Abstract
Background and Aim:
Poultry is becoming an increasingly important source of protein in the Nepalese diet. The Chitwan region of Nepal is the hub of the emerging poultry industry. Little is known about the prevalence of non-typhoidal Salmonella (NTS) on poultry farms or the role of farm management practices that may contribute to the presence of NTS on farms. The role of poultry in the transmission of Salmonella enterica to humans is also poorly defined. This descriptive study seeks establish baseline data through estimation of the prevalence of NTS on broiler and layer operations in various farms of the Chitwan district of Nepal.
Materials and Methods:
Based on district documents on poultry production and meat marketing, a purposive sampling of 18 commercial poultry farms comprising ten broilers farms and eight layers farms was conducted. Environmental samples including water, litter, feces, feed, farm, and eggshell swabs were randomly collected from each farm. Samples were cultured and tested for the presence of NTS; positives were serotyped, and antimicrobial susceptibility determined. A comprehensive farm and practice questionnaire was administered to each farm manager.
Results:
The farm level point prevalence rate was 55% (10 of 18 farms) for S. enterica. Of the total 288 farm environmental samples collected, 26 samples (9%) were positive. The rate of isolation varied according to the origin of samples: Water (27.5%), feces (10.6%), litter (8.6%), farm swabs (5%), feed (1.8%), and eggshells (0%). Farm management variables/risk factors are summarized and categorized as non-modifiable and modifiable for analysis. Broiler operations were more likely to be positive than layer operations as were poultry houses with two or less open sides. All-in/all-out management style was found to be protective. Due to the small sample size (18 farms), no associations reached statistical significance.
Conclusion:
Based on environmental sampling results, NTS is highly prevalent on the poultry farms in the Chitwan district of Nepal. Certain risk factors are associated with finding NTS on farms. Our findings are generally in agreement with other studies in similar countries with rapidly emerging poultry industries. The identification of risk factors provides owners, technicians, and veterinarians with some guidance to help reduce the prevalence of NTS on farms. This baseline data are critical to understanding the epidemiology of zoonotic strain of NTS in the region and are necessary for the design of future studies and mitigation plans and underlines the need for a one-health approach to protect public health-related to Salmonella spp. from poultry farms.
Keywords: farm risk factors, Nepal, non-typhoidal Salmonella, poultry, Salmonella enterica
Introduction
Non-typhoidal Salmonella (NTS) infections are estimated to cause around 153 million cases of gastroenteritis and 57,000 deaths globally each year, making it one of the leading causes of bacterial diarrhea worldwide [1]. Infections of humans with NTS are frequently associated with the consumption of contaminated food and are considered the second largest cause of food-borne illnesses after Campylobacter species [2-5]. This highlights the need for a global effort with a management and monitoring framework to control antibiotic resistance to protect public health related to Salmonella spp. from poultry farms. Although several Salmonella enterica serovars are consistently found at a high incidence within the poultry industry, geographic and temporal variation play a prominent role in the distribution [6,7]. Poultry is increasingly playing a major role in the human food chain. Due to increased globalization, modern poultry industries have created more complex opportunities for the spread of S. enterica, especially linked to international travel, livestock trade, and human migration [6-10].
Although there has been extensive research on S. enterica spp. among poultry farms globally, there is a lack of data in developing countries particularly in South Asia, including Nepal. Nepal is an agriculture-based country with around two-thirds of the population depending on agriculture for their livelihood. The livestock sector has contributed 26.8% of agricultural gross domestic production (AGDP) and 11% of gross domestic production (GDP). Poultry alone contributed 3.5% of GDP in 2014 [11,12] and 4.0% in 2020 with worth over NRs 50 billion [13]. Annual per capita meat consumption from all livestock and poultry in Nepal increased from 10.2 kg (in 2002) to 12.2 kg in 2011 [11] driving the rapid expansion of the poultry industry. According to the Food and Agriculture Organization data based on imputation methodology, the production of poultry meat increased rapidly from 20 million chickens to over 77 million from 2008 to 2018, almost a four-fold increase [14]. Due to the economic importance of this sector and the increasing reliance on poultry as a source of nutrition, food-borne pathogens such as S. enterica are of increasing concern as they pose a potential threat to both livestock production and human health. Despite this expansion, very little research has been conducted at the farm level regarding the risk factors which may increase the chances of acquisition, spread, and maintenance of NTS on the poultry farms in Nepal.
This study aims to establish baseline data through estimation of the prevalence of NTS on broiler and layer operations in various farms of the Chitwan district of Nepal. Farm-level management practices that may contribute to acquisition, spread, and maintenance of NTS were collected as well as environmental samples from 18 farms in Chitwan, Nepal’s leading poultry producing district.
Materials and Methods
Ethical approval and informed consent
Although the village and district of each farm were recorded, the identity and exact location of each farm were coded to ensure anonymity. Oral permission was obtained from the owner of each poultry farm before collecting environmental samples from the farm. No live animals were touched or harmed in this study. This study was reviewed by the Michigan State University Institutional Animal Use and Care Committee and the Human Subject Review Committee and ruled “Exempt” from review by both Boards.
Study area and period
This cross-sectional study was conducted in the Chitwan district, located in southwestern part of Narayani Zone, Central Development Region of Nepal with Latitude: 27° 34’ 59.99” N and Longitude: 84° 30’ 59.99” E (Figure-1). All samples were collected in May and early June, 2019. Chitwan district has emerged as a major income-generating enterprise in the agricultural sector over the past 35 years, contributing 39.5% of Nepal’s poultry population, 21.8% of poultry meat production, and 36.5% overall egg production [15,16].
Figure-1.
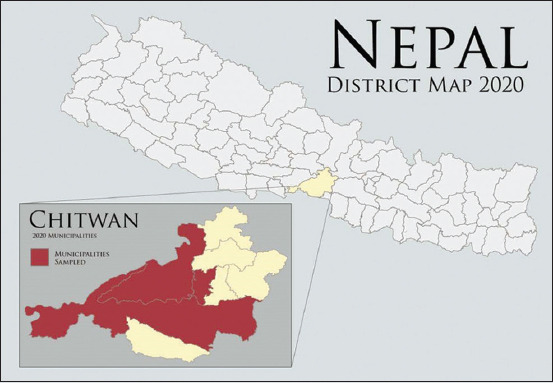
District map of Nepal (as per new political map of Nepal approved by the upper house of Nepal’s Parliament in 2020) highlighting the district of study and rural municipalities. Border within Chitwan is based on municipality borders from 2020.
Design: Data collection and sampling
Based on the review of district documents on poultry production and meat marketing, Ministry of Livestock production documents, previous research and personal communications, purposive sampling of 18 commercial poultry farms (ten broilers and eight layers) was made. Farms were categorized based on size, type (broiler/layer), and geographical location – the samples were collected from 13 major villages and towns of the municipalities. Farms of different sizes were selected to ensure an adequate representation of the poultry industry. Farms were categorized into small (200-500 birds; n=4), medium (501-1000 birds; n=6), and large (>1000 birds; n=8) farms. Not every village in the district had all sizes and types of commercial poultry farms present. Within the farm size, type, and geographic location categories, random number selection was used to select farms for sampling. Selected farms were in various stages of production and in good health at the time of sampling. Samples were collected over an 18-day period, (May 17-June 3, 2019). A total of 288 environmental samples were collected from various sources, as shown in Table-1. The number of samples was dictated by budget restrictions. Sample number and type decisions were based on prior research studies and designed to provide a robust opportunity to identify the presence of NTS, if present on a farm. Within the limit of 288 samples, we restricted the number of farms to 18. Thus, 18 became the unit of analysis for the farm management survey portion of the study.
Table-1.
Number of environmental samples collected by source and farm size.
| Environmental samples | Four small farms (200 500 birds) | Six medium farms (501 1000 birds) | Eight large farms (>1000 birds) | Total |
|---|---|---|---|---|
| Water (n = 1, 2, or 3)a | 4 | 12 | 24 | 40 |
| Soil/Litter/Bedding (n = 2, 3, or 4)a | 8 | 18 | 32 | 58 |
| Adult bird feces (n = 2, 3, or 4)a | 8 | 18 | 32 | 58 |
| Young bird feces (n = 2, 3, or 4)a (only in mixed age populations)b | 4 | 0 | 4 | 8 |
| Feed (n = 2, 3, or 4)a | 6 | 18 | 32 | 56 |
| Farm swabs (n = 1, 2, or 3)c | 4 | 12 | 24 | 40 |
| Eggshell swabs (n = 2, 4, or 6)d | 2 | 8 | 18 | 28 |
| Total Samples | 36 | 86 | 166 | 288 |
Environment samples represented by name and parentheses represents number of samples from each small, medium, and large farm, respectively.
Additional feces were collected on farms with mixed age groups.
Farm swabs were taken from three different poultry contact surfaces within the poultry house.
Eggshell swabs were taken only from layer farms with birds in laying stage
A questionnaire including open- and closed-ended questions was prepared, pilot-tested with one non-participating farm manager then revised. All questionnaires were administered by the same two veterinarians to ensure consistency and translated into the local language during survey administration. The questionnaire included data concerning farm, poultry and poultry house characteristics, management of birds, biosecurity practices, presence of domestic animals and wildlife, feeding and watering practices, cleaning and disinfection procedures, vaccination and antibiotic use, and more. If farm biosecurity protocol prevented the veterinarians from entering the poultry houses, the farm staff was verbally instructed on how to collect samples and observed through the fence. “Farm” swabs involved wetting the swab in sterile water and streaking three different poultry-contact surfaces within the poultry house and egg swabs involved swabbing three different eggshells. The end of the swab was then broken off into 25 mL of sterile water. For all other samples, approximately 25 g of material (bedding, feces, feed, and water) in contact with poultry, were randomly collected throughout the farm.
Testing: Isolation and identification of NTS enterica
Samples were pre-enriched with 1:9 concentration of buffered peptone water at 37°C for 18-20 h after preparation of the original homogenate in accordance with ISO 6579 protocol for pre-enrichment [17]. For each sample, 2 mL of the pre-enriched homogenate was then transferred to another sterile container and sent to the Laboratory of National Zoonoses and Food Hygiene Research Centre (NZFHRC) in Kathmandu, Nepal, for selective culture, isolation and biochemical analysis, and antimicrobial resistance testing on positive cultures.
Isolation of Salmonella spp. was carried out according to protocol ISO 6579 [17] with slight modifications, used for selective culture and biochemical identification. Pre-enriched samples were inoculated in Rappaport-Vassiliadis (RV) broth at 41°C for 24 h and Salmonella shigella agar was used for selective culture. Suspected colonies were subcultured and isolated colonies were cultured on nutrient agar slants for further identification and biochemical characterization.
The initial identification step was done using Gram’s stain and oxidase test; all isolates showing Gram’s stain positive and/or oxidase-positive were discarded. Then, other isolates were biochemically tested using Indole, Methyl red, Voges–Proskauer, Citrate utilization, Triple sugar iron (TSI), and urease tests as per the protocol described by Ewing [18]. The colonies showing Salmonella specific IMViC pattern (− + − +) were further inoculated on TSI slants, and colonies that produced alkaline slant (pink) and acidic butt (yellow) with or without H2S production (blackening) were tested for urea hydrolysis on urea agar slants. All the urease negative isolates were considered as biochemically confirmed Salmonella isolates. Positive isolates were submitted for serovar testing by Kauffmann-White-Le Minor scheme at the Nepal Agricultural Research Council in Lalitpur, Nepal.
Statistical analysis
Microsoft Excel 2016® (Microsoft Corporation, WA, USA) was used for data entry and management and IBM SPSS v25 (IBM Corp., NY, USA) was used for statistical analysis. The map of the sample area was created withArcGIS v10.3.1 (ESRI, CA, USA) using administrative borders fromHumanitarian Data Exchange v.1.43.5 (available at https://data.humdata.org/dataset/administrative-bounadries-of-nepal) and edited with Adobe Illustrator v16 (Adobe Inc, CA, USA). Farms were identified as positive based on at least one environmental sample collected on the farm testing positive for NTS. Due to the small sample size (n=18) variables were collapsed into two categories for association testing. To measure the impact of each factor individually on the presence of NTS on the farm, a two-tailed Fisher’s exact test was used. Odds ratio (OR) and 95% confidence intervals (CI) were calculated. Values of p<0.05 were considered statistically significant. Due to the small sample size, statistical significance was difficult to reach and some risk factors that were not statistically significant were included in the discussion as they may offer valuable information and be of interest in future studies.
Results
Farm-level NTS prevalence by farm type and size
Ten (55.5%) of the 18 poultry farms were found to have at least one environmental sample test positive for NTS. The point prevalence rate varied according to the type of farm: 80% (8 of 10) of broiler farms and 25% (2 of 8) of layer farms were positive; and by size of farm: 75% (6 of 8) large farms 0% (0 of 6) medium and 100% (4 of 4) small farms were found positive for NTS.
Environmental sample testing
Out of the total 288 samples taken, 9% (26/288) were positive. The isolation rate varied according to the origin of samples: 27.5% (11 of 40) of water samples; 10.6% (7 of 66) feces samples; 8.6% (5 of 58) of bedding samples; 5% (2 of 40) of farm swabs (n=40); 1.8% (1 of 56) of feed samples; and 0% (0 of 28) of eggshell swabs were positive. Note: Serovar testing data are presented in the 2nd paper in this series (www.veterinaryworld.org/Vol.14/February-2021/15.pdf).
Farm questionnaire summary results
Farms were selected based on type (broiler and layer) and size and distributed geography. Detailed farm questionnaire results can be found in Table-2. Within these selection parameters, most of the farm owners were male (83%) and all were literate with 22% having completed graduate-level education. Most (89%) poultry houses had concrete floors and none were fully enclosed. Most (56%) of the farms were exclusively poultry (no other domestic animals) and half reported seeing wildlife near their poultry houses daily. All farmers reported using rice husk bedding, most (72%) reported using pelleted feed (vs. mash) and most (94%) reported feeding birds manually. None of the farmers were breeding their own birds nor were they marketing directly to consumers. Over half the farms (61%) were following all-in/all-out flock management while 39% were mixing ages.
Table-2.
Summary of farm questionnaire results (n=18 farms).
| Owner information | Number | % |
|---|---|---|
| Gender of farm owner | ||
| Male | 15 | 83% |
| Female | 3 | 17% |
| Owner education level | ||
| Illiterate | 0 | 0% |
| Primary | 7 | 39% |
| Secondary | 7 | 39% |
| Graduate | 4 | 22% |
| Age of farm owner | ||
| 35 years or younger | 6 | 33% |
| 36 50 years | 6 | 33% |
| 51 years or older | 6 | 33% |
| Owner experience in poultry | ||
| 10 years or less | 10 | 56% |
| 11 years or more | 8 | 44% |
| Gender of primary caretaker | ||
| Male | 6 | 33% |
| Female | 4 | 22% |
| Both | 8 | 44% |
| Facility information | No. | % |
| Distance of farm to the road | ||
| 100 m or less | 7 | 39% |
| 101 350 m | 5 | 28% |
| 351 m or more | 6 | 33% |
| Distance to the nearest farm | ||
| 100 m or less | 6 | 33% |
| 101 200 m | 7 | 39% |
| 201 m or more | 5 | 28% |
| Floor material | ||
| Concrete | 16 | 89% |
| Clay/Dirt | 2 | 11% |
| Cage | 0 | 0% |
| Style of chicken house | ||
| Two Sides Open | 7 | 39% |
| Three Sides Open | 5 | 28% |
| Four Sides Open | 6 | 33% |
| Closed | 0 | 0% |
| Age of the chicken houses | ||
| 4 years or less | 6 | 33% |
| 5 11 years | 6 | 33% |
| 12 years or older | 6 | 33% |
| Other domestic animals present | ||
| No | 10 | 56% |
| Yes | 8 | 44% |
| Frequency wildlife observed near poultry | ||
| Frequently (every day) | 9 | 50% |
| Semi frequently (once per week) | 3 | 17% |
| Rarely (once/mo) | 4 | 22% |
| Never | 2 | 11% |
| Feed, water, bedding information | No. | % |
| Type of bedding | ||
| Rice Husks | 18 | 100% |
| Type of feed | ||
| Pellet | 13 | 72% |
| Mash | 5 | 28% |
| Style of feeding | ||
| Automatic | 1 | 6% |
| Semi automatic | 0 | 0% |
| Manual | 17 | 94% |
| Source of water for farm | ||
| Tank | 7 | 39% |
| Tap | 9 | 50% |
| Well | 2 | 11% |
| Surface | 0 | 0% |
| Water delivery to poultry | ||
| Nipple type | 2 | 11% |
| Bell type | 15 | 83% |
| Open | 1 | 6% |
| Poultry information - general | Number | % |
| Poultry breeding on farm | ||
| No | 18 | 100% |
| Type of flock | ||
| Layer | 7 | 39% |
| Broiler | 10 | 56% |
| Mixed | 1 | 6% |
| Destination of meat/eggs | ||
| Consumer direct | 0 | 0% |
| Retailer | 6 | 33% |
| Wholesaler | 4 | 22% |
| Mixed | 8 | 44% |
| Collector | 0 | 0% |
| Feed restriction prior to sale | ||
| No | 17 | 94% |
| Yes | 1 | 6% |
| Poultry cohort management | ||
| All in all out | 11 | 61% |
| Staggered/mixed ages | 7 | 39% |
| Poultry health information | No. | % |
| Frequency of vet/paravet visit | ||
| Weekly | 5 | 28% |
| > once/year | 5 | 28% |
| Once/year | 7 | 39% |
| Never | 1 | 6% |
| Medical records kept | ||
| Yes | 9 | 50% |
| No | 9 | 50% |
| Vaccination frequency | ||
| At hatching | 2 | 11% |
| Regularly | 10 | 56% |
| Both | 6 | 33% |
| Vaccine administered by | ||
| Farm owner | 4 | 22% |
| Employee | 1 | 6% |
| Vet or paravet | 1 | 6% |
| Mixed | 11 | 61% |
| N/A | 1 | 6% |
| Farm biosecurity | No. | % |
| Type of PPE used | ||
| Boots | 16 | 89% |
| None | 2 | 11% |
| Quarantine area | ||
| No | 10 | 56% |
| Yes | 8 | 44% |
| Handwashing area | ||
| Yes | 18 | 100% |
| Soap present at handwash station | ||
| Yes | 16 | 89% |
| No | 2 | 11% |
| Disinfectants present | ||
| Yes | 9 | 50% |
| No | 9 | 50% |
| Footbath used | ||
| No | 16 | 89% |
| Yes | 2 | 11% |
| Cleaning frequency | ||
| Deep clean after slaughter | 7 | 39% |
| Spot cleaning weekly | 1 | 6% |
| Both | 8 | 44% |
| No cleaning | 1 | 6% |
| Time the house is kept empty between flocks | ||
| 10 days or less | 6 | 33% |
| 11 21 days | 5 | 28% |
| 22 90 days | 7 | 39% |
| Litter disposal location | ||
| Off site | 6 | 33% |
| Onsite near housing | 8 | 44% |
| Onsite away from housing | 4 | 22% |
Only one farm reported never having a veterinarian or a paravet visit the farm, and five farms (28%) reported weekly visits; half of the farmers were keeping medical records, and all birds were reported as being vaccinated at some point in time although the researchers could not determine exactly which vaccines were being used when. Common S. enterica specific vaccination practices in the Chitwan area for commercial layer farms include using live vaccine at 6 weeks of age and killed vaccine at 15 weeks of age. Broilers are not commonly vaccinated for S. enterica. S. enterica serovars Gallinarum, Enteritidis, and Typhimurium are included in commonly used vaccines in the Chitwan District.
Biosecurity practices include having an area available for handwashing (100%) with soap present on most (89%) and wearing boots (89%). Disinfectant was present on half the farms and two farms (11%) were using footbaths. Note: Antibiotic use and administration practices, as well as antibiogram data, are presented in the 2nd paper in this series.
Risk factors
Although this study is descriptive in nature, some analysis was possible. The results of selected Fisher’s Chi-square (two-tailed) tests show several possible associations between farm practice/risk variables and finding NTS on participating farms. These variables plus additional variables of interest are summarized in Table-3 (non-modifiable factors) and Table-4 (modifiable factors). Among the non-modifiable factors, “Farm Type” and “House Type” showed an association with finding NTS on the farm. Farm Type – boiler operations (vs. layer) were found to have a higher odds of being positive for NTS (OR 12, 95% CI 1.3-111.3) as were having poultry houses with only two sides open (vs. three or more sides open) (OR 10.5 95% CI 0.9-121.4). Among the modifiable risk factors, only using pelleted feed (vs. mash) showed a notable association (OR 9, 95% CI 0.8-108.3). No associations were found to be statistically significant at the p<0.05 level. This vast amplitude in the CI may be due to small sample size. Additional association testing results are provided for variables considered to be potential risk factors or protective factors for S. enterica presence on poultry farms in general, and these potential (but not statistically significant) findings may indicate the need for additional research to clarify the relationship between variables.
Table-3.
Associations between non modifiable risk factors and finding NTS on farms.
| Variable/Risk factor | NTS + | NTS | Odd ratio (95% CI) | p value |
|---|---|---|---|---|
| Farm type | ||||
| Broiler | 8 | 2 | 12 (1.3 111.3) | 0.05 |
| Layer | 2 | 6 | ||
| House type | ||||
| Two sides open | 6 | 1 | 10.5 (0.9 121.4) | 0.07 |
| Three or more sides open | 4 | 7 | ||
| Education Level of farmer | ||||
| Secondary or Higher | 8 | 3 | 6.7 (0.8 55.0) | 0.14 |
| Primary only | 2 | 5 | ||
| Flock size | ||||
| Large (1200 or more) | 6 | 2 | 4.5 (0.6 34.6) | 0.19 |
| Small (<1200) | 4 | 6 | ||
| Frequency of wildlife/rodent/Pest seen | ||||
| Rare to never | 4 | 8 | 4 (0.5 33.3) | 0.32 |
| Weekly to daily | 4 | 2 | ||
| Age of houses | ||||
| Over 10 years old | 6 | 3 | 2.5 (0.4 16.9) | 0.64 |
| <10 years old | 4 | 5 | ||
| Age of farm owner | ||||
| Under 45 years | 6 | 3 | 2.5 (0.4 16.9) | 0.64 |
| 45 years old or above | 4 | 5 | ||
| Age of chickens | ||||
| Younger than 60 days | 6 | 3 | 2.5 (0.4 16.9) | 0.64 |
| 60 days or older | 4 | 5 | ||
| Floor material | ||||
| Clay/Soil | 1 | 0 | 2.7 (0.1 75.1) | 1.00 |
| Concrete | 9 | 8 | ||
| Water delivery method | ||||
| Others | 2 | 1 | 1.75 (0.1 23.7) | 1.00 |
| Bell type waterer | 8 | 7 | ||
| Distance from farm to road | ||||
| <100 m | 4 | 3 | 1.1 (0.2 7.5) | 1.00 |
| More than 100 m | 6 | 5 | ||
| Source of Water | ||||
| Municipal/Tap | 5 | 4 | 1.0 (0.2 6.4) | 1.00 |
| Well/Tank | 5 | 4 |
Table-4.
Associations between modifiable risk factors and finding NTS on farms.
| Variable/Risk factor | NTS + | NTS | Odd ratio (95% CI) | p value |
| Food Type | ||||
| Pellets | 9 | 4 | 9 (0.8 108.3) | 0.12 |
| Mash | 1 | 4 | ||
| Management style | ||||
| Mixed/Staggered age | 5 | 2 | 3 (0.4 22.7) | 0.37 |
| All in/All out | 5 | 6 | ||
| Presence of other domestic animals | ||||
| No | 7 | 3 | 3.9 (0.5 27.8) | 0.34 |
| Yes | 3 | 5 | ||
| Antibiotic use | ||||
| Yes | 9 | 5 | 3.6 (0.3 50.3) | 0.54 |
| No | 1 | 2 | ||
| Frequency of cleaning | ||||
| Frequent/spot cleaning | 7 | 2 | 5.8 (0.7 48.9) | 0.35 |
| Infrequent/between flocks | 3 | 5 | ||
| Houses empty between flocks | ||||
| >2 weeks | 7 | 4 | 2.3 (0.3 16.2) | 0.63 |
| 2 weeks or less | 3 | 4 | ||
| Keeping medical records | ||||
| Yes | 5 | 4 | 1.0 (0.2 6.4) | 1.00 |
| No | 5 | 4 | ||
| Use of PPE (boots) | ||||
| No | 2 | 1 | 1.8 (0.1 2.4) | 1.00 |
| Yes | 8 | 7 | ||
| Use of footbath | ||||
| No | 9 | 7 | 1.3 (0.1 25) | 1.00 |
| Yes | 1 | 1 | ||
| Frequency of visit by Vet/Paravet | ||||
| More than 1/year | 6 | 4 | 1.5 (0.2 9.8) | 1.00 |
| Annually or less frequently | 4 | 4 |
Discussion
Although there are many studies focused on the prevalence of NTS in poultry meat, slaughterhouse samples and postmortem samples of birds in Nepal [19-23], there are very few focusing on the prevalence of NTS from environmental samples of poultry farms.
Of the 288 environmental samples taken, 26 (9%) were found to be positive for NTS. This is only slightly lower than a previous study done in the Chitwan district by Dhakal and Manandhar in 2005 [24], who showed 12% NTS positive samples from the litter, food, and water from poultry farms in the Chitwan district. Some factors that might account for this variation include region sampled, methods used for sample collection and testing, types of facilities chosen for sampling, and the season in which testing was performed. When compared with the research conducted in a neighboring country, India, which has similar poultry rearing practices to Nepal, the frequency of isolating S. enterica in the environmental samples ranged widely from 7.9 to 95.7% [25]. The sample collection period for this study was in the pre-monsoon season with generally dry and extremely hot weather with average maximum daytime temperature being 30.9°C.
The prevalence of NTS found in our study varied depending on the source of the environmental sample with a high of 27.5% (water) to a low of 0% from eggshell swabs. Similar research from Algeria showed a lower prevalence of Salmonella among environmental samples; 2.18% of water samples, 3.12% of feces samples, 3.93% of farm swabs, and 6.25% for wipes, while all the feed samples were free of Salmonella [26]. Research from Nigeria showed S. enterica prevalence to be; feces (23%), feed (22.7%), litter (20.3%), and dust (18.9%) with water samples having the lowest prevalence at 15.1% [27]. Another study from North Carolina, USA found the prevalence of Salmonella in fecal samples was 38.8%, feed was 27.5% while none of the water samples were positive [28]. Interestingly, our study found water to have the highest rate of positivity at 27%. In addition, for two of the study farms, water was the only positive environmental source on the farm. This was unexpected given the results from the above-mentioned studies measuring similar parameters and introduces the question of water being a potential source of introduction of NTS onto the farm. The unexpectedly high prevalence in water may also be due to the collection of water samples directly from the water feeder which may have collected biofilms or contamination from the environment. This discrepancy could also be the result of water from the feeders not being processed through any filter or chlorinated to remove environmental contaminants. In addition, the level of contamination in water seemed to be unaffected by other factors such as layer/broiler farm type, water source, cleaning regimen, or biosecurity protocols. Because water samples were not expected to test positive at this rate, survey questions surrounding water administration were limited to water source and feeder type, and did not include well type, cleaning protocols for water dispensers, and frequency the water is changed or the distance from the water feeders to the ground which could result in contamination. The fact that these samples were taken in the dry season could also have an impact, as the moisture around the poultry waterers could provide a more hospitable environment for NTS growth and biofilm accumulation when compared with the dry, hot surroundings.
In this study, the prevalence of NTS isolates from all eggshell swabs was negative. This was also an unexpected result as previous research has shown eggshell contamination to be commonplace [29-35]. There are several possible explanations for this discrepancy. Not all the layer farms tested were in the egg production stage, reducing the number of farms from which eggshell swabs could be taken. In addition, all but one of the layer facilities that were producing eggs, were negative on all other samples, suggesting a low prevalence of NTS among layer farms. The small sample size makes it difficult to draw any definitive conclusions.
Non-modifiable risk factors
Broilers versus layers
Samples taken from broiler farms were far more likely to be contaminated with S. enterica than samples taken from layer farms (OR=12; 95 CI 1.13-111.3 and p=0.05). These findings are similar to research conducted in other countries where broiler flocks were found to be positive for S. enterica at higher rates than layer flocks [36]. A possible explanation suggested by previous researchers could be a difference in procedures for cleaning and disinfection of broiler farms when compared with layers [36-38]. As broilers have a shorter period of rearing (around 36-50 days in Nepal), farmers are trying to maximize the number of rearing cycles per year and minimizing the time between cycles. This might lead to a shorter time available for cleaning and disinfection before new stock is introduced to the farm, making it more difficult to follow the “all in-all out” management principle [36,39]. In this study, we found 64% of all farms reported practicing all-in/all-out management. Additional factors contributing to NTS contamination could be that the material used for construction of the house may not allow for satisfactory cleaning and house design may allow bacterial contamination from surrounding wildlife such as insects or rodents. The ability of S. enterica to resist desiccation allows it to survive for long periods in the environment [40,41].
The lower prevalence of infection found in layer flocks could be due to the declining rate of S. enterica colonization and fecal shedding 2 weeks post-infection in laying chickens from pullet and layer flocks [35]. Moreover, in some farms, the birds may be infected with S. enterica without showing signs of the illness, which means the presence of a sub-clinical infection in the flock. Feces from these flocks may contain S. enterica in low numbers [41]. In addition, vaccination against S. enterica in layer flocks (but not in broiler flocks) in Nepal may also lead to lower rates of infection. These vaccines are less common among broiler farms, although our current survey did not reveal any specific link between our nonspecific “vaccination” variable and NTS prevalence.
Poultry house design
Samples taken from poultry houses with two sides open were more likely to be contaminated than houses with three or more sides open which might be attributed a lack of adequate ventilation (OR=10.5; 95 CI 0.9-121.4 and p=0.07). Various studies suggest that infection could occur by oral ingestion of dust from external surfaces that can be contaminated by airborne movement during the time of feeding or pecking [42,43]. There are also other findings showing a relationship between low rate of airflow causing dead pockets to be formed in litter/manure and increasing the counts of S. enterica [44]. It has been shown that S. enterica remains in the dust of ventilation filters for several months [45]. Other studies have suggested airflow directly impacts litter dampness and that broiler growth improves with improvement of ventilation [44,46-48].
Education level of farmer
Another non-modifiable risk factor considered was the education level of the farmer (OR 6.67; 95 CI 0.8-55.0 and p=0.07). It is interesting to note that the farmers with higher education were more likely to have NTS contamination on their farms. One possible explanation could be that farmers with higher levels of education may be less inclined to spend their time on the farm and farm management may be relegated to other people with less stake in the health of the flock. People with higher education may also have other sources of income, splitting their responsibilities between farming and other off-farm duties. The results could also be due to spurious causes. For example, higher educated farmers may be more likely to own broiler farms, and the contamination is due to broiler management and unrelated to the level of education directly. Additional research related to education of farm owners, managers, and workers would be needed to properly explore this finding.
Modifiable risk factors
Pelleted feed
Due to small sample size (n=18 farms), risk factors could not be strongly correlated with any specific modifiable practice. However, there were two factors of interest that may warrant further study. Among poultry farms feeding pellet food, 69% of these farms (9 of 13) had at least one environmental sample positive for S. enterica compared to 20% of farms feeding mash (OR 9; 95 CI 0.8-108.3 and p=0.12). It is important to note that these test results do not correlate to farms with feed that tested positive, but any environmental sample. Only 1 of the 56 feed samples tested positive and this feed was mash. It is also important to note that the sample size is very small, particularly in the number of farms feeding mash. It is possible, for example, that pellet food is a more popular feed for broiler farms, and other factors related to broiler poultry management are responsible.
The incidence of S. enterica in poultry feed and feed ingredients is known to be highly variable ranging from 0 to 78% [49-51]. Various factors including source and quality of feed ingredients, and storage conditions could contribute to this variation. The recovery of S. enterica in feed using meat and bone meal has been described in the previous studies [49]. Higher incidence of S. enterica in meat and bone meal could be due to improper sterilization of these ingredients and suggest that they may be the major source of S. enterica in compound feed. In Nepal, mash feed prepared by the farmer at the farm level usually do not include bone or meat meal, but pellet feed prepared in industry level uses it regularly. Further feed analysis could reveal meaningful data, but larger samples sizes would be needed as well as specific feed composition and origin. The previous studies also showed that S. enterica has been recovered from dry products even after they have been processed at relatively high temperatures [52]. This could be the result of post-processing contamination or the ability of S. enterica to survive extremely well when heated in media of reduced water activity [52-54]. Dry animal feeds can also play an important role in the epidemiology of human salmonellosis [55]. Another study on S. enterica contamination in US swine feed reported higher pathogen presence in pelleted commercial feed when compared to on-farm mixed mash products [56].
Poultry cohort management
The other factor worth noting was the farm management style. Samples taken from farms who had mixed age chickens on site had higher odds of having positive isolates of S. enterica than farms that practiced all-in all-out style of management, where all the chickens managed on the farm enter and leave at the same time (OR=3; 95 CI 0.4-22.7 and p=0.37). This finding agreed with the previous studies looking at the effect of multiage management on the farm with the occurrence of S. enterica [57-59].
All other modifiable risk factors including biosecurity measures, cleaning procedures, frequency of veterinary visits, and presence of other domestic animals on the farm did not show a significant relationship to finding NTS on the farm. This is likely due to several factors. Many of the poultry farms visited had similar biosecurity measures. Most had separate boots for entering the chicken house. Most farms used similar protocols for cleaning, which included a mixture of VirkonS, lime powder, fumigation, and incineration. This lack of variation provided no negative grouping for comparison.
As previously mentioned, the major limitation of this study is the small number of farms available for analysis. Collecting samples during the dry season also may have decreased the likelihood of finding positive environmental samples. However, the inclusion of multiple farm sizes and types from 13 villages provides a good snapshot of the current farm practices in this important and rapidly expanding industrial hub.
Conclusion and Recommendations
To the best of the authors’ knowledge, this is the first systematic, descriptive study with a comprehensive survey to evaluate the various risk factors that may contribute to NTS acquisition, maintenance, and spread among Nepal’s poultry farms. This study provides important baseline data on the prevalence of NTS among 18 poultry farms located in 13 villages in Chitwan, Nepal’s leading poultry producing district. The in-depth questionnaire data provide a picture of common poultry farming practices, biosecurity measures, and farmer demographics for this region. Although the small sample size precluded robust analysis, some interesting trends emerged that warrant further investigation. This descriptive cross-sectional study suggests some farm management practices that may reduce the likelihood of acquiring and maintaining NTS on the farm. This data will be useful to improve farm management practices, direct future epidemiological studies, and ultimately reduce the spread of zoonotic strains of NTS from poultry to humans.
For ensuring S. enterica -free feed and water, regular microbiological examination of feed and water should be done to estimate the microbial load so that use of sanitizers/ additives can be done promptly. Furthermore, regular, and proper cleaning of feed and water containers is important to reduce biofilm formation. Proper ventilation also ensures less growth of microbes, which can reduce the chances of infection.
NTS contamination was higher among broiler operations than layer operations. Broiler farmers should take more care during cleaning and disinfection. The risk of NTS contamination was also higher when flocks were reared on farms with multiage management rather than on farms with all-in/all-out management practice. Applying “all in-all out” procedures, with appropriate biosecurity measures against animated (owner, worker rodent/pest/wild animals) or unanimated vectors (dust, litter, feed, and water) can be effective to reduce contamination of S. enterica on the farm. However, further detailed studies on the correlation of levels of biosecurity and incidence of S. enterica in poultry farms in this region of Nepal are needed.
Finally, there are some other important risk factors which were not considered in this research but should be considered in future studies. Risk factors to explore include: Biofilm development in farm equipment, vertical transmission of S. enterica, feed composition, rodent/pest control strategies, the role of temperature and humidity, cleaning and disinfecting practices with relation to the previous infection in the same poultry house, and seasonality at the time of sample collection. Most of these risk factors have been already studied in other countries, but not yet in Nepal.
Serotyping results and antibiotic resistance profiles of S. enterica isolates can be important to fully understanding the epidemiology, ecology, and distribution of this pathogen in the poultry farms of Nepal (see accompanying paper). Repeating this study would help estimate the patterns and distributions over time, including data about emerging serotypes and provide information on epidemiology that can help design future mitigation plans.
It can be concluded that there is a high prevalence of NTS found in environmental samples collected from the poultry farm environment in the Chitwan district of Nepal. Therefore, infection of birds with NTS from environmental sources is highly possible if no additional preventive strategies are taken. The present study was designed to be descriptive in nature and revealed fairly consistent farm practices within the Chitwan region. Regarding farm management practices, there is room for improvement regarding the use of footbaths, flock cohort management, cleaning and disinfection protocols and medical record keeping, making these easy targets for farmer/worker education campaigns.
Finally, findings from this study underlines the need for a “One-Health” approach that uses human, animal, and environmental health resources to solve this dynamic issue at the intersection of the human-animal-environment interface to avoid more human illness and diseases along with future outbreaks.
Authors’ Contributions
SS1 and PDF actively worked on the sample collection, questionnaire design and initial sample processing and draft writing. DKP supervised sample handling and completed the process of identification and isolation of S. enterica strains. PF and MW participated actively in study design and data analysis, while SS2 contributed in part to the study design, draft writing, data interpretation, and revising along with SS1, PF, and MW. SS2 and MJW carefully monitored each level of research. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to acknowledge the valuable contributions of Minu Sharma and the NZFHRC for providing the original idea, facilitating the laboratory component of this study and the isolation and identification of bacteria. This study has been carried out with the financial support of Michigan State University College of Veterinary Medicine, Endowed Research Funds: the Kenneth Eskelund Fund, the Michael Scott Endowment Fund, and the Food Animal Health Fund; proposal ID ERF-6115650175.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Healy J.M, Bruce B.B. CDC Traveler's Health. Retrieved on 3-7-2019. Available form: https://www.nc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/salmonellosis-nontyphoidal .
- 2.Ejo M, Garedew L, Alebachew Z, Worku W. Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. Biomed. Res. Int. 2016 doi: 10.1155/2016/4290506. DOI:10.1155/2016/4290506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakeri S.A, Yasin R.M, Koh Y.T, Puthucheary S.D, Thong K.L. Genetic diversity of human isolates of Salmonella enterica serovar Enteritidis in Malaysia. J. Appl. Microbiol. 2003;95(4):773–780. doi: 10.1046/j.1365-2672.2003.02033.x. [DOI] [PubMed] [Google Scholar]
- 4.Mead P.S, Slutsker L, Dietz V, McCaig L.F, Bresee J.S, Shapiro C, Griffin P.M, Tauxe R.V. Food-related illness and death in the United States. J. Environ. Health. 2000;62(7):9–18. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garai P, Gnanadhas D.P, Chakravortty D. Salmonella enterica serovars Typhimuriumand Typhi as model organisms:Revealing paradigm of host-pathogen interactions. Virulence. 2012;3(4):377–388. doi: 10.4161/viru.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour E.K, Ayyash D.B, Alturkistni W, Alyahiby A, Yaghmoor S, Iyer A, Yousef J, Kumosani T, Harakeh S. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. J. Infect. Dev. Ctries. 2015;9(1):1–7. doi: 10.3855/jidc.5065. [DOI] [PubMed] [Google Scholar]
- 7.Gast R.K. Paratyphoid Infections. In: Swayne D.E, editor. Diseases of Poultry. 12th Edition. Ames, IA: Wiley-Blackwell; 2003. pp. 583–599. [Google Scholar]
- 8.Gast R.K, Guraya R, Guard J. Salmonella enteritidis deposition in eggs after experimental infection of laying hens with different oral doses. J. Food. Prot. 2013;76(1):108–113. doi: 10.4315/0362-028X.JFP-12-268. [DOI] [PubMed] [Google Scholar]
- 9.Hendriksen R.S, Bangtrakulnonth A, Pulsrikarn C, Pornreongwong S, Hasman H, Song S.W, Aarestrup F.M. Antimicrobial resistance and molecular epidemiology of Salmonella Rissen from animals, food products, and patients in Thailand and Denmark. Foodborne Pathog. Dis. 2008;5(5):605–619. doi: 10.1089/fpd.2007.0075. [DOI] [PubMed] [Google Scholar]
- 10.Hendriksen R.S, Vieira A.R, Karlsmose S, Lo Fo Wong D.M.A, Jensen A.B, Wegener H.C, Aarestrup F.M. Global monitoring of Salmonella serovar distribution from the world health organization global food-borne infections network country data bank:Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog.Dis. 2011;8(8):887–900. doi: 10.1089/fpd.2010.0787. [DOI] [PubMed] [Google Scholar]
- 11.FAO. Poultry Sector Nepal. FAO Animal Production and Health Livestock Country Reviews. No. 8. Rome: FAO; 2014. [Google Scholar]
- 12.MOLD. Annual Report of Ministry of Livestock Development. Singhdurbar, Kathmandu: MOLD; 2016. [Google Scholar]
- 13.Nepal Poultry Federation. Nepal Poultry International Expo. 2020. Retrieved on 30-8-2020. Available from: http://www.nepalpoultryexpo.com .
- 14.FAOSTAT. Retrieved on 30-8-2020. Available from: http://www.fao.org/faostat/en/#data/QL .
- 15.MOALD. Statistical Information on Nepalese Agriculture [2018/19] Singhdurbar, Kathmandu: Ministry of Agriculture and Livestock Development; 2020. [Google Scholar]
- 16.MOALD. Statistical Information on Nepalese Agriculture. Singhdurbar, Kathmandu: Ministry of Agriculture and Livestock Development; 2018. [Google Scholar]
- 17.ISO 6579. Horizontal Method for the Detection of Salmonella spp. 4th ed. Geneva: The International Organization for Standardizations; 2002. Microbiology of food and animal feeding stuffs; pp. 1–4. [Google Scholar]
- 18.Ewing W.H. Edwards and Ewing's Identification of Enterobacteriaceae. New York: Elsevier; 1986. [Google Scholar]
- 19.Adhikari S.K, Gyawali A, Shrestha S, Shrestha S.P, Prajapati M, Khanal D.R. Molecular confirmation of Salmonella Typhimurium in poultry from Kathmandu Valley. J. Nepal. Agric. Res. Counc. 2018;4(1):86–89. [Google Scholar]
- 20.Sharma A, Tripathi P. Seasonal prevalence of poultry diseases and their interaction with IBD and Mycotoxicosis in Chitwan, Nepal. Res. Gate. 2015;2015 DOI:10.13140/RG.2.2.27054.41283. [Google Scholar]
- 21.Bahadur D.L, Prasad D.I, Kumar Y.S, Zohorul I. Prevalence and antibiotic resistance profile of Salmonella from livestock and poultry raw meat, Nepal. Int. J. Mol. Vet. Res. 2016;6(1):1–22. [Google Scholar]
- 22.Gompo T.R, Pokhrel U, Shah B.R, Bhatta D.D. Epidemiology of important poultry diseases in Nepal. Nepal Vet. J. 2019;36:8–14. [Google Scholar]
- 23.Bhandari N, Nepali D, Paudyal S. Assessment of bacterial load in broiler chicken meat from the retail meat shops in Chitwan, Nepal. Int. J. Infect. Microbiol. 2013;2(3):99–104. [Google Scholar]
- 24.Dhakal I.P, Manandhar P. Isolation of Salmonella in the pooled samples of litter, food and water in Chitwan poultries. Proc. Natl. Poult. Expo. 2005;2005:43–46. [Google Scholar]
- 25.Wales A, Breslin M, Davies R. Semiquantitative assessment of the distribution of Salmonella in the environment of caged layer flocks. J. Appl. Microbiol. 2006;101(2):309–318. doi: 10.1111/j.1365-2672.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 26.Djeffal S, Mamache B, Elgroud R, Hireche S, Bouaziz O. Prevalence and risk factors for Salmonella spp. Contamination in broiler chicken farms and slaughterhouses in the Northeast of Algeria. Vet. World. 2018;11(8):1102–1108. doi: 10.14202/vetworld.2018.1102-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagbamila I.O, Barco L, Mancin M, Kwaga J, Ngulukun S.S, Zavagnin P, Lettini A.A, Lorenzetto M, Abdu P.A, Kabir J, Umoh J, Ricci A, Muhammad M. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017;12(3):1–15. doi: 10.1371/journal.pone.0173097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alali W.Q, Thakur S, Berghaus R.D, Martin M.P, Gebreyes W.A. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne Pathog. Dis. 2017;7(11):1363–1371. doi: 10.1089/fpd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 29.Peresi J.T.M, Almeida I.A.Z, Lima S.I, Marques D.F, Rodrigues E.C, Fernandes, S A.D, Gelli S, Irino K. Foodborne disease outbreaks caused by Salmonella enteritidis. Rev. Saude Publica. 1998;32(5):477–483. doi: 10.1590/s0034-89101998000500011. [DOI] [PubMed] [Google Scholar]
- 30.Adesiyun A, Webb L, Musai L, Louison B, Joseph G, Stewart-Johnson A, Samlal S, Rodrigo S. Survey of Salmonella contamination in chicken layer farms in three Caribbean countries. J. Food Prot. 2014;77(9):1471–1480. doi: 10.4315/0362-028X.JFP-14-021. [DOI] [PubMed] [Google Scholar]
- 31.CDC. Update: Salmonella enteritidis infections and shell eggs-United States, 1990. MMWR Morb. Mortal. Wkly. Rep. 1990;39(50):909. [PubMed] [Google Scholar]
- 32.Indar-Harrinauth L, Daniels N, Prabhakar P, Brown C, Baccus-Taylor G, Comissiong E, Hospedales J. Emergence of Salmonella enteritidis phage Type 4 in the Caribbean :Case-control study in Trinidad and Tobago, West Indies. Clin. Infect. Dis. 2001;32(6):890–896. doi: 10.1086/319344. [DOI] [PubMed] [Google Scholar]
- 33.Much P, Berghold C, Krassnig G, Schweighardt H, Wenzl H, Allerberger F. An Austrian outbreak of Salmonella enteritidis phage Type 36 in 2004. Wien. Klin. Wochenschr. 2005;117(17):599–603. doi: 10.1007/s00508-005-0415-y. [DOI] [PubMed] [Google Scholar]
- 34.Oboegbulem S.I, Collier P.W, Sharp J.C.M, Reilly W.J. Epidemiological aspects of outbreaks of food-borne Salmonellosis in Scotland between 1980, 1989. Rev. Sci. Tech. Int. DES Epizoot. 1993;12(3):957–967. doi: 10.20506/rst.12.3.725. [DOI] [PubMed] [Google Scholar]
- 35.Hrivniakova L, Schmid D, Luckner-Hornischer A, Lassnig H, Kornschober C, Angermayer J, Allerberger F. Salmonellosis outbreak due to Salmonella enteritidis phage Type 14b resistant to nalidixic acid, Austria, September 2010. Eurosurveillance. 2011;16(34):19952. [PubMed] [Google Scholar]
- 36.Morshed R, Peighambari S.M. Salmonella infections in poultry flocks in the vicinity of Tehran. Int. J. Vet. Res. 2010;4(4):273–276. [Google Scholar]
- 37.Namata H, Welby S, Aerts M, Faes C, Abrahantes J.C, Imberechts H, Vermeersch K, Hooyberghs J, Méroc E, Mintiens K. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Prev. Vet. Med. 2009;90(3-4):211–222. doi: 10.1016/j.prevetmed.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Angen Ø, Skov M.N, Chriél M, Agger J.F, Bisgaard M. A retrospective study on Salmonella infection in Danish broiler flocks. Prev. Vet. Med. 1996;26(3-4):223–237. [Google Scholar]
- 39.Brown D.J, Olsen J.E, Bisgaard M. Salmonella enterica: Infection, cross-infection and persistence within the environment of a broiler parent stock unit in Denmark. Zentralblatt Fur. Bakteriol. 1992;277(1):129–138. doi: 10.1016/s0934-8840(11)80881-9. [DOI] [PubMed] [Google Scholar]
- 40.Kim A, Lee Y.J, Kang M.S, Kwag S.I, Cho J.K. Dissemination and tracking of spp. in integrated broiler operation. J. Vet. Med. Sci. 2007;8(2):155–161. doi: 10.4142/jvs.2007.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendriksen R. Glob Salm-Surv. 4th ed. Geneva: A Glob Surveill Lab Support Project World Health Organization; 2003. Isolation of Salmonella. [Google Scholar]
- 42.Namata H, Méroc E, Aerts M. Salmonella in Belgian laying hens:An identification of risk factors. Prev. Vet. Med. 2008;83(3-4):323–336. doi: 10.1016/j.prevetmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Gast R.K, Mitchell B.W, Holt P.S. Airborne transmission of Salmonella enteritidis infection between groups of chicks in controlled-environment isolation cabinets. Avian Dis. 1998;42(2):315. [PubMed] [Google Scholar]
- 44.Mallinson E.T, De Rezende C.E, Tablante N.L, Carr L.E, Joseph S.W. A management technique to identify prime locations of Salmonella contamination on broiler and layer farms. J. Appl. Poult. Res. 2000;9(3):364–370. [Google Scholar]
- 45.Kim A, Young J.L, Min S.K, Sang I.K, Jae K.C. Dissemination and tracking of Salmonella spp. In integrated broiler operation. J. Vet. Sci. 2007;8(2):155–161. doi: 10.4142/jvs.2007.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacy M.P, Czarick M. Tunnel-ventilated broiler houses:Broiler performance and operating costs. J. Appl. Poult. Res. 1992;1(1):104–109. [Google Scholar]
- 47.Bottcher R.W, Magura J.R, Young J.S, Baughman G.R. Effects of tilt angles on airflow for poultry house mixing fans. Appl. Eng. Agric. 1995;11(5):721–730. [Google Scholar]
- 48.Weaver W.D, Jr, Meijerhof R. The effect of different levels of relative humidity and air movement on litter conditions, ammonia levels, growth, and carcass quality for broiler chickens. Poult. Sci. 1991;70(4):746–755. doi: 10.3382/ps.0700746. [DOI] [PubMed] [Google Scholar]
- 49.Saravanan S, Purushothaman V, Ramasamy T. Molecular epidemiology of non-typhoidal Salmonella in poultry and poultry products in India :Implications for human health. Indian J. Microbiol. 2015;55(3):319–326. doi: 10.1007/s12088-015-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward J, Griffin M, Egan J. Newbury, UK: Process Monitor procedure Rapid Detect Methods Tech; 1996. Evaluation of Some Rapid Methods for the Detection of Salmonella in Poultry Carcasses, Feed and Environmental Samples; pp. 123–127. [Google Scholar]
- 51.Veldman A, Vahl H.A, Borggreve G.J, Fuller D.C. A survey of the incidence of Salmonella species and Enterobacteriaceae in poultry feeds and feed components. Vet. Rec. 1995;136(7):169–172. doi: 10.1136/vr.136.7.169. [DOI] [PubMed] [Google Scholar]
- 52.Juven B.J, Cox N.A, Bailey J.S. Survival of Salmonella in dry food and feed. J. Food Prot. 1984;47(6):445–448. doi: 10.4315/0362-028X-47.6.445. [DOI] [PubMed] [Google Scholar]
- 53.Corry J.E.L. The effect of sugars and polyols on the heat resistance of Salmonellae. J. Appl. Bacteriol. 1974;37(1):31–43. doi: 10.1111/j.1365-2672.1974.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 54.Goepfert J.M, Iskander I.K, Amundson C.H. Relation of the heat resistance of salmonellae to the water activity of the environment. Appl. Environ. Microbiol. 1970;19(3):429–433. doi: 10.1128/am.19.3.429-433.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sillikrt J.H. The Salmonella problem:Current status and future direction. J. Food Prot. 1982;45(7):661–666. doi: 10.4315/0362-028X-45.7.661. [DOI] [PubMed] [Google Scholar]
- 56.Davies P.R, Scott Hurd H, Funk J.A, Fedorka-Cray P.J, Jones F.T. The role of contaminated feed in the epidemiology and control of Salmonella enterica in pork production. Foodborne Pathog. Dis. 2004;1(4):202–215. doi: 10.1089/fpd.2004.1.202. [DOI] [PubMed] [Google Scholar]
- 57.Huneau-Salaün A, Chemaly M, Le Bouquin S. Risk factors for Salmonella enterica subsp. Enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 2009;89(1-2):51–58. doi: 10.1016/j.prevetmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Snow L.C, Davies R.H, Christiansen K.H, Cook A.J.C, Evans S.J. Investigation of risk factors for Salmonella on commercial egg-laying farms in Great Britain, 2004-2005. Vet. Rec. 2010;166(19):579–586. doi: 10.1136/vr.b4801. [DOI] [PubMed] [Google Scholar]
- 59.Denagamage T, Jayarao B, Patterson P, Wallner-Pendleton E, Kariyawasam S. Risk factors associated with Salmonella in laying hen farms:Systematic review of observational studies. Avian Dis. 2015;59(2):291–302. doi: 10.1637/10997-120214-Reg. [DOI] [PubMed] [Google Scholar]


