Abstract
Checkpoint inhibitor immunotherapy has significantly advanced treatment of a growing number of advanced malignancies. A consequences of immune system activation that leads to tumor cell destruction by checkpoint inhibitor therapy is the development of immune-related adverse events, some of which can be life threatening. There are limited data on the use of checkpoint inhibitor therapy in patients with preexisting autoimmunity owing to concerns that underlying autoimmune disease may be exacerbated by checkpoint inhibitor treatment. Decisions to treat these patients are made after careful consideration of the risks and benefits of treatment. We describe a patient with active and severe ulcerative colitis with metastatic melanoma who underwent elective colectomy prior to initiation of anti-PD-1 and anti-CTLA-4. The patient had excellent tumor response without flare of his ulcerative colitis suggesting that in select patients with high-risk inflammatory bowel disease, elective colectomy may be an effective treatment option.
Keywords: Immunotherapy, Checkpoint, Inflammatory bowel disease, Colitis, Complications
Introduction
Checkpoint Inhibitor (CPI) immunotherapy has changed the landscape of cancer treatment, demonstrating efficacy and improved survival in a growing number of cancer types. Checkpoint blockade results in activated CD8+ T cells that can kill tumor cells but can also result in Immune-Related Adverse Events (irAEs) affecting most organ systems, most commonly gastrointestinal, skin, liver and endocrine glands [1]. Gastrointestinal irAEs are a frequent and potentially fatal complication of CPI immunotherapy. Autoimmune colitis is more common with anti-CTLA-4 alone or in combination with PD-1 inhibitors than anti-PD-1 alone and toxicity with anti-CTLA-4 is dose dependent [2]. The risk of CPI-induced autoimmune colitis is also potentially increased in melanoma patients treated with anti-PD-1 compared to patients with Non-Small Cell Lung Cancer (NSCLC) and Renal Cell Carcinoma (RCC) [2]. CPI-induced colitis differs from Crohn’s disease and Ulcerative Colitis (UC) and is typically reversible [2]. Cessation of immunotherapy and immunosuppression with glucocorticoids or other therapies may be required [3].
There is a lack of data regarding the risk of colitis exacerbation in patients with preexisting Inflammatory Bowel Disease (IBD), as they have been excluded from clinical trials. Case series suggest that subjects with preexisting autoimmune conditions may potentially be safely treated with CPIs [4–7]. In general, although underlying autoimmune conditions can be exacerbated, in the face of life-threatening cancer, the risks and benefits must be carefully considered in these patients. Importantly, only a small number of patients in these case series have had preexisting IBD and a minority had active disease, as exacerbation of IBD can be fatal [4]. One study observed that 39% of patients with preexisting IBD had an exacerbation of their disease including a life threatening bowel perforation [4].
Here we report a case of a patient with severe, active UC with metastatic melanoma who underwent colectomy prior to CPI therapy. He responded to treatment and did not have exacerbation of his underlying IBD, suggesting that in select cases of life-threatening cancer and high-risk preexisting colitis, prior colectomy may be a safe and effective option. Despite having other reversible irAEs, he remains in response and off systemic therapy for 18 months.
Material and Methods
Cytokine array was performed on serum samples by Eve Technologies (Calgary, Canada) using the Human Cytokine 65-Plex Discovery Assay. Values shown are averages of observed concentrations for replicate analytes for each sample. For values out of range (below the 4 or 5 parameter logistic standard curve) the value was either designated as 0 pg/mL or as the lowest value obtained for that particular analyte when available.
Results
History and clinical course
A 41-year-old man with a history of severe ulcerative pancolitis presented to our oncology clinic in January 2018 with recently diagnosed malignant melanoma. He was on loperamide, mesalamine, 30 mg/day prednisone and had received one dose of vedolizumab for his colitis. He has a family history of melanoma in his mother and maternal grandmother and no family history of autoimmune disease.
One month prior to presentation, he noted a mass in his right axilla and on his right forearm. Biopsy of a right axillary lymph node was positive for malignant melanoma. Staging PET/CT revealed FDG uptake in a right axillary mass, left hilar node, subcutaneous lesions in the right arm and chest as well as increased uptake in the colon consistent with his known pancolitis.
He was found to have multiple supra-and infratentorial metastasis up to 9 mm on brain MRI. Subsequent skin evaluation identified a lesion on the right arm that was most likely primary tumor. Tumor profiling was positive for BRAFV600K mutation and it was determined that he required systemic therapy, preferably with immunotherapy given the relatively short duration of response of BRAFV600K mutated melanomas to BRAF/MEK inhibitors [8].
Extensive discussion was held with the patient, his gastroenterologist and members of the melanoma tumor board regarding the risks and benefits of immunotherapy. He had been diagnosed with UC at the age of 19 and required various therapies over the years including asacol, tofacitinib, infliximab, and prednisone. Most recently, he had received one dose of vedolizumab, with initial symptomatic improvement but remained on 30 mg/day of prednisone. He did not have organ involvement outside the Gastrointestinal (GI) tract. Colonoscopy in January 2018 demonstrated grade 3 pancolitis with a few scattered pseudopolyps and low-grade dysplasia in a background of diffuse active chronic pancolitis affecting the entire colon. Given the concern for exacerbation of colitis, a potentially lethal complication with risk of perforation, and the presence of low-grade dysplasia, the decision was made to proceed with colectomy prior to initiation of immunotherapy. The option of continuing treatment with vedolizumab alongside checkpoint inhibition was briefly considered, given that vedolizumab is a gut specific inhibitor unlikely to result in systemic immunosuppressive effects that would impede anti-tumor response. The presence of dysplasia on colonoscopy played a role in the decision to proceed with colectomy.
Approximately 1 week prior to colectomy he underwent Stereotactic Radiosurgery (SRS) to all 28 brain metastases and received levetiracetam for seizure prophylaxis. He underwent laparoscopic total colectomy with ileostomy placement without complications. Given his history of UC he was initially started on nivolumab only at 3 mg/kg for 2 cycles which he tolerated well except for some mild skin pruritis. With cycles 3 and 4 he also received ipilimumab 1 mg/kg which he tolerated well except for mild joint discomfort and dry mouth. His scans following 4 cycles showed response with no new metastases and a decrease in size of right axillary metastases. Following cycle 4, his Thyroid-Stimulating Hormone (TSH) decreased to 0.01 μIU/mL consistent with thyroiditis and he became hypothyroid 6 weeks later with a TSH of 89 μIU/mL for which he was started on levothyroxine. He experienced grade 1 transaminitis following cycle 5 of nivolumab and further ipilimumab was held. After 9 cycles of treatment, liver function tests were again elevated, with an Alanine Transaminase (ALT) of 107 U/L, requiring holding treatment and a short course of prednisone. Following cycle 12 of nivolumab he was admitted with CPI-induced pneumonitis requiring steroids with slow taper with subsequent resolution of his pneumonitis. Due to the pneumonitis, CPIs were no longer administered. He responded well to treatment with scans 12 months after the initiation of CPI therapy showing an ongoing response (Figure 1). Interestingly, in December 2019, one year after stopping treatment, his axillary mass, the only residual site of disease, was resected; one of 18 nodes contained totally necrotic tumor. He did not have exacerbation of his UC in the colonic stump or any other stigmata of UC such as arthritis, uveitis, pyoderma gangrenosum, pleuritis, erythema nodosum, and ankylosing spondylitis, during his treatment course. He has subsequently had successful reversal of his colostomy and pouch closure, and is experiencing normal, formed bowel movements.
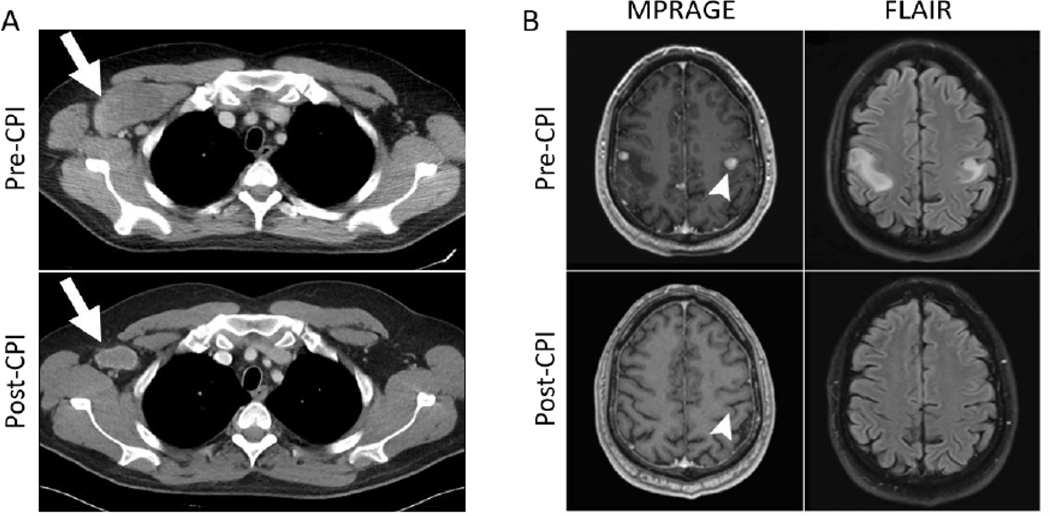
Figure 1:
CT and MRI scans pre-and post-checkpoint inhibitor therapy; (A) Pretreatment CT chest scan showing a 7.2 cm right axillary metastasis. Decrease in the size of metastases and ongoing response was observed at 1 year (following 12 cycles of therapy) with imaging showing a stable node measuring 3.5 cm (white arrows); (B) Post-SRS and pre-CPI brain MRI scan demonstrating right and left sided supratentorial metastatic lesions on contrast-enhanced MPRAGE imaging and corresponding edema on FLAIR. Post-CPI treatment scan shows no evidence of residual metastasis at 1 year. Arrow head shows resolution of the largest lesion on the left.
Pathological and laboratory examination
The subtotal colectomy specimen consisted of colon, appendix and a cuff of terminal ileum. On gross examination the serosa of the colon was unremarkable, while the mucosal aspect showed diffuse erythema, friability and granularity, with superficial ulcerations. The ascending colon was remarkable for a sessile polypoid lesion measuring 10.5 cm in greatest dimension (Figure 2A). Microscopic sections throughout the colon revealed classic features of chronic active colitis with mucosal regeneration limited to the mucosa (Figure 2B). Sections from the sessile lesion revealed extensive polypoid and flat low-grade dysplasia in a background of chronic colitis (Figure 2C and 2D). The gross and microscopic findings were characteristic of dysplasia arising in the setting of UC.
Figure 2:
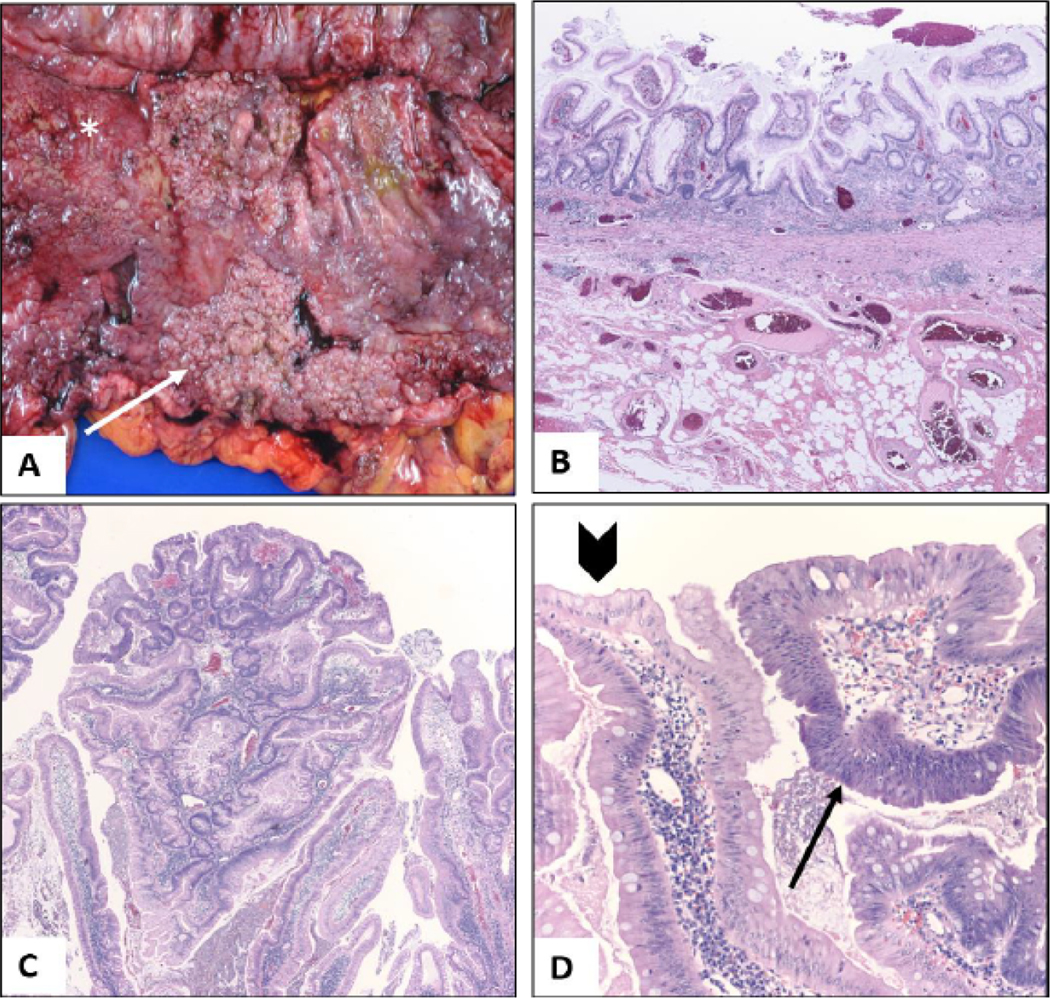
Pathology of colectomy specimen; (A) Gross appearance of sessile carpet-like polypoid lesion in the ascending colon (arrow) from the colectomy specimen. The adjacent mucosa is irregular and erythematous with loss of normal mucosal folds (asterisk); (B) On microscopy, the colonic mucosa was diffusely involved by chronic colitis, characterized by crypt distortion, basal plasmacytosis, and regenerative changes. The inflammation is limited to the mucosa (H and E, 4x); (C) Low power view of the ascending colon sessile lesion reveals proliferative mucosa forming polypoid structures (H and E, 4x); (D) On higher magnification, multiple areas of low-grade dysplasia (arrow) are seen. Compared to adjacent reactive epithelium (arrowhead) dysplastic foci show increased nuclear size, hyperchromasia, stratification and mucodepletion (H and E, 20X).
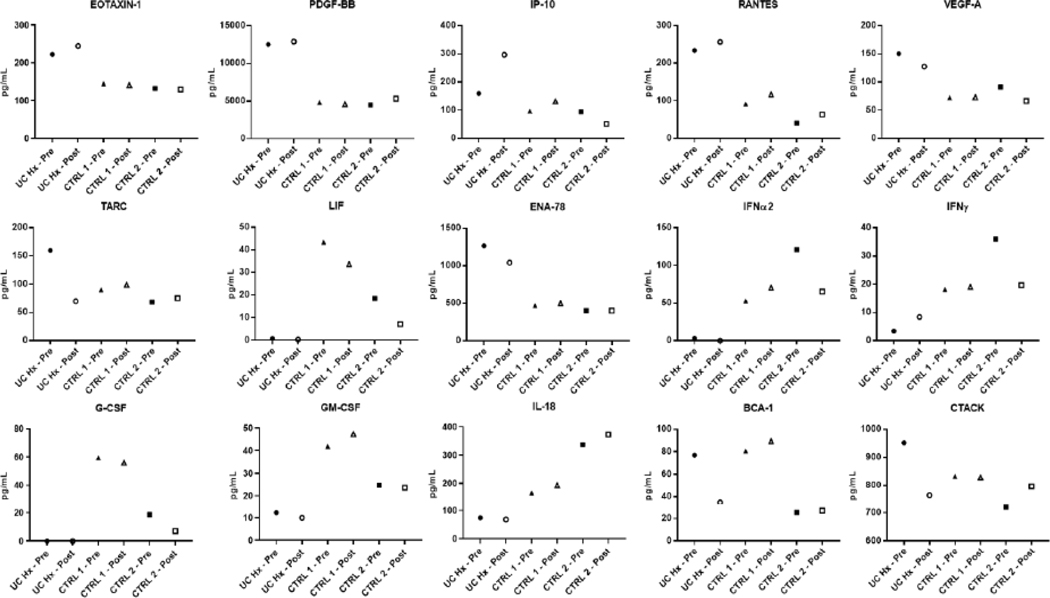
Circulating cytokines have been suggested as markers for disease severity in patients with IBD and are modified by immunomodulatory therapies [9,10]. Baseline and post treatment levels of various cytokines (IL-17, TGF-β, IL-10, IL-6, IL-8, IFNγ, IL-18) have also been proposed to be associated with toxicity or tumor response following CPI therapy [11]. Serum IL-17 levels, for example, have been found to be higher in patients who develop colitis with ipilimumab compared to those who do not, and levels decreased with symptom resolution [12]. A multiplex system was used to assess cytokine levels in our case patient pre-and post-CPI therapy compared to control patients with metastatic melanoma undergoing similar treatment but without underlying IBD to identify potential differences in serum cytokines at baseline and following CPI therapy (Figure 3 and Supplementary Figure 1). Several cytokines and chemokines previously shown to be elevated in either tissue or sera from patients with IBD were elevated in our UC case patient including EOTAXIN-1, VEGF, PDGF-BB, interferon gamma-induced protein 10 (IP-10), Thymus and Activation Regulated Chemokine (TARC), Epithelial-Derived Neutrophil-Activating Peptide 78 (ENA-78), and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) [13–18]. Several other cytokines with a potential pathologic role in IBD were lower in the UC patient including Leukemia Inhibitory Factor (LIF), IFNα2, FGF-2, IFNγ, IL-18, G-CSF, and GM-CSF [9,13,14,19]. It is possible that these changes were related to prior immunomodulatory therapies, including infliximab and prednisone, or potentially modified by prior colectomy. A few cytokines changed following CPI treatment differentially in our UC case patient compared to controls, including IP-10, TARC, B Cell-Attracting Chemokine 1 (BCA-1), and Cutaneous T-Cell-Attracting Chemokine (CTACK). IP-10 has been shown to increase following nivolumab treatment [20] and increased more in the UC case patient.
Figure 3:
Cytokine levels pre- and post-CPI therapy for case patient with ulcerative colitis (UC hx) and two control (CTRL) patients without underlying colitis or other autoimmune diseases. CTRL 1 is a 39-year-old male with metastatic melanoma to the parotid gland and lymph nodes on nivolumab (pre-and post-treatment samples are 4 weeks apart, 1 cycle of nivolumab) and CTRL 2 is a 36-year-old male with metastatic melanoma to lymph nodes on nivolumab (pre-and post-treatment samples are 4 weeks apart, 1 cycle of nivolumab). The pre-treatment sample for the UC case was 25 days after colectomy. Pre-and post-treatment samples for the UC case patient are 4 weeks apart (2 cycles of nivolumab). None had significant complications at the time of the post-treatment collection. Data represent average of replicate analytes.
Discussion
Despite the growing use of CPIs in patients with advanced malignancies little is known about use of these therapies in patients with underlying autoimmune disease. Here we report successful treatment in a patient with active severe UC following elective colectomy.
Although early CPI trials did not include patients with underlying autoimmunity, there are reports of successful treatment of patients with underlying autoimmune diseases and several retrospective studies have provided some insight into the safety and efficacy of CPI therapy in these patients. Understanding safety of CPIs in patients with preexisting IBD is particularly important as bowel perforation can be one of the most severe and potentially fatal complications of CPI therapy.
In a retrospective study, Johnson et al. assessed irAEs and autoimmune flares in patients with advanced melanoma treated with ipilimumab with underlying autoimmune disease, 43% of which were on immunosuppressive therapy at the initiation of CPI. 27% of patients experienced an autoimmune flare and 33% a conventional grade 3–5 irAE [7]. Six of the patients in this study had preexisting IBD. Two patients with UC had prior colectomies including one patient who had a colectomy immediately prior to initiating ipilimumab and one patient with Crohn’s disease had a prior partial colectomy. The other 3 patients were receiving aminosalicylates or topical hydrocortisone. Two of the 6 patients experienced CPI-induced colitis or IBD exacerbation, one was an UC patient with prior colectomy suggesting that even with colectomy, colitis remains a risk with immunotherapy. One patient’s colitis flare resolved with infliximab and another with methylprednisolone. A patient with prior colectomy without symptoms had evidence of hyperemia and rectal stump thickening at the colectomy site on surveillance CT. It is important to note that the patients in this series had minimal to no symptoms related to their IBD prior to starting therapy, whereas our patient had active UC (Table 1).
Table 1:
Summary of studies describing IBD exacerbations following CPI therapy.
| Cancer type | CPI therapy | #of patients with AID | Post CPI autoimmune complications | Status of AID at CPI start | Severity of flares/irAEs | #of patients with preexisting IBD | Frequency of IBD flares | Additional irAEs in IBD patients | |
|---|---|---|---|---|---|---|---|---|---|
| Johnson et al [7] | Advanced melanoma | Ipilimumab | 30 | 27% had exacerbation of AID. 33% had conventional grade 3–5 irAE | 43% were on immunosuppressive therapy. Minimal to no symptoms prior to CPI start | Most autoimmune complications managed with corticosteroids | 6 total: 2 UC patients and one Crohn’s patient had prior colectomies. All were asymptomatic or minimally symptomatic prior to CPI start | 2/6 (33%) developed CPI induced colitis or IBD flare. 1 patient with UC with prior colectomy developed AID exacerbation. 1 patient with Crohn’s developed CPI induced colitis | |
| Menzies et al. [5] | Advanced melanoma | Anti-PD-1 | 52 | 38% had AID flares | 29% had active symptoms. 62% were not on immunosuppression at baseline | Generally mild and only 2 (4%) discontinued therapy due to flare. Flares more frequent in those with active symptoms or on immune-suppressants | 6 with underlying GI disease: UC with colectomy: 2, Crohn’s: 3, Celiac: 1 | None | |
| Gutzmer et al. [22] | Advanced melanoma | Anti-PD-1 | 19 | 42% had AID flare | 6/19 (32%) were on immunosuppressive therapy Preexisting autoimmunity controlled in all patients | All flares were managed by immunosuppressive and/or symptomatic therapy and did not require CPI termination | 1 patient with UC on sulfasalazine and budesonide | 1/1 patient had IBD flare – managed with steroids and interruption of anti-PD-1 and subsequently retreated without further exacerbation | |
| Abdel-Wahab et al. [4] (a) | Metastatic melanoma, lung cancer, RCC, Merkel cell cancer | Ipilimumab, anti-PD-1, anti-PD-L1, or combination ipilimumab + anti-PD-1 | 123 | 50% had exacerbation of AID. 75% had exacerbation of AID, irAE, or both | 46.2% had active AID. 43.6% were on treatment for AID. No differences observed in flares between those with active vs inactive AID at baseline | Most flares and irAEs managed with steroids. 16% required other immunosuppressives. More than half of patients improved without CPI discontinuation | 13 total: UC: 8 Crohn’s: 55 had active disease. 4 were receiving treatment | 5/13 (39%) had IBD flare. 3/5 with active disease did not have flare. One patient with UC experienced life- threatening perforation | Overall 62% had adverse event (exacerbation, new irAE or both). irAEs: De novo colitis, toxic epidermal necrolysis |
| Leonardi et al. [6] | NSCLC | Anti-PD-1 or anti-PD-L1 | 56 | 55% had AID flare and/or an irAE 13 (23%) had flare of preexisting AID | 18% had active AID symptoms 20% were on immunomodulatory agents Minority had symptoms, all low-grade severity 50% who were symptomatic vs 18% who were asymptomatic developed AID flare post CPI | Exacerbations were generally mild 4/13 patients who developed exacerbation of AID required systemic corticosteroids | 6 total: UC: 3 Crohn’s: 3 One patient was symptomatic at the start of therapy | None | 3/6 developed other irAEs: 2 pneumonitis, one leukopenia |
| Kahler et al. [21] | Metastatic melanoma | Ipilimumab | 41 | 12/41 (29.2%) experienced AID flare | 11 (26.8%) were on immunosuppressants | Most were manageable | 3 total: UC: 2 Crohn’s: 1 | 1/3 (33%) had IBD flare | Pruritis grade 3 and maculopapular exanthema grade 2 in Crohn’s patient |
This study is a systematic review of the literature that includes cases from Johnson et al. and Gutzmer et al. Abbreviations: AID: Autoimmune Disease; CPI: Checkpoint Inhibitor; GI: Gastrointestinal; IBD: Inflammatory Bowel Disease; irAE: Immune Related Adverse Event; NSCLC: Non-Small Cell Lung Cancer; RCC:Renal Cell Carcinoma; UC: Ulcerative Colitis
Another retrospective study reported on the safety of anti-PD-1/PD-L1 in 56 patients with NSCLC and underlying autoimmune disease. A minority of patients in this study had symptoms related to their autoimmune disease at the start of therapy and in all, severity was low-grade. This study included six patients with IBD, only one was symptomatic at the start of immunotherapy, and none experienced disease flare during treatment. Among the three IBD patients who developed irAEs unrelated to their preexisting autoimmune disease, two developed pneumonitis [6]. Menzies et al. reported 52 patients with advanced melanoma treated with anti-PD-1 with autoimmune disease [5]. Six patients had underlying gastrointestinal disease; none had a disease flare. In a systematic review of the literature, Abdel-Wahab et al. found that of 123 patients with underlying autoimmune disease treated with CPIs, 50% had exacerbation of their autoimmune disease. There were 13 patients with underlying IBD. One patient experienced life-threatening perforation after a single dose of ipilimumab which was not responsive to corticosteroids [4]. Kahler et al. published a series of patients with metastatic melanoma treated with ipilimumab [21]. Three had IBD and one of those had disease flare. Gutzmer et al. described a patient with IBD which flared on anti-PD-1, successfully managed with steroids and intermittent administration of anti-PD-1 [22].
Collectively these studies suggest that~23%−50% of patients with underlying autoimmune disease experience a disease flare. Many of the patients with IBD had mild or no disease flares. Importantly in patients with underlying IBD, the majority did not have active disease and/or require immunosuppressive agents at the time of CPI initiation in contrast to our patient, who had active, symptomatic UC requiring multiple therapies. Although not consistent in all studies, it appears that flares are more likely to occur in patients with symptomatic/active disease or those that required immunosuppressive agents at the time of CPI initiation. In a few patients concurrent immune suppression did not prevent colitis, although in murine models prophylactic TNF blockade eliminated colitis without affecting anti-tumor response [23].
There is no dependable way to predict who will develop CPI-induced colitis, including in patients with underlying IBD [2]. There is also no known treatment modality to prevent CPI- induced colitis. Although studies are limited, prophylactic steroids do not prevent CPI-induced colitis [24]. A case report describes complete tumor response and no Crohn’s flare in a patient treated simultaneously with vedolizumab and anti-PD-1 for metastatic melanoma [25], but additional data are sorely needed.
The decision to proceed with colectomy in our patient was multifactorial, including underlying dysplasia, his youth and the aggressive nature of his melanoma with 28 brain metastases. It was also made thoughtfully through contributions from a multidisciplinary team. As a result, he had an excellent clinical response without colitis in the rectal stump. He did experience other irAEs including thyroiditis followed by hypothyroidism, hepatitis and pneumonitis. However, he did not develop extra-colonic manifestations of UC. A few of the cases in the literature describe patients with IBD who develop tracheobronchitis or interstitial lung disease with CPI treatment. Pneumonitis is a relatively common irAE occurring with PD-1/PD-L1 blockade (incidence 0%−10%) and occurs more frequently than with CTLA-4 blockade (incidence<1%). Pneumonitis also occurs more frequently with combination therapy than monotherapy (10% vs 3%) [3]. As more cases of IBD patients treated with CPIs emerge it would be interesting to assess whether these patients are at higher risk for known extra-intestinal complications associated with IBD.
Although we noted some potentially interesting differences in cytokine levels pre-and post-CPI in our patient with UC compared to control patients, additional studies are needed. There are several caveats in interpreting these results including potential lasting effects of prior immunosuppressive agents on cytokine levels in our IBD patient. There may potentially be an effect of colectomy itself on cytokine levels and we do not have serum prior to colectomy for analysis. Furthermore, although changes in circulating cytokine levels have been described in response to CPI treatment, there is no established role for changes in cytokines in assessing response to treatment or predicting complications [11].
Conclusion
While further studies are needed to better elucidate treatment approaches in patients with IBD, this case illustrates that in select cases prophylactic colectomy may be an excellent means of preventing exacerbation of colitis. However, decisions should be carefully made on a case-by-case basis with careful consideration of risk factors for developing other irAEs, IBD history (i.e. duration, severity, need for immunosuppressive therapy, evidence of dysplasia), choice of CPI, and the malignancy.
Supplementary Material
Acknowledgement
Financial support
This work was supported in part by R01 CA227473 (K Herold and H Kluger, PIs), and the Yale SPORE in Skin Cancer P50 CA121974 (M Bosenberg and H Kluger, PIs).
Abbreviations
- ALT
Alanine Transaminase
- BCA-1
B Cell-Attracting Chemokine 1
- CPI
Checkpoint Inhibitor
- CTACK
Cutaneous T-Cell-Attracting Chemokine
- CTLA-4
Cytotoxic T-Lymphocyte-Associated Protein 4
- ENA-78
Epithelial-Derived Neutrophil-Activating Peptide 78
- GI
Gastrointestinal
- IBD
Inflammatory Bowel Disease
- IP-10
Interferon Gamma-Induced Protein 10
- irAEs
Immune-Related Adverse Events
- LIF
Leukemia Inhibitory Factor
- NSCLC
Non-Small Cell Lung Cancer
- PD-1
Programmed Cell Death Protein 1
- PD-L1
Programmed Death-Ligand 1
- RANTES
Regulated on Activation, Normal T Cell Expressed and Secreted
- RCC
Renal Cell Carcinoma
- SRC
Stereotactic Radiosurgery
- TARC
Thymus and Activation Regulated Chemokine
- TSH
Thyroid-Stimulating Hormone
- UC
Ulcerative Colitis
Footnotes
Conflicts of interest
H. Kluger reports research grants from Merck, Bristol-Myers Squibb, and Apexigen during the conduct of the study, and personal fees from Corvus, Nektar, Biodesix, Roche-Genetech, Pfizer, Iovance, Immunocore, and Celldex, Array Biopharma, Bristol-Myers Squibb, Clinigen and Merck, outside of the submitted work.
References
- 1.Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 2.Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, et al. (2018) Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut 67: 2056–2067. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Lacchetti C, Thompson JA (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract 14: 247–249. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME (2018) Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 168: 121–130. [DOI] [PubMed] [Google Scholar]
- 5.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, et al. (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28: 368–376. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, et al. (2018) Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 36: 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, et al. (2016) Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2: 234–240. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan RJ, Flaherty KT (2013) Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer 49: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 9.Muzes G, Molnar B, Tulassay Z, Sipos F (2012) Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol 18: 5848–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa K, Matsumoto T, Esaki M, Torisu T, Iida M (2012) Profiles of circulating cytokines in patients with crohn’s disease under maintenance therapy with infliximab. J Crohns Colitis 6: 529–535. [DOI] [PubMed] [Google Scholar]
- 11.Bridge JA, Lee JC, Daud A, Wells JW, Bluestone JA (2018) Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front Med (Lausanne) 5: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan MKYA, Tandon S (2011) Evaluation of serum IL-17 levels during ipilimumab therapy: Correlation with colitis. J Clin Oncol 29: 2505. [Google Scholar]
- 13.Coburn LA, Horst SN, Chaturvedi R, Brown CT, Allaman MM, et al. (2013) High-throughput multi-analyte luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS One 8: e82300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, et al. (2001) VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: A novel mechanism of chronic intestinal inflammation. Am J Gastroenterol 96: 822–828. [DOI] [PubMed] [Google Scholar]
- 15.Krzystek-Korpacka M, Neubauer K, Matusiewicz M (2009) Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin Biochem 42: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi A, Watanabe K, Narumi S, Yamagami H, Fujiwara Y, et al. (2007) The production of interferon-gamma-inducible protein 10 by granulocytes and monocytes is associated with ulcerative colitis disease activity. J Gastroenterol 42: 947–956. [DOI] [PubMed] [Google Scholar]
- 17.Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK (2012) Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 18: 2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajuebor MN, Swain MG (2002) Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology 105: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egea L, Hirata Y, Kagnoff MF (2020) GM-CSF: A role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol 4: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, et al. (2016) Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res 22: 5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahler KC, Eigentler TK, Gesierich A, Heinzerling L, Loquai C, et al. (2018) Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother 67: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutzmer R, Koop A, Meier F, Hassel JC, Terheyden P, et al. (2017) Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab- triggered autoimmunity. Eur J Cancer 75: 24–32. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, et al. (2019) Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569: 428–432. [DOI] [PubMed] [Google Scholar]
- 24.Weber J, Thompson JA, Hamid O, Minor D, Amin A, et al. (2009) A randomized, double-blind, placebo- controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 15: 5591–5598. [DOI] [PubMed] [Google Scholar]
- 25.Frohne CC, Llano EM, Perkovic A, Cohen RD, Luke JJ (2019) Complete response of metastatic melanoma in a patient with Crohn’s disease simultaneously receiving anti-alpha4beta7 and anti-PD1 antibodies. J Immunother Cancer 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.