5+ cation (as TFPB– salt), 5+ ⊂ 1 (6.4 × 104 M–1) and 5+ ⊂ 2 (20 ± 5 M–1). A close inspection of the DFT-optimized structure of the pseudo[2]rotaxane 42+ ⊂ 1 (Figure 4) evidenced the crucial role played by C–H···π and cation···π interactions in the stabilization of the complex. The prism[5]arene macrocycle presents an extended π-electron-rich aromatic cavity (Figures 4g–i) in which these interactions are enhanced. In fact, when the N,N-dimethyl-N,N-dipentylammonium 8+·TFPB– salt was mixed with 1 in CD2Cl2, the pseudo[2]rotaxane 8+ ⊂ 1 was formed (Figure 4d) with an association constant of 2.0 × 104 M–1, a value significantly higher than that found for the analogous pseudo[2]rotaxane 7+ ⊂ 1 (9600 M–1) obtained with the secondary dipentylammonium 7+ axle. This result confirms the crucial role played by the +NC–H···π interactions for the stabilization of the complex between ammonium axles and the prism[5]arene. Finally, the prism[5]arene 1 forms ammonium-based complexes thermodynamically more stable than its C-confused isomer 2.
In conclusion, a novel class of macrocyclic hosts, named prismarenes, have been obtained by a templated approach of a thermodynamically controlled synthesis. The prism[n]arenes here described show a good affinity for ammonium guests and form pseudorotaxane architectures stabilized by cation···π and +NC–H···π interactions. Considering the current enormous interest directed to the synthesis of novel macrocyclic hosts, which have already found interesting nanotechnological and supramolecular applications,5 and considering the peculiar features of the prismarenes, it is conceivable that the above results will pave the way to a quick expansion of this new area of research in supramolecular chemistry.
Acknowledgments
The authors acknowledge the University of Salerno for the financial support (FARB 2018).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b12216.
Detailed synthetic procedures, 1D and 2D NMR spectra of prismarenes and their complexes, HR mass spectra, details on stability constant determination, DFT calculation details. X-ray figures, and tables of crystal data (PDF)
X-ray crystallographic data for α form of prism[5]arene 1 (CIF)
X-ray crystallographic data for β form of prism[5]arene 1 (CIF)
X-ray crystallographic data for γ form of prism[5]arene 1 (CIF)
X-ray crystallographic data for α form of 1,4-confused-prism[5]arene 2 (CIF)
X-ray crystallographic data for β form of 1,4-confused-prism[5]arene 2 (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pedersen C. J. Cyclic Polyethers and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. 10.1021/ja01002a035. [DOI] [Google Scholar]
- Schalley C. A.; Vögtle F.; Dötz K. H.. Templates in Chemistry II; Springer: Berlin, Heidelberg, 2005. [Google Scholar]
- Ogoshi T.; Kanai S.; Fujinami S.; Yamagishi T.; Nakamoto Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- Jia F.; He Z.; Yang L.-P.; Pan Z.-S.; Yi M.; Jiang R.-W.; Jiang W. Oxatub[4]arene: A Smart Macrocyclic Receptor With Multiple Interconvertible Cavities. Chem. Sci. 2015, 6, 6731–6738. 10.1039/C5SC03251B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ke H.; Yang L.-P.; Xie M.; Chen Z.; Yao H.; Wei J. Shear-Induced Assembly of a Transient Yet Highly Stretchable Hydrogel Based on Pseudopolyrotaxanes. Nat. Chem. 2019, 11, 470–477. 10.1038/s41557-019-0235-8. [DOI] [PubMed] [Google Scholar]; b Ogoshi T.; Kakuta T.; Yamagishi T.-a. Applications of Pillar[n]arene-Based Supramolecular Assemblies. Angew. Chem., Int. Ed. 2019, 58, 2197–2206. 10.1002/anie.201805884. [DOI] [PubMed] [Google Scholar]; c Wang M.; Zhou J.; Li E.; Zhou Y.; Li Q.; Huang F. Separation of Monochlorotoluene Isomers by Nonporous AdaptiveCrystals of Perethylated Pillar[5]arene and Pillar[6]arene. J. Am. Chem. Soc. 2019, 141, 17102–17106. 10.1021/jacs.9b09988. [DOI] [PubMed] [Google Scholar]
- Recently, oxatubarene macrocycles have been reported by Jiang and co-workers, in which the naphthalene units were bridged by CH2OCH2 groups: see ref (4).
- Thanks are due to Prof. Wei Jiang for a critical discussion and for suggesting “prismarenes” as the name to be given to these molecules. In fact, an inspection of the DFT structures in Figure 4 clearly reveals that in the ammonium complexes macrocycle 1 adopts a structure very similar to a prism with a pentagonal base.
- Cram D. J.; Cram J. M. In Container Molecules and Their Guests; Monographs in Supramolecular Chemistry; Stoddart J. F., Ed.; Royal Society of Chemistry: Cambridge, 1994. [Google Scholar]
- Ogoshi T.; Ueshima N.; Akutsu T.; Yamafuji D.; Furuta T.; Sakakibara F.; Yamagishi T.-a. The Template Effect of Solvents on High Yield Synthesis, Co-Cyclization of Pillar[6]arenes and Interconversion Between Pillar[5]- and Pillar[6]arenes. Chem. Commun. 2014, 50, 5774. 10.1039/C4CC01968G. [DOI] [PubMed] [Google Scholar]
- Furlan R. L. E.; Otto S.; Sanders J. K. M. Supramolecular templating in thermodynamically controlled synthesis. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4801–4804. and reference therein 10.1073/pnas.022643699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a da Pian M.; de Lucchi O.; Strukul G.; Fabris F.; Scarso A. Cation Templated Improved Synthesis of Pillar[6]arenes. RSC Adv. 2016, 6, 48272–48275. 10.1039/C6RA07164C. [DOI] [Google Scholar]; For other examples of templated synthesis of pillararenes see:; b Ogoshi T.; Ueshima N.; Sakakibara F.; Yamagishi T.-a.; Haino T. Conversion from Pillar[5]arene to Pillar[6–15]arenes by Ring Expansion and Encapsulation of C60 by Pillar[n]arenes with Nanosize Cavities. Org. Lett. 2014, 16, 2896–2899. 10.1021/ol501039u. [DOI] [PubMed] [Google Scholar]; c Da Pian M.; Schalley C. A.; Fabis F.; Scarso A. Insights Into the Synthesis of Pillar[5]arene and Its Conversion Into Pillar[6]arene. Org. Chem. Front. 2019, 6, 1044–1051. 10.1039/C9QO00176J. [DOI] [Google Scholar]; d Cao D.; Kou Y.; Liang J.; Chen Z.; Wang L.; Meier H. A Facile and Efficient Preparation of Pillararenes and A Pillarquinone. Angew. Chem., Int. Ed. 2009, 48, 9721–9723. 10.1002/anie.200904765. [DOI] [PubMed] [Google Scholar]
- Furlan R. L. E.; Otto S.; Sanders J. K. M. Supramolecular Templating in Thermodynamically Controlled Synthesis. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4801–4804. and reference therein 10.1073/pnas.022643699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi T.; Aoki T.; Kitajima K.; Fujinami S.; Yamagishi T.-a.; Nakamoto Y. Facile, Rapid, and High-Yield Synthesis of Pillar[5]arene from Commercially Available Reagents and Its X-ray Crystal Structure. J. Org. Chem. 2011, 76, 328–331. 10.1021/jo1020823. [DOI] [PubMed] [Google Scholar]
- This result is in accord with data previously reported in which methylene-bridged naphthalene acyclic oligomers were obtained by reaction between 2,6-dialkoxynaphthalenes and paraformaldehyde at rt in CH2Cl2 and using p-TsOH as acid:Pan S.-J.; Ye G.; Jia F.; He Z.; Ke H.; Yao H.; Fan Z.; Jiang W. Regioselective Synthesis of Methylene-Bridged Naphthalene Oligomers and Their Host–Guest Chemistry. J. Org. Chem. 2017, 82, 9570–9575. 10.1021/acs.joc.7b01579. [DOI] [PubMed] [Google Scholar]; See, also:Jia Y.; Dong M.; Li C. Synthesis and Host–guest Properties of Acyclic Pillar[n]naphthalenes. Front. Chem. 2019, 7, 828. 10.3389/fchem.2019.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regarding derivative 2 we use here the nomenclature 1,4-confused-prism[5]arene (abbreviated 1,4-C-) in order to make a distinction between the two isomeric prismarenes 1 and 2. The term “confused” was used for the first time in the nomenclature of the tetraphenylporphyrins in order to distinguish the isomer in which one pyrrole ring is linked through its α–β′ position instead of a more normal α–α′ linkage:Furuta H.; Asano T.; Ogawa T. ″N-Confused Porphyrin″: A New Isomer of Tetraphenylporphyrin. J. Am. Chem. Soc. 1994, 116, 767–768. 10.1021/ja00081a047. [DOI] [Google Scholar]; More recently the nomenclature “confused” was used in calixpyrrole systems; see:Depraetere S.; Smet M.; Dehaen W. N-Confused Calix[4]pyrroles. Angew. Chem., Int. Ed. 1999, 38, 3359–3361. . [DOI] [PubMed] [Google Scholar]
- Talotta C.; De Simone N. A.; Gaeta C.; Neri P. Calix[6]arene Threading With Weakly Interacting Tertiary Ammonium Axles: Generation of Chiral Pseudorotaxane Architectures. Org. Lett. 2015, 17, 1006–1009. 10.1021/acs.orglett.5b00115. [DOI] [PubMed] [Google Scholar]
- Hirose K. iIn Analytical Methods in Supramolecular Chemistry; Schalley C. A., Ed.; Wiley-VCH: Weinheim, 2007; pp 17– 54. [Google Scholar]
- Cao L.; Šekutor M.; Zavalij P. Y.; Mlinarić-Majerski K.; Glaser R.; Isaacs L. Cucurbit[7]uril·Guest Pair with an Attomolar Dissociation Constant. Angew. Chem., Int. Ed. 2014, 20, 988–993. 10.1002/anie.201309635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.