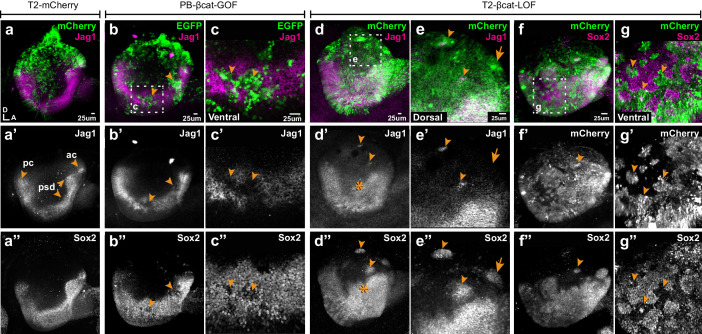
Figure 3. Wnt signalling antagonises prosensory specification.
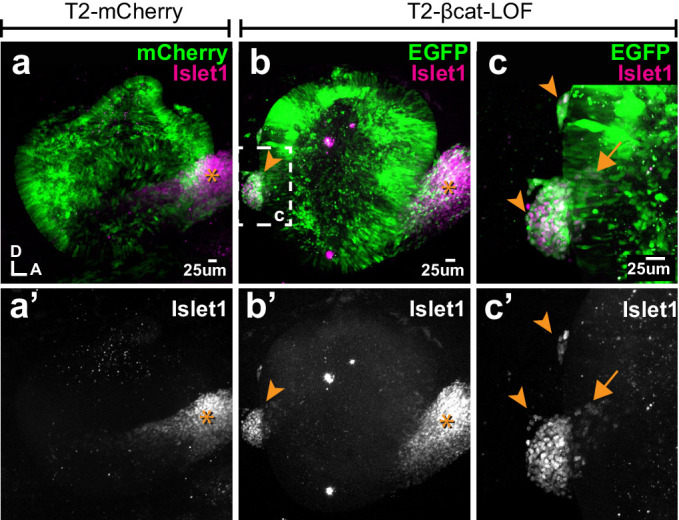
Whole-mount views of E4 chicken otocysts electroporated at E2 and immunostained for Jag1 and Sox2 expression. (a–a”) Control sample electroporated with T2-mCherry. Jag1 and Sox2 are expressed in a U-shaped ventral common prosensory-competent domain (psd) and prospective prosensory domains (pc = posterior crista; ac=anterior crista). (b–c”) βcat-GOF overexpression induces a mosaic down-regulation of Sox2 and Jag1 expression (arrowheads) in the ventral half of the otocyst. High magnification views of transfected cells (arrowheads in c–c’’) and analysis of mean fluorescence values of Sox2 and βcat-GOF (Figure 3—figure supplement 1a–e) show that this effect is cell-autonomous. (d–g”) Otocysts transfected with T2-βcat-LOF exhibit a dorsal expansion of Jag1 and Sox2 expression (star in d’–d’’) and ectopic prosensory patches dorsally (arrowheads in d’–d’’, f’’) and high magnification views in (e–e’’). Note that some ectopic Sox2-positive patches are Jag1-negative (arrows in e’–e’’). The prosensory effects of βcat-LOF were dependent on Notch activity (Figure 3—figure supplement 2a–b’”). In contrast, in the ventral-most aspect of the otocyst, βcat-LOF overexpressing cells exhibit reduced Sox2 expression (arrowheads in high magnification views g–g’’). Overexpression of βcat-LOF elicits the formation of ectopic Islet-1 expressing otic neurons in the posterior and dorsal aspect of the otocyst (Figure 3—figure supplement 3).
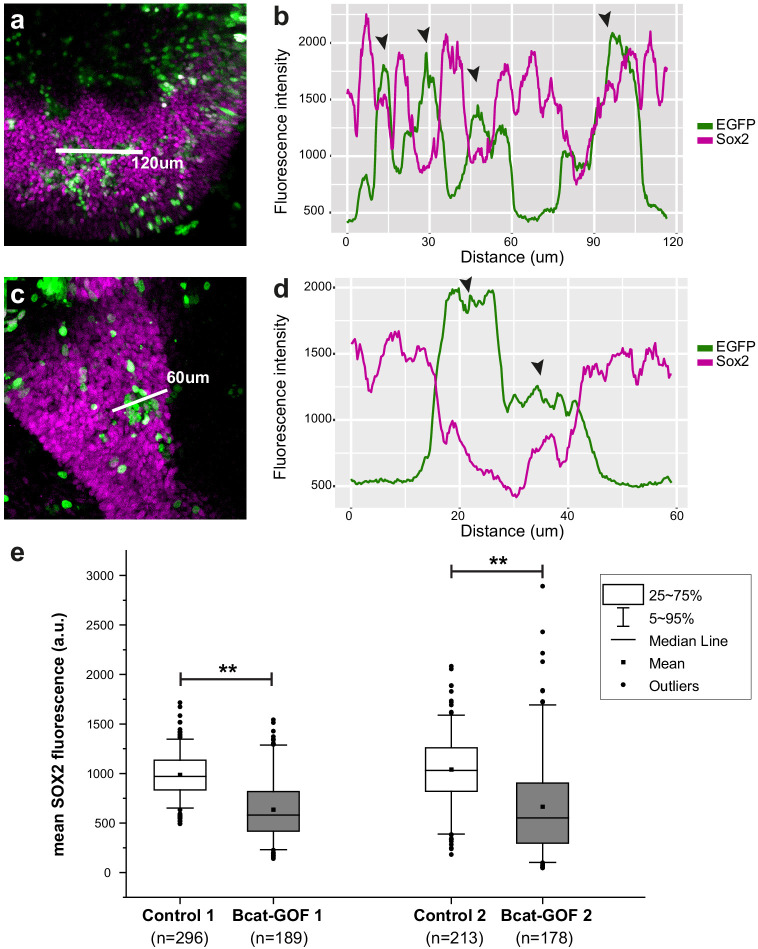
Figure 3—figure supplement 1. Analyses of Sox2 (magenta) and βcat-GOF (EGFP, green) fluorescence intensity levels in transfected prosensory regions.
Figure 3—figure supplement 2. Effects of simultaneous loss of Wnt and Notch activity on prosensory specification.
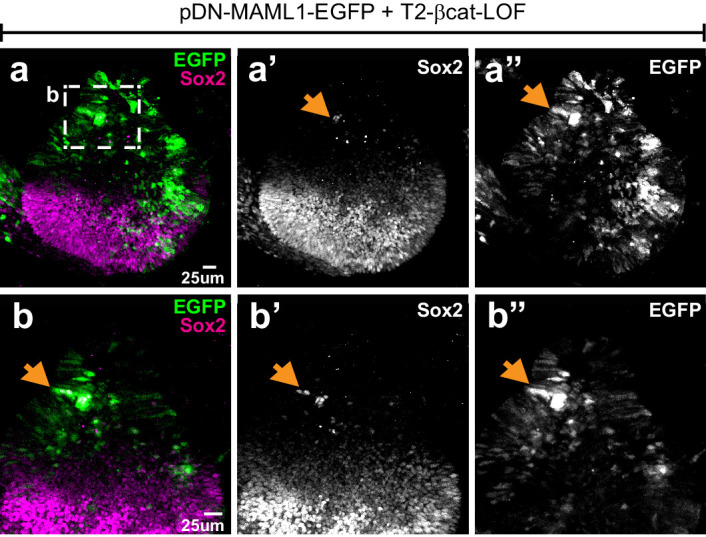
Figure 3—figure supplement 3. Blocking Wnt signalling triggers ectopic neurogenesis.