Abstract
Voltage-dependent anion channel (VDAC) is the most ubiquitous channel at the mitochondrial outer membrane, and therefore is believed to be the pathway for calcium entering or leaving the mitochondria. Therefore, understanding the molecular mechanisms of how VDAC regulates calcium influx and efflux from the mitochondria is of particular interest for mitochondrial physiology. When the Parkinson’s disease (PD) related neuronal protein, alpha-synuclein (αSyn), is added to the reconstituted VDAC, it reversibly and partially blocks VDAC conductance by its acidic C-terminal tail. Using single-molecule VDAC electrophysiology of reconstituted VDAC we now demonstrate that, at CaCl2 concentrations below 150 mM, αSyn reverses the channel’s selectivity from anionic to cationic. Importantly, we find that the decrease in channel conductance upon its blockage by αSyn is hugely overcompensated by a favorable change in the electrostatic environment for calcium, making the blocked state orders-of-magnitude more selective for calcium and thus increasing its net flux. These findings demonstrate that the phenomenon of “charge inversion” is taking place at the level of a single polypeptide chain. Measurements of ion selectivity of three VDAC isoforms in CaCl2 gradient show that VDAC3 exhibits the highest calcium permeability among them, followed by VDAC2 and VDAC1, thus pointing to isoform-dependent physiological function. Mutation of the E73 residue – VDAC1 purported calcium binding site – shows that there is no measurable effect of the mutation in either open or αSyn-blocked VDAC1 states. Our results confirm VDACs involvement in calcium signaling and reveal a new regulatory role of αSyn, with clear implications for both normal calcium signaling and PD-associated mitochondrial dysfunction.
Keywords: Voltage dependent anion channel, intrinsically disordered proteins, ion selectivity, beta-barrel channels, single-molecule measurement, calcium signaling, charge inversion
Graphical Abstract
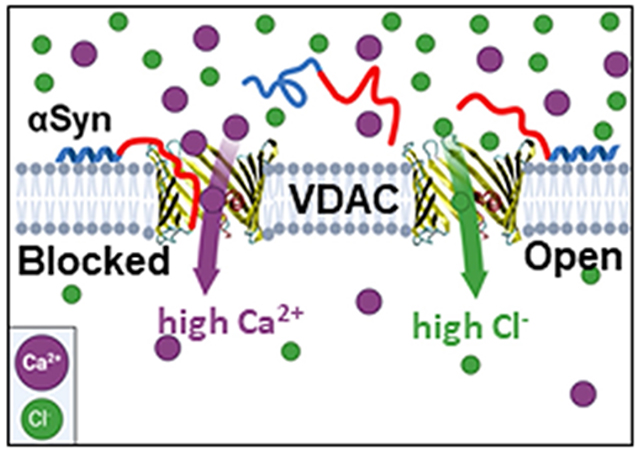
1. Introduction
The mitochondrial outer membrane (MOM), traditionally regarded as a barrier between the mitochondrial space and cytosol, also functions as the interface transmitting signals between the tightly controlled mitochondrial inner membrane (MIM) housing the electron transport chain and the rest of the cell. As the most abundant channel protein in the MOM, the Voltage Dependent Anion Channel (VDAC) controls the permeability of the MOM to ions, water soluble solutes, and metabolites [1–3]. Calcium ions (Ca2+) are no exception: with the absence of Ca2+ specific transporter in the MOM, Ca2+ must diffuse through VDAC to cross the MOM [4], though this certainly does not exclude Tom40, the channel protein of the translocase of the outer membrane (TOM) complex, as another passive Ca2+ pathway across MOM [5]. Ca2+ is well known as a secondary messenger in the mitochondria. Given VDAC’s unique role as the main conduit for the flux of small molecules and metabolites across MOM, there has been considerable interest in the biophysics of Ca2+ transport through VDAC and its regulation. For organellular ion channels like VDAC, the quantitative determination of channel properties is best of all achieved via reconstitution of these channel proteins into planar lipid bilayers [6]. Gincel et al first directly demonstrated that VDAC reconstituted into planar lipid bilayer in high concentration CaCl2 solutions is permeable to Ca2+ and gates properly under applied voltage [7]. Later, the same group [8, 9] suggested a possible Ca2+ binding site at the residue E73, now known from the VDAC1 structure to be imbedded within the lipid membrane [10, 11]. Notably, E73 residue is not very conserved among VDAC isoforms and different species: mammalian VDAC isoforms 1 and 2 have it, but mammalian VDAC3 along with VDACs from yeast [12] and fungi [13] have a non-charged glutamine Q73 (see discussion in [14]). Later, Tan and Colombini confirmed the characteristic gating of reconstituted VDAC in CaCl2 solutions concluding that channel functions normally with or without Ca2+ [15].
Gating, the VDAC’s eponymous phenomenon, is a spontaneous process by which the channel’s unique high conductance “open” state converts into a variety of lower conducting so called “closed” states under relatively high applied voltages of > 30 mV [6, 16]. Physiological relevance of VDAC gating phenomena is ensured by the fact that its closed states – which are still quite conducting for small ions – are virtually impermeable for ATP [17]. Tan and Colombini showed that VDAC closed states, that are typically more cation selective than the open state, have higher permeability to Ca2+ [15]. By extrapolating their results to physiologically low concentration of Ca2+ of 1 μM, they estimated that Ca2+ flux through the closed states could be 4 to 10 times higher than through the anion-selective open state. This study opened the possibility that rather than being an inert pathway for Ca2+, VDAC alone or in a complex with other proteins could actively modulate Ca2+ signals to or from the mitochondria. Following the work of Tan and Colombini, and taking into account that moving VDAC to its closed states requires application of significant transmembrane voltages, we were interested in learning whether VDAC interactions with protein partners could affect Ca2+ flux through the channel.
VDAC has been shown to interact both with other MOM proteins including the cholesterol transporter, TSPO, Bcl-2-family proteins, and various cytosolic proteins such as glycolytic enzymes, hexokinase, dimeric tubulin, and neuronal proteins [18–22]. These protein-protein interactions have the potential to modify Ca2+ flux through VDAC and thus affect mitochondrial Ca2+ homeostasis.
The Parkinson’s Disease (PD) associated protein, α-Synuclein (αSyn) is one of the known potent VDAC regulators [22]. It is an intrinsically disordered protein highly expressed in the central nervous system and directly involved in mitochondrial dysfunction in neurodegeneration [23]. αSyn is a relatively short 140-residue polypeptide consisting of two distinct parts: a weakly positively charged 95-residue N-terminal lipid-binding domain and a highly negatively charged 45-residue C-terminal domain [24]. In experiments with VDAC reconstituted into planar lipid membranes it was shown that αSyn is able to block VDAC conductance with nanomolar efficiency [22]. The blockage is reversible and partial, with the blocked state still retaining about 40% of the open state conductance. The molecular mechanism is that the negative voltage at the side of αSyn addition drives the negatively charged C-terminal tail of αSyn into the net-positive VDAC pore inducing fast (on the order of milliseconds) characteristic blockages of channel conductance, producing fluctuations of ion current through the channel between its open and well-defined blocked states [25]. There are three immediate physiological consequences of this αSyn-VDAC interaction: i) under certain conditions such as a relatively high applied voltage, αSyn can translocate through the VDAC pore and target respiratory complexes in MIM causing impairment of mitochondrial function [26]; ii) when the negatively charged C-terminal domain is transiently captured inside the VDAC pore, the blocked state becomes cation selective [27, 28], which should prevent translocation of the negatively charged metabolites (ATP and ADP) due to an electrostatic barrier and steric hindrance; iii) the more cation-selective αSyn-blocked state may suggest that Ca2+ permeation is higher in this state than in the open state of VDAC, which is anionic. The later implication is supported by the data obtained with αSyn overexpression and exogenous addition in HeLa cells where an increase in mitochondrial uptake of Ca2+ released by the endoplasmic reticulum (ER) was detected [29]. However, direct experiments demonstrating the effect of αSyn on Ca2+ flux through the VDAC channel were not available yet.
Another important open question in VDAC physiology is the role of each of the three mammalian VDACs (VDAC1, 2, and 3) in mediating mitochondrial Ca2+ signaling. In a key cellular study by De Stefani et al 2012, overexpression or silencing of one of the three mammalian VDAC isoforms was shown to enhance or attenuate the uptake of Ca2+ after histamine-evoked release from the ER [30]. Overexpression or silencing of VDAC2 or VDAC3, had slightly greater effect on mitochondrial Ca2+ uptake than those of VDAC1. Therefore, the authors suggested that this effect may arise from the differences in channel transport properties between isoforms; however, this hypothesis was not explored further. The authors identified that VDAC1 was the only isoform involved in a previously reported mitochondria-ER contact site (MERCS) complex involving the IP3 receptor (IP3R) and Glucose-Regulated Protein 75 (GRP75) [31]. This complex was shown to selectively transmit Ca2+ signals between the ER and mitochondria. Following the identification of the IP3R-GRP75-VDAC1 complex, other protein complexes with VDAC were found to alter Ca2+ signaling, including the Ryanodine Receptor 2 (RyR2) in complex with VDAC2, and most recently the lysosome calcium channel, TRPML1, in complex with VDAC1 [32, 33]. Peng et al reported that a mutation in the proposed Ca2+ binding site [7], E73Q, reduced Ca2+ uptake into the mitochondria.
Here we address the questions of VDAC permeability to Ca2+ by utilizing single-molecule electrophysiology on recombinant VDACs reconstituted into planar lipid membranes. We measure ion selectivity of the VDAC open and αSyn-blocked states in CaCl2 salt gradient. Using the experimental values of reversal potential and conductance of the VDAC open and αSyn-blocked states in different CaCl2 gradients, we calculate Ca2+ currents through these states, demonstrating that, notwithstanding channel conductance reduction in the blocked state, αSyn interaction with VDAC actually is able to enhance VDAC-facilitated flux of Ca2+ by more than an order of magnitude. We compare the Ca2+ selectivity of each individual VDAC isoform as well as of VDAC1 E73Q, where the purported Ca2+ binding residue is mutated, demonstrating that while there are significant differences in Ca2+ permeability between the isoforms, there is no measurable effect of the E73Q mutation in either the open or αSyn-blocked states. We thus conclude that αSyn interaction with VDAC might have important consequences for Ca2+ flux through the pore, while the E73 residue does not seem to play any role in mitochondrial Ca2+ uptake. These results have implications for both normal mitochondrial function and PD’s associated dysfunction. It is also worth mentioning that in pursuit of these findings we established that the phenomenon of “charge inversion” is able to take place not only in a single protein channel [34, 35], but at the level of a single polypeptide chain.
2. Materials and Methods
2.1. Protein Purification
Recombinant mouse VDAC1 was a kind gift of Dr. Adam Kuszak (NIDDK, NIH). Protocols for expression, refolding, and purification of VDAC1 were based on the work of Dr. Jeff Abramson’s lab [10] as described previously [36]. Recombinant mouse VDAC1 E73Q mutant and human VDAC3 were the kind gifts of Jeff Abramson (UCLA). VDAC3 was expressed, refolded, and purified following protocol in [37] and VDAC1 E73Q mutant was purified and refolded as described in [14]. Recombinant human VDAC2 was the kind gift of Dr. Tsyr-Yan Yu (Academia Sinica, Taiwan). VDAC2 was expressed, refolded, and purified following protocol in [38]. All VDAC samples stock solution was 5 mg/ml in 0.1% LDAO. Recombinant WT α-synuclein (αSyn) was the generous gift of Dr. Jennifer Lee (NHBLI, NIH). αSyn was expressed, purified, and characterized as described previously [39] and stored at −80°C.
2.2. VDAC reconstitution and conductance measurements
Planar lipid membranes were formed from diphytanoyl-phosphatidylcholine (DPhPC, Avanti Polar Lipids, Alabaster, AL) in 5 mg/ml pentane by apposition of two monolayers of across a ~70 μm aperture in the 15-μm-thick Teflon partition that separates two ~1.5-mL compartments, as previously described [40]. Channel insertion was achieved by adding 0.1-2 μL of VDAC sample diluted l00x in buffer containing 10 mM Tris, 50 mM KCl, 1mM EDTA, 15% (v/v) DMSO, 2.5% (v/v) Triton X-100, pH 7.0 to the aperture on the cis compartment side of the membrane. Potential is defined as positive when it is greater at the side of VDAC addition (cis side). Current recordings were performed as described previously [40] using an Axopatch 200B amplifier (Axon Instruments, Inc.) in the voltage-clamp mode. Unless otherwise noted, single-channel data were filtered by a low pass 8-pole Butterworth filter (Model 900 Frequency Active Filter. Frequency Devices, Inc.) at 15 kHz and saved with a sampling frequency of 50 kHz and analyzed using pClamp 10.7 software (Axon Instruments, Inc.).
VDAC ion selectivity was measured in different CaCl2 gradients and in 300 mM (cis) / 60 mM (trans) KCl gradient buffered with 5 mM HEPES at pH 7.4, as described previously [41]. In all experiments VDAC was always added to the cis (grounded) compartment. VDAC ion selectivity was inferred from the potential corresponding to the intersection of the current-voltage plot with the zero-current level, the reversal potential. Open state reversal potential was calculated for single and multichannel reconstitutions by applying either a 30 mHz or 50 mHz triangular wave of amplitude 60 mV and/or by measuring conductance acquired at different applied voltages typically at 5 mV intervals. Measurements of the αSyn-blocked state selectivity were carried out only for cases where a single channel was reconstituted. After measuring open state conductance and selectivity, αSyn was added to the membrane-bathing solution to the final 50 nM concentration to the side of higher salt concentration, or both compartments. Data were acquired at different applied voltages 10-20 min after αSyn addition. Data for selectivity measurements were filtered with low pass 8-pole Bessel filter at 100 Hz for channel open state and at 500 Hz for the αSyn-blocked state to avoid loss of fast αSyn blockage events. Conductance of the open and αSyn-blocked states were determined by the mean of a gaussian fit carried out in ClampFit 10.7. In all cases the current-voltage data were fit with a linear regression to determine both conductance and reversal potential. The measured reversal potential was corrected by the liquid junction potential calculated from Henderson’s equation [34] to obtain the final reversal potential Ψrev. The permeability ratios between Cl− and Ca2+, PCl /Pca and between Cl− and K+, PCl /PK were calculated according to the Goldman–Hodgkin–Katz equation [42]:
| (1) |
| (2) |
where r = acis /atrans is the ratio of ionic activities of the cis and trans solutions; φ = Ψrev (F/RT), where R, T, and F have their usual meaning of universal gas constant, absolute temperature, and Faraday constant. As the Ψrev approaches the Nernst potential, the calculated values for PCl /PCa approach infinity. These outliers were excluded for the average value for PCl /PCa shown in Table 1.
Table 1.
Reversal potential and conductance of VDAC1 open and αSyn-blocked states measured in different CaCl2 and KCl gradients. Reversal potential values are corrected for the liquid-junction potential. The ratios of anion to cation permeabilities, P−/P+, and currents, I−/I+ are calculated according to Eqs. (1–2) and (6), respectively, at 0 mV. Each data point is a mean ± S.E.. Number of independent experiments is shown in brackets. All conditions are as in Fig. 3.
| Salt gradient cis/trans, mM | Single channel conductance, nS | Reversal potential, mV | Permeability ratio, P−/P+ | Ion currents ratio at 0 mV, I−/I+ |
|---|---|---|---|---|
| CaCl2 gradient | ||||
| 500/100 Open (10) |
1.40 ± 0.2 | 25.5 ± 3.4 | 11.5 ± 3.7 | 7.5 ± 2.7 |
| 500/100 Blocked (3) |
0.58 ± 0.10 | 24.8 ± 4.1 | 11.0 ± 4.2 | 7.0 ± 2.4 |
| 100/500 Open (11) |
1.58 ± 0.33 | −26.4 ± 2.2 | 11.9 ± 2.6 | 8.3 ± 2.1 |
| 100/500 Blocked (3) |
0.55 ± 0.03 | −27.0 ± 1.1 | 12.5 ± 1.8 | 8.9 ± 1.1 |
| 150/30 Open (11) |
0.58 ± 0.12 | 31.4 ± 0.9 | 40.7 ± 9.8 | 26.9 ± 7.3 |
| 150/30 Blocked (4) |
0.36 ± 0.09 | −5.5 ± 3.2 | 0.60 ± 0.20 | 0.59 ± 0.20 |
| 30/150 Open (10) |
0.56 ± 0.16 | −28.4 ± 1.0 | 20.3 ± 4.3 | 13.9 ± 2.4 |
| 30/150 Blocked (4) |
0.39 ± 0.06 | 8.9 ± 1.4 | 0.35 ± 0.07 | 0.39 ± 0.08 |
| KCl gradient | ||||
| 300/60 Open (12) |
0.59 ± 0.09 | 18.5 ± 3.2 | 3.7 ± 1.1 | 2.97 ± 0.18 |
| 300/60 Blocked (3) |
0.39 ± 0.06 | −17.7 ± 2.5 | 0.31 ± 0.06 | 0.35 ± 0.06 |
2.3. Statistics
For the statistical analysis of mean values, the difference between two groups of data were analyzed by a one-way ANOVA test using p < 0.05 as the criterion of significance. Differences between many groups were analyzed using the Holm-Sidak multiple comparison tests. All statistical analysis was performed using SigmaPlot. Each experiment was performed a minimum of three times.
3. Results
We first verified the previous observations on VDAC channel properties in pure CaCl2 solutions [7, 15]. Figure 1A shows a representative current record obtained with single VDAC1 channel reconstituted into a planar bilayer formed from diphytanoyl-phosphatidylcholine (DPhPC) that separated 100 mM (cis) and 500 mM (trans) (100/500) CaCl2 solutions (see a schematic of experimental setup in Fig. 2A). Under these conditions VDAC1 single channel conductance is 1.6 ± 0.3 nS (mean ± S.D., numbers of experiments are shown in Table 1) and under high applied voltage (−50 mV as in trace in Fig. 1A) the channel moves to a low-conducting or closed state, which is a typical gating behavior of VDAC reconstituted in DPhPC membranes in monovalent salts [43].VDAC characteristic gating in CaCl2 gradient could also be monitored under different experimental protocol when a triangular voltage wave of 30 mHz frequency and ± 60 mV amplitude (Fig. 1B, bottom trace) was applied to a single channel inducing a stepwise characteristic channel closure at high voltages (Fig. 1B, upper trace). It can be seen in the Fig. 1A trace that in 100/500 mM CaCl2 gradient the reversal potential, Ψrev, (the voltage corresponding to zero current shown in dash-dotted lines in Figs. 1A and 2B) is close to −25 mV (raw data, uncorrected for liquid junction potentials). In the experiment presented in Fig. 1B, where CaCl2 gradient was reversed to 500 mM (cis) and 100 mM (trans) (500/100), the sign of Ψrev is reversed correspondingly to about +25 mV. Note that VDAC was always inserted from the cis side or the grounded side of the planar membrane (Fig. 2A). The average data of the reversal potential obtained on at least 10 independent channels in 500/100 and 100/500 CaCl2 gradients are shown in Fig. 3A and Table 1. Note that all Ψrev values presented here are corrected for the liquid-junction potentials arising at the KCl salt bridges of Ag/AgCl electrodes, which were calculated using Henderson’s equation [34]. Considering that VDAC, as well as other β-barrel channels, such as α-hemolysin [44] or the bacterial porin OmpF [45], is known to predominantly insert into planar membranes in one orientation when added to the same (cis in our case) side of the membrane [40], our data show that Ψrev does not depend on channel orientation in 500/100 CaCl2 gradient. The corresponding permeability ratios between Cl− and Ca2+, PCl /PCa, calculated by Eq. (1) are ~11.6 (Table 1) for both gradient directions.
Figure 1.
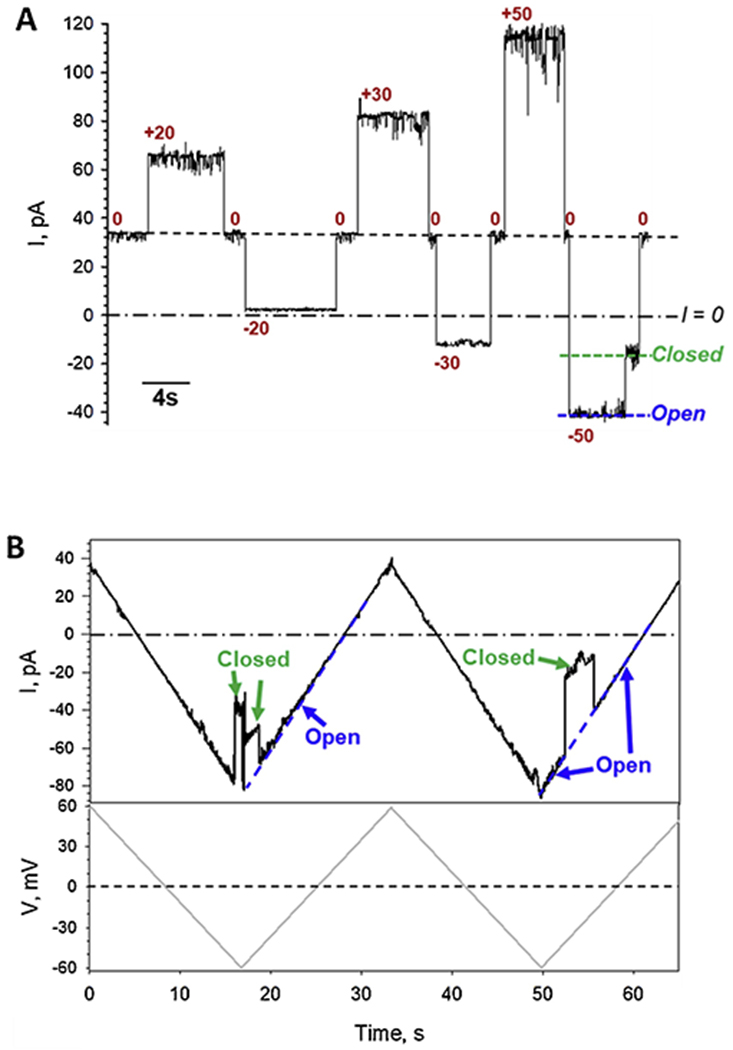
VDAC1 forms typical anion-selective channels in CaCl2 solutions and characteristically gates under applied voltage. Representative single-channel current traces obtained with reconstituted VDAC1 in 100 mM (cis)/500 mM (trans) CaCl2 gradient at applied voltages as indicated (A) and in response to applied triangular voltage wave in reverse 500 mM (cis)/100 mM (trans) CaCl2 gradient (B). Applied voltage wave of 30 mHz frequency and ±60 mV amplitude is shown in the bottom panel in (B). Here, and elsewhere, the dashed line indicates current at zero applied voltage (V = 0) and the dash-dotted line indicates zero current (I = 0). Note a substantial current at 0 mV in (A). VDAC open and closed states are indicated by dashed blue and green lines, respectively (A, B). Current records were digitally filtered at 100 Hz using a low-pass Bessel (8-pole) filter. Here, and elsewhere, planar membranes were formed from DPhPC and membrane-bathing solutions were buffered with 5 mM HEPES at pH 7.4.
Figure 2.
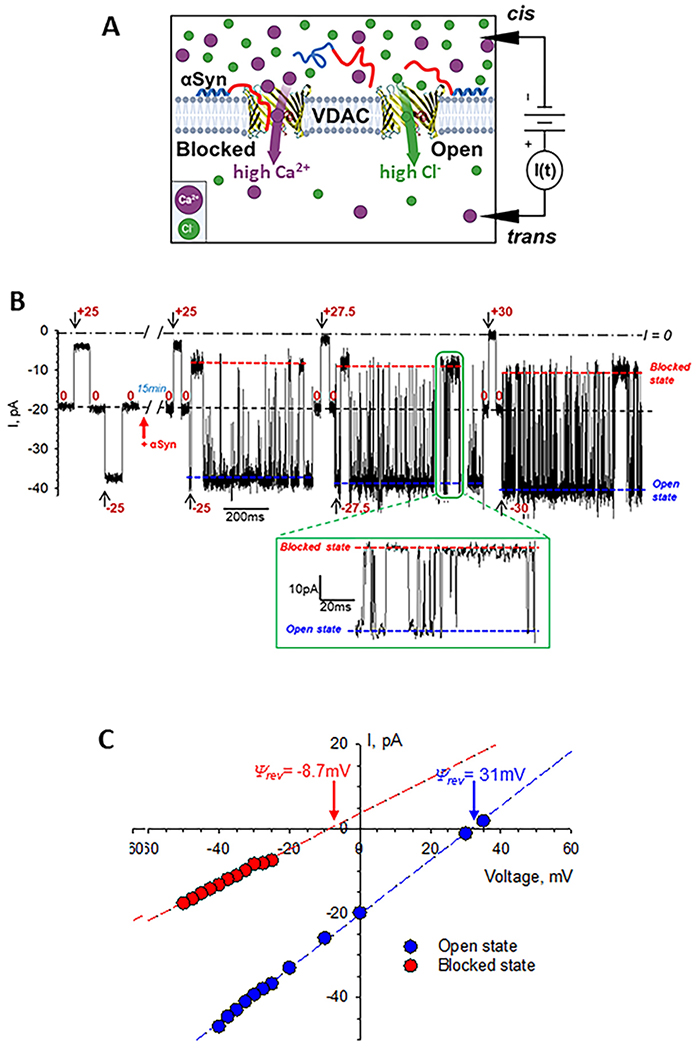
Measurements of ion selectivity of the VDAC open and αSyn-blocked states in CaCl2 gradient. A – A schematic (not to scale) of experimental setup to measure VDAC ion selectivity with high CaCl2 salt in the cis and low salt in the trans side. αSyn is drawn as a “diblock-copolymer” with the N-terminal membrane binding domain shown in blue and acidic pore-blocking C-terminal domain shown in red. B – Representative single-channel trace obtained in 150 mM (cis) /30 mM (trans) CaCl2 gradient before (leftmost trace) and 15 min after addition of 50 nM αSyn to the cis compartment at the indicated voltages. Inset shows a fragment of current record at −27.5 mV at a finer time scale. Fast fluctuations of current through the channel between the open and blocked states correspond to individual blockage events induced by αSyn. Blue and red dashed lines indicate the VDAC open and αSyn-blocked states. Current record was digitally filtered using a 1 kHz low-pass Bessel (8-pole) filter for presentation. C - I/V curves obtained from the traces example of which is shown in (B) for the open (blue symbols) and αSyn-blocked (red symbols) VDAC states. Linear regressions (dashed lines) allow calculation of the reversal potential Ψrev (indicated by arrows) for each state. A positive Ψrev corresponds to anion and negative to cation selectivity.
Figure 3.
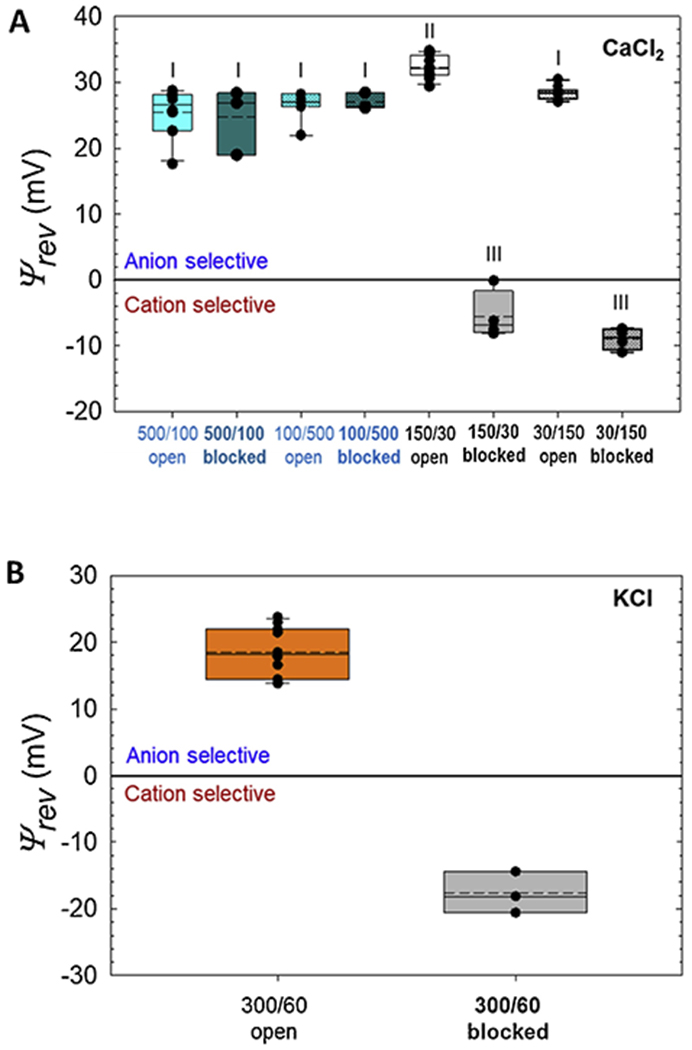
Reversal potentials of the VDAC1 open and αSyn-blocked states measured in CaCl2 (A) and KCl (B) gradients. Salt gradients are shown as in cis/trans in mM. Reversal potential (Ψrev) values are corrected for the liquid-junction potential. Note that the sign of Ψrev in experiments in (A) with the lower CaCl2 concentration in the cis side (100/500 mM and 30/150 mM) has been reversed for making comparison of Ψrev values easier. Here the ends of the boxes define the 25th and 75th percentiles, with a black line at the median, the mean displayed as dashed black lines, and error bars defining the 10th and 90th percentile. Error bars indicate SD with the numbers of experiments for each condition shown in Table 1. Each different Roman numeral indicates a potential that is significantly different (p < 0.05) from other marked potentials as determined via one-way ANOVA with Holm-Sidak multiple comparison testing. Potentials sharing the same numeral do not differ significantly.
Next, we measured VDAC1 open state selectivity in reduced CaCl2 concentration of 150 mM cis/ 30 mM trans (150/30) and in the reverse gradient of 30 mM cis/ 150 mM trans CaCl2 (30/150). As expected from the previous studies of VDAC selectivity for monovalent ions [46–48], selectivity is more anionic in low CaCl2 concentration than in high salt and corresponds to 4-fold higher PCl /PCa (40.7 ± 9.8) in 150/30 CaCl2 gradient than in 500/100 (Fig. 3A and Table 1). Similarly to the previous findings with OmpF [34], in low salt concentrations the selectivity depends on channel orientation. Specifically, in 30/150 CaCl2 gradient it is PCl /PCa = 20.3 ± 4.3 (Fig. 3A and Table 1), only half of that in the opposite orientation. These results are in accord with the earlier data obtained for VDAC in CaCl2 solutions [7, 15], confirming that first, VDAC1 gates normally and, second, its open state is anion selective.
Previously we showed that the VDAC αSyn-blocked state is either cationic or non-selective depending on which part of the αSyn molecule is trapped inside the pore at a given time [27, 28]. On average, the αSyn-blocked state is essentially non-selective with PCl /PK = 1.0 ± 0.2 measured in 1.0 M cis / 0.2 M trans KCl gradient [22]. Such a reduction in anionic selectivity and in conductance (blocked state conductance is ~40% of the open state) creates strong steric and weak electrostatic barriers for ATP translocation through the VDAC pore suggesting significant reduction or complete abolishment of the transport of large negatively charged metabolites through VDAC due to αSyn-VDAC interaction. Ca2+ is a small ion in comparison with ATP or ADP and it might be that even a considerably reduced conductance of the blocked state is still sufficiently large to allow significant Ca2+ flux through the blocked pore.
To investigate Ca2+permeability through the αSyn-blocked state, we measured ion selectivity of the blocked state in CaCl2 gradients. The schematic of these experiments is shown in Fig. 2A. 50 nM of αSyn was always added to the side with higher CaCl2 concentration. A representative experiment with VDAC1 reconstituted in 150/30 mM CaCl2 is shown in Fig. 2B. The current records were taken on the same single VDAC1 channel before (left trace in B) and 15 min after (right trace in B) αSyn addition to the cis compartment (Fig. 1A). αSyn induces characteristic time-resolved fast current fluctuations between the unaffected open state and the blocked state, which could be seen best in the inset with a finer time scale in Fig. 2B. Different voltages were applied as shown in Fig. 2B to obtain the corresponding I/V (current-voltage) plots for the open and blocked states (Fig. 2C). The anionic selectivity of the open state quantified as Ψrev = 31 mV is reversed to cationic in the blocked state with Ψrev = −8.7 mV. This corresponds to a change of the average permeability ratio PCl / PCa from 40.7 ± 9.8 for the open state to 0.58 ± 0.24 for the blocked state (Fig. 3A and Table 1), which is a 70-fold change in favor of Ca2+permeability. The average Ψrev values, obtained in two CaCl2 salt gradients, each in two cis/trans configurations, are presented in Fig. 3A and Table 1. Ψrev values for the VDAC open and αSyn-blocked states measured in monovalent KCl salt of the same as in CaCl2 5-fold gradient: 300 mM cis /60 mM trans KCl, are shown for comparison in Fig. 3B. In KCl gradient, Ψrev is reversed from 18.5 ±3.2 mV in open to −17.7 ± 2.5 mV in the blocked state with the corresponding ratios PCl/PK+ of 3.7 ± 1.11 and 0.31 ± 0.06 for the open and blocked states, respectively (Table 1).
Taken together, these results show that in low salt and at small transmembrane voltages, the αSyn-blocked state is significantly more permeable for Ca2+ than the open state. However, in high CaCl2 salt, selectivity of the αSyn-blocked state does not change, remains highly anionic as that of the open state, and does not depend on channel orientation in salt gradient.
Using the same approach, we found that in 150 cis /30 trans mM CaCl2 gradient three mammalian isoforms of VDAC differ significantly in Ca2+ permeability. All of them are indeed anionic (Fig. 4A), but with different permeability ratios of PCl/PCa = 40.7 ± 9.8 for VDAC1, 26.4 ± 11.1 for VDAC2, and 15.0 ± 6.0 for VDAC3. This makes VDAC3 nearly three-fold more permeable to Ca2+ than VDAC1, with the Ca2+ permeability series of 1 : 1.5 : 2.6 for VDAC1 : VDAC 2 : VDAC 3. respectively. To test the role of the E73 residue as the purported Ca2+ binding site, we measured Ca2+ selectivity of the VDAC1 E73Q mutant. It turned out that there is no significant difference in Ca2+ permeability of the open or αSyn-blocked states between the mutant and wild-type (WT) VDAC1 (Fig. 4B).
Figure 4.
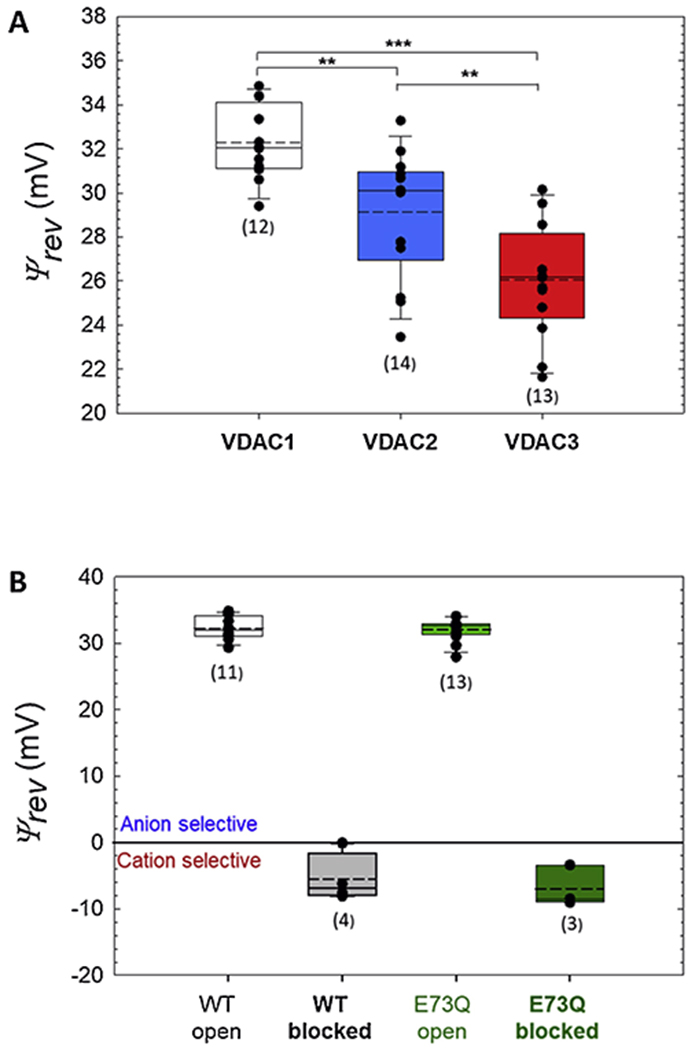
Comparison of ion selectivity of open states for three VDAC isoforms (A) and VDAC1 E73Q mutant (B) in 150/30 mM CaCl2 gradient. Reversal potentials of the open state of VDAC1, VDAC2, and VDAC3 (A) and VDAC1 E73Q mutant (B) measured in 150/30 mM CaCl2 gradient and corrected for the liquid-junction potentials. Here the ends of the boxes define the 25th and 75th percentiles, with a black line at the median, the mean displayed as dashed black lines, and error bars defining the 10th and 90th percentile. Error bars indicate SD with the numbers of experiments shown in brackets. Significance in (A) was tested using a one-way ANOVA with Holm-Sidak multiple comparison testing (***p < 0.001, **p < 0.01). Differences between data at the open and αSyn-blocked states for WT and E73Q channels in (B) are not significant: p > 0.05.
4. Discussion
The observed changes in the reversal potential allow us to explore the modulation of VDAC Ca2+ permeability by αSyn. Tan and Colombini [15] demonstrated the increase in Ca2+ flux induced by VDAC closure. Here, we use a different approach, originally developed for the study of bacterial porins selectivity (A. Alcaraz, personal communication), to calculate the total ionic current and its components carried by cations and anions in the open and blocked states from the measured single channel conductance (G) and Ψrev. We use the fact that I/V curves were linear for the salt gradients of all experiments (see an example in Fig. 2C). We also take into account that when the applied voltage equals the Nernst Potential for Ca2+ ions, VCa, their current ICa is zero. Analogously, for a bias equal to the Nernst Potential for Cl− ions, VCl, their current ICl is zero. Nernst potential Vi for ionic species i with charge number zi, is obtained from ionic activities of the cis and trans solutions as Vi = −(RT/FZi) In(acis/atrans). Total current is I = I+ − I−; the convention is that I+ > 0 means cations flowing from cis to trans while I− > 0 means anions flowing from trans to cis. Then, by considering that I(Ψrev) = 0, we get:
| (3) |
| (4) |
| (5) |
The ratio between anion and cation current at zero voltage becomes independent of the channel conductance and can be expressed as:
| (6) |
Fig. 5 displays individual ion currents through the open (A, C) and αSyn-blocked (B, D) VDAC in 150 mM cis /30 mM trans (A, B) and 30 mM cis/150 mM trans (C, D) CaCl2 gradients. The actual activity gradient corresponding to 150/30 is 74/19 [49], which yields VCa = −17.5 mV and VCl = 35.0 mV (both with the opposite sign for the reversed gradient 30/150). The change in the current ratio ICl /ICa from open to blocked states reflects the change in permeability ratio. In both gradients at zero voltages, ICl is an order of magnitude higher than ICa in the open state (Fig. 5 A, C and Table 1), whereas in the αSyn-blocked state the situation is the opposite: ICa is 1.7-2.9 times ICl (Fig. 5B, D and Table 1). It is seen that the actual ICl/ICa ratios are indeed voltage-dependent and that gradient inversion gives slightly different absolute values for the ratios. The latter is to be expected [34] due to the structural asymmetry of VDAC.
Figure 5.
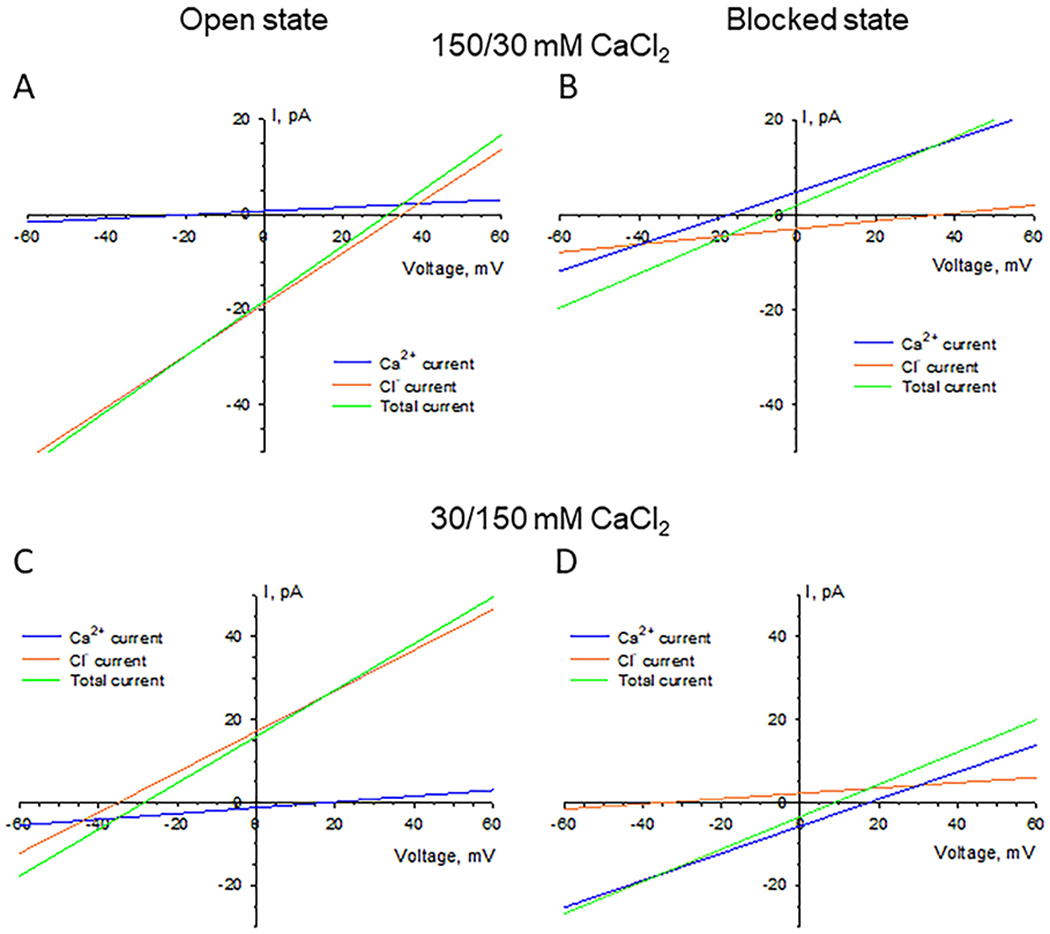
Calculated voltage dependences of individual anionic and cationic components of ion current through the open (A, C) and αSyn-blocked (B, D) VDAC in 150 mM cis /30 mM trans (A, B) and 30 mM cis/150 mM trans (C, D) CaCl2 gradients. Currents are calculated based on the average channel conductance and reversal potential (Table 1) according to Eqs. (3–5).
The increase in the Ca2+ permeability of the αSyn-blocked state resembles the enhanced Ca2+ permeability through VDAC closed states reported by Tan and Colombini [15]. Using the 80 mM/ 20 mM CaCl2 gradient, they found PCl /PCa = 24 for the open state. Voltage-induced closure of the channel reduced this value by a factor of 1.5- or 4-fold in favor of Ca2+ permeability when channel was moved to the closed states, depending on the “polarity” of the gating. From our experiments in the 150 mM/ 30 mM and in the inversed 30 mM/ 150 mM CaCl2 gradient we get the change of more than 50 times in PCl /PCa in favor of Ca2+ permeability upon channel blockage by αSyn (Table 1). Also important, it has to be mentioned here that moving VDAC to its closed state requires much higher voltage biases than those necessary for the efficient interaction with αSyn [22]. Although the existence of significant potential differences across the MOM is still under debate [2, 15], the estimates span from 10 mV [50] to as high as 50 mV [51–53], negative at the cytoplasmic side of the MOM. Considering that previous studies suggest that the cis side in our bilayer membrane setup corresponds to the cytosolic side of VDAC [54] and that the negative potential applied from the side of αSyn addition is needed to drive the anionic C-terminal tail of αSyn into the pore [22], we can assume that there is a sufficient negative potential across MOM to induce VDAC blockage by αSyn and consequently, increase Ca2+ uptake by mitochondria. Indeed, we cannot specify the direction of Ca2+ gradient across the MOM in vivo because it depends on particular physiological conditions in cell. Nevertheless, calculated I/Vcurves in Fig. 5 allow us to speculate that Ca2+ gradient between the cytosol and intermembrane space of mitochondria potentially could either favor mitochondrial Ca2+ uptake or its release depending on given physiological conditions.
From the data in Table 1 it follows that there are several ways to describe the main results of this study quantitatively. For example, under the 150/30 mM Ca+2 conditions, the blockage can be characterized by the calcium-favoring change (a) in the permeability ratio P−/P+ of 67.8; (b) in the ion current ratio I−/I+ at zero voltage of 45.6; (c) in the current ratio prorated by the conductance reduction of 45.6×(0.36/0.58) = 28.3, and (d) in the permeability ratio prorated for the conductance reduction of 67.8×(0.36/0.58) = 42.08. To add even more complexity, it is necessary to mention that these numbers depend on the particular experimental conditions, as clearly demonstrated by Table 1 and Fig. 5. Nevertheless, all the ratios imply that at small concentrations the VDAC-facilitated Ca+2 flux is drastically, by more than an order of magnitude, increased by the VDAC interaction with αSyn, notwithstanding the channel conductance reduction in the αSyn-blocked state.
The main goal of the present study was to understand how VDAC interaction with αSyn modifies Ca2+ flux through the channel. Indeed, there are two competing factors: (i) an additional steric constraint due to the presence of the αSyn C-terminal tail in the VDAC pore, which reduces its conductance, and (ii) a more favorable electrostatic environment for Ca2+ due to the negative charge of the tail. We established that the latter factor dominates, thus increasing the net flux of Ca2+. We also showed an interesting effect of the blocked state selectivity reversal by varying Ca2+ concentration. Comparison of the data in Fig. 3A and Table 1 shows that the channel is cation selective at the smaller Ca2+ concentrations and anion selective at the higher. We relate this to the so-called “charge inversion’’, the phenomenon attracting scientists’ vivid attention in many disciplines [55–58]. Though it was previously found for a bacterial β-barrel porin, OmpF [34, 35], now, using reconstituted VDAC as a nanopore sensor we demonstrate this phenomenon for a single polypeptide chain – the C-terminal tail of αSyn, the part of the protein that is responsible for the channel selectivity change [28].
One of the immediate implications of these findings to cell physiology is that αSyn could be a potent regulator of mitochondrial Ca2+ fluxes by acting through its dynamic interaction with VDAC. Previously reported increase of Ca2+ transport from the ER to mitochondria induced by αSyn in permeabilized cells [29] provides support to this conclusion. Notably, the αSyn effect described by Cali et al, required the presence of the C-terminal domain of αSyn. In accord with this observation, our previous data showed that the C-terminal tail of αSyn is a prerequisite for its interaction with the VDAC pore [22]. As postulated by Cali et al, WT αSyn may play a physiological role in altering mitochondria Ca2+ uptake. This is supported by later evidence suggesting that at physiological levels, αSyn can enhance mitochondrial respiration [59]. In contrast, pathogenic expression of αSyn resulting in excess mitochondrial Ca2+ uptake may result in mitochondrial dysfunction and cell death. However, one must be extremely careful in direct extrapolations of in vitro results to the situation in cell. Genetic evidence is needed to decisively conclude about the role of VDAC in αSyn-induced modulation of Ca2+ crosstalk between mitochondria and the ER. Interestingly, a putative antiarrhythmic small-molecule drug, efsevin, was found to exert its function by enhancing the Ca2+ permeability of VDAC2 in zebrafish [60, 61]. We can speculate that cardiac function may alter through modification of VDAC Ca2+ permeability by αSyn or another protein.
Our experiments with VDAC isoforms suggest that each isoform may differentially mediate Ca2+ signaling. VDAC3 was found to be the most permeable to Ca2+, followed by VDAC2 and VDAC1. These data are in agreement with De Stefani and coauthor’s hypothesis that slight differences in mitochondrial Ca2+ uptake upon overexpression of an individual VDAC isoform are due to differences in isoform Ca2+ permeability [30]. In their study they found VDAC3 and VDAC2 to enhance Ca2+ uptake in comparison to VDAC1. It is tempting to speculate further suggesting that enhanced permeability of VDAC3 to Ca2+ may be related to its high expression level in the testes and sperm, where VDAC3 knockout in mice leads to male infertility and sperm defects [62]. Mitochondrial dynamics and energetics are being increasingly recognized to play an important role in spermatogenesis [63]. Likewise, Ca2+ signaling plays an important role in various sperm functions including capacitation and the acrosome reaction [64].
The results of the present study are in odds with the hypothesis that the E73 residue of VDAC is a Ca2+ binding site [8, 9]. VDAC1 structure shows that E73 faces the hydrophobic lipid medium [10, 11] making its accessibility for Ca2+ extremely low if not impossible. We recently showed that this residue is not implicated in VDAC1 gating [14]. E73 has been found as cholesterol and allopregnanolone binding site [65, 66], and binding of a hydrophobic molecule can influence VDAC gating properties [67]. This may give some ground for explanation of why the E73Q mutation in VDAC1 caused a reduction of mitochondrial Ca2+ uptake from lysosomes [33].
Importantly, αSyn is not the only cytosolic protein known to directly interact with the VDAC pore. For example, dimeric tubulin is another known potent VDAC inhibitor [19] which, by blocking the VDAC pore with its C-terminal disordered anionic tail, also reverses VDAC selectivity to cationic [47]. This allows us to speculate that tubulin interaction with VDAC could also increase its Ca2+permeability, thus opening an intriguing possibility that yet undiscovered VDAC cytosolic protein partners could regulate mitochondrial Ca2+ fluxes in an αSyn-like fashion.
5. Conclusions
Here we report that a potent cytosolic regulator of VDAC, the cytosolic protein αSyn implicated in PD, modifies this channel properties towards substantially higher Ca2+ permeability. The functional interaction between VDAC and αSyn, discovered in our lab earlier, is realized through the partial blockage of the channel by the negatively charged C-terminus of αSyn. We now show that the reduction in channel conductance in the blocked state is significantly overcompensated by a favorable change in the electrostatic environment for Ca2+ created by the presence of the αSyn C-terminus in the VDAC pore. This results in the orders-of-magnitude increased selectivity of VDAC to Ca2+ and thus in the significantly enhanced Ca2+ net flux through the channel blocked state. Thus, we identify the αSyn interaction with VDAC as a new molecular mechanism of regulation and signaling in normal neural physiology and in what may underly mitochondrial dysfunction in PD. The importance of these results stems from the increasingly recognized role of mitochondrial Ca2+ homeostasis in diseases ranging from cardiac arrythmia to neurodegeneration and in the numerous cell processes associated to apoptosis. In addition, in experiments with VDAC isoforms, we uncover a key biophysical difference whereupon VDAC3 exhibits an enhanced Ca2+ permeability followed by VDAC2 and VDAC1, pointing to their varied physiological function.
Highlights.
Neuronal protein α-synuclein, implicated in Parkinson’s disease, dynamically binds to VDAC, and modifies its pore properties towards substantially higher Ca2+ permeability.
α-Synuclein-VDAC interaction is a new molecular mechanism underlying regulation of calcium uptake by mitochondria.
VDAC3 exhibits the highest calcium permeability among the three VDAC isoforms, followed by VDAC2 and VDAC1.
Contrary to predictions, the E73 residue does not affect calcium permeability of VDAC1.
The phenomenon of “charge inversion” is observed at the level of a single polypeptide chain.
Acknowledgements
W.M.R., T.K.R and S.M.B were supported by the Intramural Research Program of the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). V.M.A received support from the Government of Spain (PID2019-108434GB-I00 AEI/FEDER, UE), Generalitat Valenciana (AICO/2020/066), and Universitat Jaume I (UJI-B2018-53).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare that they have no competing interests with the contents of this article.
References
- 1.Rostovtseva TK, Bezrukov SM, VDAC regulation: role of cytosolic proteins and mitochondrial lipids, J Bioenerg Biomembr. 40 (2008) 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombini M, VDAC: The channel at the interface between mitochondria and the cytosol, Mol Cell Biochem. 256 (2004) 107–115. [DOI] [PubMed] [Google Scholar]
- 3.Lemasters JJ, Holmuhamedov E, Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box, Biochim Biophys Acta. 1762 (2006) 181–90. [DOI] [PubMed] [Google Scholar]
- 4.Hajnoczky G, Csordas G, Yi M, Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria, Cell Calcium. 32 (2002) 363–77. [DOI] [PubMed] [Google Scholar]
- 5.Hill K, et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins [see comment], Nature. 395 (1998) 516–21. [DOI] [PubMed] [Google Scholar]
- 6.Colombini M, Voltage gating in the mitochondrial channel, VDAC, J Membr Biol. 1ll (1989) 103–111. [DOI] [PubMed] [Google Scholar]
- 7.Gincel D, Zaid H, Shoshan-Barmatz V, Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function, Biochem J. 358(2001) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V, The voltage-dependent anion channel-1 modulates apoptotic cell death, Cell Death Differ. 12 (2005) 751–60. [DOI] [PubMed] [Google Scholar]
- 9.Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V, Localization of the voltage-dependent anion channel-1 Ca2+-binding sites, Cell Calcium. 41 (2007) 235–244. [DOI] [PubMed] [Google Scholar]
- 10.Ujwal R, et al. The crystal structure of mouse VDAC1 at 2.3 angstrom resolution reveals mechanistic insights into metabolite gating, Proc Natl Acad Sci USA. 105 (2008) 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G, Solution structure of the integral human membrane protein VDAC-1 in detergent micelles, Science. 321 (2008) 1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messina A, Reina S, Guarino F, De Pinto V, VDAC isoforms in mammals, Biochim Biophys Acta. 1818(2012) 1466–1476. [DOI] [PubMed] [Google Scholar]
- 13.Colombini M, The published 3D structure of the VDAC channel: native or not?, Trends Biochem. Sci 34 (2009) 382–389. [DOI] [PubMed] [Google Scholar]
- 14.Queralt-Martin M, Bergdoll L, Jacobs D, Bezrukov SM, Abramson J, Rostovtseva TK, Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating, Biochim Biophys Acta Bioenerg. 1860 (2019) 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W, Colombini M, VDAC closure increases calcium ion flux, Biochim Biophys Acta. 1768 (2007) 2510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostovtseva TK, Bezrukov SM, Function and Regulation of Mitochondrial Voltage-Dependent Anion Channel, In Electrophysiology of Unconventional Channels and Pores, Delcour AH (Ed.), Springer: Switzerland, 2015: pp. 3–31. [Google Scholar]
- 17.Rostovtseva T, Colombini M, ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane, J Biol Chem. 271 (1996) 28006–8. [DOI] [PubMed] [Google Scholar]
- 18.Al Jamal JA, Involvement of porin N, N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane, Protein J. 24 (2005) 1–8. [DOI] [PubMed] [Google Scholar]
- 19.Rostovtseva TK, et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration, Proceedings of the National Academy of Sciences. 105 (2008) 18746–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH, Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier, Proceedings of the National Academy of Sciences. 89 (1992) 3170–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magri A, et al. Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability, Sci Rep. 6 (2016) 34802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostovtseva TK, et al. alpha-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease, J Biol Chem. 290 (2015) 18467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim WS, Kagedal K, Halliday GM, Alpha-synuclein biology in Lewy body diseases, Alzheimers Res Ther. 6 (2014) 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulmer TS, Bax A, Cole NB, Nussbaum RL, Structure and dynamics of micelle-bound human alpha-synuclein, J Biol Chem. 280 (2005) 9595–603. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Mechanism of alpha-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions, Nanoscale. 9(2017) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rovini A, et al. Molecular mechanism of olesoxime-mediated neuroprotection through targeting alpha-synuclein interaction with mitochondrial VDAC, Cell Mol Life Sci. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Mechanism of alpha-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions, Nanoscale. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Real-Time Nanopore-Based Recognition of Protein Translocation Success, Biophys J. 114 (2018) 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calì T, Ottolini D, Negro A, Brini M, α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions, Journal of Biological Chemistry. 287(2012) 17914–17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Stefani D, et al. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria, Cell Death Differ. 19 (2012) 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+channels, J Cell Biol. 175(2006) 901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min CK, et al. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca(2)+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart, Biochem J. 447 (2012) 371–9. [DOI] [PubMed] [Google Scholar]
- 33.Peng W, Wong YC, Krainc D, Mitochondria-lysosome contacts regulate mitochondrial Ca(2+) dynamics via lysosomal TRPML1, Proc Natl Acad Sci USA. 117 (2020) 19266–19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcaraz A, Nestorovich EM, Lopez ML, Garcia-Gimenez E, Bezrukov SM, Aguilella VM, Diffusion, exclusion, and specific binding in a large channel: a study of OmpF selectivity inversion, Biophys J. 96(2009) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Gimenez E, Lopez ML, Aguilella VM, Alcaraz A, Linearity, saturation and blocking in a large multiionic channel: divalent cation modulation of the OmpF porin conductance, Biochem Biophys Res Commun. 404(2011) 330–4. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs D, et al. Probing Membrane Association of alpha-Synuclein Domains with VDAC Nanopore Reveals Unexpected Binding Pattern, Sci Rep. 9 (2019) 4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Queralt-Martin M, et al. A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology, J Gen Physiol. 152 (2020) e201912501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu TY, Raschle T, Hiller S, Wagner G, Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs, Biochim Biophys Acta. 1818(2012) 1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfefferkorn CM, Lee JC, Tryptophan Probes at the a-Synuclein and Membrane Interface, J Phys Chem B. 114 (2010) 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM, Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes, J Biol Chem. 281 (2006) 37496–506. [DOI] [PubMed] [Google Scholar]
- 41.Teijido O, et al. Acidification asymmetrically affects voltage-dependent anion channel implicating the involvement of salt bridges, J Biol Chem. 289 (2014) 23670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hille B, Ion Channels of Excitable Membranes. Third ed. 2001: Sinauer Associates Inc. 814. [Google Scholar]
- 43.Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J, Affixing N-terminal alpha-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating, J Biol Chem. 287 (2012) 11437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merzlyak PG, Yuldasheva LN, Rodrigues CG, Carneiro CM, Krasilnikov OV, Bezrukov SM, Polymeric nonelectrolytes to probe pore geometry: application to the alpha-toxin transmembrane channel, Biophys J. 77 (1999) 3023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nestorovich EM, Danelon C, Winterhalter M, Bezrukov SM, Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores, Proceedings of the National Academy of Sciences of the United States of America. 99 (2002) 9789–9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zambrowicz EB, Colombini M, Zero-current potentials in a large membrane channel: a simple theory accounts for complex behavior, Biophys J. 65 (1993) 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurnev PA, Rostovtseva TK, Bezrukov SM, Tubulin-blocked state of VDAC studied by polymer and ATP partitioning, FEBS Lett. 585 (2011) 2363–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krammer EM, Saidani H, Prevost M, Homble F, Origin of ion selectivity in Phaseolus coccineus mitochondrial VDAC, Mitochondrion. 19 Pt B (2014) 206–13. [DOI] [PubMed] [Google Scholar]
- 49.Staples BR, Nuttall RL, Activity and Osmotic Coefficients of Aqueous Calcium-Chloride at 298.15-K, J Phys Chem Ref Data. 6 (1977) 385–407. [Google Scholar]
- 50.Lemeshko VV, Theoretical evaluation of a possible nature of the outer membrane potential of mitochondria, Eur Biophys J. 36 (2006) 57–66. [DOI] [PubMed] [Google Scholar]
- 51.Lemeshko VV, VDAC electronics: 1. VDAC-hexo(gluco)kinase generator of the mitochondrial outer membrane potential, Biochim Biophys Acta. 1838 (2014) 1362–71. [DOI] [PubMed] [Google Scholar]
- 52.Lemeshko VV, VDAC electronics: 2. A new, anaerobic mechanism of generation of the membrane potentials in mitochondria, Biochim Biophys Acta. 1838 (2014) 1801–8. [DOI] [PubMed] [Google Scholar]
- 53.Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M, pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant, Biochem Biophys Res Commun. 326 (2005) 799–804. [DOI] [PubMed] [Google Scholar]
- 54.Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, Bezrukov SM, Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin, PLoS One. 6 (2011) e25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurnev PA, Bezrukov SM, Inversion of membrane surface charge by trivalent cations probed with a cation-selective channel, Langmuir. 28 (2012) 15824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Y, Gillespie D, Boda D, Vlassiouk I, Eisenberg RS, Siwy ZS, Tuning transport properties of nanofluidic devices with local charge inversion, J Am Chem Soc. 131 (2009) 5194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasir S, et al. Ionic transport characteristics of negatively and positively charged conical nanopores in 1:1, 2:1, 3:1, 2:2, 1:2, and 1:3 electrolytes, J Colloid Interface Sci. 553 (2019) 639–646. [DOI] [PubMed] [Google Scholar]
- 58.Grosberg AY, Nguyen TT, Shklovskii BI, Colloquium: The physics of charge inversion in chemical and biological systems, Rev Mod Phys. 74 (2002) 329–345. [Google Scholar]
- 59.Ludtmann MH, Angelova PR, Ninkina NN, Gandhi S, Buchman VL, Abramov AY, Monomeric Alpha-Synuclein Exerts a Physiological Role on Brain ATP Synthase, J Neurosci. 36 (2016) 10510–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu H, et al. Mitochondrial Ca(2+) uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity, Elife. 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilting F, et al. The antiarrhythmic compound efsevin directly modulates voltage-dependent anion channel 2 by binding to its inner wall and enhancing mitochondrial Ca(2+) uptake, Br J Pharmacol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sampson MJ, et al. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3, J Biol Chem. 276 (2001) 39206–12. [DOI] [PubMed] [Google Scholar]
- 63.Varuzhanyan G, Chan DC, Mitochondrial dynamics during spermatogenesis, J Cell Sci. 133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breitbart H, Intracellular calcium regulation in sperm capacitation and acrosomal reaction, Mol Cell Endocrinol. 187 (2002) 139–44. [DOI] [PubMed] [Google Scholar]
- 65.Cheng WWL, et al. Multiple neurosteroid and cholesterol binding sites in voltage-dependent anion channel-1 determined by photo-affinity labeling, Biochim Biophys Acta Mol Cell Biol Lipids. 1864 (2019) 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Budelier MM, et al. Click Chemistry Reagent for Identification of Sites of Covalent Ligand Incorporation in Integral Membrane Proteins, Anal Chem. 89 (2017) 2636–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rostovtseva TK, Queralt-Martin M, Rosencrans WM, Bezrukov SM, Targeting the Multiple Physiologic Roles of VDAC With Steroids and Hydrophobic Drugs, Front Physiol. 11 (2020) 446. [DOI] [PMC free article] [PubMed] [Google Scholar]


