Abstract
With the emergence of new pathogens like coronavirus disease 2019 and the prevalence of cancer as one of the leading causes of mortality globally, the effort to develop appropriate materials to address these challenges is a critical research area. Researchers around the world are investigating new types of materials and biological systems to fight against various diseases that affect humans and animals. Carbon nanostructures with their properties of straightforward functionalization, capability for drug loading, biocompatibility, and antiviral properties have become a major focus of biomedical researchers. However, reducing toxicity, enhancing biocompatibility, improving dispersibility, and enhancing water solubility have been challenging for carbon-based biomedical systems. The goal of this article is to provide a review on the latest progress involving the use of carbon nanostructures, namely fullerenes, graphene, and carbon nanotubes, for drug delivery, cancer therapy, and antiviral applications.
Keywords: Graphene, Carbon nanotubes, Drug delivery, Cancer therapy, Antiviral activity
Graphical abstract
Introduction
Selecting the appropriate materials for drug delivery, cancer therapy, and antiviral applications has been a challenge for biomedical researchers. Carbon nanomaterials have appealing properties for many biomedical applications because of the diversity in their structures, large ratios of surface area to volume, facile functionalization, unusual optical properties, and biocompatibility [1]. The diversity of nanoscale carbon structures is impressive. In nanoscale dimensions, carbon nanotubes (CNTs), graphene, fullerenes, carbon dots (CDs), graphene quantum dots (GQDs), carbon fibers (CFs), nanodiamonds, carbon nanoonions, and amorphous carbon nanostructures represent alternative structures and allotropes of only one element, carbon [2,3]. The classification of carbon nanomaterials is typically determined by the number of dimensions exceeding nanoscale (100 nm) [4]. Accordingly, CDs, GQDs, and fullerenes are zero-dimensional nanomaterials. CNTs and CFs are classified as one-dimensional nanomaterials, and layered structures like graphene are classified as two-dimensional nanomaterials. Finally, nanodiamonds are classified as three-dimensional carbon nanostructures.
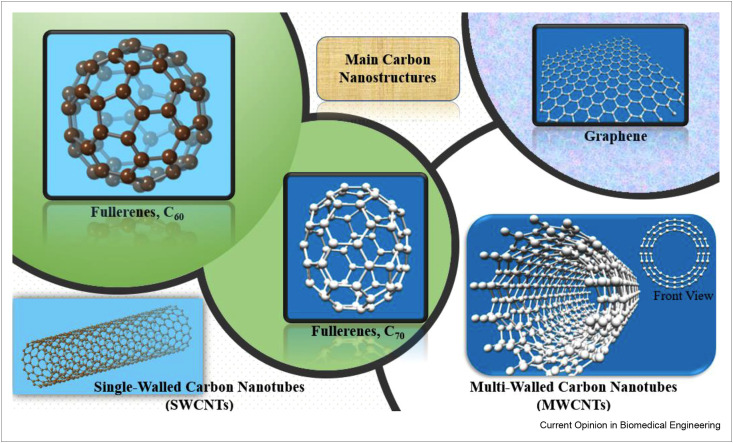
Fullerenes were first discovered in 1985; the inventors of this new carbon allotrope won the 1996 Nobel Prize in Chemistry [5]. This achievement is considered as revolutionary in the synthesis of carbon allotropes [2]. Fullerenes are sp2-carbon-atom cages of different sizes with single or double bonds (Figure 1 ) [6]. Fullerenes enjoy the highest symmetry among the various carbon nanostructures and thus exhibit a high structural and chemical stability. Fullerene surfaces can be decorated with various functional groups for targeted delivery in drug delivery, diagnosis, imaging, and biosensing applications [1,6]. The photoelectrochemical properties of fullerenes, particularly C60, make them suitable for photodynamic therapy [7]. Fullerenes are hydrophobic; the low solubility of fullerenes in polar solvents such as water is an obstacle to their use in biomedical applications, and numerous methods, including synthesizing fullerene derivatives, have been used to overcome this obstacle [6,7].
Figure 1.
The schematic of main carbon nanostructures: fullerenes (C60, C70), graphene, and carbon nanotubes (SWCNTs and MWCNTs).
Graphene is an individual layer of densely packed sp2 carbon atoms. The hexagonal arrangement of this material is indicated in Figure 1 [1]. Geim and Novoselov described the synthesis and characterization of this carbon nanostructure in 2004 [8]; thy were recognized for this effort with the 2010 Nobel Prize in Physics. In comparison with bulk structures, this nanostructure has a large relative surface area. Graphene can be functionalized chemically and dispersed in different solvents, including water. It has a great electrical and thermal conductivity as well as unique optical properties [1,9]. In graphene, all the atoms are located on the surface, which provides locations for attachment for many types of biomaterials [9]. Graphene endowed with all these features is a great candidate for biosensing, drug delivery, tissue engineering, genetic engineering, bioimaging, and therapeutics [1,9].
CNTs, as the cylindrical form of fullerenes, are rolled graphene sheets (Figure 1); these materials consist of single or multiple graphene layers called single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs), respectively [1]. Initially, CNTs were reported in 1991 by Iijima, who synthesized fullerenes through the arc discharge method [10]. CNTs are stretchable and flexible with excellent strength [11]. They have high electrical and thermal conductivity as well as significant chemical reactivity. There are some barriers that impede CNTs from large scale use such as the absence of a technique to synthesize CNTs with a repeatable structure for mass production [11].
The use of carbon nanostructures for treating new viruses such as coronavirus disease 2019 (COVID-19) and established ones such as Ebola virus along with cancer has been a focus of recent research activity. Several studies on the use of carbon nanostructures for COVID-19 therapy have recently been published. Numerous researchers have tried to bring the carbon-based materials closer to clinical drug delivery applications through functionalization and conjugation of carbon nanostructures with biological molecules. In this article, we will focus on the latest studies that involve the use of graphene, fullerene, and CNTs for advanced drug delivery, cancer therapy, and antiviral applications.
Drug delivery and cancer therapy
The biodistribution, pharmacokinetics, and excretion properties of the compound play a vital role in the efficacy of the drug delivery system [12]. Carbon nanostructures are attractive candidates for drug delivery systems because of their dimensions being close to those of viruses and other biological structures. Carbon nanostructures may linger inside biological structures with poor drainage (e.g. tumors), whereas they may not linger inside healthy cells with normal drainage [13]. As carbon nanostructures may be functionalized in a straightforward manner, they may be conjugated with chemotherapeutic agents, antibodies, antitumor drugs, and other therapeutic agents [3,13].
Fullerenes
Numerous derivatives of fullerenes with improved water solubility for advanced drug delivery systems have been developed. The size of fullerenes together with their amphiphilicity enable them to penetrate almost all biological entities and barriers. Conjugated fullerenes may be used for localized drug delivery, which avoids damage to other body organs. For instance, ibuprofen is a common prescribed drug for pain relief and inflammation with side effects such as gastrointestinal hemorrhage, ulcer, digestive aggravation, and vomiting when it is consumed orally. Recent density functional theory (DFT) calculations show that C60 fullerenes with a porphyrin-like transition metal-N4 can be used as ibuprofen carriers. Quantum studies confirmed the release of the drug in the acidic environment of unhealthy cells [14].
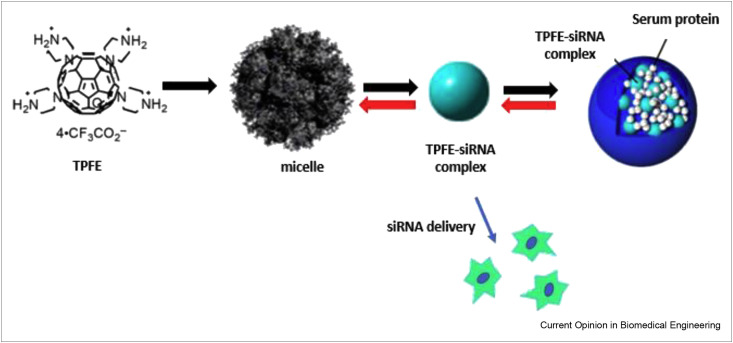
The use of fullerenes for nucleic acid delivery systems has receiving attention recently. In a recent in vitro study, tetra(piperazino)[60]fullerene epoxide (TPFE) was used for stabilization and delivery of unstable siRNA molecules [15]. In vivo studies have already shown that TPFE is nontoxic, unlike the commonly used lipofectamine 2000, for siRNA delivery and has a higher knockdown efficiency [16]. As depicted in Figure 2 , the in vitro study involved the use of TPFE-siRNA particles with a submicrometer size to deliver siRNA to the lung cells; an immediate agglutination with the plasma proteins occurred. TPFE-siRNA-plasma protein materials were initially stable and accumulated in the lung capillaries; they later became unstable after blocking the lung capillaries, which culminated in the release of siRNA into the cells. The TPFE residues in the form of micelles were under 10 nm and were removed from the lung capillaries with a high clearance rate [15].
Figure 2.
Schematic of producing TPFE-siRNA-plasma protein for siRNA delivery (adapted from Ref. [15]).
The blood–brain barrier (BBB) contains tight junctions that serve as a barrier to the delivery of highly polar drugs to the central nervous system (CNS). Fullerenes-based carriers have been shown to enable penetration of the BBB. For example, the effect of fullerene compounds on penetration of the BBB by polar hexamethonium benzosulfonate was explored. In this study, “virtually solvent-free planar lipid bilayer” phosphocholine membranes were prepared [17]. Without the fullerene, the addition of hexamethonium benzosulfonate to the lipid bilayer membrane did not produce any observable transmembrane current. However, with the presence of the fullerene compounds (IEM-2143 and IEM-2144), ion permeability through the phosphocholine bilayers, lipid disorder in the phosphocholine membranes, and movement through the lipid bilayers were enhanced [17].
Studies of the COVID-19 pandemic indicate that chloroquine (CQ) may be an efficient medicine to combat the COVID-19 infection [18]. DFT studies show that Al- and Si-doped C60 are stable CQ carriers for COVID-19 treatment because of the well-matched energetics between these species; fullerene serves as the electron acceptor and CQ serves as the electron donor [18].
Lung cancer chemotherapy methods suffer from an inadequate concentration of the drug interacting with the tumor cells and the toxicity of the drug. Various water-soluble fullerene derivatives for drug delivery were investigated for their anticancer potential as an alternative to conventional lung cancer chemotherapy [19]. It was found that a stronger cytotoxic ability of fullerene derivatives against lung cancer cells corresponds to the presence of less aliphatic single bonds attached to the fullerene cage, the absence of chlorine in the structure, and the presence of 2-phenoxyacetate residues [19].
A very effective drug for the pancreatic cancer is gemcitabine; however, this agent shows some chemoresistivity and poor distribution inside the tumor. Therefore, an alternative mechanism to deliver gemcitabine is an important focus of current research efforts. One potential solution is the conjugation of gemcitabine with [60]fullerene for improved water solubility [13]. The compound exhibits cytotoxicity that can be boosted through the oxygen species generated by blue LED-irradiated C60 [13].
C60 conjugated with serinolamide can permeate liver cancer cells; C60-serinol conjugated with paclitaxel reduces the size of tumor without the side effect of weight loss. A study of the in vivo function of C60-serinol shows that its biodistribution and excretion from kidney in mice is rapid and efficient [20]. Based on computational results, C60 can be successfully loaded with the chemotherapeutic drug doxorubicin (DOX) and antitumor agent boronic chalcone [21].
A novel drug delivery system was described by Shi et al. for advanced cancer therapy using C60 [22]. Unlike the traditional drug delivery systems, this ‘off–on’ type drug delivery system does not suffer from an uncontrolled drug release. In this approach, the fullerene is conjugated with DOX using reactive oxygen species (ROS) and a hydrophilic shell is attached to its surface, which has a tumor-targeted feature. In the ‘off’ mode, DOX is stably entrapped at environments with pH ∼5.5. However, in the ‘on’ mode, ROS are produced by fullerenes, which consequently release DOX. The switching between the ‘on’ and ‘off’ modes is controlled remotely by a 532 nm laser [22].
Some C60 derivatives are able to produce ROS under light exposure. One example is glycoconjugated C60 derivatives that are used for targeting cancer cells [23]. Cancer cells tend to accumulate glucose and lure the glycoconjugated C60 into themselves. Based on recent studies, these glycolfullerenes absorbed by cancer cells act as photodynamic cytotoxic agents when exposed to blue and green light [23].
Graphene
Although graphene has potential use as a drug carrier, it has a toxicity effect on human organs as it can aggregate in tissues and produce oxidative stress. To overcome this issue, surface modification of graphene is important. Various functional groups can be attached to the graphene surface through the deep eutectic solvent (DES) method to overcome these issues. The functionalization of graphene enables wider applications of this material for advanced drug delivery. In addition, the large surface-area-to-volume ratio of graphene is beneficial for drug carrier applications.
Zainal-Abidin et al. described DES functionalization of graphene with choline chloride and loading with DOX. This material showed good dispersibility and DOX loading capacity. It exhibits superior anticancer activity as DOX is captured by the functionalized graphene more efficiently than pristine graphene [24,25].
Graphene materials have recently been developed for pH-responsive drug delivery. Unlike normal cells, the cancer cells possess acidic environment. As such, pH-responsive materials may be useful for drug delivery systems. For example, a rubber-like nanohybrid hydrogel containing pristine graphene was developed for pH-responsive drug delivery applications [26]. This material exhibited drug release with changes in pH; in addition, the conductive nature of the hydrogel enabled tunable and pulsatile drug delivery. The pH-oscillatory response of the hydrogel was demonstrated by measuring the swelling and deswelling behavior of the hydrogel in a buffer solution as the pH of the buffer solution was adjusted between 7.8 and 1.7. This study indicated that the nanohybrid hydrogel was pH-responsive and conductive because of the acrylic acid contribution and graphene components of the structure [26].
In another study, GQDs were conjugated with the reverse transcriptase inhibitors CHI499 and CDF119 for use in human immunodeficiency virus (HIV) treatment [27]. GQDs have also been shown to permeate the BBB [28].
Lucherelli et al. attached folic acid to graphene with a PEG chain for targeted delivery of DOX; they used indocyanine green for tracking the compound inside cancer cells to observe its dispersity and anticancer activity. In vitro and in vivo studies showed reduced toxicity for the multifunctionalized graphene [29]. A novel method for fluorination of graphene by an ionic liquid was studied for curcumin delivery. The functionalized material showed higher drug loading efficiency and stronger anticancer behavior [30].
Combination drug delivery plays a pivotal role in cancer therapy as therapy methods involving only one anticancer drug are not always successful. Owing to its straightforward functionalization and large relative surface area, graphene is a promising material for combination drug delivery [31]. Computational studies show that graphene can be conjugated with paclitaxel and DOX simultaneously. Folic acid functionalized graphene is capable of co-delivery of DOX and camptothecin through strong π–π interactions [32,33].
Recent DFT calculations show that graphene with attached FeN4 serves as an excellent adsorptive carrier for ibuprofen; the ibuprofen/FeN4-graphene system possesses a high chemical bonding potential to target biological structures [34].
Carbon nanotubes
CNTs are hydrophobic materials that exhibit nonuniform dispersity in biological environments; functionalization of the CNT surface can overcome this limitation. When exposed to oxidative agents, CNTs form carboxylated surfaces. The carboxylated CNT materials are uniformly dispersed and biocompatible materials that can be loaded with drugs. Carboxylated SWCNTs can be loaded with droxidopa using amine and carboxylate groups; droxidopa is used as a treatment for orthostatic hypotension and Parkinson's disease. Molecular dynamic simulations predict good stability for this material [35].
A cell-penetrating cancer-targeting functionalized MWCNT has been described [36]. The results with material indicated showed an increase in ROS level supporting anticancer activity, elevated BBB permeability, and efficacy against glioma tumors. The BBB penetration and the selectivity of the compound toward glioma cells were associated with the antitumor functionality of the material [36].
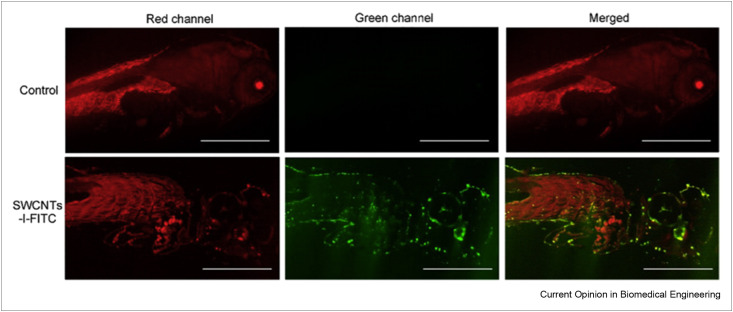
Viral nervous necrosis disease, which is caused by nervous necrosis virus (NNV), target the CNS in fish; accessing this affected tissue with drugs is a technical challenge. Fluorescein isothiocyanate (FITC) and isoprinosine, an anti-NNV agent, were conjugated with SWCNTs to boost the effectivity of isoprinosine delivery (Figure 3 ) [37]. A delivery system composed of bovine serum albumin, isoprinosine, and SWCNTs was investigated both in vitro and in zebrafish larvae and showed enhanced anti-NNV ability with significantly reduced fatality [37].
Figure 3.
Tissue section observation that confirms the effective delivery of isoprinosine using the conjugated system. The first row shows the zebrafish exposed to FITC, which served as the control material. The second row shows the zebrafish exposed to SWCNTs-I-FITC. No clear green fluorescence can be seen in the control system, whereas the green fluorescence in the conjugated system shows effective internalization (the green fluorescence corresponds to the FITC labeling and the red fluorescence corresponds to dyed cell membrane) (with permission from Ref. [37]).
SWCNTs were also successfully used to enhance the efficacy of a bath vaccine for juvenile pearl gentian grouper against iridovirus. The efficacy of the immune genes was improved through the use of SWCNTs as the vaccine carrier [38].
Besides the increasing toxicity for healthy tissues, the aggregation of CNTs prevents straightforward estimation of the cytotoxicity of an anticancer delivery system. To facilitate the uniform dispersibility and biocompatibility of CNTs, Pennetta et al. functionalized SWCNTs and MWCNTs with pyrrole polypropylene glycol (PPGP) covalently (CNT/PPGPc) and noncovalently (CNT/PPGPs) and conjugated these materials with DOX. The novel CNT/PPGP/DOX systems were associated with melanoma and lung cancer cell death at a lower dose of DOX. The uniformly distributed CNT/PPGP/DOX also facilitated easier evaluation of cytotoxicity [39].
Other novel, successful anticancer delivery systems containing CNTs and DOX include PEGylated multiwalled discrete CNTs [40], oxidization of MWCNTs by HNO3/H2SO4 and coverage by ɣ-Fe2O3 nanoparticles for magnetic drug delivery [41], and polyampholytic alternating polymer (PMT) functionalized SWCNTs [42].
CNTs exhibit exceptional capability to target a special receptor or molecule. A dual targeting system has been proposed to increase the efficiency of antibreast cancer therapy. In this system, both the glucose transporter protein and the folic acid receptor of cancer cells were targeted simultaneously through loading of CNTs with DOX-conjugated glycoblock copolymers and folic acid [43]. A summary of the recent progress in drug delivery and cancer therapy using the aforementioned carbon nanomaterials is provided in Table 1 .
Table 1.
A Summary of the recent progress in drug delivery and cancer therapy using carbon nanomaterials.
| Carbon nanomaterial | Application | New progress | Reference |
|---|---|---|---|
| Fullerene, C60 with porphyrin-like transition metal-N4 | Drug delivery, Ibuprofen | Predicts the release of Ibuprofen in acidic environment of unhealthy cells | [14] |
| Fullerene, Al- and Si-doped C60 | Drug delivery, Chloroquine | Possible COVID-19 treatment by drug delivery | [18] |
| Water-soluble fullerene derivatives | Cancer therapy | Water-soluble fullerene derivatives with cytotoxicity for lung cancer | [19] |
| [60]Fullerene-glycine derivative | Cancer drug delivery, gemcitabine | New synthetic approach for a highly water-soluble [60]fullerene-glycine derivative | [13] |
| C60-serinol | Cancer drug delivery, paclitaxel | Synthesizing a novel C60 derivative | [20] |
| C60 | Cancer drug delivery, Doxorubicin and Boronic Chalcone | The possibility of functionalizing C60 with B and N atoms and loading with Doxorubicin and Boronic Chalcone | [21] |
| Glycoconjugated C60 derivatives | Cancer therapy | Glycolfullerenes act as photodynamic cytotoxic agents | [23] |
| Functionalized graphene with choline chloride | Cancer drug delivery, Doxorubicin | First Doxorubicin delivery by graphene | [24,25] |
| Graphene with attached folic acid and indocyanine green | Cancer drug delivery, Doxorubicin | Multifunctional graphene synthesis with improved anticancer activity | [29] |
| Fluorinated graphene | Cancer drug delivery, curcumin | Synthesis of fluorinated graphene with ionic liquid for the first time and curcumin delivery | [30] |
| Folic acid functionalized graphene | Combined drug delivery, Doxorubicin and Camptothecin | Enhance the efficacy of cancer therapy | [32] |
| Graphene | Combined drug delivery, Paclitaxel and Doxorubicin | Enhance the efficacy of cancer therapy | [33] |
| Graphene with attached FeN4 | Drug delivery, Ibuprofen | High chemical bonding potential to target bio-entities | [34] |
| Carboxylated CNTs | Drug delivery, Droxidopa | Uniformly dispersed and biocompatible with great system stability | [35] |
| SWCNTs | Drug delivery, Isoprinosine | Enhanced anti-NNV ability | [37] |
| SWCNTs | Drug delivery, bath vaccine | Enhanced the efficacy of bath vaccine | [38] |
| Functionalized SWCNTs and MWCNTs with PPGP | Cancer drug delivery, Doxorubicin | Foster the uniform dispersibility and biocompatibility of CNTs, and easier evaluation of cytotoxicity | [39] |
| PEGylated multiwalled discrete CNTs | Cancer drug delivery, Doxorubicin | Successful anticancer delivery systems | [40] |
| Oxidized MWCNTs by HNO3/H2SO4 covered by ɣ-Fe2O3 nanoparticles | Cancer drug delivery, Doxorubicin | Successful anticancer delivery systems | [41] |
| Polyampholytic alternating polymers (PMT) functionalized SWCNTs | Cancer drug delivery, Doxorubicin | Successful anticancer delivery systems | [42] |
| CNTs conjugated with glycoblock copolymers and folic acid | Dual targeting system | Increase the efficiency of antibreast cancer activity | [43] |
PPGP, pyrrole polypropylene glycol.
Antiviral applications
With the emergence of new viruses such as COVID-19, the development of new antiviral systems, including drugs and vaccines, has recently attracted significant attention from the research community. Carbon nanostructures have shown promising antiviral features such as viral inhibition activity and virus enzyme blocking to combat against viruses such as HIV, influenza, herpes simplex virus (HSV), and COVID-19 [44,45]. The use of carbon nanomaterials for antiviral applications is currently at an early stage and requires more comprehensive investigation.
Fullerenes
Hydrophilic fullerenes are of special interest for antiviral applications. The biological properties of fullerenes directly correspond to the type of functional groups linked to the fullerene cage. The most water-soluble fullerene derivative is C60[P(O) (OK)2]5H with C–P connected to the cage; the synthesis of this material has recently been significantly simplified [46]. In vitro studies of the antiviral effect of C60[P(O) (OK)2]5H show strong activity against influenza A and feline coronavirus [46]. Klimova et al. reported that the water-soluble C60 (ndC60) has activity against type 1 of Herpes simplex virus (HSV-1) and human cytomegalovirus. In vitro and in vivo studies indicated that ndC60 inhibits virus entry into the host cells by blocking virus–receptor interactions [47].
The outer layer (envelope protein) of coronavirus is a hydrophobic phospholipid that is responsible for interaction with the host. The aqueous colloidal fullerene releases singlet oxygen under UVA irradiation, which damages the lipid layer of coronavirus. As such, a C60 coating may serve as an antiviral surface. In addition, the adhesion of the virus on the surface can be minimized. Fullerene-coated surfaces take advantage of the lipid structure of the virus and the hydrophobic properties of fullerenes by decreasing the contact area of the virus on the surface [45]. A computational study indicated that fulleropyrrolidine derivatives may interrupt the main mechanism by which the coronavirus protects itself against antibodies, in which the antibodies are neutralized by a receptor-binding domain of the spike protein on the coronavirus [48].
The dimensions of fullerene derivatives match well with the HIV protease active sites and can block HIV enzymes. The requirements for fullerene derivatives with efficient anti-HIV activity are hydrophilicity and a balance between cytotoxicity and antiviral action. Voronov et al. introduced five new water-soluble [60]fullerene derivatives as HIV inhibitors [49] by the reaction between C60Cl6 and dimethyl 2,2’-(1,4-phenylenebis-(oxy))diacetate. They found that the five derivatives acted against R5 and X4 strains of HIV-1 virus.
Graphene
Aside from conjugation with antiviral materials, graphene can directly interact with viruses. Graphene may interact with the virus via electrostatic and hydrophobic interactions to affect the viral envelope [50]. A newly explored direct interaction mechanism was reported by Matharu et al. [51]. They investigated the antiviral activity of graphene nanoplatelets (110 nm × 170 nm) by 24-h incubation of a suspension of graphene in Escherichia coli T4 bacteriophage, a double-stranded DNA virus. Graphene nanoplatelets significantly reduced the population of the virion and prevented infection completely within 3 h. They ascribed the antiviral effect of graphene to its morphology and the generation of ROS [51]. Another recent study indicated that a hybrid containing graphene and copper inhibited influenza A virus attachment and entry to the host cell within 30 min by destroying the virus envelope [52].
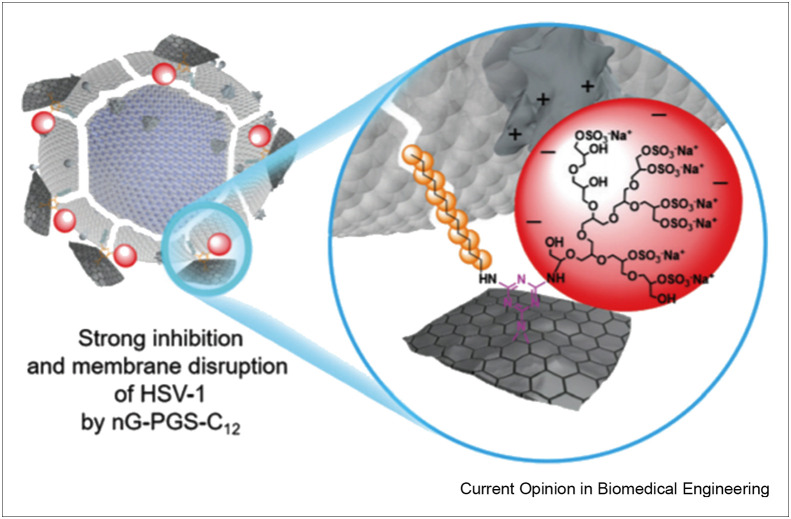
Graphene can be conjugated with negatively charged antivirals (e.g. negatively charged sulfates) [53]. This type of conjugation enhances the interaction between graphene and the positively charged residues of virions [53]. Donskyi et al. [54] functionalized graphene to enhance its antiviral activity. They synthesized graphene derivatives that were conjugated with polyglycerol sulfate and alkyl chains (C3–C18). Graphene with the C12 alkyl chain acted as the best HSV-1 inhibitor (Figure 4 ); however, it showed strong toxicity toward Vero cells. The chains shorter than C12 were non-toxic for Vero cells yet served as good HSV-1 inhibitors.
Figure 4.
A representation of inhibitory interaction of C12 with HSV-1 (with permission from Ref. [54]).
There are some suggestions regarding the use of graphene as a COVID-19 inhibitor based on computational simulations and experimental results from other RNA viruses in the coronaviruses family [55,56]. They suggested that graphene can destabilize COVID-19 virus; the strong light absorption properties of graphene may serve as a disinfectant [55,56].
Carbon nanotubes
The RNA-binding domain (RBD) of the nonstructural protein 1 (NS1) in influenza A facilitates virus survival and prevents export of the mRNA of the host cell. Molecular dynamic simulations predicted that CNTs are able to adsorb and stretch the RBD very quickly and interrupt the virus protection system [57]. The effect of nonfunctionalized SWCNTs and hydroxylated MWCNTs on influenza H1N1 strain A/Mexico/4108/2009 (IAV) in lung tissue were evaluated by Chen et al. They noted that body exposure to MWCNTs and IAV changes the antiviral response to the virus without changing the viral titers or causing significant lung injury; SWCNTs generate greater viral titers with lung damage under the same in vivo conditions [58]. MWCNTs show phagocytosis through macrophages; the excretion properties of MWCNTs are considerably faster than SWCNTs [58]. Using SWCNTs as an antiviral coating on surfaces was suggested based on the results of DFT studies [59]. According to the DFT results, Ru-, Pt-, and Cu-functionalized SWCNTs strongly adsorb H2O2 molecules, which are lethal to viruses such as coronaviruses.
Conclusions and future perspectives
In this article, recent progress in using fullerenes, graphene, and CNTs for drug delivery, cancer therapy, and antiviral activity in recent years has been reviewed. Owing to their nanoscale dimensions, these materials possess high surface-area-to-volume ratios and high chemical reactivity. These nanosized structures can penetrate many biological structures and membranes to facilitate drug delivery. There have been remarkable advancements in the chemical modification of carbon nanostructures and the introduction of new derivatives to improve solubility, enhance biocompatibility, reduce toxicity, boost antineoplastic activity, and obtain stable loading of drugs (e.g. antiviral agents). Although most studies emphasized the benefits of localized drug delivery and reducing the side effects through the use of carbon nanostructures, the toxicity and safety of these materials still need to be more carefully addressed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
This review comes from a themed issue on Biomaterials: Futures of Biomaterials
Edited by Aldo Boccaccini, Himansu Sekhar Nanda,Syam Nukavarapu and Vinoy Thomas
References
- 1.Zhang M., Naik R., Dai L. 2015. Carbon nanomaterials for biomedical applications. [Google Scholar]
- 2.Hirsch A. The era of carbon allotropes. Nat Mater. 2010;9:868–871. doi: 10.1038/nmat2885. [DOI] [PubMed] [Google Scholar]
- 3.Maiti D., Tong X., Mou X., Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:1–16. doi: 10.3389/fphar.2018.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H., Zhang L., Yan M., Yu J. Carbon nanostructures in biology and medicine. J Mater Chem B. 2017;5:6437–6450. doi: 10.1039/c7tb00891k. [DOI] [PubMed] [Google Scholar]
- 5.Kroto H, Heath J, O'Brien S, Curl R, nature RS-, 1985 undefined: C60: buckminsterfullerene. [date unknown]..
- 6.Kai-Hua Chow E., Gu M., Xu J. Elsevier Inc.; 2020. Carbon nanomaterials: fundamental concepts, biological interactions, and clinical applications. [Google Scholar]
- 7.Shi J., Yu X., Wang L., Liu Y., Gao J., Zhang J., Ma R., Liu R., Zhang Z. PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials. 2013;34:9666–9677. doi: 10.1016/j.biomaterials.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Novoselov KS, Geim AK, Morozov S V, Dubonos S V, Zhang Y, Jiang D: Room-temperature electric field effect and carrier-type inversion in graphene films. [[date unknown]].
- 9.Zhang H., Grüner G., Zhao Y. Recent advancements of graphene in biomedicine. J Mater Chem B. 2013;1:2542–2567. doi: 10.1039/c3tb20405g. [DOI] [PubMed] [Google Scholar]
- 10.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 11.Xue Y. Carbon nanotubes for biomedical applications. Ind Appl Carbon Nanotub. 2017;4:323–346. [Google Scholar]
- Su C., Liu Y., Li R., Wu W., Fawcett J.P., Gu J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv Drug Deliv Rev. 2019;143:97–114. doi: 10.1016/j.addr.2019.06.008. [DOI] [PubMed] [Google Scholar]; The authors investigated the distribution, pharmacokinetics, and the excretion of nanocarriers as the vital factors in the efficacy of the drug delivery system.
- 13.Nalepa P., Gawecki R., Szewczyk G., Balin K., Dulski M., Sajewicz M., Mrozek-Wilczkiewicz A., Musioł R., Polanski J., Serda M. A [60]fullerene nanoconjugate with gemcitabine: synthesis, biophysical properties and biological evaluation for treating pancreatic cancer. Cancer Nanotechnol. 2020;11:1–21. https://link.springer.com/article/10.1186/s12645-020-00058-4. [Google Scholar]
- 14.Alipour E., Alimohammady F., Yumashev A., Maseleno A. Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug. J Mol Model. 2020:26. doi: 10.1007/s00894-019-4267-1. [DOI] [PubMed] [Google Scholar]
- 15.Minami K., Okamoto K., Harano K., Noiri E., Nakamura E. Hierarchical assembly of siRNA with tetraamino fullerene in physiological conditions for efficient internalization into cells and knockdown. ACS Appl Mater Interfaces. 2018;10:19347–19354. doi: 10.1021/acsami.8b01869. [DOI] [PubMed] [Google Scholar]
- 16.Minami K, Okamoto K, Kent D, Harano K, reports EN-S, 2014 Undefined: siRNA delivery targeting to the lung via agglutination-induced accumulation and clearance of cationic tetraamino fullerene. nature.com [[date unknown]]. [DOI] [PMC free article] [PubMed]
- 17.Efimova S.S., Khaleneva D.A., Litasova E.V., Piotrovskiy L.B., Ostroumova O.S. The mechanisms of action of water-soluble aminohexanoic and malonic adducts of fullerene C60 with hexamethonium on model lipid membranes. Biochim Biophys Acta Biomembr. 2020;1862:183433. doi: 10.1016/j.bbamem.2020.183433. [DOI] [PubMed] [Google Scholar]
- 18.Bagheri Novir S., Aram M.R. Quantum mechanical simulation of Chloroquine drug interaction with C60 fullerene for treatment of COVID-19. Chem Phys Lett. 2020;757:137869. doi: 10.1016/j.cplett.2020.137869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.J., Kraevaya O.A., Voronov, Troshin P.A., Hsu S.H. Fullerene derivatives as lung cancer cell inhibitors: investigation of potential descriptors using qsar approaches. Int J Nanomed. 2020;15:2485–2499. doi: 10.2147/IJN.S243463. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors described the use of novel water-soluble fullerene derivatives for lung cancer cell inhibition.
- 20.Zaibaq N.G., Pollard A.C., Collins M.J., Pisaneschi F., Pagel M.D., Wilson L.J. Evaluation of the biodistribution of serinolamide-derivatized c60 fullerene. Nanomaterials. 2020;10:28–31. doi: 10.3390/nano10010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omri N., Bu Y. Azomethine ylide addition impact on functionalized [60]Fullerene and [60]Boron-Nitride: anticancer Doxorubicin and Boronic Chalcone drugs binding characteristics with mono- and bis-nanocarriers. Colloids Surf B Biointerfaces. 2020;196:111277. doi: 10.1016/j.colsurfb.2020.111277. [DOI] [PubMed] [Google Scholar]
- 22.Shi J., Wang B., Wang L., Lu T., Fu Y., Zhang H., Zhang Z. Fullerene (C60)-based tumor-targeting nanoparticles with “off-on” state for enhanced treatment of cancer. J Control Release. 2016;235:245–258. doi: 10.1016/j.jconrel.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Serda M., Ware M.J., Newton J.M., Sachdeva S., Krzykawska-Serda M., Nguyen L., Law J., Anderson A.O., Curley S.A., Wilson L.J., et al. Development of photoactive Sweet-C 60 for pancreatic cancer stellate cell therapy. Nanomedicine. 2018;13:2981–2993. doi: 10.2217/nnm-2018-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zainal-Abidin M.H., Hayyan M., Ngoh G.C., Wong W.F., Looi C.Y. Potentiating the anti-cancer profile of tamoxifen-loaded graphene using deep eutectic solvents as functionalizing agents. Appl Nanosci. 2020;10:293–304. [Google Scholar]
- 25.Zainal-Abidin M.H., Hayyan M., Ngoh G.C., Wong W.F. Doxorubicin loading on functional graphene as a promising nanocarrier using ternary deep eutectic solvent systems. ACS Omega. 2020;5:1656–1668. doi: 10.1021/acsomega.9b03709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganguly S., Ray D., Das P., Maity P.P., Mondal S., Aswal V.K., Dhara S., Das N.C. Mechanically robust dual responsive water dispersible-graphene based conductive elastomeric hydrogel for tunable pulsatile drug release. Ultrason Sonochem. 2018;42:212–227. doi: 10.1016/j.ultsonch.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Iannazzo D., Pistone A., Ferro S., De Luca L., Monforte A.M., Romeo R., Buemi M.R., Pannecouque C. Graphene quantum dots based systems as HIV inhibitors. Bioconjug Chem. 2018;29:3084–3093. doi: 10.1021/acs.bioconjchem.8b00448. [DOI] [PubMed] [Google Scholar]
- 28.Kim D., Yoo J.M., Hwang H., Lee J., Lee S.H., Yun S.P., Park M.J., Lee M.J., Choi S., Kwon S.H., et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson's disease. Nat Nanotechnol. 2018;13:812–818. doi: 10.1038/s41565-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucherelli M.A., Yu Y., Reina G., Abellán G., Miyako E., Bianco A. Rational chemical multifunctionalization of graphene interface enhances targeted cancer therapy. Angew Chemie. 2020;132:14138–14143. doi: 10.1002/anie.201916112. [DOI] [PubMed] [Google Scholar]
- 30.Jahanshahi M., Kowsari E., Haddadi-Asl V., Khoobi M., Bazri B., Aryafard M., Lee J.H., Kadumudi F.B., Talebian S., Kamaly N., et al. An innovative and eco-friendly modality for synthesis of highly fluorinated graphene by an acidic ionic liquid: making of an efficacious vehicle for anti-cancer drug delivery. Appl Surf Sci. 2020;515:146071. [Google Scholar]
- Gu Z., Zhu S., Yan L., Zhao F., Zhao Y. Graphene-based smart platforms for combined cancer therapy. Adv Mater. 2019;31:1–27. doi: 10.1002/adma.201800662. [DOI] [PubMed] [Google Scholar]; The paper describes recent advances involving graphene for combined drug delivery.
- 32.Alinejad A., Raissi H., Hashemzadeh H. Understanding co-loading of doxorubicin and camptothecin on graphene and folic acid-conjugated graphene for targeting drug delivery: classical MD simulation and DFT calculation. J Biomol Struct Dyn. 2020;38:2737–2745. doi: 10.1080/07391102.2019.1645044. [DOI] [PubMed] [Google Scholar]
- 33.Hashemzadeh H., Raissi H. Understanding loading, diffusion and releasing of Doxorubicin and Paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems. Appl Surf Sci. 2020;500:144220. [Google Scholar]
- 34.Gao J., Guo C., Wang X., Zhang W., Wang Y., Vahabi V. Porphyrin-like porous nanomaterials as drug delivery systems for ibuprofen drug. Mol Phys. 2020:118. [Google Scholar]
- 35.Yoosefian M., Jahani M. A molecular study on drug delivery system based on carbon nanotube for the novel norepinephrine prodrug, Droxidopa. J Mol Liq. 2019;284:258–264. [Google Scholar]
- 36.You Y., Wang N., He L., Shi C., Zhang D., Liu Y., Luo L., Chen T. Designing dual-functionalized carbon nanotubes with high blood-brain-barrier permeability for precise orthotopic glioma therapy. Dalt Trans. 2019;48:1569–1573. doi: 10.1039/c8dt03948h. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S., Li J., Huang A.G., Huang J.Q., Huang Y.Q., Wang G.X. Anti-betanodavirus activity of isoprinosine and improved efficacy using carbon nanotubes based drug delivery system. Aquaculture. 2019;512:734377. [Google Scholar]
- 38.Liu G.Y., Wang E.L., Qu X.Y., Yang K.C., Zhang Z.Y., Liu J.Y., Zhang C., Zhu B., Wang G.X. Single-walled carbon nanotubes enhance the immune protective effect of a bath subunit vaccine for pearl gentian grouper against Iridovirus of Taiwan. Fish Shellfish Immunol. 2020;106:510–517. doi: 10.1016/j.fsi.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Pennetta C., Floresta G., Graziano A.C.E., Cardile V., Rubino L., Galimberti M., Rescifina A., Barbera V. Functionalization of single and multi-walled carbon nanotubes with polypropylene glycol decorated pyrrole for the development of doxorubicin nano-conveyors for cancer drug delivery. Nanomaterials. 2020;10 doi: 10.3390/nano10061073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falank C., Tasset A.W., Farrell M., Harris S., Everill P., Marinkovic M., Reagan M.R. Development of medical-grade, discrete, multi-walled carbon nanotubes as drug delivery molecules to enhance the treatment of hematological malignancies. Nanomedicine Nanotechnology, Biol Med. 2019;20:102025. doi: 10.1016/j.nano.2019.102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge X., Fu M., Niu X., Kong X. Atomic layer deposition of γ-Fe2O3 nanoparticles on multi-wall carbon nanotubes for magnetic drug delivery and liver cancer treatment. Ceram Int. 2020 doi: 10.1016/j.ceramint.2020.07.123. [DOI] [Google Scholar]
- 42.Phan Q.T., Patil M.P., Tu T.T.K., Le C.M.Q., Kim G Do, Lim K.T. Polyampholyte-grafted single walled carbon nanotubes prepared via a green process for anticancer drug delivery application. Polymer. 2020;193:122340. [Google Scholar]
- Omurtag Ozgen P.S., Atasoy S., Zengin Kurt B., Durmus Z., Yigit G., Dag A. Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J Mater Chem B. 2020;8:3123–3137. doi: 10.1039/c9tb02711d. [DOI] [PubMed] [Google Scholar]; The authors proposed a novel CNT-based dual targeting system to increase the efficiency of anti-breast cancer therapy; both the glucose transporter protein and the folic acid receptor of cancer cells were targeted simultaneously.
- 44.Kraevaya O.A., Peregudov A.S., Godovikov I.A., Shchurik E.V., Martynenko V.M., Shestakov A.F., Balzarini J., Schols D., Troshin P.A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: a synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem Commun. 2020;56:1179–1182. doi: 10.1039/c9cc08400b. [DOI] [PubMed] [Google Scholar]
- 45.Siddiquie R.Y., Agrawal A., Joshi S.S. Surface alterations to impart antiviral properties to combat COVID-19 transmission. Trans Indian Natl Acad Eng. 2020;5:343–347. doi: 10.1007/s41403-020-00096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraevaya O.A., Novikov A.V., Shestakov A.F., Ershova E.S., Savinova E.A., Kameneva L.V., Veiko N.N., Schols D., Balzarini J., Kostyuk S.V., et al. Water-soluble fullerene-based nanostructures with promising antiviral and myogenic activity. Chem Commun. 2020 doi: 10.1039/d0cc03928d. [DOI] [PubMed] [Google Scholar]
- 47.Klimova R., Andreev S., Momotyuk E., Demidova N., Fedorova N., Chernoryzh Y., Yurlov K., Turetskiy E., Baraboshkina E., Shershakova N., et al. Aqueous fullerene C60 solution suppresses herpes simplex virus and cytomegalovirus infections. Fullerenes Nanotub Carbon Nanostructures. 2020;28:487–499. [Google Scholar]
- 48.Gawad A.E.-D., Bayoumy A., Ibrahim M. 2020. Molecular interactions of fullerene-based derivatives and SARS-CoV-2. [DOI] [Google Scholar]
- 49.Voronov, Martynenko V.M., Chernyak A.V., Godovikov I., Peregudov A.S., Balzarini J., Shestakov A.F., Schols D., Troshin P.A. Synthesis, characterization and anti-HIV activity of polycarboxylic [60]fullerene derivatives obtained in the reaction of C60Cl6 with a hydroquinone ether. Tetrahedron Lett. 2020;61:151598. [Google Scholar]
- 50.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., Lu Z., Chen L., He Q., Han H. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015 Sep 30;7:21571–21579. doi: 10.1021/acsami.5b06876. https://pubs.acs.org/doi/abs/10.1021/acsami.5b06876?casa_token=4GoxaQbFz2QAAAAA:AE7gIusHa2yNj0Zwb-37-IqbTWl8MTl7CYyHzwcmaPTwEf-aOD9EYv8aD3W3sNlrwZMkAWIDgk5r9-fz. [DOI] [PubMed] [Google Scholar]
- 51.Matharu R.K., Porwal H., Chen B., Ciric L., Edirisinghe M. Viral filtration using carbon-based materials. Med Devices Sensors. 2020;3:1–7. doi: 10.1002/mds3.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das Jana I., Kumbhakar P., Banerjee S., Gowda C.C. 2020. Development of a copper-graphene nanocomposite based transparent coating with antiviral activity against influenza virus. [Google Scholar]
- 53.Ziem B., Azab W., Gholami M.F., Rabe J.P., Osterrieder N., Haag R. Size-dependent inhibition of herpesvirus cellular entry by polyvalent nanoarchitectures. Nanoscale. 2017;9:3774–3783. doi: 10.1039/c7nr00611j. [DOI] [PubMed] [Google Scholar]
- Donskyi I.S., Azab W., Cuellar-Camacho J.L., Guday G., Lippitz A., Unger W.E.S., Osterrieder K., Adeli M., Haag R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale. 2019;11:15804–15809. doi: 10.1039/c9nr05273a. [DOI] [PubMed] [Google Scholar]; The authors synthesized new graphene derivatives that were conjugated with polyglycerol sulfate and alkyl chains with toxicity against HSV-1. They determined that the chain structure was non-toxic for Vero cells yet provided good inhibition of HSV-1.
- Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020;33:100883. doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors summarized the current knowledge about graphene as a potential antiviral system for COVID-19, including antiviral drug delivery, antiviral activity triggered by heat or light, surface coating with antiviral graphene, and graphene-based sensors.
- 56.Srivastava A.K., Dwivedi N., Dhand C., Khan R., Sathish N. Can graphene-based materials play a role in the fight against COVID-19? Sci Report. 2020 [Google Scholar]
- 57.Az’hari S., Mosaddeghi H., Ghayeb Y. Molecular dynamics study of the interaction between RNA-binding domain of NS1 influenza A virus and various types of carbon nanotubes. Curr Sci. 2019;116:398–404. [Google Scholar]
- 58.Chen H., Humes S.T., Rose M., Robinson S.E., Loeb J.C., Sabaraya I.V., Smith L.C., Saleh N.B., Castleman W.L., Lednicky J.A., et al. Hydroxyl functionalized multi-walled carbon nanotubes modulate immune responses without increasing 2009 pandemic influenza A/H1N1 virus titers in infected mice. Toxicol Appl Pharmacol. 2020;404 doi: 10.1016/j.taap.2020.115167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aasi A., Aghaei S.M., Moore M.D., Panchapakesan B. Pt-, Rh-, Ru-, and Cu-Single-Wall carbon nanotubes are exceptional candidates for design of anti-viral surfaces: a theoretical study. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21155211. [DOI] [PMC free article] [PubMed] [Google Scholar]