Abstract
Disentangling the separate and synergistic effects of chemicals poses methodological challenges for accurate exposure assessment and for investigating epidemiologically how chemicals affect reproduction. We investigated combined exposures to ubiquitous contemporary use pesticides, specifically organophosphates (OP) and pyrethroids (PYR), and their association with germ cell abnormalities among adult men. Fluorescence in situ hybridization was used to determine disomy in sperm nuclei and urine was analyzed for concentrations of PYR metabolites (3-phenoxybenzoic acid; 3PBA) and OP dialkyl phosphate (DAP) metabolites. Incidence rate ratios using Poisson models were estimated for each disomy type by exposure quartile of DAP metabolites and 3PBA, controlling for confounders. The shape of the associations between PYRs, OPs and disomy were frequently nonmonotonic. There were consistent interactions between OP and PYR metabolite concentrations and the risk for sperm abnormalities. Taking both chemicals into account simultaneously resulted in quantitatively different associations than what was reported previously for OPs and PYRs separately, demonstrating the importance of modeling multiple concentrations simultaneously. Methods investigating interactions using Poisson models are needed to better quantify chemical interactions and their effects on count-based health outcomes, the importance of which was shown here for germ cell abnormalities.
Keywords: Aneuploidy, Endocrine disruptors, In situ hybridization, Pesticide, Reproduction, Interactions
1. Introduction
Investigating interactions between environmental chemicals and their effects on human reproduction pose methodological challenges for environmental health and reproductive biology (Woodruff et al., 2008; Taylor et al., 2016) and considerable environmental epidemiology, toxicology, and exposure assessment expertise is being invested in developing statistical approaches to disentangle the human health effects of combined exposure to chemicals (Carlin et al., 2013; Grandjean and Landrigan, 2014; Claus Henn et al., 2014; Goodson et al., 2015; Braun et al., 2016). Real-world exposures to a specific environmental contaminant do not occur as single discrete events, but rather in combination to several toxicants at once (US EPA 2001; WHO, 2009; Zeliger, 2011). Chemical compounds interact with each other and with biological systems and they can alter the toxicity of individual compounds even in low doses (Zeliger, 2011; Tsatsakis et al., 2009; Hernández et al., 2013). Observable adverse effect levels may be different for chemical mixtures, as different toxicants may affect the body at different points along the biological pathway (Ray and Forshaw, 2000). Environmental toxicants with endocrine disrupting properties can affect spermatogenesis (Schiffer et al., 2014) and congenital abnormalities and nonviable pregnancies are related to problems during spermatogenesis, including poor sperm DNA integrity and increased human sperm aneuploidy (Jacobs, 1992; Hassold and Hunt, 2001; Cheng et al., 2011; Nagaoka et al., 2012). Because sex chromosomes (X and Y) are particularly susceptible to aneuploidy, researchers have attempted to understand the paternal role in sex chromosome disomy, the most common type of aneuploidy (Hassold and Hunt, 2001; Martin et al., 1991).
Despite advances in studying germ cell abnormalities, the exact causes of aneuploidy and/or the specific critical windows of chemical susceptibility associated with aneuploidy risk remain unknown (Herrera et al., 2008; Axelsson et al., 2010; Ashton Acton, 2013).
Regulatory assessments do not consistently identify the interactive effects that can occur between chemicals (Teuschler et al., 2004; Hernández et al., 2013; US EPA 2013; NAS, 2014). Organophosphate (OP) and pyrethroid (PYR) insecticides account for a large share of all US insecticide use. OPs are most frequently used in agriculture, recreational and commercial areas, while PYRs are regularly used in homes and gardens. Approximately 82 million US households use insecticides (US EPA, 2017).
Urinary concentrations of OP (such as dialkyl phosphates or DAPs) and PYR (3-phenoxybenzoic acid or 3PBA) metabolites have been measured in the general population (CDC, 2019). Adverse effects related to OP and PYR metabolite concentrations have been demonstrated for hormone functions (Meeker et al. 2006, 2009; Lacasaῆa et al., 2010), semen parameters and sperm DNA damage/fragmentation (Meeker et al. 2004b, 2008; Lifeng et al., 2006; Perry et al., 2007b; Recio-Vega et al., 2008; Yucra et al., 2008; Xia et al., 2008; Hossain et al., 2010; Ji et al., 2011; Toshima et al., 2012), sperm chromatin structure alteration (Sanchez-Pena et al., 2004) and sex chromosome disomy in human sperm (Padungtod et al., 1999; Recio et al., 2001; Young et al., 2013; Radwan et al., 2015).
To our knowledge, very limited information exists about the reproductive health effects of combined environmental exposures to pesticides and their association with sperm chromosomal abnormalities. In this study we examined interactions between OP and PYR metabolite concentrations and their association with the frequency of sperm sex chromosome disomy, using samples from adult men. We evaluated the hypothesis that OP and PYR interactions alter associations with sperm chromosomal abnormalities.
2. Methods
2.1. Study subjects
Participants (n = 159) were selected from a previous study of couples seeking infertility evaluation at Massachusetts General Hospital (MGH) Fertility Center. The parent study (n = 341) was conducted between January 2000–May 2003 and assessed the impact of environmental exposures on semen quality. A detailed description of the parent study has been provided elsewhere (Hauser et al., 2003). Approximately 65% of eligible subjects aged 20–54 agreed to participate in the parent study. Lack of participation was due to lack of time during their clinic visit. Men receiving treatment for infertility and/or scheduled for post-vasectomy semen analysis were excluded from the parent study. Information on demographics, medical and fertility history, and lifestyle factors were obtained by a self-administered questionnaire, and urine and semen samples were collected on the same day. Occupational exposure to pesticides or other agents was not reported (i.e., study population was selected without attention to specific occupational exposure). A prior retrospective review of anonymized non-participants’ clinical records, who met the same eligibility found no differences between participants and non-participants in regards to age or semen parameters (Duty et al., 2005). Because urine and semen from the parent study (n = 341) had been used for other analyses, inclusion in this study was based on sample availability in the biorepository (n = 159 or 47%). Signed informed consent was obtained from each participant. The parent study was approved by the Massachusetts General Hospital Human Subjects Committees and the Harvard School of Public Health. This study was approved by the Office of Human Research of the George Washington University.
2.2. Semen analysis
Semen collection and analysis have been previously described (Hauser et al., 2003). Semen samples were collected at the clinic via masturbation. Participants were asked to abstain from ejaculation for at least 48 h prior to sample collection. Semen samples were liquefied at 37 °C for 20 min before analysis. Andrologists from the MGH Andrology Laboratory analyzed the samples; they were blinded to exposure status. Volume, pH, color, and viscosity properties were determined for each semen sample. A computer-aided sperm analysis (CASA) using the Hamilton-Thorn Motility Analyzer (10HTM-IVO) was used to determine sperm count and percent motility. Two slides per sample were prepared for a morphological assessment. An oil immersion microscope lens with a 100x objective was used for this analysis (Nikon Company, Tokyo, Japan). Sperm were scored normal or abnormal using the Tygerberg Strict Criteria for morphology (< 4% normal morphology) described by Kruger et al., 1988.
2.3. Disomy analysis
In germ cells, failure of sex chromosomes (X or Y) to separate properly during meiosis results in extra or missing chromosomes, known as aneuploidy. Sex chromosome disomy, or an extra X or Y chromosome, is the most frequent form of aneuploidy observed in human sperm and was the primary outcome of interest in this study. Semen samples were stored in −80 °C without cryoprotectant until FISH analysis was performed. Disomy detection procedures are published elsewhere (McAuliffe et al., 2012). Laboratory technicians were blinded to exposure status. Fluorescence in situ hybridization (FISH) analysis was performed for chromosomes X, Y and 18 (autosomal control) to determine the presence of disomic sperm or XX18, YY18, XY18 and total sex chromosome disomy in sperm nuclei. For each FISH slide, a sequence of non-overlapping field images was taken. A fluorescence microscope was utilized to take images subsequently scored for size and shape using custom MATLAB (Mathworks Inc., Natick, MA) software. The software was designed to utilize scoring algorithms based on criteria for size and shape as previously described (Baumgartner et al., 1999). Details of the sperm FISH control procedures and validation of the semi-automated scoring method have been reported (Perry et al. 2007a, 2011).
2.4. Urinary OP and PYR measurements
Exposure to OP pesticides was estimated using six DAP urinary metabolites [dimethylphosphate (DMP); dimethylthiophosphate (DMTP); dimethyldithiophosphate (DMDTP); diethylphosphate (DEP); diethylthiophosphate (DETP); and diethyldithiophosphate (DEDTP)] according to methods described previously (Prapamontol et al., 2014). Briefly, urinary samples were analyzed using gas chromatography coupled with mass spectrometry with isotopic dilution quantification. Transformed metabolite concentrations (i.e., each DAP metabolite divided by its molecular weight) were summed and multiplied by 1,000 to obtain a total DAPs (ΣDAP) concentration in units of nmol/mL (Figueroa et al., 2015). Urinary 3PBA metabolite was used to estimate human exposure to PYR pesticides. Urine samples were analyzed using a small modification of the method described by Baker et al. (2004). The samples were spiked with an isotopically labeled analogue to enable isotope dilution quantification. The target analyte was isolated using solid phase extraction. The extract was concentrated prior to analysis by high performance liquid chromatography-tandem mass spectrometry using an Agilent 6460 triple quadruple mass spectrometer (Santa Clara, CA) with Jetstream electrospray ionization. Quality control and blank samples were analyzed jointly with unknown samples to ensure method stability and robustness. The limit of detection (LOD) for each metabolite was 0.6 ng/mL (DMP), 0.2 ng/mL (DMTP), 0.2 ng/mL (DEP), and 0.1 ng/mL (DETP, DMDTP, DEDTP, 3PBA).
Specific gravity and creatinine concentrations were measured in urine samples using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) and kinetic colorimetric assay technology with a Hitachi 911 automated chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA), respectively.
2.5. Statistical analysis
Descriptive statistics were generated for demographic and semen parameters. Semen parameters were dichotomized using the World Health Organization reference values for sperm concentration (< 15 million sperm/mL) and motility (< 32% motile sperm), and the Tygerberg Strict Criteria for morphology (< 4% normal morphology) (Kruger et al., 1988; WHO, 2010). For metabolite values below the LOD, an imputed value equal to one-half the LOD was used (Helsel, 2005). Descriptive statistics for pesticide metabolite concentrations (ng/mL) in urine were summarized. Creatinine and specific gravity adjusted summaries as well as volume-based (unadjusted) urinary values were calculated and compared. The urinary concentration results were similar when using both creatinine and specific gravity adjusted methods. Specific gravity was used in the analysis as an independent variable and crude metabolite concentrations were used in the adjusted models. Urinary metabolite concentrations were not normally distributed and were log transformed prior to examining Pearson correlations to explore associations between individual urinary metabolites.
Poisson regression (SAS GENMOD procedure) was used to model the association between each specific DAP and 3PBA volume-based urinary metabolite concentrations and the disomy measure (i.e., total sex chromosome disomy) due to the large number of sperm being scored and the relatively low frequency of disomy. The number of sperm scored and the number of disomic nuclei were summed separately for each subject; the individual subject was treated as the unit of analysis. The natural logarithm of the number of sperm counted was used as the offset variable to standardize across subjects.
Models were fitted using a disomy measure as the outcome variable (i.e., as a count of disomic cells of total sex chromosome disomy) and the metabolites of interest as the independent variables. Variables considered to have biological plausibility based on prior studies and found to be associated with aneuploidy and/or pesticide exposure were included in the adjusted models. Age, body-mass index (BMI), motility, morphology, log of sperm concentration and specific gravity were included as continuous covariates, along with categoricals smoking and race. Because sperm concentration is generally non-normal and positively skewed (Berman et al., 1996), sperm concentration was log transformed. Incidence rate ratios (IRRs) and 95% confidence intervals (95% CI) were calculated for each model (i.e., for total sex chromosome disomy by quartiles of DAP metabolite (DMP, DMTP, DMDTP, DEP, DETP) and 3PBA quartiles), and the exposure variable was included as an ordinal variable to test for trend. IRRs and 95% CIs were also calculated for total disomy by tertiles of ∑DAP by 3PBA tertiles. Statistical significance was set at p-value < 0.05.
Interactions between each DAP metabolite and 3PBA in association with each disomy outcome were examined. If an interaction was significant, the model IRRs for a specific DAP metabolite were calculated within each quartile of 3PBA. Linear tests for trend for most DAP metabolites and ∑DAP were also performed by 3PBA strata to further investigate the interaction. The interaction term was removed from the model if non-significant. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
3. Results
Table 1 shows the demographic and semen parameters of the study subjects (n = 159). The men had an average age of 35 years and a mean BMI of 28 kg/m2. The majority of the men were white (86%) and non-Hispanic (94%). Most men (74%) had never smoked and 7% were current smokers. Of the 159 men, 10% (n = 16) had sperm concentrations < 15 million/mL, 21% (n = 33) had < 32% motile sperm, and 18% (n = 28) had < 4% normally shaped sperm (Table 1). A median of 6,848 sperm nuclei were scored per subject (Table 2). The observed median percentages of XX18, YY18, XY18, and total sex chromosome disomy were 0.4%, 0.4%, 1.1%, and 1.9%, respectively. Table 3 summarizes the unadjusted urinary OP and PYR metabolite concentrations as well as the specific gravity and creatinine adjusted concentrations. The percent of samples above the LOD ranged from 57 to 89% for most metabolites. All DAP metabolites (r = 0.13–0.60) were weakly to moderately positive correlated. 3PBA was weakly correlated to DAP metabolites (r = −0.009–0.08) (Table 4). There were moderate positive correlations between Total DAPs and individual DAP metabolites (r = 0.20–0.87) and a weak correlation with 3PBA (r = 0.04).
Table 1.
Characteristics of MGH men (n =159).
| Variable | Mean ± SD |
|---|---|
| Age | 35 ± 5 |
| BMI (kg/m2) | 28 ± 5 |
| Race | N (%) |
| White | 137 (86) |
| Black | 5 (3) |
| Other | 17 (11) |
| Hispanic ethnicity No |
149 (94) |
| Yes | 10 (6) |
| Semen Concentration | |
| < 15 million/mL | 16 (10) |
| Semen Morphology | |
| < 4% normal | 28 (18) |
| Semen Motility | |
| < 32% motile | 33 (21) |
| Abstinence time | |
| < =2 days | 35 (22) |
| 3–4 days | 74 (47) |
| > =5 days | 50 (32) |
| Smoking (n = 2 missing) | |
| No | 116 (74) |
| Current smoker | 11 (7) |
| Former smoker | 30 (19) |
Table 2.
Number of sperm nuclei scored and percent disomy of men seeking infertility evaluation (n =159).
| Variable | Mean ± SD | Median | 25th | 75th |
|---|---|---|---|---|
| Nuclei (n) | 6,848 ± 4,815 | 5,503 | 2,939 | 9,976 |
| %X18 | 37.6 ± 9.1 | 39.8 | 33.3 | 44.8 |
| %Y18 | 36.5 ± 8.8 | 39.2 | 32.8 | 43.3 |
| % XX18 | 0.4 ± 0.4 | 0.3 | 0.2 | 0.5 |
| % YY18 | 0.4 ± 0.3 | 0.3 | 0.2 | 0.5 |
| % XY18 | 1.1 ± 0.8 | 0.9 | 0.6 | 1.5 |
| Total Disomy % | 1.9 ± 1.3 | 1.6 | 1.1 | 2.5 |
Table 3.
Distribution of individual pesticide metabolite concentrations in urine of men seeking infertility evaluation (n= 159).
| Metabolitea (ng/mL) | Mean ± SD | Percentile |
Range | ||||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | 95th | |||
| Unadjusted | |||||||
| DMP | 10.77 ± 25.30 | 0.30 | 4.34 | 12.48 | 29.42 | 38.60 | 0.30–270.75 |
| DMTP | 8.62 ± 17.42 | 0.68 | 3.13 | 7.28 | 21.11 | 38.71 | 0.10–148.12 |
| DMDTP | 1.18 ± 2.03 | 0.05 | 0.43 | 1.16 | 3.51 | 5.96 | 0.05–10.32 |
| DEP | 3.68 ± 8.24 | 0.10 | 1.00 | 3.65 | 8.87 | 14.82 | 0.10–63.56 |
| DETP | 1.70 ± 3.48 | 0.05 | 0.60 | 1.46 | 3.83 | 9.27 | 0.05–20.08 |
| DEDTP | 0.09 ± 0.12 | 0.05 | 0.05 | 0.05 | 0.26 | 0.39 | 0.05–0.66 |
| 3PBA | 0.89 ± 1.51 | 0.51 | 0.62 | 0.87 | 1.57 | 2.27 | 0.05–15.18 |
| SG-adjusted | |||||||
| DMP | 11.35 ± 24.04 | 0.51 | 5.17 | 15.39 | 27.51 | 43.11 | 0.20–258.98 |
| DMTP | 9.76 ± 18.07 | 0.85 | 3.73 | 9.13 | 28.62 | 44.19 | 0.05–121.76 |
| DMDTP | 1.41 ± 2.52 | 0.08 | 0.46 | 1.30 | 4.46 | 8.36 | 0.03–15.13 |
| DEP | 3.72 ± 7.48 | 0.22 | 1.33 | 4.14 | 9.33 | 13.70 | 0.05–53.78 |
| DETP | 1.77 ± 2.92 | 0.16 | 0.76 | 1.71 | 4.51 | 7.04 | 0.03–16.98 |
| DEDTP | 0.11 ± 0.13 | 0.05 | 0.06 | 0.11 | 0.29 | 0.43 | 0.03–0.72 |
| 3PBA | 1.15 ± 2.64 | 0.44 | 0.69 | 1.17 | 1.99 | 2.55 | 0.04–27.83 |
| CR-adjusted (missing = 2) | |||||||
| DMP | 7.87 ± 16.26 | 0.47 | 2.57 | 7.24 | 19.80 | 27.86 | 0.12–147.95 |
| DMTP | 7.22 ± 16.21 | 0.61 | 2.59 | 6.07 | 16.14 | 24.91 | 0.02–121.93 |
| DMDTP | 1.02 ± 2.14 | 0.07 | 0.29 | 0.96 | 2.64 | 5.32 | 0.01–18.89 |
| DEP | 2.47 ± 4.71 | 0.19 | 0.78 | 2.30 | 7.07 | 11.22 | 0.03–33.77 |
| DETP | 1.29 ± 2.20 | 0.12 | 0.51 | 1.40 | 3.80 | 5.87 | 0.01–15.60 |
| DEDTP | 0.08 ± 0.09 | 0.03 | 0.05 | 0.10 | 0.19 | 0.48 | 0.01–0.56 |
| 3PBA | 1.08 ± 3.92 | 0.28 | 0.47 | 1.01 | 1.72 | 2.24 | 0.02–46.56 |
Unadjusted urinary metabolite concentration (units) =ng/mL. LOD= 0.6 ng/mL (DMP), 0.2ng/mL (DMTP), 0.2 ng/mL (DEP), and 0.1 ng/mL (DETP, DMDTP, DEDTP, 3PBA). Percent of metabolite samples above the LOD: DMP =57% (n= 91); DMTP = 87% (n =139); DMDTP = 57% (n =90); DEP =64% (n= 101); DETP = 72% (n =114); and DEDTP =10% (n=16); 3PBA= 79% (n =126).
Table 4.
Pearson Correlation Coefficients (p-value) between individual urinary metabolite concentrations (N = 159).
| Metabolitesa | DMP | DMTP | DMDTP | DEP | DETP | DEDTP | ∑DAPs | 3PBA |
|---|---|---|---|---|---|---|---|---|
| DMP | 1.00 | |||||||
| DMTP | 0.57 (< 0.001) | 1.00 | ||||||
| DMDTP | 0.48 (< 0.001) | 0.59 (< 0.001) | 1.00 | |||||
| DEP | 0.60 (< 0.001) | 0.57 (< 0.001) | 0.50 (< 0.001) | 1.00 | ||||
| DETP | 0.33 (< 0.001) | 0.39 (< 0.001) | 0.35 (< 0.001) | 0.54 (< 0.001) | 1.00 | |||
| DEDTP | 0.17 (0.0340) | 0.14 (0.0807) | 0.13 (0.1032) | 0.20 (0.0107) | 0.22 (0.0055) | 1.00 | ||
| ∑DAPs | 0.87 (< 0.001) | 0.82 (< 0.001) | 0.63 (< 0.001) | 0.74 (< 0.001) | 0.49 (< 0.001) | 0.20 (0.0098) | 1.00 | |
| 3PBA | 0.07 (0.3799) | 0.08 (0.2875) | 0.07 (0.3660) | 0.07 (0.3974) | 0.07 (0.3942) | −0.009 (0.9089) | 0.04 (0.5784) | 1.00 |
Unadjusted urinary metabolite concentration (units) = ng/mL. The unadjusted urinary metabolite concentrations were log-transformed prior to examined Pearson correlations.
In most cases, increased disomy rates were observed only in Q1 and/or Q2 and declined in Q3 and Q4 (Table 5). DMDTP and DMTP showed U-shaped association patterns whereas the patterns for DETP showed reverse U-shapes across the quartiles of 3PBA. The highest significant associations for total disomy were observed between the third exposure quartile of DETP and second 3PBA exposure quartile for an IRR = 2.31 (95% CI: 2.02, 2.64). Statistically significant inverse associations were also observed for total disomy by concentrations of DMP and 3PBA. Increase in disomy rates occurred mainly between the second and third exposure quartiles and without substantial additional increases or decreases between the third and fourth exposure quartile, demonstrating nonmonotonic dose-response curves. Statistically significant interactions were observed for total disomy between all DAP metabolites and 3PBA in the adjusted models. The significance of all the interaction terms persisted when variables were modeled continuously (data not shown).
Table 5.
Adjusted IRRs (95% CI, p-value) for total sex-chromosome disomy by Quartiles of DAP Metabolite Concentrations (Exposure 1) by 3PBA Concentrations Quartile (Exposure 2) of men seeking infertility evaluation (n= 159).
| 3PBA Quartilea | DAP Quartileb–f | Total Disomy |
||||
|---|---|---|---|---|---|---|
| DMP IRR (95% CI) | DMTP IRR (95% CI) | DMDTP IRR(95% CI) | DEP IRR (95% CI) | DETP IRR (95% CI) | ||
| Q1 | Q1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 0.75 (0.68, 0.82) | 1.29 (1.18, 1.41) | 1.46 (1.33, 1.61) | 1.33 (1.23, 1.45) | 1.04 (0.94, 1.15) | |
| Q3 | 0.43 (0.33, 0.58) | 1.08 (0.97, 1.19) | 1.03 (0.87, 1.23) | 1.24 (1.13, 1.37) | 2.00 (1.83, 2.19) | |
| Q4 | 0.70 (0.64, 0.77) | 1.13 (0.97, 1.30) | 1.31 (1.19, 1.44) | 1.03 (0.94, 1.13) | 0.96 (0.87, 1.07) | |
| Q2 | Q1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 0.69 (0.64, 0.75) | 1.05 (0.89, 1.24) | 1.36 (1.11, 1.67) | 1.16 (1.00, 1.33) | 0.39 (0.34, 0.44) | |
| Q3 | 0.67 (0.60, 0.74) | 0.76 (0.66, 0.88) | 0.66 (0.53, 0.82) | 1.07 (0.93, 1.23) | 2.31 (2.02, 2.64) | |
| Q4 | 0.67 (0.61, 0.74) | 1.31 (1.09, 1.57) | 1.09 (1.00, 1.19) | 0.62 (0.53, 0.71) | 0.76 (0.69, 0.84) | |
| Q3 | Q1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.13 (1.06, 1.21) | 1.75 (1.48, 2.06) | 1.96 (1.59, 2.40) | 1.68 (1.48, 1.91) | 0.60 (0.53, 0.68) | |
| Q3 | 1.09 (1.02, 1.16) | 0.95 (0.83, 1.07) | 1.07 (0.88, 1.30) | 0.95 (0.84, 1.09) | 1.89 (1.65, 2.16) | |
| Q4 | 0.98 (0.89, 1.07) | 1.26 (1.06, 1.49) | 1.17 (1.08, 1.26) | 0.92 (0.80, 1.06) | 0.87 (0.80, 0.95) | |
| Q4 | Q1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 0.53 (0.48, 0.59) | 1.12 (0.96, 1.32) | 1.67 (1.36, 2.05) | 1.27 (1.11, 1.46) | 0.53 (0.46, 0.61) | |
| Q3 | 0.83 (0.76, 0.91) | 0.94 (0.83, 1.08) | 0.87 (0.71, 1.06) | 1.24 (1.07, 1.43) | 1.28 (1.08, 1.52) | |
| Q4 | 0.92 (0.84, 1.01) | 0.72 (0.61, 0.86) | 0.95 (0.83, 1.07) | 0.57 (0.49, 0.67) | 0.84 (0.76, 0.93) | |
| Interaction p-valueg | 0.0015 | < 0.0001 | 0.0022 | < 0.0001 | < 0.0001 | |
3PBA Exposure Quartiles: Q1 = X ≤ LOD (n = 33), Q2 = 0.10 < X ≤ 0.61 ng/mL (n = 46), Q3 = 0.61 < X ≤ 0.83 ng/mL (n = 39), Q4 = X > 0.83 ng/mL (n = 41).
DMP Exposure Quartiles: Q1 = X ≤ LOD (n = 68), Q2 = 0.60 < X≤ 7.95 ng/mL (n = 30), Q3 = 7.95 < X ≤ 13.39 ng/mL (n = 30), Q4 = X > 13.39 ng/mL (n = 31).
DMTP Exposure Quartiles: Q1 = X ≤ LOD (n = 30), Q2 = 0.20 < X ≤ 2.21 ng/mL (n = 43), Q3 = 2.21 < X ≤ 6.47 ng/mL (n = 42), Q4 = X > 6.47 ng/mL (n = 44).
DMDTP Exposure Quartiles: Q1 = X ≤ LOD (n = 69), Q2 = 0.10 < X ≤ 0.73 ng/mL (n = 30), Q3 =0.73 < X ≤ 1.86 ng/mL (n = 30), Q4 = X > 1.86 ng/mL (n = 30).
DEP Exposure Quartiles: Q1 = X ≤ LOD (n = 58), Q2 = 0.20 < X ≤ 1.46 ng/mL (n = 34), Q3 = 1.46 < X ≤ 3.96 ng/mL (n = 33), Q4 = X > 3.96 ng/mL (n = 34).
DETP Exposure Quartiles: Q1 = X ≤ LOD (n = 45), Q2 = 0.10 < X ≤ 0.62 ng/mL (n = 39), Q3 = 0.62 < X ≤ 1.51 ng/mL (n = 37), Q4 = X > 1.51 ng/mL (n = 38).
P-value of the interaction between DAP metabolites and 3PBA in adjusted models. IRRs were adjusted for specific gravity, age, race, BMI, smoking, sperm total concentration, motility, and morphology.
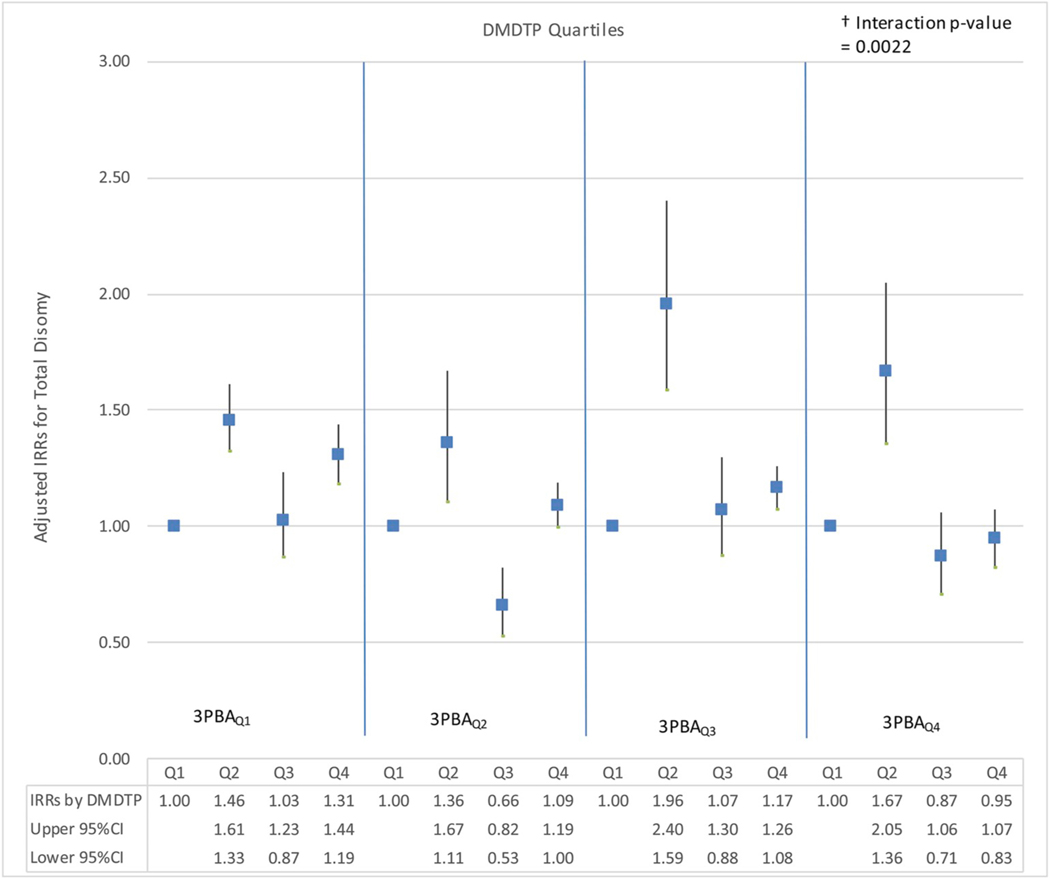
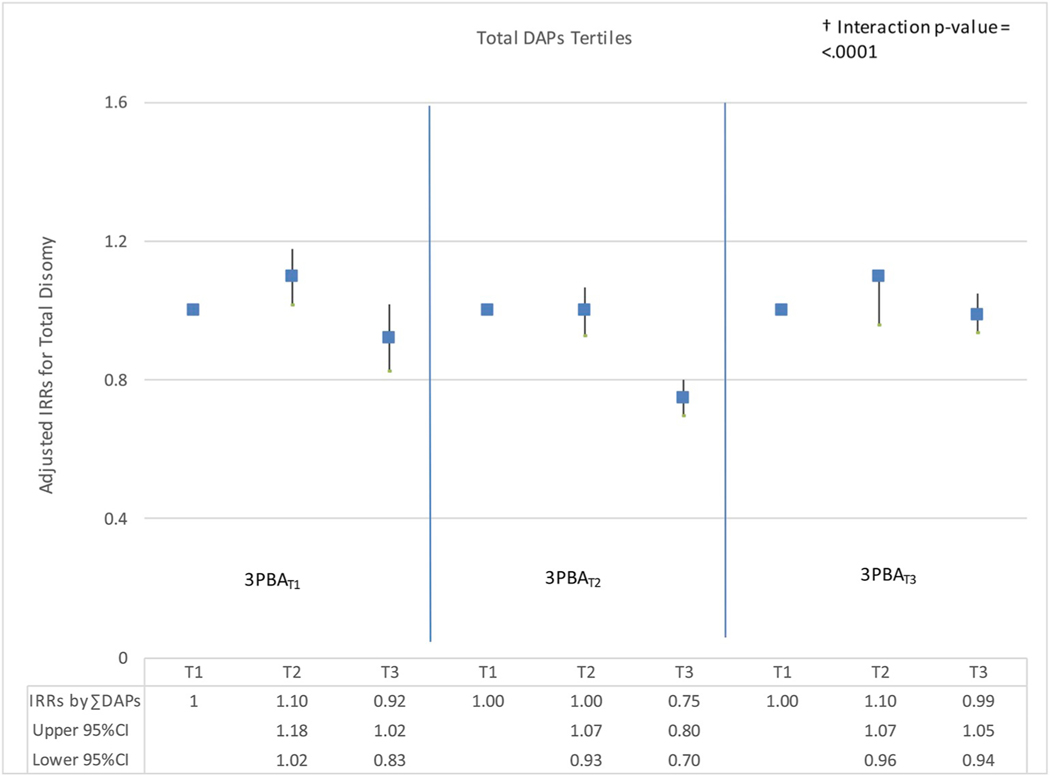
To further investigate interactions between OP and PYR metabolites, graphs of the adjusted IRRs and 95% confidence intervals were examined for total sex chromosome disomy by quartiles of DAP metabolite (DMP, DMTP, DMDTP, DEP, DETP) and 3PBA quartiles. Dose-response relationships appeared nonmonotonic across most quartiles of 3PBA with increasing individual DAP exposure. Both U-shaped and inverted-U shaped relationships were observed across 3PBA quartiles with increasing DAP metabolites. Increased risks were consistently detected for total disomy in the second DMDTP exposure quartile across all 3PBA quartiles; adjusted IRRs ranged from 1.36 (95%CI: 1.11, 1.67) to 1.96 (95%CI: 1.59, 2.40) (Fig. 1). Fig. 2 shows adjusted IRRs and 95% confidence intervals for total disomy by tertiles of ∑DAP by 3PBA tertiles. Adjusted IRRs showed mainly null associations; small or no visible changes were observed in the first and third tertiles of 3PBA with increasing ∑DAPs exposure.
Fig. 1. Adjusted IRRs for Total Disomy by Quartiles of DMDTP by 3PBA Quartiles.
Adjusted IRRs (95% CI) for Total Disomy by Quartiles of DMDTPa (Exposure 1 changing) by 3PBAb Quartiles (Exposure 2 constant). a DMDTP Exposure Quartiles: Q1 = X ≤ LOD (n = 69), Q2 = 0.10 < X ≤ 0.73 ng/mL (n = 30), Q3 = 0.73 < X ≤ 1.86 ng/ mL (n = 30), Q4 = X > 1.86 ng/mL (n=30). b 3PBA Exposure Quartiles: Q1 = X ≤ LOD (n = 33), Q2 = 0.10 < X ≤ 0.61 ng/mL (n = 46), Q3 = 0.61 < X ≤ 0.83 ng/mL (n = 39), Q4 = X > 0.83 ng/mL (n = 41).† P-value of the interaction between DAP metabolites and 3PBA in adjusted models. IRRs were adjusted for specific gravity, age, race, BMI, smoking, sperm total concentration, motility, and morphology.
Fig. 2. Adjusted IRRs for Total Disomy by Tertiles of DAPs by 3PBA Tertiles.
Adjusted IRRs (95% CI) for Total Disomy by Tertiles of DAPsa (Exposure 1 changing) by 3PBAb Tertiles (Exposure 2 constant). a ∑DAPs Exposure Tertiles: T1 = 2.76 ≤ X ≤ 35.00 (n = 53), T2 = 35.00 < X ≤ 155.00 (n = 52), T3 = X > 155.01 (n = 54). ∑DAPs is the sum of all six individual metabolites. b 3PBA Exposure Tertiles: T1 = X ≤ LOD (n = 33), T2 = 0.10 < X ≤ 0.69 (n = 68), T3 = X > 0.69 (n = 58). † P-value of the interaction between DAP metabolites and 3PBA in adjusted models. IRRs were adjusted for specific gravity, age, race, BMI, smoking, sperm total concentration, motility, and morphology.
4. Discussion
Our results showed clear evidence of interactions, resulting in complex nonmonotonic relationships changing direction within the range of exposure categories. The highest disomy rates were seen either 1) at the intermediate exposure concentration (i.e., quartiles 2 or 3) with null or no significant association observed at low and high exposures (inverse U-shaped relationship); or 2) with the highest rates observed at low and high exposures (U-shaped relationship).
Increased risk associations higher than the rates previously reported for each individual chemical class (see Supplemental Tables 1–2), were observed when assessing total disomy and OP/PYR interactions. Strong and significant interactions were observed for total disomy across all OP metabolites and PYR concentrations. The risk estimates previously reported showed inverse associations between all disomy types and 3PBA without an interaction term for OP concentrations (see Supplemental Table 1). The quantification of interactions between simultaneous pesticide exposures shown here demonstrate that main exposure effects are different from results that take interactions into account. Previously reported results showed that DAP concentrations were associated with increased disomy rates without an interaction term (see Supplemental Table 2; Figueroa et al., 2015). Notably, nonmonotonic dose-response relationships were observed between the outcomes and exposure categories for all main effects.
Adjusted IRRs for total sex chromosome disomy by ∑DAPs and 3PBA exposures showed mainly null associations when compared to the reference group. These results confirmed our previous observation that aggregating all six DAP metabolites into a composite variable of ∑DAPs conceals their separate and distinct associations with sperm disomy (see Supplemental Table 2). Similar relationships were observed for other disomy types (XX18, YY18 and XY18) when modeled by concentrations of DAP metabolites at various 3PBA concentrations. These findings suggest that modeling metabolites separately are likely to produce distinct and potentially more informative findings than using an aggregate measure (Figueroa et al., 2015).
Unadjusted 95th percentile DAP urinary concentrations were slightly higher (0.39–38.71 ng/mL) in this study when compared to the unadjusted 95th percentile DAP urinary concentrations of men surveyed in the United States (US) general population for 2007–2008 (< LOD-36.10 ng/mL) (CDC Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables 2019). The unadjusted 95th percentile 3PBA urinary concentrations were lower in this study (2.27 ng/mL) than the concentrations reported for 2009–2010 (6.50 ng/mL).
Several human studies have reported individual exposures of OP or PYR pesticides associated with decreased sperm volume and decreased sperm count, higher abnormal morphology and decreased sperm motility, sperm damage, sperm chromatin alteration, increased luteinizing hormone and decreased testosterone, and sperm aneuploidy (Padungtod et al., 1998, 1999; Recio et al., 2001; Tan et al., 2002; Kamijima et al., 2004; Xia et al., 2004; Sanchez-Pena et al., 2004; Meeker et al. 2004a, 2008; Lifeng et al., 2006; Yucra et al., 2008; Recio-Vega et al., 2008; Hossain et al., 2010; Ji et al., 2011; Young et al., 2013; Radwan et al., 2015). Less is known about the combined effects of OP/PYR pesticides and their association with human sperm parameters. Perry et al. conducted a pilot biomonitoring study to examine the relationship between environmental OP/PYR exposures and sperm concentration among Chinese men living in rural areas (Perry et al., 2007b). Results showed a high prevalence of exposure to OP/PYR pesticides and suggested that the higher exposure group had lower sperm concentration.
These findings are consistent with many other studies showing the potential low dose effects of endocrine disrupting chemicals (EDCs) affecting hormone expression and affecting the risk for adult disease (Vandenberg et al., 2012). Our findings showed complex nonmonotonic dynamics between OP/PYR exposures and disomy. Even though the effects of low doses cannot be predicted by the effects observed at high doses and sometimes low environmental exposures cannot be identified (Vandenberg et al., 2012), this study was able to detect significant associations between OP/PYR pesticides and disomy. It remains unclear how combined OP/PYR exposures could be protective for sex chromosome disomy when specific DAP metabolites are modeled within differing quartiles of 3PBA. Our results suggest that pesticide toxicity estimates may underestimate the risks associated with reproductive outcomes because they do not account for the possibility of co-occurring synergistic chemical interactions.
Acute OP toxicity involves the inhibition of the enzyme acetylcholinesterase (AChE) leading to neurotoxicity in the central and peripheral nervous system, while PYRs act via the activation and inactivation of voltage-gated sodium channels (VGSCs), causing neuronal excitability (Mandhane and Chopde, 1997; Tyler et al., 2000; Nasuti et al., 2003; US EPA, 1999; 2009; Barr et al., 2010). Because the use of PYRs in the US, particularly in residential settings, has increased dramatically over the past decade, it is important to consider their effects when combined with other pesticides that have higher mammalian toxicity such as the OPs (Williams et al., 2008; US EPA 2009; 2013; Horton et al., 2011). Exposure to CYP450-activated OP insecticides or their toxic metabolites (such as chlorpyrifos oxon) can enhance PYR toxicity by inhibiting carboxylesterases, enzymes used by the body to detoxify PYRs (Ray and Forshaw, 2000). With metabolic detoxification inhibited and sometimes irreversible even at low concentrations, PYR toxicity potency pesticides in the presence of OPs can increase due to greater tissue concentration and lower excretion rate (Wielgomas and Krechniak, 2007). Both classes of insecticides are often used simultaneously in the US and are formulated in combined products on the global market, posing the risk of unintended synergistic or potentiation effects (He et al., 2002).
Because chemical mixtures are multidimensional, there is not a standard methodology to investigate the effects of real-world exposures to multiple compounds with different modes of action (Teuschler et al., 2004, 2007; Perobelli et al., 2010; Woodruff, 2011). This highlights the difficulties with treating combined environmental exposures observed in epidemiological studies as single entities (EEA-JRC, 2013). In general, environmental exposures are highly correlated, introducing methodological issues. Several methodological advances have been made to account for issues such as collinearity, high dimensionality, and synergistic, potentiation or inhibitory effects. Advanced methods have been designed to identify subsets of mixtures, assess mixtures that are also affected by a limit of detection, accommodate joint analysis of high-dimensional biomarker data, and to model interactions (Charles et al., 2002; Moser et al., 2005, 2006; Gennings et al., 2010; Herring, 2010; Yeatts et al., 2010; Zhang et al., 2012; VanderWeele and Tchetgen, 2014). Some of these methods are relatively new and have mainly used linear and logistic regression. Methods for treating interactions are needed in non-logistic models, such as Poisson regression which is best suited to the nature of the disomy rate data examined here.
This analysis was limited by the number of men available in each quartile when stratifying by exposure category. Commonly used trend tests available to evaluate monotonic dose-response relationships present a disadvantage when evaluating nonmonotonic responses. Those tests may not fully describe the nonmonotonicity patterns often observed for EDCs. In addition, multiple statistical tests may increase the probability of finding a statistically significant association and the role of chance in these significant findings cannot be completely ruled out.
DAPs and 3PBA are non-specific urinary metabolites of OPs and PYRs insecticides. These metabolites do not retain the structure from which they were derived, and cannot be attributed to a specific original parent compound (Bravo et al., 2004). Although the use of biological measurements has been criticized due to the inability to distinguish parent compound exposures from exposures to other pre-formed breakdown products, these urinary biomarkers are still the most widely used method to estimate the internal dose of a wide range of pesticides due to their relatively low costs and their utility for interpreting health outcomes of interest (Lu et al., 2005; Sudakin, 2006, 2011; Angerer et al., 2007; Starr et al., 2008; Krieger et al., 2012).
Even though the existing multi-analyte methods are highly sensitive and able to measure low concentrations of exposure biomarkers in urine, detailed time-specific information on windows of exposure vulnerability are still needed to improve exposure assessment (Needham et al., 2007). The spermatogenic cycle lasts from 75 to 90 days and a single urinary measurement is not likely to be a robust exposure measurement as these analyses assumed that these urinary measurements are reflective of longer-term exposure. Spot urine samples may not accurately reflect cumulative pesticide exposures when the parent compounds are rapidly metabolized (Martenies and Perry, 2013). Biological half-lives of OP and PYR pesticides vary and a single urine sample may not accurately reflect exposure due to the potential changes in the concentration of chemicals from void to void and the urine volume variability (Barr et al., 2005).
Although our study population was recruited from a fertility clinic, their response to pesticide exposures is not expected to differ from men in the general population. Our sample included men with a range of semen parameters, the majority whom were above the WHO lower reference values for sperm concentration and motility, and the Tygerberg Strict Criteria for morphology. Factors specific to the male partner are thought to be responsible for 30% of infertility cases (Agarwal et al., 2015) and the majority of men in this sample were likely to have normal reproductive profiles. Extensive information was collected about potential confounders. Semen measurements reflect recent spermatogenic cycles (75–90 days), and DAP and PYR urinary metabolites reflect recent exposure to pesticides. A validated semi-automated method was used to determine disomy frequency in this study, allowing for a reliable and an objective counting of disomic sperm in a large number of samples (Perry et al., 2007b, 2011). In addition, the technicians who performed semen and disomy analyses were blinded to exposure status, preventing bias in the analysis of the outcome.
5. Conclusions
To our knowledge, this is the first epidemiologic study to explore the relationship between contemporary use pesticide interactions and germ cell abnormalities. Testing for interactions revealed new relationships, mainly showing that PYRs and OPs acted not independently, but rather interdependently in increasing the risk for germ cell abnormalities. These results demonstrate that single chemical main effect analyses miss the reality that people are exposed to multiple chemicals simultaneously and likely underestimate the synergistic and/or potentiation effects of multiple exposures.
The frequency of sperm aneuploidy is best modeled assuming a Poisson distribution, and we found few prior studies that modeled interactions in Poisson models. Methods to disentangle the combined effects of pesticides and their interactions need further development to best model outcomes that do not routinely fit linear or logistic distributions. Specific attention is needed to the methodologies assessing the combined effects of simultaneous low-concentration pesticide exposures (with different modes of action) to better quantify the reproductive impacts of environmental contaminants. Because this is the first analysis examining this combination of chemicals for this health outcome, replication of these findings is needed.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Dr. Russ Hauser for his instrumental support.
Financial support
This work was supported by the US National Institute of Health and the US National Institute of Environmental Health Sciences (grant ES 009718, grant ES 000002, grant ES 017457); and the Intramural Research Program (IRP) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the US National Institutes of Health, Bethesda, Maryland.
List of Abbreviations
- 3-PBA
3-phenoxybenzoic acid
- AChE
Acetylcholinesterase
- BMI
Body-mass index
- CASA
Computer-aided sperm analysis
- DAP
Dialkyl phosphate
- DEP
Diethylphosphate
- DETP
Diethylthiophosphate
- DEDTP
Diethyldithiophosphate
- DMP
Dimethylphosphate
- DMTP
Dimethylthiophosphate
- DMDTP
Dimethyldithiophosphate
- DNA
Deoxyribonucleic acid
- FISH
Fluorescence in situ hybridization
- GENMOD
Generalized linear model procedure
- IRR
Incidence rate ratios
- LOD
Level of detection
- MATLAB
Multi-paradigm numerical computing
- MGH
Massachusetts General Hospital
- OP
Organophosphate
- PYR
Pyrethroid
- SAS
Statistical Analysis System
- VGSC
Voltage-gated sodium channels
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2019.07.001.
Conflicts of interest
There is no conflict of interest.
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR, 2015. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton Acton Q, 2013. Sex Chromosomes—Advances in Research and Application, 2013 Edition. Scholarly Editions, Atlanta, GA. [Google Scholar]
- Axelsson J, Bonde JP, Giwercman YL, et al. , 2010. Gene-environment interaction and male reproductive function. Asian J. Androl 12, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer J, Ewers U, Wilhelm M, 2007. Human biomonitoring: state of the art. Int. J. Hyg Environ. Health 210, 201–228. [DOI] [PubMed] [Google Scholar]
- Baker SE, Olsson AO, Barr DB, 2004. Isotope dilution high-performance liquid chromatography-tandem mass spectrometry method for quantifying urinary metabolites of synthetic pyrethroid insecticides. Arch. Environ. Contam. Toxicol 46, 281–288. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, et al. , 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL, 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. Population: national health and nutrition examination survey 1999–2002. Environ. Health Perspect 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A, Hummelen P Van Lowe XR, et al. , 1999. Numerical and structural chromosomal abnormalities detected in human sperm with a combination of multicolor FISH assays. Environ. Mol. Mutagen 33, 49–58. [DOI] [PubMed] [Google Scholar]
- Berman NG, Wang C, Paulsen CA, 1996. Methodological issues in the analysis of human sperm concentration data. J. Androl 17, 68–73. [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, Barr DB, 2004. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography–tandem mass spectrometry and isotope dilution quantification. J. Expo. Anal. Environ. Epidemiol 14, 249–259. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, et al. , 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 124, A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin DJ, Rider CV, Woychik R, et al. , 2013. Unraveling the health effects of environmental mixtures—an NIEHS priority. Environ. Health Perspect 121 (1), A6–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC], 2019. National Health and Nutrition Examination Survey, Fourth Report on Human Exposure to Environmental Chemicals – 2019 Updated Tables, vol. 1 United States Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Claus Henn B, Coull BA, Wright RO, 2014. Chemical mixtures and children’s health. Curr. Opin. Pediatr 26 (2), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles GD, Gennings C, Zacharewski TR, Gollapudi BB, Carney EW, 2002. An approach for assessing estrogen receptor-mediated interactions in mixtures of three chemicals: a pilot study. Toxicol. Sci 68 (2), 349–360. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Wong EWP, Lie PPY, et al. , 2011. Environmental toxicants and male reproductive function. Spermatogenesis 1 (1), 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, et al. , 2005. Phthalate exposure and reproductive hormones in adult men. Hum. Reprod 20 (3), 604–610. [DOI] [PubMed] [Google Scholar]
- European Environment Agency-Joint Research Centre [EEA-JRC], 2013. Report on Environment and Human Health. European Union, Denmark. [Google Scholar]
- Figueroa ZI, Young HA, Meeker JD, et al. , 2015. Dialkyl phosphate urinary metabolites and chromosomal abnormalities in human sperm. Environ. Res 143 (Pt A), 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C, Sabo R, Carney E, 2010. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology 21, 77–84. [DOI] [PubMed] [Google Scholar]
- Goodson III WH, Lowe L, Carpenter DO, et al. , 2015. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis 36 (Suppl. 1), S254–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ, 2014. Neurobehavioral effects of developmental toxicity. Lancet Neurol. 13 (3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt P, 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet 2, 280–291. [DOI] [PubMed] [Google Scholar]
- Hauser R, Chen Z, Pothier L, et al. , 2003. The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p,p’-DDE. Environ. Health Perspect 111, 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Chen S, Tang X, Gan W, Tao B, Wen B, 2002. Biological monitoring of combined exposure to organophosphates and pyrethroids. Toxicol. Lett 34 (1–3), 119–124. [DOI] [PubMed] [Google Scholar]
- Helsel D, 2005. Nondetects and Data Analysis: Statistics for Censored Environmental Data. John Wiley & Sons, Inc, Hoboken, New Jersey. [Google Scholar]
- Hernández AF, Parrón T, Tsatsakis AM, et al. , 2013. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology 307, 136–145. [DOI] [PubMed] [Google Scholar]
- Herrera LA, Prada D, Andonegui MA, Dueñas-González A, 2008. The epigenetic origin of aneuploidy. Curr. Genom 9, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring AH, 2010. Nonparametric bayes shrinkage for assessing exposures to mixtures subject to limits of detection. Epidemiology 21 (Suppl. 4), S71–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, 2011. Characterization of residential pest control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol 21 (3), 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F, Ali O, D’Souza UJA, et al. , 2010. Effects of pesticide use on semen quality among farmers in rural areas of Sabah, Malaysia. J. Occup. Health 52, 353–360. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, 1992. The chromosome complement of human gametes. Oxf. Rev. Reprod. Biol 14, 47. [PubMed] [Google Scholar]
- Ji G, Xia Y, Gu A, et al. , 2011. Effects of non-occupational environmental exposure to pyrethroids on semen quality and sperm DNA integrity in Chinese men. Reprod. Toxicol 31, 171–176. [DOI] [PubMed] [Google Scholar]
- Kamijima M, Hibi H, Gotoh M, Taki K, Saito I, Wang H, Itohara S, Yamada T, Ichihara G, Shibata E, Nakajima T, Takeuchi Y, 2004. A survey of semen indices in insecticide sprayers. J. Occup. Health 46 (2), 109–118. [DOI] [PubMed] [Google Scholar]
- Krieger RI, Chen L, Ginevan M, Watkins D, Cochran RC, Driver JH, Ross JH, 2012. Implications of estimates of residential organophosphate exposure from dialkylphosphates (DAPs) and their relevance to risk. Regul. Toxicol. Pharmacol 64, 263–266. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, et al. , 1988. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil. Steril 49, 112–117. [DOI] [PubMed] [Google Scholar]
- Lacasaῆa M, Lopez-Flores I, Rodriguez-Barranco M, Aguilar-Garduno C, Blanco-Munoz J, Perez-Mendez O, Gamboa R, Bassol S, Cebrian ME, 2010. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol. Appl. Pharmacol 243, 19–26. [DOI] [PubMed] [Google Scholar]
- Lifeng T, Shoulin W, Junmin J, Xuezhao S, Yannan L, Qianli W, 2006. Effects of fenvalerate exposure on semen quality among occupational workers. Contraception 73, 92–96. [DOI] [PubMed] [Google Scholar]
- Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, Barr DB, 2005. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J. Toxicol. Environ. Health 68 (3), 209–227. [DOI] [PubMed] [Google Scholar]
- Mandhane SN, Chopde CT, 1997. Neurobehavioral Effects of Low Level Fenvalerate Exposure in Mice. [PubMed] [Google Scholar]
- Martenies SE, Perry MJ, 2013. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology 307, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RH, Ko E, Rademaker A, 1991. Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am. J. Med. Genet 39, 321–331. [DOI] [PubMed] [Google Scholar]
- McAuliffe ME, Williams PL, Korrick SA, et al. , 2012. Environmental exposure to polychlorinated biphenyls and p,p’-DDE and sperm sex-chromosome disomy. Environ. Health Perspect 120, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ryan L, Barr DB, Herrick RF, Bennett DH, Bravo R, Hauser R, 2004a. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environ. Health Perspect 112, 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Singh NP, Ryan L, Duty SM, Barr DB, Herrick RF, Bennett DH, Hauser R, 2004b. Urinary levels of insecticide metabolites and DNA damage in human sperm. Hum. Reprod 19, 2573–2580. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2006. Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reprod. Toxicol 22, 437–442. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2008. Human semen quality and sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides. Hum. Reprod. Oxf. Engl 23, 1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2009. 2009. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol 27, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, Casey M, Hamm A, Cater WH Jr., Simmons JE, Gennings C, 2005. Neurotoxicological and statistical analyses of a mixture of five organophosphorus pesticides using a ray design. Toxicol. Sci 86, 101–115. [DOI] [PubMed] [Google Scholar]
- Moser VC, Simmons JE, Gennings C, 2006. Neurotoxicological interactions of a five-pesticides mixture in preweanling rats. Toxicol. Sci 92, 235–245. [DOI] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA, 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet 13, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuti C, Cantalamessa F, Falcioni G, Gabbianelli R, 2003. Different effects of Type I and Type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 191 (2–3), 233–244. [DOI] [PubMed] [Google Scholar]
- National Academy of Science [NAS], 2014. Review of the Environmental Protection Agency’s State-Of-The-Science Evaluation of Nonmonotonic Dose-Response Relationships as They Apply to Endocrine Disrupters. The National Academies Press, National Research Council, Washington, DC. [Google Scholar]
- Needham LL, et al. , 2007. Uses and issues of biomonitoring. Int. J. Hyg Environ. Health 210, 229–238. [DOI] [PubMed] [Google Scholar]
- Padungtod C, Lasley BL, Christiani DC, Ryan LM, Xu X, 1998. Reproductive hormone profile among pesticide factory workers. J. Occup. Environ. Med 40 (12), 1038–1047. [DOI] [PubMed] [Google Scholar]
- Padungtod C, Hassold TJ, Millie E, et al. , 1999. Sperm aneuploidy among Chinese pesticide factory workers: scoring by the FISH method. Am. J. Ind. Med 36, 230–238. [DOI] [PubMed] [Google Scholar]
- Perobelli JE, Martinez MF, da Silva Franchi CA, Fernandez CDB, Viana de Camargo JL, Kempinas WDG, 2010. Decreased sperm motility in rats orally exposed to single or mixed pesticides. J. Toxicol. Environ. Health A Curr. Issues 73 (13–14), 991–1002. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Chen X, Lu X, 2007a. Automated scoring of multiprobe FISH in human spermatozoa. Cytometry A 71, 968–972. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Venners SA, Barr DB, Xu X, 2007b. Environmental pyrethroid and organophosphorus insecticide exposures and sperm concentration. Reprod. Toxicol 23, 113–118. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Chen X, McAuliffe ME, et al. , 2011. Semi-automated scoring of tripleprobe FISH in human sperm: methods and further validation. Cytometry A 79, 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapamontol T, Sutan K, Laoyang S, et al. , 2014. Cross validation of gas chromatography-flame photometric detection and gas chromatography-mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int. J. Hyg Environ. Health 217 (4–5), 554–566. [DOI] [PubMed] [Google Scholar]
- Radwan M, Jurewicz J, Wielgomas B, et al. , 2015. The association between environmental exposure to pyrethroids and sperm aneuploidy. Chemosphere 128, 42–48. [DOI] [PubMed] [Google Scholar]
- Ray D, Forshaw P, 2000. Pyrethroid insecticides: poisoning syndromes, synergies, and therapies. Clin. Toxicol 38, 95–101. [DOI] [PubMed] [Google Scholar]
- Recio R, Robbins WA, Ocampo-Gomez G, et al. , 2001. Organophosphorous pesticide exposure increases the frequency of sperm sex null aneuploidy. Environ. Health Perspect 109, 1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Vega R, Ocampo-Gomez G, Borja-Aburto V, et al. , 2008. Organophosphorus pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J. Appl. Toxicol 28, 674–680. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pena LC, Reyes BE, Lopez-Carrillo L, et al. , 2004. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol. Appl. Pharmacol 196, 108–113. [DOI] [PubMed] [Google Scholar]
- Schiffer C, Müller A, Egeberg DL, et al. , 2014. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 15, 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr J, Graham S, Stout II D, Andrews K, Nishioka M, 2008. Pyrethroid pesticides and their metabolites in vacuum cleaner dust collected from homes and day-care centers. Environ. Res 108 (3), 271–279. [DOI] [PubMed] [Google Scholar]
- Sudakin DL, 2006. Pyrethroid insecticides: advances and challenges in biomonitoring. Clin. Toxicol 44, 31–37. [DOI] [PubMed] [Google Scholar]
- Sudakin DL, Stone DL, 2011. Dialkyl phosphates as biomarkers of organophosphates: the current divide between epidemiology and clinical toxicology. Clin. Toxicol 49, 771–781. [DOI] [PubMed] [Google Scholar]
- Tan LF, Wang SL, Sun XZ, Li YN, Wang QL, Ji JM, Chen LS, Wang XR, 2002. Effects of fenvalerate exposure on the semen quality of occupational workers. Zhonghua Nan Ke Xue 8 (4), 273–276. [PubMed] [Google Scholar]
- Taylor KW, Joubert BJ, Braun JM, Dilworth C, Gennings C, Hauser R, Heindel JJ, Rider CV, Webster TF, Carlin DJ, 2016. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ. Health Perspect 124 (12), A227–A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuschler LK, Hertzberg RC, Rice GE, et al. , 2004. EPA Project-Level Research Strategies for Chemical Mixtures: Targeted Research for Meaningful Results. U.S. Environmental Protection Agency Papers Paper 180. [DOI] [PubMed] [Google Scholar]
- Teuschler LK, 2007. Deciding which chemical mixtures risk assessment methods work best for what mixtures. Toxicol. Appl. Pharmacol 223 (2), 139–147. [DOI] [PubMed] [Google Scholar]
- Toshima H, Suzuki Y, Imai K, et al. , 2012. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: a pilot study on exposure and semen quality. Int. J. Hyg Environ. Health 215, 502–506. [DOI] [PubMed] [Google Scholar]
- Tsatsakis AM, Zafiropoulos A, Tzatzarakis MN, et al. , 2009. Relation of PON1 and CYP1A1 genetic polymorphisms to clinical findings in a cross-sectional study of a Greek rural population professionally exposed to pesticides. Toxicol. Lett 186, 66–72. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Beresford N, Woning M van der Sumpter, J.P., Tchorpe K, 2000. Metabolism and environmental degradation of pyrethroid insecticides produce compounds with endocrine activities. Environ. Toxicol. Chem 19, 801–809. [Google Scholar]
- US Environmental Protection Agency [US EPA], 1999. A Science Policy on a Common Mechanism of Toxicity: Carbamate Pesticides and the Grouping of Carbamate with the Organophosphorus Pesticides. United States Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, Washington, DC. [Google Scholar]
- US Environmental Protection Agency [US EPA], 2001. General Principles for Performing Aggregate Exposure and Risk Assessments. United States Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, Washington, DC. [Google Scholar]
- US Environmental Protection Agency [US EPA], 2009. Proposed Common Mechanism Grouping for the Pyrethrins and Synthetic Pyrethroids. Draft Science Policy Paper. Office of Chemical Safety and Pollution Prevention, Washington, DC. [Google Scholar]
- US Environmental Protection Agency [US EPA], 2013. State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures. Office of Research and Development, Washington, DC. [Google Scholar]
- US Environmental Protection Agency [US EPA], 2017. Pesticides Industry Sales and Usage: 2008 and 2012 Market Estimates. Office of Chemical Safety and Pollution Prevention, Washington, DC. [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DK, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 33 (3), 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Tchetgen EJ, 2014. Attributing effects to interactions. Epidemiology 25, 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgomas B, Krechniak J, 2007. Toxicokinetic interactions of α-cypermethrin and chlorpyrifos in Rats. Pol. J. Environ. Stud 16, 267–274. [Google Scholar]
- Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, 2008. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect 116, 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Carlson A, Schwartz JM, et al. , 2008. Proceedings of the summit on environmental challenges to reproductive health and fertility: executive summary. Fertil. Steril 89, e1–e20. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, 2011. Bridging epidemiology and model organisms to increase understanding of endocrine disrupting chemicals and human health effects. J. Steroid Biochem. Mol. Biol 127, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [WHO], 2009. Assessment of Combined Exposures to Multiple Chemicals: Report of a WHO/IPCS International Workshop on Aggregate/Cumulative Risk Assessment. World Health Organization, Geneva. [Google Scholar]
- World Health Organization [WHO], 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen, fifth ed. World Health Organization, Geneva. [Google Scholar]
- Xia Y, Bian Q, Xu L, Cheng S, Song L, Liu J, 2004. Genotoxic effects on human spermatozoa among pesticide factory workers exposed to fenvalerate. Toxicology 203, 49–60. [DOI] [PubMed] [Google Scholar]
- Xia Y, Han Y, Wu B, et al. , 2008. The relation between urinary metabolite of pyrethroid insecticides and semen quality in humans. Fertil. Steril 89, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Yeatts SD, Gennings C, Wagner ED, Simmons JE, Plewa MJ, 2010. Detecting departure from additivity along a fixed-ratio mixture ray with a piecewise model for dose and interaction thresholds. J. Agric. Biol. Environ. Stat 15 (4), 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HA, Meeker JD, Martenies SE, et al. , 2013. Environmental exposure to pyrethroids and sperm sex chromosome disomy: a cross-sectional study. Environ. Health 12 (1), 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucra S, Gasco M, Rubio J, Gonzales GF, 2008. Semen quality in Peruvian pesticide applicators: association between urinary organophosphate metabolites and semen parameters. Environ. Health Global Access Sci. Source 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeliger HI, 2011. Human Toxicology of Chemical Mixtures: Toxic Consequences beyond the Impact of One-Component Product and Environmental Exposures, second ed. William Andrew Inc, Norwich, NY. [Google Scholar]
- Zhang B, Chen Z, Albert PS, 2012. Latent class models for joint analysis of disease prevalence and high-dimensional semicontinuous biomarker data. Biostatistics 13 (1), 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.