Abstract
Purpose:
Time to surgery (TTS) is a potentially modifiable factor associated with survival after breast cancer diagnosis and can serve as a proxy for quality of oncologic care coordination. We sought to determine whether factors associated with delays in TTS vary between patients who receive neoadjuvant systemic therapy (NST) vs upfront surgery and whether the impact of these delays on overall survival (OS) varies with treatment sequence.
Methods:
Women ≥18 years old with Stage I-III breast cancer were identified in the National Cancer Database (2004–2014). Multivariate linear regression stratified by treatment sequence (upfront-surgery vs NST [neoadjuvant chemotherapy {NAC}, neoadjuvant endocrine therapy {NAE}, or both {NACE}]) was used to identify factors associated with TTS. Cox proportional hazards models were used to estimate the effect of TTS on overall survival (OS).
Results:
Of 693,469 patients, 14.8% (n=102,326) received NST (NAC n=85,143, NAE n=10,004, NACE n=7179). Non-White race/ethnicity, no or government-issued insurance, more extensive surgery (i.e., mastectomy and contralateral prophylactic mastectomy vs breast-conserving surgery), and post-mastectomy reconstruction were associated with significantly longer adjusted TTS for NAC and upfront-surgery recipients, but only upfront-surgery patients had progressively worse OS with increasing TTS (>180 vs ≤30 days: HR=1.31, all p<0.001).
Conclusions:
Surgery extent, race/ethnicity, and insurance were associated with TTS across treatment groups, but longer TTS was only associated with worse OS in upfront-surgery patients. Our findings can help inform surgeon-patient communication, shared decision-making, care coordination, and patients’ expectations throughout both NST and in the perioperative period.
Keywords: breast cancer, chemotherapy, endocrine therapy, neoadjuvant, quality of care, time to treatment
Introduction
Breast cancer mortality has decreased at a rate of 1–2% per year in high-income countries over the past 30 years, and the early detection and treatment facilitated by screening mammography have contributed directly to this improvement in survival.1–4 As surgery remains the primary curative treatment for breast cancer, delays in surgical treatment should, in theory, lead to worse survival outcomes, but initial studies examining this relationship produced mixed results.5–7 However, more recent large-scale observational studies of women with breast cancer in the United States (US) have demonstrated an association between increased time-to-surgery (TTS) and worse overall and disease-specific survival.8–10
In patients with cancer, prolonged TTS can sometimes be explained by the inherent complexity associated with coordinating different components of treatment planning, such as radiologic assessment, pathological review, and coordinating operating-room availability for cases involving reconstruction. But these delays can also reflect gaps in oncologic care coordination and are observed disproportionately among vulnerable patients, with previous studies demonstrating an association between prolonged TTS and patient factors such as insurance status and race/ethnicity.9–12 More recently, there has been a trend to define TTS as a breast cancer quality measure, a development that could have implications for payment reimbursement and hospital accreditation.4,13
With increasing numbers of patients now receiving neoadjuvant systemic therapy (NST, i.e., neoadjuvant chemotherapy and/or endocrine therapy),14 it is unclear to what extent delays in TTS vary between patients who receive NST versus those who undergo upfront surgery. In this retrospective analysis, we aim to identify patient and clinical factors associated with TTS in a contemporary cohort of women with breast cancer and determine how and to what extent these factors differ between women who undergo upfront surgery as compared to those who receive NST. We also examine and compare the association between TTS and overall survival (OS) in upfront-surgery and NST recipients.
Methods
Data source
This study utilized data from the National Cancer Database (NCDB), which is jointly maintained by the American College of Surgeons and the American Cancer Society. It sources data from the hospital registries of more than 1,500 Commission on Cancer (CoC)-accredited facilities and represents approximately 70% of newly diagnosed cancer cases in the US.15
Patient cohort
Using the NCDB, we identified all female patients≥18 years old with clinical stage I-III, ductal or lobular breast cancer diagnosed between 2004 and 2015 via histological or clinical confirmation as provided by reporting facilities. Patients who did not undergo mastectomy or lumpectomy were excluded, as were patients with unknown/missing chemotherapy or endocrine therapy information, data on timing of treatment, and/or survival information (including all patients diagnosed in the last NCDB reporting year of 2015). TTS was defined as days from breast cancer diagnosis to definitive surgery. In order to examine differential delays in TTS, we grouped patients into separate cohorts based on treatment sequence: those undergoing upfront surgery (regardless of adjuvant therapy) and those receiving NST (i.e., neoadjuvant chemotherapy [NAC], neoadjuvant endocrine therapy [NAE], or both [NACE]). Time to NST was defined as the number of days from diagnosis to first neoadjuvant therapy, which we determined by comparing the time from diagnosis to first chemotherapy/endocrine therapy treatment to the time from diagnosis to definitive surgery. Continuation of systemic therapy in the adjuvant setting cannot be discerned in the NCDB, as only the start date of each therapy is available; therefore, patients who received both NAC and adjuvant chemotherapy and/or both NAE and adjuvant endocrine therapy cannot be distinguished from those who received all of their chemotherapy or all of their endocrine therapy in the neoadjuvant setting.
Patient characteristics were summarized by N (%) for categorical variables, by median (interquartile range [IQR]) for continuous variables, and by treatment sequence for all patients. T-tests and chi-square tests were used to test for inter-group differences in continuous and categorical variables, respectively. In order to facilitate interpretability of our results and after confirming non-linearity of the association between TTS and OS, ordinal TTS intervals were defined as follows and in keeping with previous literature:9 ≤30 days, 31–60 days, 61–90 days, 91–120 days, 121–180 days, and >180 days.
Statistical analysis
Multivariate linear regression was used to identify factors associated with TTS after adjustment for known covariates and stratification by treatment group (upfront-surgery, NAC, NAE, and NACE). Time to NST was included in the models for patients receiving one of the neoadjuvant treatment sequences (i.e., NAC, NAE, NACE). These models were built in the generalized estimating equations framework, and an exchangeable correlation structure was included to account for the correlation of patients treated at the same facility.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan-Meier curves were used to visualize unadjusted OS, and 5-year and 10-year OS rates and 95% confidence intervals (CIs) are reported. Cox proportional hazards models stratified by treatment group (upfront-surgery, NAC, NAE, and NACE) were used to estimate the effect of TTS (as a categorical variable; see above for increments) on OS after adjustment for known covariates. Hazard ratios (HRs) and 95% CIs are reported. A robust sandwich covariance estimator was included in all Cox proportional hazards models to account for the correlation of patients treated at the same facility.
A sensitivity analysis was conducted that included only patients who underwent mastectomy to determine if reconstruction after mastectomy was differentially associated with TTS. Results for this model did not differ significantly from the full model results, therefore only results from the adjusted linear model with lumpectomy and mastectomy patients are presented. Because HER2 status only began to be reliably collected in the NCDB in 2010, we conducted additional sensitivity analyses of patients diagnosed 2010–2014 to allow for molecular subtype (hormone-receptor-positive/HER2+, hormone-receptor-negative/HER2−, hormone-receptor-positive/HER2−, triple-negative) to be included in the multivariate linear regression models for adjusted TTS and the Cox proportional hazards models for adjusted OS (Supplemental Tables 1 and 2).
Only patients with available data for all covariates were included in each model, and effective sample sizes are reported for each table/figure. No adjustments were made for multiple comparisons. A p-value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary NC). Our study was granted exempt status by our institutional review board due to our use of de-identified patient data.
Results
Patient, disease, and treatment characteristics
693,469 patients met our inclusion criteria (Figure 1, Table 1). Median age at diagnosis was 59 years (IQR 50–69), and median follow-up time was 57.9 months (95% CI 57.7–58.0) for all patients with available follow-up data. Most patients (85.2%) underwent upfront surgery (n=591,143), and only 14.8% (n=102,326) received NST. Among NST recipients, 83.2% (n=85,143) received NAC, 9.8% (n=10,004) received NAE, and 7.0% (n=7179) received NACE. Among NAC recipients, a higher proportion were non-Hispanic Black (16.6%) compared to recipients of upfront surgery (9.3%), NAE (9.1%) and NACE (12.8%, p <0.001). Median TTS for upfront-surgery patients was 30 days (IQR 19–46), whereas TTS for NAC, NAE, and NACE patients was 180 days (IQR 154–210), 109.5 days (IQR 52–188), and 201 days (IQR 168–248), respectively.
Figure 1.
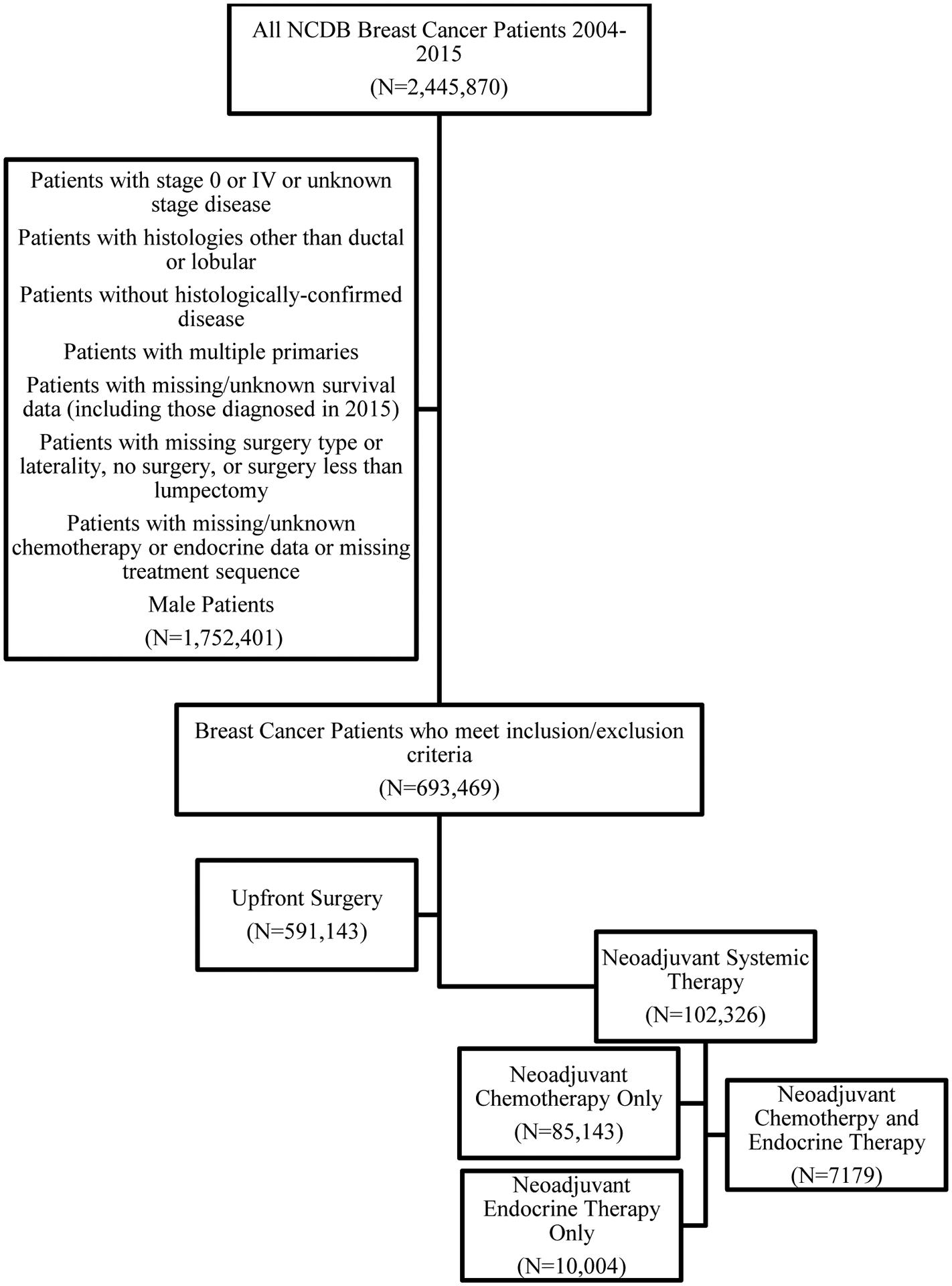
Female Patients with Stage I-III Breast Cancer by Treatment Sequence, National Cancer Database (2004–2014)
Table 1.
Female Patients with Stage I-III Breast Cancer by Treatment Sequence, National Cancer Database (2004–2014)
| All Patients (N=693,469) | Upfront Surgery (N=591,143) | NAC (N=85,143) | NAE (N=10,004) | NACE (N=7179) | P-Value | |
|---|---|---|---|---|---|---|
| Age - Median (IQR) | 59 (50 – 69) | 61 (51 – 70) | 51 (43 – 59) | 67 (57 – 77) | 53 (44 – 62) | <0.001 |
| Race/Ethnicity | <0.001 | |||||
| Non-Hispanic White | 526,836 (76%) | 457,505 (77.4%) | 56,644 (66.5%) | 7695 (76.9%) | 4992 (69.5%) | |
| Non-Hispanic Black | 70,695 (10.2%) | 54,742 (9.3%) | 14,128 (16.6%) | 906 (9.1%) | 919 (12.8%) | |
| Hispanic | 34,051 (4.9%) | 26,605 (4.5%) | 6405 (7.5%) | 485 (4.8%) | 556 (7.7%) | |
| Other | 24,553 (3.5%) | 20,061 (3.4%) | 3851 (4.5%) | 365 (3.6%) | 276 (3.8%) | |
| Charlson-Deyo Comorbidity Score | <0.001 | |||||
| 0 | 588,001 (84.8%) | 497,738 (84.2%) | 75,887 (89.1%) | 8041 (80.4%) | 6335 (88.2%) | |
| 1 | 87,081 (12.6%) | 76,909 (13%) | 7963 (9.4%) | 1496 (15%) | 713 (9.9%) | |
| ≥2 | 18,387 (2.7%) | 16,496 (2.8%) | 1293 (1.5%) | 467 (4.7%) | 131 (1.8%) | |
| Facility Type | <0.001 | |||||
| Academic | 206,750 (29.8%) | 170,560 (28.9%) | 30,240 (35.5%) | 3444 (34.4%) | 2506 (34.9%) | |
| Community | 70,331 (10.1%) | 62,271 (10.5%) | 6529 (7.7%) | 902 (9%) | 629 (8.8%) | |
| Comprehensive | 338,121 (48.8%) | 293,814 (49.7%) | 36,805 (43.2%) | 4360 (43.6%) | 3142 (43.8%) | |
| Integrated Network | 78,267 (11.3%) | 64,498 (10.9%) | 11,569 (13.6%) | 1298 (13%) | 902 (12.6%) | |
| Insurance Status | <0.001 | |||||
| Private | 387,868 (55.9%) | 321,917 (54.5%) | 57,573 (67.6%) | 3845 (38.4%) | 4533 (63.1%) | |
| Government | 282,363 (40.7%) | 251,360 (42.5%) | 22,938 (26.9%) | 5779 (57.8%) | 2286 (31.8%) | |
| Not Insured | 14,022 (2%) | 10,083 (1.7%) | 3479 (4.1%) | 195 (1.9%) | 265 (3.7%) | |
| Clinical Stage | <0.001 | |||||
| I | 419,071 (60.4%) | 405,484 (68.6%) | 8246 (9.7%) | 4296 (42.9%) | 1045 (14.6%) | |
| II | 221,872 (32%) | 165,953 (28.1%) | 47,884 (56.2%) | 4361 (43.6%) | 3674 (51.2%) | |
| III | 52,526 (7.6%) | 19,706 (3.3%) | 29,013 (34.1%) | 1347 (13.5%) | 2460 (34.3%) | |
| Tumor Size (cm) - Median (IQR) | 1.7 (1 – 2.6) | 1.5 (1 – 2.3) | 3.2 (2.1 – 5) | 2.2 (1.3 – 3.6) | 3.1 (2 – 5) | <0.001 |
| # LNs Examined - Median (IQR) | 3 (2 – 8) | 3 (2 – 7) | 8 (3 – 15) | 3 (1 – 9) | 8 (3 – 14) | <0.001 |
| # Positive LNs - Median (IQR) | 0 (0 – 1) | 0 (0 – 1) | 1 (0 – 3) | 0 (0 – 1) | 1 (0 – 3) | <0.001 |
| Molecular Subtype* | <0.001 | |||||
| HR+/HER2+ | 43,725 (9.8%) | 31,159 (8.3%) | 10,932 (19.1%) | 386 (5.6%) | 1248 (27%) | |
| HR+/HER2− | 308,548 (69.3%) | 278,443 (74%) | 21,060 (36.8%) | 6168 (89.4%) | 2877 (62.2%) | |
| HR−/HER2+ | 18,335 (4.1%) | 11,405 (3%) | 6791 (11.9%) | 7 (0.1%) | 132 (2.9%) | |
| TNBC | 52,835 (11.9%) | 36,344 (9.7%) | 16,302 (28.5%) | 37 (0.5%) | 152 (3.3%) | |
| Treatment with Chemotherapy | 325,291 (46.9%) | 231,336 (39.1%) | 85,143 (100%) | 1633 (16.3%) | 7179 (100%) | <0.001 |
| Type of Chemotherapy** | - | |||||
| Adjuvant | 232,969 (71.6%) | 231,336 (100%) | 0 (0%) | 1633 (100%) | 0 (0%) | |
| Neoadjuvant | 92,322 (28.4%) | 0 (0%) | 85,143 (100%) | 0 (0%) | 7179 (100%) | |
| Treatment with Radiation | 461,449 (66.5%) | 387,611 (65.6%) | 63,714 (74.8%) | 5362 (53.6%) | 4762 (66.3%) | <0.001 |
| Treatment with Endocrine Therapy | 476,249 (68.7%) | 416,467 (70.5%) | 42,599 (50%) | 10,004 (100%) | 7179 (100%) | <0.001 |
| Type of Endocrine Therapy*** | - | |||||
| Adjuvant | 459,066 (96.4%) | 416,467 (100%) | 42,599 (100%) | 0 (0%) | 0 (0%) | |
| Neoadjuvant | 17,183 (3.6%) | 0 (0%) | 0 (0%) | 10,004 (100%) | 7179 (100%) | |
| Surgical Approach | <0.001 | |||||
| BCS | 433,681 (62.5%) | 394,736 (66.8%) | 31,313 (36.8%) | 5481 (54.8%) | 2151 (30%) | |
| Bilateral Mastectomy for Bilateral Disease | 57 (0%) | 33 (0%) | 22 (0%) | 0 (0%) | 2 (0%) | |
| CPM | 86,506 (12.5%) | 60,765 (10.3%) | 22,646 (26.6%) | 1034 (10.3%) | 2061 (28.7%) | |
| Unilateral Mastectomy | 173,225 (25%) | 135,609 (22.9%) | 31,162 (36.6%) | 3489 (34.9%) | 2965 (41.3%) | |
| Reconstruction (% all ‖ % mastectomy)**** | 89,976 (13% ‖ 34.6%) | 68,482 (11.6% ‖ 34.9%) | 18,362 (21.6% ‖ 34.1%) | 1321 (13.2% ‖ 29.2%) | 1811 (25.2% ‖ 36%) | <0.001 |
| Time to Surgery - Categorical | <0.001 | |||||
| ≤30 Days | 303,924 (43.8%) | 302,241 (51.1%) | 548 (0.6%) | 1109 (11.1%) | 26 (0.4%) | |
| 31–60 Days | 217,683 (31.4%) | 215,027 (36.4%) | 726 (0.9%) | 1880 (18.8%) | 50 (0.7%) | |
| 61–90 Days | 55,934 (8.1%) | 52,868 (8.9%) | 1623 (1.9%) | 1364 (13.6%) | 79 (1.1%) | |
| 91–120 Days | 19,097 (2.8%) | 13,065 (2.2%) | 4818 (5.7%) | 1020 (10.2%) | 194 (2.7%) | |
| 121–180 Days | 44,847 (6.5%) | 5680 (1%) | 35,130 (41.3%) | 1924 (19.2%) | 2113 (29.4%) | |
| >180 Days | 51,984 (7.5%) | 2262 (0.4%) | 42,298 (49.7%) | 2707 (27.1%) | 4717 (65.7%) | |
| Time to Surgery (Days) - Median (IQR) | 35 (21 – 60) | 30 (19 – 46) | 180 (154 – 210) | 109.5 (52 – 188) | 201 (168 – 248) | <0.001 |
| Time to First NST (Days) - Median (IQR) | 30 (21 – 45) | - | 31 (21 – 44) | 27 (14 – 48) | 33 (21 – 51) | <0.001 |
NAC, neoadjuvant chemotherapy only. NACE, neoadjuvant chemotherapy and neoadjuvant endocrine therapy. NAE, neoadjuvant endocrine therapy only. NST, neoadjuvant systemic therapy.
Includes only patients diagnosed 2010 and after.
Out of patients who underwent chemotherapy.
Out of patients who underwent endocrine therapy.
Data only available for mastectomy recipients; proportions are calculated out of all patients and out of patients who underwent mastectomy (total n=259,788, Upfront surgery n=196,407, NAC n=53,830, NAE n=4,523, NACE n=5,028).
Percentages may not add up to 100 due to rounding or missingness.
Nearly 2/3 (62.5%) of patients underwent breast-conserving surgery (BCS, i.e., lumpectomy, n=433,681), 25.0% underwent unilateral mastectomy (UM, n=173,225), 12.5% underwent UM with contralateral prophylactic mastectomy (CPM, n=86,506), and <0.1% underwent bilateral mastectomy for bilateral disease (BM, n=57). NST patients, particularly those receiving NAC or NACE, had higher rates of CPM and UM compared to those who underwent upfront surgery (CPM: upfront-surgery 10.3%, NAC 26.6%, NACE 28.7%; UM: upfront-surgery 22.9%, NAC 36.6%, NACE 41.3%, p<0.001). Rates of post-mastectomy reconstruction ranged from 29.2% among NAE mastectomy recipients to 36% among NACE mastectomy recipients (p<0.001).
Time to surgery (TTS)
Among patients who underwent upfront surgery (Table 2), longer adjusted TTS was associated with greater extent of surgery, with both UM and CPM being associated with a nearly 6-day delay in TTS (UM: +5.8 days, 95% CI 5.46–6.14; CPM: +5.7 days, 95% CI 5.28–6.11) compared to BCS. A similar association was seen among NAC recipients, for whom both UM (+2.62 days, 95% CI 1.67–3.57) and CPM (+4.01 days, 95% CI 2.95–5.07) were associated with longer TTS vs BCS (all p<0.001). Higher stage of disease was associated with longer TTS among NAC (stage II vs I: +12.62 days, 95% CI 11.03–14.21, p<0.001), NAE (stage II vs I: +56.36 days, 95% CI 50.43–62.29; stage III v I: +90.95 days, 95% CI 81.81–100.09, both p<0.001), and NACE recipients (Stage II vs I: +9.12 days, 95% CI 1.43–16.81, p=0.02; Stage III vs I: +13.53 days, 95% CI 5.19–21.87, p=0.001). Receipt of care at an academic medical center was associated with longer adjusted TTS for all treatment sequences except NACE. Across all treatment groups, non-Hispanic Black race (vs non-Hispanic White race) was associated with longer adjusted TTS, while being Hispanic (vs non-Hispanic White) was only associated with longer TTS among those who received NAC (+3.06 days, 95% CI 1.45–4.67) or upfront surgery (+4.14 days, 95% CI 3.57–4.71, all p<0.01). Having government-issued or no insurance was associated with longer TTS for all treatment groups except NAE recipients (all p<0.001). Effect sizes in our sensitivity analysis of patients from 2010–2014 with complete biomarker information were similar for nearly all variables except as highlighted in Supplemental Table 1.
Table 2.
Linear Regression Model Predicting Time-to-Surgery (TTS) in Adjusted Days by Treatment Sequence, Female Patients with Stage I-III Breast Cancer by Treatment Sequence, National Cancer Database (2004–2014)
| Predictor | Upfront Surgery (N=513,278) | NAC (N=72,467) | NAE (N=8551) | NACE (N=6020) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-Value | Overall P-Value | Estimate (95% CI) | P-Value | Overall P-Value | Estimate (95% CI) | P-Value | Overall P-Value | Estimate (95% CI) | P-Value | Overall P-Value | |
| Age (Years) | −0.02 (−0.03–−0.01) | <0.001 | <0.001 | −0.15 (−0.19–−0.12) | <0.001 | <0.001 | 0.54 (0.29–0.79) | <0.001 | <0.001 | 0.11 (−0.1–0.31) | 0.32 | 0.32 |
| Race/Ethnicity | <0.001 | <0.001 | 0.20 | 0.04 | ||||||||
| Non-Hispanic White | REF | REF | REF | REF | ||||||||
| Hispanic | 4.14 (3.57–4.71) | <0.001 | 3.06 (1.45–4.67) | <0.001 | 0.51 (−11.18–12.2) | 0.93 | −3.51 (−12.93–5.91) | 0.47 | ||||
| Non-Hispanic Black | 7 (6.56–7.45) | <0.001 | 5.92 (4.83–7.01) | <0.001 | 8.82 (0.36–17.28) | 0.04 | 10.3 (2.59–18.01) | 0.009 | ||||
| Other | 1.35 (0.79–1.9) | <0.001 | 0.6 (−1.1–2.29) | 0.49 | 5.6 (−11.03–22.23) | 0.51 | 4.37 (−10.18–18.91) | 0.56 | ||||
| Education Level | <0.001 | 0.01 | 0.46 | 0.11 | ||||||||
| ≤83% HS Graduation Rate | REF | REF | REF | REF | ||||||||
| >83% HS Graduation Rate | −0.91 (−1.15–−0.68) | <0.001 | −1.24 (−2.19–−0.28) | 0.01 | 2.22 (−3.65–8.1) | 0.46 | −4.43 (−9.81–0.95) | 0.11 | ||||
| Insurance Type | <0.001 | <0.001 | 0.32 | <0.001 | ||||||||
| Private | REF | REF | REF | REF | ||||||||
| Government | 2.5 (2.23–2.76) | <0.001 | 3.34 (2.34–4.33) | <0.001 | 3.15 (−3.01–9.3) | 0.32 | 8.28 (2.59–13.96) | 0.004 | ||||
| Not Insured | 4.38 (3.42–5.33) | <0.001 | 7.04 (5.11–8.97) | <0.001 | 12.38 (−6.45–31.22) | 0.20 | 17.17 (6.92–27.43) | 0.001 | ||||
| Clinical Stage | <0.001 | <0.001 | <0.001 | 0.006 | ||||||||
| I | REF | REF | REF | REF | ||||||||
| II | −1.21 (−1.42–−1) | <0.001 | 12.62 (11.03–14.21) | <0.001 | 56.36 (50.43–62.29) | <0.001 | 9.12 (1.43–16.81) | 0.02 | ||||
| III | −2.81 (−3.45–−2.17) | <0.001 | 16.39 (14.68–18.1) | <0.001 | 90.95 (81.81–100.09) | <0.001 | 13.53 (5.19–21.87) | 0.001 | ||||
| Surgical Approach | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| BCS | REF | REF | REF | REF | ||||||||
| Bilateral Mastectomy for Bilateral Disease | 13.77 (3.78–23.76) | 0.007 | 1.34 (−16.35–19.02) | 0.88 | - | - | 318.99 (308.19–329.8) | <0.001 | ||||
| CPM | 5.7 (5.28–6.11) | <0.001 | 4.01 (2.95–5.07) | <0.001 | −19.71 (−29.67–−9.75) | <0.001 | 3.18 (−3.98–10.35) | 0.38 | ||||
| Unilateral Mastectomy | 5.8 (5.46–6.14) | <0.001 | 2.62 (1.67–3.57) | <0.001 | −15.65 (−21.93–−9.38) | <0.001 | 4.98 (−1.14–11.11) | 0.11 | ||||
| Underwent Reconstruction | <0.001 | <0.001 | 0.25 | 0.15 | ||||||||
| No | REF | REF | REF | REF | ||||||||
| Yes | 8.17 (7.69–8.65) | <0.001 | 4.25 (3.14–5.35) | <0.001 | −4.95 (−13.42–3.52) | 0.25 | 4.5 (−1.67–10.67) | 0.15 | ||||
| Time to First NST (Days) | - | - | - | 0.98 (0.96–1) | <0.001 | <0.001 | 1.11 (1.02–1.2) | <0.001 | <0.001 | 1.15 (1.07–1.24) | <0.001 | <0.001 |
NAC, neoadjuvant chemotherapy only. NACE, neoadjuvant chemotherapy and neoadjuvant endocrine therapy. NAE, neoadjuvant endocrine therapy only. NST, neoadjuvant systemic therapy.
This model was also adjusted for the following covariates (asterisk indicates significant associations with time-to-surgery for the treatment sequence(s) in parentheses): Year of diagnosis* (upfront surgery, NAC, NAE), Charlson-Deyo Comorbidity Score* (upfront surgery, NAC), Income Level* (upfront surgery), Facility Type* (upfront surgery, NAC, NAE), Facility Location* (upfront surgery, NAC), estrogen receptor [ER] status* (upfront surgery, NAC, NAE), progesterone receptor [PR] status* (upfront surgery), Grade* (upfront surgery, NAC, NAE), Histology* (upfront surgery), and Having Different Dates for First and Definitive Surgical Procedures* (upfront surgery, NAC, NACE).
Overall survival (OS)
Unadjusted OS rates differed significantly by treatment sequence, with patients undergoing upfront surgery having the highest 5- and 10-year unadjusted OS rates (Figure 2). After adjustment, patients who underwent upfront surgery saw a consistent decline in OS with increasing TTS (HR 1.31 for TTS>180 days vs TTS≤30 days, p<0.001, Table 3). Among patients receiving NAC and NAE, TTS was statistically associated with OS, but this association was not unidirectional, and no clear pattern was observed. Among upfront-surgery, NAC, and NACE recipients, non-Hispanic Black race was associated with worse adjusted OS (upfront-surgery HR 1.12, 95% CI 1.08–1.16; NAC HR 1.23, 95% CI 1.16–1.29; NACE HR 1.61, 95% CI 1.32–1.96) while Hispanic ethnicity was associated with improved adjusted OS across all treatment groups (all p<0.001). Among patients who received NAC, greater extent of surgery - e.g., UM (HR 1.71, 95% CI 1.62–1.79) and CPM (HR 1.33, 95% 1.25–1.40) - was associated with worse OS when compared to BCS, with bilateral mastectomy for bilateral disease being associated with the worst OS (HR 5.30, 95% CI 1.66–16.89) compared to BCS (all p<0.01). In contrast, UM (HR 0.94, 95% CI 0.92–0.97) and CPM (HR 0.81, 95% CI 0.77–0.85) were associated with slightly improved OS among patients receiving upfront surgery (both p<0.001). Receipt of care at an academic center (reference) was associated with improved OS for patients who received upfront surgery, NAC, or NACE (all p<0.05). As with TTS, effect sizes in our sensitivity analysis of patients from 2010–2014 with complete biomarker information were similar for most variables except as noted in Supplemental Table 2.
Figure 2.
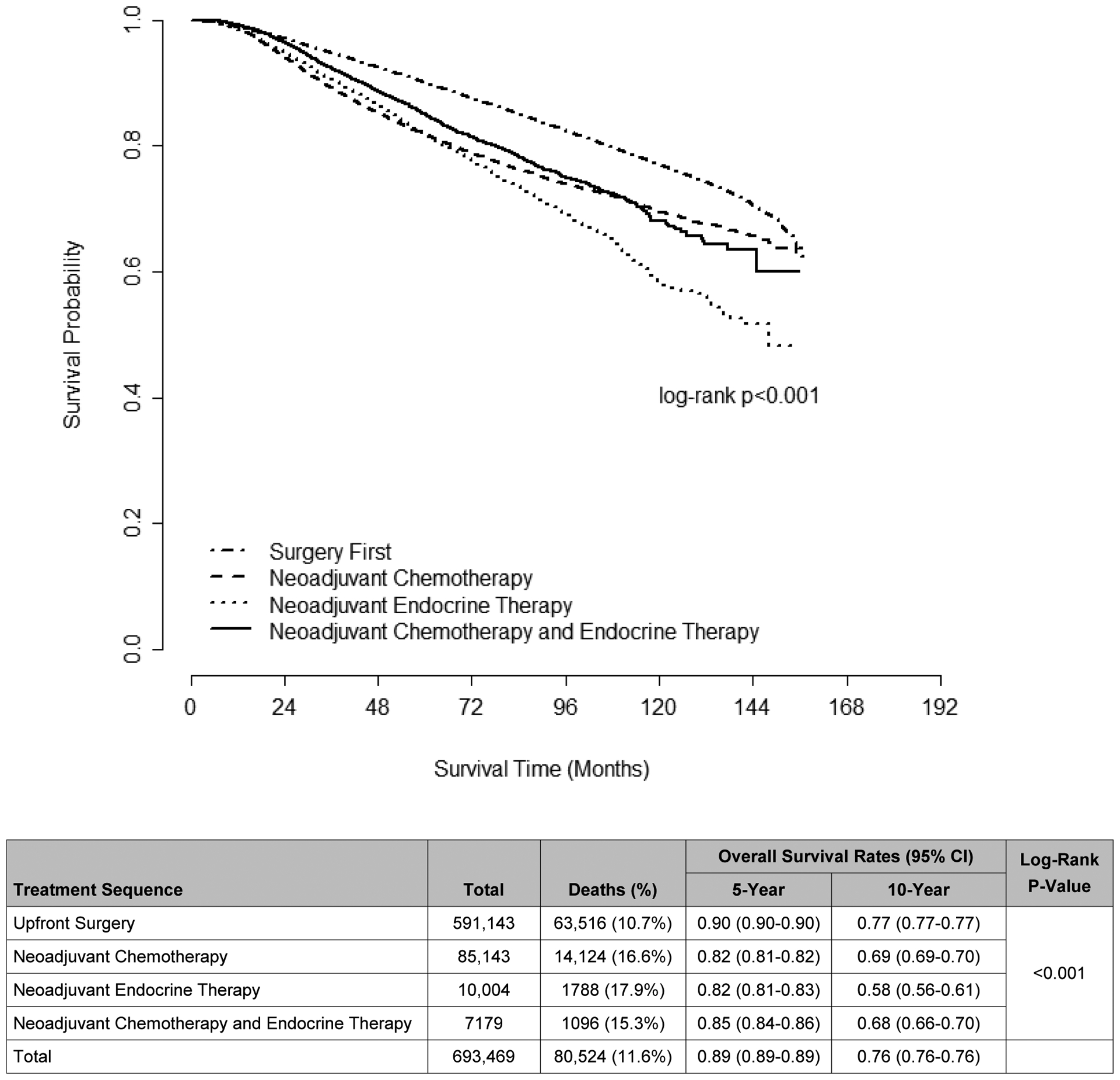
Unadjusted Overall Survival, Female Patients with Stage I-III Breast Cancer by Treatment Sequence, National Cancer Database (2004–2014)
Table 3.
Adjusted Overall Survival by Treatment Sequence, Female Patients with Stage I-III Breast Cancer, National Cancer Database (2004–2014)
| Upfront Surgery (N=507,170) | NAC (N=69,526) | NAE (N=8389) | NACE (N=5790) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-Value | Overall P-Value | Hazard Ratio (95% CI) | P-Value | Overall P-Value | Hazard Ratio (95% CI) | P-Value | Overall P-Value | Hazard Ratio (95% CI) | P-Value | Overall P-Value | |
| Time to Surgery (Days) | <0.001 | <0.001 | 0.02 | 0.25 | ||||||||
| ≤30 Days | REF | REF | REF | REF | ||||||||
| 31–60 Days | 0.98 (0.96–1) | 0.08 | 1.15 (0.82–1.59) | 0.42 | 1.17 (0.95–1.44) | 0.15 | 0.37 (0.09–1.6) | 0.18 | ||||
| 61–90 Days | 1.08 (1.04–1.12) | <0.001 | 1.24 (0.95–1.63) | 0.12 | 1.1 (0.85–1.42) | 0.47 | 1.27 (0.37–4.39) | 0.70 | ||||
| 91–120 Days | 1.19 (1.12–1.27) | <0.001 | 1.2 (0.93–1.56) | 0.16 | 1.24 (0.97–1.59) | 0.08 | 0.98 (0.29–3.3) | 0.98 | ||||
| 121–180 Days | 1.26 (1.16–1.37) | <0.001 | 1.03 (0.8–1.32) | 0.81 | 0.91 (0.73–1.13) | 0.39 | 0.82 (0.26–2.55) | 0.74 | ||||
| >180 Days | 1.31 (1.16–1.48) | <0.001 | 1.03 (0.8–1.32) | 0.83 | 1.06 (0.86–1.31) | 0.58 | 0.8 (0.26–2.51) | 0.71 | ||||
| Age (years) | 1.05 (1.05–1.05) | <0.001 | <0.001 | 1.01 (1.01–1.01) | <0.001 | <0.001 | 1.05 (1.04–1.06) | <0.001 | <0.001 | 1.01 (1–1.02) | 0.002 | 0.002 |
| Race/Ethnicity | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Non-Hispanic White | REF | REF | REF | REF | ||||||||
| Hispanic | 0.74 (0.69–0.79) | <0.001 | 0.79 (0.72–0.86) | <0.001 | 0.54 (0.37–0.78) | 0.001 | 0.67 (0.49–0.93) | 0.02 | ||||
| Non-Hispanic Black | 1.12 (1.08–1.16) | <0.001 | 1.23 (1.16–1.29) | <0.001 | 0.95 (0.78–1.16) | 0.64 | 1.61 (1.32–1.96) | <0.001 | ||||
| Other | 0.69 (0.64–0.75) | <0.001 | 0.76 (0.69–0.85) | <0.001 | 0.5 (0.3–0.81) | 0.005 | 0.92 (0.59–1.42) | 0.70 | ||||
| Charlson-Deyo Comorbidity Score | <0.001 | <0.001 | <0.001 | 0.002 | ||||||||
| 0 | REF | REF | REF | REF | ||||||||
| 1 | 1.45 (1.42–1.48) | <0.001 | 1.21 (1.14–1.28) | <0.001 | 1.58 (1.37–1.82) | <0.001 | 1.14 (0.9–1.44) | 0.27 | ||||
| ≥2 | 2.34 (2.25–2.44) | <0.001 | 1.51 (1.33–1.71) | <0.001 | 2.83 (2.33–3.44) | <0.001 | 2.03 (1.34–3.09) | <0.001 | ||||
| Income Level | <0.001 | <0.001 | 0.02 | 0.02 | ||||||||
| <$48,000 | REF | REF | REF | REF | ||||||||
| ≥$48,000 | 0.89 (0.87–0.91) | <0.001 | 0.91 (0.86–0.95) | <0.001 | 0.85 (0.75–0.97) | 0.02 | 0.82 (0.69–0.97) | 0.02 | ||||
| Insurance Type | <0.001 | <0.001 | 0.004 | <0.001 | ||||||||
| Private | REF | REF | REF | REF | ||||||||
| Government | 1.35 (1.31–1.39) | <0.001 | 1.33 (1.27–1.4) | <0.001 | 1.34 (1.13–1.6) | <0.001 | 1.41 (1.18–1.69) | <0.001 | ||||
| Not Insured | 1.52 (1.41–1.65) | <0.001 | 1.35 (1.22–1.5) | <0.001 | 1.11 (0.7–1.76) | 0.66 | 2.13 (1.52–2.98) | <0.001 | ||||
| Facility Type | <0.001 | <0.001 | 0.19 | 0.03 | ||||||||
| Academic | REF | REF | REF | REF | ||||||||
| Community | 1.21 (1.14–1.29) | <0.001 | 1.17 (1.08–1.28) | <0.001 | 1.24 (1.02–1.5) | 0.03 | 1.02 (0.77–1.35) | 0.90 | ||||
| Comprehensive | 1.15 (1.09–1.21) | <0.001 | 1.14 (1.08–1.21) | <0.001 | 1.06 (0.93–1.22) | 0.38 | 1.25 (1.06–1.48) | 0.007 | ||||
| Integrated Network | 1.11 (1.03–1.18) | 0.003 | 1.09 (1–1.18) | 0.05 | 1.01 (0.83–1.23) | 0.91 | 1.25 (1–1.56) | 0.05 | ||||
| Clinical Stage | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| I | REF | REF | REF | REF | ||||||||
| II | 1.97 (1.93–2.01) | <0.001 | 1.85 (1.67–2.06) | <0.001 | 1.69 (1.47–1.93) | <0.001 | 1.61 (1.19–2.18) | 0.002 | ||||
| III | 4.22 (4.05–4.39) | <0.001 | 3.32 (2.98–3.7) | <0.001 | 2.49 (2.05–3.01) | <0.001 | 2.87 (2.08–3.95) | <0.001 | ||||
| Treatment with Radiation Therapy | <0.001 | 0.99 | <0.001 | 0.05 | ||||||||
| No | REF | REF | REF | REF | ||||||||
| Yes | 0.62 (0.61–0.64) | <0.001 | 1 (0.95–1.05) | 0.99 | 0.63 (0.56–0.73) | <0.001 | 0.84 (0.7–1) | 0.05 | ||||
| Treatment with Chemotherapya | <0.001 | 0.57 | ||||||||||
| No | REF | REF | ||||||||||
| Yes | 0.85 (0.83–0.87) | <0.001 | 1.05 (0.88–1.26) | 0.57 | ||||||||
| Treatment with Endocrine Therapyb | <0.001 | <0.001 | ||||||||||
| No | REF | REF | ||||||||||
| Yes | 0.6 (0.59–0.62) | <0.001 | 0.65 (0.6–0.69) | <0.001 | ||||||||
| Surgery Typec | <0.001 | <0.001 | 0.40 | <0.001 | ||||||||
| BCS | REF | REF | REF | REF | ||||||||
| Bilateral Mastectomy for Bilateral Disease | 0 (0–0.01) | <0.001 | 5.3 (1.66–16.89) | 0.005 | 0 (0–0) | <0.001 | ||||||
| CPM | 0.81 (0.77–0.85) | <0.001 | 1.33 (1.25–1.4) | <0.001 | 0.83 (0.62–1.11) | 0.20 | 0.93 (0.73–1.19) | 0.57 | ||||
| Unilateral Mastectomy | 0.94 (0.92–0.97) | <0.001 | 1.71 (1.62–1.79) | <0.001 | 1 (0.88–1.14) | 0.97 | 1.51 (1.24–1.82) | <0.001 | ||||
| Time to First NST (Days) | - | - | - | 1 (1) | 0.07 | 0.07 | 1 (1–1) | 0.67 | 0.67 | 1 (0.99–1) | 0.03 | 0.03 |
NAC, neoadjuvant chemotherapy only. NACE, neoadjuvant chemotherapy and neoadjuvant endocrine therapy. NAE, neoadjuvant endocrine therapy only. NST, neoadjuvant systemic therapy.
This model was also adjusted for the following covariates (asterisk indicates significant associations with overall survival for the treatment sequence(s) in parentheses): Education Level* (upfront surgery), Facility Location* (upfront surgery, NAC), ER status* (upfront surgery, NAE), PR status* (all), Grade* (all), Histology* (NAC), Tumor Size* (all), and Having Different Dates for First and Definitive Surgical Procedures* (upfront surgery, NAC, NAE).
All patients in the NAC and NACE groups received chemotherapy so it was not included in the multivariate model for these groups.
All patients in the NAE and NACE groups received endocrine therapy so it was not included in the multivariate model for these groups.
No patients who underwent bilateral mastectomy for bilateral disease died in the neoadjuvant chemo and endocrine cohort. No (N=0) patients who underwent NAE had bilateral mastectomy for bilateral disease.
Discussion
In our examination of TTS in a large contemporary cohort of women with breast cancer, we demonstrated that Hispanic ethnicity, Black race, having no or government-issued insurance, having reconstruction, and having more extensive surgery (UM and CPM vs BCS) were associated with longer TTS in both NAC recipients and in patients who underwent upfront surgery, but a clear association between longer TTS and worse OS was only observed among patients who underwent upfront surgery.
Time from breast cancer diagnosis to surgery is an increasingly accepted quality metric for oncologic care, but in the context of increasing NST use, the value of this measure amongst patients who do not receive upfront surgery has been unclear. However, questions regarding TTS and its impact on long-term outcomes are common during pre-operative clinic visits, and we believe our results can help frame discussions regarding perioperative surgical planning and also assuage NST patients’ anxiety surrounding minor delays to surgery. By separating our patient cohort into those who underwent upfront surgery and those who received different forms of NST, we were able to show that while some factors influencing TTS are common to many patients regardless of treatment sequence and composition, important sequence-specific differences exist with regards to the clinical significance of TTS as a prognosticator and quality metric. By including patients who received both upfront surgery and NST, our study is among the first in the TTS literature that reflects the full spectrum of oncologic care received by women with breast cancer.8–9,11–12
Among patients who received upfront surgery, our study showed decreasing OS with increasing TTS beyond 60 days, with each additional TTS interval being associated with a 5–11% increased risk of mortality (HRs 1.08, 1.19, 1.26, and 1.31 for TTS 61–90, 91–120, 121–180, and >180 days, respectively, vs TTS≤30 days, all p<0.001). These results corroborate those from a previous analysis of the NCDB by Bleicher et al, though data from that study were from a less recent cohort whose members would not have benefited from some of the more effective systemic therapies (e.g., HER2-targeted immunotherapy) now available to patients.9 Notably, our analysis showed that TTS was not associated with a significant difference in OS in patients who received NST, an observation that persisted regardless of NST composition and despite the differences in TTS seen between the three NST groups. The absence of a consistent association between TTS and OS in NST recipients may reflect the efficacy of early systemic therapy in treating micrometastatic disease, which is thought to ultimately drive disease-specific survival in locally advanced breast cancer. Additionally, in patients receiving NAC, who were the youngest and had the lowest comorbidity scores in our study, disease-specific survival - which is not available in the NCDB - may be a more appropriate long-term outcome than OS for assessing the prognostic importance of TTS.
In our analysis, there appeared to be a stronger association between TTS and type of surgery among patients receiving upfront surgery than among NST recipients. Among upfront-surgery patients, those who underwent UM or CPM waited a week longer than BCS patients for their definitive surgery, and patients getting bilateral mastectomy for bilateral disease waited nearly two additional weeks. Our findings are in keeping with other studies showing that receipt of more extensive breast surgery is associated with higher rates of reconstruction and accompanying delays.16–18 We believe this observation may be explained by the increased logistical complexity of coordinating a combined set of operations with more than one surgeon.8 Concomitantly, among NST recipients, the effect of surgery type on TTS may have been less pronounced because these patients would typically have had more preoperative lead time and flexibility while they completed NST to coordinate surgical logistics.
There have been a number of retrospective single-center studies that have analyzed the impact of TTS on patients receiving NAC with mixed results.19–21 The utilization of NAC has increasingly been extended to earlier stages of breast cancer on the heels of the landmark National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18, NSABP B-27, and European Organization for Research and Treatment of Cancer (EORTC) 10902 trials, which showed equivalent oncologic outcomes for neoadjuvant and adjuvant chemotherapy.22–24 Not only is NAC useful for downstaging tumors but it has also been shown that achieving pathologic complete response (pCR) is associated with improved oncologic outcomes in patients with high-risk tumor subtypes, providing an important opportunity for on-treatment prognostication and evidence-based decisions about additional adjuvant treatment.25–28
In contrast, the role of endocrine therapy in the neoadjuvant setting is less well defined, and there is no standard duration for treatment with NAE.29 During the 2019 St. Gallen Consensus Conference, just over 30% of the international medical community believed that NAE should be administered for at least 6 months (~180 days),30 but there remain theoretical concerns that administering NAE may contribute to delayed opportunities to observe response to cytotoxic systemic therapy and may even contribute to disease progression and worse outcomes in some patients.31 Our study showed that patients who received NAE had a median TTS of 109.5 days (IQR 52–188), while patients who received NACE had the longest median TTS (201 days, IQR 168–248) of any treatment cohort. TTS increased in patients on endocrine monotherapy with more advanced stages of disease, by almost 12 weeks on average in patients with stage III disease compared to those with stage I disease, but a similar increase was not seen among patients on NACE. We believe our collective findings for NACE patients likely reflect the less linear path to surgery that might be experienced by this group of patients, many of whom will have had to change NST mid-course either because of on-treatment progression or inability to tolerate their initial systemic course.
To the best of our knowledge, our study is the largest and among the first to examine the relationship between TTS and OS among patients with both early-stage and locally advanced breast cancer who received not only upfront surgery, but also NAC or NAE, either as monotherapy or in combination. Our findings suggest that TTS thresholds may be useful measures of oncologic care quality for patients undergoing upfront surgery but may not be appropriate in those who receive preoperative systemic therapy.
Several conceptual frameworks have been developed to understand the trajectory towards treatment among patients with cancer and other diseases. One of the most commonly used is Andersen’s “General Model of Total Patient Delay,” which describes time intervals (a less value-laden term than “delays”) for each of five stages in the decisional process of seeking treatment: (1) appraisal delay (not seeking care to evaluate an unexplained symptom or abnormal imaging finding), (2) illness delay (time between recognizing illness and deciding to seek medical care), (3) behavioral delay (time between deciding that medical intervention is needed to deciding to act on this decision), (4) scheduling delay (time between deciding to act on the decision to seek care and attending a medical appointment), and (5) treatment delay (time from first appointment with a healthcare provider and actually beginning treatment).32
The “Model of Pathways to Treatment” is a revised version of Andersen’s model that grants patients more agency and also seeks to elucidate the reasons behind delays to care, breaking down the trajectory into four intervals: (1) appraisal, (2) health seeking, (3) diagnostic, and (4) pre-treatment.33 In our study, we examined the factors associated with delays in this fourth, pre-treatment interval, which begins with diagnosis and concludes with treatment. While it is impossible to distill reasons behind women’s actions at each stage of their path toward research through retrospective cohort studies, our findings indicate distinctive sociodemographic characteristics (e.g., race/ethnicity, insurance status) and treatment-related factors (e.g., extent of surgery, sequence of surgery and systemic therapy) associated with greatest risk of pre-treatment delay. Our analysis also elucidates the subset of women - namely those undergoing upfront surgery - for whom such delays in TTS are actually consequential and for whom prospective, qualitative data collection on this topic should be prioritized. Indeed, our group has recently concluded a qualitative analysis of diverse women with breast cancer and their providers to obtain their perspectives on potentially modifiable barriers to and facilitators of treatment.
But even among patients undergoing upfront surgery, use of TTS as a quality metric without appropriate contextualization can lead to inappropriate assumptions about overall quality of care. In our analysis, we found that receipt of care at an academic institution (vs a community hospital) was associated with longer TTS for upfront-surgery, NAC, and NAE patients (Table 2). Greenup et al. previously evaluated the effect of hospital volume on breast cancer outcomes using the NCDB and likewise found that treatment at high-volume centers was associated with the longest TTS of any treatment location. This prolonged TTS, however, may in part be due to shared decision-making and care coordination that cannot be discerned from registry data but that is typically a part of the integrated multidisciplinary care provided at academic sites; higher rates of preoperative genetic testing, CPM, and reconstruction also characterize these locations.34,35 Indeed, although we observed longer TTS for patients treated at academic centers in our study, receipt of care at these sites was, for the most part, associated with improved OS despite these delays. Thus, for some patients, longer TTS may represent more thorough care coordination and does not necessarily reflect undesirable delays in treatment. Further studies examining patient-reported outcomes and surgical wait-times are needed in order to evaluate the impact of differential TTS on quality of life.
Finally, our study identified ethnicity, insurance status, and race as being associated with TTS regardless of treatment sequence. The current national political discourse has highlighted the fact that racial and socioeconomic disparities often stem from systemic biases and inequities, be they in the criminal justice, education, housing, or healthcare systems. This trickle-down effect is also evident in breast cancer outcomes. It has been well documented that race, ethnicity, socioeconomic status, and insurance coverage are significantly associated with disparate breast cancer mortality.36 The origins of these associations are multifactorial, with access to healthcare services, variations in shared decision-making, and differing rates of aggressive tumor subtypes all playing major roles.36 In our study, non-Hispanic Black women had the greatest delays in TTS across all treatment groups, as well as worse OS, reflecting results from several previously published studies.37–39 Similarly, we also saw worse outcomes among patients with no/government insurance as compared to those with private insurance. If TTS is considered a proxy for access to treatment, our findings highlight and document a worrisome trend, the drivers of which must be further understood and dismantled in order to enact real systemic change. We hope our results help inform not only patient-level but also provider- and health system-level interventions in order to redress existing inequities in breast cancer treatment.
Limitations
Despite the large dataset, our study has several important limitations (e.g., selection bias) that are associated with performing retrospective analyses using national registries. The NCDB does not include disease-specific survival and does not capture recurrence. As a result, caution must be used when applying our results to patients whose OS may not be driven by breast cancer-specific survival. Furthermore, lack of information surrounding recurrence rates and response to neoadjuvant treatment limits our ability to analyse the impact of TTS delays on patients who receive NST. This impact may be most significant in patients whose benefit from NST is less clearly defined, such as patients with hormone receptor positive/HER2-negative breast cancer.25 A recent study by Sutton and colleagues examining the effect of delays in TTS on oncologic outcomes within a single-institution cohort of 463 NAC recipient concluded that TTS greater than 6 weeks was associated with worse residual cancer burden and worse recurrence-free survival.40 Together, smaller institutional studies such as that by Sutton and colleagues as well as our large-scale analysis of national data yield the type of hypothesis generation that retrospective studies are intended to produce. We hope that our results will be built upon and amplified using more granular - and ideally, prospectively collected - data.
It is also important to note that we excluded a large number of patients due to missing or unavailable data, and data for oral endocrine therapy, in particular, may not be entirely reliable. Finally, we acknowledge that among NST patients, differences in TTS from diagnosis do not necessarily reflect differences in time from completion of systemic therapy to surgery; the latter time interval cannot be characterized using the NCDB but may also represent an important period of oncologic care coordination. We have, however, included time from diagnosis to start of first neoadjuvant therapy in all adjusted models.
In summary, we believe our analysis contributes significantly to the literature by describing and comparing national patterns of care delivery between patients undergoing upfront surgery and those receiving different forms of NST. Specifically, we demonstrate the importance of differentiating TTS as a potentially appropriate measure of healthcare quality for patients undergoing upfront surgery but not for those receiving NST, a distinction that will be important for patients, physicians, and other stakeholders to appreciate.
Conclusion
Our study is among the first and largest to examine and compare the relationship between TTS and OS in patients with breast cancer who received either NST or upfront surgery. Non-Hispanic Black race, Hispanic ethnicity, insurance status, and extent of surgical treatment were associated with longer TTS across different treatment sequences. Furthermore, although we observed progressively worse OS with increasing TTS among patients who underwent upfront surgery, adjusted OS was not consistently associated with TTS in women receiving NST. We hope that our findings can help inform surgeon-patient communication, shared decision-making, care coordination, and patients’ expectations throughout both NST and in the perioperative period.
Supplementary Material
Funding:
Dr. Fayanju is supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability
The data that support the findings of this study are available from the National Cancer Data Base (NCDB) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the NCDB.
Conflicts of Interest:
Ipshita Prakash declares that she has no conflicts of interest to disclose.
Samantha M. Thomas was a consultant for AbbVie.
Rachel A. Greenup declares that she has no conflicts of interest to disclose.
Jennifer K. Plichta declares that she has no conflicts of interest to disclose.
Laura H. Rosenberger declares that she has no conflicts of interest to disclose.
Terry Hyslop was a consultant for AbbVie.
Oluwadamilola M. Fayanju declares that she has no conflicts of interest to disclose.
Ethical approval: This retrospective study involving de-identified data from human participants was conducted in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The institutional review board (IRB) at Duke University determined that our study did not need ethical approval, and our study was granted exempt status with a waiver of informed consent.
References
- 1.Siu AL. Force USPST. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016; 164:279–96. [DOI] [PubMed] [Google Scholar]
- 2.Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019; 125(9):1482–88. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Task Force On Preventive Health Care, Tonelli M, Connor GS, et al. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ 2011; 183:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosselli Del Turco M, Ponti A, Bick U et al. Quality indicators in breast cancer care. Eur J Cancer. 2010; 46(13):2344–56. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JL, Warneke CL, Mittendorf EA, et al. Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg. 2011; 254(1):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazda A, Estroff J, Euhus D et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010; 17(Suppl 3):291–96. [DOI] [PubMed] [Google Scholar]
- 7.Neal RD, Tharmanathan P, France B et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015; 112(Suppl 1):92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaglehouse YL, Georg MW, Shriver CD et al. Time-to-surgery and overall survival after breast cancer diagnosis in a universal health system. Br Cancer Res Treat. 2019; 178(2):441–50. [DOI] [PubMed] [Google Scholar]
- 9.Bleicher RJ, Ruth K, Sigurdson ER et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016; 2(3):330–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith EC, Ziogas A, Anton-Culver H et al. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013; 148(6):516–23 [DOI] [PubMed] [Google Scholar]
- 11.Eaglehouse YL, Georg MW, Shriver CD et al. Racial differences in time to breast cancer surgery and overall survival in the US military health system. JAMA Surg. 2019; 154(3):e185113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon M, Cochrane CT, Grieve DA et al. Insurance status and time to completion of surgery for breast cancer. ANZ J Surg. 2015; 86(1–2):84–87. [DOI] [PubMed] [Google Scholar]
- 13.Landercasper J, Linebarger JH, Ellis RL et al. A quality review of the timeliness of breast cancer diagnosis and treatment in an integrated breast center. J Am Coll Surg. 2010; 210(4):449–55. [DOI] [PubMed] [Google Scholar]
- 14.Murphy BL, Day CN, Hoskin TL et al. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018; 25(8):2241–48. [DOI] [PubMed] [Google Scholar]
- 15.American College of Surgeons. National Cancer Database. 1996–2016 [cited January 22, 2020]. Available at: https://www.facs.org/quality%20programs/cancer/ncdb.
- 16.Jagsi R, Jiang J, Momoh AO et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014; 32(9):919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cemal Y, Albornoz CR, Disa JJ et al. A paradigm shift in the U.S. breast reconstruction: part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013; 131-(3):320e–326e. [DOI] [PubMed] [Google Scholar]
- 18.Jabo B, Lin AC, Aljehani MA et al. Impact of breast reconstruction on time to definitive surgical treatment, adjuvant therapy, and breast cancer outcomes. Ann Surg Oncol. 2018; 25(10):3096–3105. [DOI] [PubMed] [Google Scholar]
- 19.Omarini C, Guaitoli G, Noventa S et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur J Surg Oncol. 2017; 43(4):613–18. [DOI] [PubMed] [Google Scholar]
- 20.Sanford RA, Lei X, Barcenas CH et al. Impact of time from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol. 2016; 23(5):1515–21. [DOI] [PubMed] [Google Scholar]
- 21.Suleman K, Almalik O, Haque E et al. Does the timing of surgery after neoadjuvant therapy in breast cancer patients affect the outcome? Oncology. 2020; 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15(7):2483–93. [DOI] [PubMed] [Google Scholar]
- 23.Van der Hage JA, Van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer Trial 10902. J Clin Oncol. 2001; 19:4224–4237. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 25.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–72. [DOI] [PubMed] [Google Scholar]
- 26.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG (Alliance) Z1071 Prospective Multicenter Clinical Trial. Ann Surg. 2014; 260:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg. 2018; 268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30(15):1796–804. [DOI] [PubMed] [Google Scholar]
- 29.Spring LM, Gupta A, Reynolds KL et al. Neoadjuvant endocrine therapy for estrogen-receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2(11):1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balic M, Thomssen C, Wurstlein R et al. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019; 14:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barroso-Sousa R, Silva DD, Alessi JV et al. Neoadjuvant endocrine therapy in breast cancer: current role and future perspectives. Ecancermedicalscience. 2016; 10:609. doi: 10.3332/ecancer.2016.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. The British journal of social psychology. 1995;34 (1):33–52. [DOI] [PubMed] [Google Scholar]
- 33.Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gooiker GA, van Gijn W, Post PN, van de Velde CJ, Tollenaar RA, Wouters MW. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider? Eur J Surg Oncol. 2010;36 Suppl 1:S27–35. [DOI] [PubMed] [Google Scholar]
- 35.Greenup RA, Obeng-Gyasi S, Thomas S, et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018; 267:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeder-Hayes KE, Anderson BO. Breast cancer disparities at home and abroad: a review of the challenges and opportunities for system-level change. Clin Cancer Res. 2017; 23(11):2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheppard VB, Oppong BA, Hampton R et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015; 22(9):2902–11. [DOI] [PubMed] [Google Scholar]
- 38.Tammemagi CM. Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Curr Opin Obstet Gynecol. 2007; 19:31–36. [DOI] [PubMed] [Google Scholar]
- 39.Shaves VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002; 94:332–57. [DOI] [PubMed] [Google Scholar]
- 40.Sutton TL, Schlitt A, Gardiner SK, Johnson N, Garreau JR. Time to Surgery Following Neoadjuvant Chemotherapy for Breast Cancer Impacts Residual Cancer Burden, Recurrence, and Survival. Society of Surgical Oncology (SSO) International Conference on Surgical Cancer; 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


