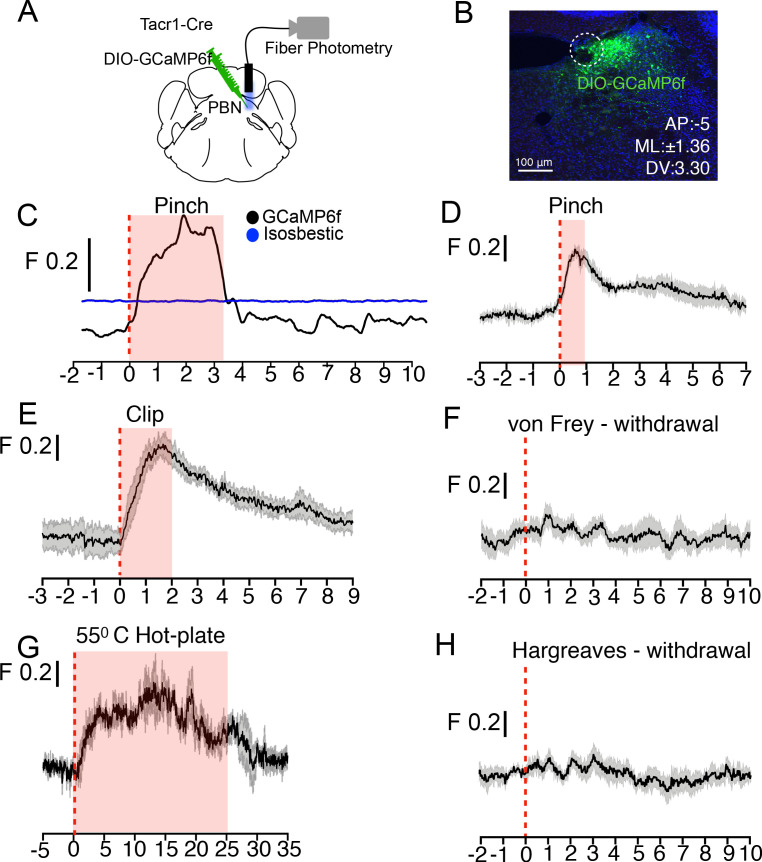
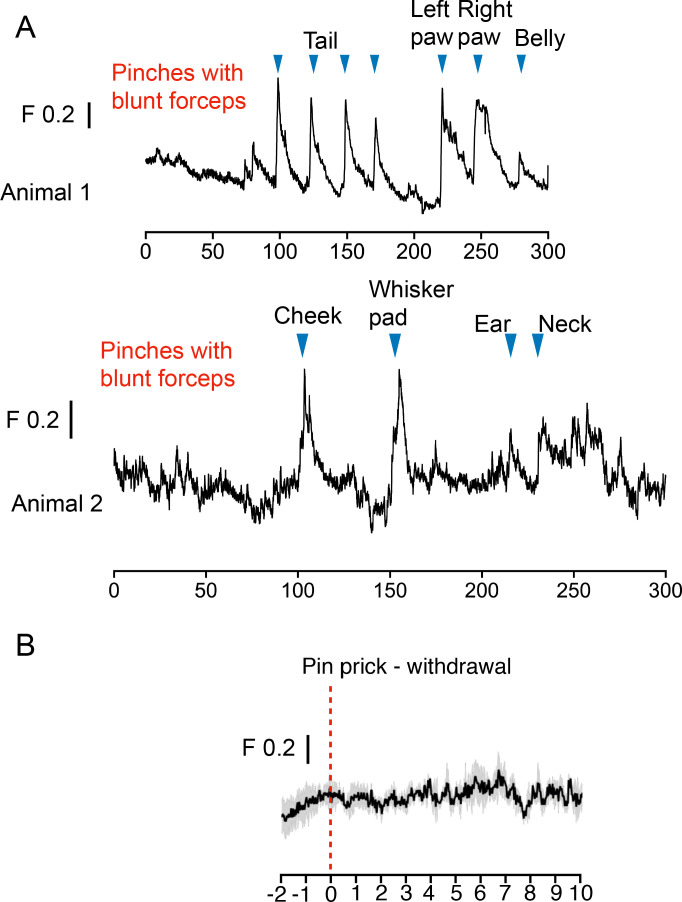
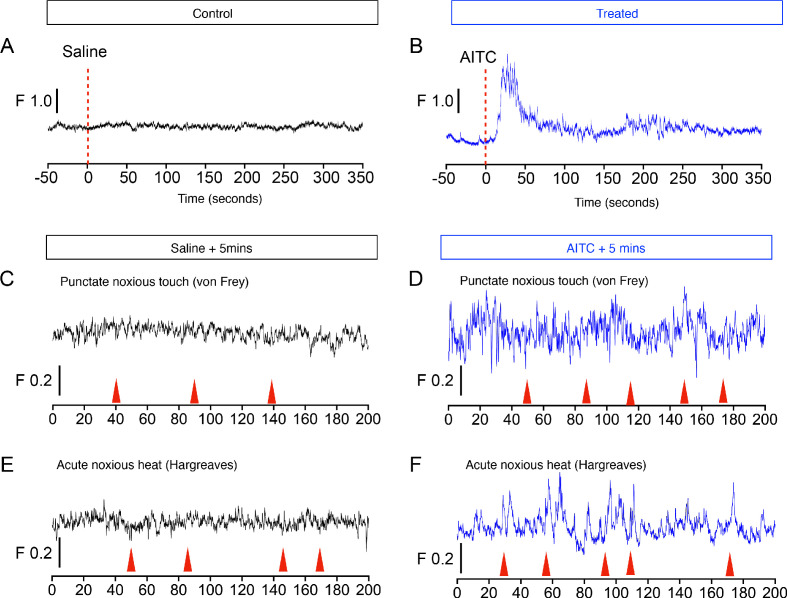
Figure 4. PBN-SLTacr1 neurons respond to sustained noxious stimulation.
(A) Cartoon depicting the approach for recording population activity in PBN-SLTacr1 neurons: a Cre-dependent viral vector encoding the genetically encoded calcium indicator GCaMP6f was injected into the PBN of Tacr1Cre mice and an optical fiber was placed over the injection site. Fiber photometry recording was used to monitor population calcium responses to different somatosensory stimuli. (B) After recording, posthoc staining and confocal imaging of PBN sections confirmed GCaMP6f expression (green) and fiber placement (dotted line). (C) An example photometry trace from a lightly anesthetized mouse showing a robust, time-locked calcium response to tail pinch with blunt forceps (stimulus onset at 0 s; dotted line). Note the response lasts the duration of the stimulation (shaded region) before returning to baseline. As a control for movement artifacts, 405 fluorescence (Blue trace; Isosbestic) was also monitored and showed no changes. (D–H) Average population calcium responses in awake mice to sustained (D, E, and G) and acute (F, H) noxious stimuli. Dark lines are means of responses from multiple animals aligned to the start of stimulation (time 0) with the standard error shown in light grey. X-axis shows time in seconds aligned to the start of stimulation; F indicates ΔF/F scaling (D) Pinching (hindpaw) with a blunt forcep (n = 6 mice). (E) Clip assay applied to hindpaw (n = 5 mice). (F) von Frey stimulation (0.6 g) aligned to paw withdrawal (n = 4 mice) (G) 55°C Hot plate test aligned to when mice are placed in a chamber and lasting 25 s (n = 3 mice). (H) Radiant heat test (Hargeaves) aligned to paw withdrawal (n = 7 mice). For all traces, time is in seconds, 0 is the start of the trial, change in fluorescence/total fluorescence is shown (black line; F).