The coronavirus disease 2019 (COVID-19) pandemic has been associated with important changes in the use of anticoagulants, including (1) the recommendation of low-molecular-weight heparin (LMWH) or fondaparinux to prevent thrombotic complications associated with COVID [1–3], and the increased use of direct oral anticoagulants (DOACs) in atrial fibrillation to prevent exposure to healthcare facilities for blood monitoring required with warfarin.
We quantified the change in global sales of anticoagulant agents after the COVID-19 pandemic declaration in March 2020.
MIDAS® data for August 2014–August 2020 were obtained from IQVIA [4], which contains retail and hospital drug purchase data for 39 countries. We extracted records for anticoagulants, including injectable anticoagulants (heparins and LMWH), vitamin K antagonists, and DOACs.
For each month and country, we calculated the number of units sold per 1000 population, and we conducted interrupted time-series analyses to examine changes in the trend of sales after the pandemic declaration in March 2020. Regression models included a continuous variable for month, an indicator variable for the period after March 2020, the interaction between them, and an indicator variable for developing countries. To quantify country-level variation, we calculated the proportion change in the number of units sold in March–August 2020 versus March–August 2019. Due to the instability of estimates for smaller countries, we constrained these analyses of variation in units for March–August 2020 versus March–August 2019 to countries with a population of at least 20 million (n = 17 countries).
Before 2020, sales of anticoagulants increased by an annual average of 6%, driven by an annual average increase in sales of DOACs of 26%. Sales of vitamin K antagonists decreased by an annual average of 8%, and sales of injectable anticoagulants remained constant (Fig. 1). After the pandemic declaration in March 2020, there was a significant increase in sales of all anticoagulant products (p-value for level change = 0.006) and DOACs (p-value for level change = 0.019). Global sales of injectable products remained constant, while sales of vitamin K antagonists increased after the pandemic declaration, but the change was not significant (p = 0.133).
Fig. 1.
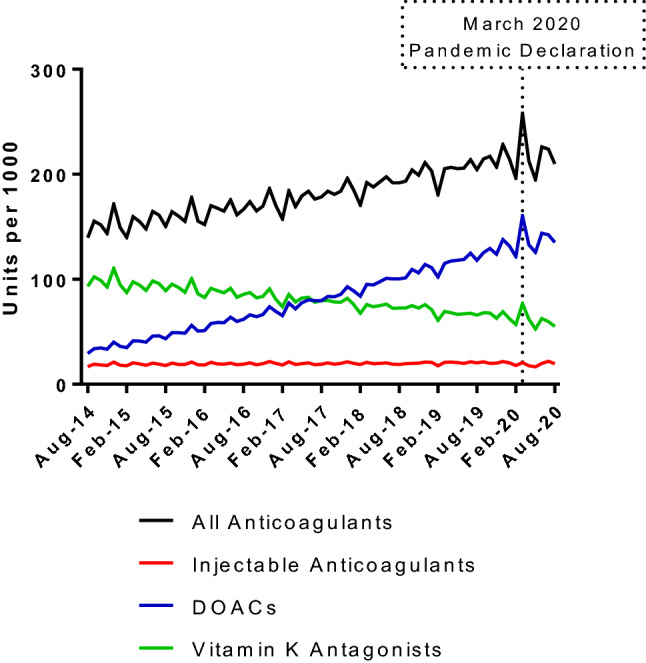
Trends in anticoagulant sales, August 2014–August 2020
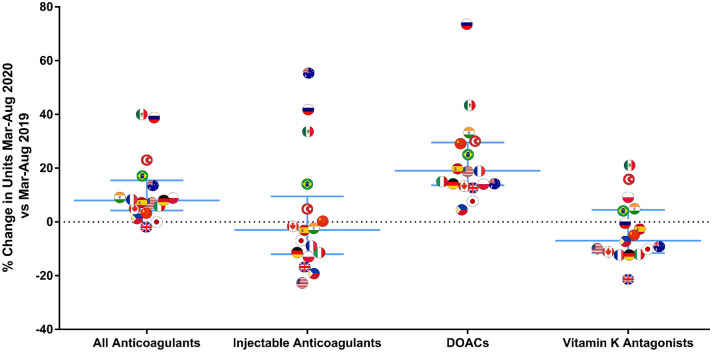
On average, countries purchased 11% more anticoagulant units in March–August 2020 than in March–August 2019 (Fig. 2). Russia and Mexico had the largest increases in sales of anticoagulant products, with increases of 39% and 40%, respectively. The UK was the only country with a decrease in sales of anticoagulant products (− 2%). Country-level variation in changes in anticoagulant sales was particularly large for injectable products. While global sales of injectable anticoagulants were lower in March–August 2020 than in March–August 2019, five countries increased their demand, most remarkably Australia (55%), Russia (42%), and Mexico (34%). Russia and Mexico also presented the greatest increases in purchases of DOACs (73% and 43%, respectively), while the US had the largest decrease in purchases of injectable anticoagulants (− 22%).
Fig. 2.
Country-level variation in change in units March–August 2020 versus March–August 2019. The figure represents the proportion change between the number of units sold per 1000 in March–August 2020 and March–August 2019 at the country level. The lines represent median and interquartile range. Analyses were performed for the subset of countries with a population of at least 20 million (n = 17), including China, India, US, Brazil, Russia, Japan, Mexico, the Philippines, Germany, Turkey, the UK, France, Italy, Spain, Poland, Australia, and Canada. Countries excluded, with a population of < 20 million included Austria, Belarus, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Finland, Hungary, Kazakhstan, Lithuania, The Netherlands, New Zealand, Norway, Portugal, Romania, Slovakia, Sweden, Switzerland and Tunisia. DOACs direct oral anticoagulants
Our paper is subject to four main limitations. First, we did not include fondaparinux in analyses because of missing data for some countries. Second, because we did not have access to drug utilization data, it is possible that changes in purchases of anticoagulants did not translate into changes in their clinical use. Additionally, it is unclear what indications anticoagulants were used for. Third, we used March 2020 as the start of the pandemic period, even though the pandemic peak did not happen simultaneously for all countries. Fourth, our analyses included countries with widely different healthcare systems, where changes in purchases of anticoagulants may not similarly translate into changes in their use.
Nevertheless, our study is an important contribution to the literature on the impact of COVID on the demand for medications. Sales of DOACs presented a significant increase in March 2020, which could reflect patients’ tendency to hoard medications in the early months of the COVID-19 pandemic for chronic indications of anticoagulants or switches from warfarin to DOACs to avoid exposing patients to healthcare facilities for blood testing. Although injectable anticoagulants have been recommended to prevent thrombotic complications in hospitalized COVID-19 patients since May 2020, global sales remained constant through August 2020. Only a few countries increased demand for these agents, most remarkably Australia, Russia, and Mexico. Interestingly, the US presented the strongest decrease in purchases of injectable anticoagulants. This could reflect the marked decrease in non-COVID-19 hospital admissions observed in the US following the pandemic outbreak [5], or the long periods with postponed elective procedures, for which injectable anticoagulants are often recommended. Additionally, country-level variation in purchases of injectable anticoagulants could be due to variability in national and institutional stockpile reserves and in the implementation of policies in response to COVID. For example, in January 2020, Russia released special rules for the import, pricing, and distribution of pharmaceutical products [6], which could partially explain the high increase in anticoagulant purchases observed. Finally, international variability in purchases of anticoagulants could represent differential speed in the uptake of new scientific evidence and guideline recommendations on COVID-19 treatments. Future research using patient-level data should examine to what extent the decrease in purchases of injectable anticoagulants observed in the US was due to the decrease in demand for inpatient care for non-COVID-19 conditions versus a delay in the use of oral anticoagulants to prevent thrombotic complications associated with COVID-19.
Funding
Inmaculada Hernandez is funded by Grant number K01HL142847.
Declarations
Conflicts of interest
Inmaculada Hernandez has received consulting fees from Pfizer and Bristol Myers Squibb, outside of the submitted work, and has served on an advisory board for Bristol Myers Squibb. Mina Tadrous, Jared W. Magnani, Jingchuan Guo, and Katie J. Suda have no conflicts of interest to declare.
Human and animal rights
The presented work constitutes no human subject research, as no human data were used.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data were obtained through a data user agreement that does not allow data sharing.
Code availability
Available upon request to corresponding author.
Role of funder/Sponsor statement
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, the US government, or IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this publication are based in part on data obtained under license from IQVIA as part of the IQVIA Institute’s Human Data Science Research Collaborative.
References
- 1.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: chest guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Throm Haesmost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. 2020;324(24):2548–2549. doi: 10.1001/jama.2020.23422. [DOI] [PubMed] [Google Scholar]
- 4.IQVIA. 2019 ACTS Annual Report. Statistical Quality Assurance applied to IQVIA’s Information Offerings. https://www.iqvia.com/-/media/iqvia/pdfs/library/publications/2019-acts-annual-report.pdf?_=1607795623443. Accessed 17 Feb 2021.
- 5.Heist T, Schwartz K, Butler S. Trends in Overall and Non-COVID-19 Hospital Admissions. 2020. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions/. Accessed 25 Jan 2021.
- 6.Pestryakova A. Special rules for pharma sector in Russia during the covid-19 emergency 2020. https://www.lexology.com/library/detail.aspx?g=4e8693b0-aef0-4dad-b8e0-d7e5b75c6330. Accessed 9 Jan 2021.