Abstract
Background: The Bhagarva surrogate molecular subtype definitions classify invasive breast cancer into seven the different subgroups based on immunohistochemical (IHC) criteria according to expression levels of markers as ER, PR, HER2, EGFR and/or basal cytokeratin (CK5/6) which are different in prognosis and responsiveness to adjuvant therapy. Purpose: The present study aimed to classify primary breast cancers and directly compares the prognostic significance of the intrinsic subtypes. Methods: The current study was conducted on 522 breast cancer patients who had surgery, but had not received neoadjuvant chemotherapy, from 2011 to 2014. The clinicopathologic characteristics were recorded. IHC staining was performed for ER, PR, HER2, Ki67, CK5/6, EGFR and D2-40 markers. All breast cancer patients were stratified according to Bhagarva criteria. The followed-up patients’ survival was analyzed by using Kaplan-Meier and Log-Rank models. Results: The luminal A (LUMA) was observed at the highest rate (32.5%). Non-basal-like triple negative phenotype (TNB-) and Luminal A HER2-Hybrid (LAHH) were the least common (3.3% in both). LUMA and luminal B (LUMB) were significantly associated with better prognostic features compared to HER2, basal-like triple negative phenotype (TNB+) and TNB-. Statistically significant differences were demonstrated between overall survival (OS), disease-free survival (DFS) and molecular subtypes (P<0.05), of which LUMB and LUMA had the highest rate of OS and DFS being 97.2 and 93.7%; and 97.2 and 90.5%, respectively. Conversely, HER2 revealed the worst prognosis with the lowest prevalence of OS and DFS (72.5 and 69.9%, respectively). Conclusion: The molecular subtypes had a distinct OS and DFS. The intrinsic stratification displayed inversely to clinicopathological features in breast cancer.
Keywords: Breast cancer, molecular subtype, immunohistochemistry, prognosis
Introduction
Breast cancer (BC) is a heterogeneous disease. Even breast tumors with similar histopathological features may have different clinical manifestations, degrees of malignancy, systematic therapeutic responses, and survival outcomes. One possible reason is that tumor cells of the same morphologic type have different origins (stem cells). Previous studies have shown that relying on conventional parameters may have limitations for patient-tailored treatment strategies and would result in a certain percentage of patients being treated incorrectly [1,2]. Accordingly, a more precise tool is needed to identify patients who would benefit from systematic adjuvant treatment after BC surgery, especially in the early stages of the disease [3]. Studies of gene expression and its association with diverse phenotypic characteristics have altered the classification of BC and other cancers at the molecular level. Recently, “molecular portraits” of breast tumors have been discovered through the “hierarchical clustering” technology of gene groups based on similarity in gene-expression patterns [4-7]. The molecular types of BC differ markedly by race/ethnicity, distribution of risk factors, prognosis, response to therapy, clinical outcome, and disease-free survival (OS and DFS) [6,8,9]. Novel molecular studies have opened a broad field in cancer research that allows basic and translational researchers to look for new potential targets. Analyses of BC with new molecular techniques now hold promise for the development of more accurate tests to predict recurrence.
Many studies have used immunohistochemistry to study BC molecules and implement BC molecular classification. However, there are many classifications with different IHC standards [9-15]. In daily practice, in parallel with identifying histopathologic types of BC, it is necessary to perform immunohistochemical (IHC) stains with a known panel to classify BC into different molecular types. Routine IHC evaluation of BCs may provide crucial information to guide clinical management and represent a valid alternative to costly genotyping assays, stratifying patients who need adjuvant treatment and giving accurate prognostic information. Vietnam is a developing country, and most BC patients cannot afford expensive molecular tests. Thus, it is important to select risk classification tools that are suitable in terms of both expense and value forselecting the appropriate adjuvant treatment. Thus, treatments used to manage Vietnamese patients with BC must be validated. The molecular categories of BC using IHC may be a good candidate, and this classification has not been applied yet in Vietnam to identify the different molecular groups. Hence, the present study aimed to classify primary BCs and directly compare the intrinsic subtypes’ prognostic significance.
Materials and methods
Participants and sample collection
All female patients had a first primary diagnosis of invasive BC and underwent surgery to remove tumor and axillary lymph node dissection between 2012 and 2014 at the National Cancer Hospital, Vietnam, belonging to stage I to IIIA. We recruited 522 treatment-naïve patients with breast cancer aged 14-87 for the current retrospective study with follow-up, excluding those who did not meet all the above selective criteria and presented with second or recurrent malignant tumors, or carcinoma in situ. The patients’ clinical information was recorded, including age, tumor site, and date of initial diagnosis, which were extracted from medical patient charts and records. All patients underwent surgery to remove the tumor by modified radical mastectomy or conservative surgery, combined with axillary lymph node dissection. Tumors were measured as to maximum diameter. The pTNM staging of breast cancer was based upon criteria from the American Joint Committee on Cancer (AJCC, 7th edition) [16]. Tumor and nodal samples were done by histopathologic tests.
Patients received systemic adjuvant chemotherapy postoperatively. Those with hormone-receptor-positive tumors also underwent endocrine therapy. Among HER2 positive BCs, only two patients could pay for all targeted treatment with trastuzumab. All individual information was anonymized for patient privacy.
Histopathology
Immediately after surgery, all specimens were transferred to the pathology department. Samples were fixed in 10% neutral formalin for 24 hours. Nodal and tumor samples were obtained by routine pathological techniques, such as hematoxylin and eosin staining (H&E). Experienced pathologists evaluated all histopathological features, such as tumor size, histopathological type, grade, nodal status, tumor necrosis, and peritumoral lymph-vascular invasion (LVI). Immunohistochemical staining was used with a D2-40 marker to confirm LVI. Histopathologic types were classified according to the 2012 WHO classifications [17]. Histologic grades were assigned according to Elston and Ellis [18]. The Nottingham Prognostic Index (NPI) was performed [19].
Detection of hormone receptor status, HER2, Ki67, CK5/6, and EGFR by immunohistochemistry
All IHC stainings were tested for sections of formalin-fixed, paraffin-embedded tissue. The IHC method was performed by Ventana automated machine, using ER, PR, HER2, Ki67, D2-40, CK5/6, and EGFR markers. We used available primary antibodies from Ventana in a ready-to-use condition, as the primary monoclonal mouse anti-human estrogen receptor (Ventana-SP01), anti-progesterone receptor (PR) (1E2) rabbit monoclonal primary antibody, monoclonal mouse anti-human c-erbB-2 oncoprotein, rabbit monoclonal (Ventana-4B5), anti-Ki67 monoclonal rabbit antibody (Ventana-30-9), podoplanin (D2-40) mouse monoclonal antibody, anti-cytokeratin 5/6 mouse monoclonal primary antibody (Ventana-D5/16B4), and anti-EGFR rabbit monoclonal primary antibody (Ventana-5B7), respectively. All IHC stained slides were also stained as positive (internal or external control) and negative controls to ensure exact staining. Regarding HER2, CK5/6, EGFR, and D2-40, a positive control with a tissue sample known to express the antigen of interest, was included on each slide.
The staining locations are consistent with their distribution, such as nuclear (ER, PR, Ki67), cytoplasmic/membranous (HER2, EGFR, D2-40), or cytoplasm (CK5/6, EGFR, D2-40). ER and PR slides were assessed by the percentage of positive cells and the intensity level (0 = none, 1 = weak, 2 = moderate, 3 = strong). We used the H-score scoring method to determine hormone receptor expression. Like the Allred score, the H-score scale is based on the intensity and positive proportion of tumor nuclei. The sum of these two parameters calculates the Allred scale, but in the H-score scale, these two parameters were multiplied each other by the formula: 3 × percentage of strongly staining nuclei + 2 × percentage of moderately staining nuclei + percentage of weakly staining nuclei, giving a range of 0 to 300 points, and positive endocrine receptors is an additional 10 points [20]. The UK recommendations were used to assess HER2 expression. Numerous different cutpoints for Ki67 have been proposed. At the 2013 St. Gallen consensus meeting, the cutpoint of the Ki67 index was set at 20 percent [21]. For CK5/6, EGFR, and D2-40, any stained invasive tumor cells were considered to be positive [14,22].
Detection of IHC HER2 equivocal by fluorescencein situ hybridization
A HER2 score of 2 plus was considered equivocal HER2. Ninety-six patients (19.2%) who had an IHC HER2 score of 2 plus were tested by FISH (Fluorescence in situ hybridization) (with a ratio of HER2 to chromosome 17 centromeric region >2.2, using PathVysion Vysis dual-color FISH by Vysis Inc., Downers Grove, Ill) to identify the amplification of the HER2 gene. Equivocal FISH result (1.8:2.2 ratio) was considered negative for HER2 in this study [23]. There were 21.9% HER2 gene amplification by FISH.
All IHC or FISH stained slides were analyzed and scored independently by two investigators. The investigators re-evaluated scores where they disagreed to reach consensus. In the present study, 9.5% of cases needed reassessment to resolve the disagreement because various IHC scores of the same slide between the different pathologists occurred.
Definition of BC molecular subtypes and risk categories
All patients were classified into molecular subtypes based on IHC data. This approach uses IHC criteria for its definitions of ER and PR, the detection of HER2 overexpression and/or amplification, and high molecular-weight keratin such as CK5/6, or EGFR marker to identify molecular subtypes. Molecular types that follow IHC criteria of Bhargava’s classification are Luminal A (LUMA: ER score is 200 or more and negative HER2), Luminal B (LUMB: ER score is ranged from 11 to 199, PR score is more 10 and loss of HER2), basal-like TNP (Triple-negative phenotype) (TNB+: ER and PR are up to 10, and neither HER2 overexpression nor amplification, CK5 or EGFR is positive), non-basal-like TNP (TNB-: ER and PR are up to 10, and HER2 negativity, CK5 or EGFR is negative) HER2-enriched (HER2: ER and PR are up to 10, and either HER2 overexpression or amplification), Luminal A HER2-Hybrid (LAHH: ER score is equal or more 200 and positive HER2), and Luminal B HER2-Hybrid (LBHH: ER score is ranged from 11 to 199, PR score is more 10 and either HER2 overexpression or amplification) [24] (Figure 1). Risk categories were grouped by following St Gallen 2007 [25].
Figure 1.

Algorithm of Bhagarva’s classification for molecular subtyping.
Follow-up and outcomes
Overall survival (OS) was defined as the date of initial diagnosis to the day of death due to BC or the last available time before being lost to follow-up [26]. Patients were excluded if they did not die of BC. Death dates were displayed on the death documents, such as certificates issued by the Vietnamese government. The recurrence and dates were demonstrated by image analytic and/or morphological data. Patients would be excluded until the death date if they had no relapse [26]. DFS was the length of time between the date of BC surgery and the diagnosis of the recurrent BC, or GC specific death, including locoregional and distant relapses [26]. Since the number of followed-up patients with TNB- and LAHH subgroups was very low (nine cases and four cases, respectively), therefore OS and DFS were evaluated based on TNP (including TNB- and TNB+) and luminal HER2 - hybrid (LUMHH) (including LAHH and LBHH) groups.
Statistical analysis
The Pearson chi-square test, Likelihood Ratio, and Fisher’s exact tests were performed to determine the clinicopathologic differences with seven molecular subtypes. Among of them, Fisher’s exact test was employed when sample sizes were small. The Kaplan-Meier model was used to investigate the five-year OS and DFS, according to the intrinsic subgroups. Survival curves of BC molecular subtypes were compared by performing a log-rank test. A difference was considered significant if the p-value was less than 0.05 or 0.001. In multivariate analysis, Cox proportional hazards regression models were employed to determine hallmarks independently associated with OS and DFS. All of the analyses were conducted using the statistical software of SPSS version 19.0.
Results
Baseline clinicopathologic features of all participants
The present study investigated all 522 BC patients who had undergone operation. Table 1 shows the patients’ baseline clinicopathologic characteristics. All study subjects exhibited good-moderate clinical prognostic features. Tumors were found at a high rate in the 50-59 Y-O group (35.9%), left side (54.6%), stage II (58.6%), moderate risk category (68.8%). Half the tumors were diagnosed at ≤2 cm in size; in the stage of negative lymph node (63.2%) and low recurrent percentage (1.5%). Similarly, the histopathologic features of all participants revealed quite good prognostic parameters such as morphologic type of infiltrating duct, NOS (71.6%), the moderate NPI (53.8%), 51.9% of low Ki67, absent LVI (64.6%). However, more than half of histologic grades were grade III (52.9%).
Table 1.
Baseline clinicopathologic characteristics of breast cancer participants
| Feature | No. of patients (%) n=522 |
|---|---|
| Age group | |
| <40 | 69 (13.2) |
| 40-49 | 144 (27.6) |
| 50-59 | 188 (35.9) |
| 60-69 | 86 (16.5) |
| ≥70 | 35 (6.7) |
| Age | |
| Young (≤40 Y-O) | 81 (69.8) |
| Older (≥70 Y-O) | 35 (30.2) |
| Tumor location | |
| Right | 234 (44.8) |
| Left | 285 (54.6) |
| Bilateral | 3 (0.6) |
| Tumor size (cm) | |
| ≤2 | 261 (50.0) |
| >2-5 | 242 (46.4) |
| >5 | 19 (3.6) |
| pTNM stage | |
| I | 134 (25.7) |
| II | 306 (58.6) |
| III | 82 (15.7) |
| Risk category | |
| Low | 24 (4.6) |
| Intermediate | 359 (68.8) |
| High | 139 (26.6) |
| Outcome | |
| Die | 24 (12.3) |
| Survival | 171 (87.7) |
| Recurrence | |
| No Relapse | 192 (98.5) |
| Relapse | 3 (1.5) |
| Histopathologic type | |
| Invasive ductal, NOS | 374 (71.6) |
| Mucinous | 16 (3.1) |
| Lobular | 89 (17.0) |
| Other | 43 (8.2) |
| Histologic grade | |
| I | 56 (10.7) |
| II | 190 (36.4) |
| III | 276 (52.9) |
| Lymph node status | |
| Negative | 330 (63.2) |
| 1-3 positive node (s) | 121 (23.2) |
| >3 positive nodes | 71 (13.6) |
| Nottingham PI | |
| Good | 122 (23.4) |
| Moderate | 281 (53.8) |
| Poor | 119 (22.8) |
| LVI | |
| Negative | 337 (64.6) |
| Positive | 185 (35.4) |
| Tumor necrosis | |
| Negative | 429 (82.2) |
| Positive | 93 (17.8) |
| Ki67 index | |
| Low (≤20%) | 271 (51.9) |
| High (>20%) | 251 (48.1) |
Correlation of the molecular subtypes and clinicopathologic features
Table 2 shows the basic clinicopathologic characteristics and relationships with the molecular subgroups. All tumors were immunohistochemically stained to classify biologic subtypes by using IHC criteria of Bhargava, the prevalence of the LUMA, HER2, TNB+ (Figure 2), LUMB, LBHH, TNB-, and LAHH subtypes were in descending order as 32.5, 19.2, 17.8, 15.7, 8.2, 3.3, and 3.3, respectively. The percentage order of subcategories were LUMA > HER2 > TNB+ > LUMB > LBHH > TNB- > LAHH.
Table 2.
Associations between baseline clinicopathologic features and BC molecular subtype
| Characteristics | No. of patients (%) n=522 | The Bhargava molecular subtypes | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| LUMA 170 (32.5) | LUMB 82 (15.7) | TNB- 17 (3.3) | TNB+ 93 (17.8) | HER2 100 (19.2) | LAHH 17 (3.3) | LBHH 43 (8.2) | |||
| Age group | <0.001a | ||||||||
| <40 | 69 (13.2) | 18 (10.6) | 11 (13.4) | 5 (29.4) | 16 (17.2) | 10 (10.0) | 0 (0.0) | 9 (20.9) | |
| 40-49 | 144 (27.6) | 40 (23.5) | 26 (31.7) | 7 (41.2) | 29 (31.2) | 24 (24.0) | 2 (11.8) | 16 (37.2) | |
| 50-59 | 188 (35.9) | 58 (34.1) | 27 (32.9) | 3 (17.6) | 31 (33.3) | 50 (50.0) | 7 (41.2) | 12 (27.9) | |
| 60-69 | 86 (16.5) | 36 (21.2) | 14 (17.1) | 2 (11.8) | 13 (14.0) | 11 (11.0) | 6 (35.3) | 4 (9.3) | |
| ≥70 | 35 (6.7) | 18 (10.6) | 4 (4.9) | 0 (0.0) | 4 (4.3) | 5 (5.0) | 2 (11.8) | 2 (4.7) | |
| Age | 0.022a | ||||||||
| Young (≤40 Y-O) | 81 (69.8) | 20 (52.6) | 14 (77.8) | 5 (100) | 20 (83.3) | 13 (72.2) | 0 (0.0) | 9 (81.8) | |
| Older (≥70 Y-O) | 35 (30.2) | 18 (47.4) | 4 (22.2) | 0 (0.0) | 4 (16.7) | 5 (27.8) | 2 (100.0) | 2 (18.2) | |
| Lateral | 0.634a | ||||||||
| Right | 234 (44.8) | 75 (44.1) | 35 (42.7) | 8 (47.1) | 38 (40.9) | 45 (45.0) | 11 (64.7) | 22 (51.2) | |
| Left | 285 (54.6) | 93 (54.7) | 47 (57.3) | 9 (52.9) | 55 (59.1) | 55 (55.0) | 6 (35.3) | 20 (46.5) | |
| Bilateral | 3 (0.6) | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | |
| Tumor size (cm) | 0.018b | ||||||||
| ≤2 | 261 (50.0) | 99 (58.2) | 40 (48.8) | 8 (47.1) | 45 (48.4) | 35 (35.0) | 11 (64.7) | 23 (53.5) | |
| >2-5 | 242 (46.4) | 66 (38.8) | 40 (48.8) | 7 (41.2) | 45 (48.4) | 58 (58.0) | 6 (35.3) | 20 (46.5) | |
| >5 | 19 (3.6) | 5 (3.0) | 2 (2.4) | 2 (11.8) | 3 (3.2) | 7 (7.0) | 0 (0.0) | 0 (0.0) | |
| Histopathologic type | <0.001b | ||||||||
| NOS | 374 (71.6) | 108 (63.5) | 61 (74.4) | 9 (52.9) | 68 (73.1) | 80 (80.0) | 12 (70.6) | 36 (83.7) | |
| Mucinous | 16 (3.1) | 10 (5.9) | 6 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Lobular | 89 (17.0) | 38 (22.4) | 12 (14.6) | 5 (29.4) | 13 (12.6) | 13 (13.0) | 5 (29.4) | 5 (11.6) | |
| Other | 43 (8.2) | 14 (8.2) | 3 (3.7) | 3 (17.6) | 7 (6.8) | 7 (7.0) | 0 (0.0) | 2 (4.7) | |
| Histologic grade | <0.001 | ||||||||
| I | 56 (10.7) | 33 (19.4) | 10 (12.2) | 1 (5.9) | 4 (4.3) | 3 (3.0) | 1 (5.9) | 4 (9.3) | |
| II | 190 (36.4) | 67 (39.4) | 41 (50.0) | 3 (17.6) | 24 (25.8) | 30 (30.0) | 7 (41.2) | 18 (41.9) | |
| III | 276 (52.9) | 70 (41.2) | 31 (37.8) | 13 (76.5) | 65 (69.9) | 67 (67.0) | 9 (52.9) | 21 (48.8) | |
| Lymph node status | <0.001 | ||||||||
| Negative | 330 (63.2) | 133 (78.2) | 52 (63.4) | 8 (47.1) | 54 (58.1) | 48 (48.0) | 11 (64.7) | 24 (55.8) | |
| 1-3 positive node (s) | 121 (23.2) | 27 (15.9) | 25 (30.5) | 0 (0.0) | 24 (25.8) | 29 (29.0) | 3 (17.6) | 13 (30.2) | |
| >3 positive nodes | 71 (13.6) | 10 (5.9) | 5 (6.1) | 9 (52.9) | 15 (16.1) | 23 (23.0) | 3 (17.6) | 6 (14.0) | |
| Nottingham PI | <0.001 | ||||||||
| Good | 122 (23.4) | 60 (35.3) | 19 (23.2) | 2 (11.8) | 14 (15.1) | 10 (10.0) | 4 (23.5) | 13 (30.2) | |
| Moderate | 281 (53.8) | 90 (52.9) | 52 (63.4) | 6 (35.3) | 53 (57.0) | 53 (53.0) | 9 (52.9) | 18 (41.9) | |
| Poor | 119 (22.8) | 20 (11.8) | 11 (13.4) | 9 (52.9) | 26 (28.0) | 37 (37.0) | 4 (23.5) | 12 (27.9) | |
| Ki67 index | 0.000 | ||||||||
| Low (≤20%) | 271 (51.9) | 121 (71.2) | 54 (65.9) | 6 (35.3) | 32 (34.4) | 35 (35.4) | 6 (35.3) | 17 (39.5) | |
| High (>20%) | 251 (48.1) | 49 (28.8) | 28 (34.1) | 11 (64.7) | 61 (65.6) | 65 (64.6) | 11 (64.7) | 26 (60.5) | |
| LVI | 0.001 | ||||||||
| Negative | 337 (64.6) | 127 (74.9) | 59 (72.0) | 11 (61.1) | 50 (53.8) | 53 (54.3) | 10 (58.8) | 27 (64.3) | |
| Positive | 185 (35.4) | 43 (25.1) | 23 (28.0) | 7 (38.9) | 42 (46.2) | 48 (45.7) | 7 (41.2) | 15 (35.7) | |
| Tumor necrosis | 0.004 | ||||||||
| Negative | 429 (82.2) | 152 (89.4) | 64 (78.0) | 12 (70.6) | 68 (73.1) | 78 (78.0) | 16 (94.1) | 39 (90.7) | |
| Positive | 93 (17.8) | 18 (10.6) | 18 (22.0) | 5 (29.4) | 25 (26.9) | 22 (22.0) | 1 (5.9) | 4 (9.3) | |
| pTNM stage | 0.000 | ||||||||
| I | 134 (25.7) | 66 (38.8) | 17 (20.7) | 1 (5.9) | 20 (21.5) | 12 (12.0) | 6 (35.3) | 12 (27.9) | |
| II | 306 (58.6) | 87 (51.2) | 61 (74.4) | 10 (58.8) | 57 (61.3) | 61 (61.0) | 6 (35.3) | 24 (55.8) | |
| III | 82 (15.7) | 17 (10.0) | 4 (4.9) | 6 (35.3) | 16 (17.2) | 27 (27.0) | 5 (29.4) | 7 (16.3) | |
| Risk | 0.000b | ||||||||
| Low | 24 (4.6) | 19 (11.2) | 4 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | |
| Inter | 359 (68.8) | 140 (82.4) | 73 (89.0) | 8 (47.1) | 54 (58.1) | 49 (49.0) | 11 (64.7) | 24 (55.8) | |
| High | 139 (26.6) | 11 (6.4) | 5 (6.1) | 9 (52.9) | 39 (41.9) | 51 (51.0) | 6 (35.3) | 18 (41.9) | |
| Survival | 0.015a | ||||||||
| Died | 24 (12.3) | 4 (6.3) | 1 (2.8) | 0 (0.0) | 6 (21.4) | 8 (24.2) | 0 (0.0) | 5 (22.7) | |
| Survived | 171 (87.7) | 59 (93.7) | 35 (97.2) | 9 (100) | 22 (78.6) | 25 (75.8) | 4 (100) | 17 (77.3) | |
| Recurrence | 0.881a | ||||||||
| No Relapse | 192 (98.5) | 61 (96.8) | 36 (100) | 9 (100) | 28 (100) | 32 (97.0) | 4 (100) | 22 (100) | |
| Relapse | 3 (1.5) | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) | |
Fisher exact test;
Likelihood Ratio.
Figure 2.
Photomicrographs of the TNB+ subtype. A. Stained by H&E method showing invasive BC, TNP (magnification of 20×). B. Photomicrograph indicates CK5/6 positivity of tumor cell cytoplasm and/or cytoplasmic membrane (magnification of 20×). C. Cytoplasmic membrane of tumor cells was positive for EGFR (magnification of 20×).
Regarding the basic clinical features, the frequency distribution of age groups, young and older BC in the molecular subtypes revealed a significant difference (P<0.001 or 0.05). TNB- breast cancer was more common among women under 40 and those 40 to 49 years old (29.4% and 41.2%, respectively), and presented only in the younger BC group (100.0%). The opposite trends were observed in patients 60-69 and ≥70. LAHH and LUMA were more common than the remaining subtypes, with percentages of 35.3% and 11.8%, respectively in LAHH; and 21.2% and 10.6%, respectively, in the LUMA subgroup. Similarly, a difference in the tumor size in the molecular subcategories was significant (P<0.05). HER2 subtype accounted for the lowest proportion in patients with up to 2 cm tumor (35.0%). On the contrary, in the large tumor group (more than 5 cm in size), TNB- was more common than other subgroups, amounting to 11.8%. Nevertheless, there was no significant difference in tumor location in the seven molecular subtypes (P>0.05). Differences among molecular subtypes concerning characteristics of pTNM and risk category were observed (P<0.001). In stage I BC, LUMA was obtained at the highest rate (38.8%); meanwhile, LUMB accounted for the largest percentage in stage II (74.4%). By contrast, TNB- was observed in the percentage of cases with stage III (35.3%). Considering breast cancer risk stratification, LUMA’s frequency was the most common (11.2%) in low-risk BCs, and no cases were seen in the TNB-, TNB+, HER2, and LAHH groups in this risk category. While a moderate risk, LUMB had the highest prevalence, with 82.4%. BC women with the TNB- and HER2 subtypes of the high-risk group had higher rates (52.9% and 51.0%, in turn), as opposed to the LUMA and LUMB. These subgroups had very low percentages of 6.4% and 6.1%, respectively.
Table 1 displays the relationships between histopathologic measures and biological groups. There were significant differences between the seven subtypes. Some parameters, such as histopathologic type, histologic grade, lymph node status, NPI, LVI, and nucleus proliferative index (Ki67), had a p-value lower than 0.001. In BC specific types, the mucinous type is illustrated only in LUMA and LUMB. Meanwhile, TNB- subgroup had a higher prevalence in the other specific types, such as medullary or metaplastic types (17.6%). The percentage of cases with low histologic grade in the LUMA group was significantly higher in other groups with 19.4%. In grade II BCs, LUMB accounted for the highest proportion (50.0%). On the contrary, TNB-, TNB+, and HER2 were the most common in the high-grade BCs, being 76.5%, 69.9%, and 67.0%, respectively. Concerning the lymph node status, LUMA was rare in metastatic axillary lymph nodes, accounting for 78.2%. LUMB had a high rate (63.4%). In BC patients with more 3 positive lymph nodes, TNB- subtype was observed at highest frequency (52.9%), and the percentage of LUMA and LUMB was the lowest (5.9% and 6.1%, respectively). Assessment of Nottingham Prognostic Index (NPI), in the BC patients with a favorable index, showed that both LUMA and LAHH were at the higher prevalences, representing 35.3% and 30.2%, respectively. Nevertheless, HER2 and TNB- subgroups were less common, with percentages of 10.0% and 11.8%, respectively. The opposite trend was observed with a poor NPI. TNB- and HER2 were more common than the remaining subtypes (52.9% and 37.0%, respectively); meanwhile, LUMA and LUMB were less common at 11.8% and 13.4%, respectively. In moderate NPI, the rate of LUMB was the largest (63.4%). When evaluating mitotic proliferation, LUMA tumors almost always exhibited a low Ki67 index, accounting for 71.2%. Conversely, TNB+, TNB- and LAHH, HER2, and LBHH were greater in the high Ki67 group, being 65.6, 64.7 (both TNB- and LAHH), 64.6%, and 60.5%, respectively.
For survival, it was clear that survival was different among the molecular categories of BC. The proportion of the HER2 positive patients with death was the largest (24.2%). LBHH and TNB+ accounted for 22.7% and 21.4%, respectively. The differences of IHC classification and survival were statistically significant (P<0.05). Three patients had recurrence; LUMA occurred in two cases (3.2%), and one was a HER2 subtype (3.0%). However, the difference was not significant (P>0.05).
Multivariate analysis was performed to demonstrate whether the molecular subtypes and several other measures that were of prognostic value by univariate analysis suggested being independent prognostic factors. Nevertheless, Table 3 revealed they were not independent prognostic indicators.
Table 3.
Estimated hazard ratios (HRs) for OS and DFS-multivariate analysis
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
|
|
|
|||
| HR | p-value | HR | p-value | |
| Molecular subgroup | 0.450 | 0.277 | ||
| LUMA vs other | 0.646 | 0.552 | 0.878 | 0.844 |
| LUMB vs other | 0.236 | 0.201 | 0.227 | 0.188 |
| TNP vs other | 0.722 | 0.599 | 0.735 | 0.620 |
| HER2 vs other | 1.374 | 0.586 | 1.714 | 0.346 |
| LVI | 2.073 | 0.202 | 1.127 | 0.797 |
| Present vs absent | ||||
| NPI | 1.027 | 0.973 | 0.941 | 0.934 |
| Poor vs other | ||||
| Histologic Grade | 1.853 | 0.263 | 2.037 | 0.158 |
| III vs other | ||||
| Lymph node status | 1.986 | 0.356 | 2.507 | 0.220 |
| >3 vs 0-3 nodes | ||||
| Risk group | 2.231 | 0.245 | 1.747 | 0.399 |
| High vs other | ||||
| TNM | 0.873 | 0.804 | 0.771 | 0.636 |
| III vs other | ||||
Survival
At the end of the follow-up, 12.3% of the patients died. The mean five-year OS of patients with breast cancer who underwent surgery were 84.6±1.5 months. Meanwhile, the median DFS was lower at 83.8±1.5 months. Overall survival in patients with luminal A and B subtypes was significantly longer than in those with the remaining subtypes (87.6±2.1 and 86.8±1.1 months, respectively). At the same time, HER2 had the lowest mean OS 76.8±4.5 months). Similarly, the average DFS of LUMA and LUMB displayed a longer period of 86.0±2.3 and 86.8±1.1 months, respectively. However, the mean for HER2 subtype was still the lowest (75.6±4.6 months).
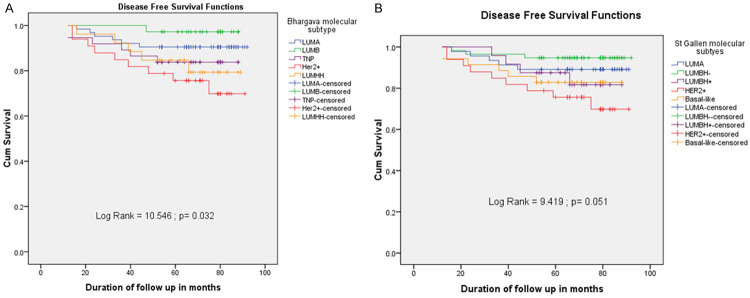
OS curves, according to molecular subtype, are summarized in Figure 3. Survival in patients with LUMA subtype was significantly longer than in those with other subtypes. Figure 3A demonstrates that the log-rank test for equality of overall survivor function showed a significant difference between the IHC subgroups (P<0.05). It was similar to the OS of St. Gallen 2013 molecular subtypes (Figure 3B). Patients who were classified in LUMB and LUMA had a better prognosis, with an OS rate in the five-year follow-up of 97.2% and 93.7%, in turn. It is clear that TNP and LUMHH BCs were present in a lower proportion of OS (83.8% and 79.3%, respectively), and HER2 accounted for the lowest frequency at 72.6%. The DFS curves, according to Bhargava IHC categories, are illustrated in Figure 4A. The difference was significant between DFS curves according to molecular subtype (P<0.05). The DFS prevalence in the five-year follow-up of the BC patients with the LUMB was the highest, accounting for 97.2% and the percentage of LUMA was the second (90.5%). On the contrary, the DFS rate of HER2 was lower, at 69.8%. LUMHH and TNP subgroups were also at a lower proportion than LUMA and LUMB (79.3% and 83.8%, respectively). The DFS findings were the same as the DFS of St. Gallen 2013 molecular subcategories (Figure 4B). However, the differences between DFS curves and St. Gallen 2013 molecular subgroups were not significant (P>0.05). Assessment of OS and DFS, according to Bhargava IHC subtypes, revealed that the prognostic order of molecular subgroups was LUMB ≥ LUMA > TNP > LUMHH > HER2. Similarly, for Gallen 2013 molecular classification, this order was LUMBH- > LUMA > Basal-like ≥ LUMBH+ > HER2.
Figure 3.
A. Five-year relative overall survival of expression of Bhagarva molecular subtypes for invasive breast cancer. B. Five-year relative overall survival of St. Gallen 2013 intrinsic categories for infiltrating breast cancer. The Log-rank test indicates that there was a significant difference between the five survival curves of two different classifications.
Figure 4.
Five-year relative disease-free survival of Bhagarva molecular subgroups in invasive breast cancer (A). Chart (B) shows five-year relative disease free survival of St. Gallen 2013 intrinsic categories for infiltrating breast cancer. The Log-rank test demonstrates a significant difference between the five survival curves for Bhagarva molecular classification and St. Gallen 2013 stratification, as well.
Discussion
Surrogate gene analysis is widely applied to the molecular classification of BC with IHC markers [9,12-15,21,27,28]. Therefore, based on gene profiling, Bhargava used the IHC method to formulate criteria for classifying molecular types of BC [24]. This subgrouping is necessary to study endocrine receptors’ application, the co-expression of endocrine receptors, and HER2 in assessing response to BC treatment, prognosis, and survival. By using the simple IHC criteria of Bhargava, the study findings demonstrated that LUMA, which expressed high ER (≥200 scores), had the highest rate, and next to the HER2 subtype, TNB+, LUMB; the remaining subtypes were at lower percentages (LBHH, LAHH, and TNB-). The current findings were similar to Bhargava’s study [10]. The different frequency of molecular subtypes, compared to the previous studies, might be explained by the method and criteria of the IHC interpretation, but LUMA was still observed. Deyarmin and associates [29] have suggested that the classification of ER-low tumors as Luminal may be inappropriate, especially LUMA. Bhargava hypothesis echoes that of Cheang and coworkers [30], but the luminal category of BC has been subdivided into LUMA, LUMB, LAHH, and LBHH based on ER expression level and HER2 status. The present research investigated the prevalence of various molecular subtypes in BC patients and also evaluated the differences in clinicopathologic characteristics between these subtypes. The findings showed that there were significantly different trends between most clinicopathologic features, except tumor location, and younger vs. older BC patients, and the different molecular subtypes had a p-value <0.05 or 0.001. Among IHC subtypes, LUMA had the strongest correlation with good prognostic measures. These findings were also demonstrated in many of the previous cohorts. Compared to LUMA, the BC patients with LUMB are characterized by a higher proliferation index, larger tumor size, lower differentiation, higher positive lymph node, and poorer NPI. Clinically, luminal B tumors also had a more advanced pTNM stage, higher risk category. Our previous study was conducted on the same participants of the present work has revealed three main risk categories of BC had adistinct OS and DFS, and showed the adverse clinicopathologic features [31]. Because of the complexity of immunophenotypes in BC, tumors with HER2-positive and positive endocrine receptors should be divided into separate hybrid groups called the HER2 hybrid luminal groups [10,24]. The luminal B type, according to other authors with the positive hormonal receptor and HER2+, is classified as luminal A and B hybrid-HER2 subtypes (LAHH and LBHH) according to Bhargava’s criteria. These types show a worse prognosis than LUMA and LUMB. Regarding the clinicopathologic characteristics, luminal hybrid-HER2 subtypes (LAHH and LBHH) have intermediate prognostic values between group of endocrine receptor-positive tumors (LUMA and LUMB) and endocrine receptor-negative BCs (TNB-, TNB+, and HER2 subtypes).
Kreike et al. showed that, based on the gene-expression profiling, basal-like subtype tumors (classified as TNP tumors in their study) are heterogeneous and can be subdivided into at least five distinct subtypes [32]. An efficient panel of antibodies has been demonstrated for detecting the basal-like variant among triple-negative carcinomas is CK5/6, EGFR, CK14, CK17, and vimentin [14,33]. The previous studies showed that including positive EGFR, and CK5/6 markers [14,33,34] for the basal subtype results in a significantly better identification of a high-risk group [10,24,30,35,36], whose outcome more closely matches that expected by gene-expression profiling [4,5,7] than was achieved using a triple-negative (ER-PR-HER2-) definition. Bertucci et al. also revealed that TNB+ is a more homogeneous group than TNP. The incomplete concordance between TNB+ and TNP has been reported by using various IHC definitions, including the ER-, HER2-, EGFR+, and/or CK5/6+ IHC profile, which is currently considered to be the most reliable definition [37]. By adding these markers to identify the basal phenotype to the set of markers in clinical use, it was possible to subdivide TNP cases into a “true basal” group defined as HER2 negative, absent ER, and either EGFR or CK5/6 positive (TNB+) which exhibited less benefit from anthracyclines than the group negative for all of these markers (TNB-) [30]. TNB- type is the normal or unclassified breast type following other classifications [24]. According to Bhargava’s classification, the molecular types with various clinical manifestations, histopathology, and immunophenotypes were identified to apply suitable therapy regimens. In Engstrøm’s study, these two subtypes had significantly differing BC survival [11].
In the present work, the prevalence of TNB+ was more common than TNB- subtype. The frequencies of TNB- and TNB+ have ranged from 17.1% to 30.5% and 8.0% to 55.7%, respectively, depending upon the definition or criteria used [10,38]. In Cheang et al.’s data from 3.744 cases, 17% were TNP, and 9% were TNB+, using the five-marker method [30]. The frequent difference might be explained by the method and the definition or criteria of the IHC interpretation. Previous studies have shown that the expression of basal markers (basal cytokeratins and EGFR) in TNP also correlates with a worse prognosis and identifies a clinically distinct subgroup within the TNP BCs [30,39,40]. Similarly, Cheang’s results from the multivariable Cox regression analyses strongly suggest that, among the triple-negative cases, a poor prognosis is conferred almost entirely to the subset of tumors that are positive for EGFR or basal cytokeratins (TNB+) [30]. Kim et al. also reported the clinicopathologic significance of the TNB+ based on the expression of basal cytokeratins indicating that TNB+ were associated with high histologic and/or nuclear grades. However, there were no significant survival differences between TNB+ and those of other subtypes [41]. On the contrary, Engstrøm et al. illustrated the five negative phenotypes (TNB-) subtypes had poorer prognoses, although they comprised a higher proportion of histologic grade 2 tumors [11]. In most previous studies, the basal status of many TNP was thought to confer a poorer clinical outcome when compared to non-basal TNP; however, our results suggested that the TNB- may be primarily responsible for poor clinical outcomes such as large tumor size, high grade, metastasis to lymph nodes, poor NPI, advanced stage, or higher risk category. This finding was concordant to Choi et al.’s and Engstrøm et al.’s studies [11,36]. The number of followed-up patients in the TNB- subgroups were very low (9 cases), so it is impossible to compare the prognostic values between TNB accurately- and TNB+ by OS and DFS in this study. With 951 BC cases, Choi et al. observed that TNP showed a poor OS prognosis, showing higher nuclear and/or histologic grade, next to the HER2 subtypes. The worse OS was in TNB- among TNP [36]. Choi et al. also demonstrated the TNP clinicopathologic characteristics were maintained in TNB+ after dividing into two groups (TNB+ and TNB-), but the poor prognosis of the TNP was primarily due to the TNB- [36]. The finding that TNB- does worse with regard to DFS and OS than TNB+ is different from the findings by Cheang et al. [30], Carery et al. [9], Rakha et al. [42], and Choi et al. [36]. The present findings were consistent with the studies mentioned above. Validation studies will reveal whether this finding is consistent or not. This difference might be explained by the fact that TNB+ reportedly benefit from adjuvant chemotherapy rather than TNB- as offered today [11,22,30,36].
Regarding prognosis in terms of survival, the molecular classification of BCs included in the present series yielded results comparable to previous studies [1,11]. Evaluating the death rate of molecular subgroups in 24 BC patients after a follow-up period of up to 92 months, we found that HER2 type accounted for one-third of all cases (8 out of 24 cases), followed by the TNP group (6 cases), LUMHH group with five cases, and the LUMA subtypes with four cases. LUMB was the lowest, in which only one patient died. Concerning the OS and DFS ratios of BC patients according to Bhargava molecular subtypes, the present findings revealed these rates decreased as follows: LUMB > LUMA > TNP > LUMHH > HER2. This order was similar to the molecular subtypes of the St. Gallen 2013 classification, in which the LUMB and LUMHH (Bhargava’s classification) types correspond to the LUMB HER2- and LUMB HER2+ (St Gallen 2013), respectively. According to this scheme, although the LUMA type was behind LUMB. The mean survival months of BC patients with LUMA was higher than LUMB in this study. The reason may be because the number of followed-up BC patients with LUMB was less than half of that with LUMA. We also found that HER2-positive BC patients (LUMHH and HER2) had lower OS and DFS rates than the TNP group. Bhargava et al. showed that, despite having the best response to neoadjuvant chemotherapy, HER2 tumors and triple-negative tumors showed the worst DFS and OS (5-year survival of 65% for stage II and 45% stage III). The survival differences were not apparent among the ER+ tumors but were significantly different between ER+ and ER-negative “molecular” classes [10]. The present findings revealed the lowest OS and DFS rate in the HER2 subtype, next to LUMHH. The finding is consistent with previous reports [1,11,43]. One reason for this difference is that HER2-positive breast cancers often have very poor prognoses [44] without targeted treatment. In our study, only two patients received anti-HER2 regime by trastuzumab (one case of HER2 and one of LUMHH), and both of these patients were alive at the time of last follow-up. However, the use of trastuzumab in the neoadjuvant and adjuvant setting was mainly limited to clinical trials before 2005 and Bhargava’s study as well [10]. Spitale et al. showed that, after the stratification of HER2 subtype based on the treatment with trastuzumab (17 treated versus 20 non-treated patients), the 2-year OS differences became more significant (log-rank test =13.5, P=0.0089). In particular, untreated Her2/neu patients showed the worst survival probability (85%) [1]. In HER2-positive breast cancers, the LUMHH group had a higher proportion of OS and DFS than the HER2 group because LUMHH breast cancer was also treated with adjuvant hormonal therapy. Hayashi et al. demonstrated that the HER2 subtype had the poorest 5-year survival, supporting the significance of ER status in determining survival. It has been shown that despite problems associated with crosstalk between ER and HER2, Luminal B (HER2+) benefits from anti-hormonal treatment [44]. Unfortunately, the present study has not demonstrated an independent prognostic role of the molecular subgroup using both St. Gallen 2013 and Bhagarva’s. This is also a limitation of the current study.
Additional limitations to the current study include the following. Not all participants were followed up because the patient database was not systematically managed on the computer system. Also, patients tend to change their phone numbers frequently in Vietnam. Therefore, it was hard to keep in contact with them after they completed their treatment. Only two HER2 positive patients received anti-HER2 therapy because most Vietnamese patients are poor, and insurance companies do not cover all therapy expenses. Therefore, their families cannot pay for all regimens of the trastuzumab treatment. If all patients had received targeted treatment, their survival rates would have been higher. Continued follow-up and analysis of all patients are planned.
Conclusions
The simple IHC-based categorization of breast cancer was inversely correlated to clinical outcome, with a different OS and DFS. Semiquantitative IHC assays of hormone receptors help better classify breast tumors than a mere positive or negative HR result. These findings suggest that the IHC molecular subtypes could be used to improve therapeutic decisions and provide valuable information for the treatment and prognosis of Vietnamese patients with breast cancer.
Acknowledgements
The authors would like to thank Assoc. Prof. To Van Ta, MD, PhD, who is Head of the biomolecular and pathology center, National Cancer Hospital, and Assoc. Prof. Roanh Dinh Le, MD, PhD, the Director of CREDCA, for their assistance and support with this study. The authors would like to thank ENAGO, USA for their assistance of language edit, too. The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure of conflict of interest
None.
References
- 1.Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20:628–635. doi: 10.1093/annonc/mdn675. [DOI] [PubMed] [Google Scholar]
- 2.Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. 2014;5:412–424. doi: 10.5306/wjco.v5.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falck AK, Fernö M, Bendahl PO, Rydén L. St Gallen molecular subtypes in primary breast cancer and matched lymph node metastases--aspects on distribution and prognosis for patients with luminal A tumours: results from a prospective randomised trial. BMC Cancer. 2013;13:558. doi: 10.1186/1471-2407-13-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 10.Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, Brufsky AM, Lembersky BC, Ahrendt GM. Immunohistochemical surrogate markers of breast cancer molecular classes predict response to neoadjuvant chemotherapy: a single Institutional experience with 359 cases. Cancer. 2010;116:1431–1439. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 11.Engstrøm MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA, Vatten LJ, Bofin AM. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140:463–473. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, members P. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;10:1093. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao YH, Chou MC, Fowler C, Mason JT, Man YG. Breast cancer heterogeneity: mechanism, proof, and implications. J Cancer. 2010;1:6–13. doi: 10.7150/jca.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 15.Tang P, Wang J, Bourne P. Molecular classifications of breast carcinoma with similar terminology and different definitions: are they the same? Hum Pathol. 2008;39:506–513. doi: 10.1016/j.humpath.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Comption CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2010. [Google Scholar]
- 17.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Vijver MJ. WHO classification of tumors of the breast. Lyon, France: IARC; 2012. [Google Scholar]
- 18.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 19.NHS Cancer Screening Programmes jointly with The Royal College of Pathologists. Pathology Reporting of Breast Disease: Joint Document Incorporating the Third Edition of the NHS Breast Screening Programme’s Guidelines for Pathology Reporting in Breast Cancer Screeningand the Second Edition of The Royal College of Pathologists’ Minimum Dataset for Breast Cancer Histopathology. London: NHSBSP Pub. No. 58.; 2005. [Google Scholar]
- 20.Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, Bell J, Elston CW, Robertson JF, Blamey RW, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalinfixed tissue samples. Hum Pathol. 1995;26:291–294. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 21.Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, Möbus V, von Minckwitz G, Loibl S, Beckmann MW, Blohmer JU, Costa SD, Decker T, Diel I, Dimpfl T, Eiermann W, Fehm T, Friese K, Jänicke F, Janni W, Jonat W, Kiechle M, Köhler U, Lück HJ, Maass N, Possinger K, Rody A, Scharl A, Schneeweiss A, Thomssen C, Wallwiener D, Welt A. 13th St. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a german team of experts (Zurich 2013) Breast Care. 2013;8:221–229. doi: 10.1159/000351692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conforti R, Boulet T, Tomasic G, Taranchon E, Arriagada R, Spielmann M, Ducourtieux M, Soria JC, Tursz T, Delaloge S, Michiels S, Andre F. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker study from two randomized trials. Ann Oncol. 2007;18:1477–1483. doi: 10.1093/annonc/mdm209. [DOI] [PubMed] [Google Scholar]
- 23.Wolff DJ, Bagg A, Cooley LD, Dewald GW, Hirsch BA, Jacky PB, Rao KW, Rao PN. Guidance for fluorescence in situ hybridization testing in hematologic disorders. J Mol Diagn. 2007;9:134–143. doi: 10.2353/jmoldx.2007.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhagarva R, Esposito NN, Dabbs DJ. Immunohistology of the Breast. In: Dabbs DJ, editor. Diagnostic immunohistochemistry: theranostic and genomic applications. USA: Saunders; 2010. [Google Scholar]
- 25.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury KR, Yagle KJ, Swanson PE, Krohn KA, Rajendran JG. A robust automated measure of average antibody staining in immunohistochemistry images. J Histochem Cytochem. 2010;58:95–107. doi: 10.1369/jhc.2009.953554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adly S, Hewedi IH, Mokhtar NM. Clinicopathologic significance of molecular classification of breast cancer: relation to nottingham prognosis index. J Egypt Natl Canc Inst. 2010;22:209–215. [PubMed] [Google Scholar]
- 28.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deyarmin B, Kane JL, Valente AL, Laar Rv, Gallagher C, Shriver CD, Ellsworth RE. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 30.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 31.Chu NV, Quang NT, Ha VTN, Huyen PT, Hoa NTP, Duong NN, Roanh LD. Application of St gallen categories in predicting survival for patients with breast cancer in vietnam. Cancer Control. 2019;26:1–10. doi: 10.1177/1073274819862794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreike B, Kouwenhove MV, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 34.Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, Chang-Claude J, Hamann U, Cornelisse CJ, Devilee P, Beckmann MW, Nestle-Krämling C, Daly PA, Haites N, Varley J, Lalloo F, Evans G, Maugard C, Meijers-Heijboer H, Klijn JG, Olah E, Gusterson BA, Pilotti S, Radice P, Scherneck S, Sobol H, Jacquemier J, Wagner T, Peto J, Stratton MR, McGuffog L, Easton DF Breast Cancer Linkage Consortium. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 35.Bhargava R, Beriwal S, McManus K, Dabbs DJ. CK5 is more sensitive than ck5/6 in identifying the “basal-like” phenotype of breast carcinoma. Am J Clin Pathol. 2008;130:724–730. doi: 10.1309/AJCP3KFF1LTYWQIY. [DOI] [PubMed] [Google Scholar]
- 36.Choi YL, Oh E, Park S, Kim Y, Park YH, Song K, Cho EY, Hong YC, Choi JS, Lee JE, Kim JH, Nam SJ, Im YH, Yang JH, Shin YK. Triple-negative, basal-like, and quintuple-negative breast cancers: better prediction model for survival. BMC Cancer. 2010;10:507. doi: 10.1186/1471-2407-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 38.Fadare O, Tavassoli FA. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol. 2007;14:358–373. doi: 10.1097/PAP.0b013e31814b26fe. [DOI] [PubMed] [Google Scholar]
- 39.Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan DS, Marchió C, Jones RL, Savage K, Smith IE, Dowsett M, Reis-Filho JS. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Rakha EA, Tan DS, Foulkes WD, Ellis IO, Tutt A, Nielsen TO, Reis-Filho JS. Are triple-negative and basal-like breast cancer synonymous? Clin Cancer Res. 2008;14:618–619. doi: 10.1158/1078-0432.CCR-07-1943. [DOI] [PubMed] [Google Scholar]
- 43.Hadizadeh M, Arani HZ, Olya M. Expression of breast cancer subtypes based on the most important biomarkers: comparison of clinicopathological factors and survival. Iran Red Crescent Med J. 2018;20:e57931. [Google Scholar]
- 44.Hayashi N, Niikura N, Yamauchi H, Nakamura S, Ueno NT. Adding hormonal therapy to chemotherapy and trastuzumab improves prognosis in patients with hormone receptor-positive and human epidermal growth factor receptor 2-positive primary breast cancer. Breast Cancer Res Treat. 2013;137:523–531. doi: 10.1007/s10549-012-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]