Abstract
Background and Aim:
Previous studies recorded the prevalence of gastrointestinal nematodes (GIN) in Limpopo Province. However, the studies did not address the seasonal patterns of infection and did not cover all districts of Limpopo Province, namely; Capricorn, Sekhukhune, Waterberg, Mopani, and Vhembe. It is, therefore, important to provide up to date information on the prevalence and seasonal occurrence data of GIN in all districts of Limpopo province. The present study was conducted to determine the occurrence of anthelmintic resistance (AR) and document the prevalence of GIN infecting sheep in five districts of Limpopo Province, South Africa.
Materials and Methods:
Forty animals in each district were used for fecal egg count reduction test (FECRT) to determine AR against ivermectin (0.2 mg/kg), levamisole (LEV) (5 mg/kg), and albendazole (7.5 mg/kg). Egg hatch test (EHT) was used to determine AR against thiabendazole (TBZ) and micro-agar larval development test (MALDT) was used for both TBZ and LEV. Naturally, infected sheep (n=780) were sampled for prevalence across five districts of Limpopo. FAMACHA© eye-color score estimations were also performed for each study animal.
Results:
FECRT showed occurrence of AR in most of the districts and a few with suspected resistance. EHT results showed AR development against TBZ for all districts, while the MALDT showed no AR against LEV in all districts, but detected AR against TBZ in Sekhukhune, Capricorn, and Waterberg. Haemonchus contortus was the most resistant species. A high nematode prevalence (88-100%) and 1210-1861 eggs per gram (EPG) was observed in all districts during the hot wet season, decreasing to 75-80% (453-1202 EPG) during the cold dry season. The sheep revealed a FAMACHA© mean score of 3, indicating mild anemia during the hot wet season except for Vhembe district that revealed a FAMACHA© mean score of 4 during the hot wet season, indicating anemia.
Conclusion:
AR recorded in Limpopo Province may be due to under-dosing caused by lack of weighing equipment and high treatment frequencies due to lack of proper training on anthelmintic use. The detection of AR in Limpopo is an important finding because it will help in outlining effective management systems against GIN.
Keywords: anthelmintic resistance, fecal egg counts, FAMACHA, seasonal prevalence, sheep
Introduction
Gastrointestinal nematodes (GIN) are responsible for substantial losses in the animal production industry [1]. Anthelmintics have been used as the primary control measure for nematode parasites in sheep [2]. However, over the years, there has been continuous and significant development of anthelmintic resistance (AR) by the parasitic worms infecting livestock [3]. AR can be defined as the ability of parasites to survive doses of drugs that would normally kill parasites of the same species and stage. AR is inherited and selected for because the survivors of treatments pass genes for resistance onto their offspring [4]. AR has been reported for all anthelmintic classes currently available, namely, benzimidazoles (BZ) (e.g., flubendazole, albendazole, and fenbendazole), imidazothiazoles (e.g., levamisole [LEV]), and macrocyclic lactones (ML) (e.g., ivermectin) [5]. In South Africa, AR has been reported in sheep [6,7] and goats [7-9] in both the commercial and resource-poor farming systems. The overall prevalence of AR in South Africa and elsewhere in Africa has, however, not been extensively investigated.
Research conducted in many parts of the world, particularly in countries such as the UK, Australia, South Africa, and New Zealand, has resulted in a growing awareness of the challenges brought about by AR, but also recognition of potential resistance-delaying strategies, which can be used on farms [10]. The three main factors for the selection for AR are high treatment frequency [11], under-dosing and management such as poor pasture management can accelerate the development of AR [12]. Anthelmintic drugs currently used in South Africa include BZ (Valbazen®, Zoetis United States), ML (Ivomec®, Merial, United States), LEV (Tramisol Ultra®, Coopers and Intervet), and praziquantel + LEV + oxfendazole + abamectin combination (Triple A plus, Virbac, New Zealand). The pathogenic implications of GIN infections on host welfare are clear [13]. It is, therefore, crucial to monitor the prevalence and distribution of livestock helminth species to plan sustainable control, especially because prevalence studies may become rapidly outdated and there is no structured mechanism in place to monitor livestock helminth prevalence over time [14,15]. Moreover, environmental conditions, particularly temperature and humidity, affect the distribution of species and the presence of parasites. Seasonal variations also play an important role in prevalence of GIN. As such, changes in humidity and temperature can influence development, survival, and transmission of parasites in the external environment because they provide favorable environmental conditions for transmission of GIN [1].
The aim of this study was to determine the occurrence of AR and document the prevalence of GIN of sheep in selected flocks in all districts Limpopo Province, South Africa.
Materials and Methods
Ethical approval
The study was approved by the scientific committee of Integrated Pest Management, North-West University, with reference no: NWU-01252-19-A9 and Department of Agriculture, Forestry and Fisheries (DAFF) in terms of section 20 of the Animal Diseases Act (35 of 1984). Participating resource-poor farmers granted permission to collect study samples from their sheep.
Study area, period and animal management
The study was conducted on small-scale farming locations in five districts of Limpopo Province, namely, Capricorn, Sekhukhune, Waterberg, Mopani, and Vhembe (Figure-1). The sheep in these locations were managed under an extensive system where communal grazing was shared among the resource-poor farmers. Meteorological data for the collection sites starting from January 2017 to November 2018 were obtained from South African Weather Service. The data included weather elements such as temperature, rainfall, and humidity.
Figure-1.
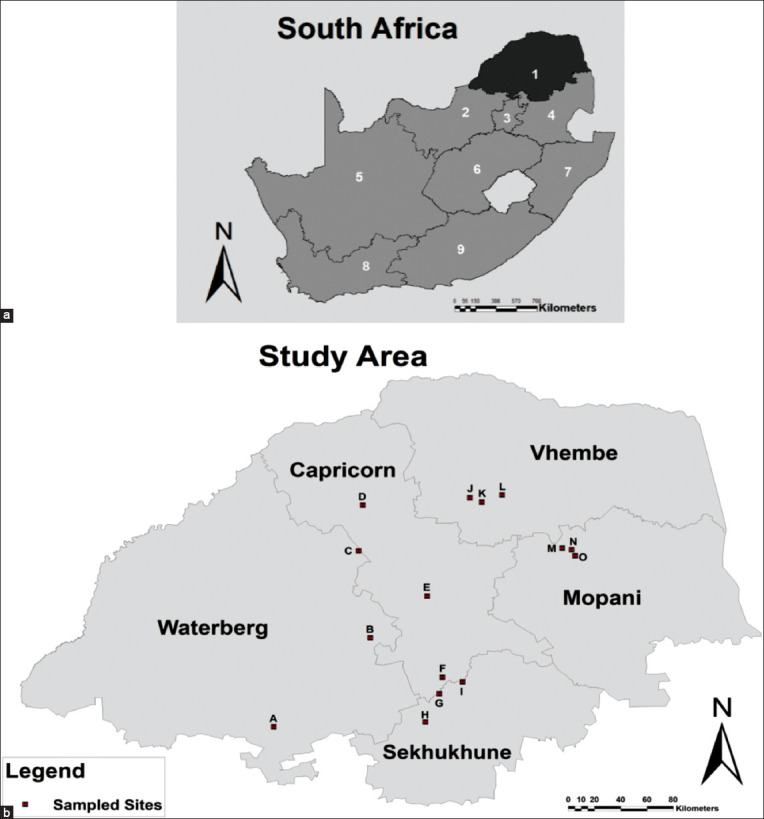
(a) Map showing nine provinces of South Africa with Limpopo Province shown in a dark grey color. (b) Locality map depicting study sites from Limpopo districts for seasonal prevalence of gastrointestinal nematodes: (A) Bela-Bela (B) Mokopane and (C) Ga-Ramela village from Waterberg district; (D) Ga-Kobe village, (E) Makotse village, and (F) Tooseng village from Capricorn district; (G) Malope village, (H) Tompi Seleka College, and (I) Strydkraal village from Sekhukhune district; (J) Madodonga village, (K) Ha-Ramantsha village and (L) Louis Trichardt from Vhembe district; (M) Ga-Maupa village, (N) Mamokgadi village and (O) Mamatlepa village from Mopani district. Villages circled in red show where anthelmintic resistance studies were conducted.
For the selection of animals to be included in the AR part of the study, the resource-poor farmers provided information regarding anthelmintic classes they use in their flocks, how often they treat their livestock, how often they change the active ingredients, and whether they weigh their animals before treatment with anthelmintics. Experimental animals for fecal egg count reduction test (FECRT) were selected from the flocks that had a long history of anthelmintic treatment and high treatment frequencies. Five flocks with the highest treatment frequencies were selected from the 77 flocks that were registered in the extension officer’s database. In each district, 40 animals belonging to one farm were divided into groups of ten animals per group. Furthermore, only animals that had not been treated for GIN for at least 8 weeks before sampling were selected. Sheep were reared under extensive grazing systems whereby they were released during the day to graze on communal lands and kept in holding pens at night.
To determine prevalence of GIN, 468 fecal samples were collected on a bi-monthly basis between March 2017 and January 2018 for Capricorn, Sekhukhune, and Waterberg districts. A further 312 samples were collected between January and November 2018 for Mopani and Vhembe districts. All in all, a total of 780 naturally infected sheep comprising of Pedi sheep and Pedi × Dorper cross breeds were sampled. The average weight of the study animals (1-2 years old) was 29.5 kg and 35.2 kg for females and males, respectively. The animals were ear tagged so that samples could be taken from the same animals throughout the duration of the study period. FAMACHA© eye-color score estimations [16,17] were also performed for each study animal.
Fecal collection and coprological examination
Fecal samples were collected directly from the rectum of sheep into clean, labeled plastic bags and stored in a cooler box at 2-4°C. They were transported immediately to the Helminthology Laboratory at the Agricultural Research Council, Onderstepoort Veterinary Institute for coprological examination. The fecal samples were examined for the presence of GIN eggs; nematode egg counts were made using the McMaster technique with minimum detection limit of 100 EPG [18]. Briefly, 2 g of feces was mixed thoroughly with 58 mL of 40% sugar solution ensuring that the fecal pellets were disrupted. A Pasteur pipette was used to transfer aliquots of the mixture into the two chambers of a McMaster slide. The slide was left to stand for 5-10 min to allow the eggs to float to the surface of the medium. The slide was examined using a dissecting microscope (Nikon Eclipse E100 Company, Japan) at 10× and/or 40×. Nematode eggs were counted in each chamber of the McMaster slide and the EPG was calculated using the following formula
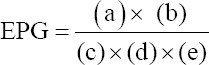
where (a) = (total number of eggs counted);
(b) = 60 (total volume of fecal suspension);
(c) = 2 (the number of chambers counted);
(d) = 2 (grams of feces);
(e) = 0.15 mL (standard volume of the chamber).
In vivo assays
FECRT
The FECRT as described by Coles et al. [19] was used to determine the presence of AR. The minimum egg count for inclusion in the pre-treatment group, excluding the control group, was 1,000 EPG. Group 1 was treated subcutaneously with ivermectin (Ivomec®, Merial, United States, 0.2 mg/kg bw), Group 2 was orally dosed with LEV (Tramisol Ultra®, Coopers and Intervet, New Zealand, 5 mg/kg bw), and the Group 3 was orally dosed with albendazole (Valbazen®, Pfizer, United States, 7.5 mg/kg bw). Group 4 represented the untreated control. Most of the nematode eggs are morphologically indistinguishable; hence, cultures were prepared from feces collected both before and after treatment to identify nematode genera as described by Van Wyk and Mayhew [20].
In vitro assays
Egg hatch test
The egg hatch test and Micro-argar larval development test (MALDT) were performed as described by Coles et al. [19] for the in vitro determination of BZ and LEV resistance, respectively. For both assays, nematode eggs were recovered from the same fecal samples that were collected before treating the animals in the FECRT using the nematode egg recovery method as described by Maphosa et al. [21] with some minor modifications.
For egg hatch test (EHT), ca. 100 nematode eggs were pipetted into a 96-well microtiter plate and then 10 μL of thiabendezole (TBZ) solution added [22]. The final concentrations of TBZ were 0.05, 0.1, 0.2, 0.3, and 0.5 μg/mL dissolved in dimethyl sulfoxide (DMSO). In addition, a negative control (distilled water) was tested. All tests were duplicated. The plates were covered and incubated under humidified conditions for 48 h at 27°C. A drop of Lugol’s iodine solution was added to each well to stop further hatching and the number of unhatched eggs and the first stage larvae (L1) present per well were counted. Inhibition percentages were calculated using a formula described by Cala et al. [23].
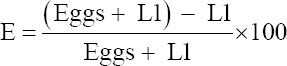
The discriminating dose (DD) for the EHT is a dose that prevents the hatching of 99% of susceptible eggs; all the eggs that hatch are resistant [19]. The level of resistance was determined by the number of eggs hatching in the DDs of 0.1 μg/mL TBZ. The percentage of hatched eggs is thus a direct measure of BZ resistance.
MALDT
For the MALDT, stock solutions of TBZ/LEV were prepared by pre-dissolving the drugs in DMSO with subsequent dilution in distilled water (1:4). One hundred nematode eggs were incubated for 7 days at 27°C in 96-well microtiter plates with culture medium (yeast extract with Earle’s Balanced Salt Solution and physiologic salt solution) in an aquatic solution of various concentrations (range from 0.0006 to 1.28 μg/mL) of thiabendazole (TBZ)/LEV [19]. After 7 days, the numbers of unhatched eggs and L1 – L3 larvae in each well were counted under an inverted microscope. The rate of L3 development in the DD (0.02 μg/mL and 0.5 μg/mL for TBZ and LEV, respectively) compared to the control was used to determine if resistance was present. The number of larvae developing from L1 to L3 stage in the DD of 0.02 μg/mL TBZ and 0.5 μg/mL LEV was an indication of resistance. The test was performed in two replicates for each drug concentration.
Prevalence was determined using the following formula [24]:
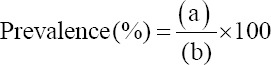
Where, “a” = Number of individuals having a disease at a particular time; “b” = Number of individuals in the population at risk at that point in time. Fecal cultures were prepared to identify nematode genera [20] and determine distribution of nematode genera in Limpopo Province. The L3 of Trichostrongylus spp. of small ruminants are difficult to differentiate from those of Teladorsagia spp. because they are similar in length and can only be differentiated generically by tail morphology after exsheathment. Consequently, they were grouped during the count.
Statistical analysis
The FECRT (FECRT %) was calculated using the formula of Kochapakdee et al. [25]. FECRT % = 100 × (1−(T2/T1), where T2 represents FEC post-treatment and T1 represent FEC pre-treatment [25]). SAS Statistics (Version 9.4) was used to analyze FECRT data for confidence limits and the Pearson’s correlation coefficient (Pearson’s r) was used to measure the linear correlation between variables.
Resistance was said to be present if the FECRT % was less than 95% and the lower limit of the 95% confidence interval was less than 90%. If only one condition was met, then resistance was only suspected [8,19,26].
Instead of using the traditional threshold values (LC 50 or LC 99), the threshold discriminating concentrations were used for both EHT and MALDT because it is faster, simpler, and cheaper [27]. Furthermore, the LC 50 criterion is not able to provide early detection during the development of resistance [28].
Results
Using the analysis described by Kochapakdee et al. [25], the results revealed development of AR against all the anthelmintic classes with percentages of ≤95% and ≤90% lower confidence limit (LCL) in all the districts of Limpopo Province except for Sekhukhune, where AR was suspected against all the three anthelmintic classes (Table-1). Furthermore, AR was also suspected against ML in Capricorn and LEV in Vhembe districts flocks at 92% FECRT with LCL of 90% and 90% FECRT with a 78% LCL, respectively. However, there was no significant difference (p>0.05) in the FECRT results between all the three anthelmintic classes tested.
Table-1.
Fecal egg count reduction test and lower limits of 95% confidence level calculated on the basis of individual animal’s egg counts before and after treatment on the same sheep using method of Kochapakdee et al. [27]. FECRT%=100 × (1−(T2/T1).
| Study site | Anthelmintic used | FEC1 (Range) | FEC2 (Range) | FECRT% | Lower limit of 95% confidence | Interpretation of results |
|---|---|---|---|---|---|---|
| Sekh | BZ | 1940 (1455-2425) | 80 (60-100) | 96 | 93 | Suspected resistance |
| LEV | 2080 (1560-2600) | 150 (112-187) | 94 | 90 | Suspected resistance | |
| ML | 2030 (1522-2537) | 120 (90-150) | 96 | 90 | Suspected resistance | |
| Control | 2080 (1560-2200) | 2060 (1840-2560) | - | - | - | |
| Capr | BZ | 1790 (895-3580) | 200 (100-400) | 92 | 85 | Resistant |
| LEV | 1660 (830-3320) | 130 (65-260) | 93 | 88 | Resistant | |
| ML | 1840 (920-3680) | 120 (60-240) | 92 | 90 | Suspected resistance | |
| Control | 1750 (1430-2080) | 1940 (1850-2300) | - | - | - | |
| W/berg | BZ | 2840 (2200-3500) | 1300 (400-2300) | 56 | 40 | Resistant |
| LEV | 2960 (1300-5000) | 580 (200-900) | 79 | 72 | Resistant | |
| ML | 4440 (2200-7200) | 1340 (0-2000) | 65 | 50 | Resistant | |
| Control | 1570 (1410-1600) | 1580 (1380-1670) | - | - | - | |
| Mopani | BZ | 1960 (500-5000) | 620 (300-880) | 47 | 26 | Resistant |
| LEV | 1620 (400-2300) | 480 (100-900) | 70 | 61 | Resistant | |
| ML | 1280 (500-2400) | 320 (100-900) | 72 | 51 | Resistant | |
| Control | 1180 (850-1250) | 1180 (900-1250) | - | - | - | |
| Vhembe | BZ | 2660 (500-4400) | 340 (0-1000) | 89 | 82 | Resistant |
| LEV | 2525 (400-7800) | 50 (0-500) | 90 | 78 | Suspected resistance | |
| ML | 1060 (500-2000) | 160 (0-300) | 90 | 80 | Resistant | |
| Control | 1290 (1100-1350) | 1280 (1080-1580) | - | - | - |
Sekh=Sekhukhune, Capr=Capricorn, W/berg=Waterberg, BZ=Benzimidazole (Valbazen®), LEV=Levamisole (Tramisol Ultra®), ML=Macrocyclic lactones (Ivomec®), FEC1=Fecal egg count pre-treatment, FEC2=Fecal egg count 14 days post-treatment
The EHT results showed that there was resistance against TBZ, which is a BZ, because it had a minimal ovicidal effect that resulted in more than 1% egg hatchability on nematode eggs at a DD of 0.1 μg/mL in all 5 (100%) flocks that were investigated in Limpopo. Figure-2 shows the hatchability percentages at the DD of 0.1 μg/mL TBZ. In the negative controls (water), the percentage of eggs hatching was >95% for all flocks.
Figure-2.
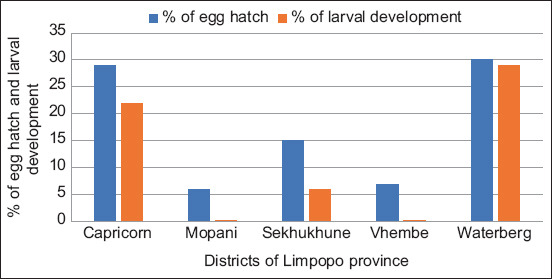
The percentage of eggs that hatched at a discriminating dose (DD) of 0.1 µg/mL thiabendazole and eggs that developed to third stage (infective) larvae in the DD of thiabendezole (0.02 μg/mL) in the macro-agar larval development test.
The MALDT showed no AR against LEV as 100% larval development inhibition was recorded in all the investigated flocks at 0.5 μg/mL DD. However, AR was detected against TBZ as development of the larvae from L1 to L3 was recorded in Sekhukhune, Capricorn, and Waterberg flocks at 6, 22, and 28%, respectively, at 0.02 μg/mL DD (Figure-2).
Table-2 shows that the fecal examination test for the presence of nematode eggs in the pre-treatment fecal samples revealed that all sheep (100%) were positive for strongyle eggs. Nematode genera/species that were identified pre-treatment from the larval cultures included Haemonchus contortus, Teladorsagia/Trichostrongylus, and Oesophagostomum columbianum. The H. contortus was identified as the most frequently encountered resistant nematode post-treatment in all five flocks. On the other hand, among all the nematode genera identified pre-treatment, O. columbianum was the only species that was not detected post-treatment in all the anthelmintic treatments (Table-2).
Table-2.
Results of the percentage of gastrointestinal nematode genera/species identified from the larval cultures at day 0 pre-treatment and day 14 post-treatment.
| Anthelmintic group | Haemonchus contortus | Tel/Trich spp. | Oesophagostomum columbianum | |||
|---|---|---|---|---|---|---|
| Sekhukhune | D 0 | D 14 | D 0 | D 14 | D 0 | D 14 |
| BZ | 74 | 90 | 23 | 10 | 3 | 0 |
| LEV | 75 | 75 | 20 | 25 | 5 | 0 |
| ML | 87 | 96 | 10 | 4 | 3 | 0 |
| Control | 83 | 84 | 15 | 16 | 2 | 0 |
| Capricorn | ||||||
| BZ | 93 | 97 | 4 | 3 | 3 | 0 |
| LEV | 87 | 93 | 10 | 7 | 3 | 0 |
| ML | 88 | 93 | 10 | 7 | 2 | 0 |
| Control | 82 | 86 | 15 | 14 | 3 | 0 |
| Waterberg | ||||||
| BZ | 89 | 100 | 9 | 0 | 2 | 0 |
| LEV | 83 | 94 | 11 | 0 | 6 | 0 |
| ML | 85 | 100 | 10 | 0 | 5 | 0 |
| Control | 91 | 92 | 7 | 8 | 2 | 0 |
| Mopani | ||||||
| BZ | 88 | 100 | 12 | 0 | 0 | 0 |
| LEV | 87 | 100 | 13 | 0 | 0 | 0 |
| ML | 95 | 100 | 5 | 0 | 0 | 0 |
| Control | 95 | 92 | 4 | 5 | 1 | 1 |
| Vhembe | ||||||
| BZ | 90 | 100 | 8 | 0 | 2 | 0 |
| LEV | 90 | 100 | 5 | 0 | 5 | 0 |
| ML | 92 | 100 | 7 | 0 | 1 | 0 |
| Control | 93 | 92 | 6 | 4 | 1 | 2 |
Tel=Teladorsagia, Trich=Trichostrongylus, BZ=Benzimidazole, LEV=Levamisole, ML=Macrocytic lactones, D0=Treatment day 0, D14=Treatment day 14
Distribution of GIN in Limpopo Province was continuous throughout the year with the highest EPGs recorded during the hot wet season between November and January, with the highest peak observed in Vhembe district in November 2018 (EPG 1887) and in Waterberg district in November 2017 (EPG 1811). On the contrary, the lowest EPGs during the cold dry season were recorded between July and September, with the lowest EPG counts recorded for Capricorn district (EPG 299) in September 2017 (Figure-3).
Figure-3.
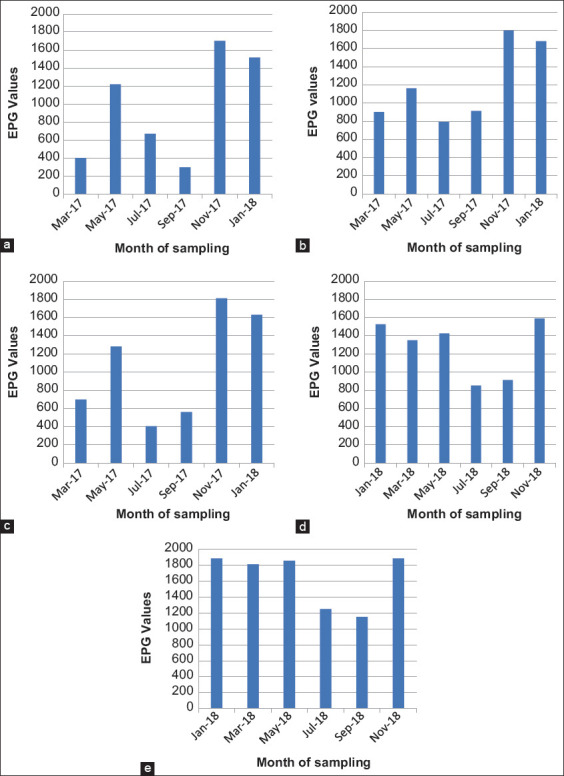
Seasonal incidence of gastrointestinal nematodes of sheep in Limpopo (a) Capricorn district, (b) Sekhukhune district, (c), Waterberg district, (d) Mopani district, and (d) Vhembe district.
Figure-4 details a high nematode prevalence ranging from 64% to 100% during the hot wet season and a decrease to a range of 75-80% prevalence during the cold dry season. However, there was no significant difference (p>0.05) in the mean EPGs between the different seasons and so was for the nematode prevalence in different seasons.
Figure-4.
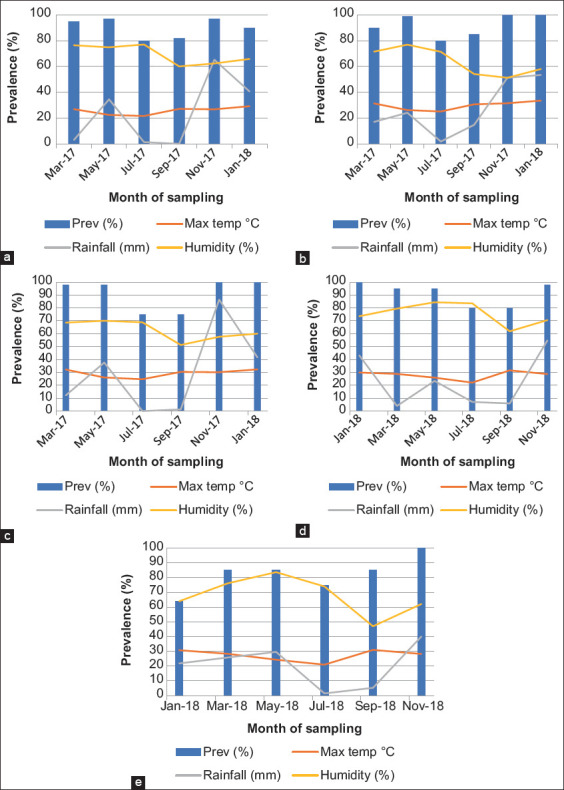
The relationship between rainfall, humidity, and maximum temperature on the prevalence of gastrointestinal nematodes per month/season in Limpopo (a) Capricorn district, (b) Sekhukhune district, (c), Waterberg district, (d) Mopani district, and (e) Vhembe district.
Furthermore, a high nematode prevalence correlated with the high rainfall experienced during November in all the districts, being particularly high in Waterberg and Capricorn districts with 68.4 mm and 65.2 mm, respectively (Figure-4). Figure-5 shows a positive correlation between mean minimum temperature and mean FEC (r=0.482; p=0.007) and between mean rainfall and mean FEC (r=0.755; p=≤0. 0001) in all the districts of Limpopo.
Figure-5.
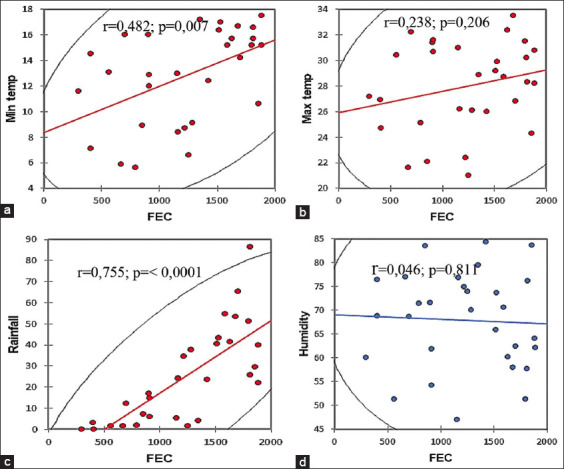
Correlation between minimum temperature and fecal egg counts (a), maximum temperature and fecal egg counts (b), rainfall and fecal egg counts (c), and humidity and fecal egg counts (d) in the five districts of Limpopo Province.
However, an overall poor correlation existed between mean humidity and mean FEC (r=0.046; p=0.811) in all the districts. In addition, the sheep examined in Vhembe district during the hot wet season revealed a FAMACHA© mean score of 4, indicating anemia. In all other districts, the sheep revealed a mean score of 3, indicating mild anemia, during the hot wet season (Table-3).
Table-3.
Mean maximum temperature, rainfall, FEC, and FAMACHA© scores during hot wet and cold dry seasons.
| District | Maximum temp | Rainfall (mm) | FEC | FAMACHA© Score | ||||
|---|---|---|---|---|---|---|---|---|
| Hot wet | Cold dry | Hot wet | Cold dry | Hot wet | Cold dry | Hot wet | Cold dry | |
| Capricorn | 26.3a | 24.4a | 35.8a | 0.7a | 1210a | 485a | 3a | 2a |
| Sekhukhune | 27.9a | 30.6a | 36.4a | 8.3a | 1386a | 853a | 3a | 2a |
| Waterberg | 30.0a | 27.5a | 44.4a | 0.7a | 1356a | 483a | 3a | 1a |
| Mopani | 28.3a | 26.8a | 31.3a | 6.6a | 1473a | 882b | 3a | 2b |
| Vhembe | 28.0a | 26.0a | 29.3a | 3.3b | 1861a | 1202b | 4a | 2b |
Means with the same letter (a-b) are not significantly different (p=0.05). FEC=Fecal egg count
In general, there was a strong correlation (r=0.959; p=≤0. 0001) between FEC and clinical anemia in sheep. On the other hand, the sheep examined in all the districts during the cold dry season were non-anemic, as indicated by the FAMACHA© score of 1 for sheep in the Waterberg and a score of 2 for the animals in all the other districts. Higher EPGs and FAMACHA© scores were observed during the hot wet season, while the opposite was seen during the cold dry season, which indicated that worm burden was responsible for anemia in sheep (Table-3).
The results of the composite fecal cultures study revealed the highest prevalence of the very prolific worm H. contortus. Figure-6 shows the mean percentage of GIN genera in the five districts of Limpopo Province during hot wet and cold dry season. The H. contortus was the dominant nematode species, representing 70-93% of the total larval recovery throughout the entire study period in all the districts of Limpopo Province, with the highest percentage of 93% recorded for Mopani in November 2018.
Figure-6.
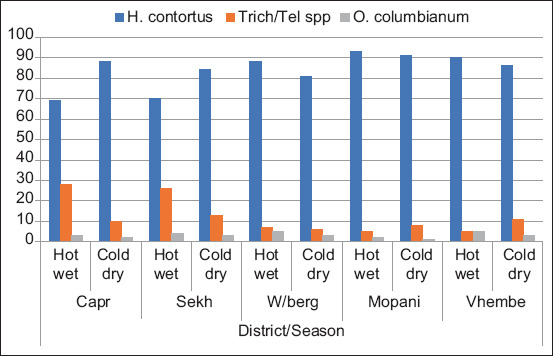
Mean percentage of gastrointestinal nematode genera in five districts of Limpopo Province during different seasons of the year.
Trichostrongylus/Teladorsagia spp. were the next most prevalent parasites, which represented 5-28% of the total infective larvae harvested from the composite fecal cultures during the study period. The O. columbianum was detected in low percentages, never exceeding 5% throughout the study period.
Discussion
Based on the results of the FECRT, resistance to BZ was detected in all the flocks except for those in the Sekhukhune district. Resistance was also confirmed by the hatching of eggs in the in vitro egg hatch test (EHT), which is regarded as highly sensitive [29]. An overwhelming majority of farmers, ranging from 45% in Mopani to 95% in Sekhukhune districts, used BZ to treat GIN of sheep in their flocks and many never changed the anthelmintic class. Similar results were obtained in the southern part of Ethiopia where albendazole was the most commonly used anthelmintic drug used by resource-poor farmers [30].
FECRT% of 56 and an LCL of 40% against BZ in a flock from Waterberg were obtained, which correlates with the highest number of hatching eggs (30%) and the highest number of larvae that developed (29%) as compared to all the other four flocks. The occurrence of resistance to BZ in Waterberg district was not unexpected since nine out of 11 farmers (82%) in that district never change the anthelmintic class, which in this case is BZ which was previously used in 55% of all the sampled flocks. The same could not be said for ML; however, because the FECRT results showed that there is resistance against this anthelmintic class even though it was the least used in all the districts of Limpopo Province, with no farmer in Sekhukhune and Waterberg ever mentioning its use in their flocks. This could be attributed to the fact that genes conferring AR are thought to be present in a small portion of individuals in the population even before the worms are exposed to a drug for the first time [12].
Earlier studies revealed that correlation between in vivo and in vitro tests is not always good [27]. Maharshi et al. [31] observed a poor correlation between FECRT results and EHT results for detecting BZ resistance. This is probably because in vitro tests are more sensitive than in vivo tests [28]. Even though some conflicting results were observed between the in vitro and in vivo assays in the present study, there are also outstanding cases where the results of both tests totally agree on flock resistance status. One such positive correlation is the non-development of larvae from L1 to L3 in the MALDT for detecting LEV resistance and high efficacy obtained from FECRT in Vhembe district flock. Field testing results indicate that H. contortus has remained generally susceptible to LEV for a longer period than to the other major drugs. LEV is used rarely as compared to BZ in Capricorn and Vhembe district flocks as it is to the rest of South Africa and many European countries [32]. It is against this backdrop that with proper use that includes administering the correct dosage for weight of the animal, pasture rotation, and other management tools, anthelmintics can still be used with maximum benefits [33].
The FECRT results obtained in the present study for the three tested anthelmintic classes coupled with EHT and MALDT results indicate occurrence of resistance in all the flocks except for Sekhukhune district flock where AR was suspected against all the anthelmintic classes. Another exception was for Vhembe district flock where AR was only suspected against LEV and ML in Capricorn. These results compare favorably with those of work done in Limpopo Province almost two decades previously, revealed LEV was <95% effective and BZ, only between 75 and 85% effective [6].
The predominance of Haemonchus spp. both pre- and post-treatment in the sheep found in the present study is consistent with the results obtained from studies conducted in Mpumalanga and Limpopo Province [6], North West Province [9], and Gauteng [7] where the most prevalent species was H. contortus. The reduction of Trichostrongylus/Teladorsagia spp. and O. columbianum and increase of H. contortus post-treatment could be attributed to high levels of pasture infectivity as a result of shorter generation interval of H. contortus which allows for greater contamination of pastures and re-infection of animals [34].
The seasonal dynamics of FEC in the present study followed the well-known pattern influenced primarily by temperature and moisture as they were highest in the hot-wet season and lowest in the cold-dry season [35].
The high prevalence of GIN seen in the current study could be due to the availability of suitable climatic conditions such as relatively high temperatures and rainfall that support the prolonged survival and development of an infective larval stage of most nematodes [36]. In the present study, H. contortus was the most prevalent nematode, followed by Trichostrongylus/Teladorsagia spp and O. columbianum. These results are comparable to those of earlier studies conducted in South Africa on the epidemiology of GIN that also reported Haemonchus spp. as the most dominant nematodes infecting sheep under commercial farming system [37] and under small-scale resource-poor farming conditions in South Africa [7,38]. These findings also corroborate well with those of studies conducted in Ethiopia and Brazil which showed H. contortus as the most prevalent nematode in small ruminants [39,40].
The observed results, of highest EPGs with the largest percentages of H. contortus L3 in fecal cultures during the rainy seasons, were in accordance with studies in other countries in Africa with distinct rainy and dry seasons, that is, Ghana [41], Kenya [42], and Zimbabwe [43]. This can be attributed to the high biotic potential of H. contortus, resulting in this parasite rapidly taking up dominance at times when environmental conditions on pasture are ideal for the development and survival of the free-living stages. The high biotic potential and shorter generation interval of Haemonchus spp. allow for greater contamination of pastures and re-infection of animals [34], which probably accounts for its higher prevalence than Trichostrongylus/Teladorsagia spp. and O. columbianum encountered in this study. In addition to the high biotic potential and shorter generation interval, a mature female of Haemonchus spp. can produce 5000-7000 eggs per day while Trichostrongylus spp. produce only 100-200 eggs per day; this shows a difference in their prolific reproduction abilities [44]. On the other hand, Oesophagostomum sp. has a very long generation interval of 45 days that cause its prevalence to be much lower [45].
Anemia observed in this study can be attributed to the GIN, predominated by H. contortus adult worms that cause harm as they cut to the mucosal lining in the abomasum, enabling them to suck blood from the mucosa of the GIT, causing blood loss, and development of anemia in sheep [46]. These observations agree with the results of previous studies where a relationship between EPG level and FAMACHA© categories was elucidated and FAMACHA© scoring was positively correlated with FEC levels in sheep [47,48]. However, the present study was in contrast with the study conducted by Mohammed et al. [49] that showed no correlation between EPG and FAMACHA© score meaning that in their study, an increase in anemia did not necessarily result in an increased EPG which could have been due to mild infection in their study animals.
Conclusion
The high prevalence of GIN in Limpopo Province could be due to conducive climatic conditions that support prolonged survival of infective larvae such as high temperatures and rainfall that prevailed in the province during the study period. High prevalence observed in this study translates to high numbers of animals with anemia and high nematode egg counts. It is for this reason that the FAMACHA© system is recommended to accurately identify animals requiring anthelmintic treatment and consequently slow down the development of AR. The correlation of FAMACHA scores and FEC makes it a necessity to train resource-poor sheep farmers in Limpopo on the use of the FAMACHA system, to determine the correct timing of deworming. The use of the FECRT, EHT, and MALDT provided an identification of the occurrence of resistance against anthelmintics in Limpopo Province. Under–dosing, which could be due to lack of weighing equipment, and high treatment frequencies due to lack of proper training on anthelmintic use may have contributed in the findings of this study. The use of a heart girth measurement tape similar to those used in cattle and pigs is, therefore, recommended as this would certainly provide resource-poor farmers with a practical tool to use in determining the live weight of their small stock.
Authors’ Contributions
MM, AMT, RM, and OMMT planned the study. MM conducted the experiments and drafted the manuscript. DMK generated the GIS maps. AMT, RM, DMK, and OMMT reviewed the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
The authors are grateful to the sheep farmers in the five District Municipalities of Limpopo Province, extension officers, and animal health technicians in the Limpopo Department of Agriculture for their cooperation. We thank the animal production interns at Tompi Seleka College of Agriculture for their assistance during data collection.
The first author is supported by a grant holder bursary of the Collaborative Postgraduate Training Grant of National Research Foundation (NRF) of South Africa (GUN: 105271) made available to OMMT. This study was made possible by the funding from Afrivet Chair on Primary Animal Health Care (University of Pretoria) research grant made available to RM and the NRF incentive grant for rated researchers (GUN94187) made available to OMMT.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Squire S.A, Robertson I.D, Yang R, Ayi I, Ryana U. Prevalence and risk factors associated with gastrointestinal parasites in ruminant livestock in the Coastal Savannah zone of Ghana. Acta Trop. 2019;199:105126. doi: 10.1016/j.actatropica.2019.105126. [DOI] [PubMed] [Google Scholar]
- 2.Claerebout E, de Wilde N, van Mael E, Casaert S, Velde F.V, Roeber F, Veloz P.V, Levecke B, Geldhof P. Anthelmintic resistance and common worm control practices in sheep farms in Flanders, Belgium. Vet. Parasitol. Reg. Stud. Reports. 2020;20:100393. doi: 10.1016/j.vprsr.2020.100393. [DOI] [PubMed] [Google Scholar]
- 3.Burke J.M, Miller J.E. Sustainable approaches to parasite control in ruminant livestock. Vet. Clin. North Am. Food Anim. Pract. 2020;36(1):89–107. doi: 10.1016/j.cvfa.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Mphahlele M, Tsotetsi-Khambule A.M, Moerane R, Mashiloane M.L, Thekisoe O.M.M. Risk factors associated with occurrence of anthelmintic resistance in sheep of resource poor farmers of Limpopo province, South Africa. Trop. Anim. Health Prod. 2019;51(3):555–563. doi: 10.1007/s11250-018-1724-2. [DOI] [PubMed] [Google Scholar]
- 5.Herrera-Manzanilla F.A, Ojeda-Robertos N.F, González-Garduño R, Cámara-Sarmiento R, Torres-Acosta J.F.L. Gastrointestinal nematode populations with multiple anthelmintic resistance in sheep farms from the hot humid tropics of Mexico. Vet. Parasitol. 2017;9:29–33. doi: 10.1016/j.vprsr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 6.van Wyk J.A, Stenson M.O, van der Merwe J.S, Vorster R.J, Viljoen P.G. Anthelmintic resistance in South Africa:Surveys indicate an extremely serious situation in sheep and goat farming. Onderstepoort J. Vet. Res. 1999;66(4):273–284. [PubMed] [Google Scholar]
- 7.Tsotetsi A.M, Njiro S, Katsande T.C, Moyo G, Baloyi B, Mpofu J. Prevalence of gastrointestinal helminths and anthelmintic resistance on small-scale farms in Gauteng province, South Africa. Trop. Anim. Health Prod. 2013;45(3):751–761. doi: 10.1007/s11250-012-0285-z. [DOI] [PubMed] [Google Scholar]
- 8.Vatta A.F, Letty B.A, van der Linde M.J, van Wijk E.F, Hansen J.W, Krecek R.C. Testing for clinical anaemia caused by Haemonchus spp. in goats farmed under resource-poor conditions in South Africa using an eye colour chart developed for sheep. Vet. Parasitol. 2001;99(1):1–14. doi: 10.1016/s0304-4017(01)00446-0. [DOI] [PubMed] [Google Scholar]
- 9.Bakunzi F.R. Anthelmintic resistance of nematodes in communally grazed goats in a semi-arid area of South Africa. J. S. Afr. Vet. Assoc. 2003;74(3):82–83. doi: 10.4102/jsava.v74i3.516. [DOI] [PubMed] [Google Scholar]
- 10.Mphahlele M, Molefe N, Tsotetsi-Khambule A.M, Thekisoe O. Anthelmintic Resistance in Livestock, Helminthiasis. Omolade Olayinka Okwa, Intech Open; 2019. Retrieved on 27-08-2019. Available from: https://www.intechopen.com/books/helminthiasis/anthelmintic-resistance-in-livestock . [Google Scholar]
- 11.Vijayasarathi M.K, Sreekumar C, Venkataramanan R, Raman M. Influence of sustained deworming pressure on the anthelmintic resistance status in strongyles of sheep under field conditions. Trop. Anim. Health Prod. 2016;48(7):1455–1462. doi: 10.1007/s11250-016-1117-3. [DOI] [PubMed] [Google Scholar]
- 12.Babják M, Königová A, Dolinská M.U, Vadlejch J, Várady M. Anthelmintic resistance in goat herds-in vivo versus in vitro detection methods. Vet. Parasitol. 2018;254:10–14. doi: 10.1016/j.vetpar.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Besier R.B, Kahn L.P, Sargison N.D, van Wyk J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016;93:95–143. doi: 10.1016/bs.apar.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Morgan E.R, Aziz N.A, Blanchard A, Charlier J, Charvet C, Claerebout E, Geldhof P, Greer A.W, Hertzberg H, Hodgkinson J, Höglund J, Hoste H, Kaplan R.M, Martínez-Valladares M, Mitchell S, Ploeger H.W, Rinaldi L, Samson-Himmelstjerna G, Sotiraki S, Schnyder M, Skuce P, Bartley D, Kenyon F, Thamsborg S.M, Vineer H.R, de Waal T, Williams A.R, van Wyk J.A, Vercruysse J. 100 questions in livestock helminthology research. Trends Parasitol. 2019;35(1):52–71. doi: 10.1016/j.pt.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Charlier J, Ghebretinsae A.H, Levecke B, Ducheyne E, Claerebout E, Vercruysse J. Climate-driven longitudinal trends in pasture-borne helminth infections of dairy cattle. Int. J. Parasitol. 2016;46(13-14):881–888. doi: 10.1016/j.ijpara.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Bath G.F, van Wyk J.A. Using the FAMACHA©system on commercial sheep farms in South Africa. Proceedings, 5th International Sheep Veterinary Congress, Stellenbosch, South Africa, 21-25 January 2001. 2001 [Google Scholar]
- 17.Soto-Barrientos N, Chan-Pérez J.I, España-España E, Novelo-Chi L.K, Palma-Ávila I, Ceballos-Mendoza A.C, Sarabia-Hernández J.A, Santos-Ricalde R.H, Cámara-Sarmiento R, Torres-Acosta J.F.T. Comparing body condition score and FAMACHA©to identify hair-sheep ewes with high faecal egg counts of gastrointestinal nematodes in farms under hot tropical conditions. Small Rumin. Res. 2018;167:92–99. [Google Scholar]
- 18.Reinecke R. Veterinary Helminthology. 1st ed. Durban: Butterworths; 1983. p. 392. [Google Scholar]
- 19.Coles G.C, Jackson F, Pomroy W.E, Prichard K, Samson H.G, Silvestre A, Taylor A, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136(3-4):167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.van Wyk J.A, Mayhew E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle:A practical lab guide. Onderstepoort J. Vet. Res. 2013;80(1):539. doi: 10.4102/ojvr.v80i1.539. [DOI] [PubMed] [Google Scholar]
- 21.Maphosa V, Masika P.J, Bizimenyera E.S, Eloff J.N. In vitro anthelmintic activity of crude aqueous extracts of Aloe ferox, Leonotis leonorus and Elephantorrhiza elephantine against Haemochus contortus. Trop. Anim. Health Prod. 2010;42(2):301–307. doi: 10.1007/s11250-009-9421-9. [DOI] [PubMed] [Google Scholar]
- 22.Soulsby E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. London: Bailliere Tindall; 1992. pp. 212–218. [Google Scholar]
- 23.Cala A.C, Chagas A.C.S, Oliveira M.C.S, Matos A.P, Borges L.M.F, Sousa L.A.D, Souza F.A, Oliveira G.P. In vitro anthelmintic effect of Melia azedarach L, Trichilia classenii C. against sheep gastrointestinal nematodes. Exp. Parasitol. 2012;130(2):98–102. doi: 10.1016/j.exppara.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Thrusfield M. Veterinary Epidemiology. 3rd ed. Sussex, UK: Black-Well Publishing; 2005. p. 183. [Google Scholar]
- 25.Kochapakdee S, Pandey VS, Pralomkan W, Chlodumrongkul S, Ngampongsai W, Lawpetchara A. Anthelmintic resistance in goats in Southern Thailand. Vet. Rec. 1995;137(5):124–125. doi: 10.1136/vr.137.5.124. [DOI] [PubMed] [Google Scholar]
- 26.Coles G.C, Bauer C, Borgsteede F.H.M, Geerts S, Klei T.R, Taylor M.A, Waller P.J. World association for the advancement of veterinary parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44(1-2):35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 27.Dolinska M, Königová A, Letková V, Molnár L, Várady M. Detection of ivermectin resistance by a larval development test-back to the past or step forward? Vet. Parasitol. 2013;198(1-2):154–158. doi: 10.1016/j.vetpar.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Dolinska M, Ivanisinova O, Königová A, Várady M. Anthelmintic resistance in sheep gastrointestinal nematodes in Slovakia detected by in vitro methods. BMC Vet. Res. 2014;10:233. doi: 10.1186/s12917-014-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherill A.F, Craig T, Kaplan R.M, Miller J.E, Navarre C, Rings M. Anthelmintic resistance of gastrointestinal parasites in small ruminants. J. Vet. Intern. Med. 2006;20(2):435–444. doi: 10.1892/0891-6640(2006)20[435:arogpi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Kumsa B, Debela E, Megersa B. Comparative efficacy of albendazole, tetramisole and ivermectin against gastrointestinal nematodes in naturally infected goats in Ziway, Oromia Regional State (Southern Ethiopia) J. Anim. Vet. Adv. 2010;9(23):2905–2911. [Google Scholar]
- 31.Maharshi A.K, Swarankar C.P, Singh D, Manohar G.S, Ayub M. Status of anthelmintic resistance in gastrointestinal nematodes of sheep in Rajasthan. Indian J. Anim. Sci. 2011;81(2):110–115. [Google Scholar]
- 32.Ploeger H.W, Everts R.R. Alarming levels of anthelmintic resistance against gastrointestinal nematodes in sheep in the Netherlands. Vet Parasitol. 2018;262:11–15. doi: 10.1016/j.vetpar.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Merlin A, Chauvin A, Lehebel A, Brisseau N, Froger S, Bareille N, Chartier C. End-season daily weight gains as rationale for targeted selective treatment against gastrointestinal nematodes in highly exposed first-grazing season cattle. Prev. Vet. Med. 2017;138:104–112. doi: 10.1016/j.prevetmed.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Muchiut M.S, Fernández A.S, Steffan P.E, Luque S.E, Cardozo P.A, Bernat G.A, Riva E, Fiel C.A. Recovery of fenbendazole efficacy on resistant Haemonchus contortus by management of parasite refugia and population replacement. Vet. Parasitol. 2019;271:31–37. doi: 10.1016/j.vetpar.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Islam M.S, Hossain M.S, Dey A.R, Alim M.A, Akter S, Alam M.Z. Epidemiology of gastrointestinal parasites of small ruminants in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2017;4(4):356–362. [Google Scholar]
- 36.Shearer C.L, Ezenwa V.O. Rainfall as a driver of seasonality in parasitism. Int. J. Parasitol. Parasites Wildl. 2020;12:8–12. doi: 10.1016/j.ijppaw.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horak I.G. Parasites of domestic and wild animals in South Africa. V. Helminths in sheep on dryland pasture on the Transvaal Highveld. Onderstepoort J. Vet. Res. 1978;45(1):1–5. [PubMed] [Google Scholar]
- 38.Tsotetsi A.M, Mbati P.A. Parasitic helminths of veterinary importance in cattle, sheep and goats on communal farms in the North Eastern Free State, South Africa. J. S. Afr. Vet. Assoc. 2003;74(2):45–48. doi: 10.4102/jsava.v74i2.503. [DOI] [PubMed] [Google Scholar]
- 39.Zeryehun T. Helminthosis of sheep and goats in and around Haramaya, Southeastern Ethiopia. J. Vet. Med. Anim. Health. 2012;4(3):48–55. [Google Scholar]
- 40.Vanessa D.V, Feitosa T.F, Vilela V.L.R, Azevedo S.S, Net J.L.D, Dayana F.D.M, Ana R.C.R, Athayde A.C.R. Prevalence and risk factors associated with goat gastrointestinal helminthiasis in the Sertão region of Paraíba State, Brazil. Trop. Anim. Health Prod. 2014;46(2):355–361. doi: 10.1007/s11250-013-0496-y. [DOI] [PubMed] [Google Scholar]
- 41.Agyei A.D, Sapong D, Probert A.J. Periparturient rise in faecal nematode egg counts in West African dwarf sheep in Southern Ghana in the absence of arrested strongyle larvae. Vet. Parasitol. 1991;39(1-2):79–88. doi: 10.1016/0304-4017(91)90064-3. [DOI] [PubMed] [Google Scholar]
- 42.Nginyi J.M, Duncan J.L, Mellor D.J, Stear M.J, Wanyangu S.W, Bain R.K, Gatongi P.M. Epidemiology of parasitic gastrointestinal nematode infections of ruminants on smallholder farms in Central Kenya. Res. Vet. Sci. 2001;70(1):33–39. doi: 10.1053/rvsc.2000.0438. [DOI] [PubMed] [Google Scholar]
- 43.Pandey V.S, Ndao M, Kumar V. Seasonal prevalence of gastrointestinal nematodes in communal land goats from the highveld of Zimbabwe. Vet. Parasitol. 1994;51(3-4):241–248. doi: 10.1016/0304-4017(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 44.Westers T, Jones-Bitton A, Menzies P, van Leeuwen J, Poljak Z, Peregrine A.S. Identification of effective treatment criteria for use in targeted selective treatment programs to control haemonchosis in periparturient ewes in Ontario, Canada. Prev. Vet. Med. 2016;134:49–57. doi: 10.1016/j.prevetmed.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Wahab A.R, Adanan C.R. Observations on the worm egg counts and their nematode species in goats from the North-East district of Penang Island, Peninsular Malaysia. Pertanika J. Trop. Agric. 1992;15(3):221–224. [Google Scholar]
- 46.Brik K, Hassouni T, Elkharrim K, Belghyti D. A survey of Haemonchus contortus parasite of sheep from Gharb plain, Morocco. Parasite Epidemiol, Control. 2019;4:e00094. doi: 10.1016/j.parepi.2019.e00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storey B.E, Williamson L.H, Howell S.B, Terrill T.H, Berghaus. R, Vidyashankar A.N, Kaplan R.M. Validation of the FAMACHA©system in South American camelids. Vet. Parasitol. 2017;243:85–91. doi: 10.1016/j.vetpar.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Seyoum Z, Getnet K, Chanie M, Derso S, Fentahun S. Morbidity parameters associated with gastrointestinal tract nematodes in sheep in Dabat district. Northwest Ethiopia. Biomed. Res. Int 2018. 2018;(1):1–7. doi: 10.1155/2018/9247439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammed K, Abba Y, Ramli N.S.B. The use of FAMACHA© in estimation of gastrointestinal nematodes and total worm burden in Damara and Barbados Blackbelly cross sheep. Trop. Anim. Health Prod. 2016;48(5):1013–1020. doi: 10.1007/s11250-016-1049-y. [DOI] [PubMed] [Google Scholar]


