Abstract
Objective:
To estimate the association between physical activity and risk of subclinical and clinical pregnancy loss among women with a history of pregnancy loss.
Design:
Prospective cohort study as a secondary analysis of the Effects of Aspirin in Gestation and Reproduction randomized controlled trial of preconception-initiated low-dose aspirin among women with one or two prior pregnancy losses.
Setting:
Four U.S. clinical centers, 2007–2011.
Patient(s):
Women with confirmed pregnancy (n = 785) as determined from hCG testing in longitudinally collected biospecimens.
Main Outcome Measure(s):
Subclinical loss of pregnancy detected only by hCG testing and clinically recognized loss.
Result(s):
Among 785 women (mean [SD] age, 28.7 [4.6] years) with an hCG-confirmed pregnancy, 188 (23.9%) experienced pregnancy loss. In multivariable models adjusted for confounders, compared with the first tertile of physical activity (median = 7.7 metabolic equivalent of task hours/week), there was a roughly twofold higher risk of subclinical loss in the second (risk ratio = 2.06; 95% confidence interval, 1.03–4.14) and third tertiles (risk ratio = 1.92; 95% confidence interval, 0.94–3.90), with median metabolic equivalent of task hours/week of 27.8 and 95.7, respectively. No relations were observed between physical activity and clinically recognized loss.
Conclusion(s):
Risk related to physical activity is different for pregnancy failure close to the time of implantation compared with that for later, clinical pregnancy loss. Higher physical activity levels were associated with an elevated risk of subclinical loss (i.e., pregnancies detected only by hCG, n = 55); however, no relationship was observed with clinically recognized loss. Further work is required to confirm these findings, assess generalizability to women without prior losses, and evaluate mechanisms.
Ethical approval:
Each participating center’s Institutional Review Board approved the study, and participants provided written informed consent. The trial was registered on ClinicalTrials.gov (NCT00467363), and a Data Safety and Monitoring Board provided oversight.
Keywords: Physical activity, pregnancy loss, longitudinal, biospecimen, epidemiology
Abstract
Objetivo:
Estimar la asociación entre la actividad física y el riesgo de pèrdida gestacional subclínica y clínica entre mujeres con historia de pèrdida gestacional.
Diseño:
Estudio prospectivo de cohortes como análisis secundario de un estudio aleatorizado de los efectos de aspirina en la gestación y la reproducción, utilizando bajas dosis de aspirina desde la preconcepción entre mujeres con una o dos pèrdidas gestacionales previas.
Lugar:
Cuatro clínicas en Estados Unidos, entre 2007–2011.
Pacientes:
Mujeres con embarazo confirmado (n= 785) determinado por pruebas de hCG en muestras biológicas recogidas longitudinalmente.
Principales medidas de resultado(s):
Pérdida gestacional subclínica detectada sólo por la prueba de hCG y la reconocida clínicamente.
Resultados:
Entre 785 mujeres (media de edad [Deviación standard], 28,7 [4,6] años) con una gestación confirmada con hCG, 188 (23.9%) que tuvieron pérdida gestacional. En un modelo multivariante ajustado para factores de confusión comparado con el primer tercil de actividad física (media= 7,7 equivalentes metabólicos de trabajo horas/semana) hubo un riesgo bruto dos veces mayor de pérdida subclínica en el segundo (relación de riesgo= 2,06; Intervalo de confianza 95%, 1,03–4,14) y el tercer tercil (Relación de riesgo = 1,92 Intervalo de confianza 95%, 0,94–3,9) con una media metabólica equivalente de trabajo horas/semana de 27,8 y 95,7 respectivamente. No hubo relación entre actividad física y pérdidas gestacionales clínicas.
Conclusiones:
El riesgo asociado a actividad física es diferente para fallo gestacional próximo al momento de la implantación comparado con el más tardío de pérdida gestacional clínica. Niveles mayores de actividad física estuvieron asociados con un riesgo elevado de pérdida subclínica (por ejemplo, embarazos detectados solo por hCG, n=55); sin embargo, no se observó relación con pérdida clínicamente reconocida. Se necesitan más trabajos para confirmar estos hallazgos, evaluando de forma general mujeres sin pérdidas gestacionales previas, y evaluar sus mecanismos.
Aprobación ética:
El comité de revisión institucional de cada centro participante aprobó el estudio y los participantes dieron por escrito su consentimiento informado. El ensayo fue registrado en ClinicalTrial.gov (NCT00467363), y el comité de seguridad y monitorización de los datos dio su aprobación
Pregnancy loss is a common event, affecting up to 30% of conceptions (1). Established risk factors for pregnancy loss include age and prior pregnancy loss (2). The effect of modifiable factors, such as physical activity (PA), on pregnancy outcomes may vary across gestation due to timing of physiologic changes and stage of development of the fetus. Because the etiology of very early subclinical loss may differ from that of clinical pregnancy losses, attention to timing is warranted in study design to evaluate risk factors for these outcomes. Particularly for women with prior pregnancy losses, identification of modifiable behavior and lifestyle factors related to risk of pregnancy loss is important to improve conception and live birth probability.
The American College of Obstetricians and Gynecologists recommends an exercise program leading to an eventual goal of moderate intensity exercise for at least 20–30 minutes per day on most days of the week for all women in pregnancy, in the absence of obstetric or medical complications or contraindications. These PA recommendations are based on established benefits for general health and well-being and some evidence of positive effects on pregnancy outcomes like gestational diabetes mellitus (3). However, evidence from epidemiologic research regarding impacts on pregnancy loss is unclear. Small studies assessing the relationship of leisure-time PA and exercise with risk of clinical pregnancy loss have observed no association (4), or a slightly reduced risk (5), whereas a significantly increased risk was observed among women in the Danish National Birth Cohort, particularly related to high-impact exercise (6). Research of PA and risk of subclinical loss—which requires prospective collection of detailed information and biospecimens—is particularly limited. Increased risk of subclinical loss has been linked to physical strain in a small prospective study (7), but data on PA with regard to subclinical and clinical pregnancy loss are scarce.
In order to address this gap, we evaluated the relationship between PA and pregnancy losses in a large preconception prospective cohort with extensive longitudinal biospecimen collection. Through the use of the Effects of Aspirin in Gestation and Reproduction (EAGeR) cohort, we leveraged detailed information on timing to assess pregnancy loss by stage of gestation, separately evaluating risk of subclinical losses detected by biochemical assay (for hCG) and clinically recognized pregnancy losses.
MATERIALS AND METHODS
Study Setting, Population, and Protocol
For this analysis, we used data from the EAGeR trial, described in detail elsewhere (8). Briefly, EAGeR was a multisite, randomized controlled trial of daily low-dose aspirin among women ages 18–40 years with a history of one to two pregnancy losses who were in a committed relationship and trying to conceive without intervention. A total of 1,228 women were randomized after recruitment at one of four U.S. university medical centers from 2007 to 2011 (Fig. 1). Each participating center’s Institutional Review Board approved the study, and participants provided written informed consent.
FIGURE 1.
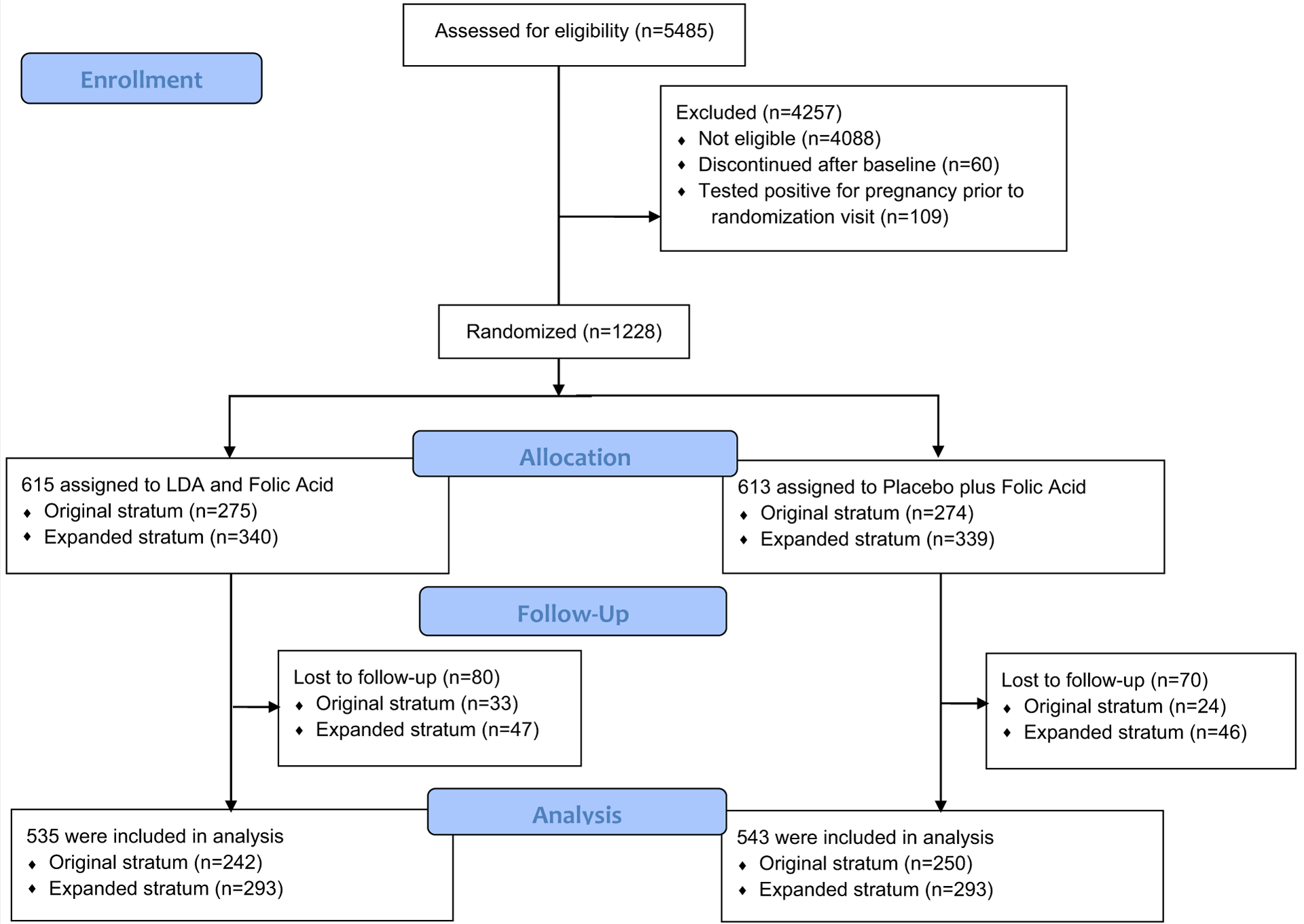
CONSORT flow diagram for the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial.
Women were followed for up to 6 months while trying to conceive. During the first two cycles of follow-up, participants provided daily first-morning urine collection. During subsequent cycles, women used home pregnancy tests and provided biospecimens at clinic visits once per menstrual cycle.
Pregnancy was determined initially by a clinic and/or home urine pregnancy test. Participants who became pregnant provided daily first-morning urine collected at home for the first month of pregnancy and spot urine samples at monthly clinic visits until the pregnancy ended. Ultrasound was performed for women with positive pregnancy tests at 6–7 weeks’ gestation to confirm ongoing pregnancy. If signs of pregnancy were absent from the sonogram, the case was considered a periconceptional loss and the participant continued on the nonpregnancy follow-up schedule. A woman’s study participation ended after completing six menstrual cycles in the trial without becoming pregnant or after having two periconception losses or other pregnancy outcome.
At the baseline visit, women completed several questionnaires assessing information on demographics, health and reproduction, lifestyle, occupation, and the short form version of the International Physical Activity Questionnaire (IPAQ-SF). Specifically, information was collected from participants on age, marital status, high school education, race, income, parity, number of prior documented miscarriages, smoking in the past year, alcohol consumption in the past year, current partner’s age, and time from last loss to randomization. Biospecimens, including spot urine samples from self-collection and those taken during clinic visits, were frozen and stored at −80°C. Body mass index (BMI) and waist-hip (W-H) ratio were calculated using measurements taken during the baseline anthropometric assessment.
Assessment of PA
PA was assessed at baseline using the self-administered, last 7-day IPAQ-SF (9). The IPAQ-SF contains seven questions that measure the frequency and duration of walking, moderate intensity activity, and vigorous intensity activity that occurred for at least 10 minutes at a time over the previous week, in addition to time spent in sedentary behavior. Vigorous intensity PA was defined as “activities that take hard physical effort and make you breathe much harder than normal.” Moderate intensity PA was defined as “activities that take moderate physical effort and make you breathe somewhat harder than normal.” Walking included “[walking] at work and at home, walking to travel from place to place, and any other walking that you might do solely for recreation, sport, exercise, or leisure.” Responses to activity questions from the IPAQ-SF were first used to calculate total metabolic equivalent of task (MET) minutes/week using standard approaches (9).
Responses from the IPAQ-SF were used to determine baseline hours/week of activity (vigorous, moderate, and walking), and intensity scores were used to determine MET hours/week. To address implausibly high values (>3 hours/day), such observations were truncated and all were set to 3 hours/day, permitting a maximum of 21 hours/week in any activity category, as per IPAQ guidelines (9). The IPAQ-SF was administered only at baseline, and thus PA at baseline was used for this study as a proxy for PA throughout the preconception period.
Outcome Measures
The primary outcome in this analysis was pregnancy loss, subcategorized by timing of loss as clinical pregnancy loss or subclinical pregnancy loss. Pregnancies were confirmed by gestational sac on ultrasound, clinical recording of fetal heart tones, or a later-stage confirmatory sign. Clinically recognized pregnancy loss was defined as a pregnancy loss of a confirmed pregnancy occurring by 20 weeks’ gestation and included a small number of ectopic pregnancies that were observed (8, 10).
Subclinical pregnancy losses were identified in two ways: first, a positive urine pregnancy test at the clinical site (Quidel Quickvue, Quidel Corporation, sensitive to 25 mIU/mL hCG) followed by the absence of signs of clinical pregnancy, with or without missed menses; second, using batched augmented urine hCG testing that was performed later in the laboratory on the last 10 days of each woman’s first and second cycle of study participation (using daily first-morning urine collected at home) and on spot urine samples collected at all postcycle visits. Overall pregnancy loss was defined as either clinically recognized loss or hCG detected loss (10).
Statistical Analysis
Characteristics of participants were compared by tertiles of PA at baseline using analysis of variance, χ2 tests, and Fisher’s test, as applicable. Log-binomial models were used to estimate risk ratios (RRs) and 95% confidence intervals (CIs) of the association between PA and incidence of pregnancy loss considering additional factors as covariates. Time to pregnancy loss or conception in relation to PA was not considered for this analysis but has been assessed with these data previously (11). The IPAQ-SF assessed baseline hours/week of activity (vigorous, moderate, and walking); intensity scores and reported duration were used to determine MET hours/week. PA was operationalized as tertiles of METs to evaluate the potential for threshold effects. Log-binomial models of PA and pregnancy loss were first restricted to women who had achieved an hCG-detected pregnancy (10). To address confounding, potential confounders were identified based on a review of published data and consideration of proposed relations with both PA (exposure) and pregnancy loss (outcome) as an initial step. Next, these potential confounders were evaluated for relations with PA and the pregnancy loss outcomes to inform multivariable model specification. Confounders were considered variables associated with both PA and pregnancy loss, whereas those associated only with pregnancy loss were considered nonconfounding risk factors. As a sensitivity analysis for unmeasured confounding, we estimated e-values to determine how strongly a variable would need to be related to PA and to loss to explain observed results (12).
In statistical analysis of factors that may influence pregnancy outcomes, biases can arise when the exposure of interest influences both the likelihood of becoming pregnant and outcomes of those pregnancies. We used sensitivity analyses to address this potential selection bias to estimates based on a sample restricted to those who became pregnant. For these sensitivity analyses, the sample was expanded to include all EAGeR participants, and inverse probability weighting for pregnancy was also used (10).
RESULTS
Among the 1,088 women who completed the EAGeR trial, our primary analysis included the 785 women who achieved an hCG+ pregnancy (63.9%; Table 1). The mean ± SD age of participants was 28.7 ± 4.6, and BMI was 25.5 ± 6.2. EAGeR participants reported past-week PA levels ranging between 0 and 275.1 MET hours/week. Women were divided into PA categories based on tertiles of MET hours/week. The ranges of METs in the tertiles were 0–16.6, tertile 1 (T1); 16.6–50.8, tertile 2 (T2); and 51.3–275.1, tertile 3 (T3). There were statistically significant, but small, differences in alcohol consumption, race, education, and parity by PA tertile. Characteristics were similar between PA groups with regard to age, BMI, W-H ratio, and other covariates. Older age was related to increased risk of pregnancy loss, and a small, borderline significant association was observed with BMI and W-H ratio (data not shown). However, no variables were observed to represent confounders as factors related to both PA and pregnancy outcomes. Age and measures of adiposity were included in multivariable models as nonconfounding risk factors.
TABLE 1.
Characteristics of women with positive pregnancy tests (N = 785) in the Effects of Aspirin in Gestation and Reproduction study (2007–2011) by tertile of physical activity at baseline.
| Characteristics | METs tertile 1 (0–16.6) n = 261 |
METs tertile 2 (16.6–50.8) n = 262 |
METs tertile 3 (51.3–275.1) n = 262 |
P valuea |
|---|---|---|---|---|
| Age | 28.7 ± 4.7 | 29.1 ± 4.8 | 28.3 ± 4.3 | .17 |
| BMI, kg/m2 | 25.5 ± 6.4 | 25.3 ± 5.9 | 25.8 ± 6.2 | .64 |
| BMI categoryb | .92 | |||
| Underweight (<18.5) | 10 (3.9) | 9 (3.5) | 12 (4.6) | |
| Normal (≥18.5 and <25) | 143 (55.6) | 138 (53.5) | 132 (50.6) | |
| Overweight (≥25 and <30) | 57 (22.2) | 63 (24.4) | 62 (23.8) | |
| Obese (≥30) | 47 (18.3) | 48 (18.6) | 55 (21.1) | |
| Waist-hip ratio | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | .39 |
| Current partner’s age | 30.2 ± 5.7 | 30.4 ± 5.2 | 29.8 ± 5.1 | .45 |
| Parity | .03 | |||
| Nulliparous | 95 (36.4) | 127 (48.5) | 98 (37.4) | |
| 1 | 110 (42.2) | 89 (34.0) | 101 (38.6) | |
| 2+ | 56 (21.5) | 46 (17.6) | 63 (24.1) | |
| Marital status | .15 | |||
| Living with partner | 12 (4.6) | 10 (3.8) | 6 (2.3) | |
| Married | 241 (92.3) | 249 (95.0) | 254 (97.0) | |
| Other | 8 (3.1) | 3 (1.2) | 2 (0.8) | |
| >High school education | 228 (87.4) | 242 (92.4) | 228 (87.0) | .09 |
| White race | 246 (94.3) | 256 (97.7) | 256 (97.7) | .04 |
| Annual income (US $) | .28 | |||
| ≥100,000 | 97 (37.2) | 118 (45.0) | 106 (40.5) | |
| 75,000–99,999 | 41 (15.7) | 43 (16.4) | 29 (11.1) | |
| 40,000–74,999 | 40 (15.3) | 36 (13.7) | 40 (15.3) | |
| 20,000–39,999 | 62 (23.8) | 53 (20.2) | 68 (26.0) | |
| ≤19,999 | 21 (8.1) | 12 (4.6) | 19(7.3) | |
| Smoking in past year | .82 | |||
| Never | 228 (88.0) | 234 (90.0) | 236 (90.1) | |
| <6 times/week | 20 (7.7) | 19 (7.3) | 16 (6.1) | |
| Daily | 11 (4.3) | 7 (2.7) | 10 (3.8) | |
| Alcohol consumption in past year | .01 | |||
| Never | 168 (64.9) | 163 (62.7) | 194 (75.2) | |
| Sometimes | 85 (32.8) | 86 (33.1) | 62 (24.0) | |
| Often | 6 (2.3) | 11 (4.2) | 2 (0.8) | |
| No. of prior miscarriagesc | .93 | |||
| 1 | 174 (66.7) | 171 (65.3) | 171 (65.3) | |
| 2 | 87 (33.3) | 91 (34.7) | 91 (34.7) | |
| Time since most recent pregnancy loss, months | .55 | |||
| ≤4 | 148 (58.3) | 167 (64.7) | 150 (58.1) | |
| 5–8 | 43 (16.9) | 43 (16.7) | 49 (19.0) | |
| 9–12 | 17 (6.7) | 17 (6.6) | 19 (7.4) | |
| >12 | 46 (18.1) | 31 (12.0) | 40 (15.5) | |
| Walking, hours/week | 1.0 (0–5.0) | 2.3 (0–15.0) | 9 (0–21.0) | < .001 |
| Moderate activity, hours/week | 0 (0–4.0) | 1.5 (0–10) | 10.3 (0–21.0) | < .001 |
| Vigorous activity, hours/week | 0 (0–2.0) | 1.0 (0–5.8) | 2.0 (0–21.0) | < .001 |
| Sitting, hours/day | 6.0 (0–18.0) | 5.0 (0–14.0) | 3.0 (0–15.0) | < .001 |
Note: Values are n (%) for categorical variables and mean ± SD or mean (range) for continuous variables. Data on covariates were missing for BMI (n = 9), current partner’s age (n = 8), smoking in past year (n = 4), alcohol consumption in past year (n = 8), and time from last loss to randomization (n = 15). BMI = body mass index; MET = metabolic equivalent.
P values were calculated from analysis of variance, χ2 test, or Fisher’s exact test, as applicable.
BMI classification (World Health Organization).
Clinically recognized pregnancy losses were documented using a combination of verification from a woman’s physician and medical records.
A total of 188 pregnancy losses were observed: there were 55 subclinical losses as detected only from hCG testing and 133 losses of clinically detected pregnancies of which six were determined to be ectopic. Log-binomial models of PA and pregnancy loss were run unadjusted as well as adjusted for age and W-H ratio. Compared with the first tertile of PA, the RR for subclinical loss was 2.06 (95% CI, 1.03–4.14) for the second and 1.92 (95% CI, 0.94– 3.90) for the third tertile of PA in models adjusted for age and W-H ratio (Table 2). These correspond to absolute risks of roughly 4%, 9%, and 8% for T1–T 3, respectively. No relations were observed between PA and clinically recognized loss or pregnancy loss overall. Models adjusted for BMI in lieu of W-H ratio did not change the above findings. PA may influence conception probability and thus present a potential for bias. To address this possibility of selection bias, log-binomial models of hCG-detected loss, clinically recognized loss, and pregnancy loss overall were run in the full population. In line with models run among women with hCG+ pregnancies only, estimates of MET groups and hCG-detected loss showed an approximately twofold increased risk (T2 RR = 2.05: 95% CI, 1.01 to 4.14; T3 RR = 1.89: 95% CI, 0.92 to 3.87) relative to T1 with adjustment for age and W-H ratio (Supplemental Table 1). Our findings from analyses of risk of clinically recognized loss, and of pregnancy loss risk overall, were null. Models were run additionally adjusting for other covariates including parity, but results were essentially unchanged. Additionally, models using inverse probability of pregnancy weighting were run to address potential selection bias; however, results of this sensitivity analysis were not meaningfully different from the primary analysis (data not shown).
TABLE 2.
Association between physical activity and pregnancy loss among women in the Effects of Aspirin in Gestation and Reproduction study (N = 785).
| Pregnancies (N = 785) | Physical activity tertile (T) [MET, hours/week] | Cases | Unadjusted RR (95% CI) | Age-adjusted RR (95% CI) | Model 2a RR (95% CI) |
|---|---|---|---|---|---|
| Any pregnancy loss (n = 188) | T1 [0–16.6] | 59 | Referent | Referent | Referent |
| T2 [16.6–50.8] | 66 | 1.11 (0.82, 1.51) | 1.09 (0.80, 1.48) | 1.10 (0.81, 1.50) | |
| T3 [51.3–275.1] | 63 | 1.06 (0.78, 1.45) | 1.08 (0.79, 1.47) | 1.09 (0.80, 1.49) | |
| Clinical loss (n = 133) | T1 [0–16.6] | 48 | Referent | Referent | Referent |
| T2 [16.6–50.8] | 43 | 0.89 (0.61, 1.30) | 0.87 (0.60, 1.26) | 0.88 (0.60, 1.28) | |
| T3 [51.3–275.1] | 42 | 0.87 (0.60, 1.27) | 0.89 (0.61, 1.29) | 0.90 (0.62, 1.31) | |
| hCG-detected loss (n = 55) | T1 [0–16.6] | 11 | Referent | Referent | Referent |
| T2 [16.6–50.8] | 23 | 2.08 (1.04, 4.19) | 2.08 (1.04, 4.18) | 2.06 (1.03, 4.14) | |
| T3 [51.3–275.1] | 21 | 1.90 (0.94, 3.86) | 1.90 (0.94, 3.87) | 1.92 (0.94, 3.90) |
Note: CI = confidence interval; MET = metabolic equivalent; RR = risk ratio; W-H ratio = waist-hip ratio.
Model 2 includes age and W-H ratio.
DISCUSSION
We observed a roughly twofold greater risk of subclinical pregnancy loss comparing women who were active to the lowest category of PA. In contrast, we observed no relationship between PA and loss of clinically recognized pregnancies. Taken together, these results support the notion that the periconceptional period represents a time of vulnerability for loss associated with PA. Because of the frequent, longitudinal biospecimen collection in the EAGeR study, we were able to evaluate relations of PA with subclinical loss as well as loss of clinically recognized pregnancies. Subclinical losses identified only through biochemical assays after the end of follow-up were unrecognized at the time and may have been perceived instead as conception delays.
Prior studies of PA and pregnancy loss have yielded inconsistent findings, which may be related to small sample size and study design. In a small prospective study (n = 119), no significant differences among PA groups were observed for clinical or subclinical pregnancy loss (4). A case-control study comparing exercise among women with chromosomally normal losses (n = 173) to that in women with chromosomally abnormal losses (n = 173) observed reduced risk of clinical pregnancy loss with higher PA based on retrospective report of exercise (5). In contrast, in a study of 92,671 Danish women, an increased risk of miscarriage for women who exercised >7 hours per week compared to non-exercisers (hazard ratio = 3.7; 95% CI, 2.9–4.7) was observed, but subclinical losses were not considered in this study (6). In a prospective preconception study of 430 couples with longitudinal specimen collection used to determine subclinical and clinical pregnancy losses, a twofold higher risk was observed for those with high strain scores compared with low based on self-reported daily physical strain (7). This result is similar to what we observed in our analysis, but for PA in general rather than strain in particular. In addition to general strain, PA has been proposed to have potential impacts on pregnancy in the peri-implanation period through mechanisms including blood flow, elevated body temperature, intra-abdominal pressure, and endocrine effects (13, 14). In animal studies, embryo resorption has been observed among both mice (15) and rats (16) that were exercised during gestation; mechanisms proposed for these effects of PA in pregnancy included increased core temperature and catecholamine release (17) and negative impacts on uteroplacental exchange (18). Results of inverse-probability weighted sensitivity analysis suggest that the observed effects are not due to PA increasing total implantation and thus opportunity for implantation failure.
The EAGeR study protocol entailed pregnancy tests performed at home and at clinic visits, assays for free β-hCG performed using first-morning urine samples collected daily over the last 10 days of each participant’s first and second observed menstrual cycles, and spot urine samples collected at all clinic visits thereafter. The frequent, longitudinal biospecimen collection provided a unique opportunity to assess factors associated with subclinical loss. However, the number of pregnancy losses (n = 188) limited statistical power; the small number of ectopic pregnancies observed in EAGeR precluded analysis of this outcome and consideration of a possible role of PA on embryo transport. In addition, although the IPAQ-SF is widely used and considered to have good reliability and agreement with accelerometer (19) and other instruments (20), in our study, the IPAQ-SF was measured only at baseline, which was used to represent activity levels in later pregnancy, whereas a simpler one-question measure of activity was used for longitudinal assessment of activity. Analysis of these data suggested that PA was stable over the preconception period (data not shown). Nevertheless, some misclassification of PA levels based on baseline self-report as in our data is inevitable (21).
Notably, pregnancy loss is a complex, multifactorial outcome, and it is important to consider the potential role of confounding for nonrandomized factors, as considered here. Available data included many potential confounding factors, and we adjusted for age and adiposity, but we observed no indication of confounding by variables considered. However, confounding related to factors not assessed in EAGeR, such as diet, cannot be ruled out. To address this important consideration, we used sensitivity analyses to evaluate the possible impact of uncontrolled confounding (12, 22); this approach estimates an e-value—the minimum RR linking a confounder to PA and risk of loss for results to be completely explained by confounding. This analysis yielded a value of 3.5, indicating that uncontrolled confounders would have to have very strong relations with both PA and implantation failure to completely explain the observed estimate. This e-value exceeds observed relations of dietary patterns with risk of loss (23) and with PA (24). Nevertheless, future studies should consider assessment of additional risk factors for implantation failure to address potential confounding. The unique nature of the study participants—women with a history of one or two pregnancy losses—may limit the generalizability of results. Because pregnancy loss is a strong risk factor for loss in subsequent pregnancies, the participants represent a high-risk, clinically relevant population for whom identification of modifiable behavior risk factors for loss is of particular importance.
Conclusions
In summary, in this study of women with a history of one or two pregnancy losses, higher PA was associated with an increased risk of hCG-detected loss but was unrelated to risk of clinical pregnancy loss. These results suggest that higher levels PA may have adverse effects on implantation and the earliest stages of pregnancy. Women with a history of pregnancy loss and/or facing conception difficulties or delays may be advised to consider changes in PA during this time but follow normal PA guidelines in the case of clinically recognized pregnancies. Additional research is warranted to evaluate this relation in general populations and to address unanswered questions regarding possible mechanisms.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contracts HHSN267200603423, HHSN267200603424, and HHSN267200603426).
Footnotes
L.M.R. has nothing to disclose. B.W.W. has nothing to disclose. J.R.F. has nothing to disclose. S.L.M. has nothing to disclose. L.A.S. has nothing to disclose. N.J.P. has nothing to disclose. K.C.S. has nothing to disclose. J.G. has nothing to disclose. R.M.S. has nothing to disclose. E.F.S. has nothing to disclose.
REFERENCES
- 1.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–9. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Platt RW. Reproductive and Perinatal Epidemiology. Oxford: Oxford University Press; 2011. [Google Scholar]
- 3.Committee on Practice Bulletins—Gynecology. The American College of Obstetricians and Gynecologists Practice bulletin no. 650. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol 2015;126:e135–42. [DOI] [PubMed] [Google Scholar]
- 4.Clapp JF III. The effects of maternal exercise on early pregnancy outcome. Am J Obstet Gynecol 1989;161:1453–7. [DOI] [PubMed] [Google Scholar]
- 5.Latka M, Kline J, Hatch M. Exercise and spontaneous abortion of known karyotype. Epidemiology 1999;10:73–5. [PubMed] [Google Scholar]
- 6.Madsen M, Jørgensen T, Jensen ML, Juhl M, Olsen J, Andersen PK, et al. Leisure time physical exercise during pregnancy and the risk of miscarriage: a study within the Danish National Birth Cohort. Br J Obstet Gynecol 2007;114:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, et al. Spontaneous abortion and physical strain around implantation: a follow-up study of first-pregnancy planners. Epidemiology 2000; 11:18–23. [DOI] [PubMed] [Google Scholar]
- 8.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol 2013;27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Physical Activity Questionnaire. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ): short and long forms; 2005. Available at: http://www.ipaq.ki.se. Accessed July 1, 2019.
- 10.Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM, et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod 2016; 31:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo LM, Whitcomb BW, Mumford SL, Hawkins M, Radin RG, Schliep KC, et al. A prospective study of physical activity and fecundability in women with a history of pregnancy loss. Hum Reprod 2018;33: 1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 13.Magann EF, Evans SF, Weitz B, Newnham J. Antepartum, intrapartum, and neonatal significance of exercise on healthy low-risk pregnant working women. Obstet Gynecol 2002;99:466–72. [DOI] [PubMed] [Google Scholar]
- 14.Clapp JF. Exercise and fetal health. J Dev Physiol 1991;15:9–14. [PubMed] [Google Scholar]
- 15.Terada M Effect of physical activity before pregnancy on fetuses of mice exercised forcibly during pregnancy. Teratology 1974;10:141–4. [DOI] [PubMed] [Google Scholar]
- 16.Lazo-Osório RA, Pereeira R, Christofani JS, Russo AK, Machado M, Ribeiro W, et al. Effect of physical training on metabolic responses of pregnancy rats submitted to swimming under thermal stress. J Res Med Sci 2009; 14:223–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Avery ND, Wolfe LA, Amara CE, Davies GAL, McGrath MJ. Effects of human pregnancy on cardiac autonomic function above and below the ventilatory threshold. J Appl Physiol 2001;90:321–8. [DOI] [PubMed] [Google Scholar]
- 18.McMurray RG, Mottola MF, Wolfe LA, Artal R, Millar L, Pivarnik JM. Recent advances in understanding maternal and fetal responses to exercise. Med Sci Sports Exerc 1993;25:1305–21. [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 20.Hartley S, Garland S, Young E, Bennell KL, Tay I, Gorelik A, et al. A comparison of self-reported and objective physical activity measures in young Australian women. JMIR Public Health Surveill 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport 2000;71:1–14. [DOI] [PubMed] [Google Scholar]
- 22.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018;29:e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaskins AJ, Rich-Edwards JW, Hauser R, Williams PL, Gillman MW, Penzias A, et al. Prepregnancy dietary patterns and risk of pregnancy loss. Am J Clin Nutr 2014;100:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loprinzi PD, Smit E, Mahoney S. Physical activity and dietary behavior in US adults and their combined influence on health. Mayo Clin Proc 2014;89: 190–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


