Abstract
In the era of combined antiretroviral therapy (cART), human immunodeficiency virus type 1 (HIV-1) is considered a chronic disease with an inflammatory component that specifically targets the brain and causes a high prevalence of HIV-1-associated neurocognitive disorders (HAND). The endocannabinoid (eCB) system has attracted interest as a target for treatment of neurodegenerative disorders, due to the potential anti-inflammatory and neuroprotective properties of cannabinoids, including its potential therapeutic use in HIV-1 neuropathogenesis. In this review, we summarize what is currently known about the structural and functional changes of the eCB system under conditions of HAND. This will be followed by summarizing the current clinical and preclinical findings on the effects of cannabis use and cannabinoids in the context of HIV-1 infection, with specifically focusing on viral load, cognition, inflammation, and neuroprotection. Lastly, we present some potential future directions to better understand the involvement of the eCB system and the role that cannabis use and cannabinoids play in neuroHIV.
Keywords: antiretroviral therapy, cannabinoid type 2 receptor, cannabinoid type 1 receptor, cannabis, C-C motif chemokine receptor 5 (CCR5), C-X-C motif chemokine receptor 4 (CXCR4), Δ9-tetrahydrocannabinol, endogenous cannabinoid system, fatty acid amide hydrolase, G-protein coupled receptor (GPCR) GPR18, HIV-associated neurocognitive disorders, inflammation, microglia, MJN110, monoacylglycerol lipase, neurodegeneration, neuroHIV, PF3845, synaptodendritic degeneration
Graphical Abstract

1. Background
In the context of human immunodeficiency virus type 1 (HIV-1) infection, cannabis use is an important topic and is one of the most commonly used drugs among people living with HIV-1 (PWH). Cannabis use has been reported to be higher in PWH compared to the general population [178], potentially to manage HIV-1 symptoms such as pain, nausea, and appetite loss, despite the negative effects [20, 178, 240, see also Table 1]. Additionally, certain cannabinoids are emerging as therapeutically promising neuroprotective agents in several neurodegenerative diseases, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis due to their anti-inflammatory, anti-oxidative, and anti-excitotoxic properties [156, 226]. To enhance our understanding about the role of the endocannabinoid (eCB) system in neuroHIV, the current review focuses on how the eCB system is altered by neuroHIV and how cannabinoids affect HIV-1 infection and specifically HIV-1-associated neurocognitive disorders (HAND).
Table 1:
Human clinical findings
| Major effects | Species | HIV pathogen | ART | Target | Ligand | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Neuroinflammation | Human (postmortem tissue) | HIV, encephalitis | Yes | CB1R CB2R |
Anti- CB1R and anti- CB2R antibodies | ↑ CB1R in white matter microglia and perivascular cells ↑ CB2R microglia, astrocytes and perivascular macrophages |
[52] |
| Human | HIV-1 | Yes | pDC | Δ9-THC | ↓ IFN-α by pDC | [99] | |
| Human | HIV-1 | Yes | T-cell | Δ9-THC | IFN-α ↑ IL-7R-α expression in T cells IFN-α ↑ IL7-induced phosphorylation of STAT5 in CD4+ and CD8+ cells CD3/CD28/IFN-α-induced proliferation was ↑ by IL-7 and ↓ by THC |
[98] | |
| Human and primary leukocytes (in vitro) | HIV-1 | CD16 and IP-10 levels | Cannabis | HIV+ users – ↓ circulating CD16 monocytes and plasma IP-10 than HIV- nonusers HIV+ users – no CD16 expression when treated with in vitro IFNa THC treatment of PBMC and purified monocytes ↓ IP-10 levels |
[197] | ||
| Cognitive performance | Human | HIV-1 | Yes | Effect on neurocognitive impairment | Cannabis | Lower likelihood of neurocognitive impairment | [233] |
| Human | HIV-1 | No info | Effect on cognitive performance, CD4 count and viral loads | Cannabis | HIV+ patients – lower neurocognitive performance than control Moderate-to-heavy HIV+ users – low learning/memory performance than moderate-to-heavy HIV- users HIV+ light users – more verbal fluency than HIV- light users HIV+ cannabis users had lower viral loads and higher CD4 count than non-users |
[228] | |
| Humans | HIV-1 | No info | Effect on brain structure and cognitive performance | Cannabis | Heavy users – smaller volumes in the entorhinal cortex and fusiform gyrus HIV+/- smaller thickness of the cingulate HIV- light-users had better cognitive performance than HIV+ |
[227] | |
| Humans | HIV-1 | Some subjects on ART | Effects on cognition and brain metabolites | Cannabis | No effect on cognition HIV+ non-users – ↓ N-acetyl aspartate in parietal white matter and ↑ choline compound in basal ganglia Cannabis users (HIV+ and HIV-) – ↓ basal ganglia N-acetyl aspartate, choline compound, and glutamate, ↑ thalamic creatine HIV+ cannabis users – ↓ glutamate in frontal white matter |
[35] | |
| Humans | HIV-1 | No info | Effects on cognitive function | Cannabis | Frequent users reported more symptoms of depression and anxiety No significant difference effects of marijuana on CD4 levels Impact of marijuana was greater on delayed memory in severe HIV disease No difference in attention, learning or memory due to marijuana use |
[58] | |
| Viral load / Immune cells | Human | HIV-1 | Yes (Indinavir or Nelfinavir) | HIV-1 RNA levels, CD4+ and CD8+ cells subset, PK analyses of protease inhibitor | Δ9-THC | Does not elevate viral load in patients on stable antiretroviral regimens No effect on CD4+ or CD8+ cell counts No clinical interaction of cannabinoid with protease inhibitors |
[1] |
| Human | HIV-1 | Yes | HIV-1 RNA levels, CD4+ and CD8+ cells | Dronabinol or cannabis | No negative changes No changes in CD4+ and CD8+ cell levels |
[29] | |
| Human | HIV-1 | No | HIV-1 RNA viral loads | Cannabis | ↓ Plasma HIV-1 RNA viral loads | [158] | |
| Human | HIV-1 | Yes | HIV-1 viral suppression | Cannabis | No viral suppression in daily or less than daily cannabis users | [175] | |
| Human | HIV-1 | Yes | HIV-1 RNA levels in blood and semen | Cannabis | ↑ HIV-1 RNA levels in semen | [83] | |
| Human | HIV-1 | Yes | HIV-1 viral load | Cannabis | Daily and nearly daily cannabis users show viral load suppression | [218] | |
| Human | HIV-1 | Yes | Inflammatory immune cell frequency | Δ9-THC | ↓ Frequency of HLA-DR+, CD38+, CD4+, and CD8+ cells ↓ Monocytes subset ↓ IL-23 and TNF-α |
[146] | |
| Human | HIV-1 | Yes (Azidothymidine and/or Dideoxyinosine | Effects of marinol on HIV-1 progression | Marinol | ↓ Clinical indicators, amylase, lipase, ALT and AST (not significant) | [236] | |
| Human | HIV-1 | Yes | Effects of cannabis on inflammatory and circulating monocytes | Cannabis | ↓ Inflammatory, nonclassical, activated classical and activated-inflammatory monocytes | [33] | |
| Human | HIV-1 | Yes | Effects of cannabis use on BMI, CD4+ cells and HIV-1 RNA suppression | Cannabis | No changes in BMI and CD4+ cell count Cannabis users had detectable viral loads |
[133] | |
| Human | HIV-1 | No info | CD4+ and CD8+ cell counts | Δ9-THC | ↑ CD4+ and CD8+ | [120] | |
| ART adherence | Human | HIV-1 | Yes | ART adherence and HIV-1 symptom | Cannabis | Cannabis dependent group: Had low adherence than non-users and non-dependent users Had higher viral loads Had frequent and severe HIV symptoms/ ART side effects |
[26] |
| Human | HIV-1 | Yes | ART adherence | Cannabis | No relationship between cannabis use and adherence Cannabis use for reducing nausea resulted in ART adherence |
[62] | |
| Human | HIV-1 | Yes | ART adherence | Cannabis | Recreational users showed low ART adherence Therapeutic users showed no association with ART adherence |
[145] | |
| Human | HIV-1 | Yes | Retention outcomes | Cannabis | Not associated with IOM retention outcome Associated with missing next appointment |
[125] | |
| Human | HIV-1 | Yes | ART adherence | Cannabis | Use led to nonadherence | [231] | |
| Appetite and/or Mood | Human | HIV-1 | Yes | Effects on caloric intake | Dronabinol, Δ9-THC | ↑ Caloric intake Minor effects on cognitive performance |
[90] |
| Human | HIV-1 | No info | AIDS-related anorexia | Dronabinol | ↑ Appetite above baseline Mood improvement ↓ Nausea |
[16] | |
| Human | HIV-1 | Yes | Effects of caloric intake, mood, and sleep | Dronabinol, Δ9-THC | ↑ Caloric intake No cognitive impairment Only Δ9-THC improved sleep |
[89] | |
| Human | HIV-1 | No info | Effect on nutritional status | Dronabinol | ↑ Percent body fat ↑ Weight gain ↑ Prealbumin ↓ Symptom distress Improved appetite |
[222] | |
| Human | HIV-1 | Yes | Effects of high dose | Dronabinol | ↑ Food cravings Improved sleep Mood improvement |
[18] | |
| Human | HIV-1 | Yes | Effect on appetite hormones | Δ9-THC | ↑ Plasma levels of ghrelin, leptin ↓ Plasma levels of PYY No effect on insulin |
[196] | |
| Humans | HIV-1 | No info | Over all effects | Δ9-THC | ↓ Anxiety and/or depression Improved appetite Pain relief |
[189] | |
| Human | HIV-1 | Yes | Long-term effects of dronabinol | Dronabinol | Safe to use for anorexia associated weight loss in patients with AIDS | [17] | |
| Human | HIV-1 | Yes | HIV-1 wasting syndrome with anorexia | Dronabinol Megestrol acetate |
Dronabinol alone did not affect weight High dose of megestrol acetate + dronabinol ↑ weight |
[229] | |
| Human | HIV-1 | Yes | Effect of appetite and weight gain | Dronabinol | Improves appetite Reverses weight loss |
[63] | |
| Human | HIV-1 | Zidovudine in 6 patients | Effects on weight | Dronabinol | ↑ Body weight | [86] | |
| Human | HIV-1 | N/A | Effects of HIV-1 symptoms | Δ9-THC | ↑ Appetite ↓ Muscle pain ↓ Nausea ↓ Anxiety ↓ Nerve pain ↓ Depression ↓ Paresthesia |
[240] | |
| Neuropathic pain | Human | HIV-1 and symptomatic HIV-SN | Yes | Effect of smoked cannabis on HIV-associated neuropathy | Δ9-THC | ↓ Chronic neuropathic pain from HIV-associated sensory neuropathy | [2] |
| Human | HIV-DSPN | Yes | Effect of smoked cannabis on HIV-associated neuropathy | Δ9-THC | ↓ Pain Improved mood and daily functioning |
[73] |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ALT, alanine transaminase; ART, antiretroviral therapy; AST, aspartate transaminase; BMI, body mass index; CB1R, cannabinoid type 1 receptor; CB2R, cannabinoid type 2 receptor; CBR, cannabinoid receptor; Δ9-THC, delta-9-tetrahydrocannabinol; HIV-SN, HIV-associated sensory neuropathy; HIV-DSPN, HIV-associated distal sensory predominant polyneuropathy; HLA-DR+, human leukocyte antigen – DR isotope; IFN-α, Interferon alpha; IL-7, interleukin 7; IL-23, interleukin 23; IL-7R-α, IL-7R-α receptor; IOM, Institute of Medicine; IP-10, IFN-γ-inducible protein 10; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells; PK, pharmacokinetics; PYY, peptide YY; STAT5, signal transducer and activator of transcription 5; TNF-α, tumor necrosis factor alpha
Criteria for exclusion from this Table: (1) Studies on cannabinoids and HIV effects not directly related to the central nervous system. (2) Studies on the effects of cannabinoids on other diseases/disease pathogens.
1.1. HIV-1 associated neurocognitive disorders (HAND)
HIV-1 associated neurocognitive disorders (HAND) was introduced in 2007 [9] and is an umbrella term for a group of neurocognitive disorders that include three subtypes; HIV-associated dementia (HAD), minor neurocognitive disorder (MND), and asymptomatic neurocognitive impairment (ANI) [72]. Before the availability of HIV-1 therapy, more than 15% of infected patients developed the more severe form HAD and autopsy usually revealed pathological and inflammatory changes to the brain, also known as HIV encephalitis (HIVE) [47, 72]. With the introduction of combined antiretroviral therapy (cART), which is very effective in suppressing HIV-1 replication and restoring the immune system [12], HAD has significantly declined (< 5%) and hardly any HIVE cases are reported at autopsy [72, 96]. However, as ART medication does not eradicate the virus, low levels of viral replication and chronic immune activation still linger, specifically in the brain due to low brain penetration of cART [148]. The difficulty of efficient delivery of cART to the central nervous system (CNS) [24, 177, 195] results in the prevalence of the milder forms of HAND to remain high. Up to 50% of cART treated PWH exhibit MND or ANI that can interfere with daily life [9, 69, 72, 80, 96, 206], involving problems in executive function, memory consolidation, decision-making, attention [60, 82, 91, 96, 214], and/or mood [25, 149, 176].
The brain mechanisms underlying HAND involve two pathways, including HIV-1 induced neuroinflammation within the brain that indirectly affect neuronal health and continued production of neurotoxic HIV-1 proteins that can target neurons directly.
Chronic neuroinflammation within the brain appears to predominate and significantly contribute to the onset of HIV-1 associated neuronal injury and thus, HAND [81, 92, 118]. Shortly after infection, HIV-1 can enter the brain within infected macrophages, monocytes, and T cells [75, 103, 116, 212, 238] and as cell-free virus that establish central reservoirs by infecting microglia, brain endothelial cells or astrocytes [8, 28, 44, 95, 127, 131, 134]. As the virus itself is not able to infect neurons the release and production of neurotoxic factors such as inflammatory mediators from HIV-1 infected cells contribute indirectly to neuronal dysfunction and injury [3, 44, 84, 104]. HIV-1 has been demonstrated to cause neurotoxicity by stimulating the production of proinflammatory cytokines and chemokines in the brain, inducing the release of TNF-α, RANTES/CCL5, and MCP-1/CCL2 from infected microglia and macrophages [71, 217, 235] and IL-8, IL-1ß, and TNF-α from infected astrocytes [37].
Additionally, HIV-1 contributes to HAND through the continued production of neurotoxic HIV-1 proteins from cellular reservoirs within the CNS that can target neurons directly [76, 77, 137, 155, 188]. HIV-1 proteins, such as the transactivator of transcription (Tat) and the envelope glycoprotein 120 (gp120) are likely agents of the observed neuronal loss in PWH and have been measured in the CNS of PWH under cART [97, 115, 153]. Besides their indirect effects on neurons via actions on microglia and astrocytes [38, 70, 119, 123, 130, 136], Tat and gp120 have direct effects on neurons by activating glutamatergic NMDA receptors [76, 77, 94, 142, 151], altering chemokine receptor signaling [gp120; 101, 154, 155], and interacting with the lipoprotein receptor-related protein [Tat; 137]. The effects of Tat on NMDA receptors in neuronal cultures has been demonstrated to potentiate glutamate-induced excitotoxicity [139], leading to increases in neuronal intracellular calcium levels, dendritic damage, and synapse loss [76, 94, 151, 215]. Similarly, blocking chemokine receptor signaling has been shown to prevent gp120-induced neuronal apoptosis in the absence of non-neuronal cells [155]. Thus, even though HIV-1 itself is not able to infect neurons, the release of HIV-1 proteins from HIV-1 infected cells can contribute to neurotoxicity via direct mechanisms on neurons.
The persistence of HAND in the era of cART has raised questions about the causes and treatment of HIV-1-related brain disorders and about the extent to which HAND and its underlying structural changes are reversible.
1.2. The endocannabinoid (eCB) system
As HAND is a group of neurodegenerative cognitive disorders with an inflammatory component, the eCB system, which regulates both cognition and immune function, presents a promising therapeutic target for treating the consequences of HIV-1 infection on the CNS.
The eCB system, constituted of endogenous cannabinoids (‘endocannabinoids’), cannabinoid receptors, and enzymes which synthesize or degrade cannabinoids, has attracted interest as a target for treatment of neurodegenerative disorders, due to the potential anti-inflammatory and neuroprotective properties of cannabinoids. A schematic presentation of the eCB system in a healthy individual is depicted in Figure 1A. The two main eCB ligands are N-arachidonoylethanolamine (AEA, also known as anandamide) and 2-arachidonoylglycerol (2-AG). These ligands as well as exogenous cannabinoids, such as delta-9-tetrahydrocannabinol (Δ9-THC) found in cannabis, act predominantly via cannabinoid type 1 and/or cannabinoid type 2 receptors (CB1R and CB2R, respectively), but can also activate the transient receptor potential vanilloid (TRPV) ion channels [102, 117], peroxisome proliferator-activated receptors (PPARs) [174, 224], and/or other G-protein-coupled receptors, including GPR55 and GPR18 [42, 50, 93, 202].
Figure 1.
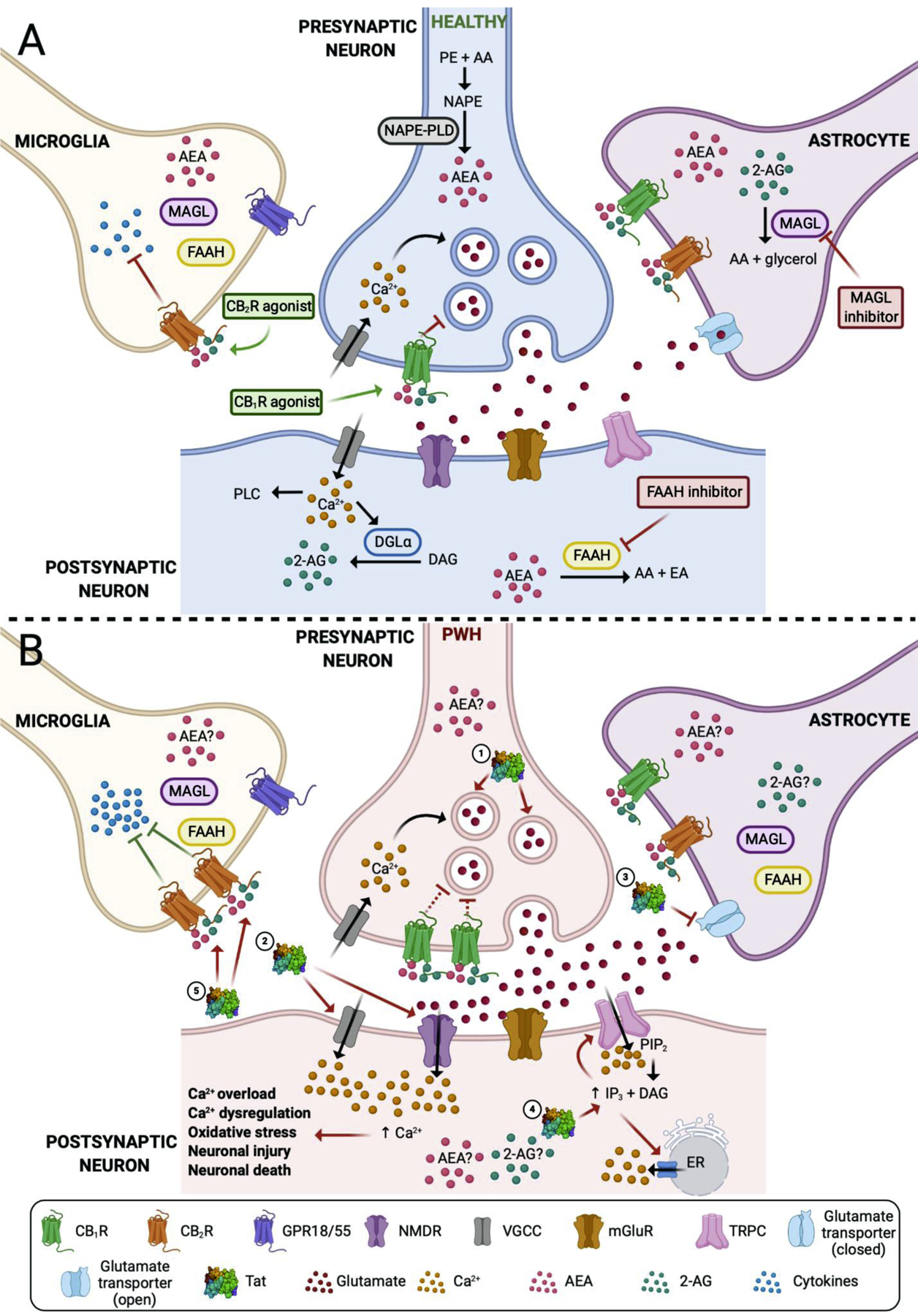
A schematic presentation of the endocannabinoid (eCB) system. (A) Represents the eCB system in a healthy individual. CB1Rs are present on presynaptic neurons. The influx of Ca2+ into the presynaptic neuron causes release of glutamate in the synapse and interacts with postsynaptic receptors (i.e. NMDR, mGluR). Excess of glutamate is taken up by the glutamate transporter present on astrocytes. CB1R agonists block the release of glutamate and decrease excitotoxicity. CB2Rs are predominantly expressed on microglia and their activation by CB2R agonists decreases neuroinflammation by blocking the production of proinflammatory cytokines. It has been shown that neurons, but also glial cells, produce eCBs AEA and 2-AG which are hydrolyzed by the enzymes MAGL and FAAH, respectively. (B) Shows the possible mechanism of action of Tat and its effects on the eCB system in PWH. (1) Tat causes an excess of glutamate release into the synapse and (2) abnormally increases Ca2+ influx by acting on the NMDR, VGCC, and TRPC. (3) Tat blocks the glutamate transporter which further increases glutamate concentration in the synapse. TRPC channel is a non-selective cation channel that is also permeable for Ca2+. (4) Tat increases the IP3 concentration which activates the TRPC and leads to Ca2+. Additionally, the increased IP3 levels cause intracellular Ca2+ release from the ER. This excess of intracellular Ca2+ concentration causes Ca2+ overload, Ca2+ dysregulation, oxidative stress which leads to neuronal injury and eventually neuronal death. Tat also leads to the upregulation of CB1Rs and CB2Rs. Even though the CB1R is upregulated in the presynaptic neuron, it is currently debated whether its inhibitory function is impaired or enhanced (represented by the broken inhibitor line). The negative effects of Tat are counteracted at the microglia as the overexpression of CB2R blocks proinflammatory cytokines more effectively. Whether the endogenous ligands AEA and 2-AG are affected by Tat and their levels are upregulated in the brain of PWH is still not known (represented by ?). Abbreviations; 2-AG, 2-arachidonoylglycerol; AA, arachidonic acid; AEA, arachidonoyl ethanolamine (anandamide); Ca2+, calcium; DAG, diacylglycerol; DGL, diacylglycerol lipase; eCB, endocannabinoid; EA, ethanolamine; ER, endoplasmic reticulum; FAAH, fatty acid amide hydrolase; IP3, inositol triphosphate; mGluR, metabotropic glutamate receptor; MAGL, monoacylglycerol lipase; NAPE-PLD, N-arachidonoyl phosphatidylethanolamine phospholipase D; NAPE, N-arachidonoyl phosphatidylethanolamine; NMDR, N-methyl-D-aspartate receptor; PE, phosphatidylethanolamine; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; PWH, people living with HIV; Tat, transactivator of transcription; TRPC, transient receptor potential cation channel; VGCC, voltage-gated calcium channel. Created with BioRender.com.
The CB1R is the most abundant G-protein-coupled receptor in the CNS, mainly expressed on neurons [150] and is responsible for the psychoactive effects of Δ9-THC, which is a primary compound of cannabis [65, 220]. CB1R agonists have demonstrated promising protective effects, such as inhibiting excitotoxic neurotransmission by blunting presynaptic glutamate release [43, 107, 147] and decreasing intracellular calcium [173]. However, therapeutic use of direct CB1R agonists is limited due to the psychoactive side effects associated with activation of CB1Rs, including sensorimotor, affective and cognitive disturbances [65, 162, 220].
In turn, CB2Rs are predominantly expressed by cells of the immune system [30, 163, 225] but can also be found in the CNS on immune-activated glia [163, 221]. CB2Rs represent a promising therapeutic target as their activation has been shown to induce anti-inflammatory signaling in astrocytes [219], regulate microglial migration and cytokine production [4, 11, 144], and reduce oxidative stress and apoptosis in neurons [232].
Another line of research has focused on the development of drugs targeting enzymes regulating the biosynthesis and degradation of AEA and 2-AG [6, 135, 187]. Because eCBs are neuromodulators that are synthetized locally on demand, the inhibition of their degradation is a therapeutic strategy that will cause their elevation only in locations where they are being actively produced to evoke their local neuroprotective effects, e.g. at the site of injury. Thus, in contrast to CB1R or CB2R agonists that are associated with side effects resulting from lack of site specificity and affecting receptors in the entire body, eCB catabolic enzyme inhibitors have high therapeutic potential as they are targeting ‘on site’ produced eCBs and inhibit eCB degradation [67, 184]. There is strong preclinical evidence that selective inhibitors of the main AEA-metabolizing enzyme, fatty acid amide hydrolase (FAAH), and of the main 2-AG enzyme, monoacylglycerol lipase (MAGL) can ameliorate the unwanted effects in a variety of different laboratory animal models of neurodegenerative diseases [165, 184]. Hydrolytic enzyme inhibitors of AEA (i.e. AM5206) and 2-AG (i.e. URB602, JZL184) have demonstrated to produce neuroprotective effects in vitro [41, 165, 209] and in vivo [124, 165, 166]. Additionally, the new generation of hydrolytic enzyme inhibitors, such as FAAH inhibitor PF3845 and MAGL inhibitors MJN110 and Abx-1431 show highly improved selectivity, potency and produce less side effects compared to previously available compounds [5, 27, 46, 64, 110, 111, 114, 171, 182].
Overall, targeting the eCB system appears to present a promising strategy to alleviate inflammatory and neurodegenerative consequences of HIV-1 infection on the CNS, which is reviewed in detail below.
2. The endocannabinoid (eCB) system in HAND
Most of what is known about the effects of HIV-1 infection on the eCB system is derived from protein expression studies for cannabinoid receptors, eCB ligands, and their enzymes, but little is known about the extent to which HIV-1 might disrupt their function. A schematic presentation of how the eCB system is potentially altered by HIV-1 Tat in PWH is depicted in Figure 1B.
Effects on CB1R expression in the context of neuroHIV have been variable, with reports ranging from no effects to upregulating effects. Brain tissue analysis from frontal cortex of simian immunodeficiency virus (SIV) rhesus macaques [21] or whole brain samples from HIV humanized mice [85] demonstrated no alterations in CB1R protein or mRNA expression levels, respectively. However, when assessing cell-type specific changes, CB1R upregulation levels have been reported [52, 112, 239]. CB1R upregulation was noted in perivascular cells and white matter macrophages in brains of PWH with HIVE [52]. Further, CB1R levels were reported to be increased in neurons in the infralimibic region in a HIV-1 Tat transgenic mouse model and was shown to be associated with behavioral deficits in an inhibitory control task [112]. The upregulation of CB1R expression levels upon Tat exposure was also supported in mouse primary prefrontal cortex neuronal cultures in vitro, which demonstrated a time-course dependent linear increase of CB1R protein expression over a 24 h time period [244]. Whether the upregulation of CB1R expression levels observed in the female Tat(+) mice is a compensatory response to the Tat-induced observed behavioral deficits or contributes to the seen deficits in the behavioral Go-No-Go task needs to be further investigated [112]. Modified expression of CB1R levels in other diseases has been negatively correlated with the prognosis of the symptoms [157]. For example, in neuropathic pain and multiple sclerosis, increases in CB1R expression is associated with reductions of symptoms and/or dampened disease progression, suggesting a neuroprotective role [185], which is also confirmed in psychiatric disorders [169].
Most of the findings for changes of CB2R expression levels provide evidence for increased expression levels of CB2Rs in the context of neuroHIV [21, 52, 193]. Clinical postmortem studies demonstrated CB2Rs upregulation in white matter microglia, astrocytes, and perivascular macrophages [52, 193], which was specifically high in HIVE tissue and differed from HIV+ brains without HIVE [52]. This finding was confirmed in brain cortical tissue of SIV-infected rhesus macaques, demonstrating cell-type specific upregulation of CB2Rs in perivascular monocytes/macrophages and microglia [21]. The increase of CB2R expression levels in HIV-1 is suggested to be due to the inducible nature of CB2R upon microglial cell activation under pathological conditions [10, 15, 23, 49, 157] and has been associated with an anti-inflammatory function in various disease models [10, 113, 179]. Interestingly, the upregulation of CB2R expression appears to be specific to inflammation-driven neurodegeneration [48], as substantially more pronounced increases in CB2R expression levels have been noted in response to HIV-1 infection or other bacterial/viral inflammatory mediators compared to direct neurotoxins that cause neuronal injury from within the cell, e.g. via oxidative stress [23, 49].
Much less is known about changes of protein expression levels of eCB ligands and their degrading enzymes in the context of neuroHIV. Fatty acid amide hydrolase (FAAH) was found to be overexpressed in perivascular astrocytes and astrocytic processes of cortical SIV tissue samples [21]. It has been previously shown that the FAAH protein is selectively overexpressed in neuritic plaque-associated astrocytes in Alzheimer’s disease brains [22]. FAAH upregulation in astrocytes appears to contribute to proinflammatory effects, as they are involved in converting AEA to arachidonic acid [56, 152], thus, providing a potential source of inflammatory processes.
Further, it is not known if endogenous ligands such as AEA or 2-AG are upregulated in the brain of PWH. A recent in vitro study, in primary prefrontal cortex neuronal cultures was unable to demonstrate a significant upregulation of AEA upon Tat exposure [100] but more clinical and preclinical studies are necessary to assess whether HIV-1 significantly alters eCB levels in the CNS.
Lastly, hardly any studies have investigated the effects of HIV-1 on eCB’s regulatory function. It is not quite clear whether eCB signaling is diminished or enhanced in the context of neuroHIV. A recent study reported Tat-induced reduction of the inhibiting effects exerted on glutamatergic neurotransmission by cannabinoids in hippocampal cultures, including Δ9-THC and 2-AG, due to impaired CB1R-mediated presynaptic inhibition of glutamate release [241]. In turn, another study has shown that the downregulating effects of eCBs on glutamatergic transmission are enhanced in the context of neuroHIV [112]. Upregulating AEA via the FAAH enzyme inhibitor PF3845 demonstrated increased inhibition of glutamate release in prefrontal cortex brain slices of male Tat(+) transgenic mice compared to their control counterparts [112]. The discrepancy between results may be due to brain region-specific differences as the prefrontal cortex and hippocampus have been shown to display differential sensitivities to Tat [45].
Overall, additional studies are necessary to understand the effects of HIV-1 on the eCB system in more detail, specifically eCB signaling, and the underlying mechanisms involved.
3. Clinical and preclinical evidence of therapeutic properties of cannabinoids in HAND
3.1. Effects of cannabinoids on viral load
The effects of cannabinoids on viral load, HIV-1 replication, and CD4+ cell count have been assessed over the last years [1, 29, 83, 133, 160, 175, 186, 193, 198, 203, 228, 239, see also Tables 1 and 2].
Table 2:
Preclinical animal studies (in vivo)
| Major Effects | Species | HIV Pathogen | ART | Target | Ligand | Effect | Receptor Involved | Reference |
|---|---|---|---|---|---|---|---|---|
| Neuronal activity | Mice | HIV-1IIIB Tat1–86 | No | PF3845 | Tat(+) female mice – inhibitory control deficits, ↑ CB1R in infralimbic cortex Negative correlation between inhibitory control and infralimbic CB1R expression ↑ sEPSC in Tat(+) mice PF3845 ↓ sEPSC |
CB1R | [112] | |
| Neuroinflammation and Immune cells | Rhesus macaques | SIVmac251, encephalitis | No info | CB1R, CB2R, FAAH | Anti- CB1R & anti- CB2R antibodies | ↑ CB2R microglia, perivascular macrophages and T-lymphocytes ↑ FAAH in perivascular astrocytes and astrocytic processes |
CB1R, CB2R | [21] |
| Mouse | pVRCgp120 | No info | Immune cells | Δ9-THC | ↑ or ↓ gp120 specific T cell responses depending on magnitude of IFN-γ response | No | [40] | |
| Mouse | pVRCgp120 | No info | Immune cells | Δ9-THC | ↑ gp120 specific INF-γ and IL-2 response with gp120 derived peptide 81 Δ9-THC ↑ gp120-specific T cell activation in WT but not CB1-/- and CB2-/- mice |
CB1R, CB2R | [39] | |
| Rhesus macaques | SIV | No info | CD4+ and CD8+ T lymphocytes | Δ9-THC | Chronic administration: No difference in lymphocyte subtypes, proliferation or apoptosis ↑ T lymphocyte CXCR4 expression of both CD4+ and CD8+ cells |
No | [132] | |
| Rhesus macaques | SIVmac251 | No info | miR | Δ9-THC | No differences in plasma viral loads ↑ Striatal BDNF ↓ TNF-α mRNA expression in THC/SIV group miRs modulation |
No | [216] | |
| Neurogenesis and Neuroinflammation | Mouse | GFAP/ gp120 | No | Deletion of FAAH gene | None | ↑ Neurogenesis by ↑ expression of COX-2 and PGE-2 ↓ Astrogliosis |
No | [13] |
| Mouse | GFAP/ gp120 | No | CB2R | AM1241 | ↑ Neurogenesis in hippocampus ↓ Astrogliosis and gliogenesis |
CB2R | [14] | |
| Viral load and disease progression | Rhesus macaques | SIVmac251 | No | HIV-1 RNA levels CD4+ and CD8+ cells |
Δ9-THC | No effect on disease progression, morbidity, and mortality ↓ Plasma SIV-RNA viral load and lengthened survival ↓ Classic markers of SIV disease |
No | [161] |
| Rhesus macaques | SIVmac251 | No | Effect of chronic Δ9-THC on viral load and inflammation | Δ9-THC | In lymph nodes and spleen: ↓ Viral replication ↓ Viral gag RNA ↓ INF-γ and IL-6 |
No | [160] | |
| Rhesus macaques | SIVmac251 | No | Effect of chronic Δ9-THC on plasma viral load | Δ9-THC | Tolerance to disruptive effects of Δ9-THC ↓ CB1R and CB2R levels in the hippocampus No effect on viral load in the plasma, CSF or brain tissue ↓ Neuropathology and opportunistic infections Lower expression of inflammatory cytokine MCP-1 |
No | [239] | |
| Mice (huPBL-SCID) | HIV-1NL4–3 | No | Effect of Δ9-THC on HIV-1 progression | Δ9-THC | ↑ HIV-infected peripheral blood leukocytes 50-fold ↑ viral load Upregulation of CCR5 and CXCR4 |
No | [203] | |
| Mice (huPBL/HIVE) | HIV-1ADA | No | Effect of CB2R agonist | Gp1a | ↑ CB2R expression in perivascular microglial cells and lymphocytes Gp1a ↓ infiltration of human cells in the mouse brain and HLA DQ activation Gp1a ↓ CCR5 expression on human cells in spleen ↑ Fas ligand expression |
CB2R | [85] | |
| Rhesus macaques | SIVmac251 | No | Viral load, CD4+ and CD8+ cells, IgE+ B cells | Δ9-THC | No difference in plasma viral load ↓ CD4+/CD8+ ratio ↓ IgE+ B cells |
No | [234] | |
| BBB impairment | HBMEC and human astrocyte cocultures (in vitro), mouse (in vivo) | gp120MN | No | TJ ZO-1, Claudin-5 expression | CP55,940, ACEA, URB597 |
In vitro – CP55,940 and ACEA prevented BBB permeability and prevented ZO-1 and claudin-5 downregulation in HBMEC In vivo – ACAE inhibited BBB permeability and prevented ZO-1 and claudin downregulation |
CB1R | [140] |
| Nociception | Rat | gp120MN | No | FAAH | URB597, PF3845 | ↓ Nociception in rat HIV neuropathy model ↓ Cold and tactile allodynia |
CB1R, CB2R | [167] |
| Rat | gp120IIIB | No info | None | WIN55,212–2, AMD3100 | ↓ Analgesic effectiveness AMD3100 restores the analgesic effects of WIN55,212–2 |
No | [180] |
Abbreviations: BBB, blood-brain barrier; BDNF, brain derived neurotropic factor; CB1R, cannabinoid type 1 receptor; CB2R, cannabinoid type 2 receptor; CBR, cannabinoid receptor; COX-2, cyclooxygenase-2; CSF, cerebrospinal fluid; Δ9-THC, delta-9-tetrahydrocannabinol; FAAH, fatty acid amide hydrolase; GFAP, glial fibrillary acidic protein; HBMEC, human brain microvascular endothelial cells; IFN-γ, Interferon gamma; IgE, immunoglobulin E; IL-2, interleukin 2; ; IL-6, interleukin 6; miR, microRNA; PGE-2, prostaglandin E2; SIV, simian immunodeficiency virus; sEPSC, spontaneous excitatory postsynaptic current; TJ ZO-1, tight junction zonula occludens-1; TNF-α, tumor necrosis factor alpha; WT, wild-type; ZO-1, zonula occludens-1
Criteria for exclusion from this Table: (1) Studies on cannabinoids and HIV effects not directly related to the central nervous system. (2) Studies on the effects of cannabinoids on other diseases/disease pathogens.
Enhancing effects of cannabis use on HIV-1 replication and viral load are more observational in nature [26, 83] and have been reported to be potentially due to low ART adherence [133], which has been demonstrated in a number of studies [61, 141]; even though others have found no association with cannabis use [201] or even enhanced ART adherence if cannabis use was associated with medical use [62, 145].
On the other hand, previous short-term randomized placebo-controlled studies reported no effects of cannabis, smoked or taken orally, on viral load, CD4+, and/or CD8+ cell counts in PWH [1, 29], which has also been confirmed in some studies for recreational cannabis use in PWH [36, 175, 236].
Nevertheless, the vast majority of literature reports a downregulation of viral load and HIV-1 replication by cannabinoids, including clinical studies [158, 228], and preclinical studies in vivo [160] and in vitro [186, 193, 198]. Clinical studies have found anti-viral effects of cannabinoids, with PWH cannabis users demonstrating lower viral load and higher CD4+ counts than PWH non-users [228]. This has also been confirmed in PWH injection drug users that use cannabis at high intensities [158] or daily and near-daily use [218], as well as in an underrepresented group of black PWH [120]. Preclinical studies in SIV-infected rhesus macaques further support clinical findings [160]. Prolonged period of Δ9-THC treatment prior to and during SIV infection resulted in lower viral load in lymph nodes and spleen as well as decreased inflammation (i.e. lower INF-γ and IL-6 protein in lymph nodes and spleen) [160, 239].
Direct effects of cannabinoids on viral replication have been demonstrated in vitro by the reduced numbers of SIV-infected cells in culture incubated with Δ9-THC prior to infection [161]. Interestingly, various preclinical in vitro studies have found CB2Rs to be involved in inhibiting viral expression [186, 193, 198]. Specifically it has been shown that CB2R activation with the agonists JWH144 and O-1966 decreased viral replication by affecting the long term repeat (LTR) of HIV-1 [193], potentially due to the CB2R-induced inhibition of cellular transcription factors involved in the transactivation of the HIV-1 LTR [193].
Another possible mechanism for suppression of viral replication includes the interaction of cannabinoids with HIV-1 coreceptors, CXCR4 and CCR5 [53, 85, 198], that are upregulated upon HIV-1 infection [132, 203]. Whereas, CB2R agonists JWH144 and O-1966 did not alter surface protein or gene expression of CXCR4 or CCR5 in HIV-1 infected monocyte-derived macrophages [193], other studies have shown an inhibitory effect of Δ9-THC on CCR5 and CXCR4 in HIV-1 infected monocyte-derived macrophages [237], WIN55,212–2 inhibitory effects on CCR5 in microglia [198], and CB2R agonist inhibitory effects in CD4+ T cells on CCR5 [85] or CXCR4 [53]. Specifically, it has been suggested that CB2R activation in CD4+ T cells can inhibit actin reorganization with decreasing F-actin levels, which is an important contributor to productive HIV-1 infection [53].
Overall, findings indicate that the activation of CB2Rs appear to potentially downregulate HIV-1 infection, with proposed mechanisms including CB2R-induced inhibition of cellular transcription factors involved in the transactivation of the HIV-1 LTR or CB2R cross-regulation of HIV-1 coreceptors, CXCR4 and CCR5, via inhibitory crosstalk.
3.2. Cannabinoid effects on cognition
A number of clinical studies have assessed cannabinoid effects in PWH. Specifically, cannabis use has been investigated and found to affect various biological processes, including cognitive performance [58, 228, 233], appetite [63, 90, 222], mood [16, 18], and neuropathic pain [2, 73, 167]. More detailed information about these studies is provided in Table 1, with this subsection specifically focusing on cannabis effects on cognition. It is known that cannabis use, in general, has negative (psychoactive) effects on memory and executive functions in healthy individuals [57, 194]; however, its effect in PWH remains unclear and divided.
The relationship between HIV-1 infection and cannabis use and their interactive effects on neurocognitive functioning is complex and various variables have been considered, including disease progression, the amount of cannabis use, and the cognitive domain assessed.
Some studies show that the effects of cannabis use in PWH depend upon disease stage [35, 58]. Cannabis use resulted in cognitive dysfunction in PWH with an advanced stage of infection (symptomatic phase) but no effects were noted for HIV negative cannabis users or asymptomatic PWH cannabis users [58]. Additionally, frequency of cannabis use was associated with greater cognitive impairment among symptomatic PWH, which appeared to be primarily related to performance on memory tasks [58]. The lack of synergistic or interactive effects of HIV-1 and cannabis use on cognitive performance for the asymptomatic stage was confirmed in another study, which did not observe any significant abnormalities for neuroasymptomatic PWH cannabis users compared to PWH non-users on standard neuropsychological tests when correcting for group differences in age, education, or depression scores [35]. Since both, HIV-1 infection and cannabis use, have effects on the immune system, there is a likelihood that their interactions are specifically exacerbated in PWH with greater immune suppression.
Other studies have demonstrated that the amount of cannabis use determines what type of effects cannabis exhibits on cognition in PWH [26, 227, 228]. For example, significant negative effects for HIV-1 symptoms and ART side effects have been reported for cannabis dependent use, whereas no differences were noted between non-cannabis use and non-dependent cannabis use [26]. Similarly, a recent study found that independent of HIV-1 status moderate-to-heavy users consistently performed worse than light users on cognitive functioning tasks (global score) [228]. In a follow up study, the finding that the amount of cannabis use has significant effects on global cognition in PWH was not confirmed and based on their results the authors concluded that cannabis use beyond 1.43 g/week had more adverse effects on neurocognitive performance in HIV negative users compared to PWH users [227]. However, caution should be exercised with this conclusion as a shortcoming of the study was that HIV negative non-users started out with significant higher cognitive performance compared to PWH non-users but then were significantly negatively affected by higher cannabis use (>1.43 g/week), which was not seen for PWH cannabis users [227]. Thus, baseline differences might have contributed to the seen adverse effects of high cannabis use in HIV negative individuals compared to PWH.
In addition to HIV-1 disease stage and the amount of cannabis use, the interactive effects of HIV-1 and cannabis also seem to depend on the cognitive domain assessed. Whereas moderate-to-heavy cannabis use significantly worsened overall cognitive performance independent of HIV-1 status (i.e. HIV negative individuals and PWH) PWH were most severely affected by moderate-to-heavy use for the learning and memory domain, displaying significantly lower scores in learning and memory performance compared to all other comparison groups [228]. It is known that the psychoactive effects of cannabis can be attributed to Δ9-THC, which is known to negatively impact memory function [87, 213] specifically at higher doses [109]. On the other hand, the study further reported that when the effects of light cannabis use were compared in PWH and HIV negative healthy controls, PWH light users outperformed HIV negative light users on verbal fluency [228]. This is an important finding as it indicates that depending on amount of cannabis use and the cognitive domain assessed, cannabis can have detrimental or beneficial effects on disease progression. The beneficial effects of cannabis use on verbal fluency find support in a recent study, that demonstrated cannabis use, defined as history of cannabis substance use disorder and cannabis use in the past year, lowered odds of neurocognitive impairment in PWH regardless of age and viral levels or disease stage, with PWH cannabis use being specifically associated with higher performance in verbal fluency [233]. The differential cannabinoid receptor and/or ligand distribution across brain regions could contribute to the distinct effects seen for cannabis use depending on the cognitive domain assessed [79, 181, 210].
Overall, based on the studies outlined above interactive effects of cannabis use and HIV-1 infection on cognition appear to be specifically seen in PWH with more advanced symptomatic stages of HIV-1 infection, and further seem to depend on the amount of cannabis use and the cognitive domain assessed; with high cannabis use exhibiting more negative effects, specifically in the learning and memory domain, whereas light cannabis use might have some beneficial effects for domains such as verbal fluency. However, additional research is needed to investigate more in detail what role different cannabis types play on cognition in PWH, including cannabis use with high versus low Δ9-THC content, as well as the components of cannabis that are non-psychoactive, including cannabinol or cannabidiol.
3.3. Anti-inflammatory properties of cannabinoids with neuroprotective benefits
The anti-inflammatory and immune modulatory properties of cannabinoids are well known [7, 59, 126, 164, 170] and have been reviewed in the context of HIV-1 infection [19, 54, 106, 242, see also Tables 1–3]. Cannabis use has been reported to lower inflammatory responses in HIV-1 infection from immune cells [33, 146, 197] and the peripheral nervous system in PWH and animal models [34, 160, 239], with this section focusing on the effects of cannabinoids on neuroinflammatory processes and its consequence on neuronal health.
Table 3.
Preclinical in vitro findings
| Major Effects | Species | Sample | HIV Pathogen | ART | Target | Ligand / Antibody | Effect | Receptor Involved | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Neuronal activity | Rat | Hippocampal neurons | Tat1–86 (clade B) | No | Effects of Tat on eCB system | WIN55,212–2, 2-AG, JZL184, Δ9-THC | Tat ↓ DSE 2-AG ↓ EPSCs JZL184 did not affect 2-AG mediated EPSCs WIN55,212–2 did not affect EPSC Δ9-THC ↓ EPSC |
CB1R | [241] |
| Neuronal damage and neuroinflammation | Mouse | PFC neuronal cultures | Tat1–86 (clade B) | No | FAAH | PF3845 | ↑ Neuronal survival ↓ [Ca2+]i ↑ Dendritic volume ↑ AEA, PEA |
CB1R | [100] |
| Mouse | PFC neuronal cultures | Tat1–86 (clade B) | No | Neurons | AEA, 2-AG | ↑ Neuronal survival ↓ [Ca2+]i ↓ Dendritic injury ↓ sEPSCs |
CB1R | [244] | |
| Human | Mesencephalic neuronal/glial culture | gp120LAV/IIIB (clade B) | No | Dopaminergic neurons | WIN55,212–2 | ↓ Neuronal damage ↓ Microglial damage ↓ Superoxide production ↓ Chemokine and cytokine production |
CB2R | [105] | |
| Human | Primary Müller cell culture | Tat (clade B) | No | Müller glia | AEA, 2-AG | AEA and 2-AG: Suppress Müller cell activation by ↓ inflammatory cytokines Control Tat-induced proinflammatory cytokines through MAPK phosphorylation Inhibit NF-KB signalosome AEA induces MKP- independent of MEK necessary for ↑ anti-inflammatory and ↓ pro-inflammatory cytokines |
CB1R, CB2R | [129] | |
| Human | Primary Müller cell culture | Tat (clade B) and Tat (clade C) | No | Müller glia | AEA | HIV-1 clade Tat B and C act differently Tat B suppresses through MKP-1 and Tat C through MEK-1 ↑ PBMC attachment |
CB1R, CB2R | [128] | |
| GABA | Mouse | Mouse brain slices | Tat1–86 (clade B) | No | GABA | WIN55,212–2, AEA | AEA ↓ GABAergic neurotransmission (mIPSCs) in PFC | CB1R | [243] |
| Cyto / Neurotoxicity | Rat | C6 glioma cells | Tat1–86 | No | NO synthase | WIN55,212–2, AEA | ↓ Cytotoxicity | CB1R | [74] |
| Human and murine | Human and murine NPCs | gp120IIIB (X4 strain) and gp120Ba-L (R5 strain) | No | CB2R | AM1241 | ↓ Neurotoxicity and apoptosis ↑ Differentiation of NPCs to neuronal cells ↑ Neurogenesis in vivo |
CB2R | [14] | |
| Synapse loss and neuroinflammation | Rat | Primary neuronal cultures | gp120IIIB | No | MAGL | JZL184 | ↓ Synapse loss ↓ Prostaglandins signaling Blocks potentiation of NMDARs |
CB2R | [245] |
| Rat | Hippocampal neuronal culture | gp120IIIB | No | Synapse | WIN55,212–2 | Inhibits synapse loss Blocks IL-1β release in microglia |
CB2R | [122] | |
| Cell migratory and/or adhesion response | Human | Leukemic monocyte lymphoma cell line | Tat1–86 | No | Migration of U937 towards Tat | Δ9-THC, CP55,940, O-2137 | ↓ Migration of U937 microphage-like cells towards Tat | CB2R | [191] |
| Human | Leukemic monocyte lymphoma cell line | Tat1–86 | Tat enhanced monocyte-like cell adhesion | Δ9-THC, CP55,940 | ↓ Attachment of U937 cells to ECM proteins by altering b-integrin expression and distribution of polymerized actin | CB2R | [192] | ||
| Mouse | BV-2 microglial-like cells | Tat1–86 | No | Migration of BV-2 | Δ9-THC, CP55,940, 2-AG, | ↓ Migration of BV-2 cells towards Tat | CB2R | [78] | |
| Inhibition of viral expression | Human | Microglial culture | None | No | HIV-1 | WIN55,212–2 | ↓ HIV-1 viral expression | CB2R | [198] |
| Human | Primary monocytes | None | No | HIV-1 | JWH133, GP1a, O-1966 | ↓ Activity of HIV-1 LTR Partially ↓ expression of HIV-1 pol |
CB2R | [193] | |
| Human | HIV-1 infected CD4+ lymphocyte and microglial cultures | None | No | HIV-1 | WIN55,212–2 | ↓ Viral expression in both CD4+ lymphocyte and microglial cultures | [186] | ||
| HIV infection | Human | CD4+ T cells | HIVNL-GI | No | CD4+ T cells | JWH-133, JWH-150, AM630 | CB2R activation in CD4+ cells inhibit actin reorganization which prevents infection of CXCR4-tropic HIV-1 in CD4+ T cells | CB2R | [53] |
| Human | Primary human monocyte cell lines | None | No | HIV-1 | Δ9-THC | ↓ HIV-1 infection of macrophages ↓ Cell surface receptors CD4, CCR5, and CXCR4 which ↓ viral entry |
CB2R | [237] | |
| Human | MT-2 cells | None | No | Syncytia formation | CP-55,940, Δ9-THC, WIN-55,212,2, WIN-55,212,3 | Cannabimimetic drugs ↑ HIV-1 infection | CB1R, CB2R | [172] |
Abbreviations: AEA, N-arachidonoylethanolamine; 2-AG, 2-Arachidonoylglycerol; ART, antiretroviral therapy; [Ca2+]i, intracellular calcium concentration; CB1, cannabinoid type 1 receptor; CB2, cannabinoid type 2 receptor; CBR, cannabinoid receptor; Δ9-THC, delta-9-tetrahydrocannabinol; DSE, depolarization-induced suppression of excitation; ECM, extracellular matrix; eCB, endocannabinoid system; EPSC, excitatory postsynaptic currents; FAAH, fatty acid amide hydrolase; GABA, gamma aminobutyric acid; LTR, long terminal repeat; MAGL, monoacylglycerol lipase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; MKP-1, mitogen-activated protein kinase phosphatase-1; NF-KB, nuclear factor kappa-light-chain-enhancer of activated B cells; NMDAR, N-Methyl-D-aspartic acid receptor; NO, nitric oxide; NPCs, neuronal progenitor cells; mIPSC, miniature inhibitory postsynaptic current; PBMC, peripheral blood mononuclear cells; PEA, palmitoylethanolamide; PFC, prefrontal cortex; sEPSC, spontaneous excitatory postsynaptic current
Criteria for exclusion from this Table: (1) Studies on cannabinoids and HIV effects not directly related to the central nervous system. (2) Studies on the effects of cannabinoids on other diseases/disease pathogens.
Various studies in the context of HIV-1 have demonstrated that the anti-inflammatory effects of cannabinoids in the CNS are related to the specific activation of CB2R [14, 32, 53, 105] which also has been reviewed previously [183, 190]. A preclinical mouse study reported downregulation of astrogliosis and gliogenesis in the hippocampus of GFAP/Gp120 transgenic mice when treated with the CB2R agonist AM1241 which was further accompanied by improved neurogenesis in the hippocampus [14]. In vitro studies demonstrated that cannabinoids, such as Δ 9-THC, CP55,940, and 2-AG, can inhibit the migration of mouse BV-2 microglial-like cells to viral products, such as HIV-1 Tat, which was linked functionally to the CB2R potentially due to reducing CCR3 levels and altering its intracellular compartmentation [78]. Interestingly, recent studies have shown that CB2R and chemokine CXCR4 are able to form heterodimers and display negative-crosstalk and cross-antagonism, thus, resulting in decreased CXCR4-mediated cell migration, invasion, and adhesion [168, 211].
The downregulation of inflammatory responses derived from microglia by cannabinoids is specifically relevant as chronic activation of brain microglia is a major contributing factor for HIV-associated brain disease [159, 199]. Using a culture model from human primary microglia, WIN55,212–2 inhibited the migration of gp120-activated microglia, thus suppressing the toxic activity of gp120 on the CNS [105]. Further, CB2R activation inhibited gp120-induced superoxide production in purified human microglial cells and reduced gp120-induced production of chemokine and cytokine (CCL2, CX3CL1, IL-1β, CXCL10) in the human mesencephalic neuronal/glial cultures [105]. The protective effects of CB2R activation are further supported in a neuronal/glial hippocampal culture model that reported inhibition of gp120-induced IL-1β production by WIN55,212–2, which subsequently let to reduced loss of synapses and was reversed by a CB2R antagonist [122].
Cannabinoid-induced attenuation of neuroinflammation has also been demonstrated by upregulating eCB levels via the direct application of 2-AG or AEA or via enzyme inhibitors such as MAGL or FAAH [13, 129, 245]. The genetic deletion of the FAAH enzyme in GFAP/gp120 transgenic mice, and thus upregulation of AEA, but not 2-AG in whole brain, demonstrated downregulation of astrogliosis as well as enhanced neurogenesis [13]. As FAAH inhibition also upregulates levels of non-eCB-related lipids (i.e. palmitoylethanolamide, PEA; oleoylethanolamide, OEA) additional mechanisms beside AEA might be involved in the anti-inflammatory effects of FAAH inhibition, including PEA and OEA’s effects on AEA metabolism by binding to PPAR-α or to TRPV1 [66, 88, 138]. In a different study the upregulation of 2-AG via the MAGL inhibitor JZL184 reduced gp120-induced prostaglandin E2 and IL-1β production, which prevented synapse loss and was attributed to CB2R activation [245]. In contrast to AEA, which acts only as a weak partial agonist toward CB1R and CB2R, 2-AG has been shown to act as a full agonist toward both receptors, CB1Rs and CB2Rs [223].
I sum, the findings suggest that cannabinoids have anti-inflammatory effects with neuroprotective benefits in the context of neuroHIV, potentially via CB2R activation, but additional mechanisms might be involved. Further, enzyme inhibitors targeting MAGL and FAAH have great therapeutic potential as they allow for selective elevation of eCB signaling, which enables investigation of physiological actions of particular eCBs as well as reveal therapeutic potential of such precise modulation [67, 184].
3.4. Neuroprotective effects of cannabinoids via presynaptic mechanisms
Even though the anti-inflammatory properties of cannabinoids appear to be the predominant mechanism for the displayed neuroprotective benefits of cannabinoids in neuroHIV, some studies have demonstrated cannabinoids-regulating effects against HIV-1 protein toxicity directly on neurons via presynaptic mechanisms [100, 244].
Direct neuroprotective effects of cannabinoids have been demonstrated in primary prefrontal cortex neuronal cultures that assessed the effects of eCB ligands against HIV-1 Tat toxicity [244]. Findings indicated that the two endogenous cannabinoid ligands 2-AG and AEA significantly decreased Tat-induced intracellular calcium, neuronal excitability, dendritic injury, and neuronal death [244]. The protective effects of both eCBs were attributed to CB1R synaptic function with CB1R but not CB2R protein levels being significantly upregulated [244]. Similarly, a different study demonstrated that enhanced AEA levels via the FAAH enzyme inhibitor PF3845 displayed protective effects against Tat-induced intracellular calcium, dendritic injury, and neuronal death in prefrontal cortex neurons in vitro [100]. Importantly, whereas CB1R played a role for downregulating the immediate effects of Tat on intracellular calcium production, CB2R appeared to be involved in the more long-term PF3845-induced protective effects on dendritic changes and neuronal survival, potentially due to the 10% astrocyte contribution in the neuronal culture model [100].
Additional cannabinoid-regulating effects on neuronal function in neuroHIV have been demonstrated on glutamatergic neurotransmission, including spontaneous, miniature, and evoked excitatory postsynaptic currents (sEPSCs, mEPSCs, and eEPSCs, respectively) [112, 241, 244]. The 2-AG- and AEA-induced downregulation of EPSCs has been shown to be related to a presynaptic CB1R-related mechanism [241, 244] with neuroprotective effects against excitations an neuronal injury [244]. It has been demonstrated that the mechanisms involved in Tat-induced synapse loss include calcium influx via NMDARs [121]. Interestingly, cannabinoid-induced glutamatergic hypofunction has been shown to involve coupling of CB1Rs with NMDAR NR1 subunit by forming heterodimers [200, 207, 208]. Note however, whether CB1R-related signaling is impaired or enhanced during neuroHIV is currently debated [112, 241] and needs to be investigated in more detail.
For inhibitory neurotransmission, not much is known about the effect of eCBs on HIV-1/HIV-1 protein-induced alterations. In one study HIV-1 Tat-induced decreases in inhibitory gamma-aminobutyric acid (GABA)ergic neurotransmission in prefrontal cortex mouse brain slices were occluded by cannabinoids, such as WIN55,212–2 and AEA, via a presynaptic CB1R-related mechanism [243]. Further, a recent study demonstrated normal CB1R signaling at inhibitory synapses in the presence of Tat [241].
Overall, there is evidence that presynaptic CB1R-related mechanisms contribute to neuroprotective effects against HIV-1 protein-induced toxicity. Additional studies are necessary to understand the underlying mechanisms involved in eCB’s potential ability to attenuate or reverse HIV-1-induced synapse decline and altered neurotransmission on glutamatergic and potentially GABAergic neurons.
4. Conclusion and future directions
Here we have reviewed the specific involvement of the eCB system in HIV-1 disease progression and its potential use as a therapeutic target to decrease HAND pathology. The review shows the specific involvement of CB2R in neuroHIV due to the inflammatory nature of the disease and indicates to be a promising therapeutic target via its anti-inflammatory effects on the immune and CNS systems, with additional beneficial effects on viral load potentially due to CB2R and CXCR4 heterodimerization. Further alternative cannabinoid receptors, including GPR55 and GPR18, are worth investigating in more detail to understand their regulating effects on the CNS and how they may contribute to or attenuate HAND pathogenesis.
In contrast, CB1R involvement in neuroHIV is less clear. Besides the known psychoactive effects via CB1R, and thus limited therapeutic use, potential protective effects via inhibition of HIV-induced excitatory neurotransmission by pre-synaptic CB1R mechanisms have been reported but more studies are necessary to assess the role of CB1R and/or cannabinoids on the inhibitory system in neuroHIV, and whether CB1R signaling is enhanced or inhibited. Additionally, as viral load and the distribution of the eCB system vary across brain regions, systematic studies are necessary to assess brain-region specific differences.
In turn, the use of eCB degrading enzyme inhibitors as a tool to alter eCB tone in neuroHIV seems a promising future avenue. Enhancing AEA or 2-AG via FAAH or MAGL enzyme inhibitors, respectively, has high therapeutic potential as they are targeting ‘on site’ produced eCBs and inhibit eCB degradation [67, 184]. While there is debate about the safety of FAAH inhibition [68, 108] the first-class MAGL inhibitor Abx-1431 [46, 64, 114] has entered clinical phase 2 for the study of Tourette syndrome or chronic motor tic disorder and indicates positive effects in these patients (www.clinicaltrials.gov). Moreover, the combination of different mechanisms of action to enhance eCB tone has started to receive attraction in other diseases [230] and is worth investigating in more detail in the context of neuroHIV.
Noteworthy, an important point to consider is the interaction of cannabinoids and cART medication. Not a lot of information is currently available but clinical studies are being conducted to investigate their interactive effects [55]. A past study has reported no clinical interaction of cannabinoid with protease inhibitors [1]. If these findings are confirmed in future studies cannabinoid-based treatment will have high therapeutic potential in the context of neuroHIV.
Further, evidence indicates that the eCB system appears to be altered in neuroHIV but more research is necessary to evaluate its impact on the CNS in PWH. Specific factors that should be taken into account are differences in HIV strains/variants, human/genetic variability, pharmacokinetics, and sex, which all could contribute to disease progression and degree of response to cannabinoid treatment. For example, inhibitory crosstalk between HIV-1 coreceptor CXCR4 and CB2R has been reported to play a potential role in viral suppression, whereas CB2R cross-regulation of CCR5 is less clear. Further, sex differences have been reported for the eCB system [51, 205] as well as for the severity of neurocognitive impairments in HIV [31, 143, 204], thus indicating the importance of including sex as a biological variable in clinical and preclinical studies. Lastly, when thinking about identifying components of the eCB system as useful biomarkers the use of in vivo neuroimaging studies, such as PET and fMRI, is highly encouraged and would provide very relevant data on eCB-related alteration in neuroHIV as well as cannabinoid treatment opportunities.
Highlights.
HIV neuropathogenesis alters the endocannabinoid system
Effects of cannabis use on cognition in people living with HIV-1 depend on various factors, including disease progression, the amount of cannabis use, and the cognitive domain assessed
Cannabinoid type 2 receptors and endocannabinoid catabolic enzymes are promising therapeutic targets in people living with HIV-1
Funding
This work was supported by the National Institute on Drug Abuse (NIDA): R01 DA045596 (SF) and R21 DA041903 (SF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare that they have no competing interests.
References
- [1].Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M, Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial, Ann Intern Med 139 (2003) 258–266. [DOI] [PubMed] [Google Scholar]
- [2].Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL, Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial, Neurology 68 (2007) 515–521. [DOI] [PubMed] [Google Scholar]
- [3].Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F, Neuronal apoptosis in HIV infection in adults, Neuropathol Appl Neurobiol 21 (1995) 218–227. [DOI] [PubMed] [Google Scholar]
- [4].Aghazadeh Tabrizi M, Baraldi PG, Borea PA, Varani K, Medicinal Chemistry, Pharmacology, and Potential Therapeutic Benefits of Cannabinoid CB2 Receptor Agonists, Chem Rev 116 (2016) 519–560. [DOI] [PubMed] [Google Scholar]
- [5].Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF, Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain, Chem Biol 16 (2009) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ahn K, McKinney MK, Cravatt BF, Enzymatic pathways that regulate endocannabinoid signaling in the nervous system, Chem Rev 108 (2008) 1687–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Almogi-Hazan O, Or R, Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back, International journal of molecular sciences 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].An SF, Groves M, Gray F, Scaravilli F, Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals, J Neuropathol Exp Neurol 58 (1999) 1156–1162. [DOI] [PubMed] [Google Scholar]
- [9].Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE, Updated research nosology for HIV-associated neurocognitive disorders, Neurology 69 (2007) 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ashton JC, Glass M, The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration, Current neuropharmacology 5 (2007) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Atwood BK, Mackie K, CB2: a cannabinoid receptor with an identity crisis, Br J Pharmacol 160 (2010) 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J, Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease, Science 277 (1997) 112–116. [DOI] [PubMed] [Google Scholar]
- [13].Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Wood J, Wang L, Masliah E, Avraham S, Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme, Br J Pharmacol 172 (2015) 4603–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, Masliah E, Avraham S, The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis, Br J Pharmacol 171 (2014) 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Basu S, Dittel BN, Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease, Immunol Res 51 (2011) 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV, Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS, J Pain Symptom Manage 10 (1995) 89–97. [DOI] [PubMed] [Google Scholar]
- [17].Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF, Mosdell KW, Shepard KV, Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia, J Pain Symptom Manage 14 (1997) 7–14. [DOI] [PubMed] [Google Scholar]
- [18].Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M, Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study, Psychopharmacology (Berl) 212 (2010) 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beji C, Loucif H, Telittchenko R, Olagnier D, Dagenais-Lussier X, van Grevenynghe J, Cannabinoid-Induced Immunomodulation during Viral Infections: A Focus on Mitochondria, Viruses 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Belle-Isle L, Hathaway A, Barriers to access to medical cannabis for Canadians living with HIV/AIDS, Aids Care 19 (2007) 500–506. [DOI] [PubMed] [Google Scholar]
- [21].Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J, A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis, J Neurosci 25 (2005) 2530–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J, Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains, J Neurosci 23 (2003) 11136–11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J, Cannabinoid CB2 receptors in human brain inflammation, Br J Pharmacol 153 (2008) 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bertrand L, Nair M, Toborek M, Solving the Blood-Brain Barrier Challenge for the Effective Treatment of HIV Replication in the Central Nervous System, Curr Pharm Des 22 (2016) 5477–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M, Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States, Archives of general psychiatry 58 (2001) 721–728. [DOI] [PubMed] [Google Scholar]
- [26].Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA, Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms, Journal of behavioral medicine 37 (2014) 1–10. [DOI] [PubMed] [Google Scholar]
- [27].Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, Boger DL, Cravatt BF, Lichtman AH, The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice, Br J Pharmacol 165 (2012) 2485–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brack-Werner R, Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis, AIDS 13 (1999) 1–22. [DOI] [PubMed] [Google Scholar]
- [29].Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI, Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients, J Clin Pharmacol 42 (2002) 82S–89S. [DOI] [PubMed] [Google Scholar]
- [30].Buckley NE, The peripheral cannabinoid receptor knockout mice: an update, Br J Pharmacol 153 (2008) 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL, Sex-based differences in neurocognitive functioning in HIV-infected young adults, AIDS 32 (2018) 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cabral GA, Griffin-Thomas L, Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation, Expert Rev Mol Med 11 (2009) e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Castro FOF, Silva JM, Dorneles GP, Barros JBS, Ribeiro CB, Noronha I, Barbosa GR, Souza LCS, Guilarde AO, Pereira A, Guimaraes RF, Oliveira TF, Oliveira SEF, Peres A, Romao PRT, Pfrimer IAH, Fonseca SGD, Distinct inflammatory profiles in HIV-infected individuals under antiretroviral therapy using cannabis, cocaine or cannabis plus cocaine, AIDS 33 (2019) 1831–1842. [DOI] [PubMed] [Google Scholar]
- [34].Chandra LC, Kumar V, Torben W, Vande Stouwe C, Winsauer P, Amedee A, Molina PE, Mohan M, Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques, J Virol 89 (2015) 1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chang L, Cloak C, Yakupov R, Ernst T, Combined and independent effects of chronic marijuana use and HIV on brain metabolites, J Neuroimmune Pharmacol 1 (2006) 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chao C, Jacobson LP, Tashkin D, Martinez-Maza O, Roth MD, Margolick JB, Chmiel JS, Rinaldo C, Zhang ZF, Detels R, Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men, Drug Alcohol Depend 94 (2008) 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen NC, Partridge AT, Sell C, Torres C, Martin-Garcia J, Fate of microglia during HIV-1 infection: From activation to senescence?, Glia 65 (2017) 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen P, Mayne M, Power C, Nath A, The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases, J Biol Chem 272 (1997) 22385–22388. [DOI] [PubMed] [Google Scholar]
- [39].Chen W, Crawford RB, Kaplan BL, Kaminski NE, Modulation of HIVGP120 Antigen-Specific Immune Responses In Vivo by Delta9-Tetrahydrocannabinol, J Neuroimmune Pharmacol 10 (2015) 344–355. [DOI] [PubMed] [Google Scholar]
- [40].Chen W, Kaplan BL, Pike ST, Topper LA, Lichorobiec NR, Simmons SO, Ramabhadran R, Kaminski NE, Magnitude of stimulation dictates the cannabinoid-mediated differential T cell response to HIVgp120, J Leukoc Biol 92 (2012) 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen X, Zhang J, Chen C, Endocannabinoid 2-arachidonoylglycerol protects neurons against beta-amyloid insults, Neuroscience 178 (2011) 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chevaleyre V, Takahashi KA, Castillo PE, Endocannabinoid-mediated synaptic plasticity in the CNS, Annu Rev Neurosci 29 (2006) 37–76. [DOI] [PubMed] [Google Scholar]
- [43].Chiarlone A, Bellocchio L, Blazquez C, Resel E, Soria-Gomez E, Cannich A, Ferrero JJ, Sagredo O, Benito C, Romero J, Sanchez-Prieto J, Lutz B, Fernandez-Ruiz J, Galve-Roperh I, Guzman M, A restricted population of CB1 cannabinoid receptors with neuroprotective activity, Proc Natl Acad Sci U S A 111 (2014) 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR, Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia, Ann Neurol 66 (2009) 253–258. [DOI] [PubMed] [Google Scholar]
- [45].Cirino TJ, Harden SW, McLaughlin JP, Frazier CJ, Region-specific effects of HIV-1 Tat on intrinsic electrophysiological properties of pyramidal neurons in mouse prefrontal cortex and hippocampus, J Neurophysiol 123 (2020) 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cisar JS, Weber OD, Clapper JR, Blankman JL, Henry CL, Simon GM, Alexander JP, Jones TK, Ezekowitz RAB, O’Neill GP, Grice CA, Identification of ABX-1431, a Selective Inhibitor of Monoacylglycerol Lipase and Clinical Candidate for Treatment of Neurological Disorders, Journal of medicinal chemistry 61 (2018) 9062–9084. [DOI] [PubMed] [Google Scholar]
- [47].Clifford DB, Ances BM, HIV-associated neurocognitive disorder, The Lancet. Infectious diseases 13 (2013) 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Concannon RM, Dowd E, Central CB2 receptors in inflammation-driven neurodegeneration: dysregulation and therapeutic potential, Neural Regen Res 11 (2016) 1409–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Concannon RM, Okine BN, Finn DP, Dowd E, Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease, Exp Neurol 283 (2016) 204–212. [DOI] [PubMed] [Google Scholar]
- [50].Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME, Activation of GPR18 by cannabinoid compounds: a tale of biased agonism, Br J Pharmacol 171 (2014) 3908–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cooper ZD, Craft RM, Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective, Neuropsychopharmacology 43 (2018) 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cosenza-Nashat MA, Bauman A, Zhao ML, Morgello S, Suh HS, Lee SC, Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities, Neuropathol Appl Neurobiol 37 (2011) 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Costantino CM, Gupta A, Yewdall AW, Dale BM, Devi LA, Chen BK, Cannabinoid receptor 2-mediated attenuation of CXCR4-tropic HIV infection in primary CD4+ T cells, PLoS One 7 (2012) e33961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Costiniuk CT, Jenabian MA, Cannabinoids and inflammation: implications for people living with HIV, AIDS 33 (2019) 2273–2288. [DOI] [PubMed] [Google Scholar]
- [55].Costiniuk CT, Saneei Z, Routy JP, Margolese S, Mandarino E, Singer J, Lebouche B, Cox J, Szabo J, Brouillette MJ, Klein MB, Chomont N, Jenabian MA, Oral cannabinoids in people living with HIV on effective antiretroviral therapy: CTN PT028-study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation, BMJ Open 9 (2019) e024793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB, Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides, Nature 384 (1996) 83–87. [DOI] [PubMed] [Google Scholar]
- [57].C. RD Crean NA; Mason BJ, An Evidence Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions, J Addict Med 5 (2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cristiani SA, Pukay-Martin ND, Bornstein RA, Marijuana use and cognitive function in HIV-infected people, J Neuropsychiatry Clin Neurosci 16 (2004) 330–335. [DOI] [PubMed] [Google Scholar]
- [59].Croxford JL, Yamamura T, Cannabinoids and the immune system: potential for the treatment of inflammatory diseases?, J Neuroimmunol 166 (2005) 3–18. [DOI] [PubMed] [Google Scholar]
- [60].Cysique LA, Maruff P, Brew BJ, Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts, J Neurovirol 10 (2004) 350–357. [DOI] [PubMed] [Google Scholar]
- [61].D’Souza G, Matson PA, Grady CD, Nahvi S, Merenstein D, Weber KM, Greenblatt R, Burian P, Wilson TE, Medicinal and recreational marijuana use among HIV-infected women in the Women’s Interagency HIV Study (WIHS) cohort, 1994–2010, J Acquir Immune Defic Syndr 61 (2012) 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].de Jong BC, Prentiss D, McFarland W, Machekano R, Israelski DM, Marijuana use and its association with adherence to antiretroviral therapy among HIV-infected persons with moderate to severe nausea, J Acquir Immune Defic Syndr 38 (2005) 43–46. [DOI] [PubMed] [Google Scholar]
- [63].DeJesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D, Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients, J Int Assoc Physicians AIDS Care (Chic) 6 (2007) 95–100. [DOI] [PubMed] [Google Scholar]
- [64].Deng H, Li W, Monoacylglycerol lipase inhibitors: modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders, Acta Pharm Sin B 10 (2020) 582–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Di Marzo V, Targeting the endocannabinoid system: to enhance or reduce?, Nat Rev Drug Discov 7 (2008) 438–455. [DOI] [PubMed] [Google Scholar]
- [66].Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Martin BR, Cannabimimetic fatty acid derivatives: the anandamide family and other endocannabinoids, Curr Med Chem 6 (1999) 721–744. [PubMed] [Google Scholar]
- [67].Di Marzo V, Stella N, Zimmer A, Endocannabinoid signalling and the deteriorating brain, Nat Rev Neurosci 16 (2015) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dider S, Ji J, Zhao Z, Xie L, Molecular mechanisms involved in the side effects of fatty acid amide hydrolase inhibitors: a structural phenomics approach to proteome-wide cellular off-target deconvolution and disease association, NPJ Syst Biol Appl 2 (2016) 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ, Changes to AIDS dementia complex in the era of highly active antiretroviral therapy, AIDS 13 (1999) 1249–1253. [DOI] [PubMed] [Google Scholar]
- [70].El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF, Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-κB trafficking and transcription, PLoS One 3 (2008) e4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ, HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse, J Virol 89 (2015) 1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ellis R, Langford D, Masliah E, HIV and antiretroviral therapy in the brain: neuronal injury and repair, Nat Rev Neurosci 8 (2007) 33–44. [DOI] [PubMed] [Google Scholar]
- [73].Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH, Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial, Neuropsychopharmacology 34 (2009) 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Esposito G, Ligresti A, Izzo AA, Bisogno T, Ruvo M, Di Rosa M, Di Marzo V, Iuvone T, The endocannabinoid system protects rat glioma cells against HIV-1 Tat protein-induced cytotoxicity. Mechanism and regulation, J Biol Chem 277 (2002) 50348–50354. [DOI] [PubMed] [Google Scholar]
- [75].Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J, CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection, J Neurovirol 7 (2001) 528–541. [DOI] [PubMed] [Google Scholar]
- [76].Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF, Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload, J Neurosci 34 (2014) 12850–12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fontana G, Valenti L, Raiteri M, Gp120 can revert antagonism at the glycine site of NMDA receptors mediating GABA release from cultured hippocampal neurons, J Neurosci Res 49 (1997) 732–738. [DOI] [PubMed] [Google Scholar]
- [78].Fraga D, Raborn ES, Ferreira GA, Cabral GA, Cannabinoids inhibit migration of microglial-like cells to the HIV protein Tat, J Neuroimmune Pharmacol 6 (2011) 566–577. [DOI] [PubMed] [Google Scholar]
- [79].Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M, Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling, Eur J Neurosci 19 (2004) 2682–2692. [DOI] [PubMed] [Google Scholar]
- [80].Gannon P, Khan MZ, Kolson DL, Current understanding of HIV-associated neurocognitive disorders pathogenesis, Current opinion in neurology 24 (2011) 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gartner S, HIV infection and dementia, Science 287 (2000) 602–604. [DOI] [PubMed] [Google Scholar]
- [82].Garvey LJ, Yerrakalva D, Winston A, Correlations between computerized battery testing and a memory questionnaire for identification of neurocognitive impairment in HIV type 1-infected subjects on stable antiretroviral therapy, AIDS Res Hum Retroviruses 25 (2009) 765–769. [DOI] [PubMed] [Google Scholar]
- [83].Ghosn J, Leruez-Ville M, Blanche J, Delobelle A, Beaudoux C, Mascard L, Lecuyer H, Canestri A, Landman R, Zucman D, Ponscarme D, Rami A, Viard JP, Spire B, Rouzioux C, Costagliola D, Suzan-Monti M, Evarist AEPSG, HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens, Clin Infect Dis 58 (2014) 1763–1770. [DOI] [PubMed] [Google Scholar]
- [84].Glass JD, Fedor H, Wesselingh SL, McArthur JC, Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia, Ann Neurol 38 (1995) 755–762. [DOI] [PubMed] [Google Scholar]
- [85].Gorantla S, Makarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L, Immunoregulation of a CB2 receptor agonist in a murine model of neuroAIDS, J Neuroimmune Pharmacol 5 (2010) 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gorter R, Seefried M, Volberding P, Dronabinol effects on weight in patients with HIV infection, AIDS 6 (1992) 127. [DOI] [PubMed] [Google Scholar]
- [87].Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T, Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study, Journal of the International Neuropsychological Society : JINS 9 (2003) 679–689. [DOI] [PubMed] [Google Scholar]
- [88].Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D, Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha), J Biol Chem 279 (2004) 27849–27854. [DOI] [PubMed] [Google Scholar]
- [89].Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW, Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep, J Acquir Immune Defic Syndr 45 (2007) 545–554. [DOI] [PubMed] [Google Scholar]
- [90].Haney M, Rabkin J, Gunderson E, Foltin RW, Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood, Psychopharmacology (Berl) 181 (2005) 170–178. [DOI] [PubMed] [Google Scholar]
- [91].Hardy DJ, Vance DE, The neuropsychology of HIV/AIDS in older adults, Neuropsychol Rev 19 (2009) 263–272. [DOI] [PubMed] [Google Scholar]
- [92].Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium HIVN, Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment, AIDS 25 (2011) 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Harkany T, Mackie K, Doherty P, Wiring and firing neuronal networks: endocannabinoids take center stage, Curr Opin Neurobiol 18 (2008) 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Haughey NJ, Holden CP, Nath A, Geiger JD, Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat, J Neurochem 73 (1999) 1363–1374. [DOI] [PubMed] [Google Scholar]
- [95].He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D, CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia, Nature 385 (1997) 645–649. [DOI] [PubMed] [Google Scholar]
- [96].Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors, J Neurovirol 17 (2011) 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, McArthur J, Letendre S, Steiner J, Kashanchi F, Nath A, Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy, AIDS 33 Suppl 2 (2019) S145–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Henriquez JE, Rizzo MD, Crawford RB, Gulick P, Kaminski NE, Interferon-alpha-Mediated Activation of T Cells from Healthy and HIV-Infected Individuals Is Suppressed by Delta(9)-Tetrahydrocannabinol, J Pharmacol Exp Ther 367 (2018) 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Henriquez JE, Rizzo MD, Schulz MA, Crawford RB, Gulick P, Kaminski NE, Delta9-Tetrahydrocannabinol Suppresses Secretion of IFNalpha by Plasmacytoid Dendritic Cells From Healthy and HIV-Infected Individuals, J Acquir Immune Defic Syndr 75 (2017) 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hermes DJ, Xu C, Poklis JL, Niphakis MJ, Cravatt BF, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S, Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS, Neuropharmacology 141 (2018) 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R, CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons, Curr Biol 7 (1997) 112–121. [DOI] [PubMed] [Google Scholar]
- [102].Higgins A, Yuan S, Wang Y, Burrell BD, Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids, Mol Pain 9 (2013) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, Wietgrefe S, Caro-Vegas C, Madden V, Sharpe G, Haase AT, Eron JJ, Garcia JV, Macrophages sustain HIV replication in vivo independently of T cells, J Clin Invest 126 (2016) 1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hong S, Banks WA, Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications, Brain, behavior, and immunity 45 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hu S, Sheng WS, Rock RB, CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120, PLoS One 8 (2013) e77577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hu S, Sheng WS, Rock RB, Immunomodulatory properties of kappa opioids and synthetic cannabinoids in HIV-1 neuropathogenesis, J Neuroimmune Pharmacol 6 (2011) 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Huang CC, Lo SW, Hsu KS, Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons, J Physiol 532 (2001) 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Huggins JP, Smart TS, Langman S, Taylor L, Young T, An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee, Pain 153 (2012) 1837–1846. [DOI] [PubMed] [Google Scholar]
- [109].Hunault CC, Mensinga TT, Bocker KB, Schipper CM, Kruidenier M, Leenders ME, de Vries I, Meulenbelt J, Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC), Psychopharmacology (Berl) 204 (2009) 85–94. [DOI] [PubMed] [Google Scholar]
- [110].Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, Lichtman AH, Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice, J Pharmacol Exp Ther 353 (2015) 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, ST ON, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH, In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects, Br J Pharmacol 171 (2014) 1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jacobs IR, Xu C, Hermes DJ, League AF, Xu C, Nath B, Jiang W, Niphakis MJ, Cravatt BF, Mackie K, Mukhopadhyay S, Lichtman AH, Ignatowska-Jankowska BM, Fitting S, Inhibitory Control Deficits Associated with Upregulation of CB1R in the HIV-1 Tat Transgenic Mouse Model of Hand, J Neuroimmune Pharmacol 14 (2019) 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Javed H, Azimullah S, Haque ME, Ojha SK, Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease, Frontiers in neuroscience 10 (2016) 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Jiang M, van der Stelt M, Activity-Based Protein Profiling Delivers Selective Drug Candidate ABX-1431, a Monoacylglycerol Lipase Inhibitor, To Control Lipid Metabolism in Neurological Disorders, Journal of medicinal chemistry 61 (2018) 9059–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A, Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein, Proc Natl Acad Sci U S A 110 (2013) 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R, HIV-1 target cells in the CNS, J Neurovirol 21 (2015) 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kano M, Control of synaptic function by endocannabinoid-mediated retrograde signaling, Proc Jpn Acad Ser B Phys Biol Sci 90 (2014) 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kaul M, Garden GA, Lipton SA, Pathways to neuronal injury and apoptosis in HIV-associated dementia, Nature 410 (2001) 988–994. [DOI] [PubMed] [Google Scholar]
- [119].Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA, HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection, Cell Death Differ 14 (2007) 296–305. [DOI] [PubMed] [Google Scholar]
- [120].Keen L 2nd, Abbate A, Blanden G, Priddie C, Moeller FG, Rathore M, Confirmed marijuana use and lymphocyte count in black people living with HIV, Drug Alcohol Depend 198 (2019) 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim HJ, Martemyanov KA, Thayer SA, Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death, J Neurosci 28 (2008) 12604–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kim HJ, Shin AH, Thayer SA, Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss, Mol Pharmacol 80 (2011) 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kim S, Hahn YK, Podhaizer EM, McLane VD, Zou S, Hauser KF, Knapp PE, A central role for glial CCR5 in directing the neuropathological interactions of HIV-1 Tat and opiates, Journal of neuroinflammation 15 (2018) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH, Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects, J Pharmacol Exp Ther 345 (2013) 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kipp AM, Rebeiro PF, Shepherd BE, Brinkley-Rubinstein L, Turner M, Bebawy S, Sterling TR, Hulgan T, Daily Marijuana Use is Associated with Missed Clinic Appointments Among HIV-Infected Persons Engaged in HIV Care, AIDS Behav 21 (2017) 1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Klein TW, Cannabinoid-based drugs as anti-inflammatory therapeutics, Nat Rev Immunol 5 (2005) 400–411. [DOI] [PubMed] [Google Scholar]
- [127].Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R, Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus, Virus Res 111 (2005) 194–213. [DOI] [PubMed] [Google Scholar]
- [128].Krishnan G, Chatterjee N, Differential immune mechanism to HIV-1 Tat variants and its regulation by AEA [corrected], Scientific reports 5 (2015) 9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Krishnan G, Chatterjee N, Endocannabinoids affect innate immunity of Muller glia during HIV-1 Tat cytotoxicity, Mol Cell Neurosci 59 (2014) 10–23. [DOI] [PubMed] [Google Scholar]
- [130].Kutsch O, Oh J, Nath A, Benveniste EN, Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes, J Virol 74 (2000) 9214–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA, Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation, J Neurovirol 2 (1996) 423–432. [DOI] [PubMed] [Google Scholar]
- [132].LeCapitaine NJ, Zhang P, Winsauer P, Walker E, Vande Stouwe C, Porretta C, Molina PE, Chronic Delta-9-tetrahydrocannabinol administration increases lymphocyte CXCR4 expression in rhesus macaques, J Neuroimmune Pharmacol 6 (2011) 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lee JT, Saag LA, Kipp AM, Logan J, Shepherd BE, Koethe JR, Turner M, Bebawy S, Sterling TR, Hulgan T, Self-reported Cannabis Use and Changes in Body Mass Index, CD4 T-Cell Counts, and HIV-1 RNA Suppression in Treated Persons with HIV, AIDS Behav 24 (2020) 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Li W, Henderson LJ, Major EO, Al-Harthi L, IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner, J Immunol 186 (2011) 6771–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lichtman AH, Blankman JL, Cravatt BF, Endocannabinoid overload, Mol Pharmacol 78 (2010) 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lipton SA, Human immunodeficiency virus-infected macrophages, gp120, and N-methyl-D-aspartate receptor-mediated neurotoxicity, Ann Neurol 33 (1993) 227–228. [DOI] [PubMed] [Google Scholar]
- [137].Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ, Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands, Nat Med 6 (2000) 1380–1387. [DOI] [PubMed] [Google Scholar]
- [138].Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D, The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide, Mol Pharmacol 67 (2005) 15–19. [DOI] [PubMed] [Google Scholar]
- [139].Longordo F, Feligioni M, Chiaramonte G, Sbaffi PF, Raiteri M, Pittaluga A, The human immunodeficiency virus-1 protein transactivator of transcription up-regulates N-methyl-D-aspartate receptor function by acting at metabotropic glutamate receptor 1 receptors coexisting on human and rat brain noradrenergic neurones, J Pharmacol Exp Ther 317 (2006) 1097–1105. [DOI] [PubMed] [Google Scholar]
- [140].Lu TS, Avraham HK, Seng S, Tachado SD, Koziel H, Makriyannis A, Avraham S, Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells, J Immunol 181 (2008) 6406–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD, Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy, American journal of epidemiology 163 (2006) 412–420. [DOI] [PubMed] [Google Scholar]
- [142].Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A, Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity, Ann Neurol 37 (1995) 373–380. [DOI] [PubMed] [Google Scholar]
- [143].Maki PM, Martin-Thormeyer E, HIV, cognition and women, Neuropsychol Rev 19 (2009) 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Malek N, Popiolek-Barczyk K, Mika J, Przewlocka B, Starowicz K, Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures, Neural Plast 2015 (2015) 130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mannes ZL, Burrell LE 2nd, Ferguson EG, Zhou Z, Lu H, Somboonwit C, Cook RL, Ennis N, The association of therapeutic versus recreational marijuana use and antiretroviral adherence among adults living with HIV in Florida, Patient Prefer Adherence 12 (2018) 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, Cheu RK, Collier AC, Funderburg NT, Martin JN, Wu MC, Isoherranen N, Hunt PW, Klatt NR, Heavy Cannabis Use Associated With Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals, Clin Infect Dis 66 (2018) 1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B, CB1 cannabinoid receptors and on-demand defense against excitotoxicity, Science 302 (2003) 84–88. [DOI] [PubMed] [Google Scholar]
- [148].Martinez-Picado J, Deeks SG, Persistent HIV-1 replication during antiretroviral therapy, Curr Opin HIV AIDS 11 (2016) 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Massie MJ, Prevalence of depression in patients with cancer, J Natl Cancer Inst Monogr (2004) 57–71. [DOI] [PubMed] [Google Scholar]
- [150].Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, Structure of a cannabinoid receptor and functional expression of the cloned cDNA, Nature 346 (1990) 561–564. [DOI] [PubMed] [Google Scholar]
- [151].Mattson MP, Haughey NJ, Nath A, Cell death in HIV dementia, Cell Death Differ 12 Suppl 1 (2005) 893–904. [DOI] [PubMed] [Google Scholar]
- [152].McKinney MK, Cravatt BF, Structure and function of fatty acid amide hydrolase, Annu Rev Biochem 74 (2005) 411–432. [DOI] [PubMed] [Google Scholar]
- [153].Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, Mokhtari M, Moreau H, Tamalet C, Brunet C, Paul P, Dignat-George F, Stein A, Brouqui P, Spector SA, Campbell GR, Loret EP, Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein, Infect Disord Drug Targets 12 (2012) 81–86. [DOI] [PubMed] [Google Scholar]
- [154].Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ, Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity, Proc Natl Acad Sci U S A 95 (1998) 14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Meucci O, Fatatis A, Simen AA, Miller RJ, Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival, Proc Natl Acad Sci U S A 97 (2000) 8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Milano WT, Capasso MF, A Cannabinoids Involvement in Neurodegenerative Diseases, Current Neurobiology 8 (2017) 135–144. [Google Scholar]
- [157].Miller LK, Devi LA, The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications, Pharmacol Rev 63 (2011) 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Milloy MJ, Marshall B, Kerr T, Richardson L, Hogg R, Guillemi S, Montaner JS, Wood E, High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs, Drug and alcohol review 34 (2015) 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C, The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis., Journal of the Neurological Sciences 202 (2002) 13–23. [DOI] [PubMed] [Google Scholar]
- [160].Molina PE, Amedee A, LeCapitaine NJ, Zabaleta J, Mohan M, Winsauer P, Vande Stouwe C, Cannabinoid neuroimmune modulation of SIV disease, J Neuroimmune Pharmacol 6 (2011) 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, Stouwe CV, Troxclair D, McGoey R, Varner K, Byerley L, LaMotte L, Cannabinoid administration attenuates the progression of simian immunodeficiency virus, AIDS Res Hum Retroviruses 27 (2011) 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Moreira FA, Grieb M, Lutz B, Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression, Best Pract Res Clin Endocrinol Metab 23 (2009) 133–144. [DOI] [PubMed] [Google Scholar]
- [163].Munro S, Thomas KL, Abu-Shaar M, Molecular characterization of a peripheral receptor for cannabinoids, Nature 365 (1993) 61–65. [DOI] [PubMed] [Google Scholar]
- [164].Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M, Cannabinoids as novel anti-inflammatory drugs, Future Med Chem 1 (2009) 1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Naidoo V, Nikas SP, Karanian DA, Hwang J, Zhao J, Wood JT, Alapafuja SO, Vadivel SK, Butler D, Makriyannis A, Bahr BA, A new generation fatty acid amide hydrolase inhibitor protects against kainate-induced excitotoxicity, J Mol Neurosci 43 (2011) 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Nallapaneni A, Liu J, Karanth S, Pope C, Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorophosphate, Neurotoxicology 29 (2008) 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Nasirinezhad F, Jergova S, Pearson JP, Sagen J, Attenuation of persistent pain-related behavior by fatty acid amide hydrolase (FAAH) inhibitors in a rat model of HIV sensory neuropathy, Neuropharmacology 95 (2015) 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Nasser MW, Qamri Z, Deol YS, Smith D, Shilo K, Zou X, Ganju RK, Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion, PLoS One 6 (2011) e23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Navarrete F, Garcia-Gutierrez MS, Jurado-Barba R, Rubio G, Gasparyan A, Austrich-Olivares A, Manzanares J, Endocannabinoid System Components as Potential Biomarkers in Psychiatry, Frontiers in psychiatry 11 (2020) 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Nichols JM, Kaplan BLF, Immune Responses Regulated by Cannabidiol, Cannabis Cannabinoid Res 5 (2020) 12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Niphakis MJ, Cognetta AB 3rd, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF, Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors, ACS chemical neuroscience 4 (2013) 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Noe SN, Nyland SB, Ugen K, Friedman H, Klein TW, Cannabinoid receptor agonists enhance syncytia formation in MT-2 cells infected with cell free HIV-1MN, Adv Exp Med Biol 437 (1998) 223–229. [DOI] [PubMed] [Google Scholar]
- [173].Nogueron MI, Porgilsson B, Schneider WE, Stucky CL, Hillard CJ, Cannabinoid receptor agonists inhibit depolarization-induced calcium influx in cerebellar granule neurons, J Neurochem 79 (2001) 371–381. [DOI] [PubMed] [Google Scholar]
- [174].O’Sullivan SE, An update on PPAR activation by cannabinoids, Br J Pharmacol 173 (2016) 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Okafor CN, Zhou Z, Burrell LE 2nd, Kelso NE, Whitehead NE, Harman JS, Cook CL, Cook RL, Marijuana use and viral suppression in persons receiving medical care for HIV-infection, Am J Drug Alcohol Abuse 43 (2017) 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Orlando M, Burnam MA, Beckman R, Morton SC, London AS, Bing EG, Fleishman JA, Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study, Int J Methods Psychiatr Res 11 (2002) 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Osborne O, Peyravian N, Nair M, Daunert S, Toborek M, The Paradox of HIV Blood-Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies, Trends Neurosci 43 (2020) 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Pacek LRT, L. S; Hobkirk AL; Nash D; Goodwin RD, Frequency of Cannabis Use and Medical Cannabis Use Among Persons Living With HIV in the United States: Findings From a Nationally Representative Sample, AIDS Educ Prev 30 (2018) 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernandez-Ruiz J, Guzman M, Galve-Roperh I, Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity, Brain 132 (2009) 3152–3164. [DOI] [PubMed] [Google Scholar]
- [180].Palma J, Narasimhan M, Guindon J, Benamar K, Supraspinal interaction between HIV-1-gp120 and cannabinoid analgesic effectiveness, Naunyn Schmiedebergs Arch Pharmacol 391 (2018) 1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Palomaki VA, Lehtonen M, Savinainen JR, Laitinen JT, Visualization of 2-arachidonoylglycerol accumulation and cannabinoid CB1 receptor activity in rat brain cryosections by functional autoradiography, J Neurochem 101 (2007) 972–981. [DOI] [PubMed] [Google Scholar]
- [182].Parker LA, Niphakis MJ, Downey R, Limebeer CL, Rock EM, Sticht MA, Morris H, Abdullah RA, Lichtman AH, Cravatt BF, Effect of selective inhibition of monoacylglycerol lipase (MAGL) on acute nausea, anticipatory nausea, and vomiting in rats and Suncus murinus, Psychopharmacology (Berl) 232 (2015) 583–593. [DOI] [PubMed] [Google Scholar]
- [183].Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, Tuma R, HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions, Brain, behavior, and immunity 25 Suppl 1 (2011) S61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Pertwee RG, Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications, The Proceedings of the Nutrition Society 73 (2014) 96–105. [DOI] [PubMed] [Google Scholar]
- [185].Pertwee RG, Emerging strategies for exploiting cannabinoid receptor agonists as medicines, Br J Pharmacol 156 (2009) 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR, Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures, J Neuroimmunol 147 (2004) 123–126. [DOI] [PubMed] [Google Scholar]
- [187].Petrosino S, Di Marzo V, FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels, Curr Opin Investig Drugs 11 (2010) 51–62. [PubMed] [Google Scholar]
- [188].Piller SC, Jans P, Gage PW, Jans DA, Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology, Proc Natl Acad Sci U S A 95 (1998) 4595–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM, Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting, J Acquir Immune Defic Syndr 35 (2004) 38–45. [DOI] [PubMed] [Google Scholar]
- [190].Purohit V, Rapaka RS, Rutter J, Cannabinoid receptor-2 and HIV-associated neurocognitive disorders, J Neuroimmune Pharmacol 9 (2014) 447–453. [DOI] [PubMed] [Google Scholar]
- [191].Raborn ES, Cabral GA, Cannabinoid inhibition of macrophage migration to the trans-activating (Tat) protein of HIV-1 is linked to the CB(2) cannabinoid receptor, J Pharmacol Exp Ther 333 (2010) 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Raborn ES, Jamerson M, Marciano-Cabral F, Cabral GA, Cannabinoid inhibits HIV-1 Tat-stimulated adhesion of human monocyte-like cells to extracellular matrix proteins, Life Sci 104 (2014) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ramirez SH, Reichenbach NL, Fan S, Rom S, Merkel SF, Wang X, Ho WZ, Persidsky Y, Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists, J Leukoc Biol 93 (2013) 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ranganathan M, D’Souza DC, The acute effects of cannabinoids on memory in humans: a review, Psychopharmacology (Berl) 188 (2006) 425–444. [DOI] [PubMed] [Google Scholar]
- [195].Rao KS, Ghorpade A, Labhasetwar V, Targeting anti-HIV drugs to the CNS, Expert Opin Drug Deliv 6 (2009) 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Riggs PK, Vaida F, Rossi SS, Sorkin LS, Gouaux B, Grant I, Ellis RJ, A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men, Brain Res 1431 (2012) 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Rizzo MD, Crawford RB, Henriquez JE, Aldhamen YA, Gulick P, Amalfitano A, Kaminski NE, HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-gamma-inducible protein 10 levels compared with nonusing HIV patients, AIDS 32 (2018) 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Rock RB, Gekker G, Hu S, Sheng WS, Cabral GA, Martin BR, Peterson PK, WIN55,212–2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors, J Neuroimmune Pharmacol 2 (2007) 178–183. [DOI] [PubMed] [Google Scholar]
- [199].Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK, Role of microglia in central nervous system infections, Clin Microbiol Rev 17 (2004) 942–964, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Rodriguez-Munoz M, Sanchez-Blazquez P, Callado LF, Meana JJ, Garzon-Nino J, Schizophrenia and depression, two poles of endocannabinoid system deregulation, Translational psychiatry 7 (2017) 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Rosen MI, Black AC, Arnsten JH, Goggin K, Remien RH, Simoni JM, Golin CE, Bangsberg DR, Liu H, Association between use of specific drugs and antiretroviral adherence: findings from MACH 14, AIDS Behav 17 (2013) 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ross GR, Lichtman A, Dewey WL, Akbarali HI, Evidence for the putative cannabinoid receptor (GPR55)-mediated inhibitory effects on intestinal contractility in mice, Pharmacology 90 (2012) 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC, Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse, Life Sci 77 (2005) 1711–1722. [DOI] [PubMed] [Google Scholar]
- [204].Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, Maki PM, Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms, Curr Psychiatry Rep 21 (2019) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Rubino T, Parolaro D, Sexually dimorphic effects of cannabinoid compounds on emotion and cognition, Front Behav Neurosci 5 (2011) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L, HIV-associated cognitive impairment before and after the advent of combination therapy, J Neurovirol 8 (2002) 136–142. [DOI] [PubMed] [Google Scholar]
- [207].Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J, The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia, Front Pharmacol 4 (2014) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Sanchez-Blazquez P, Rodriguez-Munoz M, Vicente-Sanchez A, Garzon J, Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate, Antioxid Redox Signal 19 (2013) 1766–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Sanchez-Rodriguez MA, Gomez O, Esteban PF, Garcia-Ovejero D, Molina-Holgado E, The endocannabinoid 2-arachidonoylglycerol regulates oligodendrocyte progenitor cell migration, Biochem Pharmacol 157 (2018) 180–188. [DOI] [PubMed] [Google Scholar]
- [210].Savonenko AV, Melnikova T, Wang Y, Ravert H, Gao Y, Koppel J, Lee D, Pletnikova O, Cho E, Sayyida N, Hiatt A, Troncoso J, Davies P, Dannals RF, Pomper MG, Horti AG, Cannabinoid CB2 Receptors in a Mouse Model of Abeta Amyloidosis: Immunohistochemical Analysis and Suitability as a PET Biomarker of Neuroinflammation, PLoS One 10 (2015) e0129618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Scarlett KA, White EZ, Coke CJ, Carter JR, Bryant LK, Hinton CV, Agonist-induced CXCR4 and CB2 Heterodimerization Inhibits Galpha13/RhoA-mediated Migration, Molecular cancer research : MCR 16 (2018) 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R, HIV-1 replication in the central nervous system occurs in two distinct cell types, PLoS pathogens 7 (2011) e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Schoeler T, Bhattacharyya S, The effect of cannabis use on memory function: an update, Subst Abuse Rehabil 4 (2013) 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I, H.I.V.N.R.C. Group, Neurocognitive consequences of HIV infection in older adults: an evaluation of the “cortical” hypothesis, AIDS Behav 15 (2011) 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Shin AH, Kim HJ, Thayer SA, Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat, Br J Pharmacol 166 (2012) 1002–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Simon L, Song K, Vande Stouwe C, Hollenbach A, Amedee A, Mohan M, Winsauer P, Molina P, Delta9-Tetrahydrocannabinol (Delta9-THC) Promotes Neuroimmune-Modulatory MicroRNA Profile in Striatum of Simian Immunodeficiency Virus (SIV)-Infected Macaques, J Neuroimmune Pharmacol 11 (2016) 192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Sippy BD, Hofman FM, Wallach D, Hinton DR, Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS, J Acquir Immune Defic Syndr Hum Retrovirol 10 (1995) 511–521. [PubMed] [Google Scholar]
- [218].Slawek DE, Arnsten J, Sohler N, Zhang C, Grossberg R, Stein M, Cunningham CO, Daily and near-daily cannabis use is associated with HIV viral load suppression in people living with HIV who use cocaine, AIDS care (2020) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Smith SR, Terminelli C, Denhardt G, Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice, J Pharmacol Exp Ther 293 (2000) 136–150. [PubMed] [Google Scholar]
- [220].Smith TH, Sim-Selley LJ, Selley DE, Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?, Br J Pharmacol 160 (2010) 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Stella N, Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas, Glia 58 (2010) 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG, Effect of dronabinol on nutritional status in HIV infection, Ann Pharmacother 27 (1993) 827–831. [DOI] [PubMed] [Google Scholar]
- [223].Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K, Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells, J Biol Chem 275 (2000) 605–612. [DOI] [PubMed] [Google Scholar]
- [224].Sun Y, Bennett A, Cannabinoids: a new group of agonists of PPARs, PPAR Res 2007 (2007) 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Tahamtan A, Tavakoli-Yaraki M, Rygiel TP, Mokhtari-Azad T, Salimi V, Effects of cannabinoids and their receptors on viral infections, J Med Virol 88 (2016) 1–12. [DOI] [PubMed] [Google Scholar]
- [226].Tanveer R, Macguinness N, Daniel S, Gowran A, Campell VA, Cannabinoid receptors and neurodegenerative diseases, WIREs Membr Transp Signal 1 (2012) 633–639. [Google Scholar]
- [227].Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, Hammond A, Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV-adults, Drug Alcohol Depend 170 (2017) 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP, Combined effects of HIV and marijuana use on neurocognitive functioning and immune status, AIDS care 28 (2016) 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G, The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative, AIDS Res Hum Retroviruses 13 (1997) 305–315. [DOI] [PubMed] [Google Scholar]
- [230].Toczek M, Malinowska B, Enhanced endocannabinoid tone as a potential target of pharmacotherapy, Life Sci 204 (2018) 20–45. [DOI] [PubMed] [Google Scholar]
- [231].Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL, Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection, Am J Med 114 (2003) 573–580. [DOI] [PubMed] [Google Scholar]
- [232].Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M, Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway, J Neurosci 29 (2009) 4564–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Watson CW, Paolillo EW, Morgan EE, Umlauf A, Sundermann EE, Ellis RJ, Letendre S, Marcotte TD, Heaton RK, Grant I, Cannabis Exposure is Associated With a Lower Likelihood of Neurocognitive Impairment in People Living With HIV, J Acquir Immune Defic Syndr 83 (2020) 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wei Q, Liu L, Cong Z, Wu X, Wang H, Qin C, Molina P, Chen Z, Chronic Delta(9)-Tetrahydrocannabinol Administration Reduces IgE(+)B Cells but Unlikely Enhances Pathogenic SIVmac251 Infection in Male Rhesus Macaques of Chinese Origin, J Neuroimmune Pharmacol 11 (2016) 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE, Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry, J Neuroimmunol 74 (1997) 1–8. [DOI] [PubMed] [Google Scholar]
- [236].Whitfield RM, Bechtel LM, Starich GH, The impact of ethanol and Marinol/marijuana usage on HIV+/AIDS patients undergoing azidothymidine, azidothymidine/dideoxycytidine, or dideoxyinosine therapy, Alcohol Clin Exp Res 21 (1997) 122–127. [PubMed] [Google Scholar]
- [237].Williams JC, Appelberg S, Goldberger BA, Klein TW, Sleasman JW, Goodenow MM, Delta(9)-Tetrahydrocannabinol treatment during human monocyte differentiation reduces macrophage susceptibility to HIV-1 infection, J Neuroimmune Pharmacol 9 (2014) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA, Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS, J Exp Med 193 (2001) 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, Walker EM, Birke LL, Stouwe CV, Howard JM, Leonard ST, Moerschbaecher JM, Lewis PB, Tolerance to chronic delta-9-tetrahydrocannabinol (Delta(9)-THC) in rhesus macaques infected with simian immunodeficiency virus, Exp Clin Psychopharmacol 19 (2011) 154–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A, Cannabis use in HIV for pain and other medical symptoms, J Pain Symptom Manage 29 (2005) 358–367. [DOI] [PubMed] [Google Scholar]
- [241].Wu MM, Thayer SA, HIV Tat Protein Selectively Impairs CB1 Receptor-Mediated Presynaptic Inhibition at Excitatory But Not Inhibitory Synapses, eNeuro 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Wu MM, Zhang X, Asher MJ, Thayer SA, Druggable targets of the endocannabinoid system: Implications for the treatment of HIV-associated neurocognitive disorder, Brain Res 1724 (2019) 146467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S, Cannabinoids Occlude the HIV-1 Tat-Induced Decrease in GABAergic Neurotransmission in Prefrontal Cortex Slices, J Neuroimmune Pharmacol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Xu C, Hermes DJ, Nwanguma B, Jacobs IR, Mackie K, Mukhopadhyay S, Lichtman AH, Ignatowska-Jankowska B, Fitting S, Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein, Mol Cell Neurosci 83 (2017) 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Zhang X, Thayer SA, Monoacylglycerol lipase inhibitor JZL184 prevents HIV-1 gp120-induced synapse loss by altering endocannabinoid signaling, Neuropharmacology 128 (2018) 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


