Abstract
Background:
Keloids are an abnormal proliferation of scars that can involve large areas of tissue beyond the original injury site. Hypertrophic scars are similar clinically, but do not exceed the original scar limits. These scarring abnormalities can cause noxious symptoms such as pain, tenderness, itching, and ulcerations. The aim of this review is to discuss current therapies for both types of abnormal scarring, and to determine if guidelines can be provided for excisional treatment with adjuvant therapies versus non-excisional methods.
Methods:
A systematic literature search was performed through the Web of Science database. The search revolved around keywords such as “keloid,” “hypertrophic scars,” and “treatment.” Articles were reviewed and screened for inclusion and exclusion criteria. The review focuses on an analysis and summarization of randomized control trials regarding keloid or hypertrophic scar treatments.
Results:
The original searches produced 1161 and 1275 articles for keloid and hypertrophic scars, respectively. In total, 316 duplicates were found. After accounting for 2014–2019 publication time, 655 keloid and 893 hypertrophic scar articles were reviewed. This resulted in 15 articles that pertained to treatment and randomized control trials.
Conclusions:
Keloids and hypertrophic scars present a clinical challenge. Based on qualitative review of recurrence, neither excision plus adjuvant therapy or nonsurgical treatments can be recommended preferentially at this time. More research is needed to determine if recurrence rate bias exists between the treatment regimens, as excisional treatment plus adjuvant therapy is reserved for refractory scars.
INTRODUCTION
Scars may be caused by a variety of conditions. When the normal physiological response is distorted, a keloid or hypertrophic scar may result. A keloidal scar can appear within weeks of an injury or years later, whereas hypertrophic scars (HTS) typically develop in 4–8 weeks. Keloids are elevated, bulky, and extend beyond the edges of the injury. Due to the pain, tightness, pruritus, and disfigurement, keloids can have a significant impact on a patient’s quality of life. HTS, on the other hand, remain within the boundaries of the injury and tend to regress over years. In this scoping review, we aim to provide an up-to-date list on various treatments and explore recommendations for excisional therapy combined with adjuvant treatments.
METHODS
Criteria for Article Inclusion
Articles published within the last 5 years (2014–2019) that characterized keloid or HTS treatment were included in this scoping review. Randomized control trials (RCTs) were sought to guide possible surgical guidelines regarding keloids and HTS.
Criteria for Article Exclusion
Articles with keywords such as “burn,” “thermal,” or “pathogenesis,” or with “case studies” in the title or abstract were removed. Through title and abstract review, epidemiology studies and scars resulting from side effects of other treatments were also excluded.
Search Methodology
A literature search for articles using the Web of Science was completed in October 2020. The language of articles was limited to English. Two separate searches were completed for “keloid” and “HTS.” The exact search terminology used can be found in Table 1. The search initially yielded 1161 and 1275 articles pertaining to keloids and HTS, respectively, for a combined total of 2436 articles. In total, 1548 articles were reviewed by title and abstract for exclusion criteria. To increase consistency among reviewers, this search was conducted independently by 2 of the authors. Both searches yielded the same results.
Table 1.
Search Strategies for the Web of Science Database
| 1. TS = (keloid or keloidal scar) AND TS = (treatment) PY = (2014–2019) AND LANGUAGE: (English) AND DOCUMENT TYPES (Article) |
| 2. TS (hypertrophic scars) AND TS = (treatment) PY = (2014–2019) AND LANGUAGE: (English) AND DOCUMENT TYPES (Article) |
An estimated 398 articles remained to be reviewed by full text. All RCT articles (n = 11) were included in the review at this eligibility point. Non-RCT articles with <8 citations were excluded. A total of 15 articles were included in this scoping review. The selection process for included articles is shown in Figure 1 (see also Table 2).
Fig. 1.
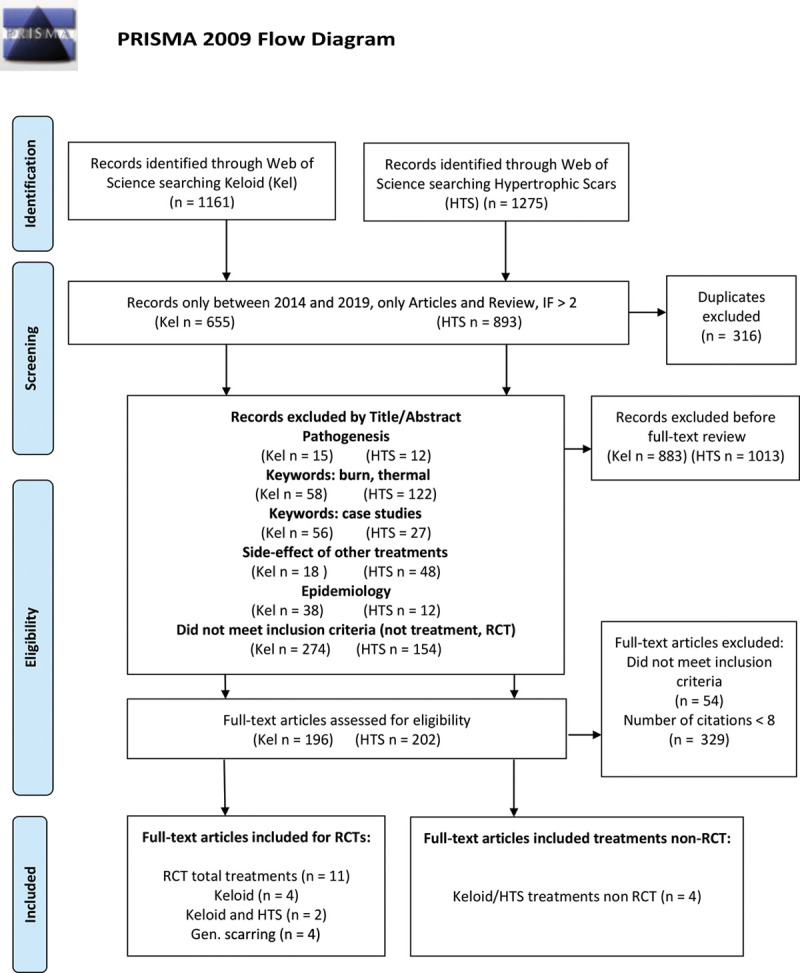
PRISMA flow diagram of systematic review.
Table 2.
Summary of RCTs in the Last 5 Years (between 2014 and 2019) Discovered in this Literature Search
| Reference | Intervention | Study Size (n) | Scar Type | Scar Location | Median Follow-up | Outcomes |
|---|---|---|---|---|---|---|
| Surgical and adjuvant treatment | ||||||
| Khalid et al1 | Excision followed by 5-FU/TAC or radiotherapy | 60 | Keloid | Ear | 20 mo | 5-FU/TAC: recurrence 26.67% at 7 mo |
| Radiotherapy: recurrence 56.6% at 6 mo | ||||||
| Bijlard et al2 | Primary keloid or resistant keloid excision followed by TAC, intralesional cryotherapy, or brachy therapy | 26 | Keloid | Thorax, ear, extremities, back, abdomen, cheek, and neck | 1 y | Primary TAC: 80% recurrence |
| Primary cryotherapy: 25% recurrence | ||||||
| Resistant brachytherapy: 0% recurrence | ||||||
| Resistant cryotherapy: 22% | ||||||
| Jensen et al3 | Excision followed by anti-CTGF oligonucleotide injection (EXC 001) or placebo injection | 21 | Hypertrophic | Breast | 24 wk | 86% reduced scar severity by Patient and Observer Scar Assessment Scale |
| Lin et al4 | Excision followed by silicon sheets or silicone gel | 32 | Non-pathologic | Cesarean incision | 12 mo | Keloid and hypertrophic scar subgroup (n = 7) had no statistical difference in VSS for either treatment |
| Nonsurgical treatment | ||||||
| Khalid et al5 | Intralesional TAC or intralesional TAC/5-FU | 69 | Keloid and hypertrophic | Pre-sternal, head, neck, ears | 22 mo | TAC: recurrence 39.2% at 10 mo |
| TAC/5-FU: recurrence 17.5% at 10 mo | ||||||
| Hietanen et al6 | Intralesional TAC or intralesional 5-FU | 50 | Keloid | Chest, shoulder, back, abdomen | 6 mo | TAC: remission rate 60% at 6 mo |
| 5-FU: remission rate 46% at 6 mo | ||||||
| Abedini et al 7 | Intralesional verapamil or intralesional corticosteroids | 50 | Keloid and hypertrophic | Chest, extremities, back | 3 mo | VSS scores did not change significantly with the use of either therapy after 3 mo; verapamil not considered an effective alternative to TAC |
| Karmisholt et al 8 | AFXL or no laser treatment | 11 | Normotrophic and hypertrophic | Cesarean incision | 6 mo | Erythema with AFXL peaks at 1 mo, pigmentation is still apparent at 3 mo, and improvement witnessed after 6 mo |
| Keaney et al9 | KTP laser or PDL laser | 20 | Erythematous and hypertrophic | Abdomen, breast, back, extremities, and chest | 12 wk | KTP treatment: statistically significant improvement of vascularity of VSS score; overall KTP laser safe and as effective as PDL laser for surgical scars |
| van Drogee et al10 | Ablative fractional CO2 laser or no laser treatment | 25 | Atrophic and hypertrophic | Breast, extremities, back, and facial | 6 mo | Ablative fractional CO2 laser not effective for every scar type; efficacy of this treatment could not be determined |
AFXL, ablative fractional laser; POSAS, Patient and Observer Scar Assessment Scale.
RESULTS
Nonsurgical Treatments
In this section, we describe the nonsurgical treatments for keloid and HTS. We include the proposed mechanism of action, typical dosage recommendations, rates of recurrence, and possible side effects of treatment.
Intralesional Triamcinolone Acetonide
The most widely used treatment for both pathologic scar types is intralesional triamcinolone acetonide (TAC) at concentrations of 10–40 mg/mL.11 Recurrence rates with TAC can be as high as 50%, in 5 years.12 TAC reduces collagen synthesis and, thus, can lead to a softening and reduction in the scar size. Adverse effects are common and include skin atrophy, hypopigmentation, and telangiectasias at the injection site.
An RCT by Khalid et al compared the use of intralesional TAC with that of intralesional TAC/5-Fluorouracil (5-FU) combination. The treatment arm that received the combination therapy of TAC/5-FU (a weekly injection of 4 mg of TAC and 45 mg of 5-FU) had a lower recurrence rate of 17.5% at 10 months versus 39.2% at 10 months with the use of 10-mg intralesional TAC alone.5 Scar height in the combination treatment group was significantly improved compared with that in the only TAC injections group, and both treatments resulted in a scar height reduction of >50%.5
Intralesional Fluorouracil
5-FU is the second line injectable for resistant scarring.13 Concentrations of 5-FU used for injections are 50 mg/mL. Recurrence rates between 25% and 47% are reported.14 5-FU is thought to induce apoptosis and reduce TGF-beta signaling, limiting the amount of collagen type 1 formation.13 Reported side effects of 5-FU injection include skin necrosis, pain, wound dehiscence, and ulcerations.15,16
Hietanen et al performed an RCT comparing treatment of keloids with intralesional TAC versus 5-FU injections. The TAC treatment group had a remission rate of 60% at 6 months compared with a remission rate of 46% at 6 months after 5-FU injections.6 On average, lesions were 1400 mm2, but the degree of flattening was not measured. Limitations of this study are the follow-up time of 6 months and the fact that 60% of the treatment groups had received TAC treatment previously.6
Verapamil
Verapamil is a calcium channel blocker commonly used for controlling hypertension and migraines, and possibly has an expanded role in scar treatment. Verapamil was shown to increase procollagenases but reduce the expression of collagen and fibronectin, as well as the depolymerization of actin fibers.17,18 Many studies indicate little to no side effects from the use of verapamil compared with other treatments, other than pain at the injection site or marked recurrence.7 Its efficacy as an agent for treating keloids and HTS, has varied across several studies.
In a 2018 RCT by Abedini et al, verapamil was compared with intralesional corticosteroids. Keloids and HTS were included in this study. It was found that verapamil barely improved Vancouver Scar Scale (VSS) scores by 7.33% compared with TAC, which showed a VSS score improvement of 68.81% for both scar types. In conclusion, verapamil did not appear to be capable of treating either HTS or keloidal scars.7
Cryotherapy
Cryotherapy is the delivery of liquid nitrogen to the scar tissue without damaging the epidermis or surrounding areas, via an uninsulated cannula through the length of the scar.19 Treatment consists of freeze and thaw cycles lasting 10–30 seconds that are repeated 3 times per session and occur every 4–6 weeks.20 Mechanism of action involves cellular damage and local ischemic necrosis. Side effects of cryotherapy include skin atrophy and hyperpigmentation.21 One hospital-based uncontrolled study by Barara et al, with a sample of 30 patients, showed that after 3 or 6 sessions of cryotherapy, the lesions flattened by 30.76% or 58.13%, respectively. It was concluded that cryotherapy treatments are best suited for treating newly formed keloids and smaller lesions.22 An RCT by Bijlard et al compared intralesional cryotherapy versus excision with brachytherapy, and found that cryotherapy was inferior to this treatment, leading to termination of the study.2
Silicone Gel
Silicone gel sheets or silicone gel may be placed over the scars to assist with wound healing. This is accomplished by maintaining the moisture content of the stratum corneum or by possibly reducing the infiltration of mast cells.23,24 Reported side effects of silicone include skin itching, skin maceration, and skin irritation.25,26
In a 1999 study by Berman et al with a sample of 32 patients, using silicone gel cushions and silicone gel sheets to treat keloids and HTS, the lesion volumes of both scar types decreased up to 53.0% using gel cushions, but there was no statistical difference between the 2 silicone treatments. These were smaller lesions measuring between 0.44 and 1.35 mm3.27 The use of silicone in this study appeared to improve moderate to severe pruritus.27 An RCT to prevent HTS with silicone gel, published in 2004 by Chan et al, showed that sternotomy scars treated with silicone gel scored lower on VSS, with statistically significant improvements in all parameters.25
Pressure Therapy
Pressure therapy may be used independently or in conjunction with surgical removal. Pressure therapy is thought to reduce oxygenation of the wound through vessel obstruction, leading to less fibroblast proliferation and sequential reduction in collagen deposition.28 Anatomical locations where the pressure therapy is the most suitable are the limbs and the trunk, but areas of flexion or concavity such as the abdomen do not achieve the optimal pressures needed for treatment benefits.29 Pressure (in mm Hg) has not been scientifically determined for best scar outcomes, but most articles indicate a capillary pressure of 25 mm Hg or greater.30 A 2011 RCT comparing pressure therapy alone or in combination with silicone sheets showed that pressure alone therapy was equivalent to the combination therapy.31 More research is required to determine the adequate pressures required and efficacy of this treatment.
Radiotherapy
Radiotherapy as a solo treatment is controversial. Modalities for radiation therapy administration range from x-rays, electron beam, to brachytherapy.32 No specific radiation dosage or administration type has been accepted as the gold standard. Recurrence rates of radiotherapy as a single treatment modality range from 50% to 100%.33 Side effects of radiation therapy include dermatitis, fibrosis, and telangiectasias, but the most concerning is malignancies, which can occur years later.2
Laser Therapy
The lasers that are commonly used for keloids and HTS are the pulsed dye laser (PDL), 1064-nm Neodym:YAG laser, and Fractional carbon dioxide lasers.13 PDLs are the gold standard treatment for treating HTS and provide improvement to erythema, thickness, and pruritus.9 The mechanism of action involves collagen remodeling and decreased fibroblasts in the tissue treated.34 Fractional carbon dioxide lasers function through photothermolysis to further wound healing and help replace old disorganized collagen with physiologically normal collagen.35 Collagen remodeling is hypothesized to occur due to increased levels of Matrix metallopeptidases-9.36 Side effect profiles from lasers range from mild erythema, purpura, hypopigmentation, or hyperpigmentation, to possible ulceration.9,10
Keaney et al performed an RCT comparing the 532-nm Potassium Titanyl Phosphate (KTP) laser with the 595 nm PDL laser for erythematous and HTS. Patients received both treatments to different scar areas or to separate areas of 1 scar if its length was >5 cm. The study showed that KTP was as effective as PDL for surgical scars. However, the KTP had limitations such as a higher likelihood of hypopigmentation due to its ability to affect melanin and was a more painful modality. KTP laser did outperform the PDL in terms of VSS score with a statistically significant improvement in vascularity.9
Three studies were reviewed regarding fractional carbon dioxide (CO2) lasers. Van Drooge et al reviewed the ablative fractional CO2 laser versus no laser treatment in an intra–individual split-lesion RCT to determine its efficacy for various types of scars. HTS represented 48% of the sample size, and keloidal scars were excluded. From the study, the efficacy of ablative fractional CO2 laser could not be concluded.10
Karmisholt et al completed an RCT comparing ablative fractional CO2 laser with no laser treatment in an intra-individual split-scar study of cesarean scars. In this study, 36.4% of scars were hypertrophic in character. They concluded that laser-related skin color changes mask the overall scar improvement compared with no treatment up to 3 months after laser therapy.8
Azzam et al performed a prospective randomized intra-individual comparative clinical trial to explore the effects of fractional CO2 lasers on keloids and HTS through histology. Laser treatment induced collagen remodeling and reduced mean collagen percentages.35
Surgical Treatments
Surgical excision is currently indicated for keloids that are resistant to nonsurgical approaches. Surgical approaches should be combined with another therapy due to high recurrence rates of 50%–80%.37 Below we review the various combinations of excision and adjuvant therapies found in our literature search, and we discuss other relevant studies.
Excision Combined with Adjuvant Intralesional TAC
Excision followed by TAC injections is widely used as a treatment for keloids and HTS. Recurrence rates of excision followed by postoperative TAC are reported to range between 8% and 50% over 5 years.33 An RCT by Bijlard et al compared intralesional cryotherapy with excision with corticosteroid injections for primary keloids (no prior intervention). The postoperative TAC treatment arm had a recurrence rate of 80% compared with the intralesional cryotherapy with a recurrence of 25%. However, the treatment satisfaction with the excision and TAC was higher.2
Excision Combined with Adjuvant Intralesional Fluorouracil
Khalid et al completed an RCT that compared 5-FU in combination with TAC following excision versus brachytherapy following excision to determine the efficacy of 5-FU. This study showed that recurrence rate after surgical excision of ear keloids treated with the 5-FU/TAC combination was 26.67% at 7 months compared with 56.6% reoccurrence at 6 months for brachytherapy.1 A study by Kare et al treating 24 ear keloid patients post-excisionally with 5-FU injections maintained a low recurrence rate of 3.57%.16
Excision Combined with Adjuvant Verapamil
Danielsen et al completed a double-blinded RCT comparing excision followed by verapamil or excision followed by TAC. It was determined that recurrence of keloidal scars after treatment with verapamil is significantly higher than with TAC.18
Excision Combined with Adjuvant Silicone Gel
A 1994 study with 8 patients used topical silicone gel following surgical removal of keloids and HTS with a CO2 laser, and compared recurrence with only surgical removal via CO2 laser. The patients who received combination therapy had keloid recurrence of 12.5% compared with 37.5% who did not receive silicone gel postoperatively.38 In phase III of the study, 80% of keloid patients thought that there was only a minimal scar thickness change.38 In another study, Lin et al completed an RCT comparing silicone sheets versus silicone gel on scars and indicated no statistical difference between both treatment for Keloids and that for HTS.4
Excision Combined with Adjuvant Pressure Therapy
Park et al treated helical rim keloids by surgical excision followed by pressure therapy of magnets and silicone gel sheets, which resulted in a reoccurrence rate of 5% after 18 months.39
Excision Combined with Radiation Therapy
Electron beam radiotherapy has the ability to penetrate 2–6 cm and is ideal for skin treatments. Ogawa et al studied 370 keloid and HTS excisions with postoperative radiation and recommends varying doses per treatment area. Keloids and resistant HTS at an elevated risk for recurrence require 20 grays (Gy) × 4 fractions within a 4-day period. Ear keloids can be effectively treated with 10 Gy × 2 for a 2-day period. This study found a total recurrence with excision followed by electron beam radiation of 14.0%.40
Bijlard et al treated patients with high-dose-rate brachytherapy after excision. Patients were treated with 2 × 9 Gy, 3 × 6 Gy or 2 × 6 Gy, and no statistical difference for recurrence was found between the fractionations of treatment. Thus, it was concluded that high-dose-rate brachytherapy (2 × 6 Gy) with a dose of 20 Gy (biologically equivalent dose) should be recommended based on an overall recurrence rate of 8.3% and low complications rates.40
An RCT by Bijlard et al compared intralesional cryotherapy with excision with brachytherapy for resistant keloids.2 With brachytherapy, in this study, none of the excised keloids recurred within 1-year of follow-up. In summary, from both of the Bijlard et al studies, it was found that resistant keloids improve with brachytherapy after excisional treatment.
Other Treatments Used Post-surgical Incisions
Other treatment modalities such as injectables and topicals are emerging in RCTs for various types of scars. Jensen et al completed a within-subject RCT comparing postexcisional treatment with anti-connective tissue growth factor oligonucleotide injection versus placebo injection on breast HTS revision. Patients were treated with anti-connective tissue growth factor on 1 breast and placebo injection on the incision site of the other. Per Patient and Observer Scar Assessment Scale score, scar severity was reduced by 86% with anti-connective tissue growth factor.3
Anesthetic for Keloid and HTS Treatment
Injection treatments for keloids and HTS can be excruciatingly painful, which may limit patient compliance and ability to continue treatment. An RCT by Usanakornkul and Burusapat41 compared the use of topical lidocaine, injected lidocaine, placebo controls, and a combination of topical and injected lidocaine in order to determine the efficacy of each treatment in pain management. The study concluded that injected lidocaine provided no relief, and topical lidocaine provided needle stick pain relief. Vibrational anesthesia for pain reduction during intralesional steroid injection for keloids was explored by Park et al. The study concluded that vibrator-assisted anesthesia was well tolerated, and patient self-reported pain score reduced significantly compared with no vibrational anesthesia.42
DISCUSSION
After reviewing recent RCTs, prospective studies, and retrospective studies, we cannot recommend any treatment modality over the other: nonsurgical or surgical with adjuvant therapy. In our review, however, scars treated with surgical removal are typically secondary or refractory scars. This may lead to higher recurrence rates in the surgical studies, making recurrence rates appear comparable to nonsurgical treatments. We speculate that comparing recurrence rates between nonsurgical and surgical scar correction may not be entirely accurate. Discretion must also be utilized when comparing outcome or effectiveness of treatment. Surgical treatment inherently remove the scar, whereas nonsurgical treatments result in relative degrees of flattening, not resolution. This should be taken into account in nonsurgical studies, as a way to determine treatment efficacy in addition to recurrence rates. Therefore, we agree with the conclusions of Khansa et al in their brief review of keloid treatment, that a multimodal treatment is necessary for keloids.43
At the University of Colorado Division of Plastic Surgery, all keloids are treated aggressively to limit recurrence after excision. Excision is followed by intralesional 5-FU injections, which were commenced 2 weeks after excision and continued weekly up to 7 times. The concentration of 5-FU used for injections is 50 mg/mL, with a dosing of 4–8 mg/cm of scar length. If a hypertrophic scar forms in the excision site, treatment with 5-FU may be extended. The last treatment provided at the 9-week mark is a combination of 5-FU and TAC to provide steroidal benefits to the scar bed. TAC is not performed throughout the weekly treatments due to the high likelihood of skin atrophy, which would lead to an unfavorable aesthetic outcome. Twenty-six keloids from 15 patients have been treated with this protocol and returned for a median follow-up time of 20 months. Four of the keloids treated developed into hypertrophic scarring, and no keloid recurrence was noted (Fig. 2).
Fig. 2.

Schematic of Keloid Treatment Protocol employed by the University of Colorado School of Medicine Plastic and Reconstructive Surgery.
Here, we propose that further clinical research is needed to explore treatments for primary keloids with surgical excision followed by adjuvant 5-FU or brachytherapy compared with nonexcisional therapy. Surgical treatments are typically reserved for refractory keloids due to a concern that keloid excision has high recurrence rates. Brachytherapy and 5-FU have shown promising results but have not been compared directly after excisional therapy. A direct comparison of these surgical regimens with adjuvant treatment to nonexcisional management may identify patients who may benefit more from surgery than from other therapies and may streamline their care.
Footnotes
Published online 22 March 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Khalid FA, Farooq UK, Saleem M, et al. The efficacy of excision followed by intralesional 5-fluorouracil and triamcinolone acetonide versus excision followed by radiotherapy in the treatment of ear keloids: a randomized control trial. Burns. 2018; 44:1489–1495. [DOI] [PubMed] [Google Scholar]
- 2.Bijlard E, Timman R, Verduijn GM, et al. Intralesional cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid treatment: randomised controlled trials. J Plast Reconstr Aesthet Surg. 2018; 71:847–856. [DOI] [PubMed] [Google Scholar]
- 3.Jensen J, Gentzkow G, Berman G, et al. Anti-CTGF oligonucleotide reduces severity of postsurgical hypertrophic scars in a randomized, double-blind, within-subject, placebo-controlled study. Plast Reconstr Surg. 2018; 142:192e–201e. [DOI] [PubMed] [Google Scholar]
- 4.Lin YS, Ting PS, Hsu KC. Does the form of dressings matter?: a comparison of the efficacy in the management of postoperative scars between silicone sheets and silicone gel: a randomized controlled trial. Medicine (Baltimore). 2018; 97:e11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Hietanen KE, Järvinen TA, Huhtala H, et al. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections – a randomized controlled trial. J Plastic, Reconstr Aesthet Surg. 2019; 72:4–11. [DOI] [PubMed] [Google Scholar]
- 7.Abedini R, Sasani P, Mahmoudi HR, et al. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: a randomized controlled trial. Burns. 2018; 44:1482–1488. [DOI] [PubMed] [Google Scholar]
- 8.Karmisholt KE, Taudorf EH, Wulff CB, et al. Fractional CO2 laser treatment of caesarean section scars—A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg Med. 2017; 49:189–197. [DOI] [PubMed] [Google Scholar]
- 9.Keaney TC, Tanzi E, Alster T. Comparison of 532 nm potassium titanyl phosphate laser and 595 nm pulsed dye laser in the treatment of erythematous surgical scars: a randomized, controlled, open-label study. Dermatol Surg. 2016; 42:70–76. [DOI] [PubMed] [Google Scholar]
- 10.van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015; 41:371–377. [DOI] [PubMed] [Google Scholar]
- 11.Lumenta DB, Siepmann E, Kamolz LP. Internet-based survey on current practice for evaluation, prevention, and treatment of scars, hypertrophic scars, and keloids. Wound Repair Regen. 2014; 22:483–491. [DOI] [PubMed] [Google Scholar]
- 12.Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018; 11:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013; 6:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment: a systematic review. Acta Derm Venereol. 2015; 95:778–782. [DOI] [PubMed] [Google Scholar]
- 15.Saha AK, Mukhopadhyay M. A comparative clinical study on role of 5-flurouracil versus triamcinolone in the treatment of keloids. Indian J Surg. 2012; 74:326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khare N, Patil SB. A novel approach for management of ear keloids: results of excision combined with 5-fluorouracil injection. J Plast Reconstr Aesthet Surg. 2012; 65:e315–e317. [DOI] [PubMed] [Google Scholar]
- 17.Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol. 2008; 74:343–348. [DOI] [PubMed] [Google Scholar]
- 18.Danielsen PL, Rea SM, Wood FM, et al. Verapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trial. Acta Derm Venereol. 2016; 96:774–778. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg G, Luber AJ. Use of intralesional cryosurgery as an innovative therapy for keloid scars and a review of current treatments. J Clin Aesthet Dermatol. 2013; 6:23–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. A prospective consecutive trial of case series. Arch Dermatol. 1993; 129:1146–1151. [PubMed] [Google Scholar]
- 21.van Leeuwen MCE, Bulstra AEJ, Ket JCF, et al. Intralesional cryotherapy for the treatment of keloid scars: evaluating effectiveness. Plastic Reconstr Surg Glob Open. 2015; 3:e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barara M, Mendiratta V, Chander R. Cryotherapy in treatment of keloids: evaluation of factors affecting treatment outcome. J Cutan Aesthet Surg. 2012; 5:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CC, Kuo YF, Chiu HC, et al. Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res. 1995; 59:705–711. [DOI] [PubMed] [Google Scholar]
- 24.Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target of mast cells. J Dermatolog Treat. 2003; 14:248–252. [DOI] [PubMed] [Google Scholar]
- 25.Chan KY, Lau CL, Adeeb SM, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005; 116:1013–1020. [DOI] [PubMed] [Google Scholar]
- 26.Westra I, Pham H, Niessen FB. Topical silicone sheet application in the treatment of hypertrophic scars and keloids. J Clin Aesthet Dermatol. 2016; 9:28–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Berman B, Flores F. Comparison of a silicone gel-filled cushion and silicon gel sheeting for the treatment of hypertrophic or keloid scars. Dermatol Surg. 1999; 25:484–486. [DOI] [PubMed] [Google Scholar]
- 28.Alkhalil A, Carney BC, Travis TE, et al. Key cell functions are modulated by compression in an animal model of hypertrophic scar. Wounds. 2018; 30:353–362. [PubMed] [Google Scholar]
- 29.Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014; 40:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puzey G. The use of pressure garments on hypertrophic scars. J Tissue Viability. 2002; 12:11–15. [DOI] [PubMed] [Google Scholar]
- 31.Steinstraesser L, Flak E, Witte B, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg. 2011; 128:306e–313e. [DOI] [PubMed] [Google Scholar]
- 32.Mankowski P, Kanevsky J, Tomlinson J, et al. Optimizing radiotherapy for keloids: a meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann Plast Surg. 2017; 78:403–411. [DOI] [PubMed] [Google Scholar]
- 33.Mustoe TA, Cooter RD, Gold MH, et al. ; International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002; 110:560–571. [DOI] [PubMed] [Google Scholar]
- 34.Alster T. Laser scar revision: comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003; 29:25–29. [DOI] [PubMed] [Google Scholar]
- 35.Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016; 31:9–18. [DOI] [PubMed] [Google Scholar]
- 36.Reilly MJ, Cohen M, Hokugo A, et al. Molecular effects of fractional carbon dioxide laser resurfacing on photodamaged human skin. Arch Facial Plast Surg. 2010; 12:321–325. [DOI] [PubMed] [Google Scholar]
- 37.Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993; 26:245–251. [DOI] [PubMed] [Google Scholar]
- 38.Gold MH. A controlled clinical trial of topical silicone gel sheeting in the treatment of hypertrophic scars and keloids. J Am Acad Dermatol. 1994; 30:506–507. [DOI] [PubMed] [Google Scholar]
- 39.Park TH, Rah DK. Successful eradication of helical rim keloids with surgical excision followed by pressure therapy using a combination of magnets and silicone gel sheeting. Int Wound J. 2017; 14:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bijlard E, Verduijn GM, Harmeling JX, et al. Optimal high-dose-rate brachytherapy fractionation scheme after keloid excision: a retrospective multicenter comparison of recurrence rates and complications. Int J Radiat Oncol Biol Phys. 2018; 100:679–686. [DOI] [PubMed] [Google Scholar]
- 41.Usanakornkul A, Burusapat C. A topical anesthetic and lidocaine mixture for pain relief during keloid treatment: a double-blind, randomized controlled trial. Dermatol Surg. 2017; 43:66–73. [DOI] [PubMed] [Google Scholar]
- 42.Park KY, Lee Y, Hong JY, et al. Vibration anesthesia for pain reduction during intralesional steroid injection for keloid treatment. Dermatol Surg. 2017; 43:724–727. [DOI] [PubMed] [Google Scholar]
- 43.Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2016; 138(3 suppl):165S–178S. [DOI] [PubMed] [Google Scholar]


