Abstract
We report onset, course, correlations with comorbidities, and diagnostic accuracy of nasopharyngeal swab in 539 individuals suspected to carry SARS-COV-2 admitted to the hospital of Crema, Italy. All individuals underwent clinical and laboratory exams, SARS-COV-2 reverse transcriptase-polymerase chain reaction on nasopharyngeal swab, and chest X-ray and/or computed tomography (CT). Data on onset, course, comorbidities, number of drugs including angiotensin converting enzyme (ACE) inhibitors and angiotensin-II-receptor antagonists (sartans), follow-up swab, pharmacological treatments, non-invasive respiratory support, ICU admission, and deaths were recorded. Among 411 SARS-COV-2 patients (67.7% males) median age was 70.8 years (range 5–99). Chest CT was performed in 317 (77.2%) and showed interstitial pneumonia in 304 (96%). Fatality rate was 17.5% (74% males), with 6.6% in 60–69 years old, 21.1% in 70–79 years old, 38.8% in 80–89 years old, and 83.3% above 90 years. No death occurred below 60 years. Non-invasive respiratory support rate was 27.2% and ICU admission 6.8%. Charlson comorbidity index and high C-reactive protein at admission were significantly associated with death. Use of ACE inhibitors or sartans was not associated with outcomes. Among 128 swab negative patients at admission (63.3% males) median age was 67.7 years (range 1–98). Chest CT was performed in 87 (68%) and showed interstitial pneumonia in 76 (87.3%). Follow-up swab turned positive in 13 of 32 patients. Using chest CT at admission as gold standard on the entire study population of 539 patients, nasopharyngeal swab had 80% accuracy. Comorbidity network analysis revealed a more homogenous distribution 60–40 aged SARS-COV-2 patients across diseases and a crucial different interplay of diseases in the networks of deceased and survived patients. SARS-CoV-2 caused high mortality among patients older than 60 years and correlated with pre-existing multiorgan impairment.
Introduction
Since December 2019 outbreak in China of the novel coronavirus infection, designated SARS-CoV-2 and termed Covid-19, the disease has quickly overflowed worldwide SARS-CoV-2 causes a clinical syndrome encompassing asymptomatic or oligosymptomatic flu-like course, gastrointestinal disturbances, mild pneumonia, acute respiratory distress and death [1–6].
After the first Italian patient was notified on February 18th, 2020 at the hospital of Codogno, 25 km far from Crema, the total number of SARS-CoV-2 patients exponentially increased in the area. The municipality of Crema, with about 34,000 inhabitants, experienced a rapid overload of the healthcare structures due to a massive influx of suspected SARS-CoV-2 patients.
We sought to perform a systematic analysis of hospitalized SARS-CoV-2 patients soon after the outbreak and outline possible correlations with comorbidities and drug intake. To this aim, we investigated all consecutive individuals suspected to harbor SARS-CoV-2 and admitted to the General Hospital of Crema between February 21st and March 13rd, 2020 and followed-up until March 19th, 2020. We provided data on onset, clinical history, laboratory and radiological findings, course of the disease, age and sex-stratified fatality rate, and diagnostic accuracy of the nasopharyngeal swab. The predictive power of not collinear variables was investigated in death incidence, in the need of non-invasive ventilation/continuous positive airway pressure and in the need of intensive unit care. We applied a network analysis [7, 8] to assess the interactions between comorbidities across age groups and outcomes.
Materials and methods
On February 19th, 2020 the General and Health Directorate of the Hospital of Crema met to update and carry out the available procedures to cope with the hospitalization of patients with a potential viral spread, based on 2009 H1N1 pandemic strategic plan revised on December 2014 after Ebola outbreak. Starting on February 21st, 2020 the Emergency Department set up a triage for any individual either reporting or presenting with fever (above 37.5°C), cough or dyspnea, or having had contact with SARS-CoV-2 carriers. All individuals admitted to the hospital underwent body temperature and pulse oximetry (SO2) recording, hematological screening, chest X-ray and/or computed tomography (CT) and nasopharyngeal swab. Swabs were stored at +4°C and immediately shipped to one of the laboratory of virology accredited by the Lombardy Region for diagnostic SARS-COV-2 real-time polymerase chain reaction assay. Based on clinical, laboratory and radiological findings, patients were discharged to home in quarantine or hospitalized.
Demographic data, date of onset and type of symptoms, comorbidities (e.g. hypertension, cardiovascular disorders, diabetes, pulmonary diseases, active and previous malignancies, renal insufficiency and any other known condition), current pharmacological treatments, use of angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin-II-receptor antagonists (sartans), and number of drugs were recorded. All available clinical data including hematological and radiological exams, treatments, respiratory support with continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV), intensive care unit (ICU) admission and deaths were recorded. Chest X-ray and CT scan were scored as positive or negative based on radiologists’ written report. Acute reticular pattern at X-ray and the presence of single or multiple ground-glass and/or consolidative lung opacities were considered indicative of interstitial pneumonia (X-ray and CT positive). Pleural and pericardial effusion, and lymphadenopathy at CT scan were also recorded. For each patient the Charlson comorbidity index was calculated [9].
The hospital of Crema is fully equipped with a computerized recording system that generates a unique code for any visit and exam. All patients were anonymized and locked to the unique code assigned at the admission, and all data were included in an electronic database. The Institutional Review Board of the Hospital of Crema has approved the study. All the experiment protocol for involving human data was accordance to guidelines of national/international/institutional or Declaration of Helsinki in the manuscript. Written informed consent was obtained from all participants and legally authorized representatives of minors below 18 patients and dead patients gave consent for data that could be used for clinical studies.
Comorbidity network analysis
Two different sets of nodes were considered for the network analysis: 252 SARS-Cov-2 positive patients and 13 possible diseases (cardiovascular disease (CaD), respiratory diseases (ReD), diabetes, hypertension, renal insufficiency (ReI), cancer, arthritis, obesity, asthma, obstructive sleep apneas (OSAS), dyslipidemia, chronic obstructive pulmonary disease COPD(), atrial fibrillation (AF)) they could suffer for. A rectangular matrix patients (rows) vs diseases (columns) (PvsD) was obtained by associating to the entry PvsD(i,j) one or zero according to the fact that patient i had been suffering for the disease j or not. The information in this matrix can be used to define an adjacency matrix [7] representing a graph, where two different sets of nodes were included (patients and diseases) and for each non-zero entry of the matrix a link between two nodes was included. The resulting graph is known as a bipartite network [10] that is, a graph where nodes can be decomposed into two disjoint sets A and B such that edges connect only elements of one set with elements of the other set. A common practice for bipartite data mining is to find a projection of the whole graph over one of the two sets. The A-projection is composed of a network containing only A-nodes, where two A-nodes are connected when they have at least N common neighboring B-node [10].
Comorbidity networks are typical bipartite where one set is given by the patients and the other by their diseases [8]. In this study, it was obtained by firstly dividing the cohort of patients in three classes of age: 40–60 years, 61–80 years, and 81–100 years. The networks have been calculated as the projection of the patient-disease bipartite network on the diseases space. This means that two diseases are connected by one edge if at least one patient suffer from both and the weight of this edge is given by the total number of patients in this situation. Such final monopartite disease networks have been filtered by fixing N to the 10% of the number of patients included in each class. The three comorbidity networks have been analyzed in terms of nodes weighted degree [7], that measures a node’s total level of involvement in the network, and of modules detection [11] which iteratively optimizes local communities until global modularity can no longer be improved given perturbations to the current community state. Thereafter, the same cohort has been divided in deceased and survived patients. Comorbidity networks were obtained for the two groups in the same way described above and the same topological analysis has been done. Before any projection, the columns of the PvsD matrix were corrected for gender and age.
In order to test the statistically significance of the difference between deceased and survived networks, we decided to compare their normalized Laplacian matrices. The normalized Laplacian matrix is a transformation of the adjacency matrix, whose eigenvalues describe aspects related to the global network structure and dynamic interactions among network parts [12]. We derived, as reported in Supplementary Materials, the null model of the difference of the two normalized Laplacian matrices and calculated the p-value associated.
Statistical analysis
Descriptive statistics were provided in terms of absolute number and percentage for categorical data, and mean with standard deviation (SD) and median with interquartile range (IQR) for continuous data. The predictive power of swabs outcome in CT scan classification (positive or negative) has been tested by means of the receiver operating characteristic (ROC) curve analysis [13] of a logistic regression [14]. Accordingly, the area under the curve of the ROC curve was calculated. A multiple logistic regression analysis [15] to investigate the chances of survival, the chances to need CPAP/NIV and the chances to need ICU as a function of few predictor variables was conducted. Such variables (Sex, Hypertension, Charlson comorbidity index [9], Number of drugs assumed, White blood cells counts, Creatinine, C-reactive protein, Alanine aminotransferase, Aspartate aminotransferase, positive X-Ray, positive CT scan, ACE inhibitors and sartans consumption) were chosen among the others in order to exclude collinearities and to ensure that there was no violation of the linearity of the logit.
Comorbidity network has been analyzed by dividing the patients in three classes of age and outcome (deceased and survived) at last day of follow-up. For each group, the comorbidity network was derived according to the constraint that at least 10% of patients must share two diseases.
Results
Clinical features of the study population
We collected data from 539 consecutive patients admitted to the hospital. All underwent nasopharyngeal swab, whose result was available within 24 hours on average. Overall, 411 patients were swab positive and 128 negative. SARS-CoV-2 patients reported the onset of symptoms for 5.3±4.3 days, whereas swab negative patients for 5.7±4.1 days. Table 1 reports the prevalence of the symptoms at onset.
Table 1. Way of arrival at the hospital and symptoms reported by SARS-CoV-2 and swab negative patients.
| n (%) | Positive (411) | Negative (128) |
|---|---|---|
| Arrival at hospital | ||
| Ambulance | 196 (47.7) | 36 (28.1) |
| Own means | 215 (52.3) | 92 (71.9) |
| Fever | 346 (84.2) | 97 (75.8) |
| Cough | 151 (36.7) | 44 (34.4) |
| Dyspnoea | 131 (31.9) | 34 (26.6) |
| Syncope | 41 (9.9) | 6 (4.7) |
| Fall with trauma | 23 (5.6) | 7 (5.4) |
| Nausea | 18 (4.4) | 5 (3.9) |
| Vomit | 16 (3.9) | 8 (3.7) |
| Diarrhoea | 15 (3.6) | 5 (3.9) |
| Confusion/psychomotor restlessness | 15 (3.6) | 6 (4.7) |
| Headache | 10 (2.4) | 2 (1.6) |
| Coma | 9 (2.2) | 4 (3.1) |
| Diffuse myalgia/arthralgia | 7 (1.7) | 2 (1.6) |
| Thoracic pain | 7 (1.7) | 10 (7.8) |
| Abdominal pain | 5 (1.2) | 2 (0.9) |
| Pharyngodinia | 4 (1.0) | 7 (5.4) |
| Recent pneumonia in treatment | 4 (1.0) | 3 (2.3) |
Thirteen patients arrived in coma. Of them, 9 were SARS-CoV-2 patients and 5 died. The other 4 patients were negative at nasopharyngeal swab; 3 died and 1 was hospitalized. Another 21 patients presented with disorientation and/or with psychomotor restlessness; 15 were SARS-CoV-2 patients and 2 died. The other 6 patients were swab negative and 4 died. Notably, 41 (9.9%) SARS-CoV-2 patients reported or had at arrival one or more syncope; 14 of them had a fall with trauma and 5 died. Another 6 (4.7%) swab negative patients reported syncope and 1 died.
At admission, body temperature was slight above 37°C both in SARS-CoV-2 and swab negative patients. Nevertheless, the large majority of patients reported the intake of paracetamol during the previous hours, thus influencing the assessment at arrival. Similarly, the mean value of pulse oximetry in patients who arrived in ambulance was influenced by the early treatment with oxygen mask during transportation. The mean value of those who reached the hospital by their own means did not differ between SARS-CoV-2 (93%, range 60–100) and swab negative (92%, range 50–100) patients.
Course and outcome in SARS-CoV-2 patients
Among 411 in SARS-CoV-2 patients at nasopharyngeal swab, the median age was 70.8 years (range 5–99), with a preponderance of males (66.6%). Of 411 patients, 262 (63.7%) were hospitalized, 44 (10.7%) were transported to other regional hospitals, and 16 (3.9%) were discharged to home in quarantine.
Chest X-ray was performed in 128 (31.1%) of 411 patients and was reported as suggestive of interstitial pneumonia in 79 (61.7%) patients and negative in 49 (38.3%). Chest CT was performed in 317 (77.2%) of 411 patients and showed interstitial pneumonia in 304 (96%), whereas was negative in 13 (4%). It showed pleural effusion in 29 (9.1%) patients, lymphadenopathy in 37 (11.7%), and pericardial effusion in 27 (8.5%). Of the 38 (9.2%) patients who underwent both the exams, 31 (81.6%) had concordant positive report, 6 (15.8%) had positive CT scan and negative X-Ray report, and 1 (2.6%) had negative CT scan and positive X-Ray report (Table 2).
Table 2. Demographic and clinical characteristics in SARS-CoV-2 patients admitted to the Hospital of Crema between February 21st and March 13rd, 2020, and followed up until March 19, 2020.
| All patients (411) | Deaths (72) | ICU (28) | CPAP/NIV (112) | |
|---|---|---|---|---|
| Age | ||||
| mean (standard deviation) | 67.7 (15.0) | 81.1 (7.5) | 65.7 (12.1) | 70.9 (10.9) |
| median (IQR) | 70.8 (57.5–79.1) | 80.7 (76.4–85.6) | 69.1 (60.2–72.9) | 72.2 (64.1–79.4) |
| Sex, n (%) | ||||
| Male | 278 (67.6) | 53 (73.6) | 26 (92.9) | 91 (81.3) |
| Female | 133 (32.4) | 19 (26.4) | 2 (7.1) | 21 (18.7) |
| Cough, n (%) | 151 (36.7) | 8 (11.1) | 11 (39.3) | 33 (29.5) |
| Dyspnoea, n (%) | 131 (31.9) | 39 (54.2) | 22 (78.6) | 67 (59.8) |
| Any comorbidity, n (%) | 256 (62.3) | 61 (84.7) | 23 (82.1) | 87 (77.7) |
| Hypertension | 193 (47.0) | 48 (66.7) | 20 (71.4) | 67 (59.8) |
| Cardiovascular diseases | 93 (22.6) | 28 (38.9) | 9 (32.1) | 37 (33.0) |
| Diabetes | 67 (16.3) | 25 (34.7) | 7 (25.0) | 33 (29.5) |
| Pulmonary diseases | 48 (11.7) | 10 (13.9) | 5 (17.9) | 18 (16.1) |
| Renal insufficiency | 22 (5.3) | 11 (15.3) | 5 (17.9) | 11 (9.8) |
| Malignancies | 33 (8.0) | 9 (12.5) | 2 (7.1) | 8 (7.1) |
| Number of drugs, n (%) | ||||
| 0 | 124 (33.9) | 10 (15.9) | 4 (16.0) | 19 (19.0) |
| <3 | 89 (24.3) | 8 (12.7) | 5 (20.0) | 20 (20.0) |
| 4–6 | 74 (20.2) | 14 (22.2) | 5 (20.0) | 25 (25.0) |
| >7 | 79 (21.6) | 31 (49.2) | 11 (44.0) | 36 (36.0) |
| ACE inhibitors, n (%) | 50 (12.2) | 11 (15.3) | 4 (14.3) | 20 (17.9) |
| Sartans, n (%) | 60 (14.6) | 14 (19.4) | 9 (32.1) | 22 (19.6) |
| X-Ray, n (%) | ||||
| probable/possible | 79 (61.7) | 25 (34.7) | 11 (39.3) | 28 (25.0) |
| negative | 49 (38.3) | 3 (4.2) | 1 (3.6) | 4 (3.6) |
| not done | 283 (68.9) | 44 (61.1) | 16 (57.1) | 80 (71.4) |
| CT scan, n (%) | ||||
| positive | 304 (96.0) | 50 (100) | 23 (82.1) | 92 (82.1) |
| negative | 13 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| not done | 94 (22.8) | 22 (30.6) | 5 (17.9) | 20 (17.9) |
X-ray and CT are intended as the first exam performed and percentage in brackets refers to the number of patients who have done the exams. Sums do not add up to the total because of some missing values.
Laboratory exams at admission showed white blood cell count below 10x109/L in 82% of patients, neutrophil count below 10x109/L in 83.4%, and lymphocyte count below 1x109/L in 55.6%. C-reactive protein, aspartate aminotransferase and lactate dehydrogenase values were elevated in most patients, particularly among those with worst clinical picture and outcome (Table 3).
Table 3. Laboratory findings in SARS-CoV-2 patients admitted to the Hospital of Crema between February 21st and March 13rd, 2020, and followed up until March 19, 2020.
| All patients (411) | Deaths (72) | ICU (28) | CPAP/NIV (112) | |
|---|---|---|---|---|
| White blood cell count, x 109 per L, n (%) | ||||
| <4 | 56 (14.6) | 3 (4.8) | 0 (0.0) | 5 (4.6) |
| 4–10 | 281 (73.0) | 43 (68.2) | 20 (74.1) | 77 (71.3) |
| >10 | 48 (12.5) | 17 (27.0) | 7 (25.9) | 26 (24.1) |
| Neutrophil count, x 109 per L, n (%) | ||||
| <7.5 | 321 (83.4) | 41 (65.1) | 16 (59.3) | 71 (65.7) |
| >7.5 | 64 (16.6) | 22 (34.9) | 11 (40.7) | 37 (34.3) |
| Lymphocyte count, x 109 per L, n (%) | ||||
| <1 | 214 (55.6) | 45 (71.4) | 18 (66.7) | 69 (63.9) |
| >1 | 171 (44.4) | 18 (28.6) | 9 (33.3) | 39 (36.1) |
| C-reactive protein, mg/L, mean (SD) | 7.51 (7.1) | 13.2 (8.4) | 15.8 (8.8) | 12.9 (7.9) |
| median (IQR) | 5.1 (2.7–11.5) | 12.4 (6.4–18.1) | 13.5 (10.5–21.4) | 12.0 (6.5–18.0) |
| Creatinine, mg/dl | ||||
| <1.3 | 283 (73.9) | 29 (45.3) | 14 (51.8) | 70 (64.8) |
| >1.3 | 100 (26.1) | 35 (54.7) | 13 (48.2) | 38 (35.2) |
| Alanine aminotransferase, U/L | ||||
| <40 | 272 (71.8) | 53 (76.8) | 15 (55.6) | 64 (54.3) |
| >40 | 107 (28.2) | 16 (23.2) | 12 (44.4) | 44 (40.7) |
| Aspartate aminotransferase, U/L | ||||
| <37 | 157 (40.5) | 14 (20.3) | 3 (11.1) | 16 (14.7) |
| >37 | 231 (59.5) | 55 (79.7) | 24 (88.9) | 93 (85.3) |
| Lactate dehydrogenase, U/L, mean (SD) | 470 (839.3) | 789 (1710) | 988 (2064) | 636 (1140) |
| median (IQR) | 358 (265–498) | 486 (373–596) | 491 (356–581) | 477 (362–586) |
| Creatine kinase, U/L, mean (SD) | 401.5 (972.3) | 650 (1660) | 319 (256) | 409 (1039) |
| median (IQR) | 166 (86–395) | 283 (163–301) | 289 (125–362) | 172 (85–461) |
| Arterial blood gas | ||||
| pO2 partial pressure, mmHg, mean (SD) | 69.7 (21.4) | 64.4 (27.0) | 59.2 (18.9) | 61.2 (21.0) |
| median (IQR) | 66.6 (58.2–76.3) | 58.7 (50.3–66.7) | 53.6 (44.2–67.2) | 58.0 (49.6–67.4) |
| pCO2 partial pressure, mmHg, mean (SD) | 33.9 (54.5) | 33.7 (7.4) | 33.7 (72.3) | 33.8 (68.1) |
| median (IQR) | 33.7 (30.8–36.3) | 32.6 (28.9–36.5) | 33.0 (30.6–37.0) | 33.0 (29.9–35.9) |
| Lactate mmol/L, mean (SD) | 1.2 (0.6) | 1.7 (1.1) | 1.4 (0.6) | 1.5 (0.9) |
| median (IQR) | 1.1 (0.8–1.4) | 1.4 (1.0–2.0) | 1.4 (1.0–1.7) | 1.3 (1.0–1.7) |
Sums do not add up to the total because of some missing values.
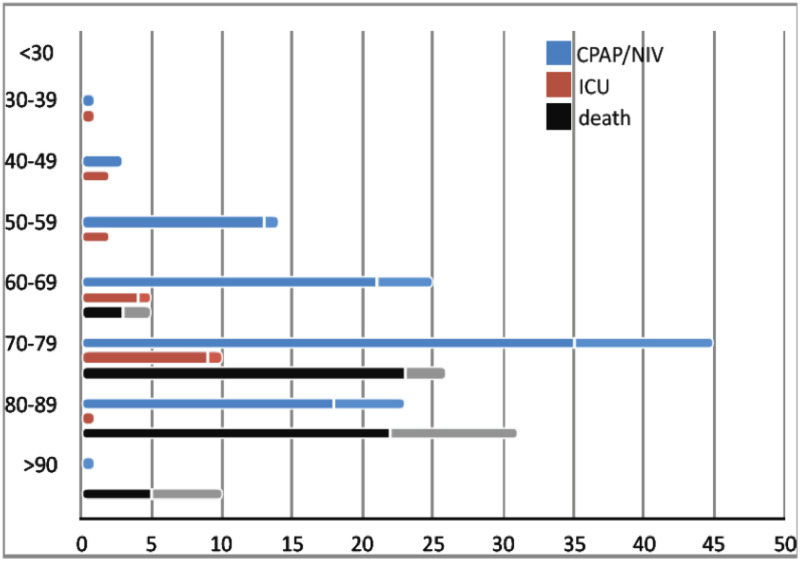
Six patients died within the first 72 hours and another 66 patients within two weeks after hospitalization, giving an overall fatality rate of 17.5%. No death occurred in patients aged below 60 years. In the older age groups, the number of deaths was 26 (88.5% males) in the decade 70–79 and 31 (71% males) in the decade 80–89 (Fig 1). Non-invasive respiratory support was needed in 112 (27.2%) patients, with 28 (6.8%) requiring ICU admission.
Fig 1. Age and sex-stratification of the outcomes (CPAP/NIV, ICU admission, and death) in SARS-CoV-2 patients.
Bars are numbers of patients. Females are represented in the upper softer bars.
Antiretroviral treatment with ritonavir plus lopinavir was started within the first 72 hours in 42.3% of patients, in combination with hydroxychloroquine sulfate in 63% of them.
Course, outcome and follow-up in nasopharyngeal swab negative patients
Among the 128 patients negative at nasopharyngeal swab, the median age was 67.7 years (range 1–98), with a preponderance of males (63.3%). Seven patients (78 to 97 years) died. Chest CT could be performed in 5 patients and it was positive in all. Of 128 patients, 82 (64%) were hospitalized and 25 (19.5%) were discharged to home in quarantine. None was moved to other regional hospitals.
Chest X-ray was performed in 59 (46%) of 128 patients and was reported as suggestive of interstitial pneumonia in 30 (50.8%) and negative in 29 (49.2%). CT scan was performed in 87 (68%) of 128 patients and showed interstitial pneumonia in 76 (87.3%), whereas was negative in 11 (12.7%). It showed pleural effusion in 6 (6.9%) patients, lymphadenopathy in 8 (9.2%), and pericardial effusion in 41 (47.1%). Of the 21 (16.4%) patients who underwent both the exams, 16 (76.2%) had concordant positive report, 3 (14.3%) had positive CT scan and negative X-Ray report, and 2 (9.5%) had concordant negative report.
Laboratory exams at admission showed white blood cell count below 10x109/L in 61% of patients, neutrophil count below 10x109/L in 67.4%, and lymphocyte count below 1x109/L in 32.2%. C-reactive protein, aspartate aminotransferase and lactate dehydrogenase values were mildly elevated in most patients, particularly those with worst clinical picture and outcome.
Two patients died within the first 72 hours and another 15 patients within two weeks after hospitalization, giving an overall fatality rate of 13.3%. Nine (53%) of the 17 patients who died had positive CT scan as compared to 25 (22.5%) in those hospitalized or discharged. Non-invasive respiratory support was needed in 22 (17.2%) patients, with 4 (3.1%) requiring ICU admission.
Antiretroviral treatment with ritonavir plus lopinavir was started in 27 (42.3%) patients, in combination with hydroxychloroquine sulfate in 82.1% of them.
Of 128 patients, 32 (25%) repeated nasopharyngeal swab (median time 8 days, range 1–26); 13 (40.6%) patients turned positive and 8 of them had either X-ray or CT positive. None was admitted to ICU and 3 died during hospitalization.
Diagnostic accuracy of nasopharyngeal swab
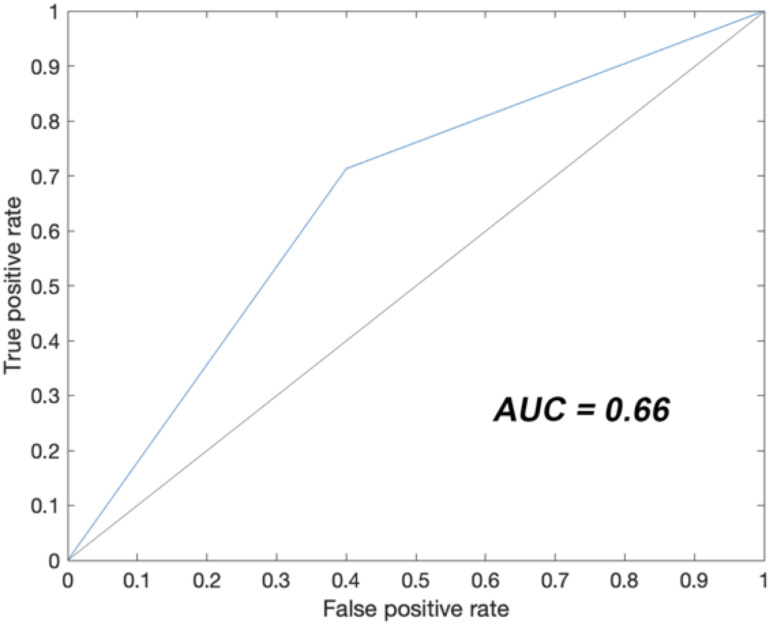
Because a number of patients had clinical and chest CT findings indicative of interstitial pneumonia, irrespective of the result of the nasopharyngeal swab, we calculated its diagnostic accuracy. To this aim, we used the report of chest CT as gold standard for the diagnosis of interstitial pneumonia and calculated the diagnostic accuracy of the first nasopharyngeal swab in entire study population of 539 patients. The sensitivity was 0.80 (CI 0.76–0.84) and the specificity was 0.45 (CI 0.25–0.65). A logistic regression was implemented to measure the predictive power of swabs outcome in CT scan classification. The associated ROC curve was calculated (Fig 2) and the AUC was 0.66.
Fig 2. Receiver operating characteristic curve analysis of the logistic regression that measured the predictive power of swabs outcome in CT scan classification.
AUC (area under the curve) estimates how a variable is far from the random predictive power (solid black line, AUC = 0.5).
Comorbidity pattern and risk profile for outcome in SARS-CoV-2 patients
Charlson comorbidity index (p<0.001) and c-reactive protein (p<0.001) were significantly associated with death (Table 4). Male gender (p<0.005), number of drugs intake (p<0.05), white blood cells count (p<0.005), c-reactive protein (p<0.05), positive CT scan (p<0.05) were significantly associated with the need of CPAP/NIV (Table 4), while only c-reactive protein (p<0.005) was associated with the need of IUC (Table 4).
Table 4. Estimated effects of selected predictors using multiple logistic regression model for three different outcomes (survival, requirement of continuous positive airway pressure (CPAP)/non-invasive ventilation (NIV), intensive care unit (ICU) admittance) in SARS-CoV-2 patients admitted to the Hospital of Crema between February 21st and March 13rd, 2020.
| Chances of Survival | Chances of CPAP/NIV requirement | Chances of ICU admittance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Intercept | 9.70 | (6.37–14.70) | <0.001*** | 0.32 | (0.24–0.42) | <0.001*** | 0.02 | (0.01–0.05) | <0.001*** |
| Sex | 1.11 | (0.78–1.56) | 0.57 | 0.68 | (0.51–0.90) | <0.005** | 0.59 | (0.28–1.23) | 0.17 |
| Hypertension | 0.97 | (0.68–1.39) | 0.89 | 1.11 | (0.83–1.47) | 0.48 | 1.54 | (0.89–2.67) | 0.14 |
| Charlson comorbidity index | 0.34 | (0.22–0.54) | <0.001*** | 1.24 | (0.91–1.67) | 0.19 | 0.58 | (0.29–1.15) | 0.13 |
| Number of drugs | 0.83 | (0.60–1.14) | 0.26 | 1.37 | (1.02–1.81) | <0.05* | 1.49 | (0.89–2.49) | 0.14 |
| White Blood Cells | 0.83 | (0.56–1.21) | 0.3372 | 1.57 | (1.16–2.13) | <0.005** | 1.22 | (0.85–1.75) | 0.31 |
| Creatinine | 0.88 | (0.65–1.19) | 0.40 | 0.88 | (0.68–1.16) | 0.39 | 1.24 | (0.77–1.98) | 0.40 |
| C-reactive protein | 0.57 | (0.41–0.78) | <0.001*** | 1.42 | (1.09–1.85) | <0.05* | 1.99 | (1.26–3.14) | <0.005** |
| Alanine aminotransferase | 1.10 | (0.78–1.54) | 0.60 | 1.17 | (0.90–1.53) | 0.28 | 1.01 | (0.66–1.53) | 0.95 |
| Aspartate aminotransferase | 0.87 | (0.64–1.21) | 0.43 | 1.19 | (0.90–1.55) | 0.21 | 1.37 | (0.95–1.96) | 0.10 |
| X-Ray | 0.77 | (0.57–1.04) | 0.10 | 1.20 | (0.92–1.56) | 0.19 | 1.32 | (0.84–2.09) | 0.25 |
| CT scan | 0.76 | (0.49–1.18) | 0.24 | 1.50 | (1.07–2.12) | <0.05* | 1.58 | (0.71–3.49) | 0.27 |
| ACE inhibitors | 1.09 | (0.79–1.51) | 0.58 | 1.12 | (0.88–1.44) | 0.39 | 0.93 | (0.57–1.53) | 0.78 |
| Sartans | 1.04 | (0.75–1.44) | 0.80 | 1.07 | (0.82–1.40) | 0.61 | 0.88 | (0.55–1.41) | 0.61 |
We reported Odds ratio values (OR), 95% Confidence Interval (CI) and p-values (* p<0.05, ** p<0.005, *** p<0.001).
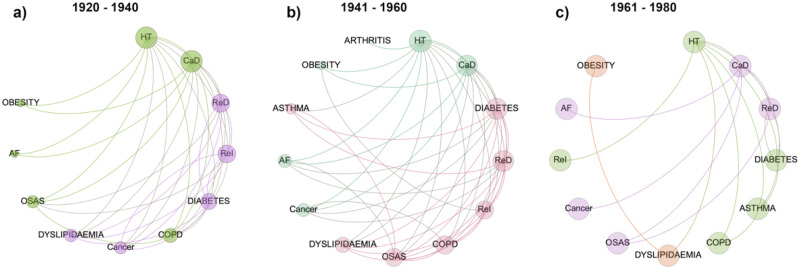
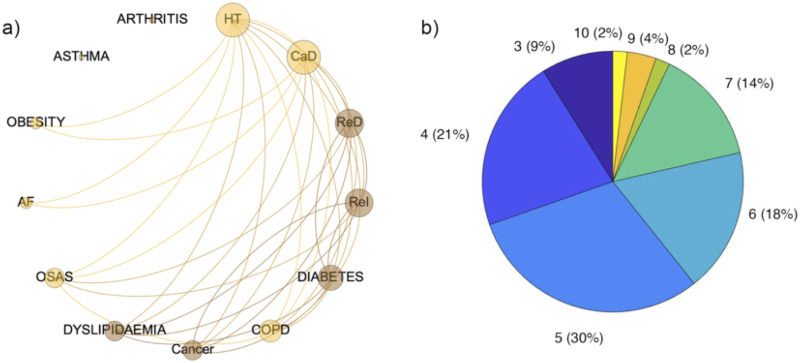
Comorbidity network analysis was performed on the reduced set cohort of SARS-CoV-2 patients born between 1920 and 1980, and suffering for at least one of the diseases included in the analysis. Subsequently, the reduced sample was divided in three age groups: 1920–1940, 1941–1960 and 1961–1980. The comorbidity networks obtained from those groups (Fig 3a–3c) showed different topological properties. The community detection algorithm was able to identify two modules for the age groups 1920–1940 and 1941–1960, whereas three communities characterized the age group 1961–1980. It means that in the older ranges of age there are two different groups of diseases (violet and green in Fig 3a and pink and light green in Fig 3b) and the number of patients who share two diseases is more similar between two nodes of the same community than between two nodes of different communities. In both the age ranges the main two nodes of the two communities are (hypertension, cardiac disease) and (diabetes and respiratory disease) respectively. The pairs of communities are essentially the same in the two networks apart COPD and OSAS that in the 1920–1940 age range network are associated with the (hypertension, cardiac disease) community while in the 1941–1960 age range network belong to the (diabetes and respiratory disease) community and cancer that in the 1920–1940 age range network is associated with the (hypertension, cardiac disease), while in the 1941–1960 age range network belongs to the (diabetes and respiratory disease) community. Arthritis and asthma appear only in the 1941–1960 age network, associated with the (hypertension, cardiac disease) and the (diabetes and respiratory disease) communities respectively. Conversely, the three communities configuration found in the 1961–1980 age group is associated with a less homogeneous distribution of the number of patients who share diseases across the whole network. In fact, we found one community composed of obesity and dyslipidemia, one community was composed of hypertension diabetes, asthma COPD and renal insufficiency, while the last community was composed of CaD, ReD, OSAS, cancer and AF. The different weighted degree (i.e. the node size in Fig 3) highlights a bimodal disease distribution among patients of the two older age ranges. Specifically, hypertension, CaD and ReD seem to be more largely present among patients, along with diabetes and chronic ReI in the 1920–1940 group, diabetes, OSAS and COPD in the 1941–1960 group. In the 1961–1980 networks edge weights seem to be similar among diseases. Finally, the same set of patients included in the analysis was divided in two groups according to the outcome deceased or survived. Notably, two modules characterized both the deceased and the survived network. The weighted degree distribution showed that in one module deceased patients shared mainly hypertension, CaD, COPD and OSAS, while in the other module ReD, ReI, diabetes dyslipidemia and cancer (Fig 4a). At the same time, survived patients show one module mainly composed of hypertension and CaD, while in the other one they shared mainly diabetes, ReD and COPD (Fig 5a). Figs 4b and 5b show the Charlson comorbidity index distribution in the deceased and survived groups respectively, highlighting the incidence of the diseases across subjects. Fig 6 shows the test of the statistically significant difference between normalized Laplacian of survived and deceased networks. The randomization approach allowed the rejection of the null hypothesis (the two graphs have the same structure) with p < 0.007.
Fig 3. Comorbidity networks.
A cohort of 270 Covid-19 patients was divided in three classes according to the year of birth: a) 1920–1940, b) 1941–1960, c) 1961–1980. For each group a comorbidity network was derived according to the constraint that at least 10% of patients must share two diseases. Nodes are ordered clockwise according to their degree (number of connection each disease has with other nodes), while node size is associated with the number of patients sharing the disease. The color of each nodes refers to the community a disease belongs to.
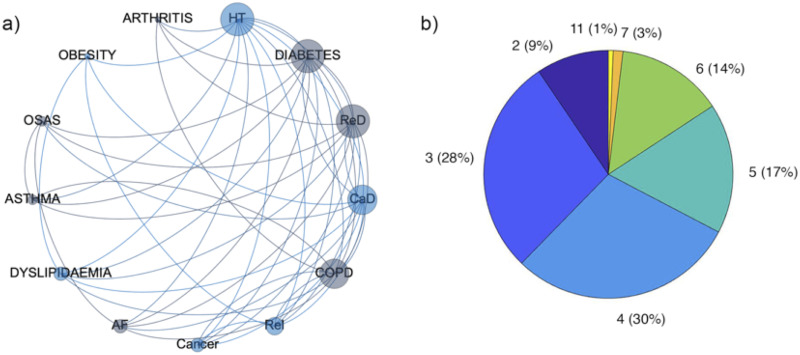
Fig 4. Comorbidity network of deceased patients.
a) The comorbidity network was obtained according to patients outcome (deceased/survived). It was derived according to the constraint that at least 10% of patients must share two diseases. Nodes are ordered clockwise according to their degree (number of connection each disease has with other nodes), while node size is associated with the number of patients sharing the disease. The color of each nodes refers to the community a disease belongs to; b) Pie chart of the Charlson Comorbidity Index calculated for each diseased patient. The frequency of patients associated with a specific value of the index is reported within the brackets.
Fig 5. Comorbidity network of survived patients.
a) The comorbidity network was obtained according to patients outcome (deceased/survived). It was derived according to the constraint that at least 10% of patients must share two diseases. Nodes are ordered clockwise according to their degree (number of connection each disease has with other nodes), while node size is associated with the number of patients sharing the disease. The color of each nodes refers to the community a disease belongs to; b) Pie chart of the Charlson Comorbidity Index calculated for each survived patient. The frequency of patients associated with a specific value of the index is reported within the brackets.
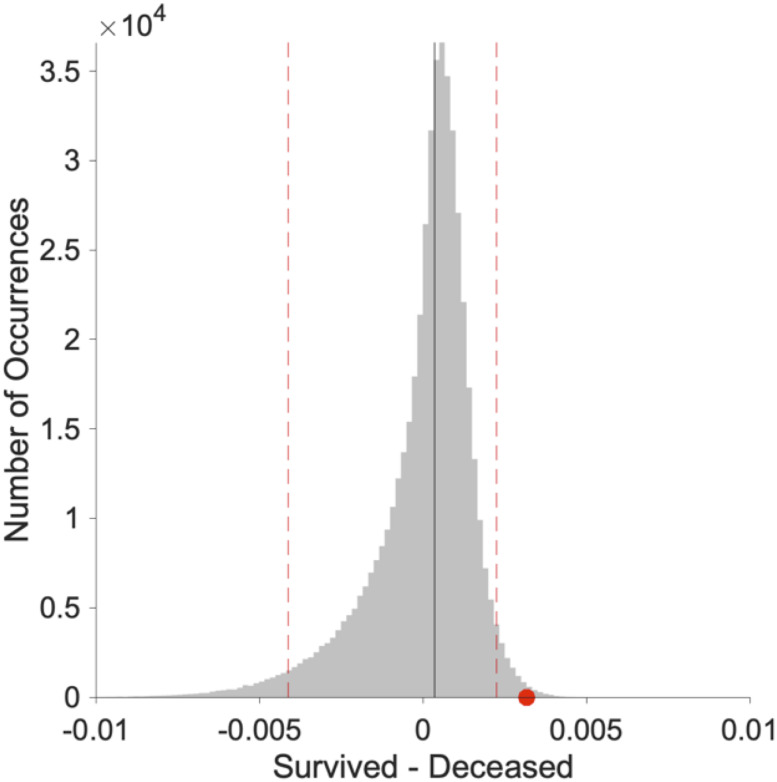
Fig 6. Statistically significant difference between normalized Laplacian of deceased and survived networks.
The difference resulted significant with p<0.007. The grey distribution is the result of the difference between 500,000 randomization of survived and deceased normalized Laplacian matrices. The red dot shows the difference between the original survived and deceased normalized Laplacian matrices. The dotted vertical lines represent the 0.05 tails of the distribution. Original image created with the software Gelphi 0.9.1 https://gephi.org/.
Randomization of a Laplacian matrix
Given a graph G(E,V), with M edges and N vertices, described by the NxN adjacency matrix A, it is always possible to obtain its Laplacian as L = D–A, where D is a matrix with the degree of the nodes on the diagonal and zeros elsewhere. L is, by construction, a semidefinite positive matrix, that is a matrix whose eigen-spectrum is composed of non-negative terms. In order to limit the eigen-spectrum in the interval [0,2], we calculated the normalized Laplacian matrix as LN = D-1/2(D-A)D-1/2. We randomized the normalized Laplacian matrix by using properties of orthogonal matrices. Given a square matrix Q, it is orthogonal if its columns and rows are orthogonal unit vectors [16]. One way to express this is QTQ = I, where QT is the transpose of Q and I is the identity matrix. It can be showed that if P is a matrix with non-negative values on the diagonal and zeros elsewhere, the matrix P’ = QPQT is symmetric and semidefinite positive, with eigenvalues given by the diagonal elements of P [16]. Accordingly, we created N = 500000 random orthogonal matrices and calculated N replicas of survived and deceased normalized Laplacian matrices. The null-distribution was obtained as the average difference of each pair of replicas (survived and deceased), and the average difference between the empirical survived and deceased normalized Laplacian matrices was tested against it.
Discussion and conclusions
We reported a systematic analysis on hospitalized SARS-CoV-2 patients soon after the outbreak in Italy. The strength of our study was the availability of homogenous data from all consecutive patients admitted to a public hospital and suspected to harbor SARS-CoV-2 infection. Therefore, we could investigate the course of any patients presenting with symptoms indicative of possible interstitial pneumonia, including those who resulted negative at nasopharyngeal swab. One limitation is the report of the events occurring soon after the outbreak, during a period in which many information on the disease course were not yet available, and with a hospital-based perspective.
We examined 411 patients with laboratory-confirmed SARS-CoV-2 at nasopharyngeal swab and compared their clinical features and course to that of 128 swab negative patients. Overall, the percentage of SARS-CoV-2 patients that arrived at hospital with an ambulance and required non-invasive respiratory support or ICU admittance was as twice as that of swab negative patients. Nevertheless, the fatality rate was only about 4% higher in SARS-CoV-2 patients. At the same time, chest CT was reported as positive in nearly 60% of swab negative patients, as previously reported [17], and mainly among those who died. These findings indicate that a number of swab negative patients were admitted to the hospital because of an interstitial pneumonia, which could be attributed to SARS-CoV-2 in 13 of the 32 patients in whom it was possible to perform the follow-up nasopharyngeal swab that resulted positive. It has been reported [18, 19] that RT-PCR on nasopharyngeal swab could be negative in patients eventually receiving the diagnosis. Since nasopharyngeal swab is currently used as screening tools in the population, its sensitivity value, namely the percentage of true positive, is important. We sought to address this issue by using chest CT as the gold standard for the diagnosis of interstitial pneumonia on the entire study population of 539 patients. In our cohort, we estimated a sensitivity of 80%. This finding was similar to what found in other studies [20].
In our cohort, SARS-CoV-2 predominantly affected males. Our rate of 66.6% males confirmed those reported in China ranging between 54.3% and 73% [1–3, 21–24]. No biological explanation for this unexpected gender distribution is currently available. Fatality rate related to the whole population of SARS-CoV-2 subjects is unreliable and of limited usefulness in the pandemic, while urgent strategies are needed to improve patients’ care and healthcare system capability. Indeed, the number of SARS-CoV-2 subjects experiencing mild, or non-respiratory symptoms (e.g. gastrointestinal), plus those asymptomatic are unknown because of the variability in testing policies. Since hospitalized patients carry the highest risk of dismal outcome, the inherent fatality rate should be used also to compare findings across countries. Our hospital-based fatality rate in SARS-CoV-2 patients was 17.5%. Previous Chinese studies on hospitalized patients reported rates of 28% in 191 patients [22] and 21.8% in 201 patients [24]. Three studies including 99 [1], 41 [2] and 137 [25] patients reported lower rates ranging between 11% [1, 25] and 14.6% [2]. Another two studies reported much lower rates of 1.4% and 4.3% [3]. We did not record any death below 60 years. Mortality increased across the decades and it was 6.6% in 60–69 years old, 21.1% in 70–79 years old, 38.8% in 80–89 years old and 83.3% above 90 years. Nearly 74% of the deaths occurred in males, which may be not surprising based on the predominantly male distribution of the diseases. Nevertheless, the fatality rate among males exceeded by 10% that of the whole population of SARS-CoV-2 patients, suggesting that the disease could more severely affect males. A similar difference between deaths and infected was reported in one [22] out of three previous studies [23, 24]. Nonetheless, our results show that the best proxies to predict adverse outcomes resulted to be the Charlson comorbidity index and amount of c-reactive protein, suggesting that the fatality rate strongly depend on the co-occurrence of several pathologies together with a high level of inflammation, especially due to pulmonary infection diseases [26, 27].
Non-invasive respiratory support was needed in 27% of SARS-CoV-2 patients and ICU admission rate was 6.8%. Previous Chinese studies [1–3, 25] reported ICU admission rates between 4.3% and 31.7%, with fatality rate between 11% and 14.6%. A recent Italian study focused on ICU patients [28] reported overall fatality rate of 26%. This comparison may suggest that a larger availability of ICU beds might be associated with lower fatality rates. To investigate this hypothesis, we analyzed the distribution of the fatality rates in the decades 60–69 (76 patients) and 70–79 (123 patients) (Fig 1). The proportion of patients in CPAP/NIV and ICU (32.9% and 6.5% in 60–69, respectively and 36.5% and 8.1% in 70–79, respectively) did not differ. Nevertheless, the fatality rate was 30% higher in the 70–79 (47%; 21/45; p = 0.02) than 60–69 decade (16%; 4/25). Among patients admitted to ICU, the fatality rate was 40% (4/10) in the 70–79 years old, the same finding reported in the Italian larger series [28].
Tentative explanation of this difference could be the generic higher risk related to aging, because patients in the two groups of age seemed to not significantly differ in terms of distribution of comorbidities and use of more than 4 drug (10% and 5% higher in 70–79 years old, respectively). Thus, even though it might be possible that higher ICU admission rate could have reduced the mortality in the older age group, leading to a lower overall fatality rate, older age per se might also play a major role on dismal outcome. Our statistical results show also that if the best proxy to predict ICU admittance is the amount of c-reactive protein as already showed by Sharifpour et al. [29], CPAP/NIV requirement is positively associated mainly with male gender and white blood cells counts, as reported also by Krause at al. [30].
Besides this descriptive and multivariate statistical findings, particularly important results have been obtained through the investigation of comorbidity patterns showed by patients stratified according to the age and to the final outcome. All previous studies [1–3, 21–25, 31, 32] reported high frequency of comorbidities mainly among patients with poorer outcome, suggesting a role in enhancing SARS-CoV-2 morbidity [33]. To better correlate relationship and frequency of comorbidities to age and outcome, we adopted a network analysis approach. Our findings showed that the composition of the two communities found in in deceased and survived patients is crucial to discriminate the potential adverse outcome. In fact, while in both the networks the main nodes of a community are (HT, CaD, COPD) in deceased patients and (HT,CaD,ReI) in survived patients, in the other community we found (Red, ReI, diabetetes) for deceased patients and (ReD,COPD,diabetes) for those who survived. As a consequence, the major co-occurrence of respiratory disease and renal insufficiency seems to be a boost for an adverse outcome, more than the HT and CaD co-occurrence. Moreover, an inhomogeneous distribution of the overall diseases co-occurrence was found in the age groups between 40 and 60 years, suggesting that a more diversified comorbidity can unlikely lead to a potential adverse outcome. Even though indirectly and in a relatively small sample size, these findings seem suggesting that SARS-CoV-2 morbidity is enhanced, and possibly even triggered by pre-existing multi-organ impairment. These findings should be confirmed in a larger and multicenter study.
Finally, a major concern was raised on the use ACE inhibitors and sartans because SARS-CoV-2 uses the ACE2 (angiotensin-converting enzyme 2) protein as the receptor to entry the cells [34]. Our results confirmed that these classes of drug did not have any influence on patients’ outcome [35, 36].
Acknowledgments
We are deeply indebted with all the nurses of the Hospital of Crema, whose passion, skills and fortitude in taking care of patients was a bastion to limit the wave of suffering that in a few weeks have overwhelmed an entire community. We are particularly grateful to Dr. Maria Lidia Sinatra, Health Management Unit and Dr. Anna Maria Bona, Nurse Management Unit. We also indebted with the staff of all the departments, for the support provided to the study.
We also wish to acknowledge our collaborators at the RADIOLOGY UNIT (Drs. Acqualagna Renato, Astorri Silvia, Bonardi Roberto, Borghetti Maurizio, Conti Maria Paola, Garavaglia Marco, Gandolfi Silvia, Maldotti Marco, Limonta Simone, Parziale Maurizio Giuseppe, Spinazzola Angelo, Padrenostro Mauro); GASTROENTEROLOGY UNIT (Drs. Alicante Saverio, Brambilla Gianfranco, Iiritano Elena, Londoni Claudio, Manfredi Guido, Menozzi Fernanda, Romeo Samanta, Pedaci Marianna EMERGENCY MEDICINE UNIT Accordino Silvia, Cacco Silvia, De Matthaeis Anna, Formagnana Pietro, La Boria Elisa, Masotti Michela, Provini Stella, Seresini Monica, Severgnini Silvia, Taverni Silvana, Valsecchi Cesare, Vezza Carlotta); PNEUMOLOGY UNIT 1 (Drs. Luca Bilucaglia, Michele Ceruti, Alessia Edallo, Fabrizio Mauri, Mara Parati, Emanuele Rana, Simona Scorsetti, Massimiliano Villani); PNEUMOLOGY UNIT 2 (Drs. Besozzi Alessandra, Chiesa Lodovico, Giunti Valeria); INTENSIVE CARE UNIT (Drs. Bianchi Fulgenzia, Biazzi Antonella, Bisicchia Maria Cristina, Bonfanti Giuseppina, Caruso Andrea, Cerisara Marco, Chiarenza Federica, Foglio Elena, Francavilla Nicola, Gigliotti Alberto, Meglio Giuseppina, Perotti Vittorio, Adriani Luca, Bergamaschini Giulio, Borromeo Raffaella, Comassi Paolo, Corallo Sandro, Lupi Giuseppe, Troiano Carmine, Villani Pier Giorgio); EMERGENCY UNIT (Drs. Guerra Claudia, Piantelli Miriam, Susca Micaela, Galmozzi Attilio, Zuccon William, Sportelli Silvia, Corti Serena, Depetri Federica, Ruini Alain, Kostihova Anna, Dolci Francesco, Bertin Elena).
Data Availability
Together with Prof. Lauria we decided to put on line the data that can be found here https://www.openicpsr.org/openicpsr/project/130561/version/V1/view or this doi version https://doi.org/10.3886/E130561V1.
Funding Statement
The Horizon 2020 Framework Programme, 952026 Humane-AI-Net, provided funding to GC. TG has been funded by the IMT project PAI (Progetto di Attività Integrata) 2018 “Chain the Brain: The Endophenotype of Serotonin Dysfunction in Violent Offenders and Psychopaths. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA—J Am Med Assoc. 2020. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COvid-19 RIsk and Treatments (CORIST) Collaboration. RAAS inhibitors are not associated with mortality in COVID-19 patients: Findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vasc Pharmacol. 2020;135:106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Castelnuovo A, COvid-19 RIsk and Treatments (CORIST) Collaboration. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30(11):1899–1913. 10.1016/j.numecd.2020.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COvid-19 RIsk and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur J Intern Med. 2020;82:38–47. 10.1016/j.ejim.2020.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldarelli G. Scale-Free Networks: Complex Webs in Nature and Technology.; 2010. 10.1093/acprof:oso/9780199211517.001.0001 [DOI] [Google Scholar]
- 8.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: A network-based approach to human disease. Nat Rev Genet. 2011. 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10.Asratian AS, Denley TMJ, Häggkvist R. Bipartite Graphs and Their Applications.; 1998. 10.1017/cbo9780511984068 [DOI] [Google Scholar]
- 11.Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech Theory Exp. 2008. 10.1088/1742-5468/2008/10/P10008 [DOI] [Google Scholar]
- 12.Chung F, Lu L, Vu V. Spectra of random graphs with given expected degrees. Proc Natl Acad Sci U S A. 2003. 10.1073/pnas.0937490100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006. 10.1016/j.patrec.2005.10.010 [DOI] [Google Scholar]
- 14.Tolles J, Meurer WJ. Logistic regression: Relating patient characteristics to outcomes. JAMA—J Am Med Assoc. 2016. 10.1001/jama.2016.7653 [DOI] [PubMed] [Google Scholar]
- 15.McDonald JH. Handbook of Biological Statistics, 3th Edition.; 2014. [Google Scholar]
- 16.Lang S. Algebra (Revised Third Edition).; 2002.
- 17.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol. 2020. 10.1128/JCM.00297-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020. 10.1002/jmv.25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamal AJ, Mozafarihashjin M, Coomes E, et al. Sensitivity of Nasopharyngeal Swabs and Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2020. 10.1017/ice.2020.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. 10.1056/nejmoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Chen X, Cai Y, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warusevitane A, Karunatilake D, Sim J, Smith C, Roffe C. Early diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C-Reactive protein. PLoS One. 2016. 10.1371/journal.pone.0150269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto H, Kasai T, Sato A, et al. Association between C-reactive protein levels at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessels. 2019. 10.1007/s00380-019-01435-9 [DOI] [PubMed] [Google Scholar]
- 28.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA—J Am Med Assoc. 2020. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifpour M, Rangaraju S, Liu M, et al. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One. 2020. 10.1371/journal.pone.0242400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause M, Douin DJ, Kim KK, Fernandez-Bustamante A, Bartels K. Characteristics and Outcomes of Mechanically Ventilated COVID-19 Patients—An Observational Cohort Study. J Intensive Care Med. 2020. 10.1177/0885066620954806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1,590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020. 10.1016/S0140-6736(20)30558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danser AHJ, Epstein M, Batlle D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension. 2020. 10.1161/HYPERTENSIONAHA.120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flacco ME, Acuti Martellucci C, Bravi F, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: A meta-analysis. Heart. 2020. 10.1136/heartjnl-2020-317336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Together with Prof. Lauria we decided to put on line the data that can be found here https://www.openicpsr.org/openicpsr/project/130561/version/V1/view or this doi version https://doi.org/10.3886/E130561V1.