Abstract
This article provides a succinct overview of sex differences in epilepsy and putative molecular mechanisms underlying sex differences in seizure susceptibility in chemical, genetic and acquired epileptogenesis. The susceptibility to excitability episodes and occurrence of epileptic seizures are generally higher in men than women. The precise molecular mechanisms remain unclear, but differences in regional morphology and neural circuits in men and women may explain differential vulnerability to seizures and epileptogenic cascades. Changes in seizure sensitivity are due to steroid hormones, including fluctuations in neurosteroids as well as neuroplasticity in their receptor signaling systems. Other potential neurobiological basis for sex differences in epilepsies include differences in brain development, neurogenesis, neuronal chloride homeostasis, and neurotrophic and glial responses. In catamenial epilepsy, a gender-specific neuroendocrine condition, epileptic seizures are most often clustered around a specific menstrual period in adult women. A deeper understanding of the molecular and neural network basis of sex differences in seizures and response to antiepileptic drugs is highly warranted for designing effective, sex-specific therapies for epilepsy, epileptogenesis and seizure disorders.
Keywords: Epilepsy, Epileptogenesis, Estrogen, Neurosteroid, Progesterone, Sex difference, Seizure
1. Introduction
Epilepsy is a chronic neurological disorder characterized by repeated, often debilitating seizures that can result in brain damage, substantial bodily injury, and sometimes death. Numerous subtypes of epilepsies exist, with each of them involving network ensemble hyperexcitability and synchrony as a result of dysfunctional neuronal mechanisms. In the United States alone, roughly 3.4 million people suffer from epilepsy, and many of these individuals endure significant socioeconomic burdens as a result of their diagnosis [1,2]. Despite recent advancements in neurology and pharmacology, more than 30% of epileptic patients experience uncontrollable seizures [3,4]. These epilepsies are said to be refractory and do not respond to current antiepileptic drugs (AEDs). Therefore, there is an urgent need for novel pharmaceutical therapies for epilepsy and other related seizure disorders.
The process of developing epilepsy, referred to as epileptogenesis, can be influenced by a myriad of factors. Of them, differences related to biological sex are among the most apparent [5]. There is a disproportionate amount of epilepsy research centered on male individuals. Although greater focus has begun shifting to the relationship between biological sex and epilepsy, there are still gaps in our understanding of sex differences in epilepsy disorders. Epileptic seizures occur randomly in most epileptic men and women. However, seizures are known to occur predictably in women suffering from catamenial epilepsy. Exploring this trend may further increase our knowledge of the mechanisms underlying sex differences in epilepsy. This would then allow us to develop more effective therapies to improve the quality of life for epileptic individuals [6,7]. This article provides a concise overview of sex differences in the epilepsies and discusses potential mechanisms underlying differential seizure susceptibility in epileptic men and women.
2. Sex differences in the epilepsies
2.1. Diagnostic trends
Sex differences in epilepsy are abundant, existing across the gamut of epilepsy syndromes. The extent of these differences varies between specific seizure disorders and is heavily influenced by age-related factors. Women are more frequently diagnosed with idiopathic generalized epilepsies than men [8,9]. In this context, idiopathic describes forms of epilepsy which are genetically based. Women are also more commonly diagnosed with cryptogenic localization-related epilepsies, with cryptogenic describing epilepsies without obvious causes [10]. In contrast, men are diagnosed more frequently with localization-related symptomatic epilepsies and injury-induced epilepsies [10,11]. More information regarding diagnostic trends in various epilepsy syndromes are listed in Table 1.
Table 1.
A breakdown of the sex-related incidence preferences of several common epilepsy syndromes.
| Epilepsy Syndrome | Male or Female Preponderance | Citation |
|---|---|---|
| Idiopathic generalized epilepsy | Female | [8,9] |
| Cryptogenic localization-related epilepsy | Female | [10] |
| PCDH19-related epilepsy | Female | [18] |
| Childhood absence epilepsy | Female | [19] |
| Photosensitive epilepsy | Female | [20] |
| Catamenial epilepsy | Only female | [21] |
| Localization-related symptomatic epilepsy | Male | [11] |
| Injury-induced epilepsy | Male | [10] |
| Status epilepticus | Male | [22] |
| Focal cortical dysplasia | Male | [23] |
| Perinodular heterotopia | Male | [23] |
| Epilepsy as a whole | Male | [12] |
There is significant data showing that males experience a higher incidence of epilepsy and thus have an increased risk of developing epilepsy over the course of their lives [12–15). However, sociological factors may influence this trend. There is evidence which suggests that more women than men may attempt to conceal their epilepsy diagnosis in certain countries where epilepsy is heavily stigmatized [16,17].
2.2. Cerebral connectivity and temporal lobe epilepsy
Sexual dimorphism with respect to cerebral connectivity is evident from birth to old age [24]. Studies indicate that the male brain is better equipped to handle intrahemispheric neuronal communication due to a higher abundance of white matter connections between cortical regions [24,25]. In contrast, the female brain exhibits greater interhemispheric connectivity and local clustering [24–26]. This discrepancy has been described in both the adolescent and adult brain and may partly explain why more women than men are diagnosed with idiopathic generalized epilepsies [8,26,27].
Variations in cerebral connectivity also exist abundantly within the limbic system and are therefore relevant to temporal lobe epilepsy (TLE) [24] (Fig.1). TLE is among the most common forms of epilepsy and is characterized by spontaneous focal seizures originating from within the limbic system [28]. These seizures are often accompanied by impaired awareness and syncope that differ in extent and nature between the sexes [28]. For example, auras are abnormal sensations which precede seizures and are often experienced by individuals with TLE. Women are known to exhibit these auras more commonly than males, although the exact mechanism by which this occurs is unclear [29]. Differences are also seen in the lateralization and generalization of seizures and may be related to differential connectivity within the limbic system (Fig.1). [29]. The typical male brain has a larger amygdala and thalamus than its female counterpart, while the female brain contains a larger hippocampus, caudate nuclei, regional gray matter, and cortices [25,24]. Within the amygdala, men have been shown to have better right-sided connectivity while women have more left-sided neuronal connections [30,31]. Taken together, these structural variations within the limbic system suggest that sex-differences in the epilepsies may be partly explained by underlying, sex-based variations in functional connectivity.
Fig. 1.
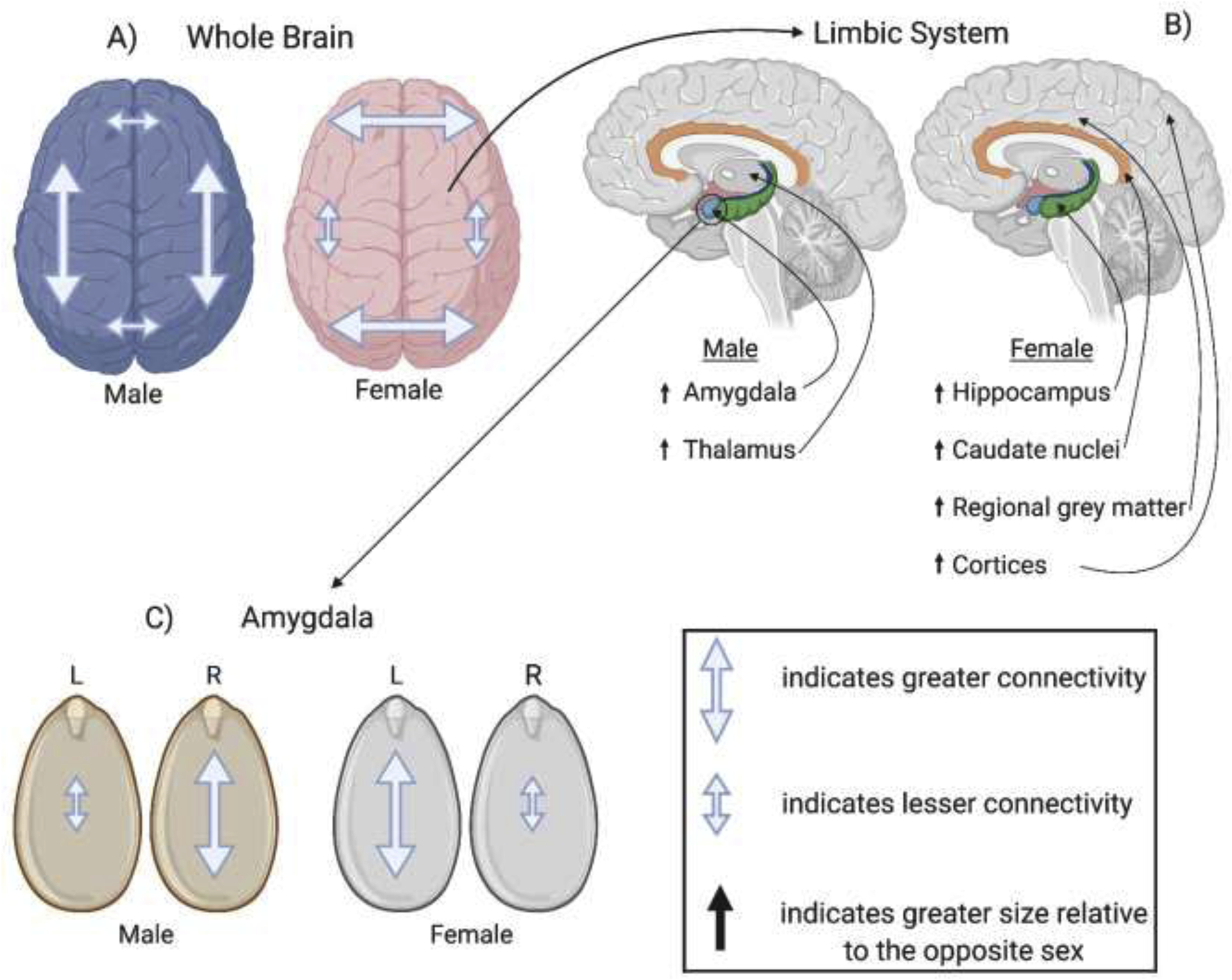
Sexual dimorphism within the brain. A) Males exhibit greater intrahemispheric connectivity, whereas females exhibit greater interhemispheric connectivity. B) Within the limbic system, males feature larger amygdalae and thalami, and females feature larger hippocampi, caudate nuclei, regional grey matter, and cortices. C) Males exhibit greater connectivity within the right amygdala than the left, whereas females exhibit greater connectivity in the left amygdala.
2.3. The relationship between epilepsy and other neurological disorders
Not only are the manifestations of epilepsy disorders affected by biological sex, but they are also influenced by several other neurodegenerative disorders such as stroke, depression, and TBI, all of which have been shown to increase the incidence risk of epilepsy [32]. However, epilepsy is not only affected by concomitant neurological conditions but can affect these conditions as well. One such example is dementia. It is well-documented that individuals suffering from dementia are at a higher risk for developing epilepsy. Yet, pre-existing long-term epilepsy places an individual at higher risk for developing dementia [33,34]. A dementia of particular significance is Alzheimer’s disease, as a recent study has discovered via transcriptome sequencing that Alzheimer’s disease and epilepsy share common pathogenesis pathways [35].
3. Potential molecular mechanisms of sex differences in epilepsy
A variety of mechanisms have been proposed to account for sex-based differences in the epilepsies and seizure sensitivity [6]. Such factors include body weight, steroid hormones, cytochrome P450 activity, neurotransmitter systems, and sexually dimorphic neuronal networks in the brain [7,36–38]. Of these, the impact of steroid hormones and endogenous neurosteroids on neuronal excitability and seizure susceptibility are among the most widely investigated [7,39–43].
3.1. Neurosteroids
The influence of neurosteroids on seizure susceptibility and epileptogenesis have been gaining recognition in recent years. Neurosteroids such as allopregnanolone (AP, brexanolone) and androstanediol mediate the activity of GABAA receptors [44]. This occurs throughout the brain and produces significant neuronal inhibition [39]. Availability of steroid hormones such as progesterone and testosterone affects the production of neurosteroids in the brain. Sex-based differences in the endocrine system therefore play a role in producing inhibitory neurosteroids. There have not been any studies to suggest that reduced levels of neurosteroids can result in epileptogenesis. However, the striking influence that neurosteroids have on neuronal inhibition serves as a likely basis for sex differences in epilepsy.
Neurosteroids are synthesized from steroid hormone precursors in the brain and act as positive allosteric modulators of inhibitory GABAA receptors [45,46]. Composed of various protein subunits (α1–6, β1–4, γ1–3, δ, ε, θ, ρ1–3), these receptors form heteropentameric chloride ionophore channels and produce the majority of inhibitory neurotransmission in the brain [47,48]. When activated, GABAA receptors induce an increased chloride influx into the cell, producing a hyperpolarization of the neuron that counteracts neuronal hyperexcitability and seizures [47].
GABAA receptors are divided into synaptic and extrasynaptic receptors based on location. Synaptic receptors exist within the synapse and are pervasive throughout the brain, producing phasic currents in response to the release of GABA from pre-formed vesicles [49]. In contrast, extrasynaptic GABAA receptors are found beyond the synaptic cleft and are expressed in specific regions of the brain (hippocampus, thalamus, amygdala, cerebellum). Due to constant concentrations of GABA outside of the synapse, GABAA receptors create non-desensitizing tonic currents that are regulated by stable levels of extracellular GABA [49].
Neurosteroids are powerful modulators of synaptic and extrasynaptic GABAA receptors. The mechanisms by which neurosteroids act varies. At low concentrations, neurosteroids allosterically potentiate GABAA receptors; at high concentrations, they can directly activate GABAA receptors [49,50]. Unlike benzodiazepines and barbiturates, neurosteroids increase both the frequency and duration of chloride channel opening when acting at GABAA receptors [51]. While the exact neurosteroid binding site remains undetermined, neurosteroids are believed to bind and interact with discrete sites on the receptor-channel complex located within the transmembrane domains of the α- and β-subunits [49]. Although neurosteroids act on all GABAA receptor isoforms, they impose substantial effects on extrasynaptic isoforms which contain δ-subunits, enabling neurosteroids to inhibit neuronal excitability to a greater extent by potentiating tonic inhibition [49,50].
3.2. Steroid hormones
Steroid hormones are synthesized and secreted from ovarian, gonadal and adrenal sources and play a key role in the neuroendocrine control of neuronal excitability and seizure susceptibility [40] (Table 2). In men, the main circulating steroids are androgenic steroids (testosterone and dihydrotestosterone) and adrenal corticosteroids (cortisol and aldosterone). Deoxycorticosterone is also released from the adrenal cortex in response to stress. In women, the primary reproductive steroid hormones are estrogens and progesterone, which are released during the menstrual cycle. The early follicular phase is associated with low levels of estrogens and progesterone. Estradiol is secreted in the second half of the follicular phase and increases to a peak at midcycle, while progesterone is elevated during the luteal phase and declines before menstruation begins.
Table 2.
List of endogenous steroids and neurosteroids that modulate seizure susceptibility.
| Anticonvulsant steroids | Proconvulsant steroids |
|---|---|
| Progesterone | Estradiol |
| Allopregnanolone | Pregnenolone sulfate |
| Pregnanolone | Dehydroepiandrosterone sulfate |
| Dihydroprogesterone | Cortisol |
| Androstanediol | 11-Deoxycortisol |
| Etiocholanone | |
| Dihydrotestosterone | |
| Deoxycorticosterone | |
| Dihydrodeoxycorticosterone | |
| Allotetrahydrodeoxycorticosterone |
Progesterone is an anticonvulsant hormone that has a relatively higher concentration in females. Progesterone acts as an anticonvulsant agent through three primary mechanisms: negatively impacting glutamatergic (excitatory) transmission, binding to progesterone receptors (PRs), and metabolization to the inhibitory neurosteroid AP. [42,52,53]. This occurs through sequential A-ring reductions and allows AP to act as a positive allosteric modulator of GABAA receptors [39,45,54,55]. Progesterone is also metabolized to 5α-dihydroprogesterone which is known to inhibit seizures. This occurs during progesterone’s conversion to AP and contributes to the anticonvulsant effects of progesterone [56,57]. Somewhat counterintuitively, it was demonstrated that PRs take longer to exhibit their anticonvulsant effects compared to the rapid conversion of progesterone to neurosteroids, suggesting that these conversions could potentially be more relevant to developing pharmaceutical treatments for various epilepsies. This conclusion is further validated by the significantly large protective ED50 of progesterone and its precipitous loss of anticonvulsant activity in the presence of finasteride, which blocks progesterone’s conversion to neurosteroids [56,58].
Estrogens can affect seizure susceptibility. In general, estrogens have proconvulsant and epileptogenic properties in animals and humans [59]. There are limited studies that support protective effects of estrogens, and it may also be anticonvulsant under some circumstances [60]. However, the effect of estrogens on seizure susceptibility is highly variable and depends on factors such as treatment duration, dosage, hormonal status, and the seizure model [61]. Early studies of estradiol administration to ovariectomized rats revealed proconvulsant effects [54]. The effect of estrogens on hippocampus seizure susceptibility is controversial [59]. While estradiol has been shown to be proconvulsant in several studies, there is also evidence which supports lack of effect or protective effect of estrogens [62–64]. The effect of circulating estrogens has been studied in female rats with epilepsy [65]. Epileptic female rats show cyclic increases in epileptiform activity that coincide with their ovarian cycle, mostly attributable to estrogens. Estradiol is well-known to exacerbate seizures in women with epilepsy [66]. An increase in the ratio of estrogen-to-progesterone levels during perimenstrual period might at least partly contribute to the development of perimenstrual catamenial epilepsy [67,68].
Like progesterone’s impact on GABAergic transmission, estrogen positively impacts glutamatergic transmission and produces proconvulsant actions in the brain [69,42]. Estradiol potentiates glutamatergic synaptic transmission in both male and female rats. This occurs through a combination of increased presynaptic glutamate release probability and increased postsynaptic sensitivity to glutamate and other changes in the dendritic structure [70,71]. Seizure susceptibility in females may subsequently be partly determined by physiological ratios of estrogen and progesterone. However, because of recent studies suggesting that estrogen may also display anticonvulsant properties primarily through the neuroprotective effects of the β-estradiol subtype, the impact of progesterone on female seizure susceptibility is much more profound [63,72,73,74]. In addition, testosterone has marked impact on seizure susceptibility by metabolism to estrogens [75].
Androgens and testosterone serve as bimodal modulators of seizure susceptibility, exhibiting both anticonvulsant and proconvulsant properties [76,77,78,79,80]. Research has shown that the metabolization of testosterone to estrogens increases seizure susceptibility in both animal and human models [66]. In contrast, testosterone can inhibit seizures through its conversion to the neurosteroid androstanediol, a positive allosteric modulator of GABAA receptors [75,81].
We recently investigated the neuroprotective effects as it pertains to sex of three neurosteroids - AP, androstanediol and ganaxolone - in the experimental status epilepticus and complex partial seizure states [82]. Results revealed emphatic sex differences in their antiseizure activity, and greater sensitivity was demonstrated in females than males. As expected from it’s substantial effect on extrasynaptic GABAA receptors, AP-enhanced tonic currents in hippocampal granule cells in normal wild type animals but not in those with the extrasynaptic receptor “knocked out.” This lays the groundwork for understanding how sexually dimorphic, GABAergic tonic circuits in the hippocampus possibly contribute to sex differences in seizure susceptibility and neurosteroid protection. These results could prove particularly important for designing personalized neurosteroid therapies for epileptic seizures such as complex partial seizures, catamenial epilepsy, and SE [45].
3.3. Brain development, neurogenesis and glial response
Sex-based differences exist throughout brain development, and organizational effects of steroid hormones are known to impact seizures [6]. For example, brain volumes differ between males and females at birth and remain different throughout an individual’s life. Early in development, organizational effects mediated by steroid hormones cause terminal differentiation of neurons and the adoption of sex-specific circuitry patterns [83]. The window for these effects is developmentally restricted but is permanent and can form dimorphic network patterns that contribute to seizure susceptibility [84].
Neurogenesis serves as a basis for sex differences in epilepsy and differs during development in a sex-specific manner. Newborn male rats display higher rates of hippocampal neurogenesis than females, whereas newborn female rats display higher rates of neurogenesis in the amygdala [85,86]. It is therefore likely that differences in neurogenesis may influence factors that cause variations in epileptogenesis. Moreover, aberrant neurogenesis is implicated as a pathologic feature of TLE in experimental models [87,88]. Although the role of neurogenesis in epilepsy is complex, sex differences in neurogenesis during epileptogenic conditions could lead to differences in the rate or incidence of epileptic seizures.
The role of glial cells, particularly astrocytes and microglia, has become highlighted in recent research as an important factor in the pathophysiology of acquired epileptogenesis and epilepsy [89,90]. Astrocytes perform many functions; they provide biochemical support for the endothelial cells which form the blood–brain barrier, provide nutrients to nervous tissue, maintain extracellular ion balance, and influence the repair and scarring processes of neuronal injuries. Microglia play a key role in the immune response of the CNS. They serve as the main neuroimmune cells in the brain and provide neuroinflammation when a potential threat is identified. When acting without restriction, microglia have the ability to phagocytize and kill healthy neurons [91]. Indeed, microglial-induced neuronal injury has been identified across several neurodegenerative disorders including Alzheimer’s and Parkinson’s diseases [92].
There is experimental evidence of differential expression of astrocytes and microglia in the brain; sex-specific differences in astrocyte levels become apparent when rats transition from prepubescence to adulthood [93,94,95]. Astrocyte organization is heavily influenced by endocrine hormones, causing male astrocytes to have a stellate morphology while female astrocytes are bipolar in shape [96]. There are sex-differences in astrocyte levels throughout the brain that include various different brain regions such as the hippocampus, amygdala, and hypothalamus [96,97]. Given the crucial role astrocytes perform in synaptic communication and neurotransmission, differences in astrocyte morphology may serve as a foundation for sex differences in epilepsy. Similar differences exist for glial cells, with the hippocampus and amygdala expressing different levels of microglia between the sexes [98]. Cultures of astrocytes and microglia from female and male rats exhibit striking sex differences in functional response and expression of inflammatory markers [99,100]. The neuroprotective inflammation and immune function of microglia may significantly affect the disease processes associated with epilepsy. Although microglia and astrocytes likely play a role in determining sex differences in epilepsy, there is limited direct evidence of their differential functional responses in animal models of epileptogenesis [6].
3.4. Chloride homeostasis
There is emerging evidence which suggests that developmental chloride homeostasis influences sex differences in seizure susceptibility and drug effects in epilepsy [36] (Fig.2). The immature brain has relatively higher concentrations of intracellular chloride ions [101]. The expression of two separate Na-KCl cotransporters (NKCC1 and KCC2) is responsible for this distinction, with the neonatal brain having increased expression of NKCC1 proteins. These proteins will eventually be converted to KCC2 cotransporters on neuronal membranes as the brain matures [36]. Chloride concentrations are therefore partly determined by ratios of NKCC1 and KCC2, as NKCC1 brings chloride ions into the cell and KCC2 extrudes them [36]. When GABA binds to its receptor in neonates, chloride ions flow out of the neuron at a higher rate, which causes neuronal depolarization. This explains why neonatal seizures are not sensitive to GABAergic drugs [101]. As the brain reaches maturity, there is a gradual shift in the predominance of NKCC1 to KCC2 on the cellular membrane (Fig.2). This change results in reduced concentrations of intracellular chloride ions. When GABA binds to its receptor in this instance, chloride ions will flow down their concentration gradient and into the neuron. This will cause neuronal hyperpolarization, resulting in electrical inhibition [36]. Experimental evidence shows that NKCC1 conversion to KCC2 differs in a sex-dependent manner [102]. Females are known to experience this conversion at a higher rate, and this difference may serve as a basis for greater seizure susceptibility in male individuals [102]. Differences in levels of endogenous steroid hormones may further promote variable seizure susceptibility, as these hormones have been shown to affect KCC2 expression in a sex-specific fashion [103,104]. Changes such as these likely contribute to variation in GABAergic inhibition, but the impact of sex-dependent neonatal mechanisms on adult individuals remains under investigation.
Fig. 2.
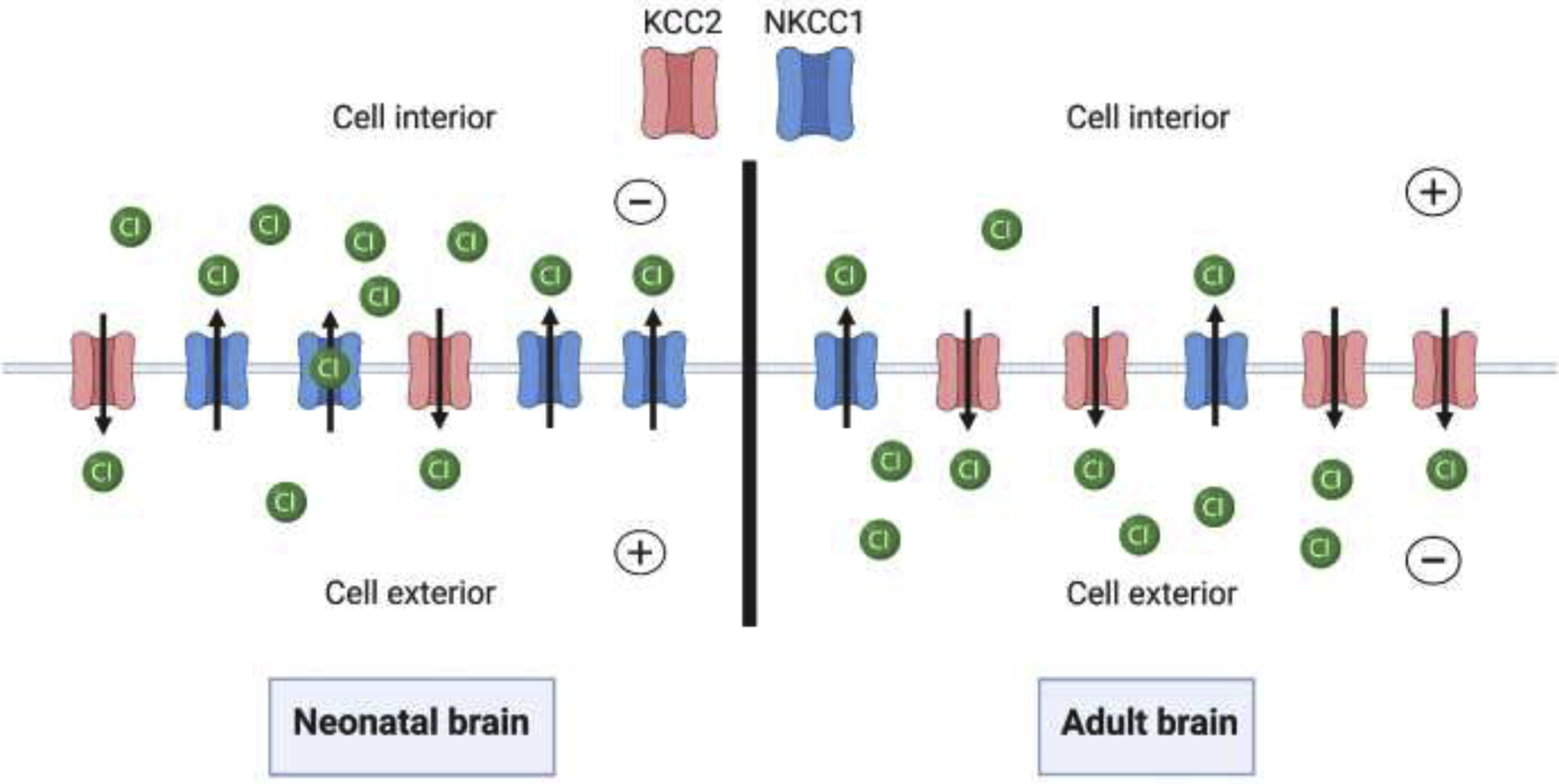
Age-dependent neuronal chloride homeostasis. The image depicts the relative abundance of NKCC1/KCC2 chloride cotransporters in neonatal/adult brains. As the brain matures, NKCC1 transport proteins convert to KCC2 proteins, reversing chloride concentrations on either side of the neuronal membrane. This figure was created using BioRender.com.
3.5. Neurotrophic factors
A variety of neurotrophic factors and inflammatory cascades are known to influence brain injury, repair, and seizure susceptibility. Brain-derived neurotrophic factor (BDNF) and its tropomyosin-related kinase B (TrkB) receptor may promote normal neuron functioning and epileptogenesis [23,105]. However, their roles in sex differences in epilepsy remain under investigation. There is great interest in BDNF and TrkB in the field of epileptogenesis, as there appears to be some noticeable sex differences in BDNF actions. In fact, the presence and pattern of sex differences in BDNF content across brain areas exhibits prominent species differences. Female rats exhibit greater BDNF in the hippocampus, cortex, and amygdala, which are associated with epileptic pathology [106,107]. The levels of BDNF in the hippocampus is higher in males, especially in the mossy fiber pathway [108]. Male mice have higher hippocampal BDNF than females, but a greater level of BDNF is noticed in the prefrontal cortex in women [109,110]. Indeed, BDNF expression is modulated by steroid hormones estradiol, progesterone, and testosterone [105]. BDNF elicits its biological effects through activation of TrkB receptors in the brain. Heterozygous BDNF knockout mice exhibit a sex difference in the TrkB receptor pathway, with greater TrkB phosphorylation, and thus increased activation of downstream signaling, in the frontal cortex and striatum of males compared with females [111]. Sex differences in acquired epileptogenesis, caused by brain injury, are associated with differences in BDNF content [112]. Overall, BDNF levels and its signal transduction may exhibit sex differences in neuronal excitability relevant to seizure susceptibility.
4. Neurobiological mechanisms in catamenial epilepsy
Catamenial epilepsy (CE) refers to cyclical series of seizure exacerbations near specific phases of the menstrual cycle in women who are already diagnosed with epilepsy [21,113,114]. CE is quite prevalent among epileptic women, affecting between 25–70% of those in reproductive age, with the large range attributed to differences in both definition and diagnostic criteria of CE [66,115]. The occurrence of epileptic seizures during particular phases of the menstrual cycle is caused by the cyclic fluctuations of hormones, and as a result, corresponding neurosteroid levels [21,116]. When the levels of inhibitory neurosteroids drop due to a lack of their steroid hormone precursor, preexisting epilepsies may manifest themselves due to an imbalance of neuronal inhibition [117]. While there is no stringent definition as to the particular types of epilepsies and seizures that are susceptible to catamenial exacerbations, studies suggest that seizures in both partial and primary generalized epilepsies can exhibit catamenial exacerbations [21]. There are currently no FDA-approved drug therapies for treating CE, so in many cases, women diagnosed with CE are prescribed common antiepileptic drugs (AEDs) [118]. However, many women still experience catamenial exacerbations despite drug treatment.
There are three types of catamenial seizures: perimenstrual (C1, most common clinical type), periovulatory (C2), and inadequate luteal phase (C3) [66,68]. Clinical diagnosis of CE is made by longitudinally assessing menstruation and seizure records. If the first day of menstrual bleeding is considered the first day of the cycle, the menstrual cycle can be divided into four phases: (a) menstrual phase, days −3 to +3; (b) follicular phase, days +4 to +9; (c) ovulatory phase, days +10 to +16; and (d) luteal phase, days +17 to −4. After counting the number of seizures in each of the 4 phases for at least two weeks, CE is diagnosed if there is a two-fold or greater increase in frequency of seizures during a particular phase of the cycle [40]. Ovulation is not required to diagnose CE, as one study found that 16.5% of subjects experienced anovulatory cycles and associated inadequate luteal-phase seizures [119].
4.1. Neuroendocrine mechanisms
The neuroendocrine explanation for CE may be understood by general aspects of the interplay between hormones and seizures. As previously discussed, the delicate balance between the normally proconvulsant molecule estradiol (and as a result, the hormone estrogen) and the anticonvulsant hormone progesterone underlies the changes in seizure susceptibility associated with CE. Estradiol levels rise during both the follicular and luteal phase of the menstrual cycle, subsequently increasing the estrogen:progesterone ratio and possibly an epileptic woman’s likelihood of displaying perimenstrual seizures [67,115,120]. The waxing and waning of progesterone levels in the menstrual cycle produce corresponding increases and decreases in seizure susceptibility, respectively [66]. Two examples are the mid-luteal and pre-menstrual phases. Seizure frequency decreases at high progesterone levels during the mid-luteal phase and increases before menstruation when progesterone levels are significantly lower [121]. Recent studies suggest that perimenstrual catamenial seizures are tightly correlated with the swift fall in progesterone near menstruation [122].
Among the variety of neurosteroids present in the brain, research indicates that AP (brexanolone), tetrahydrodeoxycorticosterone (THDOC), and androstanediol provide the most neuroprotection against epileptic seizures [81,123]. During their synthesis from steroid hormone precursors, the 5ɑ-reduction of the A-ring converts intermediates into neuroactive steroids [124]. Much of the increase in seizure susceptibility during the phases of the menstrual cycle that feature low levels of progesterone are attributed to the loss of corresponding neurosteroids and their anticonvulsant effects [66,74,116,117,125]. An intriguing phenomenon occurs during the perimenstrual period regarding the relative abundance of extrasynaptic GABAA receptors. During this period, extrasynaptic GABAA receptors are notably upregulated above normal, with possible molecular mechanisms underlying this phenomenon discussed in the next section [126]. This overexpression of extrasynaptic GABA receptors provides neurosteroids with an abundance of locations to exert their anticonvulsant effects, particularly as it relates to perimenstrual seizures in CE [50].
4.2. Experimental models
There are several features of an ideal CE model [127,128,129]. It should reflect pathophysiology similar to those of catamenial seizures in women with epilepsy, exhibit appropriate menstrual seizure phenotypes, be consistent with the neuroendocrine fluctuations of women with epilepsy, exhibit appropriate latency following steroid hormone fluctuations or withdrawal period, and respond to drug therapy with resistance to certain anticonvulsants. Because CE is so diverse in its causes and manifestations, no lone animal model can fully epitomize the gamut of human catamenial seizure phenotypes. Thus, screening novel therapeutics and investigating pathological mechanisms in a wide range of animal models is of utmost importance.
We developed both rat and mouse models, utilizing healthy and epileptic rats and mice, on the premise of creating a hormonal milieu of the perimenstrual period [129]. This was done by creating a variety of manipulations through the use of pseudopregnancies, the exogenous administration of progesterone, and the utilization of a spontaneous seizure model. Both mice and rats were subjected to studies that mimicked the surge and precipitous drop of progesterone and corresponding neurosteroids during the perimenstrual period [69,130]. These studies produced a neurosteroid withdrawal period analogous to common catamenial seizure occurrences. It was found that during this time the seizure threshold dropped significantly before returning to baseline within 72 hours. During these periods a number of therapeutic agents were tested, but neuroactive steroids proved to offer the best protective ability [130]. Furthermore, we tested the readily bioavailable synthetic neurosteroid ganaxolone (AP analog), which shifted the dose response to the left, suggesting that animals in the withdrawal phase are more sensitive to neurosteroids [131].
We recently replicated the above paradigms in two distinct mouse models to allow for more mechanistic studies [57,132]. These studies revolve around the notion that seizure susceptibility and neurosteroid levels are inversely related in females in association with specific changes in GABAA receptor subunit plasticity. First, a female hippocampal kindling model was created to mimic the chronic seizure condition. Second, these mice were administered varying levels of neurosteroids, similar to what is seen in the ovarian cycle. Finally, we adopted two separate pharmacological methods of inducing increased levels of neurosteroids: (a) chronic exogenous progesterone treatment, and (b) a gonadotropin administration to induce endogenous neurosteroid synthesis. The gonadotropin-induced neurosteroid synthesis and withdrawal paradigm appears more physiologically relevant than the exogenous progesterone treatment, as the withdrawal paradigm is induced by the neurosteroid synthesis inhibitor finasteride [132]. We have found that the mouse model of perimenstrual CE is useful for the investigation of disease mechanisms and exploring the efficacy of new therapeutic techniques. However, there are some limitations with these preclinical models stemming from differences in human vs. rodent menstrual cycles. In rodents the estrous cycle duration is 4–7 days, whereas in women it lasts roughly 28 days.
4.3. Neurosteroid replacement therapy
Since there are currently no FDA-approved therapies specifically for catamenial seizures, particularly those in the perimenstrual period, the neurosteroid withdrawal model has and is being used as a basis for developing new therapies for CE [51,133]. Consistent with the observation that women with catamenial epilepsy do not respond to common AEDs, a key result from this model is that conventional AEDs have a reduced potency in protecting against catamenial seizures, producing a form of a pharmacoresistant paradigm. However, we found that neurosteroids and their synthetic analogs have enhanced activity in the perimenstrual CE model [131,133,134]. This led to the idea that “neurosteroid replacement” may be an effective method for countering catamenial seizure exacerbations [135]. We hypothesized that a neurosteroid or synthetic analog could be administered throughout the month, at a low dose to avoid sedative side effects and produce little anticonvulsant activity during most of the menstrual cycle, to serve as a CE therapeutic.
While the molecular mechanisms underlying the enhanced neurosteroid sensitivity in catamenial epilepsy remain unclear, a delta-force hypothesis has been coined to explain this phenomenon [41,136]. We recently discovered that the extrasynaptic GABAA receptors in the dentate gyrus of a mouse perimenstrual model of CE appear to be plastic [126,137]. In summary, it is posited that the drop in neurosteroids during the perimenstrual period catalyzes a selective overexpression of ɑ4δ subunits of GABAA receptors in the dentate gyrus granule cells. This alone is not adequate to abate the increased seizure susceptibility due to the absence of AP, but this pathologic rise in ɑ4δ subunits could possibly act as a vehicle catalyst (“Trojan Horse”) for exogenously administered AP to prevent seizures. This unique sensitivity to AP displayed by women with perimenstrual CE is concordant with the results of the NIH progesterone trial, where the responder group was in fact women with perimenstrual CE [138]. This group demonstrated a significant post-treatment surge in AP, an outcome that suggests perimenstrual CE would be an ideal candidate for testing the therapeutic benefits of a brief, repetitive neurosteroid administration during the perimenstrual period [138]. Taken together, this molecular mechanism represents a molecular rationale for neurosteroid replacement therapy.
5. Conclusions and future directions
Sex differences are evident in many epilepsies and seizure conditions. The current evidence suggests that men exhibit greater overall seizure susceptibility than women, while women exhibit greater fluctuations in seizure susceptibility, such as menstrual cycle-related catamenial seizures. However, the precise changes in neuroendocrine factors and molecular mechanisms remain poorly understood. Steroid hormones and endogenous inhibitory neurosteroids are important in gender differences as it relates to susceptibility to epileptic seizures. Neurosteroids may exert sex differences in their protection against neuronal excitability and seizures. It is likely that differences in steroid hormones or neurosteroid levels in the brain in males and female may contribute to sex differences in seizure control and epileptic seizures. Besides factors like brain development, chloride homeostasis, neurotrophic factors and neurogenesis, sexual dimorphism in specific receptors and function may account for the sex differences in seizure susceptibility and resistance. It is likely that endogenous neurosteroids may mediate sex differences in seizure susceptibility. Consequently, neurosteroids exhibit greater antiseizure potency in females than males, likely due to greater abundance of extrasynaptic GABAA receptors and also differences in neural circuits that regulate seizure susceptibility. Catamenial epilepsy, which is attributed to withdrawal or loss of endogenous neurosteroids around the perimenstrual period, is a unique gender-specific epilepsy in women. Neurosteroid replacement therapy is a viable approach to effectively control catamenial seizures in women with epilepsy. Additional research is warranted to identify the structural and neuroendocrine map of sex differences in the epilepsies.
Highlights.
Sex differences exist in epilepsy and epileptogenesis, but mechanisms remain unclear.
Sex-related differences in morphology and circuits in men and women may explain differential vulnerability to seizures and epileptogenic injuries.
Changes in seizure sensitivity are due to neurosteroids as well as receptor signaling systems.
Potential neural basis for sex differences in epilepsies include differences in neurogenesis, chloride homeostasis, and neurotrophic and glial responses.
A greater understanding of sex differences is needed for designing effective therapies for epilepsy and disease-modification of epileptogenesis.
Acknowledgements
This work was supported by NIH grants NS052158 and NS051398 (to DSR). Dr. Reddy’s research work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01-NS083460, U01-NS117209 and U01-NS117278].
ABBREVIATIONS:
- AED
antiepileptic drug
- AP
allopregnanolone (brexanolone)
- BDNF
brain derived neurotrophic factor
- GX
ganaxolone
- NKCC1
Na-K-Cl cotransporter
- PCDH19
Protocadherin 19
- THDOC
tetrahydrodeoxycorticosterone
- TLE
temporal lobe epilepsy
- TrkB
tropomyosin-related kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Nothing to declare.
References
- [1].Zack MM, Kobau R, National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015, MMWR Morb. Mortal Wkly. Rep 66 (2017) 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Espinosa-Jovel C, Toledano R, Aledo-Serrano A, Garcia-Morales I, Gil-Nagel A, Epidemiological profile of epilepsy in low income populations, Seizure 56 (2018) 67–72. [DOI] [PubMed] [Google Scholar]
- [3].Rugg-Gunn F, Miserocchi A, McEvoy A, Epilepsy surgery, Pract. Neurol 20 (2020) 4–14. [DOI] [PubMed] [Google Scholar]
- [4].Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, Price PH, Epilepsy surveillance among adults–−19 States, Behavioral Risk Factor Surveillance System, 2005, Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 57 (2008) 1–20. [PubMed] [Google Scholar]
- [5].Kight KE, McCarthy MM, Using sex differences in the developing brain to identify nodes of influence for seizure susceptibility and epileptogenesis, Neurobiol. Dis 72 (2017) 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christian CA, Reddy DS, Maguire J, Forcelli PA, Sex Differences in the Epilepsies and Associated Comorbidities: Implications for Use and Development of Pharmacotherapies, Pharmacological Reviews 72 (2020) 767–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reddy DS, Brain structural and neuroendocrine basis of sex differences in epilepsy, In: Handbook of Clinical Neurology (Edited by Lanzenberger R, Kranz GS and Savic I), Elsevier, Vol. 175, (2020) pp-223–233. [DOI] [PubMed] [Google Scholar]
- [8].Savic I, Sex differences in human epilepsy, Exp. Neurol 259 (2014) 38–43. [DOI] [PubMed] [Google Scholar]
- [9].Cerulli IE, Morano A, Cocchi A, Casciato S, Fanella M, Albini M, Avorio F, Basili LM, Fisco G, Barone FA, Mascia A, D’Aniello A, Manfredi M, Fattouch J, Quarato P, Giallonardo AT, Di Gennaro G, Di Bonaventura C, Doing without valproate in women of childbearing potential with idiopathic generalized epilepsy: Implications on seizure outcome, Epilepsia 61(2020) 107–114. [DOI] [PubMed] [Google Scholar]
- [10].Christensen J, Kjeldsen MJ, Anderson H, Friis ML, Sidenius P, Gender differences in epilepsy, Epilepsia 46 (2005) 956–960. [DOI] [PubMed] [Google Scholar]
- [11].Hauser WA, Incidence and prevalence. In: Engel J Jr., Pedley TA (Eds.), Epilepsy: A Comprehensive Textbook, Lippincott-Raven Publishers, Philadelphia: (1997) pp.47–57. [Google Scholar]
- [12].McHugh JC, Delanty N, Epidemiology and classification of epilepsy: Gender comparisons, Int. Rev. Neurobiol 83 (2008) 11–26. [DOI] [PubMed] [Google Scholar]
- [13].Benamer HT, Grosset DG, A systematic review of the epidemiology of epilepsy in Arab countries, Epilepsia 50 (2009) 2301–2304. [DOI] [PubMed] [Google Scholar]
- [14].Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA, Estimating risk for developing epilepsy: A population-based study in Rochester, Minnesota, Neurology 76 (2011) 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim DW, Lee SY, Chung SE, Cheong HK, Jung KY, Clinical characteristics of patients with treated epilepsy in Korea: A nationwide epidemiologic study, Epilepsia 55 (2014) 67–75. [DOI] [PubMed] [Google Scholar]
- [16].Bangar S, Shastri A, El-Sayeh H, Cavanna AE, Women with epilepsy: clinically relevant issues, Functional neurology 31 (2016) 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N, Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies, Neurology, 88 (2017) 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Samanta D, PCDH19-Related Epilepsy Syndrome: A Comprehensive Clinical Review, Pediatr. Neurol 105 (2020) 3–9. [DOI] [PubMed] [Google Scholar]
- [19].Kessler SK, McGinnis E, A Practical Guide to Treatment of Childhood Absence Epilepsy, Paediatr. Drugs 21 (2019) 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Padmanaban V, Inati S, Ksendzovsky A, Zaghloul K, Clinical advances in photosensitive epilepsy, Brain Res. 1703 (2019) 18–25. [DOI] [PubMed] [Google Scholar]
- [21].Reddy DS, Role of neurosteroids in catamenial epilepsy, Epilepsy Res. 62 (2004b) 99–118 [DOI] [PubMed] [Google Scholar]
- [22].Carlson C, Dugan P, Kirsch HE, Friedman D; EPGP Investigators, Sex differences in seizure types and symptoms, Epilepsy Behav. 41 (2014) 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scharfman HE, MacLusky NJ, Sex differences in the neurobiology of epilepsy: a preclinical perspective, Neurobiol. Dis 72 (2014a) 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Savic I, Engel J Jr, Structural and functional correlates of epileptogenesis—does gender matter?, Neurobiol. Dis 70 (2014) 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luders E, Gaser C, Narr KL, Toga AW, Why sex matters: Brain size independent differences in gray matter distributions between men and women, J. Neurosci 29 (2009) 14265–14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R, Sex differences in the structural connectome, Proceedings of the National Academy of Sciences 111 (2014) 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tyan YS, Liao JR, Shen CY, Lin YC, Weng JC, Gender differences in the structural connectome of the teenage brain revealed by generalized q-sampling MRI, NeuroImage: Clinical 15 (2017) 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Englot DJ, Blumenfeld H, Consciousness and epilepsy: why are complex-partial seizures complex?, Progress in brain research 177 (2009) 147–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Janszky J, Schulz R, Janszky I, Ebner A, Medial temporal lobe epilepsy: Gender differences, J. Neurol. Neurosurg. Psychi 75 (2004) 773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kilpatrick LA, Zald DH, Pardo JV, Cahill LF, Sex-related differences in amygdala functional connectivity during resting conditions, NeuroImage 30 (2006) 452–461. [DOI] [PubMed] [Google Scholar]
- [31].Savic I, Lindstrom P, PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects, Proc. Natl. Acad. Sci. USA 105 (2008) 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Blank LJ, Acton EK, Thibault D, Willis AW, Neurodegenerative disease is associated with increased incidence of epilepsy: a population based study of older adults, Age Ageing 50 (2021) 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hauser WA, Lleo A, Schmolck H, Dementia and epilepsy: Not a one-way street, Neurology 95 (2020) 1074–1075 [DOI] [PubMed] [Google Scholar]
- [34].Johnson EL, Krauss GL, Kucharska-Newton A, Albert MS, Brandt J, Walker KA, Yasar S, Knopman DS, Vossel KA, Gottesman RF, Dementia in late-onset epilepsy: The atherosclerosis risk in communities study, Neurology 95 (2020) e3248–e3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang X, Lu H, Liu W, Wu Z, Wu Q, Li X, Xu Z, Hui F, Zhao Q, The overlap between Alzheimer’s disease and epilepsy uncovered by transcriptome sequencing, Clin. Transl. Med 10 (2020) e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Reddy DS, The neuroendocrine basis of sex differences in epilepsy, Pharmacology, biochemistry, and behavior 152 (2017) 97–104. [DOI] [PubMed] [Google Scholar]
- [37].Runtz L, Girard B, Toussenot M, Espallergues J, Fayd’Herbe De Maudave A, Milman A, deBock F, Ghosh C, Guérineau NC, Pascussi JM, Bertaso F, Marchi N, Hepatic and hippocampal cytochrome P450 enzyme overexpression during spontaneous recurrent seizures, Epilepsia, 59 (2018) 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Werner FM, Coveñas R, Neural Networks in Generalized Epilepsy and Novel Antiepileptic Drugs, Current pharmaceutical design, 25 (2019) 396–400. [DOI] [PubMed] [Google Scholar]
- [39].Reddy DS, Neurosteroids: Endogenous role in the human brain and therapeutic potentials, Prog. Brain Res 186 (2010) 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reddy DS, Neuroendocrine aspects of catamenial epilepsy, Hormon. Behav 63 (2013) 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reddy DS, Neurosteroids and their role in sex-specific epilepsies, Neurobiol. Dis 72 (Pt B, 2014) 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Del Río JP, Alliende MI, Molina N, Serrano FG, Molina S, Vigil P, Steroid Hormones and Their Action in Women’s Brains: The Importance of Hormonal Balance, Frontiers in public health 6 (2018) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Giatti S, Garcia-Segura LM, Barreto GE, Melcangi RC, Neuroactive steroids, neurosteroidogenesis and sex, Prog. Neurobiol 176 (2019) 1–17. [DOI] [PubMed] [Google Scholar]
- [44].Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C, Neuroactive steroids for the treatment of status epilepticus, Epilepsia, 54 (Suppl. 6, 2013), 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reddy DS, Estes W, Clinical potential of neurosteroids for CNS disorders, Trends Pharmacol. Sci 37 (2016) 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Joshi S, Rajasekaran K, Williamson J, Kapur J, Neurosteroid-sensitive δ-GABAA receptors: A role in epileptogenesis? Epilepsia 58 (2017) 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sarto-Jackson I, Seighart W, Assembly of GABA(A) receptors (Review), Mol. Membr. Biol 25 (2008) 302–10 [DOI] [PubMed] [Google Scholar]
- [48].Olsen RW, GABAA receptor: Positive and negative allosteric modulators, Neuropharmacology 136 (2018) 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reddy DS, GABA-A Receptors Mediate Tonic Inhibition and Neurosteroid Sensitivity in the Brain Vitamins and hormones 107 (2018) 177–191. [DOI] [PubMed] [Google Scholar]
- [50].Chuang S-H, Reddy DS, Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: Therapeutic insights for epilepsy, J. Pharmacol. Exp. Therap 364 (2018) 180–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Joshi S, Kapur J, Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy, Brain Res. 1703 (2019) 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J, Progesterone receptors: form and function in the brain, Frontiers in neuroendocrinology, 29 (2008) 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Motta E, Golba A, Ostrowska Z, Steposz A, Huc M, Kotas-Rusnak J, Łuszczki JJ, Czuczwar SJ, Lasoń W, Progesterone therapy in women with epilepsy, Pharmacol Reports 65 (2013) 89–98. [DOI] [PubMed] [Google Scholar]
- [54].Reddy DS, The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy, Epilepsy Res. 85 (2009a) 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carver CM, Reddy DS, Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA-A receptors, J. Pharmacol. Exp. Ther 357 (2016) 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wu YV, Burnham WM, Progesterone, 5a-dihydroprogesterone and allopregnanolone’s effects on seizures: A review of animal and clinical studies, Seizure 63 (2018) 26–36. [DOI] [PubMed] [Google Scholar]
- [57].Reddy DS, Gangisetty O, Briyal S, Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis, Neuropharmacol. 59 (2010) 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reddy DS, Castenada DA, O’Malley BW, Rogawski MA, Antiseizure activity of progesterone and neurosteroids in progesterone receptor knockout mice, J. Pharmacol. Exp. Ther 310 (2004) 230–239. [DOI] [PubMed] [Google Scholar]
- [59].Scharfman HE, MacLusky NJ, The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female, Epilepsia 47 (2006) 1423–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Velísková J, The role of estrogens in seizures and epilepsy: the bad guys or the good guys?, Neuroscience 138 (2006) 837–844. [DOI] [PubMed] [Google Scholar]
- [61].Velíšková J, Estrogens and Epilepsy: Why are we so excited?, Neuroscientist 13 (2007) 77–88. [DOI] [PubMed] [Google Scholar]
- [62].Reibel S, André V, Chassagnon S, André G, Marescaux C, Nehlig A, Depaulis A, Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat, Neurosci. Lett 281 (2000) 79–82. [DOI] [PubMed] [Google Scholar]
- [63].Veliskova J, Velisek L, Galanopoulou AS, Sperber EF, Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus, Epilepsia 41 (Suppl.6, 2000) S30–35. [DOI] [PubMed] [Google Scholar]
- [64].Velísková J, Velísek L, Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide-Y, J. Neurosci 27 (2007) 6054–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scharfman HE, Malthankar-Phatak GH, Friedman D, Pearce P, McCloskey DP, Harden CL, Maclusky NJ, A rat model of epilepsy in women: a tool to study physiological interactions between endocrine systems and seizures, Endocrinology 150 (2009) 4437–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Reddy DS, Steroid hormones and sex differences in seizure susceptibility, In: Encyclopedia of Basic Epilepsy Research (Editor, Philip Schwartzkroin: ), Vol 1. Oxford, Academic Press, (2009b) 526–533. [Google Scholar]
- [67].Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A, Unbalanced progesterone and estradiol secretion in catamenial epilepsy, Epilepsy Res. 3 (1989) 100–106. [DOI] [PubMed] [Google Scholar]
- [68].Herzog AG, Klein P, Ransil BJ, Three patterns of catamenial epilepsy, Epilepsia 38 (1997) 1082–1088. [DOI] [PubMed] [Google Scholar]
- [69].Oberlander JG, Woolley CS, 17beta-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females, J. Neurosci 36 (2016) 2677–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA, Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci 17 (1997) 1848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Woolley CS, Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus, Horm. Behav 34 (1998) 140–8. [DOI] [PubMed] [Google Scholar]
- [72].Reddy DS, Zeng YC, Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy, FASEB. J 21(2007) A1179–A11179. [Google Scholar]
- [73].Tauboll E, Sveberg L, Svalheim S, Interactions between hormones and epilepsy, ScienceDirect 28 (2015) 3–11. [DOI] [PubMed] [Google Scholar]
- [74].Pottoo FH, Javed MN, Barkat MA, Alam MS, Nowshehri JA, Alshayban DM, Ansari MA, Estrogen and serotonin: complexity of interactions and implications for epileptic seizures and epileptogenesis, Curr. Neuropharmacol 17 (2019) 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Reddy DS, Jian K, The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors, J Pharmacol Exp Ther. 334 (2010) 1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Werboff LH, Havlena J, Audiogenic seizures in adult male rats treated with various hormones, Gen. Comp Endocrinol 3 (1968) 389–397. [DOI] [PubMed] [Google Scholar]
- [77].Thomas J, McLean JH, Castration alters susceptibility of male rats to specific seizures, Physiol. Behav 49 (1991) 1177–1179. [DOI] [PubMed] [Google Scholar]
- [78].Frye CA, Reed TA, Androgenic neurosteroids: anti-seizure effects in an animal model of epilepsy, Psychoneuroendocrinology 23 (1998) 385–399. [DOI] [PubMed] [Google Scholar]
- [79].Pesce ME, Acevedo X, Bustamante D, Miranda HE, Pinardi G, Progesterone and testosterone modulate the convulsant actions of pentylenetetrazol and strychnine in mice, Pharmacol. Toxicol 87 (2000) 116–119. [DOI] [PubMed] [Google Scholar]
- [80].Mejias-Aponte CA, Jimenez-Rivera CA, Segarra AC, Sex differences in models of temporal lobe epilepsy: role of testosterone, Brain Res 944 (2002) 210–218. [DOI] [PubMed] [Google Scholar]
- [81].Reddy DS, Anticonvulsant activity of the testosterone-derived neurosteroid 3a-androstanediol, Neuroreport 15 (2004a) 515–518. [DOI] [PubMed] [Google Scholar]
- [82].Reddy DS, Carver CM, Clossen B, Wu X, Extrasynaptic γ-aminobutyric acid type A receptor-mediated sex differences in the antiseizure activity of neurosteroids in status epilepticus and complex partial seizures, Epilepsia, 60 (2019) 730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].McCarthy MM, Arnold AP, Reframing sexual differentiation of the brain, Nat. Neurosci 14 (2011) 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Arnold AP, The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues, Horm. Behav 55 (2009) 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM, Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior, Proc. Natl. Acad. Sci. USA 107 (2010) 20535–20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bowers JM, Waddell J, McCarthy MM, A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol, Biol. Sex. Differ 1 (2010) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Löscher W, Brandt C, Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research, Pharmacol. Rev 62 (2010) 668–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Löscher W, Potschka H, Sisodiya SM, Vezzani A, Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options, Pharmacological reviews, 72 (2020) 606–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Eyo UB, Murugan M, Wu LJ, Microglia-neuron communication in epilepsy, Glia 65 (2017) 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Patel DC, Tewari BP, Chaunsali L, Sontheimer H, Neuron-glia interactions in the pathophysiology of epilepsy, Nat. Rev. Neurosci 20 (2019) 282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yanuck SF, Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders, Frontiers in psychiatry 10 (2019) 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hickman S, Izzy S, Sen P, Morsett L, El Khoury J, Microglia in neurodegeneration, Nature neuroscience 21 (2018) 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Conejo NM, González-Pardo H, Pedraza C, Navarro FF, Vallejo-Seco G, Arias JL, Evidence for sexual difference in astrocytes of adult rat hippocampus, Neurosci. Lett 339 (2003) 119–122. [DOI] [PubMed] [Google Scholar]
- [94].Conejo NM, González-Pardo H, Cimadevilla JM, Argüelles JA, Díaz F, Vallejo-Seco G, Arias JL, Influence of gonadal steroids on the glial fibrillary acidic protein-immunoreactive astrocyte population in young rat hippocampus, J. Neurosci. Res 79 (2005) 488–494. [DOI] [PubMed] [Google Scholar]
- [95].Arias C, Zepeda A, Hernández-Ortega K, Leal-Galicia P, Lojero C, Camacho-Arroyo I, Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus, Horm. Behav 55 (2009) 257–263. [DOI] [PubMed] [Google Scholar]
- [96].Nelson LH, Lenz KM, The immune system as a novel regulator of sex differences in brain and behavioral development, Journal of neuroscience research, 95 (2017) 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Johnson RT, Breedlove SM, Jordan CL, Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala, J. Comp. Neurol 511 (2008) 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lenz KM, McCarthy MM, A starring role for microglia in brain sex differences, The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry, 21 (2015) 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Morizawa Y, Sato K, Takaki J, Kawasaki A, Shibata K, Suzuki T, Ohta S, Koizumi S, Cell-autonomous enhancement of glutamate-uptake by female astrocytes, Cell Mol. Neurobiol 32 (2012) 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR, Sex and estradiol influence glial proinflammatory responses to lipopolysaccharide in rats, Psychoneuroendocrinology 37 (2012) 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Moore YE, Conway LC, Wobst HJ, Brandon NJ, Deeb TZ, Moss SJ, Developmental Regulation of KCC2 Phosphorylation Has Long-Term Impacts on Cognitive Function, Frontiers in molecular neuroscience 12 (2019) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Galanopoulou AS, Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA-A receptors, J. Neurosci 28 (2008a) 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Galanopoulou AS, Sexually dimorphic expression of KCC2 and GABA function, Epilepsy Res. 80 (2008b) 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Galanopoulou AS, Moshe SL, Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra, Exp. Neurol 184 (2003) 1003–1009. [DOI] [PubMed] [Google Scholar]
- [105].Scharfman HE, MacLusky NJ, Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats, Neuropharmacology 76 (2014b) 696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF, Expression of cfos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress, Brain Res. 1051 (2005) 90–99. [DOI] [PubMed] [Google Scholar]
- [107].Bakos J, Hlavacova N, Rajman M, Ondicova K, Koros C, Kitraki E, Steinbusch HW, Jezova D, Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner, Neuroscience 164 (2009) 788–797. [DOI] [PubMed] [Google Scholar]
- [108].Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ, Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor, J. Neurosci 23 (2003) 11641–11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM, Exploring the relationship between serotonin and brainderived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression, J. Neurosci. Methods 140 (2004) 81–92. [DOI] [PubMed] [Google Scholar]
- [110].Hayley S, Du L, Litteljohn D, Palkovits M, Faludi G, Merali Z, Poulter MO, Anisman H, Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide, Neurosci. Lett 600 (2015) 12–16. [DOI] [PubMed] [Google Scholar]
- [111].Hill RA, van den Buuse M, Sex-dependent and region-specific changes in TrkB signaling in BDNF heterozygous mice, Brain Res. 1384 (2011) 51–60. [DOI] [PubMed] [Google Scholar]
- [112].Chen X, Li Y, Kline AE, Dixon CE, Zafonte RD, Wagner AK, Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury, Neuroscience 135 (2005) 11–17. [DOI] [PubMed] [Google Scholar]
- [113].Newmark ME, Penry JK, Catamenial epilepsy: a review, Epilepsia 21 (1980) 281–300. [DOI] [PubMed] [Google Scholar]
- [114].Herzog AG, Fowler KM, The NIH Progesterone Trial Study Group, Sensitivity and specificity of the association between catamenial seizure patterns and ovulation, Neurol. 70 (2008) 486–487. [DOI] [PubMed] [Google Scholar]
- [115].Maguire MJ, Nevitt SJ, Treatments for seizures in catamenial (menstrual-related) epilepsy, Cochrane Database Syst. Rev 10 (2019) CD013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Herzog AG, Progesterone therapy in women with epilepsy: a 3-year follow-up, Neurology 52 (1999) 1917–1918. [DOI] [PubMed] [Google Scholar]
- [117].Miziak B, Chroscinska-Krawczyk M, Czuczwar SJ, Neurosteroids and Seizure Activity, Front. Endocrinol. (Lausanne) 11 (2020) 541802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Joshi S, Sun H, Rajasekaran K, Williamson J, Perez-Reyes E, Kapur J, A novel therapeutic approach for treatment of catamenial epilepsy, Neurobiol Dis. 111 (2018) 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Herzog AG, Harden CL, Liporace J, Pennell PB, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M, Frequency of catamenial seizure exacerbation in women with localization-related epilepsy, Ann. Neurol 56 (2004) 431–434. [DOI] [PubMed] [Google Scholar]
- [120].Herzog AG, Reproductive endocrine considerations and hormonal therapy for men with epilepsy, Epilepsia 32 (Suppl 6, 1991) S34–S37. [DOI] [PubMed] [Google Scholar]
- [121].Najafi M, Sadeghi MM, Mehvari J, Zare M, Akbari M, Progesterone therapy in women with intractable catamenial epilepsy, Adv. Biomed. Res 2 (2013) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Navis A, Harden C, A Treatment Approach to Catamenial Epilepsy, Curr. Treat. Options Neurol 18 (2016). [DOI] [PubMed] [Google Scholar]
- [123].Reddy DS, Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy, Frontiers Endocrinol. 2 (2011) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H, Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system, Pharmacol. Rev 51 (1999) 63–81. [PubMed] [Google Scholar]
- [125].Jacono JJ, Robinson J, The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women, Epilepsia 28 (1987) 571–577. [DOI] [PubMed] [Google Scholar]
- [126].Carver CM, Wu X, Gangisetty O, Reddy DS, Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABA-A receptors mediating tonic inhibition and neurosteroid sensitivity, J. Neurosci 34 (2014) 14181–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ, Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine, Exp. Neurol 196 (2005) 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Scharfman HE, Kim M, Hintz TM, MacLusky NJ, Seizures and reproductive function: insights from female rats with epilepsy, Ann. Neurol 64 (2008) 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Reddy DS, Catamenial epilepsy: Discovery of an extrasynaptic molecular mechanism for targeted therapy, Front. Cell. Neurosci 10 (2016) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Reddy DS, Rogawski MA, Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy, Epilepsia 42 (2001) 337–44. [DOI] [PubMed] [Google Scholar]
- [131].Reddy DS, Rogawski MA, Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy, J. Pharmacol. Exp. Ther 294 (2000a) 909–915. [PubMed] [Google Scholar]
- [132].Reddy DS, Ramanathan G, Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis, Epilepsy Behav. 25 (2012) 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Reddy DS, Kim HY, Rogawski MA, Neurosteroid withdrawal model of perimenstrual catamenial epilepsy, Epilepsia 42 (2001) 328–336. [DOI] [PubMed] [Google Scholar]
- [134].Reddy DS, Rogawski MA, Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself, J. Pharmacol. Exp. Ther 295 (2000b) 1241–1248. [PubMed] [Google Scholar]
- [135].Reddy DS, Rogawski MA, Neurosteroid replacement therapy for catamenial epilepsy, Neurotherapeutics 6 (2009) 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Galanopoulou AS, The perimenstrual delta force: A trojan horse for neurosteroid effects, Epilepsy Currents 15 (2015) 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wu X, Gangisetty O, Carver CM, Reddy DS, Estrous cycle regulation of extrasynaptic δ-containing GABA-A receptor-mediated tonic inhibition and limbic epileptogenesis, J. Pharmacol. Exp. Ther 346 (2013) 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Herzog AG, Fowler KM, Smithson SD, A Kalayian L, Heck CN, Sperling MR, Liporace JD, Harden CL, A Dworetzky B, Pennell PB, Massaro JM, The NIH Progesterone Trial Study Group, Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial, Neurol. 78 (2012) 1959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


