Supplemental Digital Content is available in the text.
Keywords: antibiotic resistance, nosocomial infections, phage therapy
Abstract
Objective:
Bacterial infections caused by antibiotic-resistant pathogens are a major problem for patients requiring critical care. An approach to combat resistance is the use of bacterial viruses known as “phage therapy.” This review provides a brief “clinicians guide” to phage biology and discusses recent applications in the context of common infections encountered in ICUs.
Data Sources:
Research articles were sourced from PubMed using search term combinations of “bacteriophages” or “phage therapy” with either “lung,” “pneumonia,” “bloodstream,” “abdominal,” “urinary tract,” or “burn wound.”
Study Selection:
Preclinical trials using animal models, case studies detailing compassionate use of phage therapy in humans, and randomized controlled trials were included.
Data Extraction:
We systematically extracted: 1) the infection setting, 2) the causative bacterial pathogen and its antibiotic resistance profile, 3) the nature of the phage therapeutic and how it was administered, 4) outcomes of the therapy, and 5) adverse events.
Data Synthesis:
Phage therapy for the treatment of experimental infections in animal models and in cases of compassionate use in humans has been associated with largely positive outcomes. These findings, however, have failed to translate into positive patient outcomes in the limited number of randomized controlled trails that have been performed to date.
Conclusions:
Widespread clinical implementation of phage therapy depends on success in randomized controlled trials. Additional translational and reverse translational studies aimed at overcoming phage resistance, exploiting phage-antibiotic synergies, and optimizing phage administration will likely improve the design and outcome of future trials.
Infections due to antibiotic-resistant bacterial pathogens are a major cause of morbidity and mortality (1); the Centers for Disease Control and Prevention estimates that greater than 2.8 million antibiotic-resistant infections occur in the United States each year, and of these, 35,000 result in death (2). Despite the need, pharmaceutical companies continue to abandon antibiotic development, largely due to high costs and poor returns on investment (3). Given these challenges, antibiotic alternatives that kill bacteria in distinct ways warrant investigation. One approach is the use of bacterial viruses (bacteriophages/phages) known as “phage therapy (PT).” Promising results from laboratory studies, however, have failed to translate into improved outcomes in the few controlled human trials performed to date (4–7). The purpose of this review is to highlight the progress of PT in the context of common infections encountered in ICUs and to define the challenges that need to be overcome in future laboratory and clinical trials.
Basics of Phage Biology
Phages are naturally occurring viruses that infect and lyse bacteria. Phages are ubiquitous, diverse, and thought to shape the composition of virtually all microbial niches. In humans, phages are present in many tissues and are particularly abundant in the gut (8). Bacterial surface molecules and phage receptor binding proteins determine an individual phages tropism (9). Some phages may have relatively broad bacterial host ranges, capable of infecting strains from a few closely related species, whereas others may have narrow host ranges, limited to a few isolates within a single species.
Once adsorbed to the surface of a susceptible bacterium, phage DNA is injected into the cell, at which point the phage can undergo two prototypical lifecycles; the lytic cycle, where phages propagate inside the bacteria, induces lysis and can then infect additional cells upon release, or the lysogenic cycle, where the phage genome is incorporated into that of the host bacteria (Fig. 1). “Temperate phages” can switch between the lysogenic and lytic lifecycles based on situational cues. The integration of temperate phages into bacterial genomes poses a threat for the transfer of potentially harmful genes (i.e., toxins and antibiotic resistance) between the strains, and as such, temperate phages are rarely considered for therapy. In contrast, “lytic phages” do not have the genetic capability to exercise a lysogenic cycle and are more appropriate for PT. Of note, early bacterial lysis can occur in the absence of phage replication (termed “lysis from without”) either when a large number of phage particles are absorbed onto the bacteria or as a consequence of the activity of exogenous phage products (10). In this context, phage-encoded lysins are also currently being considered for therapeutic application (11).
Figure 1.

Prototypical phage lifecycles. Phages (red) recognize specific receptors on the surface of susceptible bacterial cells (blue). Following attachment, the phage genome is injected into the bacterial cell. Two prototypical phage lifecycles are illustrated. The lytic cycle involves phage replication, followed by cell lysis, liberating phages for subsequent infection. The lysogenic cycle involves integration of phage DNA into the bacterial chromosome. Lysogeny may facilitate the spread of harmful genes between bacteria. “Temperate phages” can switch between lysogenic and lytic cycles, and as such, they are not typically considered for therapy. “Lytic phages” can only use the lytic cycle and are more commonly used for phage therapy.
Phages for the Treatment of Antibiotic-Resistant Infections
Antibiotics are relied upon not only to treat bacterial infections, but also to facilitate modern medical practices including invasive surgeries, organ transplantations, and chemotherapy. Bacteria, however, have evolved to overcome antibiotics. The bacterial cell envelop is an imposing barrier that restricts the entry of toxic compounds. Antibiotics that penetrate can be extruded via resistance pumps, degraded or modified by specific enzymes, or lose activity due to mutation/modification of the compounds target. Common sources of antibiotics were overmined midway through the 20th century, and recently, the antibiotic development pipeline is running dry (12). Thus, there is an urgent need to develop alternative strategies to combat antibiotic-resistant bacterial infections.
Phages were first considered for therapy over 100 years ago based on their ability to lyse bacterial cells (13) and have seen continued use in countries such as Georgia, Poland, and Russia (14, 15). Early clinical data about their efficacy were conflicting (16, 17) and often attributed to a poor understanding of fundamental phage biology. This, coupled with the early success of antibiotics saw phages, falls out of favor in most other countries. Phages, however, have characteristics that may be advantageous in the age of antibiotic resistance and equipped with an improved understanding of how they work, they warrant reconsideration. They enter and destroy bacterial cells using a mechanism that is distinct from traditional antibiotics, suggesting that the threat of phage-antibiotic cross-resistance is low. They may produce enzymes that can degrade biofilms (bacterial communities encased within a protective extracellular matrix) (18). Biofilms are notorious for challenging antibiotic therapy, particularly in the cases of foreign body infection. Phages replicate in the presence of susceptible bacteria (termed “autodosing”), which is desirable when considering PT for high bacterial load infections (19). Finally, they can be highly pathogen-specific, limiting the potential for off-target microbiome disruption, which has been associated with broad-spectrum antibiotic use (20) (Fig. 2).
Figure 2.

The pearls and perils of phage therapy. Phages have many beneficial characteristics to suggest they could be effective against antibiotic-resistant bacterial infections. Many obstacles, however, need to be circumvented in order for phage therapy to reach its clinical potential.
PT is not without its own caveats. Most notably, PT may be complicated by “phage resistance,” where the therapeutic phage is unable to kill the target bacteria (akin to antibiotic resistance). Phage resistance may be intrinsic and present at the onset of therapy (4), or it may evolve during therapy (21). Bacteria may not have the appropriate surface receptor or may code for molecular systems that destroy phage DNA upon entry into the cell (i.e., clustered regularly interspaced short palindromic repeats-cas) (22) (Fig. 2). Importantly, phage resistance mechanisms are often highly specific; the emergence of resistance to one phage does not necessarily result in resistance to a second, distinct phage. The sheer abundance and diversity of phages in nature should ensure that alternative phages are found to mitigate phage resistance (21). Additionally, “cocktails” consisting of a mixture of different phages are commonly used when treating patients.
In preparation for PT, phages should be genome-sequenced to ensure that they are not temperate and do not code for harmful genes. The susceptibility of the infective bacteria to the phages should be confirmed in vitro, and the therapeutic product should be purified to eliminate residual bacterial components carried over from production (i.e., endotoxin) (23) (Fig. 3).
Figure 3.
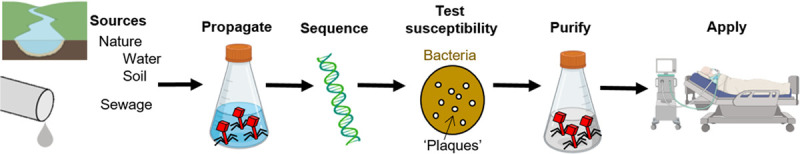
Preparing phages for therapy. Phages are ubiquitous and can be collected from a variety of sources including soil, natural water bodies, and sewage. Phages are first produced to high concentration within a susceptible bacterial host. Phages are then genome sequenced to exclude undesired genes (i.e., lysogeny, antibiotic resistance, and toxins). The susceptibility of the disease causing bacterial isolate should be tested by observing lysis plaques using double-layer agar plates or bacterial growth inhibition in liquid media. Prior to application, phage solutions should be purified to exclude potentially harmful bacterial debris (i.e., endotoxin).
Phage Therapy in Critical Care Situations
Respiratory Tract Infections
Animal Reports
Pulmonary infections are an enormous clinical challenge within the ICU (24). Using small animal models of respiratory infection, PT has consistently improved outcomes when compared with untreated controls (Table S1, http://links.lww.com/CCX/A511). In an experimental model of ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus (MRSA), IV application of phages was as effective as standard-of-care antibiotics (25). Local administration has also shown promise in the context of lung infection. Inhaled phages controlled various Gram-negative bacteria (26–28), and nebulized phages, when applied prophylactically, rescued most animals from lethal MRSA pneumonia (29). Further studies are warranted to investigate the distribution of nebulized phages during ventilation and to determine their efficacy for the treatment of established pneumonia.
Human Reports
PT for the treatment of pneumonia in humans has not been assessed in a randomized controlled trial (RCT). However, recent case studies have shown promising results. Maddocks et al (30) reported successful eradication of multidrug-resistant (MDR) Pseudomonas aeruginosa. Initial treatment with antibiotics failed to result in clinical improvement. A 7-day course of IV and nebulized phages used adjunct to antibiotics was associated with “remarkable” clinical progress. Additional reports described infection resolution following adjunct PT for two ventilated patients with pneumonia, also due to MDR P. aeruginosa (31), and a patient with lung infection due to MDR Klebsiella pneumoniae (32). Case reports have also described instances, where antibiotics and adjunct PT failed to resolve respiratory infections. In each case, therapy was complicated by phage resistance (31, 33). Nevertheless, preclinical and human case studies have reported largely positive findings, which suggest pneumonia is an appropriate setting for future RCTs assessing the safety and efficacy of PT.
Abdominal Infections
Animal Reports
The abdomen is an important point of origin for sepsis and septic shock (1, 34). Abdominal infections are often due to vancomycin-resistant enterococci (VRE) and Gram-negatives including P. aeruginosa. In a rat model of abdominal infection caused by VRE, intraperitoneal injection of phages was as effective as antibiotics (ampicillin) in rescuing animals from fatal sepsis. Importantly, PT did not appear to disrupt the gut microbiota (35). For P. aeruginosa abdominal sepsis, orally administered phages improved survival, reduced organ bacterial densities, and alleviated inflammation compared with controls (36). Patients with severe abdominal infections in the ICU, however, will typically be administered therapeutics IV, and this route is yet to be assessed using animal models.
Human Reports.
Little is known about the usefulness of PT for life-threatening abdominal infections in humans; however, a recent case-report revealed positive patient outcomes (21). The case involved a critically ill patient with a pancreatic pseudocyst infected with MDR Acinetobacter baumannii that was refractory to antibiotic therapy. After 3 weeks of antibiotic and phage coadministration, the clinical condition improved resulting in extubation and discontinuation of vasopressor support. Phage resistance did emerge; however, its impact was mitigated by the rapid isolation and purification of an additional phage from sewage, which was subsequently applied (21). Together, the case highlights the almost limitless pool of phages available in nature that may be useful for therapy, while illustrating the rapid nature with which bacteria can adapt to overcome them (Fig. 2).
Bloodstream Infections
Animal Reports
Most studies that evaluated PT for bloodstream infections in animal models revealed favorable outcomes with improved survival, reduced bacterial counts, and reduced inflammation when compared with untreated controls (Table S1, http://links.lww.com/CCX/A511). Most studies lacked an antibiotic treatment control arm for comparison. Still, the few exceptions showed that phages perform at least as efficaciously as the standard-of-care (37–39).
Human Reports
Most, but not all of the recently published cases of compassionate use PT for bloodstream infections reported a favorable outcome with either notable improvement of the clinical condition or infection resolution (32, 33, 40–42). All patients were severely ill and underwent unsuccessful antibiotic treatment prior to the start of PT. Typically, antibiotics were pursued concurrently with PT. A case series designed to assess safety and tolerability of PT in critically ill patients included patients with native or prosthetic valve endocarditis due to S. aureus (33). A positive effect was reported, reflected by a tendency toward improvement of clinical condition, lower inflammatory markers, and reduced bacterial counts. However, despite phage and antibiotic treatment, four patients succumbed to infection, likely reflecting the severity of underlying disease (33). A second case series presented three patients with aortic graft infections where PT adjunct to antibiotics controlled or eliminated the causative pathogens. For a fourth patient, local and systemic PT adjunct to IV daptomycin reduced S. aureus loads; however, the patient ultimately succumbed to S. aureus sepsis (32).
Phages were typically applied IV (33, 41, 42), with additional examples using intracavitary (40), oral, and local intraoperative administration (32). Various treatment regimens have been used, from single injection (40), to reapplication at regular intervals (33, 42), and continuous infusion (41). However, the optimal approach remains unclear. Following IV injection, phages were detected in the circulation for up to 12 hours and their number increased after repeated application, suggesting either phage autodosing, or a saturation mechanism in phage elimination (33). Elimination seems to be driven by the spleen, liver, and kidney (43); however, our understanding as to how phages are metabolized is incomplete.
In summary, data from animals and humans have shown that systemic PT seems safe, tolerable, and effective in combination with antibiotics at controlling bloodstream infections.
LESSONS FROM RECENT CLINICAL TRIALS
Much of the excitement surrounding PT for antibiotic-resistant infections stems from human case reports. It is important, however, to maintain some degree of skepticism; in most cases, patients remained on antibiotics, so the absolute impact of PT is unclear, and more generally, case reporting may be influenced by positive publication bias (i.e., failures are less likely to be published). In recent phase I/II randomized, placebo-controlled, double-blind clinical trials, the results are less promising. “PhagoBurn” (NCT02116010) assessed PT for severe burn wound infections due to P. aeruginosa using a phage product designed to comply with good manufacturing and clinical practices (4). A phage-cocktail was delivered topically once daily for 7 days (n = 12) and compared with standard-of-care sulfadiazine silver (n = 13). The primary outcome was reduced bacterial growth on agar plates. Results of the trial were disappointing; patients receiving PT took longer to reach the primary endpoint (144 vs 47 hr, p = 0.018), and fewer patients reached that end point by day 7, although this difference was not statistically significant (50% vs 85%, p = 0.0917) (4). Intravesical phages were assessed for the treatment of urinary tract infections (UTIs; NCT03140085) (6). Instillation of phages into the bladder was not superior to instilled placebo, and although phages were not statistically inferior to antibiotics, the number of patients that reached the primary end point was lower at 7 days (5/28 for phages, 13/37 for antibiotics, p = 0.11). Finally, the largest RCT performed to date, investigating oral PT for diarrhea in children due to Escherichia coli (NCT00937274), was stopped following an interim analysis (120 of 375 patients), citing “no amelioration in quantitative diarrhea” (5).
These trials illustrated some of the shortcomings for the clinical application of PT. Although these safety-driven RCTs may have been underpowered to reveal treatment efficacy outcomes, it is apparent that positive results from preclinical trials have so far failed to translate to positive patient outcomes (Fig. 4). In the context of PhagoBurn, topically applied phages were statistically superior to standard-of-care (silver nitrate) in a murine model of P. aeruginosa burn wound infection (44), which is juxtaposed to the findings of the trial. Additionally, the stability of the therapeutic was poor, which contradicted laboratory findings, meaning patients received ~10,000 times fewer phages than was intended. Although the occurrence of adverse events was not different between the treatments hinting toward safety, the importance of this finding was confounded by the use of low doses (4).
Figure 4.
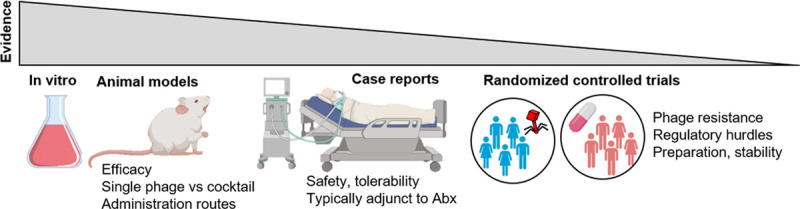
The “bench-to-bedside” nature of the evidence to support the implementation of phage therapy.
For the treatment of UTI, phages were instilled into the bladder to reduce the potential systemic effects of PT, yet, to our knowledge, this treatment approach had not been explored in experimental studies, which may represent a disconnect in the progression from “bench-to-bedside” (Fig. 4). The authors hypothesized that mechanical irrigation of the bladder may have explained any therapeutic effects based on the similar efficacy of each treatment arm to placebo (6). The high rate of spontaneous clearance (~30%) may indicate that the infection setting is not ideal for the assessment of PT.
Finally, the nature of the therapeutic product is highly important. PhagoBurn tested a predefined cocktail of 12 phages that were designed to cover a wide range of P. aeruginosa clinical isolates. However, susceptibility to the cocktail was not tested prior to randomization, and treatment failures were attributed largely to “intermediate resistance” at the start of therapy. Similarly, only half of the patients from the E. coli diarrhea trial had phage-susceptible E. coli in their stool (5). In contrast, for the UTI trial, phage sensitivity was confirmed in vitro before assigning patients to the phage treatment arm, which is a clinically important approach. However, the exact concentration and composition of the phage product were not described, which, if known, may have helped to explain the poor efficacy of PT, as data from animal models of UTI have revealed phage dose-dependencies (45).
At the very least, these foundational RCTs have highlighted some of the hurdles, both biological and regulatory, that need to be circumvented in future trials.
BLUEPRINT FOR PHAGE THERAPY IN THE ICU
The acute nature of many ICU infections presents a unique set of challenges for the application of PT. In chronic settings, there is sufficient time to tailor a personalized treatment. This may include a hunt for efficient phages in the environment and global phage biobanks, or engineering of phages for improved infectivity and persistence (46–48). In contrast, the window of time is considerably narrower for patients in the ICU. Thus, an appropriate PT treatment algorithm will need to be designed to be executed rapidly. In order to implement PT within days of a diagnosis, centralized facilities will likely need to maintain ready-to-use phage preparations. Although this approach proved challenging in the PhagoBurn RCT, the process would benefit from a better understanding of phage storage and distribution requirements and the generation of improved phage cocktails that are broadly effective in the context of local infection epidemiology. Alternatively, the increased use of whole genome sequencing in routine clinical microbiology has paved the way for computational approaches to predict positive pathogen-phage interactions (49). Once PT is initiated, its effectiveness should be continually monitored to ensure that phage resistance does not emerge. This will require the establishment of standardized definitions of resistance, akin to minimum inhibitory concentration breakpoints for antibiotics, and protocols that can be easily adopted by diagnostic microbiology laboratories.
Even when facing the current antibiotic resistance crisis, antibiotics remain a cornerstone of critical care. It is unlikely that clinicians will cease antibiotics altogether in favor of PT, even if the infection is responding poorly to the standard-of-care. Indeed, all bar one human case study has seen PT applied as an adjunct to multiple antibiotic classes (Table S1, http://links.lww.com/CCX/A511). It is then surprising that most preclinical studies and each of the RCTs detailed above did not assess phage-antibiotic combination therapies. When tested at different concentrations in vitro, some phage-antibiotic combinations can reveal synergisms (50, 51), additive effects, and, most worryingly, antagonisms (50), which may ultimately reduce treatment efficacy. These important findings should be further assessed in vivo to avoid antagonisms and exploit synergies.
Phage dosing and timing are each crucial for effective PT. Insufficient phage concentrations and delays in the initiation of treatment have been consistently associated with poor outcomes (38, 52–59). However, the available data do not yet point to clear answers as to how phages should be administered and how frequently this should occur. For the former, IV application of phages seems safe and should not be shied away from in future trials, and nebulized phages applied to mechanically ventilated subjects warrant future investigation (29, 30). For the latter, phage pharmacokinetic and pharmacodynamic (PK/PD) models need to be developed (60) and tested to systematically determine optimal phage dosing strategies.
CONCLUSIONS
Recent RCTs assessing PT failed to demonstrate efficacy in humans. Future success requires a concerted effort by the research community to perform further studies to overcome phage resistance, exploit phage-antibiotic synergies, and to optimize phage PK/PD.
ACKNOWLEDGMENTS
We thank Dr. David Berger and Dr. Luca Cioccari for their critical reading of the manuscript and helpful suggestions. Figures contain images available at https://BioRender.com.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Supported, in part, by the European Society for Intensive Care Medicine (basic science award to J.P.), the Swiss National Foundation (grants #320030_176216 to Y.-A.Q. and CR31I3_166124 to Y.-A.Q.), the Novartis Foundation (unrestricted grant to Y.-A.Q.), and the Swiss Heart Foundation (grant #FF20114 to D.R.C.).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Vincent JL, Sakr Y, Singer M, et al. ; EPIC III Investigators. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020; 323:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Antibiotic Resistance Threats in the United States, 2019. 2019, Atltanta, GA: U.S. Department of Health and Human Services, CDC [Google Scholar]
- 3.Talbot GH, Jezek A, Murray BE, et al. ; Infectious Diseases Society of America. The Infectious Diseases Society of America’s 10 × ‘20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration approved by 2020): Is 20 × ‘20 a possibility? Clin Infect Dis. 2019; 69:1–11 [DOI] [PubMed] [Google Scholar]
- 4.Jault P, Leclerc T, Jennes S, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase ½ trial. Lancet Infect Dis. 2019; 19:35–45 [DOI] [PubMed] [Google Scholar]
- 5.Sarker SA, Sultana S, Reuteler G, et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: A randomized trial in children from Bangladesh. EBioMedicine. 2016; 4:124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitner L, Ujmajuridze A, Chanishvili N, et al. Intravesical bacteriophages for treating urinary tract infections: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis. 2021; 21:427–436 [DOI] [PubMed] [Google Scholar]
- 7.Wittebole X, Opal S. Witzany G. Phagetherapy: Clinical applications – critical appraisal of randomized controlled trials. In: Biocommunication of Phages. 2020, Cham: Springer International Publishing; 371–383 [Google Scholar]
- 8.Gregory AC, Zablocki O, Zayed AA, et al. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe. 2020; 28:724–740.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakhuba DV, Kolomiets EI, Dey ES, et al. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol J Microbiol. 2010; 59:145–155 [PubMed] [Google Scholar]
- 10.Abedon ST. Lysis from without. Bacteriophage. 2011; 1:46–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012; 7:1147–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis K. The science of antibiotic discovery. Cell. 2020; 181:29–45 [DOI] [PubMed] [Google Scholar]
- 13.d'Herelle F: On an invisible microbe antagonistic to dysentery bacilli. Note by M. F. d’Herelle, presented by M. Roux. Comptes Rendus Academie des Sciences 1917. Bacteriophage. 2011; 1:3–5 [Google Scholar]
- 14.Kutateladze M, Adamia R. Phage therapy experience at the eliava institute. Med Mal Infect. 2008; 38:426–430 [DOI] [PubMed] [Google Scholar]
- 15.Międzybrodzki R, Hoyle N, Zhvaniya F, et al. Harper DR, Abedon ST, Burrowes BH, McConville ML. Current updates from the long-standing phage research centers in Georgia, Poland, and Russia. In: Bacteriophages: Biology, Technology, Therapy. 2018, Cham: Springer International Publishing; 1–31 [Google Scholar]
- 16.Krueger A, Scribner E. The bacteriophage: Its nature and its therapeutic use. JAMA. 1941; 116:2160–2167 [Google Scholar]
- 17.Eaton M, Bayne-Jones S. Bacteriophage therapy: Review of the principles and results of the use of bacteriophage in the treatment of infections JAMA. 1934; 103:1769–1776 [Google Scholar]
- 18.Hughes KA, Sutherland IW, Jones MV. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiology (Reading). 1998; 144Pt 113039–3047 [DOI] [PubMed] [Google Scholar]
- 19.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011; 1:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008; 6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized Bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017; 61:e00954–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol. 2010; 8:317–327 [DOI] [PubMed] [Google Scholar]
- 23.Pirnay JP, Blasdel BG, Bretaudeau L, et al. Quality and safety requirements for sustainable phage therapy products. Pharm Res. 2015; 32:2173–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Pascale G, Ranzani OT, Nseir S, et al. Intensive care unit patients with lower respiratory tract nosocomial infections: The ENIRRIs project. ERJ Open Res. 2017; 3:00092–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prazak J, Iten M, Cameron DR, et al. Bacteriophages improve outcomes in experimental Staphylococcus aureus ventilator-associated pneumonia. Am J Respir Crit Care Med. 2019; 200:1126–1133 [DOI] [PubMed] [Google Scholar]
- 26.Debarbieux L, Leduc D, Maura D, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010; 201:1096–1104 [DOI] [PubMed] [Google Scholar]
- 27.Hua Y, Luo T, Yang Y, et al. Phage therapy as a promising new treatment for lung infection caused by Carbapenem-resistant Acinetobacter baumannii in mice. Front Microbiol. 2017; 8:2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dufour N, Debarbieux L, Fromentin M, et al. Treatment of highly virulent extraintestinal pathogenic Escherichia coli Pneumonia with bacteriophages. Crit Care Med. 2015; 43:e190–e198 [DOI] [PubMed] [Google Scholar]
- 29.Prazak J, Valente L, Iten M, et al. Nebulized bacteriophages for prophylaxis of experimental ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Crit Care Med. 2020; 48:1042–1046 [DOI] [PubMed] [Google Scholar]
- 30.Maddocks S, Fabijan AP, Ho J, et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2019; 200:1179–1181 [DOI] [PubMed] [Google Scholar]
- 31.Aslam S, Courtwright AM, Koval C, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant. 2019; 19:2631–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubalskii E, Ruemke S, Salmoukas C, et al. Bacteriophage therapy for critical infections related to cardiothoracic surgery. Antibiotics (Basel). 2020; 9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovic Fabijan A, Lin RCY, Ho J, et al. ; Westmead Bacteriophage Therapy Team. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020; 5:465–472 [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006; 34:344–353 [DOI] [PubMed] [Google Scholar]
- 35.Gelman D, Beyth S, Lerer V, et al. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res Microbiol. 2018; 169:531–539 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe R, Matsumoto T, Sano G, et al. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 2007; 51:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oechslin F, Piccardi P, Mancini S, et al. Synergistic interaction between phage therapy and antibiotics clears pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2017; 215:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunagar R, Patil SA, Chandrakanth RK. Bacteriophage therapy for Staphylococcus aureus bacteremia in streptozotocin-induced diabetic mice. Res Microbiol. 2010; 161:854–860 [DOI] [PubMed] [Google Scholar]
- 39.Leshkasheli L, Kutateladze M, Balarjishvili N, et al. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J Glob Antimicrob Resist. 2019; 19:255–261 [DOI] [PubMed] [Google Scholar]
- 40.Chan BK, Turner PE, Kim S, et al. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health. 2018; 2018:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jennes S, Merabishvili M, Soentjens P, et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit Care. 2017; 21:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duplessis C, Biswas B, Hanisch B, et al. Refractory pseudomonas bacteremia in a 2-year-old sterilized by bacteriophage therapy. J Pediatric Infect Dis Soc. 2018; 7:253–256 [DOI] [PubMed] [Google Scholar]
- 43.Geier MR, Trigg ME, Merril CR. Fate of bacteriophage lambda in non-immune germ-free mice. Nature. 1973; 246:221–223 [DOI] [PubMed] [Google Scholar]
- 44.Kumari S, Harjai K, Chhibber S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J Med Microbiol. 2011; 60:205–210 [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa H, Yasuda M, Uchiyama J, et al. T-even-related bacteriophages as candidates for treatment of Escherichia coli urinary tract infections. Arch Virol. 2008; 153:507–515 [DOI] [PubMed] [Google Scholar]
- 46.Merril CR, Biswas B, Carlton R, et al. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci U S A. 1996; 93:3188–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilcher S, Studer P, Muessner C, et al. Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc Natl Acad Sci U S A. 2018; 115:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007; 104:11197–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leite DMC, Brochet X, Resch G, et al. Computational prediction of inter-species relationships through omics data analysis and machine learning. BMC Bioinformatics. 2018; 19:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu Liu C, Green SI, Min L, et al. Phage -antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comeau AM, Tétart F, Trojet SN, et al. Phage-antibiotic synergy (PAS): Beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007; 2:e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Hu B, Xu M, et al. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J Mol Med. 2006; 17:309–317 [PubMed] [Google Scholar]
- 53.Matsuzaki S, Yasuda M, Nishikawa H, et al. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J Infect Dis. 2003; 187:613–624 [DOI] [PubMed] [Google Scholar]
- 54.Capparelli R, Parlato M, Borriello G, et al. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother. 2007; 51:2765–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider G, Szentes N, Horváth M, et al. Kinetics of targeted phage rescue in a mouse model of systemic Escherichia coli K1. Biomed Res Int. 2018; 2018:7569645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas B, Adhya S, Washart P, et al. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun. 2002; 70:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng M, Liang J, Zhang Y, et al. The bacteriophage EF-P29 efficiently protects against lethal vancomycin-resistant Enterococcus faecalis and alleviates gut microbiota imbalance in a murine bacteremia model. Front Microbiol. 2017; 8:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchiyama J, Rashel M, Takemura I, et al. In silico and in vivo evaluation of bacteriophage phiEF24C, a candidate for treatment of Enterococcus faecalis infections. Appl Environ Microbiol. 2008; 74:4149–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W, Mi Z, Yin X, et al. Characterization of Enterococcus faecalis phage IME-EF1 and its endolysin. PLoS One. 2013; 8:e80435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin YW, Chang RY, Rao GG, et al. Pharmacokinetics/pharmacodynamics of antipseudomonal bacteriophage therapy in rats: A proof-of-concept study. Clin Microbiol Infect. 2020; 26:1229–1235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


