Abstract
Accumulating evidence on the role of Follistatin-like protein 1 (FSTL1) in tumorigenesis and cancer progression is conflicting. Nevertheless, the underlying mechanisms by which FSTL1 contributes to gastric cancer (GC) remain unknown. This study shows that FSTL1 was frequently upregulated in primary GC tissues and significantly correlated with infiltrating depth, lymph node metastasis, unfavorable tumor stage and poor prognosis of GC. Down or up-regulation of FSTL1 inhibited or increased, respectively, the proliferation by reducing apoptosis, clonogenicity, migration and invasion of GC cells in vitro. Moreover, the higher expression of FSTL1 promoted subcutaneous xenograft tumor growth and lung/liver tumor metastasis in vivo. Furthermore, we demonstrate that FSTL1 is involved in regulation of the AKT signaling through analyzing databases and experimental results. Mechanistic studies showed that FSTL1 promoted proliferation, migration and invasion in GC, at least partially, by activating AKT via regulating TLR4/CD14. In all, this study highlights the role of the FSTL1-TLR4/CD14-AKT axis, which provided novel insights into the mechanism of growth and metastasis in GC for the first time.
Keywords: Gastric cancer, FSTL1, TLR4/CD14, AKT, prognosis
Introduction
Gastric cancer (GC), one of the most common malignant tumors, is a leading cause of cancer-related mortality, especially in China [1,2]. Despite the improvement in the current diagnosis and therapeutics, a low 5-year survival rate of GC patients at an advanced stage, owing to late presentation, high recurrence and metastasis rate, remains a challenge [3]. Therefore, it is critical to unveil the molecular mechanisms of GC and find a specific biomarker for diagnosis and prognosis to improve the clinical management of advanced GC.
AKT, a serine and threonine kinase, plays a pivotal role in cancer formation and progression. Upon its activation, AKT can regulate numerous downstream targets, such as the Bcl2 family, FAK and snail, with various functions including cell growth, survival, apoptosis, migration and transformation [4]. The Bcl2 family, one of AKT downstream signaling targets, comprises activators (Bax, Bid, Bim) or inhibitor (Bcl2, Bcl-xl, Mcl1) of apoptosis, which plays an essential part in regulating cell apoptosis [5]. Akt mediates the interaction of 14-3-3 proteins and phosphorylated BAD, and thereby regulating the anti-apoptotic function of Bcl2 and Bcl-xl [6]. Furthermore, emerging evidence indicate that AKT associated signaling pathway contributes to the motility and invasion of malignant cells. The activation of AKT-FAK signaling is responsible for cancer metastasis via regulating the adhesion of epithelial cancer cells to extracellular matrix proteins [7]. AKT-snail pathway mediated epithelial to mesenchymal transition also regulates migration of tumor cells [8]. However, the key upstream factor that links AKT signaling and GC malignant progression remain largely unknown.
Follistatin-like protein 1 (FSTL1), also named as transforming growth factor (TGF)-β-induced clone 36 (TSC36) or follistatin-related protein (FRP), is a 308 amino acid secreted glycoprotein belonging to the osteonectin (SPARC) family. It contains a pair of EF-hand domains, a follistatin-like domain, and a VMFC domain [9]. Studies have shown that FSTL1 is mainly involved in systemic autoimmune disease, developmental processes, and cardiovascular diseases [10-12]. Although emerging evidence suggests a close association between FSTL1 and the development and progression of various cancer, it is controversial [11]. FSTL1 was reported to suppress tumor growth, migration, and invasiveness in ovarian, lung, endometrial, and nasopharyngeal carcinoma [13-17]. On the other hand, FSTL1 was upregulated in hepatocellular carcinoma (HCC), esophageal squamous cell carcinoma (ESCC), breast cancer, and colon cancer. Yang W et al. showed that overexpression of FSTL1 in HCC promoted proliferation and inhibited apoptosis by activating the AKT/GSK-3β pathway [18]. Similarly, FSTL1 promoted chemoresistance and metastasis via NF-kB-BMP crosstalk in ESCC [19]. To date, only one study showed that the expression of FSTL1 was upregulated in GC cell lines and FSTL-1 knockdown may promoted GC cell apoptosis via the STAT6 signaling pathway [20]. The clinical significance of FSTL1 and its associated molecular mechanism in GC development and progression require further in-depth study.
In this study, the expression profile and clinical significance of FSTL1 and its correlations with GC patient survival were investigated by analyzing multiple public databases and clinical samples. We demonstrated that FSTL1 contributed to the growth and metastasis of GC in vivo and in vitro. Importantly, FSTL1 promoted proliferation, migration, and invasion in GC, at least partially, by activating AKT via regulating TLR4/CD14, which provided novel insights into the molecular mechanism of FSTL1 in GC pathogenesis.
Materials and methods
Clinical samples
A total of primary GC and matched non-tumor tissue samples (Zhejiang cohort 1 N = 108 for qPCR; Zhejiang cohort 2 N = 30 for western blot; Zhejiang cohort 3 N = 106 for IHC) were collected from patients referred to the Zhejiang University, who provided written informed consent prior to surgical resection. Patients were diagnosed with GC based on histopathology and had not received adjuvant treatment before surgery. Tumor staging was determined according to the American Joint Committee on Cancer (eighth edition).
Cell lines and transfection
Five human GC cell lines (HGC27, SGC7901, BGC823, MKN45, and MKN28) were cultured in RPMI 1640 (Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco). All cell lines were purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China).
Human FSTL1 expression was knocked down using a specific FSTL1 siRNA (siFSTL1) or short-hairpin plasmids (shFSTL1), which were purchased from Sigma (Shanghai, China). These two siRNAs sequences were: siFSTL1-1, sense, GAAGAGCUAAGGAGCAAAUTT, antisense, AUUUGCUCCUUAGCUCUUCTT; siFSTL1-2, sense, GAGCAAUGCAAACCUCACATT, antisense, UGUGAGGUUUGCAUUGCUCTT. Plasmids and sequences of shRNAs were: shFSTL1-1 (pGpU6/Neo, GCCTGTGTGTGGCAGTAATGG), shFSTL1-2 (pGpU6/Neo, GCTGGAAGCTGAGATCATTCC). Expression of human FSTL1 was upregulated using a plasmid (pEX-3) for cell assays (Sigma, Shanghai, China) or a lentiviral vector (GV492, FSTL1 (NM_007085)) for animal assays (ov-FSTL1 and ov-NC) (Gene, Shanghai, China). siRNA and plasmids were transfected with Lipofectamine 3000 transfection reagent (Invitrogen, Carlsbad, California, USA) in Opti-MEM medium (Gibco, Carlsbad, California, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Detailed method for RT-qPCR is given in the Supplementary Methods.
Immunohistochemistry (IHC)
Detailed method for IHC assay is in the Supplementary Methods.
Cell proliferation, apoptosis, and transwell assay
Detailed methods for cell proliferation, apoptosis and transwell assay are given in the Supplementary Method.
Co-immunoprecipitation (co-ip)
Co-ip experiments were performed using the Crosslink Magnetic IP/Co-IP Kit (Thermo Scientific, America). Briefly, FSTL1 antibody (5 ug, Proteintech) was bound to Protein A/G magnetic beads for 15 minutes, crosslink antibody to beads with DSS for 30 minutes, and then incubate cell lysate with prepared beads overnight at 4°C. Finally, immunoprecipitated proteins were separated by SDS-PAGE, and visualized by Western blot.
Immunofluorescence
Cells were seeded on glass coverslips and cultured for 24 hours and fixed with 4% Polyoxymethylene for 15 minutes at room temperature. After permeabilization with 0.1% Triton X-100 for 25 minutes at room temperature, cells were blocked in 10% normal blocking serum at 37°C for 30 min, and incubated with primary antibodies against FSTL1 (10 ug/ml, AF1694, R&D) and TLR4 (1:50, ab13556, Abcam), and CD14 (1:50, ab181470, abcam) overnight at 4°C, followed by incubation with 557-conjugated Anti-Goat IgG Secondary Antibody (1:1000, NL001, R&D) or 488-conjugated anti-Rabbit IgG secondary antibodies (1:1000, #4412, CST) or 647-conjugated anti-mouse IgG secondary antibodies (1:1000, ab6563, Abcam) for 1 hour at room temperature. To visualize the nuclei, cells were stained with 6-diamidino-2-phenylindole (DAPI; 1 ug/ml; Sigma-Aldrich, USA).
Western blot
Detailed methods for western blotting is described in the Supplementary Methods. For collection of conditioned medium from GC cell lines, cells were first serum-starved for 24 hours and conditioned medium was subsequently collected.
In vivo xenograft models
Male athymic nude mice (BALB/C, 3-4 weeks) aged 3-4 weeks were purchased from Nanjing Provincial Center for Disease Control and Prevention. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Zhejiang University. For xenograft models, 7 × 106 HGC-27 or BGC823 cells stably transfected with ov-FSTL1 vector or ov-NC were injected subcutaneously in the right flank of mice (five mice per group). Tumor size in mice was measured every 7 days. Tumor volumes were calculated using the formula: V (mm3) = 0.5 × a × b2 (V, volume; a, longitudinal diameter; b, latitudinal diameter). After 5 weeks, these mice were sacrificed, tumors were weighed, and processed for hematoxylin and eosin staining.
2 × 106 BGC823 cells transfected with ov-FSTL1 or ov-NC were intravenously injected through tail vein into nude mice to study tumor metastasis in vivo. After 6 weeks, mice were sacrificed and lungs and livers were harvested for tumor nodules and IHC.
Bioinformatics
mRNA expression of FSTL1 in GC and clinical information of GC patients was analyzed by using data from the Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov) and Oncomine database (https://www.oncomine.org). The gene expression data and patients clinicopathological information was download from the TCGA via cBioPortal (https://www.cbioportal.org/) (on 4 November 2018).
The Kaplan-Meier (K-M) plotter database (http://kmplot.com/analysis/) was investigated to validate the prognostic value of FSTL1. GC patients were divided into two cohorts based on FSTL1 expression (high vs. low expression). The overall survival (OS) and progression-free survival (PFS) of GC patients were analyzed in K-M plotter database. Log rank p-value and samples counts were calculated.
Genes co-expressing with FSTL1 in GC were identified in the cBioPortal for Cancer Genomics (http://www.cbioportal.org/) and the Coexpedia (http://www.coexpedia.org/index.php). Coexpressed genes in cBioPortal were extracted based on a |Pearson’s r| ≥ 0.4 and |Spearman’s r| ≥ 0.4. Genes overlapping both in cBioPortal and Coexpedia were used for gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis in David v6.7 (http://david.abcc.ncifcrf.gov/) to elucidate enrichment in biological processes and signaling pathways.
Statistical analysis
Data were presented as mean ± standard deviation. Two or multiple groups were compared using t-test and one-way ANOVA, respectively. P value < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001). Chi-square and Yates’ continuity corrected chi-square tests were used to analyze categorical data. Survival rates were calculated using the Kaplan-Meier method, and the difference between curves was analyzed using Log-rank test. Univariate and multivariate analysis were performed using the Cox regression model. Variables with P < 0.05 were included in the multivariate analysis to identify independent factor. Western blot band intensity was analyzed by Image J. RNA fluorescence in situ hybridization images were analyzed by FV10-ASW software. Statistical analysis was performed using GraphPad Prism 8 software.
Results
FSTL1 is upregulated and correlated with poor prognosis in GC
FSTL1 expression in GC was firstly extracted from the TCGA, Oncomine databases and results showed FSTL1 mRNA in GC was upregulated and its expression was higher in stage II-IV samples than stage I (Figures 1A and S1A). This was validated in Zhejiang cohort 1 (108 GC tissues and paired normal tissues) (Figure 1B). Then the expression of FSTL1 protein was next analyzed by western blot and IHC in paired tumor tissues and corresponding normal tissues of Zhejiang cohort 2 and 3. Results showed that FSTL1 level was elevated in GC and its expression was higher in stage III-IV than stage I-II (Figures 1C-F and S1B). In addition, upregulation of FSTL1 was significantly correlated with the infiltrating depth, lymph node metastasis, and unfavorable tumor stage of GC in TCGA database and Zhejiang cohorts 1 and 3 (Tables 1 and S1, S2). As shown in Figure 2A, GC patients with overexpressing FSTL1 in K-M plotter, TCGA databases and Zhejiang cohort 3 had remarkably shorter overall survival (OS) compared to patients with low FSTL1 expression. Meanwhile, high FSTL1 level in GC was correlated with poor prognosis of PFS in K-M plotter (Figure S2A). Further stratification according to the clinical characteristics of GC patients in K-M plotter showed that high expression of FSTL1 was significantly associated with poor OS and PFS in TNM stage III and IV patients and patients with lymph node metastasis (stage N1-3), but not in stage I and II or stage N0, indicating FSTL1 was prognostic in advanced stages and lymph node metastasis (Figures 2B-E, S2B-D and Table S3). Also, multivariate analysis in Zhejiang cohort 3 showed that FSTL1 expression was an independent prognostic factors for OS (Figure 2F). Collectively, these data suggested that FSTL1 was aberrantly upregulated and associated with poor prognosis in GC, especially in the advanced stages.
Figure 1.
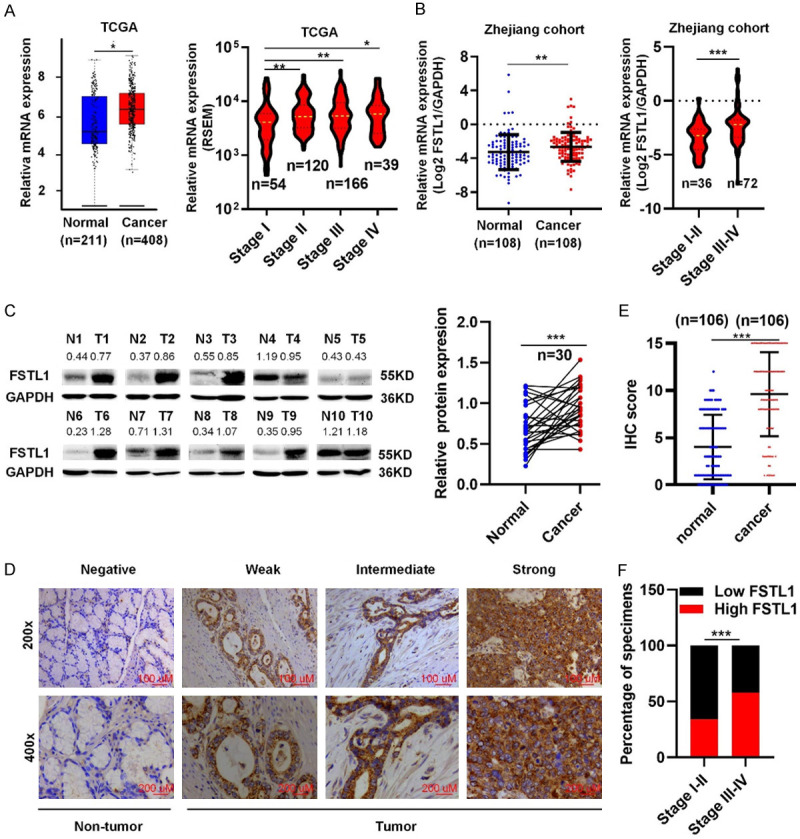
FSTL1 is overexpressed in GC. A. FSTL1 mRNA levels in GC and normal gastric tissue in TCGA database (Data from GEPIA: http://gepia.cancer-pku.cn; Match TCGA normal and GTEx data) and the mRNA expression of FSTL1 at stage I-IV of GC in TCGA database. B. FSTL1 mRNA levels in GC and normal gastric tissue and the mRNA expression of FSTL1 at stage I-II and III-IV of GC in Zhejiang cohort 1. C. Representative images of FSTL1 proteins level in Zhejiang cohort 2 (Sample 1-10). D, E. IHC analysis of FSTL1 in Zhejiang cohort 3 (SP, × 200). F. The percentage of FSTL1 at stage I-II and stage III-IV of GC in Zhejiang cohort 3. Data are shown as mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
Association between FSTL1 expression and clinicopathological features of GC in Zhejiang cohort 3
| No. patients | Low expression (n = 53) | High expression (n = 53) | P value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 83 (78.3%) | 41 (38.7%) | 42 (39.6%) | 0.8137a |
| Female | 23 (21.7%) | 12 (11.3%) | 11 (10.4%) | |
| Age | ||||
| ≤ 60 | 44 (41.5%) | 20 (18.9%) | 24 (22.6%) | 0.4304a |
| > 60 | 62 (58.5%) | 33 (31.1%) | 29 (27.4%) | |
| Grade | ||||
| G1-2 | 9 (8.5%) | 6 (5.7%) | 3 (2.8%) | 0.4895b |
| G3 | 97 (91.5%) | 47 (44.3%) | 50 (47.2%) | |
| T stage | ||||
| T1-2 | 15 (14.2%) | 12 (11.3%) | 3 (2.8%) | 0.0258b |
| T3-4 | 91 (85.8%) | 41 (38.7%) | 50 (47.2%) | |
| N stage | ||||
| N0 | 21 (19.8%) | 15 (14.1%) | 6 (5.7%) | 0.0283a |
| N1-3 | 85 (80.2%) | 38 (35.8%) | 47 (44.3%) | |
| M stage | ||||
| M0 | 89 (84%) | 48 (45.2%) | 41 (38.7%) | 0.0639a |
| M1 | 17 (16%) | 5 (4.7%) | 12 (11.3%) | |
| Tumor size | ||||
| ≤ 5 cm | 60 (56.6%) | 34 (32.1%) | 26 (24.5%) | 0.1169a |
| > 5 cm | 46 (43.4%) | 19 (17.9%) | 27 (25.5%) | |
| AJCC stage | ||||
| I-II | 30 (28.3%) | 20 (18.9%) | 10 (9.4%) | 0.0311a |
| III-IV | 76 (71.7%) | 33 (31.1%) | 43 (40.6%) |
Chi-square test;
Yates’ continuity corrected chi-square test.
Figure 2.
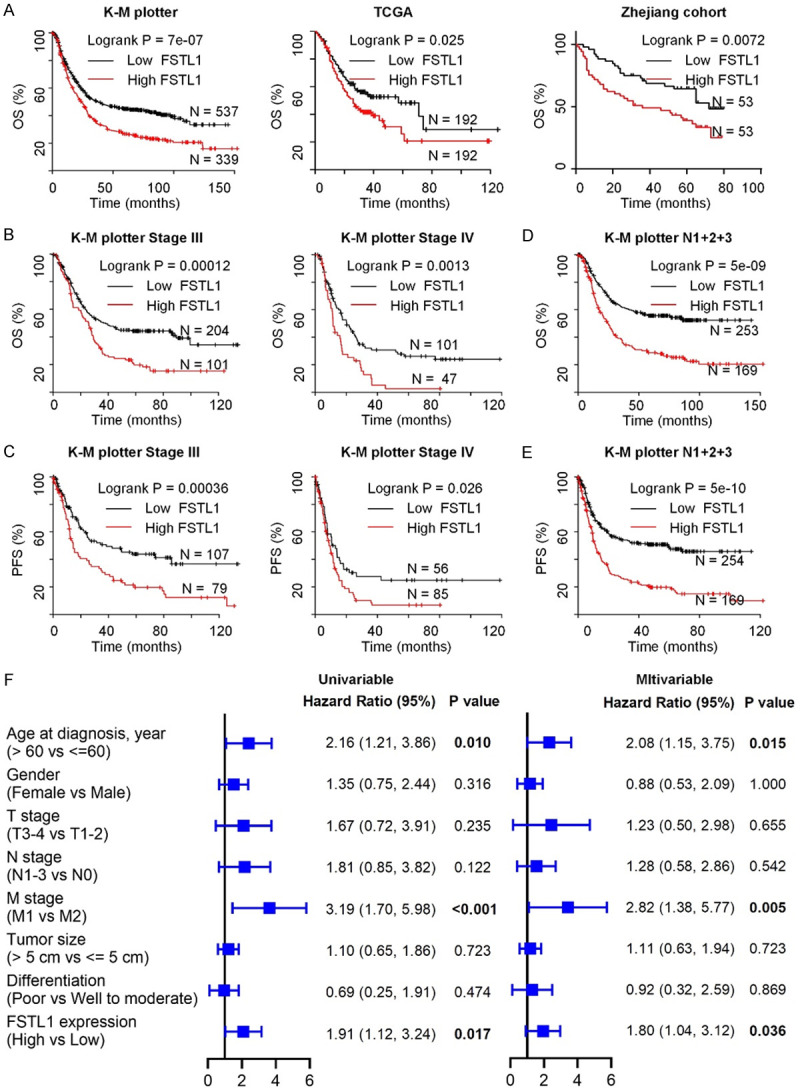
FSTL1 is associated with poor prognosis in GC. A. The association between FSTL1 expression and overall survival (OS) in GC patients assessed by K-M plotter, TCGA databases and Zhejiang cohort 3. B, C. Kaplan-Meier curves for OS and progression-free survival (PFS) of GC patients with stage III/IV using K-M plotter database. D, E. OS and PFS for GC patients with N1+2+3 disease. F. Univariable and multivariable analyses were performed in Zhejiang cohort 3. All the bars correspond to 95% confidence intervals.
FSTL1 promotes GC cells proliferation, migration and invasion in vitro
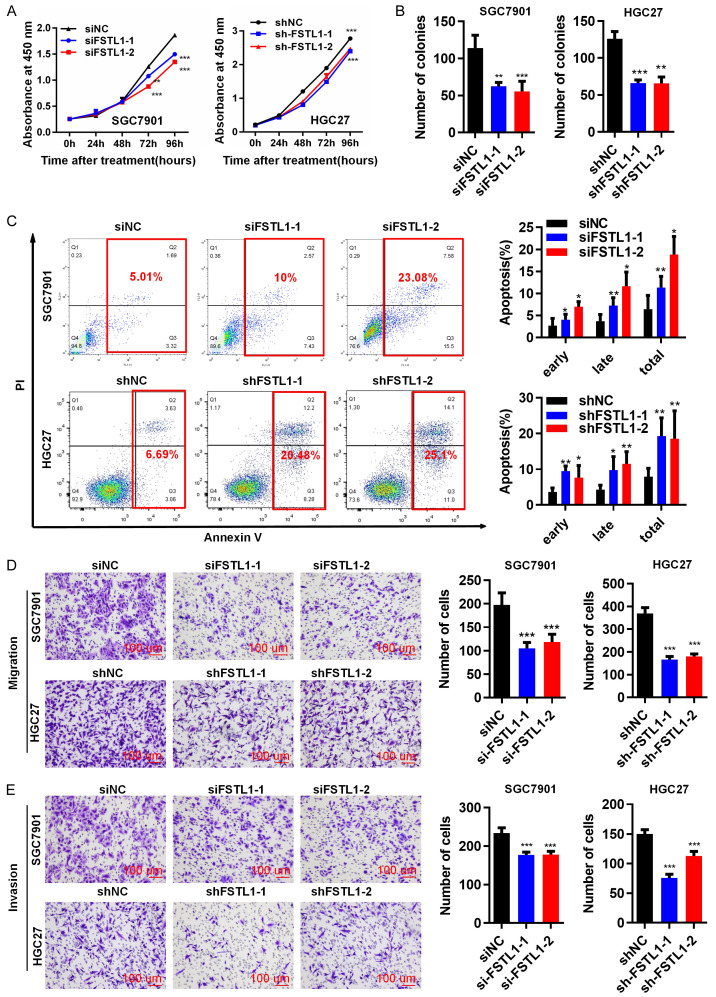
Previously, Marco et al. reported that FSTL1, as a secretory protein, was detected in condition medium of ESCC cells. Therefore, endogenous mRNA expression of FSTL1, and protein levels of five GC cell lines (SGC7901, BGC823, HGC27, MKN28 and MKN45) were measured (Figure S3A). To investigate the role of FSTL1 on the biological behavior of GC cells, we silenced FSTL1 expression using siRNA or shRNA in GC cells: SGC7901 cells were transfected with siFSTL1-1/siFSTL1-2 and normal control (siNC); HGC27 cells were transfected with shFSTL1-1/shFSTL1-2 and control (shNC). Successful knockdown of FSTL1 in GC cells was confirmed by qRT-PCR and western blotting (Figure S3B, S3C). Silencing FSTL1 by siRNA or shRNA decreased the rate of proliferation in GC cells (Figure 3A). Colony formation capacity was decreased in both siFSTL1 and shFSTL1 transfected GC cell lines compared to the respective controls (Figures 3B and S3D). Furthermore, silencing FSTL1 increased the proportion of apoptotic cells compared with NC groups (Figure 3C) indicating that down-regulation of FSTL1 promoted apoptosis, and thus inhibition of cell proliferation. Moreover, suppression of FSTL1 attenuated migration and invasiveness of SGC7901 and HGC27 cells (Figure 3D, 3E).
Figure 3.
Silenced FSTL1 inhibits GC cells proliferation, migration and invasion in vitro. (A) CCK-8 assay, (B) Colony formation assay in SGC7901 and HGC27 cells transfected with siRNA/NC or shRNA/NC. (C) Flow cytometry analysis indicating the percentages of FSTL1 silenced and control cells at different apoptosis phases. (D) Migration assay, (E) Invasion assay in SGC7901 and HGC27 cells transfected with siRNA/NC or shRNA/NC (SP, × 200). Representative images and the statistical analyses (mean ± standard deviation) were shown. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, we also analyzed the effect of FSTL1 overexpression on the growth and motility of GC cells. Over-expression of FSTL1 expression in HGC27 and BGC823 cells was confirmed by western blotting (Figure S3E, S3F). Over-expression of FSTL1 enhanced proliferation, colony formation, migration and invasiveness of HGC27 and BGC823 cells, and significantly reduced apoptosis of the GC cells (Figures 4A-E and S3G-I). Expression apoptosis related inhibited proteins (Mcl1, Bcl2, Bcl-xl) and metastasis associated proteins (p-FAK, FAK, EGFR, Snail1) were distinctly increased in response to FSTL1 overexpression in HGC27 and BGC823 cells, while the pro-apoptosis related proteins (Bax, Bim) were dramatically decreased compared with control vector groups (Figure 4D and 4F). Overall, these data demonstrated that FSTL1 expression promoted GC cells proliferation, migration and invasion in vitro.
Figure 4.
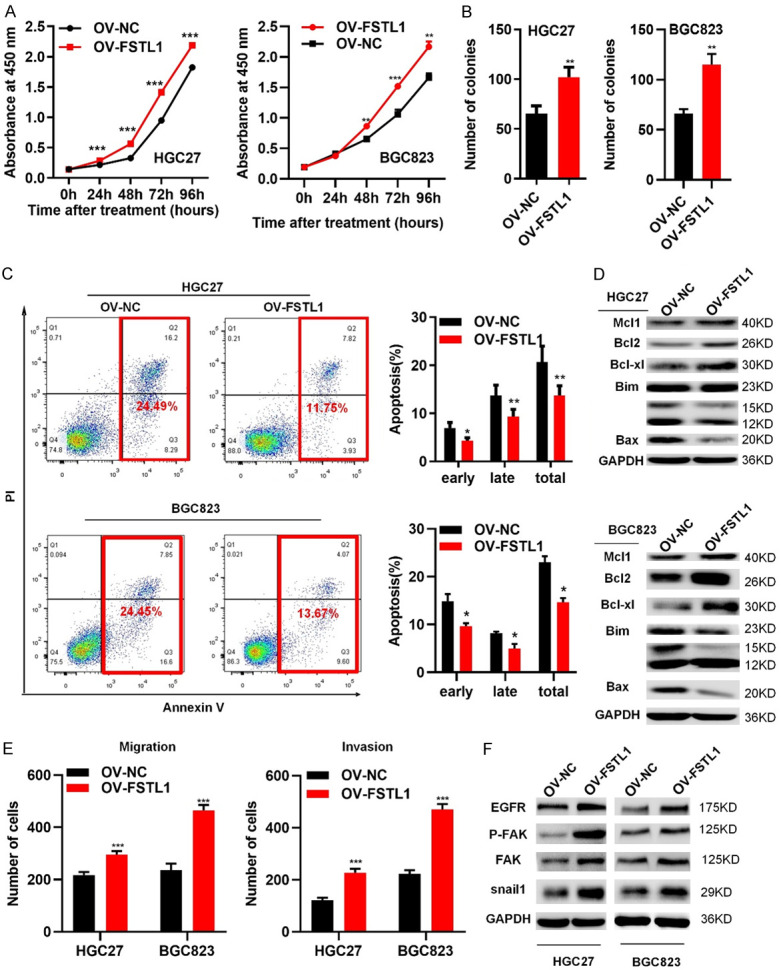
Overexpressed FSTL1 promotes GC cells proliferation, migration and invasion in vitro. (A) CCK8 assay, (B) Colony formation assay in HGC27 and BGC823 cells of overexpressing FSTL1/NC. (C) Flow cytometry showing the percentages of FSTL1 overexpressing cells and control cells at different apoptosis phases. (D) Apoptosis-associated proteins (Bim bands: 23 KD; 15 KD; 12 KD) in FSTL1 overexpressing cells by western blot. (E) Migration and invasion assay in HGC27 and BGC823 cells of elevating FSTL1. (F) Metastasis-correlated proteins in FSTL1 overexpressing cells by western blot. Representative images and the statistical analyses (mean ± standard deviation) were shown. *P < 0.05, **P < 0.01, ***P < 0.001.
FSTL1 promotes gastric cancer cell tumorigenesis and metastasis in vivo
To examined whether FSTL1 could promote GC cells tumorigenesis and/or metastasis in vivo, HGC27 and BGC823 cells stably transfected with FSTL1 overexpression lentivirus vector (ov-FSTL1/ov-NC) (Figure S4) were implanted in nude mice. Five weeks after injection, tumor weights and volumes in the ov-FSTL1 group were significantly higher than the ov-NC group (Figure 5A-F). Importantly, consistent with these finding, HE staining showed histological feature of GC and IHC revealed that enhanced Ki-67 staining in ov-FSTL1 group than the ov-NC group, further supporting the notion that FSTL1 aids the proliferation and tumorigenicity of GC cells (Figure 5G). In addition, six weeks after intravenous injection of BGC823 cells through tail vein into nude mice, higher number of tumor nodules in the lungs and liver were found in ov-FSTL1 group compared with the ov-NC group, indicating FSTL1 induced GC cells metastasis (Figure 5H, 5I). Taken together, these data revealed that FSTL1 promoted GC cell proliferation and metastasis in vivo.
Figure 5.
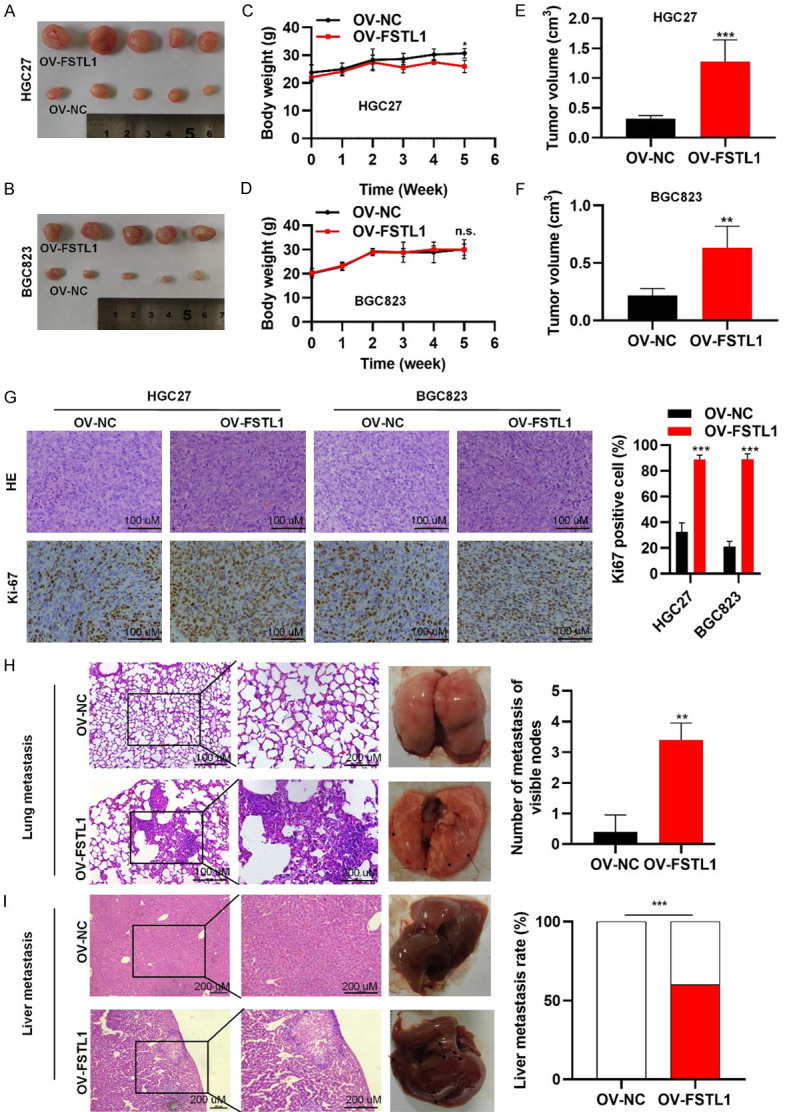
FSTL1 promotes gastric cancer cell tumorigenesis and metastasis in vivo. A, B. Representative images of subcutaneous tumors in nude mice injected with HGC27 and BGC823 cells transferred with stably OV-FSTL1/OV-NC. C-F. Tumor volumes and final body weights of nude mice injected with HGC27 and BGC823 cells transferred with stably OV-FSTL1/OV-NC. G. HE staining and Ki-67 staining with anti-Ki67 antibody in xenografted HGC27 and BGC823 tumors overexpressing FSTL1. H, I. Representative images and HE staining of lung and liver metastases in nude mice by intravenous injection of BGC823 cells through tail vein. Data were expressed as mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001. C-H. Based on Student’s t-test. I. Based on Fisher’s exact test.
Secretory FSTL1 promotes GC growth and metastasis by AKT activation
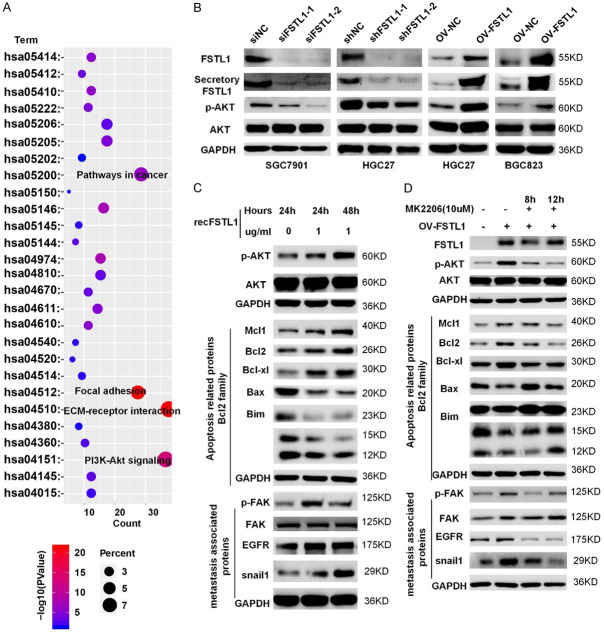
To better explore the molecular mechanisms of FSTL1-driven GC growth and progression, we selected the overlapping genes between cBioPortal and Coexpedia for GO and KEGG analyses in David v6.7 (http://david.abcc.ncifcrf.gov/) (Figure S5 and Tables S4, S5, S6). Signaling pathway analysis showed that FSTL1 was involved in AKT-associated signaling pathway (Figure 6A). Interestingly, FSTL1 knockdown reduced phosphorylation of AKT, while FSTL1 overexpression resulted in the activation of AKT (Figure 6B). As FSTL1 is a secretory glycoprotein, treating BGC823 cells with 1 ug/ml recFSTL1 protein (R&D Systems, 1694-FN-050) for 24 or 48 hours significantly increased the expression of phosphorylation of AKT, apoptosis related inhibited proteins (Mcl1, Bcl2, Bcl-xl), and metastasis associated proteins (p-FAK, EGFR, Snail1), decreased the expression of pro-apoptosis related proteins (Bax, Bim), indicating secretory FSTL1 may also play an important role in the progression of GC (Figure 6C). Inhibition of AKT by MK2206 (Selleck, USA) decreased phosphorylation of AKT and metastasis associated proteins, and rescued pro-apoptosis associated proteins resulting from elevated FSTL1 in BGC823 cells (Figure 6D). These results further confirmed that secretory FSTL1 enhanced GC cells growth and metastasis via regulating AKT-related signaling pathway.
Figure 6.
Secretory FSTL1 promotes GC growth and metastasis by AKT activation. A. KEGG pathway analysis of the genes significantly correlated with the FSTL1 expression in GC from cBioPortal and Coexpedia. KEGG, Kyoto Encyclopedia of Genes and Genomes. B. The protein expression levels of FSTL1, Secretory FSTL1, p-AKT and AKT in GC cell lines after FSTL1 silencing or overexpressing. C. The protein expression levels of p-AKT, AKT, apoptosis associated proteins (Bim bands: 23 KD; 15 KD; 12 KD) and metastasis related proteins in GC cell lines after the presence of recombinant FSTL1. D. The protein expression levels of p-AKT, AKT, apoptosis associated proteins (Bim bands: 23 KD; 15 KD; 12 KD) and metastasis related proteins in GC cell lines after the presence of recombinant FSTL1 and MK2206.
FSTL1 activates AKT by partially regulating TLR4/CD14
Previous studies have shown that FSTL1 could bind to TLR4, CD14, Follistatin, or disco-interaction protein 2 homolog A (DIP2A) [21-23]. We found that in GC FSTL1 was tightly correlated with TLR4, CD14 and Follistatin expression, but not with DIP2A in TCGA (Figure 7A). A co-ip assay verified the interaction between TLR4, CD14 and FSTL1 in GC (Figure 7B). Meanwhile, correlation studies in 30 fresh frozen GC tissues showed that FSTL1 mRNA expression was correlated positively correlated with TLR4, CD14 expression (Figure 7C). And similar results were received in the protein levels of 10 freshly GC tissues (Figures 7D and S6). Pervious study showed that TLR4 and CD14 complex is necessary for signaling in cancer cells [24,25]. In addition, immunofluorescence showed that FSTL1 and TLR4/CD14 have extensive co-localization in GC cells (Figures 7E and S7). Hence, FSTL1 interacted with TLR4/CD14 in GC. To further verify our hypothesis that TLR4 contributes to optimal activation of AKT by FSTL1, we found that inhibition of TLR4 by TAK-242 (Medchemexpress, USA) reduced the activation of AKT in BGC823 cells overexpressing FSTL1 and abrogated the recFSTL1 induced phosphorylation of AKT (Figure 7F, 7G). Overall, FSTL1 promotes activation of AKT partially dependent on TLR4 signaling.
Figure 7.
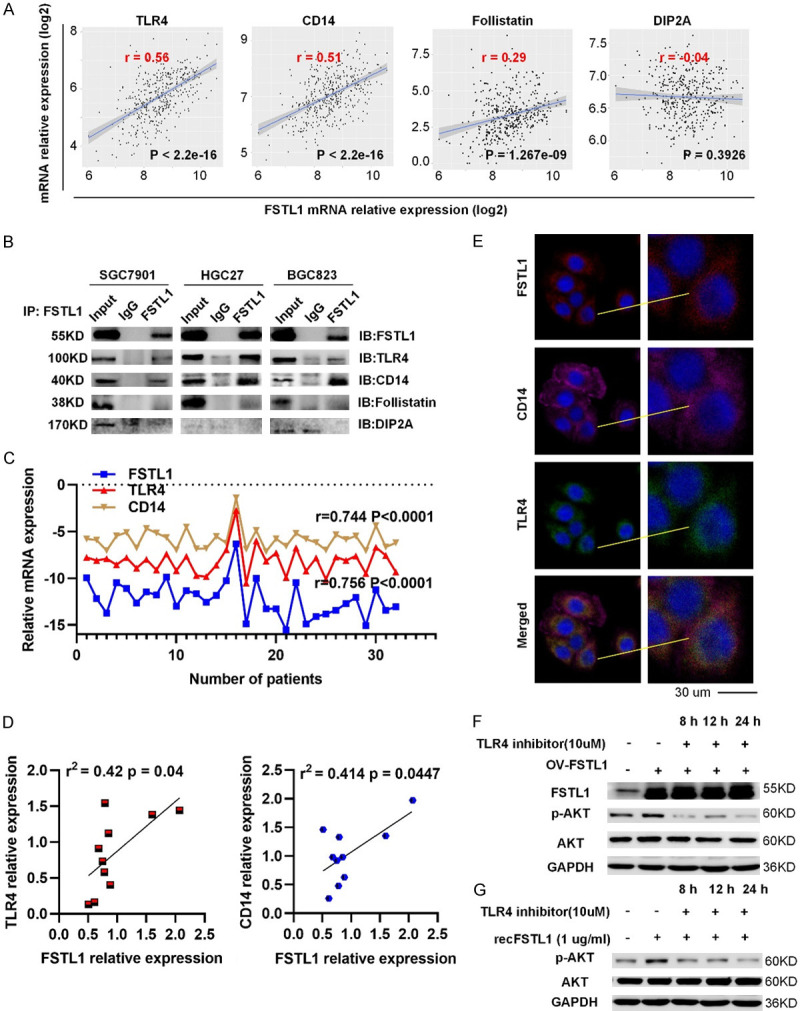
FSTL1 can promote the activation of AKT by regulating TLR4/CD14. A. Correlation analysis between TLR4/CD14/Follistatin/DIP2A expression and FSTL1 level in TCGA database. B. The physical interaction between TLR4, CD14, Follistatin, DIP2A and FSTL1 in SGC7901, HGC27 and BGC823 cells by co-immunoprecipitation. C. Correlation analysis between TLR4, CD14 mRNA expression and FSTL1 mRNA level in 30 fresh frozen GC tissues. D. Correlation analysis between TLR4, CD14 expression and FSTL1 level in 10 freshly GC tissues. E. Immunofluorescence images of TLR4, CD14, FSTL1 expression in SGC7901 cells. F, G. The protein expression levels of p-AKT, AKT proteins in BGC823 cells after overexpressed FSTL1 or the presence of recombinant FSTL1 and MK2206. A, C, D. Based on Spearman’s rank correlation test.
Discussion
GC, a global threat to human health, represents one of the most malignant tumors. Although a focus of extensive research, detailed mechanisms of GC carcinogenesis and development remain elusive. Therefore, discovery of lurking effective biomarkers that can predict the progression and prognosis of GC to provide clinical significance is urgently needed. In this study, we used a straightforward approach to reveal the expression pattern and clinicopathological features of FSTL1 in GC and its association with prognosis by integrative analysis of multiple cancer databases and clinical samples. Furthermore, functional and mechanism exploration revealed that overexpression of FSTL1 significantly inhibited the apoptosis and promoted metastatic ability of GC cells by triggering AKT-associated signaling pathway via regulating TLR4/CD14. Our results indicated that FSTL1 functions as an oncogene to drive GC and therefore is a promising therapeutic target for the intervention of GC (Figure 8).
Figure 8.
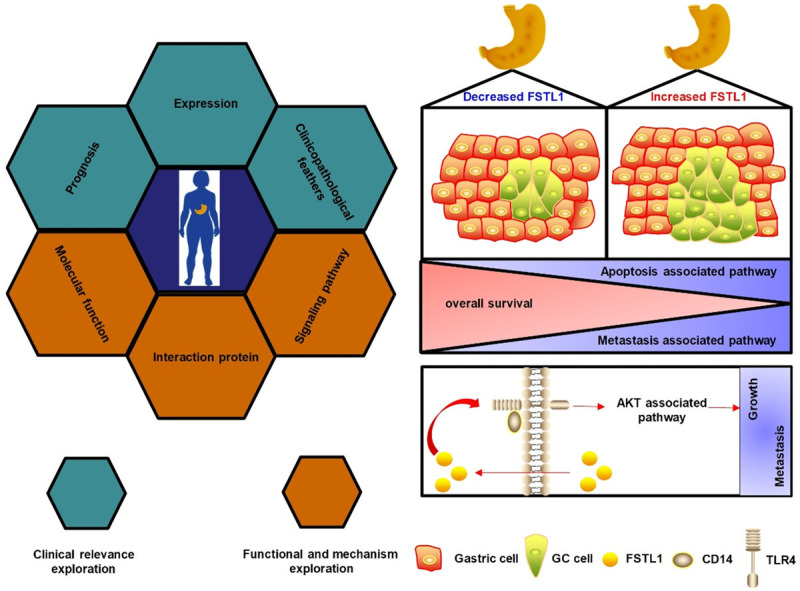
Schematic model of FSTL1-TLR4/CD14-AKT axis in GC. (Left) The integrative analytic strategy in this study. (Right) FSTL1 promotes growth and metastasis in GC by activating AKT via regulating TLR4/CD14.
Identification of proteins with diagnostic and prognostic value may contribute to tumor classification and the development of novel therapies against human cancers. The clinical relevance of FSTL1 has been well reported in various cancers, but it is highly variable with respect to its expression and prognostic value in different tumors. Compared to normal tissues, reduced expression of FSTL1 was reported in many types of cancers, such as lung adenocarcinoma, ovarian and nasopharyngeal carcinoma.
Low expression of FSTL1 is predictive of poor prognosis in lung adenocarcinoma, endometrial, and ovarian tumors. On the other hand, FSTL1 is higher in breast cancer cells, in most cases of HCC, in cancer-associated-fibroblasts of colon cancer, in ESCC cells than healthy tissues [13,18,19,26-28]. Yang W et al. also suggested that overexpressed FSTL1 in HCC was associated with larger tumors, advanced TNM stages and metastasis [18]. An J reported that FSTL1 was elevated in brain metastatic sites compared to primary breast cancer [13]. Moreover, several studies have reported a correlation between upregulated FSTL1 and poor prognosis in head and neck squamous cell carcinoma in HCC and ESCC patients [18,19,29]. In this study, we found that both mRNA and protein expression of FSTL1 was frequently upregulated in GC tissues in TCGA, Oncomine databases and three independent cohorts of 244 GC patients. Furthermore, FSTL1 expression was higher in the stages III and IV samples compared with stages I and II, indicating patients with high FSTL1 in GC are at an increased risk of tumor relapse. Higher FSTL1 was correlated with unfavorable clinical parameters, including the infiltrating depth, unfavorable tumor stage of GC and lymph node metastasis. Besides, patients with a high level of FSTL1 were accompanied by poor survival in K-M plotter, TCGA databases and GC samples. These findings suggested a potentially important role of FSTL1 in the underlying mechanisms of GC.
Considering these findings, it was logical to hypothesize that FSTL1 is involved in the pathogenesis of GC tumorigenicity and/or progression. Previous reports have shown that FSTL1 plays an important role in cancer cell proliferation and malignancy. Yang Y et al. found that FSTL1 not only accelerated breast cancer cell proliferation but also promoted angiogenesis [30]. Sundaram et al. reported that FSTL1 enabled the production of MMP9 by blocking Wnt7a-mediated repression of extracellular signal-regulated kinase phosphorylation toward metastasis [29]. To test this hypothesis, we investigated the role of FSTL1 in the regulation of GC cell proliferation and metastasis using gain- and loss-of function approaches. Our findings indicated that FSTL1 depletion inhibited proliferation by regulating cell apoptosis, migration and invasion of GC cells, while opposite results were observed in GC cells with FSTL1 overexpression in vivo and in vitro. Additionally, upregulated FSTL1 inhibited the expression of apoptosis-associated proteins (BCL2 family) and increased the expression of metastatic proteins (p-FAK, EGFR, snail). These data supported the hypothesis that FSTL1 is an important contributor to GC progression and multiple oncogenic processes.
To investigate the molecular mechanisms of FSTL1 in proliferation and metastasis of GC, we found that FSTL1 may be involved in AKT-associated pathways in GC. The AKT pathway controls basic cellular processes, including cell survival, growth, proliferation, apoptosis, and cell repair, and functions as an oncogene in many types of tumors, including GC [31,32]. Recent studies reported that FSTL1 activated the AKT pathway in various biological processes, including apoptosis of HCC, and myocardial infarction [18,33-35]. Yang W et al. reported that FSTL1 repressed cells apoptosis via AKT/GSK-3β pathway in HCC [18]. Liang et al. showed that FSTL1 repressed neuronal apoptosis and improved neurological deficits via FSTL1-DIP2A-AKT pathway [36]. In this study, we showed that FSTL1 regulated the phosphorylation of AKT. At the same time, exogenous FSTL1 enhanced the phosphorylation of AKT and regulated the apoptosis-associated proteins (BCL2 family) and metastasis-correlated proteins (p-FAK, snail, and EGFR). These changes were verified by rescue assays in BGC823 cells. Therefore, FSTL1 promoted the proliferation and metastasis in GC cells via the AKT associated signaling pathway.
Previous studies identified that FSTL1 is a secreted protein that can bind to TLR4, CD14, Follistatin, or DIP2A, which act as membrane receptors [21-23]. Murakami et al. found that FSTL1, interacting with CD14, can evoke an innate immune response via TLR4 signaling. Ouchi et al. showed that DIP2A, as an FSTL1 receptor, regulated the cardiovascular protective roles of FSTL1. In this study, we found that FSTL1 expression positively correlated with the expression of TLR4 and CD14 in TCGA and GC tissues. Furthermore, FSTL1 in GC regulated TLR4 and CD14, but not DIP2A, and Follistatin. Previous studies showed that TLR4/CD14 (CD14 is a co-Receptor for TLR4) could trigger pro-inflammatory cytokines and contribute to the tumor progression [24,25]. Meanwhile we found FSTL1 and TLR4, CD14 was extensive co-located in GC cells by Immunofluorescence assays. Based on previous reports and our results, we hypothesized that FSTL1 regulates AKT signaling partially via TLR4/CD14. In the present study, inhibition of TLR4/CD14 in by a TLR4 signaling inhibitor (TAK-242) reduced phosphorylated in FSTL1 overexpressing BGC823 cells or in response to recFSTL1. Thus, these findings indicated partially dependent on TLR4 signaling. While we were preparing the manuscript, Peng X published their work on FSTL1, and showed that FSTL1 might inhibit GC cell apoptosis via the STAT6 signaling pathway, which also gave evidence for the critical roles of FSTL1 in the development of GC [20].
Conclusions
In summary, this study reports an altered FSTL1 expression pattern in GC and demonstrates its clinical relevance. Furthermore, functional and mechanistic studies suggested a crucial function of FSTL1 in cell proliferation and metastasis through the AKT signaling pathway partially via regulating TLR4/CD14. Therefore, targeting the FSTL1-TLR4/CD14-AKT pathway might be a new therapeutic method to improve the treatment and survival of patients with GC.
Acknowledgements
This work was supported by Project of the regional diagnosis and treatment centre of the Health Planning Committee (JBZX-201903); the Key Research and Development Program of Science and Technology Department of Zhejiang Province (2018C03022); National Health and Family Planning Commission Research Fund & Zhejiang Provincial Medical and Health Major Science and Technology Plan Project (KWJ-ZJ-1802); the National Natural Science Foundation of Zhejiang Province (LQ20H160043); Programs for Science and Technology Development of Henan Province (212102310198).
Disclosure of conflict of interest
None.
Supplementary Methods, Figures S1-S7, Tables S1-S3, S5 and S6
Table S4
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Bernards N, Creemers GJ, Nieuwenhuijzen GA, Bosscha K, Pruijt JF, Lemmens VE. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann Oncol. 2013;24:3056–3060. doi: 10.1093/annonc/mdt401. [DOI] [PubMed] [Google Scholar]
- 4.Ricciardi MR, Mirabilii S, Licchetta R, Piedimonte M, Tafuri A. Targeting the Akt, GSK-3, Bcl-2 axis in acute myeloid leukemia. Adv Biol Regul. 2017;65:36–58. doi: 10.1016/j.jbior.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Cory S, Adams JM. The bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 6.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowska A, Kijewska M, Lipko M, Hibner U, Kaminska B. Downregulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and downregulates MMPs expression. Biochim Biophys Acta. 2011;1813:655–67. doi: 10.1016/j.bbamcr.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 9.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-β1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–9. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters MMC, Meijs TA, Gathier W, Doevendans PAM, Sluijter JPG, Chamuleau SAJ, Neef K. Follistatin-like 1 in cardiovascular disease and inflammation. Mini Rev Med Chem. 2019;19:1379–1389. doi: 10.2174/1389557519666190312161551. [DOI] [PubMed] [Google Scholar]
- 11.Mattiotti A, Prakash S, Barnett P, van den Hoff MJB. Follistatin-like 1 in development and human diseases. Cell Mol Life Sci. 2018;75:2339–2354. doi: 10.1007/s00018-018-2805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. doi: 10.1007/s12026-014-8526-z. [DOI] [PubMed] [Google Scholar]
- 13.An J, Wang L, Zhao Y, Hao Q, Zhang Y, Zhang J, Yang C, Liu L, Wang W, Fang D, Lu T, Gao Y. Effects of FSTL1 on cell proliferation in breast cancer cell line MDAMB231 and its brain metastatic variant MDAMB231BR. Oncol Rep. 2017;38:3001–3010. doi: 10.3892/or.2017.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 15.Bae K, Park KE, Han J, Kim J, Kim K, Yoon KA. Mitotic cell death caused by follistatin-like 1 inhibition is associated with up-regulated Bim by inactivated Erk1/2 in human lung cancer cells. Oncotarget. 2016;7:18076–18084. doi: 10.18632/oncotarget.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni X, Cao X, Wu Y, Wu J. FSTL1 suppresses tumor cell proliferation, invasion and survival in non-small cell lung cancer. J Cell Physiol. 2018;39:13–20. doi: 10.3892/or.2017.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Xiao X, Huang T, Du C, Wang S, Mo Y, Ma N, Murata M, Li B, Wen W, Huang G, Zeng X, Zhang Z. Epigenetic inactivation of follistatin-like 1 mediates tumor immune evasion in nasopharyngeal carcinoma. Oncotarget. 2016;7:16433–16444. doi: 10.18632/oncotarget.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Wu Y, Wang C, Liu Z, Xu M, Zheng X. FSTL1 contributes to tumor progression via attenuating apoptosis in a AKT/GSK-3beta - dependent manner in hepatocellular carcinoma. Cancer Biomark. 2017;20:75–85. doi: 10.3233/CBM-170132. [DOI] [PubMed] [Google Scholar]
- 19.Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, Law S, Lee NP, Cheung AL, Qin YR, Chan KW, Ning W, Guan XY, Ma S. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFkappaB-BMP signaling cross-talk. Cancer Res. 2017;77:5886–5899. doi: 10.1158/0008-5472.CAN-17-1411. [DOI] [PubMed] [Google Scholar]
- 20.Peng X, Wang P, Li S, Jiang Y, Wu C. Follistatin-like protein 1 knockdown elicits human gastric cancer cell apoptosis via a STAT6-dependent pathway. Oncol Rep. 2019;42:2806–2813. doi: 10.3892/or.2019.7334. [DOI] [PubMed] [Google Scholar]
- 21.Murakami K, Tanaka M, Usui T, Kawabata D, Shiomi A, Iguchi-Hashimoto M, Shimizu M, Yukawa N, Yoshifuji H, Nojima T, Ohmura K, Fujii T, Umehara H, Mimori T. Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett. 2012;586:319–324. doi: 10.1016/j.febslet.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Riva M, Björk P, Swärd K, Mörgelin M, Leanderson T, Ivars F. CD14 is a co-receptor for TLR4 in the S100A9-induced pro-inflammatory response in monocytes. PLoS One. 2016;11:e0156377. doi: 10.1371/journal.pone.0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S, Huang Y, Lou C, He Y, Zhang Y, Zhang Q. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin beta3/Wnt signaling under miR-137 regulation. Cancer Biol Ther. 2019;20:328–337. doi: 10.1080/15384047.2018.1529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bevivino G, Sedda S, Franze E, Stolfi C, Di Grazia A, Dinallo V, Caprioli F, Facciotti F, Colantoni A, Ortenzi A, Rossi P, Monteleone G. Follistatin-like protein 1 sustains colon cancer cell growth and survival. Oncotarget. 2018;9:31278–31290. doi: 10.18632/oncotarget.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su S, Parris AB, Grossman G, Mohler JL, Wang Z, Wilson EM. Up-regulation of follistatin-like 1 by the androgen receptor and melanoma antigen-A11 in prostate cancer. Prostate. 2017;77:505–516. doi: 10.1002/pros.23288. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram GM, Ismail HM, Bashir M, Muhuri M, Vaz C, Nama S, Ow GS, Vladimirovna IA, Ramalingam R, Burke B, Tanavde V, Kuznetsov V, Lane EB, Sampath P. EGF hijacks miR-198/FSTL1 wound-healing switch and steers a two-pronged pathway toward metastasis. J Exp Med. 2017;214:2889–2900. doi: 10.1084/jem.20170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Mu T, Li T, Xie S, Zhou J, Liu M. Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac Cancer. 2017;8:606–612. doi: 10.1111/1759-7714.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 32.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 33.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altekoester AK, Harvey RP. Bioengineered FSTL1 patches restore cardiac function following myocardial infarction. Trends Mol Med. 2015;21:731–733. doi: 10.1016/j.molmed.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Chiba A, Watanabe-Takano H, Miyazaki T, Mochizuki N. Cardiomyokines from the heart. Cell Mol Life Sci. 2018;75:1349–1362. doi: 10.1007/s00018-017-2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang X, Hu Q, Li B, McBride D, Bian H, Spagnoli P, Chen D, Tang J, Zhang JH. Follistatin-like 1 attenuates apoptosis via disco-interacting protein 2 homolog A/Akt pathway after middle cerebral artery occlusion in rats. Stroke. 2014;45:3048–3054. doi: 10.1161/STROKEAHA.114.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.