Abstract
Glioblastoma is the most aggressive and lethal tumor in the central nervous system in adult and has poor prognosis due to strong proliferation and aggressive invasion capacity. Acidic microenvironment is commonly observed in tumor tissues but the exact role of acidosis in the pathophysiology of glioblastoma and underlying mechanisms remain unclear. Acid-sensing ion channels (ASICs) are proton-gated cation channels activated by low extracellular pH. Recent studies have suggested that ASICs are involved in the pathogenesis of some tumors, such as lung cancer and breast cancer. But the effect of acidosis and activation of ASICs on malignant glioma of the central nervous system has not been reported. In this study, we investigated the expression of ASIC1 in human glioma cell lines (U87MG and A172) and its possible effect on the proliferation and migration of these cells. The results demonstrated that ASIC1 is functionally expressed in U87MG and A172 cells. Treatment with extracellular weak acid (pH 7.0) has no effect on the proliferation but increases the migration of the two cell lines. Application of PcTX1, a specific inhibitor of ASIC1a and ASIC1a/2b channels, or knocking down ASIC1 by siRNA, can abolish the effect of weak acid-induced cell migration. Together, our results indicate that ASIC1 mediates extracellular weak acid induced migration of human malignant glioma cells and may therefore serve as a therapeutic target for malignant glioma in human.
Keywords: Acid-sensing ion channels, malignant glioma, acidic microenvironment, weak acid, proliferation, migration
Introduction
Glioblastoma is a broad category of tumors derived from glial cells that support nerve cells in the brain and spinal cord. Every year, about 100,000 people worldwide are diagnosed with glioblastoma multiforme, a malignant glioma [1]. Although it represents only one to two percent of all newly diagnosed cancers, glioblastoma is one of the most disabling and lethal diseases [2]. The treatment and prognosis of glioblastoma depend on a series of factors, such as age, tumor type, and location of the tumor in the brain. The recurrence and poor prognosis of malignant glioma are mainly due to its strong ability of uncontrolled proliferation and aggressive invasion [3]. The infiltrative growth makes surgical removal very difficult, sometimes impossible. In addition, the high resistance of malignant glioma cells to therapies and the vulnerability of the central nervous system hamper the antitumor interventions [4]. It is hoped that potential new mechanisms and targets can be discovered for effective therapies to prolong the survival of glioblastoma patients.
Acidosis is one of the most common changes of extracellular microenvironment in tumors [5,6]. The pH of normal tissues is kept within tight limits under physiological conditions. In pathological situations, such as during tumor growth, ischemia and inflammation, however, local pH drop and long-lasting acidification develop. An acidosis often occurs in tumors with high metabolic rates owing to blood hypoperfusion, local hypoxia, and increased glycolytic activity [7]. The acidic microenvironment is harmful to most normal cells, but it is often favorable to solid tumor cells although the precise mechanisms remain elusive [7-9].
Acid-sensing ion channels (ASICs) are voltage-insensitive cationic channels activated by extracellular acidification. ASICs form a subfamily of the epithelial sodium channels/degenerin (ENaC/Deg) family. Four different genes encode six ASIC subunits: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4. They can form functional hetero/homotrimeric channels that are activated by acidic pH and inhibited by amiloride, a commonly used non-selective ASIC antagonist [10]. ASICs are expressed with high levels in the nervous system, which are responsible for the acid-evoked currents and act as pH sensors leading to excitation in neurons [11]. Evidence has shown that ASICs play physiological and pathological roles in many processes, such as pain sensation, learning, fear behavior, and neurodegeneration after ischemic stroke [9]. The expression of ASICs in high grade glioma cells has been reported over a decade ago [12], however, the exact role of these channels in the pathogenesis of glioma remains elusive. Although accumulating evidence showed that acidic conditions can affect tumor progression by promoting cell migration, metastasis, invasion and angiogenesis [13-16], the potential mechanism is not well known. In the present study, we demonstrated that weak acid (pH 7.0) promotes the migration of malignant glioma cells, at least in part, by activating ASIC1 channels.
Materials and methods
Reagents and antibodies
Polyclonal anti-ASIC1 antibody for Western blot was a gift from Dr. Xiangming Zha (University of South Alabama College of Medicine, Mobile, Alabama) [17]. Vybrant® MTT cell proliferation assay kit (V-13154) used for cell viability assay was purchased from Thermo Fisher Scientific (MA, USA). SiRNA targeting the human ASIC1 (Product#: NM_001095, siRNA ID: SASI_Hs01_00167899) and SiRNA negative control (SIC001) were synthesized and purified by Sigma-Aldrich (St. Louis, Mo).
Cell culture and transfection
The human glioblastoma cell lines U87MG and A172 were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured with Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), 50 units/ml penicillin and 50 μg/ml streptomycin (PS) at 37°C in humidified atmosphere incubator containing 5% CO2. SiRNAs (targeting the human ASIC1 and negative control) were transfected using Lipofectamine® RNAiMAX reagent (Invitrogen, Carlsbad, CA) in serum free DMEM medium at a final concentration of 10 nM according to the manufacturer’s instructions. After 24 hours of transfection, cells were further grown for 24 hours in growth medium before Western blotting, wound-healing and transwell migration assays.
Western blotting
Cells were lysed using M-PER Mammalian Protein Extraction Reagent with protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Rockford, USA). Samples were then centrifuged at 13200 g for 20 minutes at 4°C, protein concentrations were measured using Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA). The supernatant of lysates was transferred to a new tube with 4 × loading buffer and boiled for 10 minutes. All the samples were separated by 10% SDS-PAGE gel, and then transferred onto PVDF membranes (Millipore, Germany). Primary antibodies were diluted at the following ratios: rabbit anti-ASIC1 (1:1000), and rabbit anti-β-actin (1:8000). Blots were incubated with primary antibodies overnight at 4°C followed by the HRP-conjugated secondary antibodies (Thermo Fisher Scientifc, 1:5000) for 1 hour. The signals were identified using an ECL kit (Millipore, Germany) and the band images were acquired using Image Quant LAS 4000.
Whole-cell patch-clamp recording
Whole-cell currents were recorded by patch-clamp techniques as described in our previous study [18]. The currents were recorded with an Axopatch 200B amplifier and low-pass filtered at 2 KHz, digitized using Digidata 1320 DAC unit. Cells were clamped at a holding potential of -80 mV. The patch pipettes were made from thin walled borosilicate glass micropipettes (1.5 mm diameter, World Precision Instruments, Sarasota, FL) by a micropipette puller (PP83; Tokyo, Japan). The range of pipette resistance was 3-5 MΩ when filled with intracellular solution contained 140 mM CsF, 1 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, 11 mM EGTA, 2 mM tetraethylammonium chloride, and 4 mM MgATP; pH 7.3 adjusted with CsOH, 290 to 300 mOsm adjusted with sucrose. Cells were perfused with an extracellular fluid (ECF) contained 140 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Glucose, and 10 mM HEPES; pH 7.4 and 6.0 adjusted with NaOH, 320-330 mOsm. PcTX1 (Peptide International) was dissolved in ddH2O at 20 μM stock solution before adding to ECF. The data were recorded and analyzed with pClamp and Sigma Plot software.
Cell viability assay
Cells were seeded in 96-well plates overnight and then incubated with pH 7.4 or 7.0 ECF in the absence or presence of 20 nM PcTX1 for 3 h, and then changed to culture medium containing 5% FBS incubating for 24 h, 48 h and 72 h. After the treatments, cell viability was measured by MTT Cell Proliferation Assay Kit according to the manufacturer’s instructions. The absorbance values at 540 nm were detected by spectrometer (Molecular devices).
Wound-healing assay
Wound-healing assay was performed as described in our previous studies [19]. Cells were seeded into a 24-well plate and grown to ~80% confluence. The cell monolayer was scratched with 200 μl sterile pipette tip and treated with ECF with or without PcTX1 for 3 hours. The original wound areas at 0 hour were photographed with microscope (Olympus FSX100, 4 ×). After 3 hours, cells were then incubated with culture medium containing 1% FBS for 24 hours. 24 hours later, images were taken from the same location of the original wound areas and the migration distance was measured with Image J software. The wound-healing rate was calculated as: the ratio of wound closure = [(Area of original wound at 0 h - Area of wound after healing at 24 h)/Area of original wound at 0 h] × 100%.
Transwell migration assay
Transwell assay was performed as described in our previous studies [19]. The assay is based on two compartments separated by a membrane with an accurately defined pore size. Cell transwell chambers with 8-μm pore size of polycarbonate membrane filters (Corning, USA) were first pre-coated with poly-L-ornithine overnight. Before plating cells, chambers were incubated with serum-free culture medium for 30 minutes. After that, cells were seeded with culture medium containing 1% FBS in the upper-chambers that were immersed to 750 μl whole medium (DMEM with 10% FBS). After incubating for another 24 hours, some cells on the upper surface of the filter migrated through the membrane to the lower surface of the filter. Cells on the upper face of the filter were wiped out thoroughly with cotton swabs, and those attached on the lower surface were fixed with 4% paraformaldehyde for 15 min. They were then stained with 0.5% crystal violet for 10 min, and let the membrane air dry. The cells on the lower surface of the membrane filter were counted under a microscope in 3-4 random fields with 10 × magnification. Cell numbers in each group were normalized to the control groups. Assays were performed in at least 3 independent experiments.
Statistical analysis
The statistical significance was analyzed using the Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). The data were expressed as mean ± SEM. Groups were compared using one-way ANOVA followed by Tukey’s multiple comparison test or unpaired Student’s t test where appropriate. P < 0.05 was regarded as statistically significant.
Results
Functional ASIC1 is expressed in human glioma cells
To determine whether ASIC1 is expressed in human glioma cells, we first investigated the expression of ASIC1 protein in A172 and U87MG cells by Western blotting. Results showed that both of the two glioblastoma cell lines express ASIC1 protein, especially the U87MG cells (Figure 1A). Next, we detected the existence of acid-activated currents and the effect of pharmacologic agents on the currents. Acid-activated currents were recorded by whole cell patch-clamp as described in our previous studies [18]. As shown in Figure 1B, drop of extracellular pH from 7.4 to 6.0 can induce transient inward currents in A172 and U87MG cells. PcTX1 from venom of tarantula Psalmopoeus cambridgei is a specific blocker for homomeric ASIC1a and heteromeric ASIC1a/ASIC2b channels [20], which potently inhibits the proton-gated currents mediated by ASIC1a. For this reason, we confirmed the effect of PcTX1 on acid-activated currents. As shown in Figure 1C and 1D, the peak of acid-activated currents was decreased by 80% after several minutes of PcTX1 (20 nM) perfusion (***P < 0.001, unpaired student’s t test, n=5) indicating that the majority of currents activated by extracellular acid are mediated by ASIC1.
Figure 1.
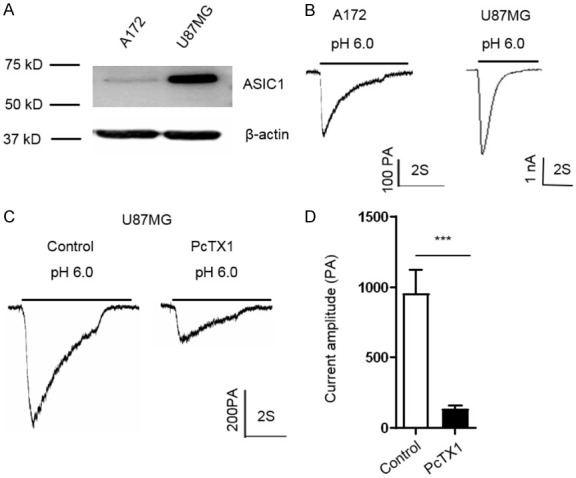
Expression of ASIC1 and electrophysiological characteristics of ASICs currents in glioma A172 and U87MG cells. (A) Representative Western blot bands showing ASIC1 proteins in A172 and U87MG cells. (B) Representative traces showing the ASIC current in A172 and U87MG cells. (C) Representative traces and (D) statistical results showing the inhibitory effect of PcTX1 (20 nM) on ASIC current in U87MG cells. Data are expressed as mean ± SEM. n=5. ***P < 0.001 by Students’ t-test.
Weak acid has no effect on proliferation of human glioma cells
Previous study showed that severe acidosis (e.g. pH 6.0) promotes the proliferation of some type of glioma cells [21]. In this study, we first tested the effect of weak acid on the proliferation of A172 and U87MG cells. We used either pH 7.4 or pH 7.0 solution to pretreat cells for 3 h, and then changed back to medium containing 5% FBS. The cell viability was measured at 24 h, 48 h and 72 h after acid treatment by MTT assay. Results showed that treatment of cells with pH 7.0 solution had no significant effect on the proliferation of A172 cells at 24 h, 48 h and 72 h, and PcTX1 had no influence on the proliferation of A172 cells (one-way ANOVA, n=3, Figure 2A and 2B). Similarly, treatment with weak acid (pH 7.0) and PcTX1 had no clear effect on the proliferation of U87MG cells at different time points (one-way ANOVA, n=3, Figure 2C and 2D).
Figure 2.
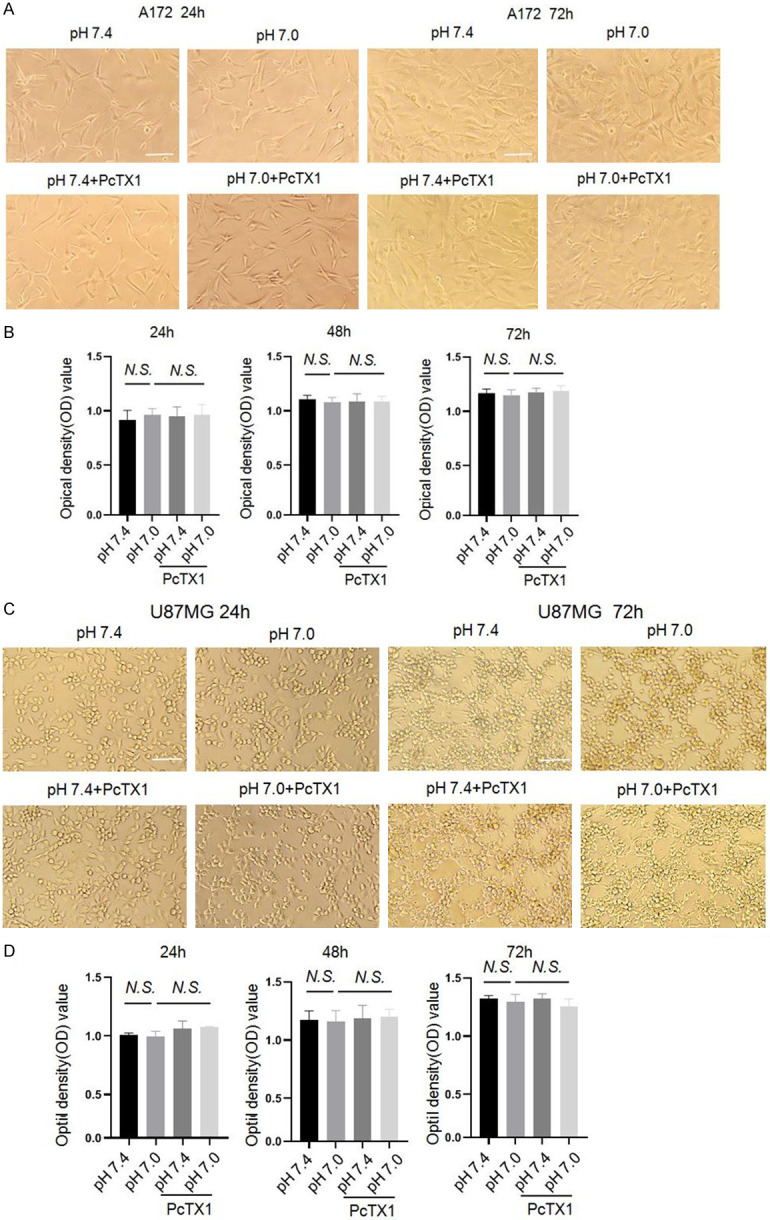
Effects of acidosis and PcTX1 on proliferation of A172 and U87MG cells. (A) Representative micrographs of 24 h and 72 h and (B) statistical results of 24 h, 48 h and 72 h showing the presence or lack of effects of extracellular weak acid pH 7.0 and PcTX1 on the proliferation of A172 cells. (C) Representative micrographs of 24 h and 72 h and (D) statistical results of 24 h, 48 h and 72 h showing the presence or lack of effect of extracellular weak acid and PcTX1 on the proliferation of U87MG cells. Data are expressed as mean ± SEM. n=3. Scale bar =100 µm. One-way ANOVA followed by Tukey’s multiple comparison test.
Weak acid promotes the wound healing of human glioma cells
Next, we investigated the effect of weak acid (pH 7.0) on the migration of A172 and U87MG cells using the wound healing assay. As shown in Figure 3A and 3B, treatment of A172 cells with weak acid (pH 7.0) for 3 h promoted the migration (would healing) by 26% measured at 24 h after the acid treatment. However, in the presence of PcTX1 (20 nM), the acid-induced increase of migration was inhibited (*P < 0.05, **P < 0.01, one-way ANOVA, n=4). Similarly, weak acid (pH 7.0) stimulated the migration of U87MG cells by 67% and PcTX1 (20 nM) blocked the acid-induced change in migration of these cells (*P < 0.05, **P < 0.01, one-way ANOVA, n=3, Figure 3C and 3D).
Figure 3.
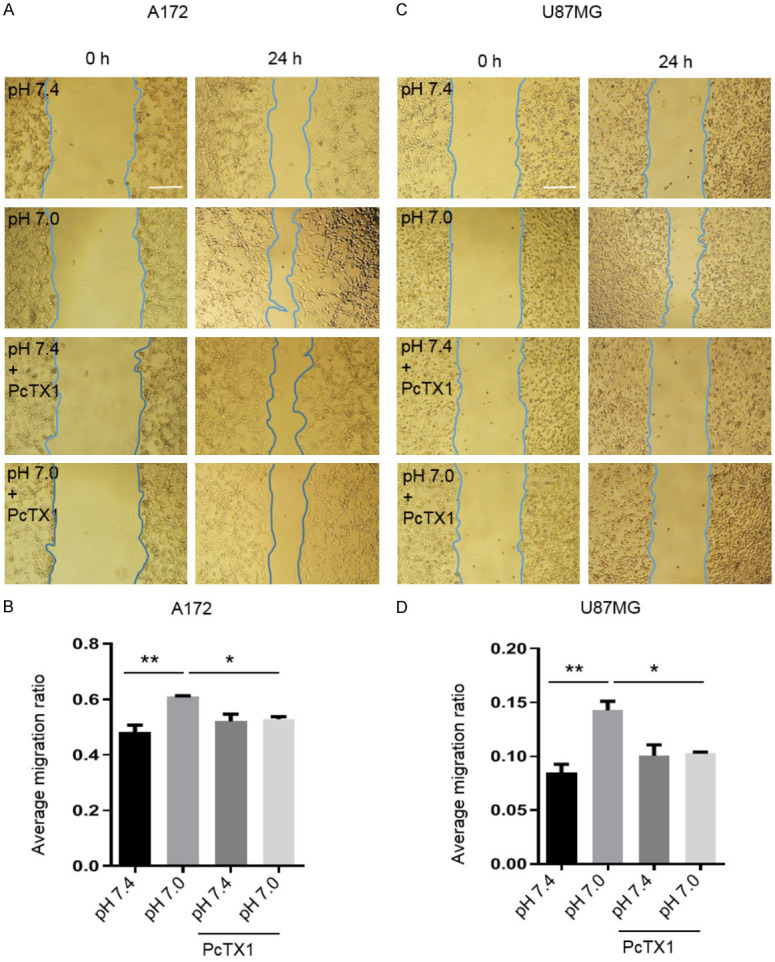
Effects of extracellular acidosis and PcTX1 on migration of A172 and U87MG cells by wound healing. (A) Representative micrographs and (B) statistical results showing the effect of extracellular weak acid and PcTX1 on migration of A172 cells by wound-healing assay. (C) Representative micrographs and (D) statistical results showing the effect of extracellular acid and PcTX1 on the migration of U87MG cells by wound-healing assay. Data are expressed as mean ± SEM. n=3-4. Scale bar =250 µm. *P < 0.05, **P < 0.01, by one-way ANOVA followed by Tukey’s multiple comparison test.
Weak acid promotes the migration of human glioma cells in transwell assay
To further examine the role of ASIC1 in glioma cell migration, we performed a transwell migration assay using inserts with an 8-μm pore size. The glioma cells were cultured in the upper chambers for 24 h, and then pretreated with pH 7.0 in the absence or presence of PcTX1 for 3 h. After 24 h, the number of migrated cells in the lower chamber was examined. Compared with pH 7.4, weak acid (pH 7.0) greatly increased the number of migrated A172 cells by 37%, and PcTX1 (20 nM) completely inhibited the acid induced migration of A172 cells (*P < 0.05, one-way ANOVA, n=5, Figure 4A and 4B). Similarly, in U87MG cells, weak acid (pH 7.0) increased the migration capacity by 47%. As anticipated, PcTX1 completely blocked the acid-induced increase of migrated U87MG cells (*P < 0.05, one-way ANOVA, n=4, Figure 4C and 4D).
Figure 4.
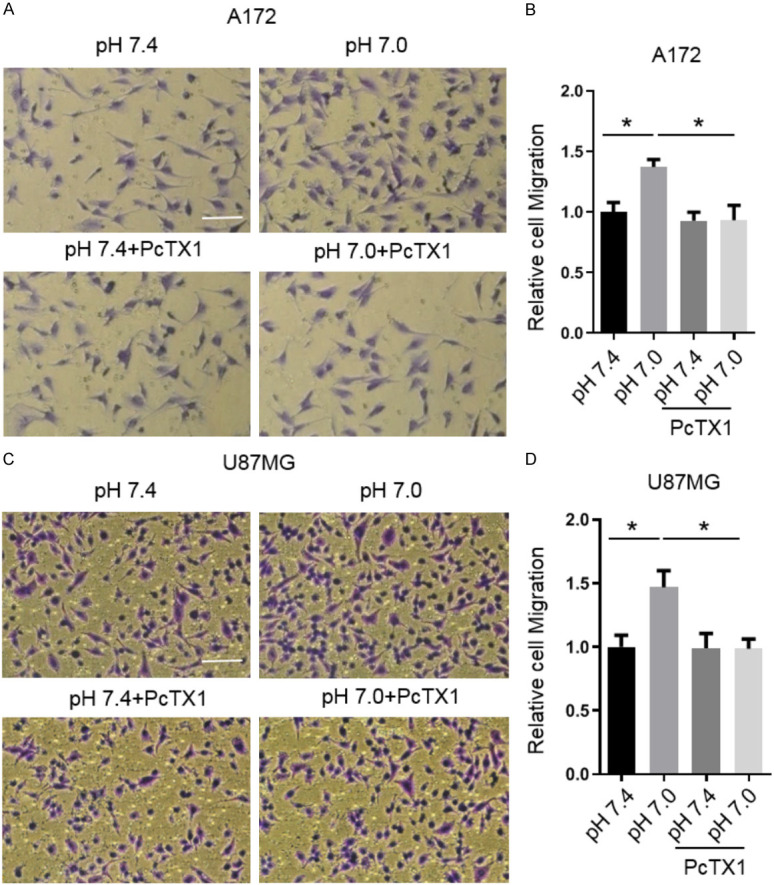
Effects of extracellular acidosis and PcTX1 on migration of A172 and U87MG cells by transwell. (A) Representative micrographs and (B) statistical results showing the effects of extracellular acid and PcTX1 on the migration of A172 cells by transwell assay. (C) Representative micrographs and (D) statistical results showing the effects of extracellular acid and PcTX1 on the migration of U87MG cells by transwell assay. Data are expressed as mean ± SEM. n=4-5. Scale bar =100 µm. *P < 0.05, by one-way ANOVA followed by Tukey’s multiple comparison test.
Knockdown of ASIC1 inhibits migration of glioma cells
Next, we used small interference RNA to silence ASIC1 expression and further determined the influence of ASIC1 protein on the migration of glioma cells under acidic condition. Owing to the low ASIC1 expression in A172 cells, we used U87MG in this experiment to test the effect of ASIC1 siRNA. As shown in Figure 5A, siRNA-ASIC1 decreased the expression of ASIC1 protein expression in U87MG cells by 72% when compared with siRNA-Control (***P < 0.001, unpaired student’s t test, n=4, Figure 5A and 5B). Next, we examined whether knockdown of ASIC1 affects cell proliferation in the presence or absence of extracellular weak acid. MTT assay was performed to detect the cell viability at 24 h and 72 h with and without acid treatment both in siRNA-Control and siRNA-ASIC1 groups. Results showed that knockdown of ASIC1 had no obvious effect on the proliferation of U87MG cells both at 24 h and 72 h either without or with the treatment of extracellular weak acid (one-way ANOVA, n=3, Figure 5C and 5D). We further examined whether knockdown of ASIC1 has an effect on the migration of U87MG cells. As shown in wound healing assay, ASIC1 knockdown did not have an effect on the migration under normal pH (7.4), but it reduced the migration by 44% under acidic condition (pH 7.0) when compared with siRNA-Control (*P < 0.05, one-way ANOVA, n=5, Figure 5E and 5F). The effect of ASIC1 knockdown on migration was also assessed by transwell. As shown in Figure 5G and 5H, weak acid (pH 7.0) produced a large increase in the migration of siRNA-Control treated U87MG cells, however, it had no effect on the migration of siRNA-ASIC1 treated cells (*P < 0.05, ***P < 0.001, one-way ANOVA, n=4). These results further suggested that ASIC1 is involved in weak acid-induced migration of malignant human glioma cells.
Figure 5.
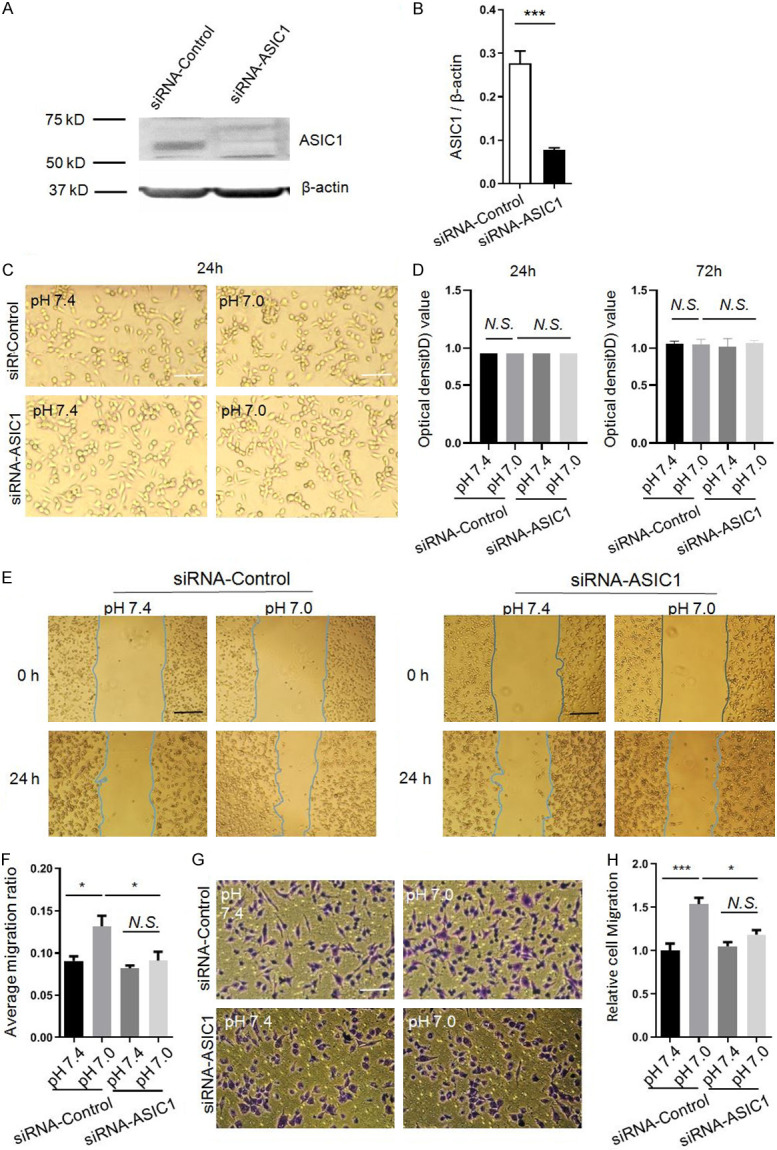
Silencing ASIC1 inhibits the migration of U87MG cells. (A) Representative Western blot bands and (B) quantification of the ASIC1 expression showing the transfection efficiency of siRNA-ASIC1 in U87MG cells. (C) Representative micrographs and (D) statistical results showing that knockdown of ASIC1 has no obvious effect on the proliferation of U87MG cells both at 24 h and 72 h without or with the treatment of extracellular weak acid. (E) Representative micrographs and (F) statistical results showing the effect of siRNA-Control or siRNA-ASIC1 on acid-induced migration of U87MG cells by wound-healing assay. (G) Representative micrographs and (H) statistical results showing the effects of siRNA-Control or siRNA-ASIC1 on acid-induced migration of U87MG cells by transwell assay. Data are expressed as mean ± SEM. n=3-5. Black bar =250 µm. White bar =100 µm. *P < 0.05, **P < 0.01, ***P < 0.001, by one-way ANOVA followed by Tukey’s multiple comparison test.
Discussion
Glioblastoma multiforme, with a high rate of morbidity and mortality, is the most aggressive type of cancer in central nervous system (CNS), and accounts for approximately 15% of all brain tumors and 60-75% of astrocytic tumors [22,23]. Due to its uncontrollable infiltrative nature and resistant to radiation and chemotherapy, the treatment effect of conventional therapies, such as radiotherapy, chemotherapy or surgery, is seriously hindered for glioma, making it one of the most serious solid tumors threatening patients’ life [24,25]. Therefore, it is of urgent need to understand the complex interaction between glioma cells and their microenvironment, identify the potential molecular mechanisms underlying their aggressive behaviors, and explore new therapeutic strategies to control them. Our present study demonstrated that ASICs were expressed in U87MG and A172 glioma cell lines and enhanced the migration capacity of these cells induced by weak acidic stimulation. Furthermore, we found that application of a commonly used ASIC1a and ASIC1a/2b channel inhibitor, PcTX1, or inducing ASIC1 knockdown strongly inhibited acid-mediated migration of U87MG cells. Taken together, our results suggest that ASIC1 mediates extracellular weak acid induced migration of malignant glioma cells.
Glioblastoma is hard to be treated owing to the complex microenvironment. Due to high metabolic rate, all the tumor microenvironment in humans and animal models is both hypoxic [26,27] and acidic [28-31]. Much work has been performed on the effect of low pH upon normal cells, and low pH microenvironment has been shown to inhibit cell proliferation, activity and survival [32]. However, different from normal cells, the tumor environment is normally acidic, especially in solid tumors [33,34]. Solid tumors grow rapidly, to maintain the sufficient supply of oxygen and nutrients, tumor cells tend to switch energy metabolism toward glycolysis [35,36]. The high endogenous metabolism rate and enhanced glycolysis of tumor cells cause the accumulation of lactic acid and carbon dioxide, leading to acidic microenvironment, with a pH as low as 5.8 [28,31]. The microenvironment plays crucial role in tumor progression, such as increased mutations [37], resistance to apoptosis [38] and enhanced invasion and metastasis [39,40]. In this study, we found that extracellular weak acid (e.g. pH 7.0) promoted the migration of U87MG and A172 glioma cells. We have also tried lower pH, e.g. pH 6.5, but it clearly affected the viability of U87MG and A172 cells (data not shown), making it difficult to study the migration property of these cells.
The poor prognosis of glioma is mainly due to the powerful ability of cellular proliferation and invasion [3]. Previous studies have shown that ASICs activation mediated by extracellular acidosis affects proliferation or migration in some tumor cells such as mammary carcinoma and lung cancer cells [12,21,34], but there are no studies on malignant glioma of the central nervous system. We provided both biochemical and electrophysiological evidence suggesting the expression of functional ASICs in two distinct human glioblastoma cell lines. We detected the ASIC1 protein expression and recorded ASIC currents in U87MG and A172 cell lines. We found that the amplitude of inward current of ASIC in A172 was lower than U87MG cells, which was probably consistent with the lower expression of ASIC1 protein in A172 cells. Further, the inward currents were blocked by PcTX1. The electrophysiological and pharmacological characterizations revealed clear evidence for the presence of functional ASIC1 channels in U87MG cells. Our results first showed that weak acid (pH 7.0) incubation had no effects on the proliferation, but clearly enhanced the migration of malignant human glioma cells (U87MG and A172).
The most aggressive tumor, glioblastoma multiforme, hampered the treatment efficacy due to the lack of control on glioma cell growth and differentiation, and the lack of detailed knowledge of how the transformed phenotype evolves and how the tumor adapts to the microenvironment to support metabolic demand, etc. [12]. It has been shown that amiloride-sensitive inward Na+ channel is constitutively activated in glioma cells, but not in normal and low grade astrocytes [41]. Additionally, the up-regulation of Cl+ and K+ channels in glioma cells is not found in normal glia, at least functionally [42,43]. Therefore, it is reasonable to assume that the specific ion transport system is closely related to the characteristics of malignant gliomas, such as significant growth and migration ability. We then explored the effect of ASIC1 activation on glioma proliferation and migration. As shown in our results, weak acid (pH 7.0) had no effect on the proliferation of A172 and U87MG cells measured both at 24 h and 72 h, but it apparently increased their capacity of migration as shown by wound-healing and transwell assays. Application of ASIC1 inhibitor, PcTX1, effectively blocked the acid-induced migration of the two cells, suggesting that weak acid stimulated glioma migration is mediated, at least in part, by ASIC1 activation. To further determine the role of ASIC1 in the migration of glioma cells, we also examined the effect of knocking down ASIC1 on glioma migration. We found that specific siRNA-ASIC1 down-regulated the expression of ASIC1 in U87MG cells and knocking down the ASIC1 channel impaired the migration ability of these cells.
Ion channels are considered to be crucial in the invasive capacity of tumor cells and formation of metastases [44]. Previous researches showed that voltage-gated K+ channels, such as KV1.3, KV10.1, and KV11.1, play a role in cell migration [45]. In addition, voltage-gated Na+ channels are expressed in metastatic tumor cells and had positive impact on cell migration and invasion [46]. Besides K+ and Na+, Ca2+ is also important for cancer cell migration [34]. As we all know, the extracellular microenvironment of tumor tissues is often acidic [47]. Extracellular pH changes are known to have impact on the activity of a variety of membrane receptors and ion channels. Acid-sensing ion channels (ASICs) are H+-gated cation channels activated by extracellular acid. The high H+ sensitivity makes them attractive signaling molecules to adapt the tumor behavior to acidic microenvironment. Recent studies have shown that acidic microenvironment activates ASICs and induces ROS generation, contributing to the pathogenesis of breast cancer [34]. ASIC-calcium signaling also plays a role in the effect of acidic microenvironment on proliferation and migration of lung cancer cells [21]. In our present study, we showed that the activation of ASIC1 mediates extracellular weak acid induced migration of malignant human glioma cells.
In summary, our study displayed the first evidence that supports a significant role of this channel in the pathogenesis of human gliomas.
Acknowledgements
We thank Ms. Tao Yang for technical assistance. Supported by National Center for Minority Health and Health Disparities of the National Institutes of Health (U54MD007602).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18:v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang L, Shen A. Numbl inhibits glioma cell migration and invasion by suppressing TRAF5-mediated NF-kappaB activation. Mol Biol Cell. 2012;23:2635–2644. doi: 10.1091/mbc.E11-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C, Perryman L, Hargrave D. Paediatric and adult malignant glioma: close relatives or distant cousins? Nat Rev Clin Oncol. 2012;9:400–413. doi: 10.1038/nrclinonc.2012.87. [DOI] [PubMed] [Google Scholar]
- 5.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 6.Rohani N, Hao L, Alexis MS, Joughin BA, Krismer K, Moufarrej MN, Soltis AR, Lauffenburger DA, Yaffe MB, Burge CB, Bhatia SN, Gertler FB. Acidification of tumor at stromal boundaries drives transcriptome alterations associated with aggressive phenotypes. Cancer Res. 2019;79:1952–1966. doi: 10.1158/0008-5472.CAN-18-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Hu X, Mo YY. Acidosis promotes tumorigenesis by activating AKT/NF-kappaB signaling. Cancer Metastasis Rev. 2019;38:179–188. doi: 10.1007/s10555-019-09785-6. [DOI] [PubMed] [Google Scholar]
- 8.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 9.Boscardin E, Alijevic O, Hummler E, Frateschi S, Kellenberger S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19. Br J Pharmacol. 2016;173:2671–2701. doi: 10.1111/bph.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 11.Vullo S, Kellenberger S. A molecular view of the function and pharmacology of acid-sensing ion channels. Pharmacol Res. 2020;154:104166. doi: 10.1016/j.phrs.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 13.Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, Gatenby RA, Gillies RJ. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008;25:411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 14.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, Gillies RJ. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 16.Peppicelli S, Bianchini F, Contena C, Tombaccini D, Calorini L. Acidic pH via NF-kappaB favours VEGF-C expression in human melanoma cells. Clin Exp Metastasis. 2013;30:957–967. doi: 10.1007/s10585-013-9595-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Leng T, Yang T, Chen F, Hu W, Xiong ZG. beta-estradiol protects against acidosis-mediated and ischemic neuronal injury by promoting ASIC1a (Acid-Sensing Ion Channel 1a) protein degradation. Stroke. 2019;50:2902–2911. doi: 10.1161/STROKEAHA.119.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Leng TD, Li MH, Shen JF, Liu ML, Li XB, Sun HW, Branigan D, Zeng Z, Si HF, Li J, Chen J, Xiong ZG. Suppression of TRPM7 inhibits proliferation, migration, and invasion of malignant human glioma cells. CNS Neurosci Ther. 2015;21:252–261. doi: 10.1111/cns.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Gao B, Xiong QJ, Wang YC, Huang DK, Wu WN. Acid-sensing ion channels contribute to the effect of extracellular acidosis on proliferation and migration of A549 cells. Tumour Biol. 2017;39:1010428317705750. doi: 10.1177/1010428317705750. [DOI] [PubMed] [Google Scholar]
- 22.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3:121. doi: 10.3978/j.issn.2305-5839.2015.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamza MA, Gilbert M. Targeted therapy in gliomas. Curr Oncol Rep. 2014;16:379. doi: 10.1007/s11912-014-0379-z. [DOI] [PubMed] [Google Scholar]
- 25.Arko L, Katsyv I, Park GE, Luan WP, Park JK. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2010;128:1–36. doi: 10.1016/j.pharmthera.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaupel P, Kallinowski F, Okunieff P. Blood fl ow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 27.Okunieff P, Dols S, Lee J, Singer S, Vaupel P, Neuringer LJ, Beshah K. Angiogenesis determines blood fl ow, metabolism, growth rate, and ATPase kinetics of tumors growing in an irradiated bed: 31P and 2H nuclear magnetic resonance studies. Cancer Res. 1991;51:3289–3295. [PubMed] [Google Scholar]
- 28.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Schornack PA, Gillies RJ. Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors. Neoplasia. 2003;5:135–145. doi: 10.1016/s1476-5586(03)80005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itai Y, Ohtomo K, Furui S, Yoshikawa K, Yashiro N, Iio M. Magnetic resonance of liver tumors: a preliminary report. Radiat Med. 1984;2:131–135. [PubMed] [Google Scholar]
- 33.Riemann A, Schneider B, Gundel D, Stock C, Gekle M, Thews O. Acidosis promotes metastasis formation by enhancing tumor cell motility. Adv Exp Med Biol. 2016;876:215–220. doi: 10.1007/978-1-4939-3023-4_27. [DOI] [PubMed] [Google Scholar]
- 34.Gupta SC, Singh R, Asters M, Liu J, Zhang X, Pabbidi MR, Watabe K, Mo YY. Regulation of breast tumorigenesis through acid sensors. Oncogene. 2016;35:4102–4111. doi: 10.1038/onc.2015.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 36.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 37.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 38.Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. [PubMed] [Google Scholar]
- 39.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 40.Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubien JK, Keeton DA, Fuller CM, Gillespie GY, Reddy AT, Mapstone TB, Benos DJ. Malignant human gliomas express an amiloride-sensitive Na+ conductance. Am J Physiol. 1999;276:C1405–1410. doi: 10.1152/ajpcell.1999.276.6.C1405. [DOI] [PubMed] [Google Scholar]
- 42.Brismar T, Collins VP. Potassium and sodium channels in human malignant glioma cells. Brain Res. 1989;480:259–267. doi: 10.1016/0006-8993(89)90191-1. [DOI] [PubMed] [Google Scholar]
- 43.Ullrich N, Sontheimer H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Physiol. 1996;270:C1511–1521. doi: 10.1152/ajpcell.1996.270.5.C1511. [DOI] [PubMed] [Google Scholar]
- 44.Riemann A, Schneider B, Gundel D, Stock C, Thews O, Gekle M. Acidic priming enhances metastatic potential of cancer cells. Pfl ugers Arch. 2014;466:2127–2138. doi: 10.1007/s00424-014-1458-6. [DOI] [PubMed] [Google Scholar]
- 45.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 46.Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28:3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Fan Z, Zhang J, Changyi Y, Huang C, Gu Y, Xu Z, Tang Z, Lu W, Wei X, Li C. Evaluating tumor metastatic potential by imaging intratumoral acidosis via pH-activatable near-infrared fl uorescent probe. Int J Cancer. 2015;136:E107–116. doi: 10.1002/ijc.29153. [DOI] [PubMed] [Google Scholar]


