Abstract
Alternative splicing (AS), a vital post-transcription process for eukaryote gene expression regulating, can efficiently improve gene utilization and increase the variety of RNA transcripts and proteins. However, AS of non-coding RNAs (ncRNAs) has not been paid enough attention to compared with that of protein-coding RNAs (mRNAs) for a long time. In fact, AS of ncRNAs, especially long noncoding RNAs (lncRNAs), also plays a significant regulatory role in the human disease. Recently, some bifunctional genes transcribed into both mRNA and lncRNA transcripts by AS have been observed. Here, we focus on the AS of lncRNAs and bifunctional genes producing lncRNA transcripts and propose a strategy for the future research of lncRNA AS.
Keywords: Alternative splicing, lncRNAs, bifunctional genes
Introduction
Eukaryotic genes are split genes that contain introns which need to be removed from pre-mRNAs by splicing to form mature RNAs [1]. Previous studies have shown that a single pre-RNA can be spliced into several mature RNAs by the process of alternative splicing [2,3]. Surprisingly, more than 90% of human genes and 60% of plant genes undergo AS, suggesting that AS occurs much more intensively than expected in eukaryotes [4-6]. Furthermore, both mRNAs and ncRNAs can be alternatively spliced [7]. However, studies have paid more attention to the AS of mRNAs than that of ncRNAs. Recently, increasing evidences have shown that alternative splicing of ncRNAs is even more common [7,8].
Until now, ncRNAs have been defined as RNA transcripts that are transcribed by the genome and devoid of protein-coding potential. In fact, recent studies have suggested that there may not be an either-or relationship between mRNAs and ncRNAs. First, some lncRNAs have been found to contain short open reading frames (sORFs) that encode small peptides [9,10]. Second, several studies have revealed that some mRNAs function directly at the RNA level independent of their proteins [11]. Additionally, it is increasingly being recognized that some genes can be transcribed into both mRNA transcripts and ncRNA transcripts [12]. Although these results revoke the strict definition between mRNAs and ncRNAs, ncRNAs appear to stand with tremendous potential to be proceeded with.
Our foci lncRNAs are heterogeneous ncRNAs with a length more than 200 nucleotides [13]. Currently, lncRNAs have been found to contribute significantly to various physiological processes and the occurrence and development of multiple diseases [14-18]. It has been repeatedly demonstrated that lncRNAs regulate gene expression as competing endogenous RNAs or by involving in numerous signal pathways [19-21]. Although with strong functions, lncRNAs were not actually discovered until the 1980s [22]. As the earliest discovered lncRNAs, XIST and H19 have been studied for a long time and have produced excellent results [23,24]. However, various lncRNAs have captivated scientists’ attention since the 21st century, when part of the work of the Encyclopedia of DNA Elements (ENCODE) Project and the human genome project showed that ncRNAs account for an overwhelming proportion of the human genome [25,26]. Thus, there is still a long way to go to fully understand the role of lncRNAs in various physiological and pathological processes. In this review, we focus on the AS of lncRNAs and bifunctional genes producing lncRNA transcripts.
Functions and mechanisms of alternative splicing
The high incidence of AS in eukaryotic genes extremely increases the variety and function of ncRNAs and proteins [4-6]. AS events play an important role in both physiology and pathology progresses. First, AS events show tissue-dependent and developmental stage-dependent manners [27,28]. For example, a mass of AS events are observed during myogenesis and the AS of Mef2d and Rock2 regulated by Rbfox2 drives myoblast fusion to promote developmental transition [29]. Similarly, Key hematopoietic regulators manifest characteristic alternative splicing patterns in hematopoietic stem cells (HSCs), and the AS of HMGA2, one of these regulators, affects HSC properties [30]. In addition, AS events play a pivotal role in embryonic development, neurodevelopmental and pediatric liver development [31-33]. Second, abnormal AS induces the switch to disease-specific transcripts to promote disease development. For instance, miR-193a-5p is reported to regulate splicing factor SRSF6-mediated AS of oxoglutarate dehydrogenase-like (OGDHL) and extracellular matrix protein 1 (ECM1) to boost the epithelial-to-mesenchymal transition (EMT) in pancreatic cancer [34]. Similarly, RNA helicase MTR4 modulates hepatocellular carcinoma relevant AS through recruiting polypyrimidine tract binding protein 1 (PTBP1) to its target pre-mRNAs, thus promoting tumorigenesis and cancer metabolic reprogramming [35]. Additionally, some genome-wide analyses have revealed that disease-specific transcripts produced by abnormal AS are enriched in extensive human diseases [36-39]. AS events also have a significant impact on the biogenesis and progression of other diseases such as type 1 diabetes and neuropathic pain [40-42]. Therefore, AS events and their relevant splicing factors are expected to be new biomarkers for early diagnosis and prognostic prediction of diseases. In consideration of the vital role of AS in physiological processes and development of diseases, it is valuable to thoroughly investigate the mechanisms of AS, and to develop novel therapeutic strategies based on the abnormal AS in diseases.
Generally, AS has seven basic patterns including exon skipping, mutually exclusive exons, intron retention, alternative 5’ splice site, alternative 3’ splice site, alternative first exons and alternative last exons [3]. Some genes have, hitherto, been reported to have only one AS pattern, such as Spo11 which has been found only cassette exon pattern [43]. While some genes have been reported to be spliced simultaneously in several AS patterns, such as CASC18 [44].
Splicing reactions are catalyzed by the spliceosome, a complex macromolecular machine consisting of at least five kinds of snRNAs and more than 200 snRNPs. In the splicing reaction, spliceosome is assembled step by step at the splice site of the pre-RNA through a complicated process [45,46]. However, splice sites only provide splicing position information during the splicing reaction. Generally, the use of splice sites is regulated by splicing regulatory cis-elements and trans-factors [47]. Cis-elements are specific RNA sequence elements of pre-RNA and exert a positive or negative effect on the use of splice site. They can be classified into the following groups: exon splicing enhancers (ESEs), exon splicing silencers (ESSs), intron splicing enhancers (ISEs) and intron splicing silencers (ISSs) [47]. Generally, cis-elements regulate splicing through recruiting trans-factors which include two classic families, serine and arginine rich splicing factor (SR proteins) and nuclear heterogeneous ribonucleoproteins (hnRNPs) [48,49]. Considering its context-dependent activities, the interaction network consisting of cis-elements and trans-factors exerts extremely complex regulatory functions to AS [47]. For example, different hnRNP family members could either facilitate splicing or inhibit splicing when they bind to the same position of a pre-RNA [50]; SR proteins suppress splicing when they bind to introns but activate splicing when they bind to exons [51]. Therefore, the overexpression of disease-associated or mutational splicing factors can lead to abnormal regulation of AS and alter the splicing pattern of downstream molecules, thus promoting the occurrence and development of diseases [52-56]. Moreover, transcription, chromatin structure, DNA methylation, histone modification and RNA modification can also affect the use of AS regulatory elements [57-61].
Furthermore, considering that lncRNAs extensively affect gene expression by interacting with their target DNAs, RNAs and proteins, they could modulate AS at multiple levels [62-64]. Mechanically, on the one hand, lncRNAs can interact with splicing factors. For example, lncRNA SNHG6 was found to bind to hnRNPA1 in order to induce hnRNPA1-coordinated specific splicing of PKM mRNA, resulting in the rise in PKM2/PKM1 ratio and the enhancement of aerobic glycolysis in colorectal cancer [65]. In addition to SNHG6, LUCAT1, LASTR and other lncRNAs have been proved to regulate AS by binding to splicing factors [42,66]. On the other hand, lncRNAs can form RNA-RNA duplexes with target pre-mRNAs to regulate their AS. This was exemplified by the interaction between Fas pre-mRNA and lncRNA Saf, which contributes to the formation of the Fas isoform lacking exon 6 [67]. Furthermore, lncRNAs could regulate AS by affecting chromatin remodeling [68,69].
Regulatory role of alternative splicing of lncRNA in human diseases
LncRNAs not only regulate AS of mRNAs, but also undergo AS similar to mRNAs, and lncRNA AS plays an important regulatory role in the occurrence and development of human diseases [7]. Systematic analysis of alternative splicing landscape across 8075 cancer patients demonstrates that tumors have up to 30% more AS events than normal samples, suggesting that AS plays an important role in carcinogenesis [70]. With the emergence of increasing studies on lncRNA AS in cancers, the regulation of lncRNA AS on carcinogenesis has been gradually revealed. Several studies have demonstrated that the regulation of lncRNAs on carcinogenesis shows a transcript-dependent manner. For instance, CRNDE-g has been reported to be the most abundant transcript among twelve transcripts of CRNDE in all detected cancer cell lines and be involved in the regulation of aberrant cell proliferation [71]. Similarly, ZNF695 transcript variant 3, one of ZNF695 lncRNA transcripts, is specifically upregulated in leukemia and has the ability to predict overall survival of B-cell acute lymphoblastic leukemia patients [72]. Surprisingly, it has been revealed that abnormal lncRNA AS could switch lncRNA splicing to the disease-specific transcripts to promote carcinogenesis. For example, the polypyrimidine tract binding protein 1 (PTBP1) mediates the alternative splicing of lncRNA LHFPL3-AS1 precursor towards LHFPL3-AS1-long transcript which upregulates BCL2 protein to suppress apoptosis of melanoma stem cells [73]. Similarly, the risk-associated single nucleotide polymorphism (SNP) rs11672691 increases PCAT19-long and decreases PCAT19-short by mediating promoter-enhancer switching, thus regulating cell proliferation, tumor growth, and metastasis in prostate cancer [74]. Furthermore, Pvt1b, a transcript of lncRNA Pvt1, was proved to be induced by p53-mediated response to stress and repress Myc transcription and inhibit cellular proliferation and tumor growth [75]. In summary, abnormal lncRNA AS induces the increase of disease-specific lncRNA transcripts to promote carcinogenesis. Therefore, a better understanding of underlying molecular mechanisms of lncRNA AS and relationships between lncRNA transcripts involved in carcinogenesis may provide us effective therapeutic targets and predictive biomarkers.
In addition, lncRNA AS plays a similar role in other diseases. For example, in the brain of multiple system atrophy, the expression of linc00320-002 transcript was significantly upregulated compared with the normal brain, but no difference was observed in the expression of linc00320-006 and 007 transcripts [76]. Moreover, lncRNA AS has also been reported to be involved in developmental and neural differentiational regulation [44,77].
Interrelationships between lncRNA transcripts in human disease regulation
As described above, lncRNAs could be spliced alternatively and lncRNA transcripts thus produced may even exceed our imagination (Table 1). For instance, CRNDE gene produces at least twelve transcripts and CASC18 gene produces seven transcripts by AS [44,71]. LncRNA transcripts of a single pre-RNA may play different roles due to their differences in exons or introns resulting from AS. In addition, they may play similar roles, such as CASC2a/b and CASC2c, which can both inhibit tumor cell proliferation [78-80].
Table 1.
LncRNAs being alternatively spliced
| LncRNA | Transcripts involved | Relationship between transcripts | Target genes or regulated genes | Other significant results | Diseases or processes | References |
|---|---|---|---|---|---|---|
| CASC18 | CASC18-A1, A2, B1, B2, C, D1, D2 | Not investigated | PAX6 | CASC18-D has potential of being a neural cell differentiation marker | Neural differentiation | [44] |
| CRNDE | CRNDE-a, b, c, d, e, f, g, h, i, j, k, l | Not investigated | Not investigated | CRNDE-g is the most expressed transcript in various cancer types | Various solid and hematopoietic malignancies | [71] |
| LHFPL3-AS1 | LHFPL3-AS1-long and LHFPL3-AS1-short | Not investigated | miR-181a-5p and Bcl-2 | PTBP1 mediates the alternative splicing of lncRNA LHFPL3-AS1 precursor towards LHFPL3-AS1-long transcript | Melanoma | [73] |
| PCAT19 | PCAT19-long and PCAT19-short | reciprocal expression and clinical relevance | HNRNPAB and a subset of cell-cycle genes | rs11672691 increases PCAT19-long and decreases PCAT19-short by mediating promoter-enhancer switching | Prostate cancer | [74] |
| PVT1 | Pvt1a and Pvt1b | Opposite roles in the regulation of Myc | Myc | Pvt1b is induced by p53-mediated response to stress | Lung cancer | [75] |
| Full-length transcript and transcript lacking exon 4 (PVT1ΔE4) | Similar roles in the proliferation of tumor cells and sponging miR-200s | miR-200s | SRSF1 up-regulates the expression of PVT1ΔE4 | Clear cell renal cell carcinoma | [85] | |
| linc00320 | linc00320-002, 006, 007 | Not investigated | Not investigated | linc00320-002 is highly expressed in the white matter compared to grey matter in the multiple system atrophy brain and normal brain | Multiple system atrophy | [76] |
| H19 | H19 variant lacking part of exon 1 | Not investigated | Not investigated | H19 variant lacking part of exon 1 is expressed in embryonic and placental not cancer issues | Embryonic and placental development | [77] |
| CASC2 | CASC2a/b and CASC2c | Similar roles in the proliferation of tumor cells | miR-18a, JNK, ERK1/2, β-catenin | None | Hepatocellular carcinoma, gastric cancer and colorectal cancer | [78-80] |
| LINC00477 | LINC00477 isoform 1, 2 and 3 | Opposite roles in the cell proliferation and migration | ACO1 | Isoform 1 suppresses the conversion ability from citrate to isocitrate by binding to ACO1 | Gastric cancer | [86] |
| PXN-AS1 | PXN-AS1-L and PXN-AS1-S | Opposite roles in the regulation of PXN and tumor development | PXN | MBNL3 increases PXN expression by inducing lncRNA-PXN-AS1 exon 4 inclusion | Hepatocellular carcinoma | [87] |
| MALAT1 | FL-MALAT1 and Δsv-MALAT1 | Unique role for each transcript | Genes involved in the PI3K/AKT pathway | The expression of Δsv-MALAT1 is likely to be regulated by YAP | Breast cancer | [93] |
| GAS5 | Full-Length (FL) and Clone 2 (C2) | Unique role for each transcript | P53, BRCA1, GADD45A | FL rescues cell cycle arrest, while C2 enhances cell apoptosis | Neuroblastoma | [94] |
| MIR503HG | at least four distinct transcripts in human placenta and multiple transcripts in other tissues | Not investigated | Not investigated | MIR503HG transcripts are downregulated in gynecological cancers | Gynecological cancers | [104] |
The main reason that different lncRNA transcripts of a single pre-RNA have the same function may be that they retain the same functional site after alternative splicing. For instance, Plasmacytoma variant translocation 1 (PVT1) is a kind of lncRNA proved to be upregulated in multiple cancers [81-84]. It has been proved that both PVT1 full-length transcript and PVT1 transcript lacking exon 4 facilitate the development of renal cancer through interacting with miR-200s, because the binding site to miR-200s is not on exon 4 of PVT1 [85].
Compared to transcripts with similar functions, we are more interested in lncRNA transcripts which exert opposite functions in the development of diseases. For example, LINC00477 is a lncRNA alternatively spliced into three transcripts in gastric cancer [86]. Compared with normal tissues, the expression of LINC00477 transcription 1 in gastric cancer tissues is down-regulated, while the expression of transcription 2 is up-regulated [86]. And transcript 1 and 2 have opposite effects on the proliferation and migration of gastric cancer cells [86]. This indicates that it is not rigorous to generalize the function of all transcripts of a single lncRNA at an overall level, because the function of different transcripts may be opposite. In addition, it was found that transcript 1 could interact with aconitase 1 (ACO1) to regulate iron metabolism and glycometabolism, while transcript 2 could not [86]. This is possibly because transcript 1 not transcript 2 has the special binding site to ACO1.
Why could different lncRNA transcripts of a single pre-RNA show opposite characters in the disease development? Researchers focused on the mechanism of functional differences between two transcripts of PXN-AS1 in their study [87]. PXN-AS1 gene contains five exons, and produces two transcripts by AS. The longer lncRNA transcript PXN-AS1-L includes exon 4, while the shorter lncRNA transcript PXN-AS1-S skips exon 4. Its antisense chain gene encodes paxillin (PXN), a focal adhesion protein upregulated in various tumors and involving in cell movement and migration by recruiting structural and signaling molecules [88]. Recent research found that splicing factor MBNL3 increases PXN expression by inducing the PXN-AS1 exon 4 retention, that is, increasing PXN-AS1-L expression and decreasing PXN-AS1-S expression (Figure 1A) [87]. In other words, these two transcripts are antagonistic in the regulation of PXN expression. Mechanically, PXN-AS1-L exon 4 binds to the PXN mRNA 3’UTR and prevents PXN mRNA degradation induced by miR-24-AGO, thereby upregulating the expression of PXN [87]. While PXN-AS1-S exon 5 inhibits PXN mRNA translation elongation by binding to its CDS, thereby reducing the expression of PXN [87]. Subsequently, another study has reported that PXN-AS1-L increases the expression of SAPCD2 protein by binding to SAPCD2 mRNA 3’UTR and inhibiting its degradation induced by miRNAs-AGO2 [89]. In this case, does PXN-AS1-S also affect the expression of SAPCD2? In addition, a recent study has unraveled that PXN-AS1 functions as a competing endogenous RNA of miR-3064 [90]. Both two transcripts contain the binding site to miR-3064, so are there any differences in the binding capacity of the two transcripts to miR-3064? Although many questions remain to be answered, these studies provide a new direction to further explore lncRNA AS. The above studies suggest that different lncRNA transcripts of a single gene produced by AS may have completely opposite effects on the downstream target molecules and even the occurrence and progression of diseases. Mechanically, this noteworthy phenomenon can be explained by the inclusion or exclusion of a certain functional site or the change of RNA secondary structure.
Figure 1.
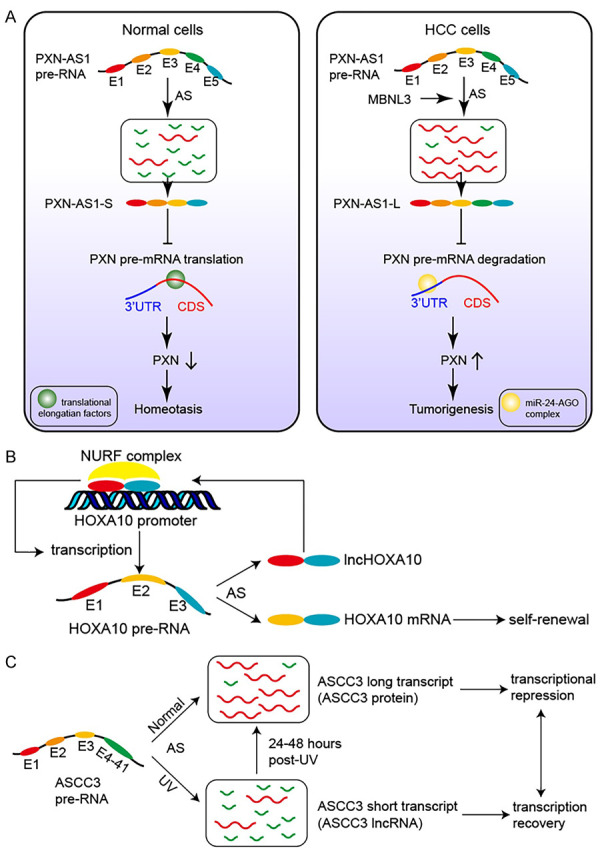
Research achievements of lncRNA alternative splicing in several investigations. A. MBNL3 increases the expression of PXN protein by inducing the inclusion of lncRNA-PXN-AS1 exon 4 in hepatocellular carcinoma (HCC) cells. B. LncHOXA10 drives the transcription initiation of HOXA10 by interacting and recruiting NURF chromatin remodeling complex to its promoter. C. UV irradiation causes a decrease in the expression of ASCC3 mRNA and an increase in the expression of ASCC3 lncRNA transcript. And the two transcripts of ASCC3 play antagonistic effects on transcriptional recovery after DNA damage.
Furthermore, another relationship has been reported that different transcripts exert disparate functions respectively, neither the same nor the opposite. For example, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a nuclear-retained lncRNA that regulates AS and transcriptional control of oncogenes [91,92]. Recently, Didier et al. found that besides full-length transcript (FL-MALAT1), MALAT1 is alternatively spliced into the other transcript, Δsv-MALAT1 which shows two deletion regions by AS [93]. From their investigation, it can be found that there is no considerable functional correlation between FL-MALAT1 and Δsv-MALAT1, in spite of their positively correlated expression correlation. First, FL-MALAT1 and Δsv-MALAT1 show notably different expression patterns. Compared with the normal breast tissue, FL-MALAT1 increases in 14.1% tumors, while Δsv-MALAT1 increases in 5.4% tumors and decreases in 18.8% tumors. Second, Δsv-MALAT1 is observed to play a critical role in aggressiveness of breast tumors, while FL-MALAT1 is not. Third, a transcriptional regulation of Δsv-MALAT1 by the transcriptional co-activator YAP is observed, while FL-MALAT1 is not. Fourth, Δsv-MALAT1 activates the PI3K/AKT pathway, while FL-MALAT1 does not. Through studying the functional differences between the two MALAT1 transcripts, the researchers emphasized the vital influence of AS on the functions of lncRNA transcripts. However, are the functional differences between the two MALAT1 transcripts caused by the excluded fragments? Does the excluded fragment simply cause a change in the sequence, or simultaneously change the RNA secondary structure? These questions remain underexplored. Similarly, full-length transcript of GAS5 rescues cell cycle arrest, while C2 transcript drives apoptosis, indicating that these two transcripts of GAS5 have their own function separately in neuroblastoma cancer [94]. In summary, different lncRNA transcripts of a single gene have relatively complex regulatory effects on physiological and pathological processes, which cannot be simply generalized at the overall level in the research.
Interrelationships between lncRNA transcripts and mRNA transcripts of bifunctional genes in human disease regulation
With the increasing understanding of gene expression, emerging data have suggested that there are some bifunctional genes that can be alternatively spliced into both mRNA and lncRNA transcripts (Table 2). For example, ZNF695 is one of the members of the largest DNA-binding protein family, zinc finger protein family (ZNF), in mammals [72]. Ricardo et al. found that ZNF695 pre-RNA produces six transcripts by AS, including ZNF695_TV1, TV3, TV4, TV5, TV6 and TV7. Among them, ZNF695_TV1, ZNF695_TV4 and ZNF695_TV5 belong to mRNA, while ZNF695_TV3, ZNF695_TV6 and ZNF695_TV7 belong to lncRNA [72]. In addition, a recent study identified that PNUTS pre-RNA is alternatively spliced into lncRNA-PNUTS besides PNUTS-mRNA and hnRNP E1 downregulates lncRNA-PNUTS expression by binding to an alternative splicing site of PNUTS pre-RNA [95]. They also found that lncRNA-PNUTS, not PNUTS-mRNA, interacts with miR-205 and decreases its bioavailability, thus playing a regulatory role in EMT [95].
Table 2.
Bifunctional genes transcribed into both mRNA and lncRNA transcripts through alternative splicing
| Gene | mRNA transcripts | LncRNA transcripts | Relationship between transcripts | Target genes or regulated genes | Other significant results | Diseases or processes | References |
|---|---|---|---|---|---|---|---|
| ZNF695 | ZNF695 transcript variant TV1, TV4, TV5 | ZNF695 transcript variant TV3, TV6, TV7 | Not investigated | Not investigated | ZNF transcript variant TV3 has the potential to predict overall survival of leukemia patients | Childhood B-cell acute lymphoblastic leukemia | [72] |
| PNUTS | PNUTS mRNA | LncRNA-PNUTS | Not investigated | miR-205 | HnRNP E1 regulates alternative splicing of PNUTS pre-RNA by binding to its splicing site | Breast cancer | [95] |
| HOXA10 | HOXA10 | LncHOXA10 | LncHOXA10 activates HOXA10 transcription | PTEN | SF3B1 modulates the alternative splicing of HOXA10; LncHOXA10 and HOXA10 promote liver tumor initiating cell self-renewal | Liver cancer | [96,100] |
| ASCC3 | ASCC3 long isoform | ASCC3 short isoform | Opposite roles in transcriptional regulation | Not investigated | Transcript elongation rates are reduced and transcriptions are restricted to the 5’ end of genes after UV irradiation | UV irradiation | [101] |
| SAT1 | SSAT1 | SSATX | SSATX functions as a melanoma tumor suppressor in a manner independent of SSAT1 | Genes involved in the Wnt/β-catenin pathway | Repression of SSATX promotes cell proliferation, invasion, migration and colony formation | Melanoma | [102] |
| SRA | SRAP | SRA | Repression of SRA has no effect on SRAP | E-cadherin, N-cadherin, β-catenin, CCL21, p38 | SRA regulates cancer cell proliferation, invasion, EMT and distant metastasis | Melanoma | [105] |
For bifunctional genes that produce both mRNA and lncRNA transcripts, the interaction between the two kinds of transcripts has become the main concern of the study. For example, Shao et al. found that Homeobox A10 (HOXA10) lncRNA transcript promotes its mRNA transcript expression [96]. HOXA10, a member of the homeobox transcription factor family, effects the development of lung adenocarcinoma, gastric cancer and other cancers [97-99]. HOXA10 gene is composed of three exons and produces two transcripts of HOXA10 mRNA (containing exon 2 and exon 3) and lncHOXA10 (containing exon 1 and exon3) by alternative splicing [96]. It was identified that lncHOXA10 drives the transcription initiation of HOXA10 by interacting and recruiting NURF chromatin remodeling complex to its promoter, thus promoting liver tumor initiating cell self-renewal and tumorigenesis (Figure 1B) [96]. Another study also found that splicing factor 3b subunit 1 (SF3B1) regulates HOXA10 splicing to affect cell proliferation, apoptosis and cell cycle arrest in gastric cancer [100]. In conclusion, the lncRNA transcript of HOXA10 gene plays its biological function by promoting the expression of its mRNA transcript. However, whether HOXA10 mRNA and its coding protein have a feedback effect on the expression of lncHOXA10 has not been reported, so the interaction between the two transcripts remains to be further studied.
In addition, mRNA and lncRNA transcripts of a single pre-RNA may exhibit antagonistic effects. For instance, Williamson et al. found that the two transcripts of ASCC3 play antagonistic effects on transcriptional recovery after DNA damage [101]. ASCC3 pre-RNA is alternatively spliced into two transcripts. The longer transcript encodes ASCC3 protein, while the shorter transcript functions as a lncRNA. They found that UV irradiation causes a decrease in the expression of ASCC3 mRNA and an increase in the expression of ASCC3 lncRNA transcript. Further study revealed that ASCC3 protein maintains transcriptional inhibition, while ASCC3 lncRNA transcript facilitates transcriptional recovery (Figure 1C) [101]. However, knockdown of one transcript has no effect on the expression of the other, indicating that the mechanism of antagonism between the two transcripts is not simply to regulate each other’s expression [101]. There are several speculations as to the specific mechanism: one is that lncRNA transcript may regulate post-translational modification of ASCC3 protein, and the other is that lncRNA transcript may recruit another transcriptional regulator to promote transcriptional recovery after UV irradiation [101].
Furthermore, mRNA and lncRNA transcripts may be independent of each other. For example, SAT1 gene is transcribed into two transcripts, SSAT1 and SSATX. SSAT1 protein is a rate-limiting enzyme in the polyamine catabolism and SSATX, the lncRNA transcript, is inserted a 110 bp exon between exon 3 and exon 4 of SSAT1 mRNA [102]. SSATX is down-regulated in melanoma, and knockdown of SSATX activates the Wnt/β-catenin signaling pathway and promotes the proliferation, invasion and migration of melanoma cells, which is not dependent on SSAT1 protein [102]. Since SSATX and SSAT1 sequences are exactly similar except for the insertion, there are several questions to be further studied: whether the tumor suppressor function of SSAT1 independent of SSATX is the function of the additional exon, and whether the two transcripts have other functions in common. Overall, the above studies indicate that there may be a promoting or inhibiting relationship between mRNA transcript and lncRNA transcript of a single gene produced by AS, or they may function independent of each other.
Strategies to study different lncRNA transcripts produced by alternative splicing
Recently, a growing body of studies have found that some lncRNAs have more than two transcripts, such as CRNDE and CASC18, which makes the comparative study of these transcripts less effective, so researchers turned to adopt the method of studying specific exons directly [44,71]. For example, PVT1 contains at least 12 exons and can produce multiple lncRNA transcripts by alternative splicing. The researchers considered PVT1 exon 9 as the research object, and focused on its function in the development of prostate cancer and in the resistance to androgen deprivation therapy [103]. This methodology suggests that for genes with several transcript types, several exons and complicated functions, it is convenient to study the function of certain exon separately as a starting point.
One excellent application object of the above methodology could be exon 2 of linc00320. Exon 2 retention is the main feature that differentiates linc00320-002 from linc00320-006 and 007 transcripts. As mentioned above, linc00320-002 transcript not linc00320-006 and 007 transcripts was upregulated in the brain of multiple system atrophy compared with the normal brain [76]. The explanation may be that exon 2 in linc00320-002 is likely to be involved in the pathogenesis of multiple system atrophy. Hence the function of exon 2 in multiple system atrophy should be studied separately in subsequent studies.
However, the separate study of functional site cannot replace the study of the whole transcript, because a certain functional site may not necessarily perform its function in the whole RNA molecule with a specific secondary structure. For example, in the PXN-AS1 study above, the researchers found both two transcripts contain exon 5 and put a noteworthy problem: why can PXN-AS1-S bind to the PXN mRNA CDS region, while PXN-AS1-L cannot? They proposed that the combination of PXN-AS1-L exon 4 and PXN mRNA 3’UTR might prevent PXN-AS1-L from binding to the PXN mRNA CDS region, and this proposition was proved by the fact that PXN mRNA binds to exon 4 more tightly than exon 5 [87]. Consequently, these results may provide potential strategies for further lncRNA AS studies: even if different transcripts contain the same functional site, their functions may vary due to the adjacent sequence of the functional site and changes in RNA structure. Therefore, the separate analysis of an exon or intron can explain the possible generality of the transcripts containing the exon or intron and provide ideas for subsequent studies, but it cannot replace the study of the whole transcript.
As described above, lncRNA-PNUTS has been found to competitively bind and inhibit miR-205 [95]. Both mRNA transcript and lncRNA transcript share the miR-205 binding site, so why cannot the former bind to miR-205 to play the regulatory role? The researchers made the following two speculations: one is that the miRNA binding site is located in the CDS region of PNUTS mRNA, and ribosomal hindrance may affect the binding of miR-205 to the target site; the other is that the two transcripts may have distinct secondary structures, and the secondary structure of lncRNA-PNUTS is more prone to binding with miR-205 [95].
In summary, RNA transcripts of a single pre-RNA may not have the same function even if they have the same functional site, because a certain functional site functions as a part of the whole RNA instead of on its own. Therefore, this review provides a relatively reasonable and rigorous strategy to study the functions of lncRNA transcripts produced by AS: for different lncRNA transcripts of a single pre-RNA produced by AS, it is necessary not only to investigate the functions of different exons or introns separately, but also to study the similarities and differences between transcripts in disease regulation based on their context and RNA structure.
Conclusion and outlook
Despite masses of studies on lncRNAs, we have paid less than half a century’s attention to lncRNAs, and our understanding of its production mechanism and function may be only a drop in the sea. In particular, the discovery of small peptides encoded by lncRNAs, bifunctional genes and independent functional mRNAs reveals the limitations of our understanding of lncRNAs [9,11,12].
Studies have shown that AS exists widespreadly during gene expression. However, researchers have been focusing on mRNA AS rather than lncRNA AS for a long time. Recently, the burgeoning research on lncRNA AS indicates that lncRNA AS plays a critical regulatory role in the occurrence and development of human diseases, especially cancers. Abnormal lncRNA AS resulting from various causes, including overexpression of disease-associated splicing factors and genotoxic and oncogenic stress, leads to a switch of lncRNA splicing to the disease-specific transcripts, thus promoting tumorigenesis [73,75,87]. Moreover, the interrelationships between transcripts in the regulation of diseases are complex. Different lncRNA transcripts of a single gene may have the same or completely opposite or even unrelated functions. Further, there may be a mutually reinforcing or inhibiting relationship between transcripts. Therefore, it is better to study functions of different transcripts separately rather than to generalize all transcripts during fundamental research in order to better apply to clinical diagnosis and treatment.
Furthermore, in order to study the functions and relationships of different transcripts of lncRNAs more clearly, we propose a reasonable and rigorous research strategy: it is necessary not only to study the function of certain exon or intron individually, but also to study the functional similarities and differences between transcripts in disease regulation based on their RNA structures.
In conclusion, lncRNA AS events and their relevant splicing factors can be used as biomarkers or therapeutic targets for various diseases, especially cancers. Further studies of lncRNA AS are required to explore the global landscape of disease-associated lncRNA AS, the regulation mechanism of abnormal lncRNA AS and the specific role of lncRNA AS in the human disease regulation, thus to translate basic science research of lncRNA AS into clinical application.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China [81672402, 82072754]; Natural Science Foundation of Jiangsu Province, China [BK20171305]; Jiangsu key R & D program social development project, China [BE2018689].
Disclosure of conflict of interest
None.
References
- 1.Sharp PA. Split genes and RNA splicing. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 2.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park E, Pan Z, Zhang Z, Lin L, Xing Y. The expanding landscape of alternative splicing variation in human populations. Am J Hum Genet. 2018;102:11–26. doi: 10.1016/j.ajhg.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 5.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquez Y, Brown JW, Simpson C, Barta A, Kalyna M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in arabidopsis. Genome Res. 2012;22:1184–1195. doi: 10.1101/gr.134106.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deveson IW, Brunck ME, Blackburn J, Tseng E, Hon T, Clark TA, Clark MB, Crawford J, Dinger ME, Nielsen LK, Mattick JS, Mercer TR. Universal alternative splicing of noncoding exons. Cell Syst. 2018;6:245–255. e245. doi: 10.1016/j.cels.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Yang Z, Yang C, Wang Z, Chen D, Xie Y, Wu Y. Identification and genetic analysis of alternative splicing of long non-coding RNAs in tomato initial flowering stage. Genomics. 2020;112:897–907. doi: 10.1016/j.ygeno.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, Zeng Z, Xiong W. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. doi: 10.1186/s12943-020-1147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polycarpou-Schwarz M, Gross M, Mestdagh P, Schott J, Grund SE, Hildenbrand C, Rom J, Aulmann S, Sinn HP, Vandesompele J, Diederichs S. The cancer-associated microprotein CASIMO1 controls cell proliferation and interacts with squalene epoxidase modulating lipid droplet formation. Oncogene. 2018;37:4750–4768. doi: 10.1038/s41388-018-0281-5. [DOI] [PubMed] [Google Scholar]
- 11.Candeias MM, Malbert-Colas L, Powell DJ, Daskalogianni C, Maslon MM, Naski N, Bourougaa K, Calvo F, Fahraeus R. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat Cell Biol. 2008;10:1098–1105. doi: 10.1038/ncb1770. [DOI] [PubMed] [Google Scholar]
- 12.Dhamija S, Menon MB. Non-coding transcript variants of protein-coding genes - what are they good for? RNA Biol. 2018;15:1025–1031. doi: 10.1080/15476286.2018.1511675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Yu W, Wang Y, Xia K, Huang Y, Xu A, Chen Q, Liu B, Tao H, Li F, Liang C. lncRNAs: function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res Ther. 2019;10:344. doi: 10.1186/s13287-019-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Dominguez JR, Lodish HF. Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood. 2017;130:1965–1975. doi: 10.1182/blood-2017-06-788695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gourvest M, Brousset P, Bousquet M. Long noncoding RNAs in acute myeloid leukemia: functional characterization and clinical relevance. Cancers (Basel) 2019;11:1638. doi: 10.3390/cancers11111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu ZY, Trenner M, Boon RA, Spin JM, Maegdefessel L. Long noncoding RNAs in key cellular processes involved in aortic aneurysms. Atherosclerosis. 2020;292:112–118. doi: 10.1016/j.atherosclerosis.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: an innate immune perspective. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194419. doi: 10.1016/j.bbagrm.2019.194419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafiee A, Riazi-Rad F, Havaskary M, Nuri F. Long noncoding RNAs: regulation, function and cancer. Biotechnol Genet Eng Rev. 2018;34:153–180. doi: 10.1080/02648725.2018.1471566. [DOI] [PubMed] [Google Scholar]
- 20.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20:5758. doi: 10.3390/ijms20225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhan A, Mandal SS. Long noncoding RNAs: emerging stars in gene regulation, epigenetics and human disease. Chem Med Chem. 2014;9:1932–1956. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 23.Lecerf C, Le Bourhis X, Adriaenssens E. The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell Mol Life Sci. 2019;76:4673–4687. doi: 10.1007/s00018-019-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loda A, Heard E. Xist RNA in action: past, present, and future. PLoS Genet. 2019;15:e1008333. doi: 10.1371/journal.pgen.1008333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ENCODE Project Consortium; Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermüller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaöz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Löytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA NISC Comparative Sequencing Program; Baylor College of Medicine Human Genome Sequencing Center; Washington University Genome Sequencing Center; Broad Institute; Children’s Hospital Oakland Research Institute. Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrímsdóttir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Szustakowki J International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 27.Furlanis E, Scheiffele P. Regulation of neuronal differentiation, function, and plasticity by alternative splicing. Annu Rev Cell Dev Biol. 2018;34:451–469. doi: 10.1146/annurev-cellbio-100617-062826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol. 2017;18:437–451. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh Ravi K, Xia Z, Bland Christopher S, Kalsotra A, Scavuzzo Marissa A, Curk T, Ule J, Li W, Cooper Thomas A. Rbfox2-coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis. Molecular Cell. 2014;55:592–603. doi: 10.1016/j.molcel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesana M, Guo MH, Cacchiarelli D, Wahlster L, Barragan J, Doulatov S, Vo LT, Salvatori B, Trapnell C, Clement K, Cahan P, Tsanov KM, Sousa PM, Tazon-Vega B, Bolondi A, Giorgi FM, Califano A, Rinn JL, Meissner A, Hirschhorn JN, Daley GQ. A CLK3-HMGA2 alternative splicing axis impacts human hematopoietic stem cell molecular identity throughout development. Cell Stem Cell. 2018;22:575–588. e577. doi: 10.1016/j.stem.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majewska M, Lipka A, Paukszto L, Jastrzebski JP, Gowkielewicz M, Jozwik M, Majewski MK. Preliminary RNA-seq analysis of long non-coding RNAs expressed in human term placenta. Int J Mol Sci. 2018;19:1894. doi: 10.3390/ijms19071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furlanis E, Traunmüller L, Fucile G, Scheiffele P. Landscape of ribosome-engaged transcript isoforms reveals extensive neuronal-cell-class-specific alternative splicing programs. Nat Neurosci. 2019;22:1709–1717. doi: 10.1038/s41593-019-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Groen BD, Bi C, Gaedigk R, Staggs VS, Tibboel D, de Wildt SN, Leeder JS. Alternative splicing of the SLCO1B1 gene: an exploratory analysis of isoform diversity in pediatric liver. Clin Transl Sci. 2020;13:509–519. doi: 10.1111/cts.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Wu P, Yang Z, Deng S, Ni L, Zhang Y, Jin L, Pan Y. miR-193a-5p promotes pancreatic cancer cell metastasis through SRSF6-mediated alternative splicing of OGDHL and ECM1. Am J Cancer Res. 2020;10:38–59. [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L, Kim J, Jiang L, Feng B, Ying Y, Ji KY, Tang Q, Chen W, Mai T, Dou W, Zhou J, Xiang LY, He YF, Yang D, Li Q, Fu X, Xu Y. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat Commun. 2020;11:708. doi: 10.1038/s41467-020-14437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyos LE, Abdel-Wahab O. Cancer-specific splicing changes and the potential for splicing-derived neoantigens. Cancer Cell. 2018;34:181–183. doi: 10.1016/j.ccell.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu M, Hong W, Ruan S, Guan R, Tu L, Huang B, Hou B, Jian Z, Ma L, Jin H. Genome-wide profiling of prognostic alternative splicing pattern in pancreatic cancer. Front Oncol. 2019;9:773. doi: 10.3389/fonc.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zong Z, Li H, Yi C, Ying H, Zhu Z, Wang H. Genome-wide profiling of prognostic alternative splicing signature in colorectal cancer. Front Oncol. 2018;8:537. doi: 10.3389/fonc.2018.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang YT, Chiu YC, Kao CJ, Hou HA, Lin CC, Tsai CH, Tseng MH, Chou WC, Tien HF. The prognostic significance of global aberrant alternative splicing in patients with myelodysplastic syndrome. Blood Cancer J. 2018;8:78. doi: 10.1038/s41408-018-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman JRB, Conesa A, Mika M, New FN, Onengut-Gumuscu S, Atkinson MA, Rich SS, McIntyre LM, Concannon P. Disease-specific biases in alternative splicing and tissue-specific dysregulation revealed by multitissue profiling of lymphocyte gene expression in type 1 diabetes. Genome Res. 2017;27:1807–1815. doi: 10.1101/gr.217984.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang L, Wu S, Lin C, Chang YJ, Tao YX. Alternative splicing of Nrcam gene in dorsal root ganglion contributes to neuropathic pain. J Pain. 2020;21:892–904. doi: 10.1016/j.jpain.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huan L, Guo T, Wu Y, Xu L, Huang S, Xu Y, Liang L, He X. Hypoxia induced LUCAT1/PTBP1 axis modulates cancer cell viability and chemotherapy response. Mol Cancer. 2020;19:11. doi: 10.1186/s12943-019-1122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesari E, Loiarro M, Naro C, Pieraccioli M, Farini D, Pellegrini L, Pagliarini V, Bielli P, Sette C. Combinatorial control of Spo11 alternative splicing by modulation of RNA polymerase II dynamics and splicing factor recruitment during meiosis. Cell Death Dis. 2020;11:240. doi: 10.1038/s41419-020-2443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehravar M, Jafarzadeh M, Kay M, Najafi H, Hosseini F, Mowla SJ, Soltani BM. Introduction of novel splice variants for CASC18 gene and its relation to the neural differentiation. Gene. 2017;603:27–33. doi: 10.1016/j.gene.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Wan R, Bai R, Shi Y. Molecular choreography of pre-mRNA splicing by the spliceosome. Curr Opin Struct Biol. 2019;59:124–133. doi: 10.1016/j.sbi.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Papasaikas P, Valcarcel J. The spliceosome: the ultimate RNA chaperone and sculptor. Trends Biochem Sci. 2016;41:33–45. doi: 10.1016/j.tibs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Fu XD, Ares M Jr. Context-dependent control of alternative splicing by RNA-binding proteins. Nat Rev Genet. 2014;15:689–701. doi: 10.1038/nrg3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong S. SR proteins: binders, regulators, and connectors of RNA. Mol Cells. 2017;40:1–9. doi: 10.14348/molcells.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y, Rio DC. Mechanisms and regulation of alternative pre-mRNA splicing. Annu Rev Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Xiao X, Zhang J, Choudhury R, Robertson A, Li K, Ma M, Burge CB, Wang Z. A complex network of factors with overlapping affinities represses splicing through intronic elements. Nat Struct Mol Biol. 2013;20:36–45. doi: 10.1038/nsmb.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Ma M, Xiao X, Wang Z. Intronic splicing enhancers, cognate splicing factors and context-dependent regulation rules. Nat Struct Mol Biol. 2012;19:1044–1052. doi: 10.1038/nsmb.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue D, Bradley RK, Abdel-Wahab O. Spliceosomal gene mutations in myelodysplasia: molecular links to clonal abnormalities of hematopoiesis. Genes Dev. 2016;30:989–1001. doi: 10.1101/gad.278424.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyun J, Sun Z, Ahmadi AR, Bangru S, Chembazhi UV, Du K, Chen T, Tsukamoto H, Rusyn I, Kalsotra A, Diehl AM. Epithelial splicing regulatory protein 2-mediated alternative splicing reprograms hepatocytes in severe alcoholic hepatitis. J Clin Invest. 2020;130:2129–2145. doi: 10.1172/JCI132691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X, Harvey SE, Zheng R, Lyu J, Grzeskowiak CL, Powell E, Piwnica-Worms H, Scott KL, Cheng C. The RNA-binding protein AKAP8 suppresses tumor metastasis by antagonizing EMT-associated alternative splicing. Nat Commun. 2020;11:486. doi: 10.1038/s41467-020-14304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamazaki T, Liu L, Lazarev D, Al-Zain A, Fomin V, Yeung PL, Chambers SM, Lu CW, Studer L, Manley JL. TCF3 alternative splicing controlled by hnRNP H/F regulates E-cadherin expression and hESC pluripotency. Genes Dev. 2018;32:1161–1174. doi: 10.1101/gad.316984.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer MA, Ashby C, Wardell C, Boyle EM, Ortiz M, Flynt E, Thakurta A, Morgan G, Walker BA. Differential RNA splicing as a potentially important driver mechanism in multiple myeloma. Haematologica. 2020 doi: 10.3324/haematol.2019.235424. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou HL, Luo G, Wise JA, Lou H. Regulation of alternative splicing by local histone modifications: potential roles for RNA-guided mechanisms. Nucleic Acids Res. 2014;42:701–713. doi: 10.1093/nar/gkt875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naftelberg S, Schor IE, Ast G, Kornblihtt AR. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu Rev Biochem. 2015;84:165–198. doi: 10.1146/annurev-biochem-060614-034242. [DOI] [PubMed] [Google Scholar]
- 59.Lev Maor G, Yearim A, Ast G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015;31:274–280. doi: 10.1016/j.tig.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Adhikari S, Xiao W, Zhao YL, Yang YG. m6A: signaling for mRNA splicing. RNA Biol. 2016;13:756–759. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3’-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu LY, Zhu YR, Dai DJ, Wang X, Jin HC. Epigenetic regulation of alternative splicing. Am J Cancer Res. 2018;8:2346–2358. [PMC free article] [PubMed] [Google Scholar]
- 63.Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci. 2019;44:33–52. doi: 10.1016/j.tibs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Romero-Barrios N, Legascue MF, Benhamed M, Ariel F, Crespi M. Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 2018;46:2169–2184. doi: 10.1093/nar/gky095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lan Z, Yao X, Sun K, Li A, Liu S, Wang X. The interaction between lncRNA SNHG6 and hnRNPA1 contributes to the growth of colorectal cancer by enhancing aerobic glycolysis through the regulation of alternative splicing of PKM. Front Oncol. 2020;10:363. doi: 10.3389/fonc.2020.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Troyer L, Zhao P, Pastor T, Baietti MF, Barra J, Vendramin R, Dok R, Lechat B, Najm P, Van Haver D, Impens F, Leucci E, Sablina AA. Stress-induced lncRNA LASTR fosters cancer cell fitness by regulating the activity of the U4/U6 recycling factor SART3. Nucleic Acids Res. 2020;48:2502–2517. doi: 10.1093/nar/gkz1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villamizar O, Chambers CB, Riberdy JM, Persons DA, Wilber A. Long noncoding RNA Saf and splicing factor 45 increase soluble Fas and resistance to apoptosis. Oncotarget. 2016;7:13810–13826. doi: 10.18632/oncotarget.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez I, Munita R, Agirre E, Dittmer TA, Gysling K, Misteli T, Luco RF. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22:370–376. doi: 10.1038/nsmb.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, Dorrestein PC, Rosenfeld MG. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell. 2011;147:773–788. doi: 10.1016/j.cell.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kahles A, Lehmann KV, Toussaint NC, Hüser M, Stark SG, Sachsenberg T, Stegle O, Kohlbacher O, Sander C Cancer Genome Atlas Research Network. Rätsch G. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell. 2018;34:211–224. e216. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma X, Zhang W, Zhang R, Li J, Li S, Ma Y, Jin W, Wang K. Overexpressed long noncoding RNA CRNDE with distinct alternatively spliced isoforms in multiple cancers. Front Med. 2019;13:330–343. doi: 10.1007/s11684-017-0557-0. [DOI] [PubMed] [Google Scholar]
- 72.Rosa R, Villegas-Ruiz V, Caballero-Palacios MC, Perez-Lopez EI, Murata C, Zapata-Tarres M, Cardenas-Cardos R, Paredes-Aguilera R, Rivera-Luna R, Juarez-Mendez S. Expression of ZNF695 transcript variants in childhood B-cell acute lymphoblastic leukemia. Genes (Basel) 2019;10:716. doi: 10.3390/genes10090716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S, Wan H, Zhang X. LncRNA LHFPL3-AS1 contributes to tumorigenesis of melanoma stem cells via the miR-181a-5p/BCL2 pathway. Cell Death Dis. 2020;11:950. doi: 10.1038/s41419-020-03141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hua JT, Ahmed M, Guo H, Zhang Y, Chen S, Soares F, Lu J, Zhou S, Wang M, Li H, Larson NB, McDonnell SK, Patel PS, Liang Y, Yao CQ, van der Kwast T, Lupien M, Feng FY, Zoubeidi A, Tsao MS, Thibodeau SN, Boutros PC, He HH. Risk SNP-mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19. Cell. 2018;174:564–575. e518. doi: 10.1016/j.cell.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 75.Olivero CE, Martinez-Terroba E, Zimmer J, Liao C, Tesfaye E, Hooshdaran N, Schofield JA, Bendor J, Fang D, Simon MD, Zamudio JR, Dimitrova N. p53 activates the long noncoding RNA Pvt1b to inhibit myc and suppress tumorigenesis. Mol Cell. 2020;77:761–774. e768. doi: 10.1016/j.molcel.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills JD, Chen J, Kim WS, Waters PD, Prabowo AS, Aronica E, Halliday GM, Janitz M. Long intervening non-coding RNA 00320 is human brain-specific and highly expressed in the cortical white matter. Neurogenetics. 2015;16:201–213. doi: 10.1007/s10048-015-0445-1. [DOI] [PubMed] [Google Scholar]
- 77.Matouk I, Ayesh B, Schneider T, Ayesh S, Ohana P, de-Groot N, Hochberg A, Galun E. Oncofetal splice-pattern of the human H19 gene. Biochem Biophys Res Commun. 2004;318:916–919. doi: 10.1016/j.bbrc.2004.04.117. [DOI] [PubMed] [Google Scholar]
- 78.Li QY, Yang K, Liu FG, Sun XG, Chen L, Xiu H, Liu XS. Long noncoding RNA CASC2c inhibited cell proliferation in hepatocellular carcinoma by inactivated ERK1/2 and Wnt/beta-catenin signaling pathway. Clin Transl Oncol. 2020;22:302–310. doi: 10.1007/s12094-019-02223-7. [DOI] [PubMed] [Google Scholar]
- 79.Pei Z, Du X, Song Y, Fan L, Li F, Gao Y, Wu R, Chen Y, Li W, Zhou H, Yang Y, Zeng J. Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8:18145–18153. doi: 10.18632/oncotarget.15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang G, Wu X, Li S, Xu X, Zhu H, Chen X. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6:26524. doi: 10.1038/srep26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He Y, Jing Y, Wei F, Tang Y, Yang L, Luo J, Yang P, Ni Q, Pang J, Liao Q, Xiong F, Guo C, Xiang B, Li X, Zhou M, Li Y, Xiong W, Zeng Z, Li G. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018;9:235. doi: 10.1038/s41419-018-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang C, Liu S, Wang H, Zhang Z, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8:5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 83.Chang Z, Cui J, Song Y. Long noncoding RNA PVT1 promotes EMT via mediating microRNA-186 targeting of Twist1 in prostate cancer. Gene. 2018;654:36–42. doi: 10.1016/j.gene.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 84.Kong R, Zhang EB, Yin DD, You LH, Xu TP, Chen WM, Xia R, Wan L, Sun M, Wang ZX, De W, Zhang ZH. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. doi: 10.1186/s12943-015-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang T, Zhou H, Liu P, Yan L, Yao W, Chen K, Zeng J, Li H, Hu J, Xu H, Ye Z. lncRNA PVT1 and its splicing variant function as competing endogenous RNA to regulate clear cell renal cell carcinoma progression. Oncotarget. 2017;8:85353–85367. doi: 10.18632/oncotarget.19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao H, He Y, Li H, Zhu A, Ye Y, Liu G, Zhao C, Zhang X. The opposite role of alternatively spliced isoforms of LINC00477 in gastric cancer. Cancer Manag Res. 2019;11:4569–4576. doi: 10.2147/CMAR.S202430. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Yuan JH, Liu XN, Wang TT, Pan W, Tao QF, Zhou WP, Wang F, Sun SH. The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nat Cell Biol. 2017;19:820–832. doi: 10.1038/ncb3538. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Colome AM, Lee-Rivera I, Benavides-Hidalgo R, Lopez E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10:50. doi: 10.1186/s13045-017-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia X, Niu P, Xie C, Liu H. Long noncoding RNA PXN-AS1-L promotes the malignancy of nasopharyngeal carcinoma cells via upregulation of SAPCD2. Cancer Med. 2019;8:4278–4291. doi: 10.1002/cam4.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan J, Jia Y, Chen H, Chen W, Zhou X. Long non-coding RNA PXN-AS1 suppresses pancreatic cancer progression by acting as a competing endogenous RNA of miR-3064 to upregulate PIP4K2B expression. J Exp Clin Cancer Res. 2019;38:390. doi: 10.1186/s13046-019-1379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y, Ma L. New insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers. 2019;11:216. doi: 10.3390/cancers11020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amodio N, Raimondi L, Juli G, Stamato MA, Caracciolo D, Tagliaferri P, Tassone P. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol. 2018;11:63. doi: 10.1186/s13045-018-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meseure D, Vacher S, Lallemand F, Alsibai KD, Hatem R, Chemlali W, Nicolas A, De Koning L, Pasmant E, Callens C, Lidereau R, Morillon A, Bieche I. Prognostic value of a newly identified MALAT1 alternatively spliced transcript in breast cancer. Br J Cancer. 2016;114:1395–1404. doi: 10.1038/bjc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazar J, Rosado A, Shelley J, Marchica J, Westmoreland TJ. The long non-coding RNA GAS5 differentially regulates cell cycle arrest and apoptosis through activation of BRCA1 and p53 in human neuroblastoma. Oncotarget. 2017;8:6589–6607. doi: 10.18632/oncotarget.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grelet S, Link LA, Howley B, Obellianne C, Palanisamy V, Gangaraju VK, Diehl JA, Howe PH. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shao M, Yang Q, Zhu W, Jin H, Wang J, Song J, Kong Y, Lv X. LncHOXA10 drives liver TICs self-renewal and tumorigenesis via HOXA10 transcription activation. Mol Cancer. 2018;17:173. doi: 10.1186/s12943-018-0921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang QS, Li B, Xu G, Yang SQ, Wang P, Tang HH, Liu YY. Long noncoding RNA LINC00483/microRNA-144 regulates radiosensitivity and epithelial-mesenchymal transition in lung adenocarcinoma by interacting with HOXA10. J Cell Physiol. 2019;234:11805–11821. doi: 10.1002/jcp.27886. [DOI] [PubMed] [Google Scholar]
- 98.Song C, Han Y, Luo H, Qin Z, Chen Z, Liu Y, Lu S, Sun H, Zhou C. HOXA10 induces BCL2 expression, inhibits apoptosis, and promotes cell proliferation in gastric cancer. Cancer Med. 2019;8:5651–5661. doi: 10.1002/cam4.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park SM, Choi EY, Bae M, Choi JK, Kim YJ. A long-range interactive DNA methylation marker panel for the promoters of HOXA9 and HOXA10 predicts survival in breast cancer patients. Clin Epigenetics. 2017;9:73. doi: 10.1186/s13148-017-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Yuan Z, Jiang Y, Shen R, Gu M, Xu W, Gu X. Inhibition of splicing factor 3b subunit 1 (SF3B1) reduced cell proliferation, induced apoptosis and resulted in cell cycle arrest by regulating homeobox A10 (HOXA10) splicing in AGS and MKN28 human gastric cancer cells. Med Sci Monit. 2020;26:e919460. doi: 10.12659/MSM.919460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williamson L, Saponaro M, Boeing S, East P, Mitter R, Kantidakis T, Kelly GP, Lobley A, Walker J, Spencer-Dene B, Howell M, Stewart A, Svejstrup JQ. UV irradiation induces a non-coding RNA that functionally opposes the protein encoded by the same gene. Cell. 2017;168:843–855. e813. doi: 10.1016/j.cell.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Q, Deng Y, Xu Y, Ding N, Wang C, Zhao X, Lou X, Li Y, Zhao H, Fang X. Knockdown of SSATX, an alternative splicing variant of the SAT1 gene, promotes melanoma progression. Gene. 2019;716:144010. doi: 10.1016/j.gene.2019.144010. [DOI] [PubMed] [Google Scholar]
- 103.Pal G, Huaman J, Levine F, Orunmuyi A, Olapade-Olaopa EO, Onagoruwa OT, Ogunwobi OO. Long noncoding RNA from PVT1 exon 9 is overexpressed in prostate cancer and induces malignant transformation and castration resistance in prostate epithelial cells. Genes (Basel) 2019;10:964. doi: 10.3390/genes10120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choudhari R, Yang B, Rotwein P, Gadad SS. Structure and expression of the long noncoding RNA gene MIR503 in humans and non-human primates. Mol Cell Endocrinol. 2020;510:110819. doi: 10.1016/j.mce.2020.110819. [DOI] [PubMed] [Google Scholar]
- 105.Hong CH, Ho JC, Lee CH. Steroid receptor RNA activator, a long noncoding RNA, activates p38, facilitates epithelial-mesenchymal transformation, and mediates experimental melanoma metastasis. J Invest Dermatol. 2020;140:1355–1363. e1. doi: 10.1016/j.jid.2019.09.028. [DOI] [PubMed] [Google Scholar]


