Abstract
Background: To explore the need for the high-risk and general population to undergo endoscopy and the best age for these two groups to do so. Material and Methods: Data on 35,525 patients who underwent endoscopy in the Endoscopic Center of Shanxi Cancer Hospital and associated medical group hospitals from January 2016 to December 2019 were collected. Two aspects of the high-risk and general population were analyzed retrospectively: 1. The detection rate of precancerous diseases. 2. The difference and distribution of the detection rate in different genders, different ages, and different pathologic types. Results: A total of 35,525 patients, 24,185 in the general population and 11,340 in the high-risk population, were examined by electronic gastroscopy and colonoscopy simultaneously. Of these, 20,659 were men and 14,866 were women. The detection rate of gastric diseases (gastric cancer, gastric polyp, gastric ulcer, chronic atrophic gastritis) in the general population was 9.27%, and that in the high-risk population was 25.18%. The detection rate of colonic polyps was 53.76% in the general population and 56.77% in the high-risk population. Conclusion: Both the high-risk and the general population should consider gastroscopy and colonoscopy as routine physical examination items. Routine gastroscopy is highly recommended for the high-risk population. The general population should pay close attention to their colonoscopy results. The best screening age for both populations is 40 years old and above.
Keywords: High-risk population, general population, digestive endoscopy, precancerous diseases
Introduction
Digestive tract cancer accounts for 15% of the cancer incidence rate and 19.3% of the death rate [1]. Early detection of gastrointestinal cancer is essential to reduce the global cancer burden [2]. The routine method of clinical diagnosis is gastroscopy or colonoscopy, followed by biopsy and histopathology. This process is regarded as the gold standard of clinical diagnosis [2]. Endoscopy can diagnose precancerous lesions and early cancer with relatively low invasiveness, thus improving the survival rate of patients [3]. People with high-risk factors for gastric cancer and colon cancer are more likely than others to develop those diseases. Many researchers studying gastrointestinal diseases focus on either high-risk groups or groups without risk factors. However, for the prevention and treatment of gastric cancer and colon cancer, it is helpful to know the diseases of the stomach and precancerous cancer in the general population and the high-risk population. Data on 35,525 patients who underwent gastroscopies and colonoscopies from January 2016 to December 2019 were collected from the Endoscopic Center of Shanxi Cancer Hospital and associated medical group hospitals. The detection rate of precancerous diseases in the high risk and general population, as well as the distribution according to gender, age, and pathologic classification were analyzed to provide a basis for clinical practice.
Materials and methods
Clinical data: collection
Patients who underwent endoscopy in the Endoscopic Center of Shanxi Cancer Hospital and related medical group hospitals from January 2016 to December 2019 were selected as the study subjects. The clinical data of simultaneous gastroscopy and colonoscopy were screened out. Of the 35,525 patients who underwent gastroscopy and colonoscopy, 24,185 were in the general population and 11,340 were in the high-risk population; 20,659 were male and 14,866 were female; 4,556 were ≤40 years old, 5,155 were 40-45 years old, 5,828 were 46-50 years old, 6,401 were 51-55 years old, 6,753 were 56-60 years old, and 6,832 were ≥61 years old.
Inclusion criteria for high-risk groups
Shanxi Cancer Hospital is a large-scale early screening base allocated by the state. Every year, high-risk groups are screened out through the community for physical examination.
Inclusion criteria for the high-risk population undergoing colonoscopy
Subjects with factors such as family history of colon cancer, long-term history of drinking and smoking, history of intestinal polyps, change of defecation habits and characteristics, positive mucous and bloody stools, and occult blood in stool are at high risk. In contrast to the high-risk population, the general population is composed of people without clinical manifestations such as hematochezia, change of defecation habits, and abdominal pain.
Inclusion criteria for high-risk groups undergoing gastroscopy: (1) Long-term eating of salted, fumigated, barbecued, fried, and high-salt food. (2) Long-term smokers and drinkers. (3)Long-term irregular diet, work, and rest; staying up late; being cautious, stressful, and anxious. (4) Long-term chemical workers, such as rubber factory workers. (5) Patients with a family history of cancer, especially those with immediate relatives who have had digestive tract tumors. (6) Gastric symptoms occur often. (7) First diagnosis. (8) Helicobacter pylori-positive patients.
Exclusion criteria: (1) Non-first-diagnosed patients. (2) Patients who have been diagnosed with precancerous lesions such as chronic atrophic gastritis and colon polyps. (3) Patients with diseases who cannot tolerate endoscopy, including coagulation dysfunction, renal insufficiency, severe cardiopulmonary disease, and fluorescein sodium allergy. (4) Pregnant and lactating women.
Precautions before examination
Precautions for gastroscopy patients
The day before the examination, smoking was prohibited to reduce the secretion of gastric acid, to prevent the patient from coughing when intubated, and to facilitate observation. Patients were instructed to fast for at least 6 hours before the examination. During the examination, two dyclonine hydrochloride gels, one bottle of dimethyl silicone oil powder were used.
Precautions for colonoscopy
One bag compound polyethylene glycol electrolyte powder (Heshuang) + 1000 ml cold water was taken on the day before the examination at 7:00 p.m. (specification 68.56 g/bag), and 2 bags compound polyethylene glycol electrolyte powder (Heshuang) + 1 bottle of dimethyl silicone oil powder + 2000 ml cold water were taken at 10:00-11:30 a.m. (specification 68.56 g/bag) on the day before the examination until water-like stool was discharged (if stool was pasty, increase water consumption or clean enema, properly cooperate with ambulation during medication, which can increase intestinal peristalsis and reduce abdominal distension). One Obucaine hydrochloride gel (or two dyclonine hydrochloride slurry) was routinely used during the examination. Patients were asked to eat less solid and semi-liquid food at lunch the day before the examination and to fast and drink more water in the morning and noon of the examination day to fully clean the bowel in preparation for the examination.
Requirements for operators
To reduce errors caused by differences in the physicians’ professional ability, all examinations were performed by the chief physician of the hospital.
Operational requirements
The gastroscopy and colonoscopy were not performed under anesthesia. A GIF-H260 or GIF-HQ290 high-definition or high-definition bifocal gastroscope was used for the gastroscopy. An OLYMPUS290 high definition colonoscope was used for the colonoscopy.
Research methods
The gender, age, microscopic diagnosis results, type of pathology, and other data of the subjects were recorded in detail, and the detection rate of various precancerous lesions, their differences in different age groups, different genders, and their pathological type distribution were analyzed. The study protocol was approved by the Clinical Research Ethics Committee of Shanxi Cancer Hospital.
Statistical methods
SPSS 26.0 statistical software was used to analyze the clinical data. The counting data were expressed by frequency and percentage. χ2 tests were used to compare the differences between groups. P<0.05 was considered significant.
Results
Gastroscopy results
A total of 35,525 patients underwent electronic gastroscopy: 11,340 were high-risk, 24,185 comprised the general population that were not high-risk. Gastric diseases were detected in 5,098 (14.35%) out of the 35,525. The proportion of patients with gastric cancer, gastric polyps, gastric ulcer, and chronic atrophic gastritis was 0.79%, 8.54%, 3.67%, and 1.35%, respectively. In the 11,340 high-risk patients, there was a total of 2,855 cases of gastric diseases (25.18%) detected. The proportion of these individuals with gastric cancer, gastric polyp, gastric ulcer, and chronic atrophic gastritis was 1.50%, 14.80%, 6.68%, and 2.20%, respectively. In the general population comprised of 24,185 individuals, there was a total of 2,243 cases of gastric diseases (9.27%) detected. The proportion of these individuals with gastric cancer, gastric polyps, gastric ulcer, and chronic atrophic gastritis was 0.45%, 5.61%, 2.26%, and 0.95%, respectively. The detection rate of precancerous diseases in the high-risk population was higher than in the general population, and the difference between the two groups was significant (χ2=8.335, P<0.05) (Table 1).
Table 1.
Distribution of gastric diseases in different populations [n]
| Grouping (Group) | Number of persons examined | gastric cancer | gastric polyps | gastric ulcer | chronic atrophic gastritis |
|---|---|---|---|---|---|
| high-risk population | 11340 | 170 | 1678 | 757 | 250 |
| general population | 24185 | 110 | 1356 | 547 | 230 |
Colonoscopy results
The detection rate of colonic polyps was 53.76% in the general population and 56.77% in the high-risk population. The difference was significant (χ2=28.343, P<0.05). The detection rate of colonic polyps between the high-risk and general populations was significantly different, Figure 1.
Figure 1.

Distribution of gastric diseases in different populations, *P<0.05.
Relationship between colonic polyps and gender
24,185 cases in the general population underwent electronic colonoscopy: 60.14% (8,369/13,915) were males, and 45.10% (4,632/10,270) were females. Of 11,340 cases in the high-risk population, 62.50% (4,215/6,744) were males and 48.37% (2,223/4,596) were females. The detection rate of colonic polyps in males was significantly higher than in females in the different populations. There was a significant difference between the two groups (general population: χ2=537.784, P<0.0001; high-risk population: χ2=222.426, P<0.0001), Figure 2.
Figure 2.

Distribution of colonic polyps of different sexes in different populations. A: Distribution of colonic polyps of different sexes in general population; B: Distribution of colonic polyps of different sex in high risk population, ****P<0.0001.
Relationship between colonic polyps and age
The general population was stratified by age into: ≤40 years old, 40-45 years old, 46-50 years old, 51-55 years old, 56-60 years old, and ≥61 years old, in which the proportion of colonic polyps detected was 35.34% (1,235/3,495), 46.12% (1,717/3,723), 49.71% (2,024/4,072), 55.82% (2,394/4,289), 63.79% (2,815/4,413), and 67.16% (2,816/4,193) respectively. The high-risk population was also stratified by age into: ≤40 years old, 40-45 years old, 46-50 years old, 51-55 years old, 56-60 years old, and ≥61 years old, in which the proportion of colonic polyps detected was 36.10% (383/1,061), 47.07% (674/1,432), 50.74% (891/1,756), 56.01% (1,183/2,112), 64.66% (1,513/2,340) and 67.98% (1,794/2,639) respectively. The distribution of colonic polyps in both groups at different ages showed that the detection rate of colonic polyps increased with age, which can be seen in Figure 3.
Figure 3.
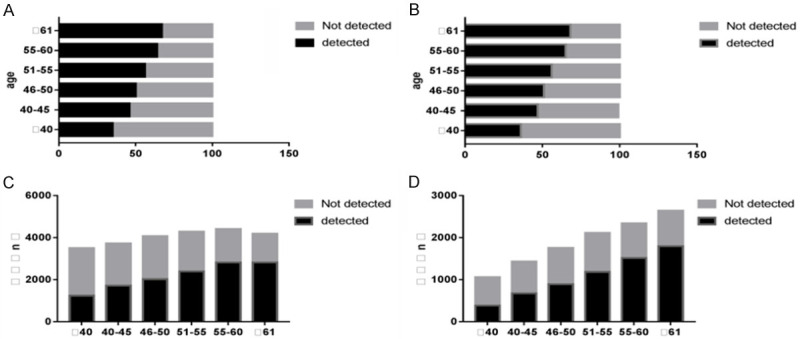
Distribution of colonic polyps in different age groups in different populations. A: 100-slice graph, distribution of colonic polyps in different age groups in general population; B: 100-slice chart, distribution of colon polyps in different age groups in high-risk population; C: Bar chart, distribution of colonic polyps in different age groups in the general population; D: Bar chart, distribution of colonic polyps in various age groups in high-risk groups.
Relationship between pathologic classification of colonic polyps and age
Adenomatous polyps are common in different age groups. The proportion of adenomatous polyps and cancerous polyps increases with age (Spearman Correlation: 1, P<0.01), while the proportion of inflammatory polyps and proliferative polyps declines (Spearman Correlation: -1, P<0.01). The composition ratio of various pathologic types in the same age group is similar in the general population and in the high-risk population (Tables 2, 3).
Table 2.
Pathologic classification of colonic polyps in different age groups of the general population/case (%)
| Grouping (age) | Number of detected cases | Adenomatous | Inflammatory | Proliferative | Cancerous |
|---|---|---|---|---|---|
| ≤40 | 1235 | 678 (54.90) | 241 (19.51) | 315 (25.51) | 1 (0.08) |
| 40-45 | 1717 | 996 (58.01) | 303 (17.65) | 370 (21.55) | 48 (2.80) |
| 46-50 | 2024 | 1243 (61.41) | 322 (15.91) | 354 (17.49) | 105 (5.19) |
| 51-55 | 2394 | 1532 (63.99) | 313 (13.07) | 387 (16.17) | 162 (6.77) |
| 56-60 | 2815 | 1858 (66.00) | 309 (10.98) | 434 (15.42) | 214 (7.60) |
| ≥61 | 2816 | 1980 (70.31) | 211 (7.49) | 330 (11.72) | 295 (10.48) |
| χ2 | 526.980 | ||||
| P | <0.001 |
Table 3.
Classification of colonic polyps in different age groups of the high-risk population/case (%)
| Grouping (age) | Number of detected cases | Adenomatous | Inflammatory | Proliferative | Cancerous |
|---|---|---|---|---|---|
| ≤40 | 383 | 217 (56.66) | 77 (20.10) | 85 (22.19) | 4 (1.04) |
| 40-45 | 674 | 403 (59.79) | 127 (18.84) | 111 (16.47) | 33 (4.90) |
| 46-50 | 891 | 561 (62.96) | 142 (15.94) | 126 (14.14) | 62 (6.96) |
| 51-55 | 1183 | 763 (64.50) | 171 (14.45) | 160 (13.52) | 89 (7.52) |
| 56-60 | 1513 | 1023 (67.61) | 181 (11.96) | 163 (10.77) | 146 (9.65) |
| ≥61 | 1794 | 1310 (73.02) | 141 (7.86) | 110 (6.13) | 233 (12.99) |
| χ2 | 288.093 | ||||
| P | 0.033 |
Detection of precancerous diseases in the general population and the high-risk population
The detection rate of gastric diseases was 9.27% in the general population, and 25.18% in the high-risk population. The detection rate of colonic polyps was 53.76% in the general population, and 56.77% in the high-risk population. The difference was significant, as shown in Figure 4.
Figure 4.

Detection of precancerous diseases in the general population and the high-risk population.
Discussion
Gastric and colon cancers are common malignant tumors. With the changes of lifestyle and habits, their incidence is gradually increasing. The incidence of gastric cancer in East Asian countries such as Japan, Korea, and China is higher than in Western countries [4]. The risk of colon cancer, which can pose a serious threat to patients’ life and health, increases with age in both Western and Asian populations [5]. Due to the lack of specific symptoms allowing for early detection of gastric and colon cancer, diagnosis can often be made only at an advanced stage of the disease, resulting in poor prognosis [6]. The best way to improve the survival rate of patients with gastric and colon cancer is early diagnosis and appropriate treatment [7]. The wide use of gastroscopy and colonoscopy for screening has improved the early diagnosis rate of gastrointestinal cancer [8]. For lesions histologically diagnosed as cancerous, endoscopists perform detailed examinations to determine the histologic type, tumor size, presence of ulcers or scars, and depth of invasion to determine treatment indications, i.e., endoscopic or surgical treatment, which greatly reduces the mortality rate of gastric and colon cancer [9]. The development from a precancerous disease or lesion to gastric or colon cancer involves multiple factors and stages. Universal screening with gastroscopy and colonoscopy is of great importance for the early detection of these precancerous diseases or lesions and the resultant early detection and treatment of early cancer. Chronic atrophic gastritis with intestinal metaplasia, dysplasia, gastric polyps, or gastric ulcer, is the main stage of precancerous lesions of gastric cancer. Patients with precancerous diseases have an increased risk of gastric cancer, and the prevalence of these precancerous diseases is closely associated with gastric cancer mortality. Chronic atrophic gastritis and epithelial cell lesions are a long-term process. Early diagnosis of chronic atrophic gastritis with gastric mucosal atrophy or intestinal metaplasia is important for the prevention of gastric cancer [10,11]. Colorectal adenomatous polyps can be considered precancerous lesions because highly proliferative epithelial cells can transform from adenoma to colon cancer [12,13]. Early diagnosis and treatment of colorectal adenomatous polyps depend on colonoscopy, which can significantly reduce the incidence of colon cancer [14]. Early detection and resection of colorectal adenomatous polyps is the key to preventing colon cancer [3].
In both the general population and the high-risk population, the polyp detection rate of male patients was higher than that of female patients. Therefore, special attention should be paid to middle-aged and elderly male patients with colonic polyps. The high prevalence of polyps in males may be related to smoking, drinking, and unhealthy living habits.
With an increase of age, the detection rate of colonic polyps, and the malignant transformation rate both increased significantly [15]. Some guidelines focus on people over 50 years of age for endoscopic screening [16], but studies have found that the incidence of colon tumors in people 40-49 years of age is close to that in people over 50 years of age [17]. A study by the American Cancer Society presented in Barcelona at the 25th United European Gastroenterology Week conference recommended that colorectal cancer screening begin at 45 years of age [18]. Studies have shown that age is one of the most important cancer risk factors. This study can conclude that the detection rate of precancerous lesions of gastric and colon cancer increases with age, suggesting that advanced age is associated with the occurrence of precancerous lesions of stomach and colon cancer. The relationship between aging and carcinogenesis is complex, but increased cancer risk is an inevitable consequence of aging [19]. This study showed that the detection rate of colonic polyps was significantly increased in individuals between 40 and 45 years of age (46.12% in the general population and 47.07% in the high-risk population), suggesting that people aged 40 and over should routinely undergo gastroscopy and colonoscopy. Colonoscopy screening is recommended from age 40. Considering the current situation of our country, we believe that increasing screening efforts, expanding the target screening population, and increasing the training of gastroenterologists can reduce the incidence and mortality rate of colon cancer.
Comprehensive gastroscopic and colonoscopic screening of different populations can provide early detection of precancerous gastrointestinal diseases and lesions, and endoscopic treatment can quickly cut off the cancer pathway, thus reducing the incidence and mortality rate of gastrointestinal tumors. Benign diseases other than precancerous diseases or lesions can also be detected by endoscopy. Although these benign lesions are not at risk of carcinogenesis, the detection of these lesions can provide patients with guidance on lifestyle and dietary habits.
Conclusion
This study shows that the detection rate of precancerous gastric diseases differs greatly between the general population and the high-risk population, while the detection rate of colonic polyps is similar between the two groups. People should routinely undergo gastroscopic and colonoscopic examinations. High-risk groups need more routine gastroscopy. The general population and high-risk groups need routine gastroscopy and endoscopy, and the general population needs to pay attention to colonoscopy so that the transformation to gastric and colon cancers is curtailed, the survival rate improves, and the mortality rate is reduced. Regardless of population, 40 years old or older is the optimal screening age.
Acknowledgements
The authors thank Three-dimensional and multi-level clinical pathway optimization research project for digestive tract cancer (national key research and development plan 2016YFC1303600). The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Disclosure of conflict of interest
None.
References
- 1.Stokłosa P, Borgström A, Kappel S, Peinelt C. TRP channels in digestive tract cancers. Int J Mol Sci. 2020;21:1877. doi: 10.3390/ijms21051877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Yu J, Zhang R, Li X, Zheng W. Two-photon excitation fluorescence lifetime imaging microscopy: a promising diagnostic tool for digestive tract tumors. J Innov Opt Health Sci. 2019;12:115–132. [Google Scholar]
- 3.Saftoiu A, Hassan C, Areia M, Bhutani MS, Bisschops R, Bories E, Cazacu IM, Dekker E, Deprez PH, Pereira SP, Senore C, Capocaccia R, Antonelli G, van Hooft J, Messmann H, Siersema PD, Dinis-Ribeiro M, Ponchon T. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy. 2020;52:293–304. doi: 10.1055/a-1104-5245. [DOI] [PubMed] [Google Scholar]
- 4.Tanahashi T, Yoshida K, Yamaguchi K. Current status and future perspective of the chemotherapy for gastric cancer. Nihon Shokakibyo Gakkai Zasshi. 2018;115:500–506. doi: 10.11405/nisshoshi.115.500. [DOI] [PubMed] [Google Scholar]
- 5.Chung RY, Tsoi KKF, Kyaw MH, Lui AR, Lai FTT, Sung JJ. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol. 2019;59:29–36. doi: 10.1016/j.canep.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Titov SE, Anishchenko VV, Poloz TL, Veryaskina YA, Arkhipova AA, Ustinov SN. Differential diagnostics of gastric cancer and precancerous changes of the gastric mucosa using analysis of expression of six microRNAS. Klin Lab Diagn. 2020;65:131–136. doi: 10.18821/0869-2084-2020-65-2-131-136. [DOI] [PubMed] [Google Scholar]
- 7.Ma TM, Sun LP, Dong NN, Sun MJ, Yuan Y. Protein expression trends of DNMT1 in gastrointestinal diseases: from benign to precancerous lesions to cancer. World J Gastrointest Oncol. 2019;11:1141–1150. doi: 10.4251/wjgo.v11.i12.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. 2020;53:142–166. doi: 10.5946/ce.2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiotani A. Gastric cancer (with special focus on studies from japan) II helicobacter pylori and related virulence factors for gastrointestinal diseases. World J Gastrointest Oncol. 2019;11:1141–1150. [Google Scholar]
- 10.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotiniotis IA, Rokkas T, Furth EE, Rigas B, Shiff SJ. Altered gastric epithelial cell kinetics in Helicobacter pylori-associated intestinal metaplasia: implications for gastric carcinogenesis. Int J Cancer. 2000;85:192–200. [PubMed] [Google Scholar]
- 12.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soylu A, Ozkara S, Alis H, Dolay K, Kalayci M, Yasar N, Kumbasar AB. Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon. BMC Gastroenterol. 2008;8:35. doi: 10.1186/1471-230X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CX, Mao Y, Du J, Xu Y, Zhu Z, Cao H. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol. 2019;19:14. doi: 10.1186/s12876-018-0918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCashland TM, Brand R, Lyden E, de Garmo P CORI Research Project. Gender differences in colorectal polyps and tumors. Am J Gastroenterol. 2001;96:882–886. doi: 10.1111/j.1572-0241.2001.3638_a.x. [DOI] [PubMed] [Google Scholar]
- 16.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 17.Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008;134:1311–1315. doi: 10.1053/j.gastro.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar RR. Cancer in the elderly: is it preventable? Clin Geriatr Med. 2004;20:293–316. doi: 10.1016/j.cger.2004.03.002. [DOI] [PubMed] [Google Scholar]


