The rapid implementation of SARS-CoV-2 vaccination is now a global health-care priority. Successful phase 3 trial outcomes have been reported for numerous vaccines that induce robust humoral and cellular immune responses against the SARS-CoV-2 spike protein.1, 2, 3, 4, 5, 6 To gain rapid control of accelerating cases and maximise public health impact, the UK Government has adopted the strategy of delaying second vaccination to 12 weeks. This policy has generated controversy, particularly among health-care workers (HCWs), the majority of whom have received BNT162b2 mRNA vaccine.7
Limited data on immune responses to single-dose vaccination with BNT162b2 are available, and vaccine responses following previous natural infection have not been assessed in clinical trials.2, 3, 4, 5, 6 We have therefore investigated immunological responses to single-dose BNT162b2 using a combination of serology, live virus neutralisation, and T-cell enzyme-linked immunospot (ELISpot) assays.
72 HCWs from Imperial College Healthcare NHS Trust, who were vaccinated between Dec 23 and Dec 31, 2020, provided blood samples at the time of receiving their first dose of BNT162b2 vaccine and 21-25 days after vaccination. Baseline samples were tested for antibodies to SARS-CoV-2 nucleocapsid and spike (anti-S) proteins using the Abbott Architect SARS-CoV-2 IgG and IgG Quant II, respectively (Abbott, Maidenhead, UK). 21 (29%) participants had evidence of previous SARS-CoV-2 infection: 16 with positive baseline serology, and five further with strong T-cell responses to non-spike antigens post-vaccination (>100 spot forming units [SFU] per 106 peripheral blood mononuclear cells [PBMC]). Although baseline ELISpot data were not available for these five participants, a cohort of 30 unvaccinated, infection-naive participants did not demonstrate reactivity to these peptide pools. 51 participants had negative baseline serology and cellular responses post-vaccine limited to spike antigens; this group was defined as infection-naive.
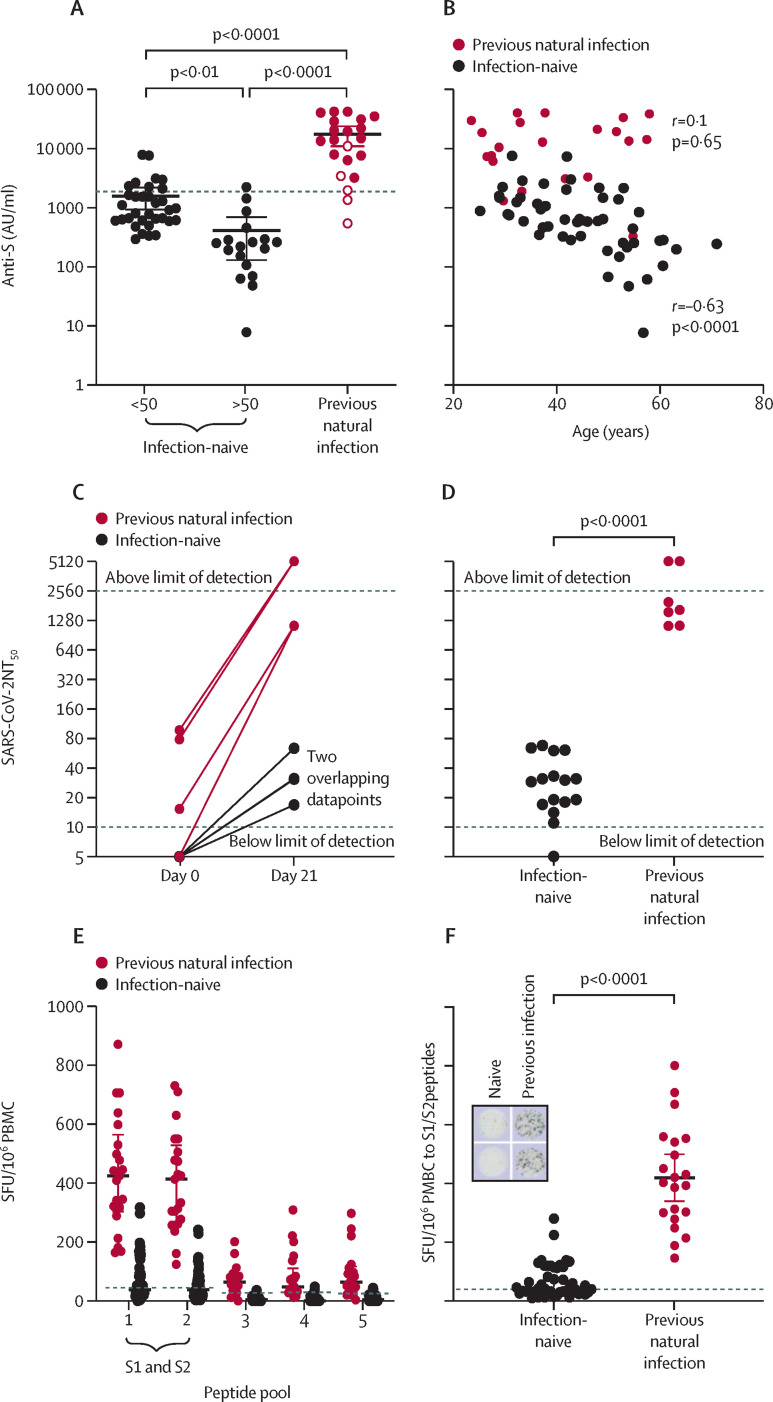
As BNT162b2 mRNA encodes the spike glycoprotein of SARS-CoV-2, we assessed immune responses to spike protein post-vaccination. Anti-S titres were significantly higher in individuals with previous natural infection than in infection-naive individuals (median 16353 arbitrary units [AU] per mL [IQR 4741–28 581] vs 615·1 AU/mL (286·4–1491), p<0·0001; figure A ). The five participants with previous natural infection yet negative serology at baseline developed post-vaccination anti-S titres that were intermediate between the infection-naive and previously infected groups (figure A). Infection-naive individuals showed an inverse correlation between post-vaccination anti-S titre and age (figure B), with individuals older than 50 years generating a significantly weaker serological response than those younger than 50 years (median 230·1 AU/mL vs 888·9 AU/mL, p<0·0001; figure A). This correlation was not seen in the group with previous natural infection (figure B).
Figure.
Immunological responses to a single dose of BNT162b2 mRNA vaccine
(A) Anti-S antibody titres 21–25 days after vaccination in individuals who were infection-naïve or had evidence of previous natural infection. Datapoints with open circles represent five individuals who, despite a negative serological test at baseline, were identified as having previous infection due to reactivity to non-spike peptides on ELISpot testing post-vaccination (which could not have been induced by vaccine alone). Dotted line indicates median anti-spike titre in a cohort of health-care workers 2–8 weeks after PCR-confirmed natural infection with SARS-CoV-2 (n=23, IQR 463–3621). (B) Correlation of post-vaccination anti-spike titre with age in infection-naïve participants. (C) SARS-CoV-2 live virus neutralising antibody titres in the eight individuals with paired results available (n=4 infection-naïve, n=4 with previous natural infection. (D) SARS-CoV-2 live virus neutralising antibody titres post-vaccination in infection-naïve individuals and individuals with previous infection. (E) T-cell responses to SARS-CoV-2 peptide pools post-vaccination in infection-naïve individuals and individuals with previous infection. Peptide pool 1 and peptide pool 2 contain spike protein peptides S1 and S2. Dotted lines indicate mean plus 3 standard deviation for each peptide pool calculated from infection naïve, unvaccinated individuals (48, 43, 26, 33, and 26 SFU/106 PBMC for peptide pools 1–5 respectively). (F) T-cell responses to spike protein peptides of SARS-CoV-2 post-vaccination in infection-naïve and previously infected participants. Inset shows example of ELISpot for an infection-naïve and a previously infected individual for the 2 spike peptide wells. Dotted line indicates mean plus 3 standard deviation for spike peptide pool reactivity calculated from infection naïve, unvaccinated individuals. All data are median with IQR. Statistical analysis was by Kruskall-Wallis test with Dunns' post-hoc correction (A), Spearman rank correlation (B) and Mann-Whitney test (D, F). SFU=spot forming unit. PBMC=peripheral blood mononuclear cells. NT50= neutralisation titres that achieved 50% neutralisation.
Anti-S titre is reported to correlate with in-vitro virus neutralisation. We therefore used a subset of samples for live SARS-CoV-2 virus (SARS-CoV-2/England/IC19/2020) neutralisation assays on Vero cells.8 Eight paired sera (n=4 infection-naive, n=4 previous natural infection) and a further 15 post-vaccination samples were included (n=12 infection-naive, n=3 previous natural infection). In individuals with previous exposure, vaccine induced very strong neutralising antibody titres even in those without detectable or very low virus neutralisation titres (NT) at baseline (median NT that achieved 50% neutralisation [NT50] 1/1635, range 1/1123·1 to beyond the 1/2560 upper limit; figure C, D). In infection naive individuals, vaccination induced detectable neutralising antibodies in 15 of 16 sera, but titres were all lower than for previously infected individuals (median NT50 1/29·50, range from below lower limit of detection to 1/68; figure C, D).
We next assessed post-vaccination T-cell responses using the T-SPOT Discovery SARS-CoV-2 (Oxford Immunotec, Oxford, UK), which includes a panel of five SARS-CoV-2 peptide pools. Post-vaccination, participants with evidence of previous SARS-CoV-2 infection at baseline (n=21) mounted very strong T-cell responses to spike peptides (median 400 SFU/106 PBMC [IQR 287–544]; figure E, F). In the infection-naive group, post-vaccination T-cell responses to spike peptides were significantly weaker than in individuals with previous infection (38 SFU/106 PBMC (IQR26–110), p<0·0001; figure E, F), and 24 (50%) of 48 participants generated T-cell responses that could be considered negative (<40 SFU/106 PBMC). Unlike humoral responses, there was no correlation between age and degree of T-cell response.
In summary, we show that individuals with previous SARS-CoV-2 infection generate strong humoral and cellular responses to one dose of BNT162b2 vaccine, with evidence of high titres of in-vitro live virus neutralisation. In contrast, most individuals who are infection-naive generate both weak T-cell responses and low titres of neutralising antibodies.
Existing studies predicting risk of re-infection based on neutralising antibody titres, or longevity of immunological responses, are highly heterogeneous.9, 10, 11, 12, 13 Evidence for the longevity and protective effect of T-cell responses is particularly limited. In particular, peptide pool selection might affect T-cell responses, meaning results cannot be compared between studies. We use S1 and S2 peptide pools, rather than peptides spanning the whole spike glycoprotein, which might underestimate the true magnitude of T-cell responses. Despite the difficulty of extrapolating immunological data to clinical protection, our findings raise important issues that warrant consideration when determining optimal use of vaccine supplies. Firstly, those with serological evidence of previous disease at baseline mount robust antibody and T-cell responses after a single dose of vaccine. Conversely, some infection-naive individuals mount very little demonstrable response to single-dose vaccination, which might not provide sufficient immunity to protect from clinical disease or viral shedding, and might not persist for a 12-week delay until second vaccine is administered. One infection-naive individual included in our study developed symptomatic, PCR-proven infection 5 weeks after one dose of vaccine; notably, their anti-S titre post-vaccination was 61·8 AU/mL.
In keeping with published reports of other vaccines, serological response to BNT162b2 inversely correlates with age.14 We found median anti-S titres post-vaccination in the infection-cohort to be lower than those seen 2–8 weeks after natural infection alone, and this difference was particularly striking in those older than 50 years. Two participants did not seroconvert, and eight participants generated antibody titres less than 250 AU/mL, which might not be sufficient for any virus neutralisation based on correlation of virus neutralisation and anti-S titre in our study. All ten of these individuals were older than 50 years. In a setting where prioritisation of groups of HCWs for second vaccination might be necessary, consideration must be given to protocolised vaccination of infection-naive individuals or those over the age of 50 (who are at increased risk of both severe COVID-19 and minimal vaccine response). These results also highlight the need for continuing rigorous use of personal protective equipment after vaccination to prevent both infection and asymptomatic spread of disease.
Acknowledgments
MP, CC, PK, and MW contributed equally. PK and MW have received support to use the T-SPOT Discovery SARS-CoV-2 from Oxford Immunotec. X-NX and PK are supported by the Department for International Development and the Wellcome Trust. MP is supported by the National Institute for Health Research (NIHR). All other authors declare no competing interests. We thank the HCWs who participated in this study. We also thank Graham Pickard and Donald Mokreri for help with the spike protein antibody assay. We acknowledge support from the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Eng J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New Eng J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Med. 2020;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Eng J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020 doi: 10.1101/2020.12.09.20245175. published online Dec 11. (preprint). [DOI] [Google Scholar]
- 7.Mahase E. Covid-19: medical community split over vaccine interval policy as WHO recommends six weeks. BMJ. 2021;372:n226. doi: 10.1136/bmj.n226. [DOI] [PubMed] [Google Scholar]
- 8.McKay PF, Hu K, Blakney AK, et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nature Comm. 2020;11 doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng W, Bao L, Liu J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyllie D, Mulchandani R, Jones HE, et al. SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: a prospective cohort study in keyworkers. medRxiv. 2020 doi: 10.1101/2020.11.02.20222778. published online Nov 4. (preprint). [DOI] [Google Scholar]
- 13.Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084–e00118. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]