Abstract
Background.
There is lack of data on feasibility and safety of kidney transplants from living donors who recovered from COVID-19.
Methods.
Here, we present a retrospective cohort study of 31 kidney transplant recipients (KTR) from living donors who recovered from polymerase chain reaction confirmed COVID-19 across 19 transplant centers in India from July 3, 2020, to December 5, 2020. We detailed demographics, clinical manifestations, immunosuppression regimen, treatment, and outcomes. Donors with a previous diagnosis of COVID-19 were accepted after documenting 2 negative polymerase chain reaction tests with complete symptom resolution for at least 28 days and significant social distancing for 14 days before surgery.
Results.
COVID-19 clinical severity in donors ranged from completely asymptomatic (71%, n = 22) to mild infection (29%, n = 9). None progressed to moderate or severe stages of the disease in the entire clinical course of home treatment. Patient and graft survival was 100%, respectively, with acute cellular rejection being reported in 6.4% (n = 2) recipient. All recipients and donors were asymptomatic with normal creatinine at median follow-up of 44 days after surgery without any complications relating to surgery and COVID-19.
Conclusions.
Our data support safety of proceeding with living donation for asymptomatic individuals with comprehensive donor, recipients screening before surgery, using a combination of clinical, radiologic, and laboratory criteria. It could provide new insights into the management of KTR from living donors who have recovered from COVID-19 in India. To the best of our knowledge, this remains the largest cohort of KTR from living donors who recovered from COVID-19.

INTRODUCTION
COVID-19 Burden in India
As of November 30, 2020, India carries the second largest COVID-19 burden in the world. India has 4.74% active COVID-19 cases undergoing treatment (n = 446 952), 93.81% of patients have been cured/discharged (n = 8 847 600), and 1.45% have died (n = 137 139). Mortality in >70% COVID-19 cases has been associated with comorbidities. Death rate is low in India compared with the United States (99 versus 823 per million population), and a total of 140 379 976 tests have been performed to date.1
Impact of COVID-19 on Transplant Rates in India
India has a predominant living donor kidney transplant program (80%). A total of 61 821 organ transplants are performed between 2013 and 2019 including 48 083 kidney (living donor = 41 197, deceased donor = 6886), 11 971 liver (living donor = 8405, deceased donor = 3566), 1082 heart, 573 lung, 100 pancreas, and 8 small bowel transplants as per Global Observatory on Donation and Transplantation data 2013–2019.2,3 As per Government advisory, the majority of transplant-related activity and evaluation other than emergency lifesaving transplants were temporarily suspended in India during the months of March–May 2020 due to the COVID-19 pandemic. Transplant activities continue to be adversely affected in the public sector, given the challenges of limited health resources, manpower, and COVID-19 health priority.4 The increased burden on dialysis care providers along with the financial constraints of the patients warrant resumption of kidney transplants. With the ongoing spread of infection, the numbers are only expected to rise.
Organ Donation During the Coronavirus Pandemic From Living Donors Who Recovered COVID-19 Is an Evolving Saga in Uncharted Waters
With resumption of transplant programs in various countries, new issues are expected to occur. Evidence based data are required to document safety of kidney transplant recipients (KTR) from living donors who recovered from COVID-19. Is it safer to be transplanted from living donors who recovered from polymerase chain reaction (PCR) confirmed COVID-19 or to remain on dialysis? What should be the optimum waiting time for living donors who recovered from COVID-19 between organ donation and COVID positive/negative report? Can COVID-19 be transmitted by organ donation/transplantation? What treatments are effective? Do we need any change in induction and maintenance immunosuppression? What are the outcomes of KTR from living donors who recovered from PCR confirmed COVID-19? At this time, we do not have specific evidence–based information about these issues. In the ongoing pandemic, our experience from living donor kidney transplant predominant program in India may allay some of the concerns.
We report on our experience of demographics, clinical manifestations, immunosuppression regimen, and outcomes in 31 kidney transplants from living donors who recovered from COVID-19 across 19 transplant centers (2 public and 17 private hospitals).
MATERIALS AND METHODS
The aim of this study was to evaluate the feasibility and safety of 31 kidney transplants from living donor who have recovered from PCR confirmed COVID-19. This is a retrospective multicenter cohort study carried out between July 3, 2020, and December 5, 2020. All transplants were performed after review by the appropriate authorization committee and therefore performed in accordance with the ethical standards laid down in the Declaration of Helsinki as well as the Declaration of Istanbul. All recipients and donors gave their informed consent before the transplants and their inclusion in the study.
Confirmed Case
The diagnosis of COVID-19 was confirmed by PCR from nasopharyngeal (nasal) and oropharyngeal (throat) swabs. The clinical severity (asymptomatic, mild) determined by Government COVID-19 clinical management protocol; all the donors underwent home isolation treatment.5,6
Study Population
Thirty-one KTR from living donors who recovered from PCR confirmed COVID-19 were included from the following institutions: (1) Rabindranath Tagore International Institute of Cardiac Sciences, Kolkata, West Bengal (n = 4), (2) Mahatma Gandhi Medical College & Hospital, Jaipur (n = 3), (3) IQRAA International Hospital and Research Centre Calicut, Kerala (n = 3), (4) Medanta Institute of Kidney and Urology, Haryana (n = 3), (5) VPS Lakeshore Hospital, Kochi, (6) BGS Global hospital, Bengaluru, Karnataka (n = 2), (7) Indraprastha Apollo Hospital, New Delhi (n = 2), (8) Institute of Kidney Diseases and Research Center, Dr HL Trivedi Institute of Transplantation Sciences (IKDRC-ITS), Ahmedabad (n = 1), (9) Muljibhai Patel Urological Hospital, Nadiad, Gujarat (n = 1), (10) Postgraduate Institute of Medical Education and Research, Chandigarh (PGIMER), Chandigarh (n = 1), (11) Virinchi Hospitals, Hyderabad, Telangana (n = 1), (12) Manipal Hospital, Bangalore (n = 1), (13) North City Hospital, Kolkata, West Bengal (n = 1), (14) Manipal Hospital, Jaipur (n = 1), (15) Kovai Medical Center and Hospital, Coimbatore (n = 1), (16) Yashoda Hospital, Secunderabad (n = 1), (17) MIT Hospital & Research Institute, Aurangabad (n = 1), (18) Manipal Hospital, Whitefeild, Bangalore (n = 1), and (19) Fortis Group of Hospitals, New Delhi (n = 1) treated from July 3, 2020, to December 5, 2020, were assessed.
Evaluation of Donors, Recipients Pair Before Transplant
All the DRP were advised to practice social distancing and hand hygiene for 14 days before surgery and using surgical facemask when going out in public.7-11 DRP should sign the fully documented written and informed consent, including risk and benefit of transplantation versus dialysis, accepting a potential risk of COVID-19 infection during hospital stay and after transplant. Healthcare worker (HCW) teams were designated to care exclusively for transplant cases (COVID free safe transplant pathway) to reduce the risk of transmission. Routine clinical and epidemiological screening for COVID-19 in DRP and HCW was performed. Routine COVID-19 PCR test of airway specimen and chest CT scan for DRP within 24–72 hours of transplants was performed. The donor with a previous diagnosis of COVID-19 was accepted with documented 2 negative PCR tests to avoid the known false negative rates of single tests in COVID-19 including negative PCR test at the time of surgery and complete symptom resolution for 28 days. Transplants were performed with documented 2 negative PCR tests and another negative test at the time of transplant surgery and complete symptom resolution for 28 days. Induction and other immunosuppressive drugs were given based on recipient’s immune risk stratification as being practiced before COVID-19. All DRP were updated on COVID-19 tests and preventive measures before transplants and enhanced follow-up either by telemedicine in the absence of clinical issues or physical exams as required due to the unknown infection risk were suggested. Potential recipient and donors who were diagnosed with COVID-19 during the pretransplant evaluation were managed as per local authority guidelines; at this time, there are no country-wide standard accepted treatment guidelines. Transplants were performed in an experienced center. We ensured a safe and technically successful transplant operation. As a safeguard, we made sure that recipients would get prompt PCR testing and treatment in unfortunate scenarios of COVID-19 presentation after transplantation. Adequate screening for donor safety was done just before and after donation and planned at discharge, 1, 2, 3, 6, and 12 months after donation. Transplant teams used a multidisciplinary approach and effective communication during COVID-19 pandemic to improve outcome.
Statistical Analysis
Continuous data are presented as mean ± SD, median (range), and categorical data are presented as percentage (numbers).
RESULTS
Demographics
We included 31 KTR from living donors who recovered from COVID-19 across 19 transplant centers (2 public and 17 private hospitals) in our analysis.
Baseline demographics of recipients/donors, comorbidities, immunosuppression regimen, and outcome of kidney transplants are summarized in Tables 1 and 2.
TABLE 1.
Baseline demographics of recipients/donors, comorbidities, immunosuppression regimen, and outcome of kidney transplants
| Study duration | July 3, 2020–December 5, 2020 |
|---|---|
| Study type | Retrospective |
| Study centers | 19 (2 public, 17 private hospitals) |
| Transplant numbers | 31 |
| Recipients | |
| Age (y), mean ± SD, median (range) | 39 ± 10, 39 (11–59) |
| Gender (M, F) | 28 M, 3 F |
| ABO blood group | AB = 1, A = 7, B = 11, O = 12 |
| Body mass index (kg/m2) | 23.1 ± 3.9, 23 (15–30) |
| Dialysis duration | 9.5 ± 6.9, 9 (0–24) mo |
| Comorbidities: yes, no | 87% (n = 27), 13% (n = 4) |
| Comorbidities | |
| Hypertension | 71% (n = 22) |
| Overweight (BMI > 25 kg/m2) | 31% (n = 9) |
| Diabetes | 30.7% (n = 8) |
| Heart disease = 6 | 24.4% (n = 7) |
| Retransplant, anemia | 6.9% (n = 2) each |
| 2D echo | |
| Left ventricular dysfunction | 19.4% (n = 6) |
| Normal | 80.6% (n = 25) |
| HLA match (A, B, DR) (n = 27) | 2.1 ± 1.8, 3 (0–6) |
| ABO compatible, incompatible | 87% (n = 27), 13% (n = 4) |
| Pretransplant evaluation COVID positive, negative | 29% (n = 9),71% (n = 22) |
| COVID severity in 9 positive recipients | Asymptomatic (n = 5), mild (n = 4) |
| PCR positive to transplant in positive recipients | 65 ± 11, 73 (34–92) d |
| Induction, dose | 74.2% (n = 26) |
| Thymoglobulin | 51.7% (n = 15), 2.7 ± 0.5, 3 (2–3) mg/kg |
| Basiliximab | 20.6% (n = 8), 20 mg on d 1 and 4 |
| Rituximab | 13.7% (n = 4), 200 mg in 4 ABOiKT |
| Grafelon | 3.8% (n = 1), 6 mg.kg |
| No induction | 17.2% (n = 5) |
| Maintenance (standard dose) | Steroid+ tacrolimus + mycophenolate |
| Donors | |
| Age (y) | 44 ± 11, 42 (28–67) |
| Gender (M, F) | M = 8, F = 23 |
| Weight (kg) | 62 ± 12, 60 (38–85) |
| Body mass index (kg/m2) | 24.1 ± 4.1, 25 (17–34) |
| ABO blood group | AB = 2, A = 4, B = 10, O = 15 |
| Relation with recipients | |
| Parents | 32.6 (n = 10) |
| Spouse, siblings | 27.6% (n = 8) each |
| Other than near relatives | 17.2% (n = 5) |
| GFR (right), mL/min/1.73 m2 | 49 ± 5, 49 (38–63) |
| GFR (left), mL/min/1.73 m2 | 49 ± 4.6, 48 (40–57) |
| Creatinine (mg/dL) | 0.8 ± 0.2, 0.8 (0.6–1.2) |
| PCR testing time | |
| PCR positive to first negative | 27 ± 17.6, 24 (5–84) d |
| First PCR negative to transplant | 27 ± 18.6, 25 (4–77) d |
| PCR just before transplant | 1.9 ± 0.6, 2 (1–3) d |
| PCR positive to transplant | 54 ± 20, 52 (28–94) d |
| COVID severity in donors | Asymptomatic (71%, n = 22), mild (29%, n = 9) |
| Home, hospital COVID treatment | 100% (n = 31), 0% (n = 0) |
| Outcome | |
| Acute rejection | 6.4% (n = 2) |
| Patient survival | 100% (n = 31) |
| Graft survival | 100% (n = 31) |
| Last follow-up creatinine (mg/dL) | 1 ± 0.2, 1 (0.7–1.7) |
| Posttransplant COVID positive | 0% (n = 0) |
| Follow-up duration (d) | 53 ± 35, 44 (10–162) |
| Follow-up duration (wks) | |
| <2 | 6.4% (n = 2) |
| 2–4 | 19.5% (n = 6) |
| 4–8 | 35.5% (n = 11) |
| 8–12 | 22.5% (n = 7) |
| >12 | 16.1% (n = 5) |
BMI, body mass index; COVID, coronavirus disease; F, female; GFR, glomerular filtration rate; M, male; PCR, polymerase chain reaction.
TABLE 2.
Baseline demographics of donors
| Donor | Age (y) | Gender | DR relation | BMI | ABO group | GFR (R) | GFR (L) | SCr | D |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | F | Wife | 27 | A | 48 | 48 | 0.6 | A |
| 2 | 36 | M | LUR | 27 | O | 54 | 50 | 0.7 | |
| 3 | 63 | F | Mother | 23 | O | 45 | 45 | 0.9 | |
| 4 | 28 | F | Wife | 31 | B | 38 | 40 | 0.9 | |
| 5 | 47 | F | Mother | 20 | O | 59 | 55 | 0.6 | |
| 6 | 40 | F | Sister | 26 | O | 43 | 44 | 0.8 | |
| 7 | 64 | M | Father | 29 | B | 48 | 51 | 0.9 | |
| 8 | 30 | M | LUR | 21 | A | 52 | 57 | 1 | |
| 9 | 44 | M | Brother | 27 | B | 54 | 52 | 0.8 | |
| 10 | 34 | F | Wife | 20 | AB | 47 | 45 | 0.9 | |
| 11 | 35 | F | Sister | 28 | B | 46 | 44 | 0.7 | |
| 12 | 28 | F | Wife | 24 | O | 51 | 48 | 0.8 | |
| 13 | 60 | M | Brother | 25 | B | 52 | 57.3 | 0.8 | |
| 14 | 48 | F | Mother | 25 | B | 50 | 50 | 0.8 | |
| 15 | 51 | M | Brother | 22 | O | 49 | 43 | 1 | Mild |
| 16 | 56 | F | Mother | 34 | O | 49 | 52 | 0.7 | |
| 17 | 46 | M | LUR | 25 | A | 50 | 47 | 1 | |
| 18 | 34 | F | Mother | 17 | AB | 63 | 51 | 0.6 | |
| 19 | 40 | F | LUR | 26 | O | 51 | 49 | 1.1 | |
| 20 | 38 | M | Brother | 26 | B | 55 | 50 | 0.8 | |
| 21 | 38 | F | LUR | 27 | O | 49 | 51 | 1.2 | |
| 22 | 37 | F | Wife | 28 | O | 47 | 48 | 0.8 | |
| 23 | 45 | F | Mother | 26 | B | 46 | 48 | 0.7 | A |
| 24 | 35 | F | Wife | 19 | B | 50 | 45 | 0.7 | |
| 25 | 35 | F | Wife | 18 | O | 49 | 56 | 0.6 | |
| 26 | 42 | F | Wife | 20 | O | 46 | 54 | 0.6 | |
| 27 | 67 | F | Mother | 21 | A | 41 | 41 | 0.7 | |
| 28 | 60 | F | Mother | 21 | O | 42 | 45 | 0.9 | |
| 29 | 55 | F | Mother | 25 | O | 46 | 47 | 0.8 | |
| 30 | 39 | F | Sister | 19 | B | 48 | 47 | 0.9 | |
| 31 | 46 | F | Sister | 24 | O | 47 | 52 | 0.8 | Mild |
A, Asymptomatic 1-14 and 23-30; BMI, body mass index (kg/m2); COVID, coronavirus disease; D, donor COVID severity; DR, donor recipient; F, female; GFR, glomerular filtration rate; L, left; LUR, other than near relatives; M, male; Mild, 15-22 and 31; R, right; SCr, serum creatinine.
Donors
Symptoms, laboratory findings of donors are summarized in Table 3.
TABLE 3.
Symptoms and laboratory findings of kidney donors
| Donor | Fever | Cough | Sputum | Myalgia | No symptoms | Hb (gm/dL) | WBC /mm3 | PMN (%) | Lymphocyte (%) | Platelet ×103 (/mm3) | CRP | PCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | – | – | – | Y | 13.5 | 5290 | 67 | 27 | 204 | N | N |
| 2 | – | – | – | – | Y | 14.5 | 7000 | 65 | 32 | 241 | N | N |
| 3 | y | 14 | 8050 | 72 | 25 | 210 | N | N | ||||
| 4 | – | – | – | – | Y | 13.2 | 8200 | 60 | 32 | 210 | N | N |
| 5 | – | – | – | – | Y | 9 | 5900 | 65 | 32 | 255 | ND | ND |
| 6 | – | – | – | – | Y | 11 | 5800 | 60 | 35 | 301 | N | ND |
| 7 | – | – | – | – | Y | 15.1 | 9690 | 60 | 33 | 363 | N | N |
| 8 | – | – | – | – | Y | 13.9 | 9800 | 76 | 24 | 230 | N | N |
| 9 | – | – | – | – | Y | 12 | 9200 | 70 | 27 | 220 | N | N |
| 10 | – | – | – | – | Y | 10.4 | 5800 | 58 | 29 | 230 | N | ND |
| 11 | – | – | – | – | Y | 11.5 | 9500 | 75 | 22 | 190 | N | N |
| 12 | – | – | – | – | Y | 12 | 7250 | 65 | 32 | 237 | N | N |
| 13 | – | – | – | – | Y | 12 | 7530 | 65 | 30 | 219 | N | N |
| 14 | – | – | – | – | Y | 12 | 8500 | 70 | 25 | 212 | N | N |
| 15 | Y | – | – | – | 13.5 | 6500 | 66 | 26 | 239 | 21 | N | |
| 16 | Y | – | – | – | 10.5 | 8800 | 55 | 36 | 325 | ND | ND | |
| 17 | Y | Y | – | Y | – | 12.6 | 7800 | 77 | 19 | 367 | 39 | N |
| 18 | Y | – | – | Y | – | 13.1 | 5000 | 56 | 35 | 234 | ND | ND |
| 19 | Y | Y | – | – | 11 | 8500 | 62 | 33 | 210 | N | N | |
| 20 | - | Y | Y | Y | – | 12 | 5900 | 80 | 15 | 220 | 56 | N |
| 21 | Y | Y | – | – | 12 | 6200 | 70 | 25 | 190 | N | N | |
| 22 | Y | – | – | – | 11.5 | 7900 | 66 | 32 | 205 | N | N | |
| 23 | Y | 12.5 | 8200 | 76 | 23 | 220 | N | ND | ||||
| 24 | – | – | – | – | Y | 11 | 8000 | 65 | 32 | 200 | N | N |
| 25 | – | – | – | – | Y | 11 | 6200 | 63 | 32 | 221 | N | N |
| 26 | – | – | – | – | Y | 11 | 7200 | 70 | 27 | 190 | N | N |
| 27 | – | – | – | – | Y | 11 | 7500 | 64 | 33 | 240 | N | N |
| 28 | – | – | – | – | Y | 11.5 | 4500 | 66 | 29 | 165 | 18 | N |
| 29 | Y | 11.2 | 6500 | 65 | 35 | 210 | N | ND | ||||
| 30 | – | – | – | – | Y | 13.4 | 4100 | 62 | 33 | 190 | N | ND |
| 31 | Y | – | – | – | 12 | 7300 | 70 | 27 | 260 | N | N |
Asymptomatic 1-14 and 23-30. CRP, C-reactive protein; Hb, hemoglobin; Mild, 15-22 and 31; N, normal; ND, not done; PCT, procalcitonin; PMN, polymorphonuclear leukocyte; WBC, white blood cell; Y, yes.
Median age of donors was 42 years, 23 were females and 8 were males. Donors did not have any comorbidities; presenting symptoms in 9 mild symptomatic donors at the time of COVID-19 presentation included fever (n = 8), cough (n = 4), myalgia (n = 3), and sputum production (n = 1); 22 donors were asymptomatic donors. The median duration of symptoms was 3 days. COVID-19 exposure was frequently related to a family cluster (n = 11), a social cluster (n = 9), a nosocomial/healthcare cluster (n = 7), and COVID exposure was unknown in 4 donors, and there was no donor-derived transmission. Clinical severity ranged from asymptomatic (n = 22, 71%) to mild (n = 9, 29%), and none of the donors progressed to moderate and severe disease during their clinical course of home treatment supported by telemedicine. None required hospitalization, ICU care, and ventilation. The duration between PCR positive to first negative was 27 ± 17.6, 24 (5–84) days, first PCR negative to transplant was 27 ± 18.6, 25 (4–77) days, and PCR positive to transplant was 54 ± 20, 52 (28–94) days. This is interval of PCR testing done and not the time required to become PCR negative.
Interval in days of donor COVID PCR positive to negative results and from negative to transplant are summarized in Figure 1.
FIGURE 1.
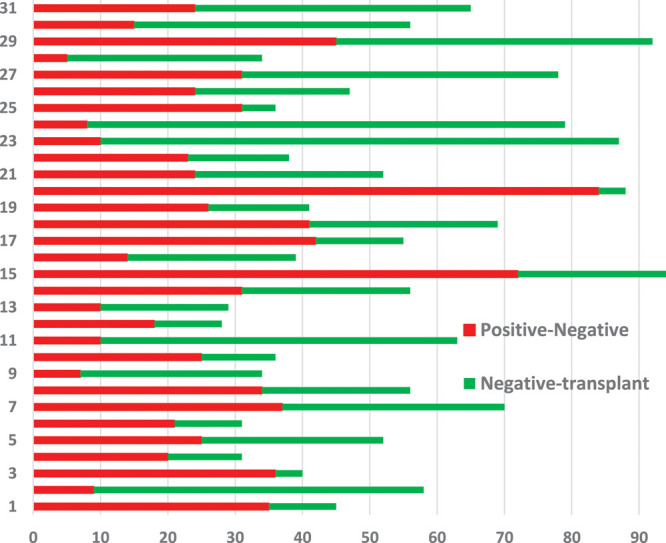
Interval in d of donor COVID PCR positive to negative and from negative to transplant. COVID, coronavirus disease; PCR, polymerase chain reaction.
Laboratory findings (Table 3) at the time of donation showed a hemoglobin of 12.1 ± 1.3, 12 (9–15.1) gm/dL, total white blood cell count was 7213 ± 1509, 7300 (4100–9800) cm, polymorphs 66 ± 6.2, 65% (55–80), lymphocyte 29 ± 5, 30% (15–36), and platelet count was 232 ± 47, 220 (165–367) × 103/mm3. Further parameters suggested normal inflammatory markers, including C reactive protein, procalcitonin, ferritin, D-dimer, lactate dehydrogenase, and IL6 level at COVID-19 presentation and at time of kidney donation. At the time of COVID-19 diagnosis, 28 donors had normal chest x-ray findings and or CT scan of the chest; abnormalities were seen in 3 donors in the form of ground-glass opacity. All donors were managed as outpatients to optimize the utilization of scarce resources during the COVID-19 pandemic without any mortality; home visits were carried out as required. Specific treatments included azithromycin (n = 27, 87%), hydroxychloroquine (n = 15, 48%), favipiravir (n = 19, 61%) along with supplemental vitamins. All the donors had normal CT scan of the chest at time of kidney donation.
Recipients
Baseline demographics of recipients, comorbidities, induction, and maintenance immunosuppression regimen are summarized in Tables 1 and 4.
TABLE 4.
Baseline demographics of recipients, comorbidities, induction, and maintenance immunosuppression regimen
| Recipient | Age (y) | Gender | Blood group | BMI, kg/m2 | Dialysis (mo) | Comorbidities | HLA match | Induction, mg/kg | SCr(mg/dL) | R | D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | A | 23 | 6 | HT | 1 | ATG, 3 | 0.8 | Neg | A |
| 2 | 28 | M | O | 24 | 24 | HT | 1 | ATG, 3 | 0.9 | ||
| 3 | 40 | M | B | 24 | 6 | No | 3 | ATG, 2 | 0.9 | ||
| 4 | 35 | M | AB | 23 | 2 | HT | ND | ATG, 2 | 1.4 | ||
| 5 | 24 | M | B | 19 | 8 | No | 3 | No | 1.7 | ||
| 6 | 33 | M | O | 16 | 6 | HT | 6 | No | 0.8 | ||
| 7 | 35 | M | B | 30 | 9 | HT | 3 | B | 0.8 | ||
| 8 | 57 | M | A | 22 | 24 | HT | 2 | G6 | 1 | ||
| 9 | 39 | M | B | 26 | 4 | No | 0 | No | 1.2 | ||
| 10 | 38 | M | A | 22 | 0 | HT | 1 | R | 1.3 | ||
| 11 | 39 | F | B | 29 | 8 | Filaria | 6 | No | 0.7 | ||
| 12 | 37 | M | O | 29 | 18 | HT | 0 | No | 1 | ||
| 13 | 50 | M | B | 24 | 12 | DM | 2 | ATG, 2 | 0.9 | ||
| 14 | 26 | M | B | 26 | 12 | HT | 3 | ATG, 2 | 1 | ||
| 15 | 47 | M | O | 22 | 13 | HTN | 2 | ATG, 3 | 1 | Mild | |
| 16 | 32 | M | O | 24 | 3 | GN | 3 | ATG, 3 | 1.2 | ||
| 17 | 56 | M | A | 26 | 21 | HTN, DM | 0 | ATG, 3 | 1 | ||
| 18 | 11 | M | A | 15 | 12 | HT | 5 | B+R | 0.8 | ||
| 19 | 59 | M | O | 22 | 12 | A, RT | 0 | B | 1.1 | ||
| 20 | 45 | M | B | 23 | 18 | HT, DM | 3 | B | 0.9 | ||
| 21 | 50 | M | O | 17 | 14 | HT, A | 0 | ATG, 3 | 1.2 | ||
| 22 | 44 | M | O | 30 | 6 | DM HTN | ND | ATG, 2 | 0.9 | ||
| 23 | 32 | M | B | 23 | 11 | HT | 3 | B | 1.1 | A | A |
| 24 | 42 | M | O | 24 | 3 | DM, HT | 0 | ATG, 3 | 1.1 | ||
| 25 | 39 | M | A | 20 | 0 | DM, HT | 0 | ATG, 3 | 1.1 | ||
| 26 | 41 | M | B | 18 | 16 | DM, HT | 0 | ATG, 3 | 1.3 | ||
| 27 | 35 | M | B | 26 | 3 | No | 3 | B+R | 1 | ||
| 28 | 38 | M | O | 23 | 11 | HT | 3 | ATG, 3 | 0.8 | Mild | |
| 29 | 26 | M | O | 20 | 12 | HT | 3 | B | 0.8 | ||
| 30 | 37 | F | A | 18 | 0 | HT, RT | 3 | R | 1.3 | ||
| 31 | 50 | F | O | 27 | 0 | DM | 3 | B | 0.9 | Mild |
A, positive but asymptomatic; A: anemia; ATG, thymoglobulin; B, basiliximab; BMI, body mass index; COVID, coronavirus disease; D, donor; D, DONORS POSITIVE: Asymptomatic: 1-14,23-30, Mild: 15-22,31; DM, diabetes; F, female; G, Grafelon; GN, Glomerulonephritis; HT, hypertension; M, mild presentation; M, male; ND, not done; PCR, polymerase chain reaction; R, RECIPIENTS NEGATIVE: 1-22, POSITIVE (asymptomatic: 23-27; mild: 28-31); R, recipient; R, rituximab 200 mg; RT, retransplant; R –PCR, recipients COVID PCR before transplant; SCr, follow-up creatinine.
Median age of recipients was 39 years, 28 were males and 3 were females. Comorbidities were present in 27 recipients (87%) and included hypertension (n = 22, 71%), overweight, body mass index (BMI) ≥25 (n = 9, 29%), diabetes (n = 8, 25.8%), heart disease (n = 7, 22.5%), retransplantation (n = 2, 6.5%), anemia (n = 2, 6.5%), filaria (n = 1, 3.2%); 4 recipients (12.9 %) had no comorbidities. Four (13%) were ABO-incompatible transplants (recipient 10, 18, 27, 30) with low baseline ABO titer ≤1:16, and 27 (87%) were ABO compatible transplants with negative lymphocyte crossmatch, flow crossmatch, and donor-specific antibody. The reasons for their transplant consent were difficult vascular access (n = 6), suboptimal outcome on dialysis due to severe left ventricular dysfunction (n = 8); the remaining majority had financial constraints to cover long-term dialysis from out-of-pocket expenditure with potential risk of COVID exposure during dialysis. Pretransplant recipient evaluation showed negative 1 (n = 3) and 2 (n = 28) COVID tests before surgery as per available resources. Twenty-two (71%) recipients were COVID-19 negative, and 9 (29%) were COVID-19 positive (asymptomatic: 23–27; mild: 28–31 COVID-19) in pretransplant evaluation. All the recipients were COVID negative at the time of transplants. Specific antiviral treatment in these 9 COVID positive recipients included azithromycin, hydroxychloroquine, favipiravir, remdesivir along with supplemental vitamins and supportive care as inpatient (n = 3) and outpatient (n = 6). The duration between PCR positive to transplant 65 ± 11, 73 (34–92) days in 9 (29%) COVID-19 positive recipients.
Outcome
The median serum creatinine (SCr) in recipient was 1 mg/dL at median follow-up of 44 days (Table 1). The follow-up duration after transplant was <2 (n = 2), 2–4 (n = 6), 5–8 (n = 11), 9–12 (n = 7), and >12 weeks (n = 5). Patient survival and graft survival was 100%. Biopsy-proven acute cellular rejections were observed in 2 (6.4%) recipients number 12, 26. Recipient 12 had a poor HLA match and underwent transplantation without prior induction therapy due to financial constraints. Both recipients with acute rejections responded well to methylprednisolone 500 mg pulses ×3 with an improvement of their renal function. Neither recipients nor donors developed fever, cough, or any other clinical symptoms of COVID-19 during their hospital stay and during their follow-up with a strict preventive strategy. All recipients that underwent testing (77%, n = 24) had a negative posttransplant COVID-19 PCR negative status. Donor survival was 100%. All recipients and donors were asymptomatic with normal creatinine at their last follow-up after surgery and absent medical or surgical complications. Data on transplant function documented normal kidney function, with absent proteinuria, hematuria with normal urine analysis before and after donation.
DISCUSSION
Over the course of 5 months, we have performed 31 kidney transplants from living donors who recovered from PCR confirmed COVID-19. Indeed, with an increasing prevalence is appears of utmost relevance to test the safety of using organs from COVID positive living donors. This is particularly in the resource-limited developing world with a high risk of COVID exposure and increased mortality rates while on dialysis. Here, we report on a retrospective multiinstitutional study of 31 KTR from living donors who recovered from COVID-19 across 19 transplant centers (2 public and 17 private sector transplant centers) in India. To the best of our knowledge, this remains the largest cohort of KTR from living donors who recovered from COVID-19 to be reported from a developing country. We have implemented steps/recommendations to ensure donor and recipient safety within available resources. Fortunately, there was no COVID-19 clinical presentation/diagnosis in any recipient in this study with median follow-up of 44 days. Our study support feasibility and safety of kidney transplants from COVID-19 recovered living donors. This approach would provide the greatest value in resource-limited, low-and middle-income countries and in countries where alternative treatment of dialysis access is limited and mortality on dialysis is higher and much higher if they become COVID-19 positive. We believe that the risk of getting coronavirus from living organ donation is low because the majority of the donors were asymptomatic (71%, n = 22) at the time of testing; those with mild disease were symptoms free for 28 days with 2 PCR tests negative indicating that it is safe to proceed with a living donation for asymptomatic individuals with 2 negative PCR tests including a negative PCR test close to donation surgery and 2 weeks significant social distancing, hand hygiene before surgery, and adherence to guidelines by the American Society for Transplantation (AST) and The National Institute for Health and Care Excellence (NICE).
The United Network for Organ Sharing/AST offers considerations for proceeding in light of a positive test from a previously infected potential donor if (1) the donor is between 21 and 90 days from initial symptoms, (2) symptoms have resolved, and (3) an infectious disease expert is consulted.7 Those recommendations acknowledge that positive PCR tests in time frame from asymptomatic patients most likely represent persistent viral shedding of little significance rather than active infection. NICE guidelines for live donors with COVID-19 infection recommend deferring transplants for 28 and 14 days of comprehensive social distancing and hand-hygiene measures. Donation should resume only after donor is clinically asymptomatic and with negative nasopharyngeal swab test result for nCoV2019 and another negative test not >3 days before donation.8 National Organ and Tissue Transplant Organization (NOTTO) guidelines suggest accepting donor with a previous diagnosis of COVID-19 with documented 2 negative COVID-19 tests and complete symptom resolution for 28 days and another negative test at the time of donation in case of life-saving transplant.9 Till date, no organ donor-derived infections or blood-borne transmission have been reported.10,11
Our study also shows that carefully selected kidney donors with asymptomatic and mild COVID-19 status can be managed at home with favorable outcomes similar to KTR as described in an Italian and UK cohort.12-14 This finding supports home treatment is feasible for mild COVID-19 potential kidney donors with relevance for countries in the developing world
There is lack of evidence based data on choosing an appropriate immunosuppression protocol during the COVID-19 pandemic. Contrary to the logic in decreasing immunosuppression during a pandemic, we continued to use an induction and maintenance immunosuppression regimen based on the recipient’s immune risk stratification as was being practiced before the COVID-19 pandemic. No changes were made based on the COVID 19 pandemic concerns.7-9 downgrading the levels of immunosuppression due to COVID concerns may lead to clinical, subclinical rejection.
Published data from dialysis centers in India have reported mortality rates between 12-37.8%.15,16 We have reported on a mortality of 11% in 250 kidney transplant recipients in a multicenter cohort study from India.17 There was no nosocomial transmission to healthcare workers or the donor-derived transmission to the recipient in unanticipated ABO-incompatible living donor liver transplantation with a COVID-19–infected donor.18
Donor Safety
Our understanding of COVID-19 is rapidly evolving. COVID-19 has been shown to cause kidney damage, including glomerulonephritis that can potentially lead to chronic kidney damage. However, kidney involvement is more common in moderate, severe COVID-19 with coexisting multiple comorbidities.19-22 SARS-CoV-2 can damage the kidney through several mechanisms, including acute lung injury, sepsis, hemodynamic alterations, cytotoxic effects, cytokine release syndrome, rhabdomyolysis, coagulopathy, microangiopathy, and collapsing glomerulopathy. There is no information at this time on occult kidney involvement in COVID-19 recovered cases with asymptomatic /mild presentation without co-existing comorbidities. Thus, large-scale prospective clinical trials are needed to document living kidney donor safety with long term follow-up.
Strength
This study reports data from 19 (2 public and 17 private hospitals) transplant centers in India that aim to include complete data with full national coverage, eliminating the sampling bias found in smaller and non–population-based studies.
Limitations
We understand that our studies have limitations as there was no uniform treatment protocol for COVID-19 positive donors and recipients and that treatment changes continued to evolve based on new evidence and new data from the growing number of COVID-19 published reports. Our report also focused on kidney transplant recipients only, and thus conclusions may not be broadly applicable to other organ transplant recipients. COVID antibody testing of DRP in transplant evaluation and routine discharge PCR in asymptomatic recipients after transplants could not be performed in all due to resource limitations. Although with comprehensive donor, recipients screening before surgery, using a combination of clinical, radiologic, and laboratory criteria, none of the recipients developed COVID-19 after transplants, there is neither definite therapy nor postexposure prophylaxis in a COVID-19 infection. Limitations of the follow-up period with small sample size represent an additional limitation. Nevertheless, we feel that it is important and relevant to communicate our results to the transplant community as we are navigating through entirely uncharted territory. Moreover, we have been unable to perform time zero biopsies of the engrafted kidneys due to resource limitations during the pandemic.
In summary, our data provide new insights into the management of KTR from living donors who have recovered from COVID-19 in India. Our data could improve outcomes in KTR globally amidst the COVID 19 pandemic. To the best of our knowledge, this remains the largest cohort of KTR from living donors who recovered from COVID-19. More on-going studies are required to refute unknown infection transmission risk in organ donation from living donors who recovered from COVID-19 recessive infection on transplantation resumption.22
ACKNOWLEDGMENTS
The authors wish to thank Stefan G. Tullius, MD, PhD, for encouragement, helpful comments, and support in editing this manuscript.
Footnotes
The authors declare no funding or conflicts of interest.
All authors have equal contribution to design of the work, acquisition, analysis, data interpretation, drafting/revision of the work, and final approval of the version to be published.
REFERENCES
- 1.Ministry of Health and Family Welfare. Ministry of Health and Family Welfare, Government of India. Available at https://www.mohfw.gov.in/. Accessed December 1, 2020.
- 2.Summary. Global Observatory on Donation and Transplantation. Available at http://www.transplant-observatory.org/summary/. Accessed November 8, 2020.
- 3.Kute V, Ramesh V, Shroff S, et al. Deceased-donor organ transplantation in India: current status, challenges, and solution. Exp Clin Transplant. 2020;18(Suppl 2):31–42. [DOI] [PubMed] [Google Scholar]
- 4.Kute V, Ramesh V, Shroff S, et al. Benefit to few versus risk to many: an ethical dilemma during coronavirus disease 2019 pandemic for deceased-donor organ transplant in a resource-limited developing country. Exp Clin Transplant. 2021;19:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division). Clinical Management Protocol: COVID-19. Available at https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf. Accessed December 1, 2020.
- 6.Ministry of Health and Family Welfare, Government of India. Revised Discharge Policy for COVID-19. Available at https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf. Accessed December 1, 2020.
- 7.COVID-19 Information. American Society of Transplantation. Available at https://www.myast.org/covid-19-information. Accessed November 15, 2020.
- 8.National Institute for Health and Care Excellence. COVID 19 rapid guideline: renal transplantation. NICE guideline [NG178]. Last updated August 19, 2020. Available at https://www.nice.org.uk/guidance/ng178/chapter/3-Transplant-donors. Accessed December 13, 2020. [PubMed]
- 9.Kute V, Guleria S, Prakash J, et al. NOTTO transplant specific guidelines with reference to COVID-19. Indian J Nephrol. 2020;30:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kates OS, Fisher CE, Rakita RM, et al. Use of SARS-CoV-2 infected deceased organ donors: should we always “just say no?” Am J Transplant. 2020;20:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossini N, Alberici F, Delbarba E, et al. ; Brescia Renal COVID task force Kidney transplant patients with SARS-CoV-2 infection: the Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15:1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benotmane I, Perrin P, Vargas GG, et al. Biomarkers of cytokine release syndrome predict disease severity and mortality from COVID-19 in kidney transplant recipients. Transplantation. 2021;105:158–169. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande R, Dash S, Bahadur MM, et al. Study of COVID-19 pandemic in representative dialysis population across Mumbai, India: an observational multicentric analysis. J Assoc Physicians India. 2020;68:13–17. [PubMed] [Google Scholar]
- 16.Trivedi M, Shingada A, Shah M, et al. Impact of COVID-19 on maintenance haemodialysis patients: the Indian scenario. Nephrology (Carlton). 2020;25:929–932. [DOI] [PubMed] [Google Scholar]
- 17.Kute VB, Bhalla AK, Guleria S, et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. [Epub ahead of print. December 21, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong HL, Kim SH, Choi DL, et al. A case of coronavirus disease 2019-infected liver transplant donor. Am J Transplant. 2020;20:2938–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassanein M, Radhakrishnan Y, Sedor J, et al. COVID-19 and the kidney. Cleve Clin J Med. 2020;87:619–631. [DOI] [PubMed] [Google Scholar]
- 20.Kudose S, Batal I, Santoriello D, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty AA, Tawhari I, Safar-Boueri L, et al. COVID-19-associated glomerular disease. J Am Soc Nephrol. 2021;32:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian X, Fan X, Wang Y. Influence of asymptomatic carriers with COVID-19 on transplantation resumption in Wuhan. Transplantation. 2020;104:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]


