Abstract
Methanol and methanol/water extracts of olive stones and seeds from Olea europaea var. meski were analyzed by reversed-phase high-performance liquid chromatography (HPLC) with diode array detection and mass spectrometry (LC-MS/MS). A total of 28 metabolites were identified; among them are hydroxycinnamic acid derivatives, phenolic alcohols, flavonoids and flavonoid glucosides, secoiridoids, and terpenes. All the extracts were screened for the inhibitory effect of key enzymes related to diabetes and obesity, such as α-amylase and lipase. An in vitro study revealed that Olea meski stone ethanol (MSE) and methanol (MSM) extracts and Olea meski seed ethanol (MSE1) and methanol (MSM1) extracts exert an inhibitory action against lipase and α-amylase. The most potent activity was observed in the StM extract with IC50 equal to 0.19 mg/ml against DPPH oxidation, 1.04 mg/ml against α-amylase, and 2.13 mg/ml against lipase. In HFFD rats, the findings indicated that the increase of body weight, LDL, TC, and glucose levels and then the decrease in HDL-C were significantly suppressed in the MSM-treated group than those in HFFD rats. Moreover, the MSM extract exhibited a prominent selective inhibitory effect against intestinal lipase and α-amylase activities. The MSM extract was also able to protect the liver-kidney functions efficiently, which was evidenced by biochemicals and histological studies.
1. Introduction
The value of Olea europaea L. has a long history in traditional medicine, especially in Mediterranean countries. Different parts of the olive tree as well as its products mainly olive oil and olive drupes have been used for centuries for their nutritional properties but also for their health protective effects. Recent research works confirm this valuable profile. Olive drupes have been known to reduce blood sugar, cholesterol, and uric acid. It has also been used to treat diabetes, hypertension, inflammation, diarrhea, respiratory and urinary tract infections, stomach and intestinal diseases, asthma, hemorrhoids, and rheumatism and as a laxative, mouth cleanser, and vasodilator [1]. These biological effects are mainly attributed to certain phenolic compounds found in the Olea genus such as phenylethanols, secoiridoids, flavonoids, and lignans [2–4].
Specifically, olives and their byproducts are recognized as a valuable source of natural phenolic antioxidants [2]. For instance, secoiridoid derivatives exhibit a diverse range of biological properties [5–8]. Oleuropein, the major secoiridoid of olive fruit and leaves, has been assessed for its antioxidant potential, anti-inflammatory, antimicrobial [9], and antiviral activities [10]. Recent reports demonstrated an antiamyloidogenic effect of oleuropein suggesting a possible protective role against Alzheimer's disease [11]. Regarding composition, several studies have been carried out mainly focusing on the phenolic composition in olive oil, the most well-known and studied product of olive tree. Much less is the information on the phenolics of other Olea organs such as olive fruits, stones, stems, and roots [12, 13]. According to the literature, little attention has been taken for Olea europaea var. meski stone and seed extracts leading to a limited knowledge about their phytochemical content.
In the current work, the chemical composition of different extracts (ethyl acetate, methanol, and methanol/water) of olive stones and seeds from Olea europaea var. meski using ultrahigh-performance liquid chromatography coupled with the high-resolution mass spectroscopy (UPLC-MS) technique was studied for the first time. In parallel, the effect of these extracts on key enzymes related to obesity, hyperlipidemia, and hyperglycemia and liver-kidney functions was undertaken.
2. Materials and Methods
2.1. Collection of Plant Material
The seeds (kernel) and stones (wood shell and endocarp) (Figure 1) of olive fruits from Olea europaea var. meski were subjected to extraction by maceration in solvents of increasing polarity: ethyl acetate, methanol, and methanol/water (80/20). This extraction procedure led to the production of six extracts (Table 1).
Figure 1.
Olive fruit organs.
Table 1.
Secondary metabolites detected in the different Olea europaea meski stone and seed extracts.
| No. | Rt (min) | Proposed EC | [M − H]−m/z | Δ in ppm | RDBeq | m/z MS2 (Relative intensity %) | Tentative identification | Olea meski stone extracts | Olea meski seed extracts | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MeOH | MeOH/H2O | MeOH | MeOH/H2O | ||||||||
| 1 | 2.54 | C14H19O8 | 315.10764 | −2.87 | 5.5 | 153.0506 (100), 287.1157, 196.7752 | Hydroxytyrosol hexoside | − | − | + | + |
| 2 | 2.77 | C8H9O3 | 153.05582 | 0.64 | 4.5 | 123.0452 (100) | Hydroxytyrosol | − | − | ++ | + |
| 3 | 3.27 | C14H19O7 | 299.11270 | 0.57 | 5.5 | Nd | Salidroside | + | + | + | + |
| 4 | 3.45 | C10H13O5 | 213.07648 | −1.73 | 4.5 | 151.0763 (100), 196.8127, 183.0658 | Hydroxylated products of the decarboxylated form of hydroxy elenolic acid(isomer I) | + | + | + | + |
| 5 | 3.55 | C10H13O5 | 213.0765 | −1.52 | 4.5 | 151.0763 (100), 196.8127, 183.0658 | Hydroxylated products of the decarboxyl elenolic acid (isomer ΙI) | + | + | + | + |
| 6 | 3.63 | C11H13O6 | 241.07126 | −2.08 | 5.5 | Nd | Elenolic acid | + | − | − | − |
| 7 | 3.78 | C16H21O11 | 389.10782 | −2.86 | 6.5 | 345.1174 (100), 287.1134, 196.8236 | Oleoside | − | − | + | + |
| 8 | 4.50 | C17H23O11 | 403.12350 | −2.67 | 6.5 | 223.0603 (100), 359.1330, 333.0810, 179.0557. | Oleoside 11-methyl ester | + | + | ++ | ++ |
| 9 | 5.82 | C25H31O14 | 555.17032 | −0.482 | 10.5 | 537.1587 (100), 456.8495, 403.1225, 393.1173, 287.0841 | Hydroxyoleuropein | + | + | + | + |
| 10 | 5.90 | C31H41O18 | 701.22791 | −1.40 | 11.5 | 539.1738 (100), 377.1226, 307.0786, 175.0913 | Neo-nuzhenide | + | + | + | + |
| 11 | 5.98 | C21H19O11 | 447.09225 | −2.30 | 12.5 | Nd | Luteolin hexosides | − | − | + | + |
| 12 | 6.12 | C29H35O15 | 623.19629 | −2.97 | 12.5 | 461.1638 (100) | Verbascoside | + | + | + | + |
| 13 | 6.17 | C29H35O15 | 623.19672 | −2.29 | 12.5 | 461.1639 (100) | Isoacteoside | + | + | + | + |
| 14 | 6.47 | C25H35O13 | 543.20715 | −2.13 | 8.5 | 525.1948 (100), 513.1946 | Dihydro oleuropein | − | − | + | + |
| 15 | 6.51 | C31H41O17 | 685.23309 | −1.06 | 11.5 | 523.1719 (100), 453.1376, 421.1480, 299.1130 | Nuzhenide | ++ | ++ | +++ | +++ |
| 16 | 7.14 | C10H11O4 | 195.06624 | −0.22 | 5.5 | Nd | Hydroxytyrosol acetate | + | − | − | − |
| 17 | 7.52 | C25H31O13 | 539.17572 | −2.39 | 10.5 | 377.1223 (100), 307.0809, 275.0913 | Oleuropein | +++ | +++ | + | + |
| 18 | 7.63 | C25H27O13 | 535.14655 | 1.55 | 12.5 | Nd | Comselogoside (p-Coumaroyl-6 oleoside) | − | − | + | + |
| 19 | 8.35 | C25H27O13 | 523.18054 | −2.97 | 10.5 | 361.1273 (100), 291.0858, 259.0962 | Ligstroside | ++ | + | + | + |
| 20 | 8.43 | C19H21O7 | 361.12854 | −2.03 | 9.5 | 291.0861 (100), 259.0966 | Monoaldehydic form of ligstroside aglycon | + | + | + | + |
| 21 | 8.81 | C15H9O6 | 285.03976 | −2.44 | 11.5 | Nd | Luteolin | − | − | + | + |
| 22 | 8.87 | C17H19O6 | 319.11795 | −2.38 | 8.5 | Nd | Oleacein | + | − | + | + |
| 23 | 8.99 | C25H35O13 | 381.15448 | −2.65 | 7.5 | 363.1430 (100), 349.1275, 331.1170, 287.121 | Hydroxytyrosol acyclodihydroelenolate | − | − | + | + |
| 24 | 10.64 | C18H33O5 | 329.23254 | 0.289 | 2.5 | 229.1438 (100), 311.2214, 293.2111, 211.1334 | 9,12,13-trihydroxy octadeca-7-enoic acid | ++ | − | − | − |
| 25 | 15.61 | C19H25O8 | 377.12372 | −1.25 | 9.5 | 345.0978 (100), 307.0822, 275.0924 | Oleuropein aglycon | + | − | − | − |
| 26 | 17.50 | C30H47O4 | 471.34674 | −1.47 | 7.5 | 423.3267 (100), 405.3052, 393.3250 | Maslinic acid | ++ | + | − | − |
| 27 | 18.11 | C18H31O3 | 295.2378 | 0.39 | 3.5 | 251.2372 (100), 277.2162, 155.1440 | Hydroxyoctadecadienoic acid | ++ | − | − | − |
| 28 | 21.01 | C30H47O3 | 455.35178 | −2.83 | 7.5 | 196.7925 (100), 287.0947, 407.3293 | Oleanolic acid | ++ | + | − | − |
| 29 | 21.19 | C20H37O2 | 309.1732 | −0.41 | 2.5 | 287.1234 (100), 196.7988 | Eicosanoic acid | ++ | − | − | − |
| 30 | 22.21 | C21H37O4 | 353.2667 | −1.35 | 4.5 | 287.0779 (100), 196.8279 | Dihydroxyheneicosanoic acid | ++ | − | − | − |
| 31 | 25.61 | C27H29O15 | 593.15009 | −1.86 | 13.5 | Nd | Luteolin-7-O-rutinoside | − | − | + | + |
Rt: retention time; EC: elemental composition; RDBeq: ring double bond equivalent; [M–H]−: m/z of the pseudomolecular ion; +,++,+++relative abondance; −not detected.
2.2. Animal Study
Male Wistar rats, with body weights of 180 to 200 g and bred in the Central Animal House and obtained from the Central Pharmacy, Tunisia, were used in this study. The animals were fed on a pellet diet (Socco, Sfax, Tunisia) and water ad libitum. The animals were maintained in a controlled environment under standard conditions of temperature and humidity with an alternating light-and-dark cycle. The handling of the animals was approved by the Tunisian Ethical Committee for the care and use of laboratory animals.
2.3. Experimental Protocol
A total of 32 rats were randomly subdivided into four experimental groups (4 × 8) and subjected to the following treatment in a period of 90 days: the C0 group—control rats fed on a standard diet (control diet (CD) group) at the start of the experiment as a reference; the C90 group—control rats fed on a standard diet (control diet (CD) group) for 90 days; the HFFD group—rats fed on high-fat high-fructose diet (HFFD) composed of 59.95% normal diet, 20% sheep fats, 20% fructose, and 0.5% cholic acid for 90 days; and HFFD + MSM group—rats fed on HFFD and received additional 100 mg/kg of body weight of MSM extract in a volume of 1 ml water daily for 90 days.
After the end of the period of 90 days, the serum was obtained from the trunk and collected after decapitation. Plasma was immediately separated by centrifugation at 4°C and 1,500 × g for 15 minutes. The samples were stored at −80°C until further use. The intestine of each rat was excised and the lumen was flushed out several times with 0.9% NaCl. The scraped off mucosal tissue was pooled, homogenized, and centrifuged at 4,000 × g, and the supernatant was frozen until use in further enzymatic assays.
2.4. Analytical Methods
The activities of intestinal lipase and α-amylase activities were determined using commercial kits from Biomagreb (Tunis, Tunisia). The activities of aspartate and alanine transaminases (AST and ALT) and lactate dehydrogenase (LDH) as well as the levels of total cholesterol, triglyceride, and HDL cholesterol in the serum were measured using commercial kits from Biomagreb (Tunis, Tunisia) and BIOLABO (Lyon, France).
2.5. UHPLC-ESI-HRMS and HRMS/MS Analyses
A Waters ultra-performance liquid chromatography (UPLC) system hyphenated to a hybrid LTQ Orbitrap Discovery mass spectrometer (Thermo Scientific, Brehmen, Germany) was employed for the analysis. The mass spectrometer was equipped with an electrospray ionization (ESI) source and operated in a negative mode. The UPLC system is composed by a Waters pump and a Waters autosampler. Stock solutions of 300 μg/ml (ACN/water 1 : 1, v/v) of all the extracts were prepared and 10 μl was injected on a Fortis C18 (Fortis Technologies) column (2.1 x 100 mm, 1.7 μm). The mobile phase used was aqueous acetic acid 0.1% (v/v) (solvent A) and methanol (solvent B). The initial conditions were 95% of solvent A and 5% of solvent B, adjusting the gradient to 100% B in 20 min. This solvent composition was maintained for 1 min (100% B) followed by a return to the initial conditions and a re-equilibration step (3 min) prior the next run. The flow rate was set to 400 μl/min. The mass tolerance was set to 5 ppm. MS Data were acquired in the ESI(−)mode, in the full-scan m/z range of 115–1000 with a resolution of 30,000. MS2 spectra were recorded using data-dependent acquisition with a CID value of 35% and a mass resolution of 7,500. Capillary temp was set at 350°C with a respective source voltage set on 2.7 kV. Tube lens and the capillary voltage were tuned at −100 and −30 V in the negative mode. Finally, nitrogen was used as sheath gas (40 arbitrary units) and auxiliary gas (10 arbitrary units). Xcalibur 2.0.7 software was used for the pre- and postacquisition of the results.
3. Results
3.1. Phytochemical Analysis
Olea europaea var. meski was cultivated in Tunisia and its detailed phytochemical content is not yet reported. The current study provides the LC-HRMS and HRMS/MS profiling of methanol and methanol/water extracts from olive stones and seeds. Interestingly, the phytochemical analysis of the aforementioned plant material revealed differences as a matter of quality and relative quantity of the identified secondary metabolites in the two plant organs. Specifically, chromatographic and spectrometric features such as retention time and HRMS/MS data allowed the detection and identification of 28 secondary metabolites as well as the performance of a comparative study for the presence of these constituents in the different parts of woody endocarp of olive fruits. The suggested elemental composition (EC) was based on the pseudomolecular ions, the HRMS/MS fragments, and the respective indicative RDBeq values which assisted drastically in the identification process. Moreover, the electrospray ionization (ESI) method, in the negative mode, was chosen due to its efficiency for the detection of secondary metabolites in olive fruits.
Noticeably, olive stones are the richest in secondary metabolites among the studied olive organs particularly for the methanol extract. 31 metabolites were identified; among them are phenolic alcohols, hydroxycinnamic acid derivatives, secoiridoids, flavonoids, terpenes, and fatty acids. Verbascoside (m/z 623.19629) was the main hydroxycinnamic acid derivative identified in all extracts with the main fragment at m/z 461.1638. Two simple phenyl alcohols hydroxytyrosol (m/z 153.05582) and its glycosylated derivative (m/z 315.10764) were detected only in Olea meski seed extracts. Hydroxytyrosol was more abundant in the methanol extract. Likewise, the glycosylated derivative of tyrosol, salidroside (m/z 299.11270), was detected in the two investigated olive organs. The main secoiridoid derivatives, oleuropein (m/z 539.17572) and ligstroside (m/z 523.18121), were found in olive stones and seeds, as long as they are hydrolyzed on glycosidic bond derivatives oleuropein aglycon (m/z 377.12378) and ligstroside aglycon (m/z 361.12854), respectively. Oleuropein was more abundant in the two olive stone extracts. The monoterpene iridoid, nuzhenide (m/z 685.23309), was found in all extracts in a significant amount and was more abundant in olive seeds. Similarly, neonuzhenide (m/z 701.22791) was also detected in all extracts but in a less abundance. Regarding flavonoids, luteolin (m/z 285.03976) and its glycoside derivatives luteolin7-O-rutinoside (m/z 593.15009) and luteolin hexoside (m/z 447.09238) were detected only in olive seeds with a weak abundance. The typical olive triterpenes, maslinic (m/z 471.34674) and oleanolic (m/z 455.35178) acids, were identified only in the olive stones and they were more abundant in the methanol extract. On the other hand, four fatty acids (9,12,13-trihydroxy octadeca-7-enoic acid, hydroxyoctadecadienoic acid, eicosanoic acid, and dihydroxyheneicosanoic acid) were identified only in the methanol extract of the olive stones (Table 1 and Figure 2).
Figure 2.
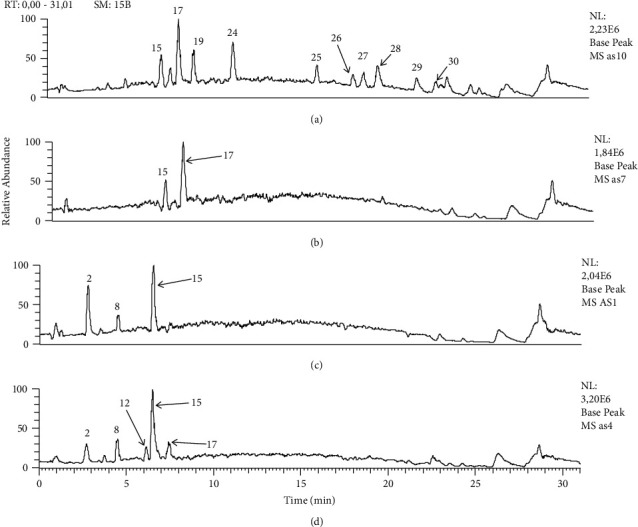
Comparative base peak chromatograms of all extracts from major identified metabolites, Olea europaea var. meski organs. (a) Stones (methanol); (b) stones (methanol/H2O); (c) seeds (methanol); (d) seeds (methanol/H2O). 2: hydroxytyrosol; 8: oleoside 11-methyl ester; 12: verbascoside; 15: nuzhenide; 17: oleuropein; 19: ligstroside; 24: 9,12,13-trihydroxy octadeca-7-enoic acid; 25: oleuropein aglycon; 26: maslinic acid; 27: hydroxyoctadecadienoic acid; 28: oleanolic acid; 29: eicosanoic acid; 30: dihydroxyheneicosanoic acid.
3.2. Effect of Methanol, Hexane, and Water Olive Byproduct Extracts on α-Amylase and Lipase Activities In Vitro
Table 2 showed that olive byproduct extracts (methanol, hexane, and aqueous) showed dose-dependent lipase and α-amylase inhibition. The methanol olive stone extract (MSE) exhibited the lowest IC50 of 1.04 mg/ml against α-amylase and IC50 of 2.13 mg/ml against lipase. The standard positive controls acarbose and atorvastatin showed an IC50 of 0.39 and 1.1 mg/ml, respectively (Table 3).
Table 2.
AST, ALT, and LDH activities and TC, LDL-C, and HDL-C in plasma levels in control, HFFD, and HFFD rats treated with MSM.
| Control (0 day) | Control (90 days) | HFFD | HFFD + MSM | |
|---|---|---|---|---|
| AST | 30.7 ± 3.5 | 36.1 ± 4.6 | 76.1 ± 9.6∗# | 47.7 ± 7.44∗#@ |
| ALT | 35.3 ± 7 | 41.3 ± 3.7 | 64.3 ± 5.9∗# | 52.1 ± 9.1∗#@ |
| LDH | 19.5 ± 1.9 | 21 ± 2.1 | 31 ± 1.9∗# | 27.9 ± 4.2∗#@ |
| TC (g/l) | 1.21 ± 0.12 | 1.34 ± 0.12 | 1.68 ± 0.12∗# | 1.39 ± 0.11∗@ |
| LDL-C (g/l) | 0.56 ± 0.07 | 0.65 ± 0.08 | 1.29 ± 0.05∗# | 0.66 ± 0.09∗@ |
| HDL-C (g/l) | 0.65 ± 0.08∗ | 0.69 ± 0.06 | 0.39 ± 0.06∗# | 0.73 ± 0.05#@ |
∗ P < 0.05 significant differences compared to controls (0 day). #P < 0.05 significant differences compared to controls (90 days). @P < 0.05 significant differences compared to HFFD rats.
Table 3.
Antilipase and anti α-amylase inhibition activities (% of inhibition) of methanol, hexane, and water olive byproducts extracts (IC50, mg/ml) at different concentrations (n = 3).
| IC50 (mg/ml) | |||
|---|---|---|---|
| Olive byproduct extracts | DPPH | α-Amylase | Lipase |
| Methanol olive stone extract (MSE) | 0.19 ± 0.01 | 1.04 ± 0.10 | 2.13 ± 0.13 |
| Methanol/water olive stone extract | 0.27 ± 0.03 | 1.94 ± 0.19 | 3.1 ± 0.11 |
| Methanol olive seed extract | 0.25 ± 0.09 | 1.56 ± 0.17 | 2.57 ± 0.11 |
| Methanol/water olive seed extract | 0.37 ± 0.09 | 2.37 ± 0.19 | 4.50 ± 0.19 |
| Standard ascorbic acid | 0.19 ± 0.09 | ||
| Standard acarbose | 0.011 ± 0.02 | 0.39 ± 0.07 | |
| Standard atorvastatin | 1.1 ± 0.10 | ||
3.3. Intestinal Lipase Activity and Plasma Lipid Concentration
Figure 1 indicates that the administration of MSM extract to HFFD rats reverted the activity of lipase in the intestine back by 37%. This supplement was also observed to bring about a considerable decrease of 17 and 48% in the TC and LDL-C concentrations, respectively, in the plasma. Moreover, while diabetes was noted to induce a decrease in the HDL-Ch concentration by 51%, the MSM supplement was observed to prevent this decrease (Figure 3 and Table 2).
Figure 3.
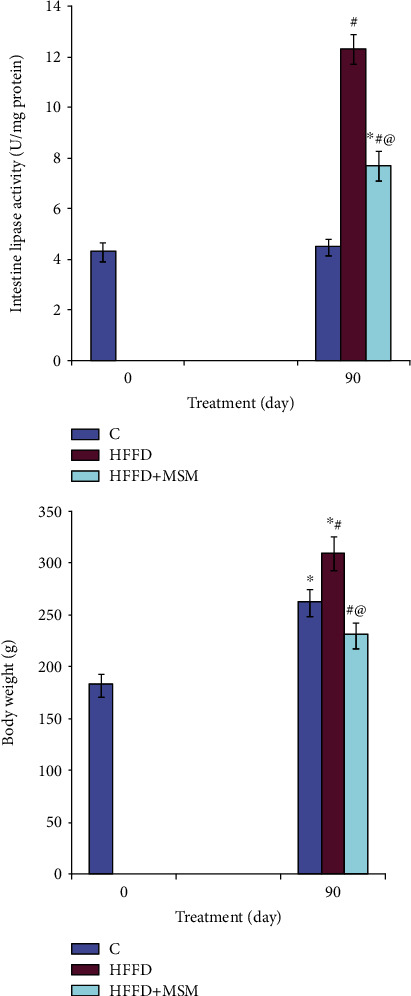
Intestinal lipase activity and body weight in HFFD rats treated with MSM. ∗P < 0.05 significant differences compared to controls (0 day). #P < 0.05 significant differences compared to controls (90 days). @P < 0.05 significant differences compared to HFFD rats.
3.4. Intestinal α-Amylase Activity and Plasma Glucose Levels
Figure 2 shows that the supplementation of MSM extract to HFFD rats was found to significantly decrease the activities of α-amylase by 26%. In addition, the administration of MSM to HFFD rats reduced the glucose concentration in plasma by 27% as compared to HFFD-untreated rats (Figure 4).
Figure 4.
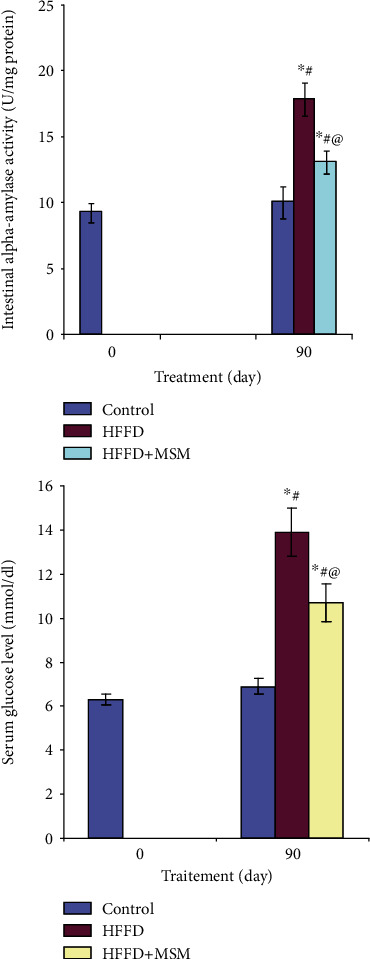
Intestinal α-amylase activity and blood glucose level in HFFD rats treated with MSM. ∗P < 0.05 significant differences compared to controls (0 day). #P < 0.05 significant differences compared to controls (90 days). @P < 0.05 significant differences compared to HFFD rats.
3.5. Hepatic Function, HFFD Diet, and MSM Extract
In HFFD rats treated with MSM extract, a clear protective effect was observed in hepatic function and metabolism. In fact, administration of MSM extract inhibited the increase of AST, ALT, and LDH in plasma of HFFD. This positive effect of this extract was confirmed by histological finding. As shown in Figure 4, fatty cysts appeared in hepatic tissues of HFFD rats. However, MSM administration to HFFD rats reduced the appearance of fat cells in the liver (Table 2 and Figure 5).
Figure 5.
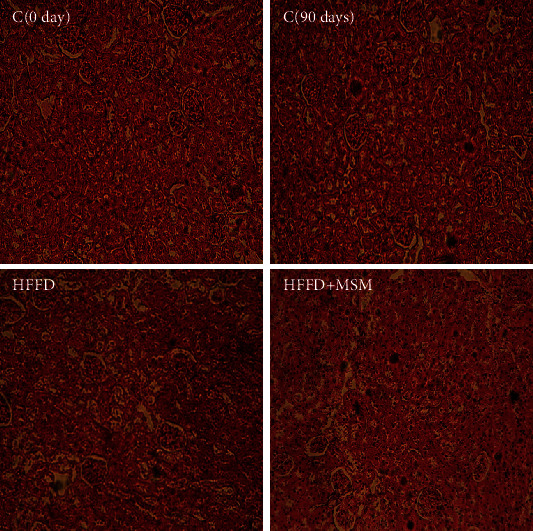
C0day and C90days: normal rat liver; HFFD rat liver: sinusoidal congestion and fatty degeneration in the form of fat lake; HFFD + MSM: diabetic treated with MSM—a positive effect was observed (H&E 100).
4. Discussion
In this study, Table 1 summarizes the results of the profiling study including some spectroscopic characteristics and major fragments of the detected compounds. All the ECs and the fragmentation patterns agree with the previous reports [1, 12]. The chemical composition of olive fruits and olive oil has been extensively studied while other Olea organs such as stems, roots, and drupe stones have received little attention leading to a limited knowledge about their phytochemical content. Indeed, recent studies related to the phytochemical investigation of olive leaves have been referred [14–16], while only few reports are available for olive drupe parts: stones and seeds [13]. According to the literature, the main components of olive roots and stems are hydroxytyrosol, tyrosol, oleuropein, and ligstroside [17] as well as verbascoside and flavonoids such as taxifolin, luteolin, and apigenin derivatives (in stem) [18]. However, the chemical composition of stone and olive extracts obtained by methanol and ethyl acetate revealed great similarities in comparing the chemical composition of other olive organs in other studies [17, 18]. Finally, a new insight into the diverse biochemical pathways in the whole tree is gained contributing to the better understanding of the nutritional and medicinal values of olive tree products.
4.1. In Vitro Study
The present study revealed that the extracts obtained from Olea meski seeds and stones using methanol or methanol/H2O mixture possess remarkable inhibitory activity on lipase and α-amylase. The most potent inhibitor effect was observed in the methanol extract from Olea meski stone (MSM) extract. This is would be explained by the presence of several metabolites well known by their antidiabetic and antihyperlipidemic activities such as oleuropein, oleuropein aglycon, and oleanolic acid [19, 20].
In HFFD rats, this study showed that the administration of MSM extract to HFFD rats inhibits key enzymes related to lipid digestion and absorption as lipase in the intestine and consequently to a decrease in TC and LDL-C and to an increase in HDL-C as compared to normal rats. In fact, phytochemical analysis showed that MSM extract contains bioactive metabolites such as hydroxytyrosol, oleuropein, oleuropein aglycon, and oleanolic acid which are known by their antihyperlipidemic effects and also by their inhibitory activity against lipase. A previous study has demonstrated that oleuropein decreased the body weight gain in vivo [21]. In this context, Park and coworkers [22] have established that oleuropein, at low concentration, attenuated hepatic steatosis induced by high-fat diet in mice [22]. More recent reports have shown that oleuropein supplementation at high concentration (0.59%) significantly decreased the body weight, decreased leptin concentration, and modulated gene expression related to obesity in mice [23].
Furthermore, our findings are in agreement with the report of Hamden and coworkers who demonstrated that flavonoids and polyphenols are able to suppress enzymes related to lipid absorption and consequently to show antiobesity and antihyperlipidemic effects [2]. In addition, it was shown in a previous investigation that administration of hydroxytyrosol-rich extract (3 mg/kg) for 17 weeks in Wistar rats fed a cholesterol-rich diet attenuated the increase in serum triglycerides and total and LDL cholesterol [24].
In addition, this study showed that administration of MSM to HFFD rats inhibited intestinal α-amylase activity and reduced the blood glucose level as compared to untreated HFFD rats. Our results are in agreement with the report of Tiss et al. [25, 26] who induced diabetes in male Wistar rats via intraperitoneal injections of alloxan monohydrate (150 mg/kg), and animals with hyperglycemia (blood glucose levels of 2 g/l after 2 weeks) were retained for experimentation. The treatment groups were given daily hydroxytyrosol by gastric gavage route which resulted in significantly decreased blood glucose levels. The hepatic toxicity indicators TBARS, bilirubin, and fatty cysts were reduced in animals receiving HT treatment. Hepatic glycogen, circulating high-density lipoprotein (HDL), and antioxidant enzymes (SOD, CAT, and GPX) in the liver and kidney were increased by HT. On the other hand, we have noticed that the increase of glucose and lipid levels caused the formation of free radicals of oxygen (ROS). The oxidative atmosphere in cells caused the damage of cells and tissues in the liver. Moreover, the oxidative atmosphere that attacks the hepatic and kidney function appeared by an increase in the levels of AST, ALT, and LDH, an indication of liver dysfunction. However, both the hypoglycemic and hypolipidemic actions of MSM prevented glucose and lipid toxicities as ROS formation. In fact, this study showed that the MSM extract exhibited a strong promising potential as a protective therapeutic agent against liver and kidney toxicities in diabetic rats. It proved remarkably efficient in the decrease of liver dysfunction indices in HFFD rats, namely, the AST, ALT, and LDH activities confirmed by histological analysis.
5. Conclusion
In conclusion, this study indicated that Olea meski stone played an effective role in the amelioration of type 2 diabetes by inhibition of α-amylase activity and the decrease of body weight and normalization of the lipid profile. In addition, Olea meski stone protects liver-kidney-heart functions and tissues.
Acknowledgments
The authors would like to express their gratitude to the Laboratory of Biochemistry, CHU Fattouma Bourguiba, University of Monastir, Tunisia. This research was supported by the Tunisian Ministry of Higher Education and Scientific Research and Technology and the Tunisian Ministry of Public Health.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethical Approval
The animal studies conducted in this work followed the International Guidelines for Animal Care Directive 86/609/EEC and were approved by the Tunisian Ethics Committee of the University of Monastir (Monastir, Tunisia) for the care and use of laboratory animals.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Saad A, Keskes H, Chaari A, Sakavitsi ME, Halabalaki M, and Allouche N collected the plant material, did the UHPLC-ESI-HRMS and HRMS/MS analyses, and wrote the paper. Tiss M and Hamden K performed the biological experiment and analysis, analyzed the data, and wrote the paper. All the authors provided consent to the publication of the manuscript to the Journal of Diabetes Research.
References
- 1.Kanakis P., Termentzi A., Michel T., Gikas E., Halabalaki M., Skaltsounis A.-L. From olive drupes to olive oil. An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. PLANTA MED. 2013;79(16):1576–1587. doi: 10.1055/s-0033-1350823. [DOI] [PubMed] [Google Scholar]
- 2.Hamden K., Allouche N., Damak M., Elfeki A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chemico-Biological Interactions. 2009;180(3):421–432. doi: 10.1016/j.cbi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Johnson R., Melliou E., Zweigenbaum J., Mitchell A. E. Quantitation of oleuropein and related phenolics in cured Spanish-style green, California-style black ripe, and Greek-style natural fermentation olives. Journal of Agricultural and Food Chemistry. 2018;66(9):2121–2128. doi: 10.1021/acs.jafc.7b06025. [DOI] [PubMed] [Google Scholar]
- 4.Olmo-García L., Kessler N., Neuweger H., et al. Unravelling the distribution of secondary metabolites in Olea europaea L.: exhaustive characterization of eight olive-tree derived matrices by complementary platforms (LC-ESI/APCI-MS and GC-APCI-MS) Molecules. 2018;23(10, article 2419) doi: 10.3390/molecules23102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanakis C. M., Kotsiou K. Olive Mill Waste. Elsevier; 2017. Recovery of bioactive compounds from olive mill waste; pp. 205–229. [DOI] [Google Scholar]
- 6.Kiritsakis K., Melliou E., Magiatis P., Gerasopoulos D. Enhancement of bioactive phenols and quality values of olive oil by recycling olive mill waste water. Journal of the American Oil Chemists' Society. 2017;94(8):1077–1085. doi: 10.1007/s11746-017-3011-1. [DOI] [Google Scholar]
- 7.Obied H. K., Karuso P., Prenzler P. D., Robards K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. Journal of Agricultural and Food Chemistry. 2007;55(8):2848–2853. doi: 10.1021/jf063300u. [DOI] [PubMed] [Google Scholar]
- 8.Ventura G., Calvano C. D., Abbattista R., et al. Characterization of bioactive and nutraceutical compounds occurring in olive oil processing wastes. Rapid Communications in Mass Spectrometry. 2019;33(21):1670–1681. doi: 10.1002/rcm.8514. [DOI] [PubMed] [Google Scholar]
- 9.Qabaha K., AL-Rimawi F., Qasem A., Naser S. A. Oleuropein is responsible for the major anti-inflammatory effects of olive leaf extract. Journal of Medicinal Food. 2018;21(3):302–305. doi: 10.1089/jmf.2017.0070. [DOI] [PubMed] [Google Scholar]
- 10.Yoon S. K. The Liver. Elsevier; 2018. Oleuropein as an Antioxidant and Liver Protect; pp. 323–335. [DOI] [Google Scholar]
- 11.Pourkhodadad S., Alirezaei M., Moghaddasi M., et al. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. The Journal of Physiological Sciences. 2016;66(5):397–405. doi: 10.1007/s12576-016-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel T., Khlif I., Kanakis P., et al. UHPLC-DAD-FLD and UHPLC-HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of Koroneiki and Chetoui varieties. Phytochemistry Letters. 2015;11:424–439. doi: 10.1016/j.phytol.2014.12.020. [DOI] [Google Scholar]
- 13.Silva S., Gomes L., Leitão F., Bronze M., Coelho A. V., Boas L. V. Secoiridoids in olive seed: characterization of nüzhenide and 11-methyl oleosides by liquid chromatography with diode array and mass spectrometry. Grasas y Aceites. 2010;61(2):157–164. doi: 10.3989/gya.087309. [DOI] [Google Scholar]
- 14.Fu S., Arráez-Roman D., Segura-Carretero A., et al. Qualitative screening of phenolic compounds in olive leaf extracts by hyphenated liquid chromatography and preliminary evaluation of cytotoxic activity against human breast cancer cells. Analytical and Bioanalytical Chemistry. 2010;397(2):643–654. doi: 10.1007/s00216-010-3604-0. [DOI] [PubMed] [Google Scholar]
- 15.Quirantes-Piné R., Lozano-Sánchez J., Herrero M., Ibáñez E., Segura-Carretero A., Fernández-Gutiérrez A. HPLC–ESI–QTOF–MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochemical Analysis. 2013;24(3):213–223. doi: 10.1002/pca.2401. [DOI] [PubMed] [Google Scholar]
- 16.Taamalli A., Arráez-Román D., Ibañez E., Zarrouk M., Segura-Carretero A., Fernández-Gutiérrez A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. Journal of Agricultural and Food Chemistry. 2012;60(3):791–798. doi: 10.1021/jf204233u. [DOI] [PubMed] [Google Scholar]
- 17.Ortega-García F., Peragón J. HPLC analysis of oleuropein, hydroxytyrosol, and tyrosol in stems and roots of Olea europaea L. cv. Picual during ripening. Journal of the Science of Food and Agriculture. 2010;90(13):2295–2300. doi: 10.1002/jsfa.4085. [DOI] [PubMed] [Google Scholar]
- 18.Japón-Luján R., Luque de Castro M. Liquid–liquid extraction for the enrichment of edible oils with phenols from olive leaf extracts. Journal of Agricultural and Food Chemistry. 2008;56(7):2505–2511. doi: 10.1021/jf0728810. [DOI] [PubMed] [Google Scholar]
- 19.Del Ben M., Nocella C., Loffredo L., et al. Oleuropein-enriched chocolate by extra virgin olive oil blunts hyperglycaemia in diabetic patients: results from a one-time 2-hour post-prandial cross over study. Clinical Nutrition. 2020;39(7):2187–2191. doi: 10.1016/j.clnu.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Dai W., Ye S. The olive constituent oleuropein exerts nephritic protective effects on diabetic nephropathy in db/db mice. Archives of Physiology and Biochemistry. 2019;22:1–8. doi: 10.1080/13813455.2019.1691603. [DOI] [PubMed] [Google Scholar]
- 21.Ahamad J., Toufeeq I., Khan M. A., et al. Oleuropein: a natural antioxidant molecule in the treatment of metabolic syndrome. Phytotherapy Research. 2019;33(12):3112–3128. doi: 10.1002/ptr.6511. [DOI] [PubMed] [Google Scholar]
- 22.Park S., Choi Y., Um S.-J., Yoon S. K., Park T. Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. Journal of Hepatology. 2011;54(5):984–993. doi: 10.1016/j.jhep.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 23.van der Stelt I., Hoek-van den Hil E. F., Swarts H. J., et al. Nutraceutical oleuropein supplementation prevents high fat diet-induced adiposity in mice. Journal of Functional Foods. 2015;14:702–715. doi: 10.1016/j.jff.2015.02.040. [DOI] [Google Scholar]
- 24.Khalili A., Nekooeian A. A., Khosravi M. B. Oleuropein improves glucose tolerance and lipid profile in rats with simultaneous renovascular hypertension and type 2 diabetes. Journal of Asian Natural Products Research. 2017;19(10):1011–1021. doi: 10.1080/10286020.2017.1307834. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed T., Souiy Z., Achour L., Hamden K. Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globularia alypumextracts. Archives of Physiology and Biochemistry. 2020;13:1–8. doi: 10.1080/13813455.2020.1773865. [DOI] [PubMed] [Google Scholar]
- 26.Tiss M., Souiy Z., Abdeljelil N., Njima M., Achour L., Hamden K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and Liver-Kidney toxicities in HFFD- rats. Journal of Functional Foods. 2020;67, article 103869 doi: 10.1016/j.jff.2020.103869. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


