Abstract
Background:
Successfully combining targeted agents with chemotherapy is an important future goal for cancer therapy. However, an improvement in patient outcomes requires an enhanced understanding of the tumor biomarkers that predict for drug sensitivity. NRG Oncology/Gynecologic Oncology Group (GOG) Study GOG-86P was one of the first attempts to combine targeted agents (bevacizumab or temsirolimus) with chemotherapy in patients with advanced endometrial cancer. Herein we performed exploratory analysis to examine the relationship between mutations in TP53, the most commonly mutated gene in cancer, with outcomes on GOG-86P.
Methods:
TP53 mutational status was determined and correlated with progression-free survival (PFS) and overall survival (OS) on GOG-86P.
Results:
Mutations in TP53 were associated with improved PFS and OS for patients that received bevacizumab as compared to temsirolimus (PFS: HR 0.48, 95% CI 0.31, 0.75; OS: HR: 0.61, 95% CI 0.38, 0.98). By contrast, there was no statistically significant difference in PFS or OS between arms for cases with WT TP53.
Conclusions:
This exploratory study suggests that combining chemotherapy with bevacizumab, but not temsirolimus, may enhance PFS and OS for patients whose tumors harbor mutant p53. These data set the stage for larger clinical studies evaluating the potential of TP53 mutational status as a biomarker to guide choice of treatment for endometrial cancer patients.
Keywords: endometrial cancer, chemotherapy, bevacizumab, p53
INTRODUCTION
The anti-angiogenic agent bevacizumab is FDA-approved for use in combination with chemotherapy in several cancer types, including ovarian, colorectal, non-small cell lung and renal cell cancers. Based on preclinical studies of bevacizumab in animal models of endometrial cancer [1, 2] and the activity of bevacizumab as a single agent in advanced/recurrent endometrial cancer,[3] two studies have evaluated the efficacy of bevacizumab in combination with chemotherapy.[4, 5] Neither study observed a significant improvement in outcome compared to chemotherapy alone.
The multi-center trial NRG Oncology/Gynecologic Oncology Group Study GOG-86P was a three-arm randomized Phase II study of bevacizumab in combination with carboplatin and paclitaxel (Arm 1), the mTOR inhibitor temsirolimus with carboplatin and paclitaxel (Arm 2) or bevacizumab with carboplatin and ixabepilone (Arm 3) in endometrial cancer patients in the frontline setting.[4] GOG-86P is an important clinical trial milestone because it represents one of the first attempts to combine chemotherapy with targeted agents, specifically bevacizumab and temsirolimus, which were both previously shown to have independent activity as single agents in advanced/recurrent endometrial cancer.[3, 6] Overall, none of the arms had statistically significantly increased progression-free survival (PFS) relative to historical controls.[4] The hazard ratios (92.2% confidence intervals) for Arms 1, 2 and 3 relative to historical controls were 0.81 (0.63 to 1.02), 1.22 (0.96 to 1.55) and 0.87 (0.68 to 1.11), respectively.[4] The END-2 study by the MITO group in Italy was a two-arm randomized Phase II trial of chemotherapy alone or in combination with bevacizumab.[5] Like GOG-86P, the bevacizumab-containing arm failed to significantly improved PFS relative to chemotherapy alone with an HR of 0.84. Both the GOG-86P and MITO END-2 study populations included patients with a biologically heterogeneous group of endometrial tumors, defined as advanced stage or recurrent, and trial eligibility criteria did not limit accrual to cases with specific biomarkers that could have predicted for response.[4, 5] However, the identification of subgroups within the total study population that may have received benefit is an important goal that will shed light on which patients should be considered for future therapy.
The Cancer Genome Atlas (TCGA) project has increased our understanding of the genomic heterogeneity of endometrial cancers.[7] Among cases represented in the TCGA, 91% of serous endometrial cancers contain TP53 mutations and 25% of grade 2/3 endometrioid cancers have TP53 mutations.[7] These are now denoted the “high copy number Cluster 4 tumors” and constitute the most lethal form of the disease.[7] In addition, such cancers share genomic similarities with high-grade serous ovarian carcinoma and triple-negative or basal-like breast cancers insofar as they also express a high frequency of mutations in TP53 (84–96% of cases) and a low frequency of PTEN mutations (only 1–2%).[8–12]
TP53 mutations are associated with poor prognosis for many types of cancer[13]. Hence, new therapeutic regimens that can improve outcomes in such cases are needed. Herein we performed an exploratory analysis to assess TP53 mutational status in patients from GOG-86P and determined the implications on clinical outcomes.
METHODS
Study cohort
GOG-86P (NCT00977574) was a three-arm randomized phase II study of paclitaxel/carbo- platin/ bevacizumab (NSC#704865, IND#7921), paclitaxel/carboplatin/temsirolimus (NSC#683864, IND#61010) or ixabepilone (NSC#710428, IND# 59699)/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB, or recurrent endometrial cancer. The control group for GOG-86P was historical, from a subset of patients assigned to the paclitaxel/carboplatin (PC) arm of GOG-209 (NCT00063999).[14] The patients enrolled on GOG-209 used as historical controls had similar disease characteristics compared to those enrolled on GOG-86P.[4, 14] The design of GOG-86P was based on PFS estimates from GOG-177 paclitaxel/doxorubicin/cisplatin (TAP) arm, in which median PFS was 8.3 months.[13]
For GOG-86P, the primary endpoint was PFS, defined as the time alive and progression-free from the date of study entry. Secondary endpoints included OS and best confirmed response using RECIST 1.1. For further details related to study design, please refer to Aghajanian et al.[4] Biospecimens were collected from patients who consented to participate in the translational research component of the study. Collection of archival formalin fixed paraffin embedded (FFPE) tumor and DNA from whole blood was coordinated by the NRG Oncology Biospecimen Bank.
TP53 mutational analysis
For the GOG-86P dataset, tumor was macro-dissected from FFPE tissues, enriching for regions with at least 60% tumor cell nuclei. DNA was extracted using standard laboratory protocols. Paired normal and tumor DNA underwent massively parallel sequencing using a custom Roche Noblemen SeqCap EZ system to enrich for targeted regions as described previously.[4] Alignment was completed using BWA-MEM[15] with duplicate reads marked and removed using Picard tools (Broadinstitute.gifhub.io/picard/). Variant calling was completed using VarScan[16] and MuTect[17] on each tumor and normal pair. False positive filtering was performed as described in the VarScan2 paper[16] and implemented for SomaticSniper.[18]
Statistical Analysis
Consistent with protocol objectives, an exploratory analysis of mutational markers identified on GOG-86P was performed. For this analysis, tumors were classified into groups based on mutational analysis [wild type (WT) vs. mutant]. Outcomes (PFS and OS) were compared between treatment arms or between mutational analysis groups using proportional hazards models. Hazard ratio estimates and 95% profile likelihood confidence limits from these models were reported. Kaplan-Meier estimates of the time to event distributions were plotted; at risk counts, medians and event counts were included in the plots. No p-values are reported. A confidence interval that excludes a hazard ratio of 1.0 was considered evidence of a relationship between the endpoint and the analysis group. Confidence intervals that included 1 did not always rule out clinically significant differences. Interpretation in the situation of low precision is made with caution when neither hypothesis can be ruled out. No correction for multiple analyses was included. Statistical analyses were generated using SAS/STAT software, Version 9.4 of the SAS System for Windows. Copyright © 2016 SAS Institute Inc.
RESULTS
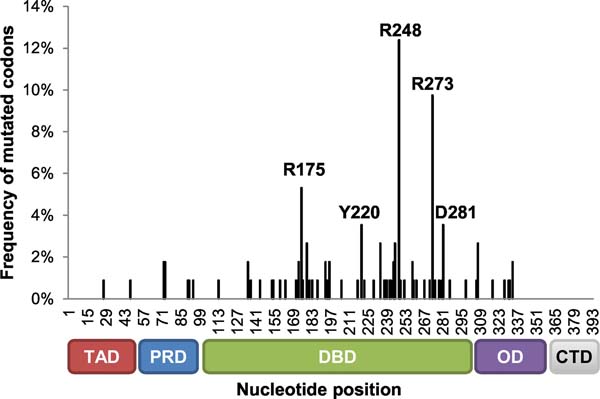
A total of 349 patients were enrolled on GOG-86P; 243 tumors were available for sequencing and had TP53 sequencing data as well as sequencing data for the catalytic subunit of DNA polymerase epsilon (POLE) and genes associated with microsatellite instability (PMS2 and MSH6). Of the 243 cases available for sub-analysis, approximately 44% harbored a mutation in TP53. The TP53 mutated group included both missense mutations as well as approximately 14 cases with mutations likely to result in a p53 null phenotype. Three TP53 mutated cases also had microsatellite instability owing to mutations in PMS2 or MSH6, and five such cases harbored a mutation in POLE. The POLE and MSI status did not preclude cases with mutations in TP53 from being so designated, i.e., such cases were still included in the TP53 mutated group. The remaining 56% of the 243 cases with IHC data had WT TP53 sequence. Among these, 55 harbored a mutation in PMS2 or MSH6 suggestive of microsatellite instability and 10 harbored a mutation in POLE. As assessed by histology, sequencing for TP53 in endometrioid grade 1 and 2 cases revealed that most were WT; TP53 status was more evenly split for patients with endometrioid grade 3 tumors, with approximately 44% of the cases with a TP53 mutation (Table 1).[4] For the 45 sequenced serous tumors, 87% had at least one mutation in TP53.[4] Reflective of previous studies from multiple tumor sites,[19] the majority of mutations in TP53 occurred in the DNA binding domain of the gene (Figure 1).
Table 1:
TP53 mutational status in each histological subtype on GOG-86P
| Not Sequenced | Mutant | Wild Type | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Histology/Grade | N | % | N | % | N | % | N | % |
| Endometrioid, grade 1 | 8 | 7.5 | 5 | 4.6 | 32 | 23.7 | 45 | 12.9 |
| Endometrioid, grade 2 | 18 | 17.0 | 14 | 13.0 | 55 | 40.7 | 87 | 24.9 |
| Endometrioid, grade 3 | 23 | 21.7 | 26 | 24.1 | 33 | 24.4 | 82 | 23.5 |
| Serous | 28 | 26.4 | 39 | 36.1 | 6 | 4.4 | 73 | 20.9 |
| Clear Cell | 9 | 8.5 | 5 | 4.6 | 2 | 1.5 | 16 | 4.6 |
| Mixed Epithelial | 7 | 6.6 | 7 | 6.5 | 3 | 2.2 | 17 | 4.9 |
| Adenocarcinoma, NOS | 4 | 3.8 | 6 | 5.6 | 1 | 0.7 | 11 | 3.2 |
| Other | 9 | 8.5 | 6 | 5.6 | 3 | 2.2 | 18 | 5.2 |
| Total | 106 | 100.0 | 108 | 100.0 | 135 | 100.0 | 349 | 100.0 |
Figure 1: The majority ofTP53 mutations in GOG-86P dataset are in the DNA binding domain.
The frequency of mutated codons was calculated as the number of variants at each nucleotide position relative to the total number of variants detected in the dataset. Mutations that occurred in ≥4 samples are annotated in the graph. R248: 14 variants; R273: 11 variants; R175: 6 variants; Y220: 4 variants; D281: 4 variants. TAD: transactivation domain; PRD: proline-rich domain; DBD: DNA binding domain; OD: tetramerization domain; CTD: C-terminal domain.
While GOG-86P was a three-arm trial consisting of the combination of paclitaxel/carboplatin/ bevacizumab (Arm 1), paclitaxel/carboplatin/temsirolimus (Arm 2), or ixabepilone/carboplatin/bevacizumab (Arm 3)[4] and since paclitaxel and ixabepilone are both microtubule stabilizing agents, data from Arms 1 and 3 were combined and compared to Arm 2. The number of patients on each arm and distribution of WT or mutant TP53 on each arm are provided in Supplementary Table I. Supplementary Tables II and III contain analyses when Arms 1 and 3 were not combined.
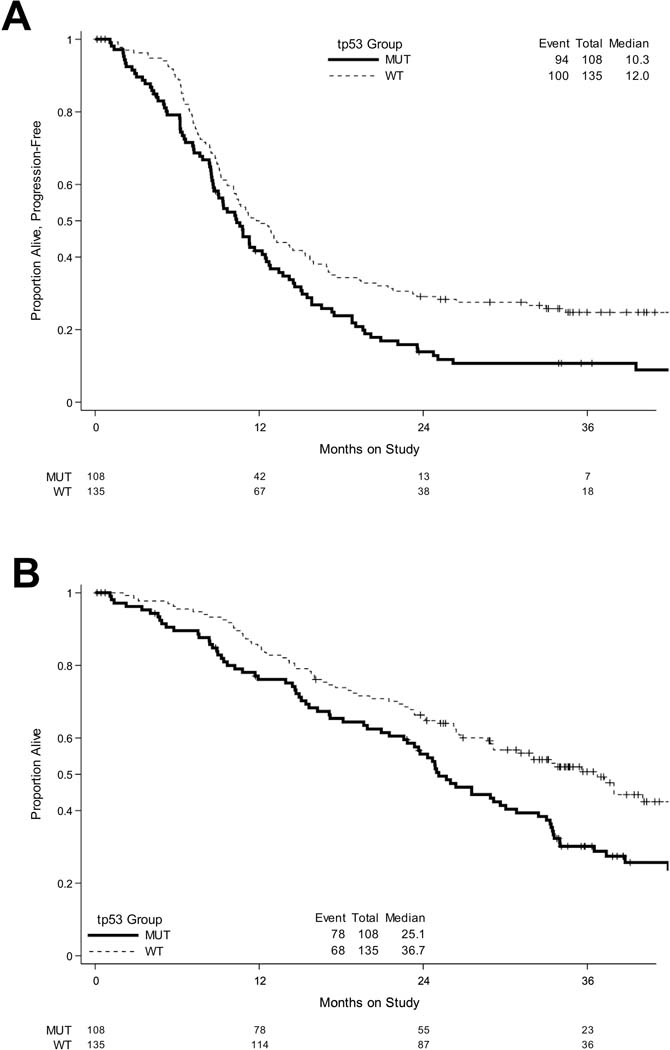
Defining the presence or absence of a TP53 mutation had clinical significance and was prognostic for poor outcomes (hazard ratio confidence interval excluded 1, Figure 2). Specifically, women whose tumors harbored a TP53 mutation had a worse median PFS compared to those with intact wild-type TP53 (10.3 months vs. 12.0 months). However, subgroup analysis based on TP53 mutational status suggests that patients with a TP53 mutations had better outcomes when bevacizumab was combined with chemotherapy (Figures 3, 4).
Figure 2: Tumors with mutant TP53 have worse outcomes as compared to tumors with WT TP53.
Kaplan-Meier survival plots of (A) PFS and (B) OS when all eligible cases on GOG-86P were subanalyzed by TP53 mutational status.
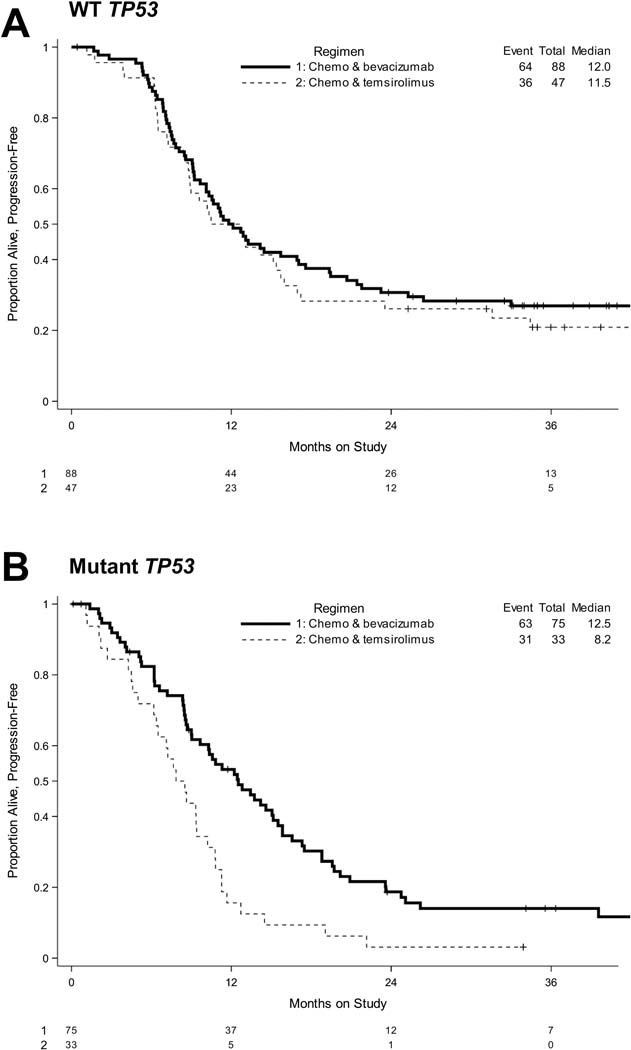
Figure 3: TP53 mutational status is associated with improved PFS on bevacizumab-containing arms.
Kaplan-Meier survival plots of PFS when cases are sub-analyzed by TP53 mutational status (A, WT; B, Mutant TP53) on Arms 1 and 3 (bevacizumab-containing arms) vs. Arm 2 (temsirolimus-containing arm).
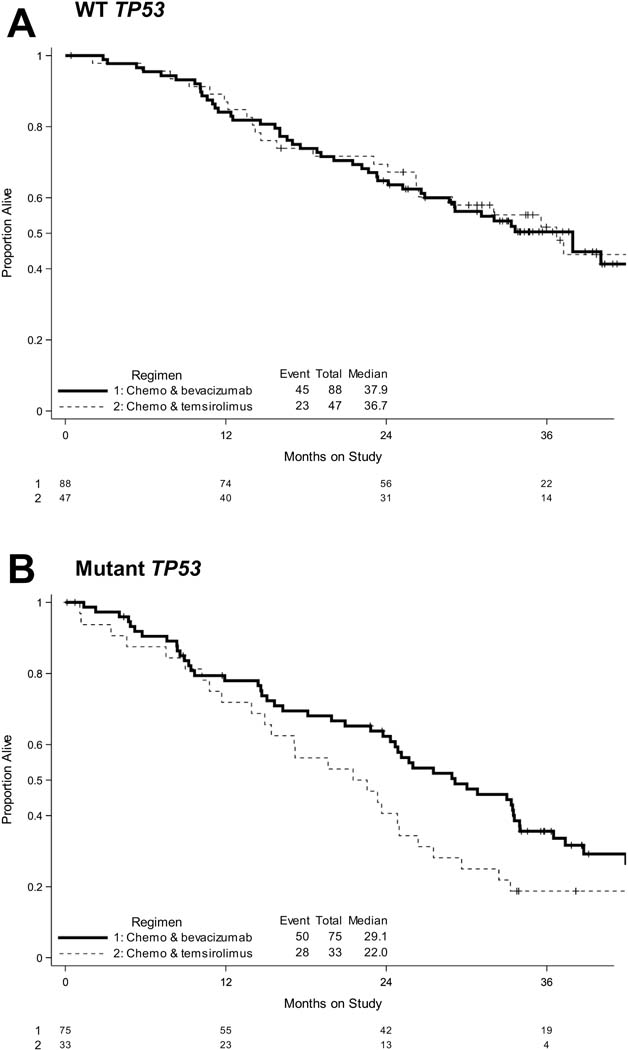
Figure 4: TP53 mutational status is associated with improved OS on bevacizumab-containing arms.
Kaplan-Meier survival plots of OS when cases are sub-analyzed by TP53 mutational status (A, WT; B, Mutant TP53) on Arms 1 and 3 (bevacizumab-containing arms) vs. Arm 2 (temsirolimus-containing arm).
First, patients with a TP53 mutation who received bevacizumab achieved a longer PFS relative to patients who received temsirolimus (Table 2, Figure 3, Supplementary Tables II and III). The median PFS for patients with a TP53 mutation was 12.5 months when they received bevacizumab-containing chemotherapy vs. 8.2 months on temsirolimus-containing chemotherapy (Table 2, Supplementary Table II). The longer PFS appeared to be the case whether the two arms with bevacizumab (bevacizumab + paclitaxel or bevacizumab + ixabepilone) were combined (HR 0.48, 95% CI 0.31, 0.75, Table 2) or not (Arm 1 vs. 2: HR 0.55, 95% CI 0.32, 0.94; Arm 2 vs. 3: HR 0.43, 95% CI 0.26, 0.71, Supplementary Table III). Second, for cases with a mutation in TP53, confidence intervals for OS hazard ratios for the bevacizumab arms relative to the temsirolimus arm excluded 1.0 when data from both bevacizumab arms were combined (HR 0.61, 95% CI 0.38, 0.98, Table 2, Figure 4).
Table 2:
Hazard ratios by TP53 mutational status on bevacizumab-containing arms (Arms 1 and 3) vs. the temsirolimus-containing arm (Arm 2).
| Endpoint | Group | Point Estimate | 95% Profile Likelihood Confidence Limits | |
|---|---|---|---|---|
| PFS | WT | 0.87 | 0.58 | 1.31 |
| Mutant | 0.48 * | 0.31 | 0.75 | |
| OS | WT | 1.05 | 0.64 | 1.77 |
| Mutant | 0.61 * | 0.38 | 0.98 | |
The number of patients in each arm and number events are provided in Supplementary Table I.
denotes statistically significant differences between groups; Arm 2 is the reference arm. WT: wild type TP53; mutant: mutation in TP53.
In contradistinction to the tumors with mutated TP53, patients with WT TP53 did not have a markedly different PFS or OS on the bevacizumab arms compared to the temsirolimus arm (Table 2, Figures 3 and 4, Supplementary Table III). However, for cases with WT TP53, the combination of bevacizumab with paclitaxel appeared to have improved OS relative to the combination of bevacizumab with ixabepilone (HR 0.48, 95% CI 0.27, 0.87, Supplementary Table III).
DISCUSSION
The national study GOG-86P was one of the first trials combining a targeted agent (either bevacizumab or temsirolimus) with standard chemotherapy for high risk or recurrent endometrial cancer.[4] Patients were enrolled on this trial based upon their clinical presentation and not based upon any biologic marker or tumor phenotype that would have been predicted to benefit most from the agents on the treatment arms. As a result, the biological makeup of the tumors was heterogeneous, and the results of the overall trial failed to show any significant benefit in PFS on any of the arms compared to historical controls (PC arm on GOG-209).[14] Similarly, patients were not pre-selected based upon expression of a biomarker in the Italian study of bevacizumab in combination with chemotherapy, MITO END-2, a trial that also did not observe improved PFS when bevacizumab was added to the chemotherapy backbone.[5]
Nevertheless, when the cases were further analyzed for potential biomarkers based upon specific hypotheses relating to the effect of each of the targeted agents, some insights have become evident.[4] In the GOG-86P study, patients with a mutation in CTNNB1, the gene that encodes β-catenin, had a slight improvement in PFS when treated with bevacizumab-containing chemotherapy (HR=0.73, 95% CI: 0.60–0.91). In this report, we demonstrate a preliminary signal that the combination of bevacizumab + chemotherapy including a mitotic spindle inhibitor (paclitaxel in arm 1 and ixabepilone in arm 3) is associated with increased PFS and OS in patients with TP53 mutated tumors relative to the addition of temsirolimus to chemotherapy. For patients with WT TP53, bevacizumab did not appear to be associated with longer PFS or OS relative to temsirolimus, but a signal was noted in favor of paclitaxel over ixabepilone when combined with carboplatin.
Despite efforts in multiple cancer types to identify pre-treatment biomarkers of response to bevacizumab when combined with chemotherapy,[20–27] no biomarker has achieved clinical utility.[27, 28] The vast majority of putative biomarkers are related to the anti-angiogenic properties of bevacizumab (e.g., circulating VEGF-A, soluble VEGFR1, Flt-3 and Ang1/Tie2 or expression of VEGF-A, VEGFR1, NRP1, and CD31 as a surrogate for microvascular density). A recent RNA expression study of a subset of ovarian tumor specimens suggests that tumors with “proliferative” or “mesenchymal” molecular features may have greater responses to bevacizumab+ chemotherapy; angiogenic factors tend to be upregulated in these molecular subtypes.[29] A recently published analysis of translational endpoints on NRG/GOG-218 failed to identify any prognostic or predictive factors; this study included analyses of CD31 (microvascular density marker).[27] Of note, the most promising data in other cancer types are related to TP53. Said et al., in a small cohort of advanced cancers of mixed histologies found that patients with mutant but not WT TP53 had longer PFS on bevacizumab-containing vs. non-bevacizumab-containing chemotherapy regimens.[30]
From a mechanistic perspective, it is interesting to speculate as to why mutated forms of TP53 might be associated with improvement in outcomes in response to bevacizumab. One possibility is the described link between the p53 protein and VEGF: wild type p53 protein inhibits transcription of angiogenic factors such as VEGF-A.[31] Mutations in TP53 that negatively impact p53 wild type transcriptional activity have been reported to relieve the transcriptional repression of VEGF-A, resulting in higher expression of the direct target of bevacizumab.[31–35] Previous studies by our group have shown that higher levels of VEGF-A may be associated with improved outcomes when patients with advanced endometrial cancer are treated with bevacizumab as a single agent.[3]
A strength of this study is our ability to detect a potential biomarker, mutated p53, which portends an improved outcome for a subset of patients being treated with bevacizumab + chemotherapy on GOG-86P. Biomarkers can be defined as either prognostic or predictive. Predictive biomarkers relate to a therapeutic outcome, whereas prognostic biomarkers are indicative of overall outcome independent of treatment.[36] We consider mutations in TP53 to be a potential predictive biomarker associated with response bevacizumab in addition to chemotherapy. A weakness of our sub-analysis was that the number of patients with mutated p53 was relatively small. We chose to use the temsirolimus arm for comparison since GOG-86P did not include a chemotherapy-only arm, and sequencing was not performed on subjects from historical controls GOG-209, which was used as the reference for comparison in the original analysis of GOG-86P. It should be noted that OS was a secondary endpoint in the original analysis of GOG-86P; event rates (and thereby precision) are lower for this endpoint than for the primary endpoint of PFS. In some cases, the confidence intervals that included 1.0 could not exclude clinically relevant hazard ratios. In addition, a proportion of all cases, but particularly serous cases, did not have available sequencing data. These are the cases that would be predicted to have the highest rate of mutations in TP53. Hence, although we report a potential signal relating to improvement in the bevacizumab arms specifically in the case of mutated p53, further confirmation in larger populations is required.
Another potential limitation of this study is our inability to analyze outcomes based upon the type of mutation in TP53. The cases included in the TP53 mutated group in this report included any mutation in this gene, whether missense or predictive of a p53 null tumor. The ability to segregate these two types of p53 abnormalities with respect to outcomes would have been interesting; however, only 14 cases demonstrated a mutation that would be clearly linked to the loss of p53 as judged by the authors. Therefore, the number of cases predicted to be null was too small and the confidence intervals too broad with these few cases to allow a definitive conclusion to be drawn as to outcomes unique to this group of patients. Both p53 null and missense mutations (sometimes referred to as gain of oncogenic function) lead to the loss of wild type p53 function as it pertains to cell cycle regulation and the transcription or repression of wild type p53-controlled genes. Whether null or missense, we hypothesize that cell cycle regulation will be disrupted and angiogenesis will be enhanced in tumor cells due to the loss of wild type p53 repression of pro-angiogenic factors including the bevacizumab target VEGFA. Hence, in both cases, p53 missense and null, there is a rationale for the benefit of bevacizumab over temsirolimus plus chemotherapy, and when analyzed as an entire group including all mutations, that is what the data demonstrate.
Given the expense and potential side effects of therapy, it is critical to identify biomarkers to define the proper subpopulation of patients to receive targeted agents such as bevacizumab. This study identified a potential tumor biomarker, mutated TP53, worthy of future investigation as a predictor of those most likely to receive benefit from bevacizumab treatment.
Supplementary Material
In previous analyses of GOG-86P, the addition of upfront bevacizumab to chemotherapy did not improve outcomes overall.
We now report that cases with mutations in the tumor suppressor TP53 experienced longer PFS and OS with bevacizumab.
OS with bevacizumab versus temsirolimus + chemotherapy doubled for cases with mutated TP53 (30 versus 14.4 months).
A mutation in TP53 is a potential biomarker for sensitivity to bevacizumab when added to chemotherapy upfront.
ACKNOWLEDGMENTS
This work was supported by National Cancer Institute grants to NRG Oncology (U10 CA 180822), NRG Operations (U10 CA180868), and NRG Specimen Bank (U24CA196067). Funding was also received from NIH 2 R01 CA99908–17 to KKL and from the Holden Comprehensive Cancer Center, the University of Iowa (NIH 2 P30 CA086862–16). DAL and CA received funding from American Recovery and Reinvestment Act (ARRA) 3 U10 CA02746929S1 and a Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Washington University School of Medicine, Duke University Medical Center, University of California Irvine Medical Center, University of Iowa Hospitals and Clinics, Women and Infants Hospital, Ohio State University Comprehensive Cancer Center, Rush University Medical Center, Walter Reed National Military Medical Center, University of Cincinnati, Cleveland Clinic Foundation, The Hospital of Central Connecticut, UCSF-Mount Zion, Memorial Sloan Kettering Cancer Center, Mayo Clinic, Cancer Research for the Ozarks NCORP, University of Texas Southwestern Medical Center, Georgia Center for Oncology Research and Education (CORE), Northwestern University, University of North Carolina at Chapel Hill, MD Anderson Cancer Center, University of Wisconsin Hospitals and Clinics, Roswell Park Cancer Institute, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Women’s Cancer Center of Nevada, University of Hawaii, Abington Memorial Hospital, University of Mississippi Medical Center, State University of New York Downstate Medical Center, Cooper Hospital University Medical Center, Carolinas Medical Center/Levine Cancer Institute, William Beaumont Hospital, Abramson Cancer Center of the University of Pennsylvania, University of Chicago, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Virginia Commonwealth University, Penn State Milton S. Hershey Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Stony Brook University Medical Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, University of Virginia, Case Western Reserve University, Yale University, Yale University, University of Texas – Galveston, Michigan Cancer Research Consortium Community Clinical Oncology Program, Delaware/Christiana Care CCOP, University of Minnesota Medical Center-Fairview, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, University of Kentucky, Moffitt Cancer Center and Research Institute, Saint Joseph’s Hospital and Medical Center, Scott and White Memorial Hospital, Kalamazoo CCOP, Northern Indiana Cancer Research Consortium, and Iowa-Wide Oncology Research Coalition NCORP.
Footnotes
CONFLICT OF INTEREST STATEMENT
Kimberly K. Leslie: Dr. Leslie attests that she has no conflicts of interest.
Virginia L. Filiaci: Dr. Filiaci is supported by institutional grants from NIH during the conduct of the study; Institutional contracts from GOG Foundation, Inc. outside the submitted work.
Adrianne R. Mallen: Dr. Mallen reports no conflicts of interest.
Kristina W. Thiel: Dr. Thiel is a co-founder of and holds equity in Immortagen, Inc.
Eric J. Devor: Dr. Devor reports no conflict of interest.
Katherine Moxley: Dr. Moxley attests that she has nothing to declare.
Debra Richardson: Dr. Richardson reports that she serves on Advisory Boards for Genentech, Tesaro/GSK, AstraZeneca, Bayer, Deciphera, Mersana and Foundation Medicine David Mutch: Dr. Mutch attests that he has no conflicts of interest.
Angeles Alvarez Secord: Dr Alvarez Secord discloses clinical trial grant funding received from AbbVie, Amgen, Astellas Pharma Inc., Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Eisai, Exelixis, Immutep Ltd, Incyte, Merck, PharmaMar, Roche/Genentech, Seattle Genetics, Inc., Tesaro/GSK, VBL Therapeutics and National Cancer Trial Network. Dr. Alvarez Secord has also received honoraria for Advisory Boards from Aravive, AstraZeneca, Clovis, Cordgenics, Eisai, Merck, Myriad, Oncoquest, Roche/Genentech and Tesaro/GSK within the past 24 months.
Krishnansu S Tewari: Dr. Tewari served as a consultant and attended advisory boards for the manufacturers of bevacizumab (i.e., Genentech/Roche)
Megan E McDonald: Dr. McDonald has no conflicts of interest to disclose.
Cara Mathews: Dr. Mathews reports grants from National Institute of Health during the conduct of the study; grants from Syros, grants from Deciphera, grants from Astra Zeneca, grants from Astellas Pharma, grants from Tesaro/GSK, grants from Seattle Genetics and grants from Regeneron outside the submitted work.
Casey Cosgrove: Dr. Cosgrove attests that she has no conflicts of interest.
Summer Dewdney: Dr. Dewdney attests that she has nothing to declare.
Yovanni Casablanca: Dr. Casablanca reports that spouse owns shares in Celsion (biotech company), but not related to this manuscript.
Amanda Jackson:
Peter G Rose: Dr. Rose attests that he has no conflicts of interest.
Xun Clare Zhou: - Dr. Zhou attests that she has no conflicts of interest.
Michael McHale: - Dr. McHale has no conflicts of interest to report.
Heather Lankes: Dr. Lankes attests that she has no conflicts of interest.
Douglas Levine: - Dr. Levine reports serving in a consulting/advisory role for Tesaro/GSK, Merck. Research funding to institution from Merck, Tesaro, Clovis Oncology, Regeneron, Agenus, Takeda, Immunogen, VBL Therapeutics, Genentech, Celsion, Ambry, Splash Pharmaceuticals. Founder Nirova BioSense, Inc.
Carol Aghajanian: Dr. Aghajanian reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, grants from Genentech, grants from AbbVie, grants from Astra Zeneca, grants from Astra Zeneca, personal fees from Eisai/Merck, personal fees from Mersana Therapeutics, personal fees from Roche, personal fees from Abbvie, outside the submitted work.
Disclosure forms provided by the authors are available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Davies S, Dai D, Pickett G, et al. Effects of bevacizumab in mouse model of endometrial cancer: Defining the molecular basis for resistance. Oncol Rep 2011;25(3):855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res 2007;13(24):7487–95. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol 2011;29(16):2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghajanian C, Filiaci V, Dizon DS, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol Oncol 2018;150(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorusso D, Ferrandina G, Colombo N, et al. Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2 - A randomized phase II trial. Gynecol Oncol 2019;155(3):406–412. [DOI] [PubMed] [Google Scholar]
- 6.Behbakht K, Sill MW, Darcy KM, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecologic Oncology 2011;123(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Integrated genomic analyses of ovarian carcinoma. Nature 2011;474(7353):609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mankoo PK, Shen R, Schultz N, et al. Time to recurrence and survival in serous ovarian tumors predicted from integrated genomic profiles. PLoS One 2011;6(11):e24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang HJ, Chun SM, Kim KR, et al. Clinical relevance of gain-of-function mutations of p53 in high-grade serous ovarian carcinoma. PLoS One 2013;8(8):e72609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502(7471):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 2004;22(11):2159–66. [DOI] [PubMed] [Google Scholar]
- 14.Miller D, Filiaci V, Fleming G, et al. Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2012;125(3):771. [Google Scholar]
- 15.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010;26(5):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koboldt DC, Zhang Q, Larson DE, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 2012;22(3):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013;31(3):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson DE, Harris CC, Chen K, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 2012;28(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 2007;28(6):622–9. [DOI] [PubMed] [Google Scholar]
- 20.Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 2013;31(9):1219–30. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Clamp A, Backen A, et al. Systematic analysis of circulating soluble angiogenesis-associated proteins in ICON7 identifies Tie2 as a biomarker of vascular progression on bevacizumab. Br J Cancer 2016;115(2):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backen A, Renehan AG, Clamp AR, et al. The combination of circulating Ang1 and Tie2 levels predicts progression-free survival advantage in bevacizumab-treated patients with ovarian cancer. Clin Cancer Res 2014;20(17):4549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han ES, Burger RA, Darcy KM, et al. Predictive and prognostic angiogenic markers in a gynecologic oncology group phase II trial of bevacizumab in recurrent and persistent ovarian or peritoneal cancer. Gynecol Oncol 2010;119(3):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurzrock R, Stewart DJ. Exploring the Benefit/Risk Associated with Antiangiogenic Agents for the Treatment of Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2017;23(5):1137–1148. [DOI] [PubMed] [Google Scholar]
- 25.Hegde PS, Jubb AM, Chen D, et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 2013;19(4):929–37. [DOI] [PubMed] [Google Scholar]
- 26.Fleming ND, Coleman RL, Tung C, et al. Phase II trial of bevacizumab with dose-dense paclitaxel as first-line treatment in patients with advanced ovarian cancer. Gynecol Oncol 2017; 10.1016/j.ygyno.2017.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tewari KS, Burger RA, Enserro D, et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J Clin Oncol 2019;37(26):2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norquist BM, Brady MF, Harrell MI, et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018;24(4):777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kommoss S, Winterhoff B, Oberg AL, et al. Bevacizumab May Differentially Improve Ovarian Cancer Outcome in Patients with Proliferative and Mesenchymal Molecular Subtypes. Clin Cancer Res 2017;23(14):3794–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Said R, Hong DS, Warneke CL, et al. P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget 2013;4(5):705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhang Ghahremani M, Goossens S, Haigh JJ. The p53 family and VEGF regulation: “It’s complicated”. Cell Cycle 2013;12(9):1331–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwaederle M, Lazar V, Validire P, et al. VEGF-A Expression Correlates with TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Antiangiogenesis Therapy. Cancer Res 2015;75(7):1187–90. [DOI] [PubMed] [Google Scholar]
- 33.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res 2001;61(18):6952–7. [PubMed] [Google Scholar]
- 34.Qin G, Kishore R, Dolan CM, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A 2006;103(29):11015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa T, Watanabe J, Kamata Y, et al. VEGF expression and its reguration by p53 gene transfection in endometrial carcinoma cells. Hum Cell 2003;16(1):47–54. [DOI] [PubMed] [Google Scholar]
- 36.Ballman KV. Biomarker: Predictive or Prognostic? J Clin Oncol 2015;33(33):3968–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.