Summary
Right ventricular hypertrophy (RVH) occurs in high pressure afterload, e.g., tetralogy of Fallot/pulmonary stenosis (TOF/PS). Such RVH is associated with alterations in energy metabolism, neurohormonal and epigenetic dysregulation (e.g., microRNA), and fetal gene reprogramming in animal models. However, comprehensive expression profiling of competing endogenous RNA in human RVH has not been performed. Here, we unravel several previously unknown circular, long non-coding, and microRNAs, predicted to regulate expression of genes specific to human RVH in the non-failing heart (TOF/PS). These genes are significantly overrepresented in pathways related to regulation of glucose and lipid metabolism (SIK1, FABP4), cell surface interactions (THBS2, FN1), apoptosis (PIK3IP1, SIK1), extracellular matrix composition (CTGF, IGF1), and other biological events. This is the first unbiased RNA sequencing study of human compensated RVH encompassing coding and non-coding RNA expression and predicted sponging of miRNAs by non-coding RNAs. These findings advance our understanding of adaptive RVH and highlight future therapeutic targets.
Subject areas: Molecular Biology, Omics
Graphical abstract

Highlights
-
•
First comprehensive transcriptomic study of human RVH via RNA expression and network analysis
-
•
First human RVH study using exclusively freshly isolated myocardium
-
•
Known hypertrophy genes are regulated the strongest by competing endogenous RNA networks in RVH
-
•
Epigenetic mRNA regulation in RVH by ncRNAs is dependent on sex and age
Molecular Biology; Omics
Introduction
Right ventricular hypertrophy (RVH) occurs in pulmonary hypertension (PH), tetralogy of Fallot/pulmonary stenosis (TOF/PS), or other conditions with high right ventricular (RV) pressure afterload. RV adaptation and remodeling in high pressure afterload involves a number of interdependent complex processes, such as altered bioenergetics (hypoxia/ischemia and mitochondrial remodeling/dysfunction) and neurohormonal and immunological activation. In concert with underlying genetic and epigenetic alterations (e.g., microRNAs), these pathobiological events ultimately lead to RV dilation and failure (“decompensated RVH”) (Agrawal et al., 2020; van der Bruggen et al., 2017). Right ventricular hypertrophy may occur in the setting of RV outflow tract obstruction (PS; normal pulmonary vascular resistance) or in hypertensive pulmonary vascular disease with anatomically normal RV outflow (Hansmann, 2017; Hansmann et al., 2019; Santens et al., 2020). Both etiologies have been studied in various animal models (Andersen et al., 2020); however, none of them fully simulate human RVH in vivo (Agrawal et al., 2020; Andersen et al., 2020; Bernardo et al., 2020; Santens et al., 2020).
The hallmarks of maladaptive decompensated cardiac hypertrophy are cell death, fibrosis, dysregulation of Ca2+-handling proteins, mitochondrial dysfunction, metabolic reprogramming, reactivation of fetal gene expression, altered sarcomere structure, and insufficient angiogenesis (Agrawal et al., 2020; Nakamura and Sadoshima, 2018). More specifically, a number of microRNAs (miRNAs) have been suggested to be involved in the pathobiology of RV hypertrophy and dysfunction in conditions of high pressure afterload (pulmonary hypertension or PS/pulmonary artery banding). However, the vast majority of such studies have been conducted on rodent heart tissues, postmortem human cardiac specimen, and/or circulating RNAs (see discussion and refs. Bittel et al., (2011); Bittel et al. (2014); Chouvarine et al., 2020; O'Brien et al. (2012); Wang et al. (2014); Yang et al. (2013); Zhang et al. (2013)), but not on freshly isolated human myocardium.
Since non-coding RNAs, especially long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), are not well conserved between species (e.g., mouse/rat versus human [Gandhi et al., 2019; Jakobi et al., 2020]), we chose an exploratory study design in two well-matched patient cohorts that are distinguished by the presence or absence of RVH due to RV outflow tract obstruction (TOF/PS versus ventricular septal defect without PS). TOF is a congenital heart disease with four anatomical features: (1) pulmonary artery stenosis, (2) ventricular septal defect (VSD), (3) deviation of the origin of the aorta (septal overriding aorta), and (4) concentric RVH.
We hypothesized that specific transcriptional programs exist in the human hypertrophied, non-failing RV of infants with TOF/PS versus the non-hypertrophied RV of infants without PS (VSD only), reflecting features of fetal gene programming and altered glucose/lipid metabolism. Comprehensive understanding of such transcriptional RNA profiles and networks requires quantification of competing endogenous RNA, which consists of a variety of non-coding RNAs and protein-coding RNAs competing as targets of miRNA regulation (Salmena et al., 2011).
To identify the transcriptional profiles characteristic of human RVH, we analyzed differentially expressed genes (DEGs) by sequencing messenger RNAs (mRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) in human RV tissue. Additionally, shorter regulatory microRNAs (miRNA) were also sequenced. Analysis of potential regulatory networks based on target specificity of the differentially expressed miRNAs to the DEGs in this study allowed us to unravel key miRNAs likely involved in transcriptional adaptation in RVH with increased pressure afterload. Apparently, most relevant regulatory miRNAs are simultaneously epigenetically regulated by several circRNAs and lncRNAs, which sponge the miRNAs interacting with them, thereby reducing the regulatory capacity of these miRNAs. Moreover, using only sets of differentially expressed RNAs, we show that less biologically relevant miRNAs are regulated to a much lower degree by lncRNAs and circRNAs, compared with the most relevant regulatory miRNAs.
Results
Demographics of the study cohort
The study included 19 infants with TOF/PS and eight controls with VSD without PS, 2–12 months old, undergoing surgical repair of the underlying congenital heart disease at three tertiary centers. Demographics data are summarized in Table 1, and individual information is provided in Table S1. The two groups were similar in terms of age and weight.
Table 1.
Characteristics of all patients with TOF/PS and VSD controls
| Patients | VSD controls n = 8 | TOF/PS n = 19 | p value |
|---|---|---|---|
| Demographics | |||
| Age (months) | 6.1 ± 3.5 | 5.4 ± 1.3 | n.s. |
| Older (7–8 months), n = 9 | 9.0 ± 2.2 | 7.2 ± 0.4 | n.s. |
| Young (2–4 months), n = 8 | 2.7 ± 1.2 | 3.4 ± 0.5 | n.s. |
| Between (5–6 months), n = 10 | 5 | 5.4 ± 0.5 | n.s. |
| Count by age, n | 8 | 19 | |
| Older (7–8 months) | 4 | 5 | |
| Young (2–4 months) | 3 | 5 | |
| Between (5–6 months) | 1 | 9 | |
| Sex, female, n (%) | 5 (63%) | 7 (37%) | |
| Older (7–8 months) | 3 (75%) | 2 (40%) | |
| Young (2–4 months) | 1 (33%) | 2 (40%) | |
| Between (5–6 months) | 1 (100%) | 3 (33%) | |
| Body height, cm | 62.6 ± 5.2 | 66.6 ± 4.4 | n.s. |
| Older (7–8 months) | 66.0 ± 3.6 | 68.5 ± 6.1 | n.s. |
| Young (2–4 months) | 60.3 ± 4.9 | 63.0 ± 4.2 | n.s. |
| Between (5–6 months) | 56 | 66.6 ± 2.8 | n.s. |
| Body weight, kg | 5.6 ± 1.5 | 6.9 ± 1.0 | 0.008 |
| Older (7–8 months) | 6.7 ± 1.2 | 7.8 ± 1.1 | n.s. |
| Young (2–4 months) | 4.6 ± 1.2 | 5.8 ± 0.2 | n.s. |
| Between (5–6 months) | 4.3 | 6.9 ± 0.9 | n.s. |
| RVOTO (RV pressure afterload) | |||
| RVOT CW Doppler, m/s | NA | 4.7 ± 1.1 | |
| Older (7–8 months) | NA | 5.0 ± 1.5 | |
| Young (2–4 months) | NA | 4.7 ± 0.8 | |
| Between (5–6 months) | NA | 4.4 ± 1.1 | |
| Oxygen saturation (SpO2), % | 99.3 ± 1.3 | 90.9 ± 8.6 | 0.0125 |
| Older (7–8 months) | 99.0 ± 1.7 | 91.6 ± 8.6 | n.s. |
| Young (2–4 months) | 99.3 ± 1.5 | 88.9 ± 10.4 | n.s. |
| Between (5–6 months) | 100 | 92.0 ± 9.1 | n.s. |
BSA, body surface area; CW, continuous wave; NA, non-applicable; n.s., not significant; RV, right ventricle; RVOT, RV outflow tract; RVOTO, RVOT obstruction; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
For individual patient data, see also Table S1.
Values are presented as mean ± SD. A Wilcoxon signed-rank test was applied. p < 0.05 was considered significant.
Cardiac mRNA expression in human RV hypertrophy has a specific profile dependent on sex and age
After subtraction of age- and sex-specific genes (see methods), a total of 500 DEGs (false discovery rate [FDR]<0.05) were detected, of which 141 were upregulated and 359 were downregulated. Additionally, we identified 23 DEGs specific to sex, i.e., differentially expressed between males and females in both TOF/PS and VSD groups, and 41 genes specific to age, i.e., differentially expressed between older (7–8 months) and younger (2–4 months) patients in both TOF/PS and VSD groups. Figure 1 shows a heatmap of differentially expressed genes with fold changes ≥2 or ≤0.5 identified by comparisons of the entire TOF/PS (labeled: TOF) and VSD cohorts; it also shows the corresponding fold changes and FDR-adjusted p values of the same genes in five additional comparisons stratified by sex and age. The differential expression profiles in males, and those in the younger subgroup (2–4 months old), generally were consistent with those of the entire cohort (Figure 1). Female-specific transcriptional regulation and the developmental changes in the older subgroup (7–8 months old) bring in additional regulatory signaling, making identification of the disease-related transcriptional regulation in these subgroups more challenging. The heatmap in Figure 1 represents the DEGs after removal of the sex- and age-specific genes (see methods for details). Many biological processes relevant to human adaptive (compensated) RVH in TOF/PS versus VSD controls are represented by the top DEGs shown in Figure 1, e.g., TGF-β regulation of extracellular matrix (SERPINA3, CEBPD, ELN, SERPINE1, LTBP2, AEBP1, IGF1, CTGF, SFRP4, ALDH1A3, UCHL1, CTSK, DDIT4, COL8A2, ID4, NRCAM, SCRG1, CFB); fibrosis regulation by small leucine-rich proteoglycan (BGN and FMOD); gap junction pathways for regulation of intercellular current flow (TUBA3E, TUBA3D, HTR2B, HTR2A); receptor for advanced glycation end (RAGE) pathway (SERPINE1, IGF1, CTGF); collagen biosynthesis (COL25A1, COL8A2, COL9A2); lipid metabolism (HMGCS2 and CTGF); etc. To identify the RVH pathways that are significantly overrepresented by DEGs in the entire cohort (with age- and sex-specific genes excluded) and the five age- and sex-stratified subgroups, we used the BioPlanet pathway annotation (the results are shown in Data S1). Several pathways were noticeably more overrepresented by the TOF/PS versus VSD DEGs in the females compared with the males. Most prominent examples of such pathways are the brain-derived neurotrophic factor (BDNF) signaling pathway (27 DEGs in females versus 15 DEGs in males) and the collagen biosynthesis and modifying enzymes pathway (15 DEGs in females versus 6 DEGs in males). Similarly, overrepresentation of pathways was also age dependent. For example, the TGF-β regulation of extracellular matrix pathway had 42 DEGs in the younger group (2–4 months) versus 15 DEGs in the older group (7–8 months). Analysis of the Gene Ontology (GO) biological process terms overrepresented by DEGs among the subgroups showed that age and sex have significant effects on the RVH-associated gene expression profile (Figure S1). To visualize the significance of the age and sex effects, compared with the RVH effect, on the overall gene expression, we performed principal-component clustering analysis that revealed all three effects as appreciable and also showed absence of outliers (Figure S2).
Figure 1.
Heatmap of differentially expressed genes shows more consistent expression profiles in male and young groups and more diverse gene expression in female and older groups
The heatmap shows 101 genes that were significantly differentially expressed (FDR<0.05) and had fold change <0.5 or >2 in the All group (based on all samples in the study), from which the age- and sex-specific genes were excluded. The age-specific genes were defined as found in both TOF_old versus TOF_young and Con_old versus Con_young comparisons. The sex-specific genes were defined as found in both TOF_m versus TOF_f and Con_m versus Con_f comparisons. The five comparisons, shown in the heatmap after the All group comparison, contain only those differentially expressed genes (regardless of the fold change) that correspond to the selection of genes in the All group. The group sizes are as follows: All: NTOF = 19, NCon = 8; Male: NTOF = 12, NCon = 3; Female: NTOF = 7, NCon = 5; Young: NTOF = 5, NCon = 3; Older: NTOF = 5, NCon = 4; Older female: NTOF = 2, NCon = 3.
miRNA expression analysis reveals potential epigenetic regulators of human RVH
To investigate gene expression regulation in RVH by miRNAs, we performed separate sequencing of mature miRNAs, which are single-stranded RNA molecules approximately 21–23 nucleotides long (Farazi et al., 2008), i.e., shorter than the other RNAs sequenced in this study (>200 nt). Differentially expressed miRNAs with fold changes and p values are presented in Table S2. The following miRNAs were significantly (FDR<0.05) differentially expressed (1) in the entire cohort: miR-31↑, miR-33b↓, miR-135a-2↓, miR-188↓, miR-216a↓, miR-323b↓, miR-371a↑, and miR-372↑; (2) in males: miR-31↑ and miR-217↓; (3) in females: miR-372↑; (4) in the young group (2–4 months): none; (5) in the older group (7–8 months): miR-184↓, miR-216a↓, miR-4423↓, miR-4999↓, and miR-5008↓; and (6) in older females: miR-33a↓, miR-216a↓, miR219a-1↓, and miR-5008↓ (the arrows show the direction of regulation). Of note, miR-31, miR-216a, miR-372, and miR-5008 co-occur in the overall cohort and several age/sex subgroups, each with the same direction of regulation; thus these four miRNAs are likely involved in strong epigenetic regulation occurring in RVH in TOF/PS. On the other hand, based on their membership in the aforementioned subgroups, these four miRNAs can be associated with RVH in certain subgroups: in males: miR-31; in females: miR-372; and in older patients: miR-216a and miR-5008.
Differential expression of sponging circRNAs and lncRNAs indicates age- and sex-specific regulation of non-coding RNAs in high pressure load conditions such as TOF/PS (RVH)
To be able to construct regulatory networks of non-coding RNAs, we conducted differential expression analysis of circRNAs and lncRNAs. lncRNAs and circRNAs are capable of sponging miRNAs, thereby reducing their availability to act as inhibitors of both sustained expression of mRNA and further translation of functional peptides/proteins. Differentially expressed circRNA isoforms and lncRNAs with fold changes and p values are reported in Tables S3 and S4, respectively. Interestingly, except for lncRNAs PAX8-AS1 and AC083843.3, the sponging molecules (lncRNAs or circRNAs) are not co-occurring in the age- or sex-stratified subgroups, suggesting age- and sex-specific non-coding RNA regulation in high pressure load conditions such as TOF/PS, via transcriptional regulation by circRNAs and lncRNAs (see Tables S3 and S4 for differential expression of circRNAs and lncRNAs in the overall cohort and subgroups).
mRNA-miRNA-circRNA/lncRNA interaction networks reflect differences in age- and sex-specific epigenetic RVH regulation
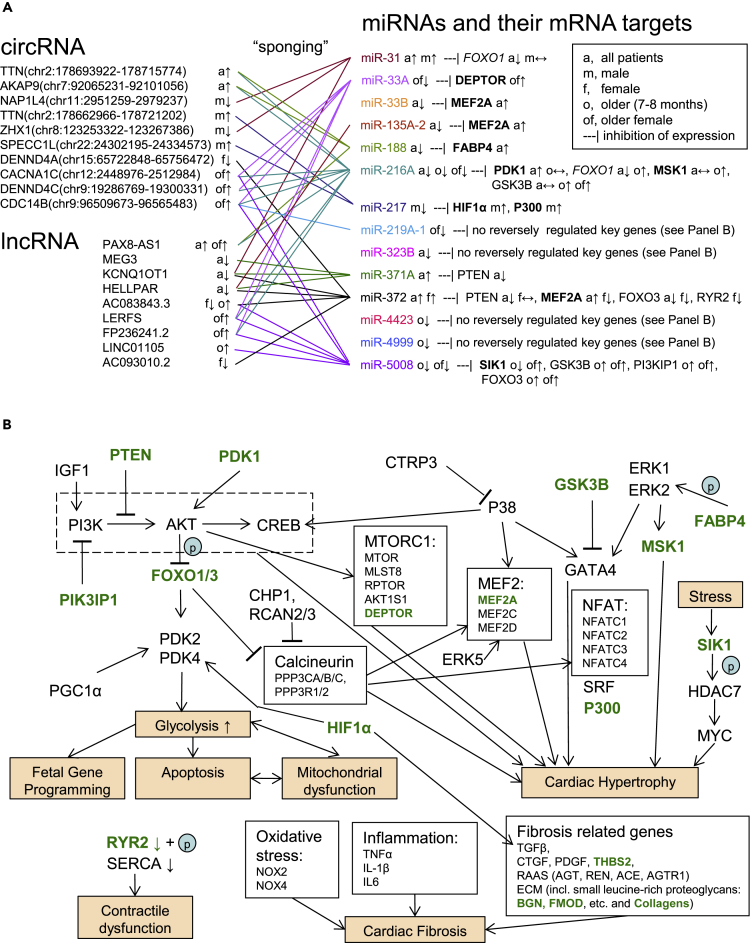
Combining the expression data with the miRNA gene target information (miRDB, TargetScan) and the circRNA/lncRNA target information obtained by the TargetScan algorithm (see methods), we were able to create a network of transcriptional regulation in the hypertrophied myocardium undergoing adaptation to high RV pressure load (Figures 2 and 3). Figure 2 shows the major regulatory effects of the differentially expressed miRNAs and their differentially expressed epigenetic regulators (circRNA and lncRNA sponges) on the genes known to mediate cardiac hypertrophy, fibrosis, cardiac dysfunction/decreased contractility, and other associated pathobiological events. We show some of these key genes if they were differentially expressed in our datasets (e.g., SIK1, FABP4, PIK3IP1), or could be tentatively called up- or downregulated based on their fold change, i.e., |Log2(Fold Change)| ≥ 0.14 (target genes in Figure 2A and genes shown in green in Figure 2B). Importantly, we observed that miRNAs with most biologically relevant key gene targets (mRNA transcripts) are also sponged by the highest number of non-coding RNAs, which reveals the significance of this level of epigenetic regulation: As evident from Figure 2A, miRNAs not targeting any known key genes are practically not regulated by lncRNAs or circRNAs. Additionally, we created regulatory networks showing DEGs (mRNA) regulated by the differentially expressed miRNAs in both the entire cohort (Figure 3A) and in older females (Figure 3B). Although the target genes in Figure 3 consist of a large number of less known genes associated with cardiac hypertrophy, their functional annotation shows strong involvement in the biological processes potentially associated with RVH (e.g., TGF-β regulation of ECM, focal adhesion, etc.). Additionally, we created the same type of interaction networks for female-, male-, and older subgroups (Figure S3). The “younger” group (2–4 months old) was not evaluated further, because no differentially expressed miRNAs were identified for this group.
Figure 2.
Epigenetic regulation of key genes involved in processes associated with cardiac hypertrophy by miRNA silencing of gene expression and the sponging effects of circular RNA and long non-coding RNA
Here, we only show predicted circRNA-miRNA-mRNA and lncRNA-miRNA-mRNA interactions, in which regulation changes direction, e.g., upregulated circRNA-downregulated miRNA-upregulated mRNA, thus suggesting involvement of the miRNA and the corresponding sponging non-coding RNA in the regulation of gene expression. The regulation direction is based on various TOF/PS versus Control comparisons (a = all patients with TOF/PS and VSD controls; m = male TOF/PS and VSD; f = female TOF/PS and VSD; o = older, i.e., 7–8 months old, TOF/PS and VSD; of = older female TOF/PS and VSD). The direction of regulation is indicated by the arrows: ↑ upregulation, ↓ downregulation, ↔ no change (|Log2(Fold Change)| < 0.14, i.e., 0.9 > Fold Change <1.1). All differentially expressed miRNAs are included in (A). Genes shown in (A) are in bold font if their expression is known to positively correlate with the effects of cardiac hypertrophy. Genes shown in italics have a double role and may be both pro- and anti-hypertrophic. Genes shown in green font in (B) are likely modulated by the circRNA/lncRNA-miRNA epigenetic control (based on our findings) as shown in (A). With the exception of miR-33B, miRNAs regulated by multiple sponging circRNA/lncRNA appear to exert more epigenetic regulation on key genes associated with cardiac hypertrophy, i.e., it appears that all available modes of epigenetic regulation are employed for quick transition to the hypertrophic cardiomyocyte state.
Figure 3.
Competing endogenous RNA regulates DEGs and their functions associated with cardiac hypertrophy
(A) Epigenetic regulation network based on the predicted interactions among differentially expressed molecules of the All group (based on all samples in the study [NTOF = 19, NCon = 8]), from which the age- and sex-specific genes were excluded. The age-specific genes were defined as found in both TOF_old versus TOF_young and Con_old versus. Con_young comparisons. The sex-specific genes were defined as found in both TOF_m versus TOF_f and Con_m versus Con_f comparisons. For space considerations only DEGs with relevant functions are presented. Genes shown with brown border may be affected by sex (were also found in TOF_m versus Con_m and/or TOF_f versus Con_f). Genes shown with green border may be affected by age (were also found in TOF_old versus Con_old and/or TOF_young versus Con_young). Genes shown with yellow border may be affected by both sex and age.
(B) The same type of an interaction network is shown for the older female group (NTOF = 2, NCon = 3). Nodes are colored blue if downregulated, and red if upregulated. LncRNAs are shown as hexagons, circRNAs are shown as octagons. Line thickness represents the miRNA target score (see methods). Functional annotation was obtained primarily from the NCATS BioPlanet (https://tripod.nih.gov/bioplanet/).
Differentially expressed lncRNAs have predicted DNA/mRNA/protein interactions, whereas no predicted interactions (circRNA-mRNA/protein) were found for the differentially expressed circRNAs
Non-coding RNA, such as lncRNAs, can affect (either promote or suppress) gene expression in cis (i.e., near the loci from which these lncRNAs are transcribed) via a variety of mechanisms (Gil and Ulitsky, 2020). Moreover, lncRNAs and circRNAs can directly interact with mRNAs (affecting their pre-mRNA splicing, RNA editing, mRNA stability control, translation activation, or prevention of miRNA-induced repression) (Szczesniak and Makalowska, 2016) and proteins (Lin et al., 2020). lncRNA/circRNA- miRNA interactions are the most straightforward to interpret, due to the expected transcript degradation or translational inhibition effect of miRNAs on their targets. However, for completeness, we also checked whether cataloged lncRNA-DNA/mRNA/protein interactions and circRNA-mRNA/protein interactions from the RNA Interactome database (RNAInter) (Lin et al., 2020) exist for the differentially expressed RNA in our study. We did not find such interactions with the circRNAs, but we found cataloged lncRNA-DNA/mRNA/protein interactions (see Data S2).
Co-expression networks in RVH (TOF/PS) compared with those in controls (VSD) identify gene clusters of RVH adaptation to high pressure load
To elucidate which of the overrepresented pathways (based on the proportion of DEGs) are truly involved in the biological processes specific to the corresponding phenotype of compensated RVH, we employed co-expression analysis; by doing so, we narrowed the list of these pathways based on the co-expression profiles of the participating genes. We performed co-expression analysis using the partial correlation coefficient with information theory (PCIT) approach (see methods for details). Subsequently we unraveled one VSD control co-expression cluster (with KCNMA1 as the “hub”) and four TOF/PS RVH clusters (with THBS3, FKBP10, BGN, and SALL3 as the “hubs”). Such gene clusters likely represent a biological process driven by directly or indirectly interacting molecules. Moreover, these clusters can be specific to a condition, e.g., TOF/PS, and span multiple pathways, thereby providing an additional level of functional relation annotation among the identified DEGs. Table S5 lists the pathways overrepresented by the DEGs from these five clusters. The pathways of the TOF/PS clusters largely recapitulate the findings of the differential expression analysis (see Data S1 and Table S5 for comparison). Additional pathways identified using this method included: (1) small leucine-rich proteoglycan (SLRP) molecules (BGN and FMOD); (2) cell adhesion molecules (CAMs; CLDN11, VCAN, NLGN2, ITGA8, NRXN2, NRCAM); (3) keratan sulfate/keratin metabolism (ACAN, CHST6, FMOD, CHST2); and (4) inflammatory response (COL1A2, FN1, THBS3).
Multiple molecules (mRNA) from the same co-expression cluster are likely targets of a single regulatory RNA (miRNA or lncRNA). Therefore, we also checked whether some differentially expressed miRNAs and lncRNAs target mRNA and/or DNA of multiple DEGs in each of the co-expression clusters. Interestingly, miR-371a, miR-372, and to a lesser degree miR-33 (all upregulated) likely downregulate several genes in the co-expression clusters (Table S5). lncRNAs BANCR and HELLPAR interact with a few mRNAs in the co-expression clusters (Table S5). lncRNAs MEG3 and RMRP have a large number of DNA interactions with DEGs of the co-expression clusters (Table S6).
Discussion
We conducted the first study of human RV cardiac hypertrophy by means of comprehensive RNA expression and RNA interaction network analysis, including predicted sponging of miRNA by circRNA and lncRNA. Previous human RVH RNA expression studies relied on postmortem control tissue to study mRNA expression (Bittel et al., 2011; O'Brien et al., 2012; Wang et al., 2014; Yang et al., 2013), expression of miRNA (Zhang et al., 2013), and expression of miRNA and small no-non coding RNA (Bittel et al., 2014; O'Brien et al., 2012; Wang et al., 2014). Therefore, the results of these studies on postmortem tissue cannot be used for direct comparison with our data that is based on intraoperatively harvested, freshly snap frozen RV tissue. Moreover, all the previous studies used microarray panels, further complicating possible comparison with our RNA sequencing results.
On the mRNA level, we report DEGs overrepresented in pathways that have been reported to be involved in cardiac hypertrophy, as further discussed in the supplemental information. Another important finding in our current study on compensated RVH (compensated RVH in TOF/PS; in the “All” group), is the possibly compensatory upregulation of FABP4, a fatty acid-binding protein known to positively regulate cardiac hypertrophy via activation of the ERK signaling pathway (Zhang et al., 2016). Consistently, we had previously demonstrated FABP4 downregulation in the RV of patients with end-stage PH with decompensated RVH and heart failure (Legchenko et al., 2018). We had also shown that the PPARγ agonist pioglitazone induces RV and cardiomyocyte FABP4 mRNA expression (Legchenko et al., 2018) that was associated with reversal of both RV dysfunction and severe PH in rats exposed to VEGFR2 blockade (SU5416) and hypoxia (Legchenko et al., 2018).
On the non-coding RNA level, we report for the first time miRNAs, lncRNAs, and circRNAs associated with human RVH (Tables S2–S4). To our knowledge, previous reports identified several miRNAs associated with either LVH in high pressure afterload models (Mohan et al., 2018; Rau et al., 2017; Wehbe et al., 2019), RVH in rodent pulmonary artery (PA) banding models (Andersen et al., 2020; Reddy and Bernstein, 2015; Reddy et al., 2012; Thum and Batkai, 2014), or human RVH with postmortem control samples (Bittel et al., 2014; O'Brien et al., 2012; Wang et al., 2014; Zhang et al., 2013). We did not find any of the miRNAs previously reported in cardiac hypertrophy of animals or humans, with the exception of miR-216a and miR-217, which were downregulated in human compensated RVH in our study (see the supplemental information for extended discussion).
lncRNAs are RNA molecules that can modulate gene expression via several mechanisms: signaling induced by transcription factors, miRNA sponging, recruiting chromatin-modifying enzymes, and molecular scaffolding resulting in histone modification (Wang and Chang, 2011). Within the scope of our study, we concentrated on the decoy function (miRNA sponging) of lncRNAs. Reportedly, Mhrt, Chast, CHRF, ROR, H19, Plscr4, and MIAT are associated with cardiac hypertrophy, and MALAT1, wisper, MEG3, and H19 are involved in ECM remodeling (reviewed in Zhou et al., 2019, and Liu and Tang, 2019). Additionally, Uca1 (Zhou et al., 2018) and Peg10 (Wen et al., 2019) have been reported as modulators of LVH via Hoxa9 in mouse TAC models, whereas Kcnq1ot1 was shown to sequester miR-30e-5p to release Adam9 thereby inducing cardiac hypertrophy in angiotensin II-stimulated hypertrophic cardiomyocyte cell culture (Wang et al., 2020). From the aforementioned list of lncRNAs compiled from the published literature on LVH, we found MEG3 and KCNQ1OT1 to be significantly downregulated in human compensated RVH in our study (further discussed in the supplemental information). Interestingly, a recent study used quantitative PCR of RV endomyocardial biopsies taken from patients with end-stage PH and postmortem or aortic valve stenosis controls, to show that the lncRNA H19 is upregulated in decompensated RVH (Omura et al., 2020). In RV pressure overload models (monocrotaline, PA banding), lncRNA H19 expression progressively increased (from the compensated to decompensated state), becoming upregulated in the decompensated but not in the compensated RV of rats with pressure overload (Omura et al., 2020). Our study subjects (infants with TOF/PS) had adaptive, compensated RVH without RV dilation and failure: The lncRNA H19 was slightly upregulated (fold change 1.17), but not differentially expressed (q-value 0.56) in the overall cohort. Thus, we speculate that lncRNA H19 in humans is progressively upregulated in the hypertrophied RV as it transitions from preserved volume and function, to dilation and heart failure.
To our knowledge, at present there are no published circRNA studies on human RVH. Comparison of our current results with published mouse LVH models is discussed in the supplemental information.
In our circ/lncRNA-miRNA-mRNA interaction network (Figure 2A), RVH-associated miRNAs targeting key cardiac hypertrophy genes appear to have the most sponging circ/lncRNA regulators/decoys. Clearly regulated key genes (Figure 2A) include PTEN, downregulated by combined action of miR-372 (with five ncRNA decoys) and miR-371A (with three ncRNA decoys); PDK1 and MSK1, upregulated by miR-216A (with eight ncRNA decoys); and GSK3B, PI3KP1, and FOXO3, upregulated by miR-5008 (with six ncRNA decoys). Such heavy reliance on epigenetic regulation indicates that the known pro-hypertrophic pathways enabled by these genes (PTEN, PDK1, GSK3B [Dorn and Force, 2005]; MSK1 [Markou et al., 2009]; PI3KIP1 [Song et al., 2015]; and FOXO3 [Ni et al., 2006]) are particularly important in human compensated RVH.
Gene co-expression analysis gave us another way to gain insight into key biological processes in human compensated RVH. An extended discussion on the uncovered pathways using this method is provided in the supplemental information. Interestingly, the miRNAs miR-371a and miR-372, described earlier, are also predicted to target multiple differentially expressed, RVH-related genes in the co-expression modules (Table S5). This discovery underlines the significance of these miRNAs (miR-371a and miR-372) as potential biomarkers and/or therapeutic targets for diseases associated with RV pressure load.
The strength of our study is the first comprehensive view of transcriptomic expression in human RVH in vivo. To the best of our knowledge, ours is the first study exploring lncRNA and circRNA expression, as well as miRNAs, in human RVH. This is particularly important because lncRNA and circRNA have poor species conservation, so that cell culture and animal studies are difficult to translate to human diseases.
Limitations of the study
Our study also has certain limitations. Owing to the large volume of data, the regulatory mechanisms that we present are based largely on in silico predictions—their validation will need to be conducted in subsequent research. The reported regulatory network presented in Figure 2A was not validated by qPCR of cDNA derived from cardiac tissue or cardiomyocyte cell culture studies. Thus we cannot completely ascertain to which degree the presented miRNAs are involved in regulation of the target genes and whether or not other mechanisms may also play a role in regulation of the key genes shown. Because cardiac surgery on the patients with TOF/PS (compensated RVH) and VSD (control) had to be performed within the first year of life, our findings may not fully reflect RVH in older patients. However, we did exclude neonates (first postnatal month) as they undergo postnatal adaption of pressure and flow in their circulation, and performed subgroup analyses by age and sex. Finding non-surgical human, freshly isolated RVH and non-RVH samples for a representative in vivo study is difficult (ethics, consent), but has been pursued in adults with scleroderma-associated or idiopathic PH, via RV septal endomyocardial biopsies (Hsu et al., 2018). Recently, a specific RNA signature of tissue fibrosis and enrichment of beneficial metabolic regulators (AMPK, PPARs) was identified in animal models of organ fibrosis, i.e., kidney, liver, and lung (Zhang et al., 2020) and the failing RV in severe PH (Legchenko et al., 2018). Of note, it has been postulated that mild to moderate fibrosis prevents dilation (cardiomyocyte overstretch) of the hypertrophied ventricles (Andersen et al., 2019). In contrast, we found only a moderate induction of a pro-fibrosis mRNA expression signature in the hypertrophied RV of patients with TOF/PS (downregulation of BGN and FMOD), probably because of the high adaptivity and plasticity of the infant myocardium, and the rather short duration of high pressure afterload.
Taken together, we provide comprehensive RNA expression profiles, signaling characteristics, and network analysis of human RVH due to high pressure afterload (compensated RVH, no RV failure). The miRNAs reported here are at the core of this transcriptomic regulation in compensated (adaptive) RVH and, as such, are potential targets of heart failure therapies and/or biomarkers of disease severity/progression. Validation of the predicted competing endogenous RNA interactions (sponging of miRNA by circRNA and lncRNA) and further investigation of the regulatory mechanisms should be a focus of future research.
Resource availability
Lead contact
Georg Hansmann, Department of Pediatric Cardiology and Critical Care, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany. E-mail: georg.hansmann@gmail.com.
Materials availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Georg Hansmann (georg.hansmann@gmail.com), according to the material transfer agreement (MTA). This study did not generate new unique reagents.
Data and code availability
This study did not generate any software, except for Perl/bash scripts for subtracting circRNA read counts from the total RNA read count data, which are deposited to https://github.com/pch-code/circ-scripts. Sequencing data are not publicly available due to consent restrictions. Upon request, the data can be made available via controlled access (National Register for Congenital Heart Defects, Berlin, Germany).
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This study was supported by the German Research Foundation (DFG; HA4348/6-2 KFO311 to G.H.). Dr. Hansmann receives additional funding from the German Research Foundation (DFG; HA4348/2-2), the Federal Ministry of Education and Research (BMBF ViP + program 03VP08053; BMBF 01KC2001B), and the European Pediatric Pulmonary Vascular Disease Network (www.pvdnetwork.org). The Competence Network for Congenital Heart Defects and the National Register for Congenital Heart Defects have received funding from the Federal Ministry of Education and Research, grant number 01GI0601 (until 2014), and the DZHK (German Center for Cardiovascular Research; as of 2015).
Author contributions
P.C. performed the data analysis and wrote the manuscript. J.P., R.C., J.S., U.M.M.B., T.P., H-H.K., S.D., and F.B. contributed to the acquisition of tissue samples and clinical data. G.H. generated the hypotheses, developed the experimental design and concept of the study, performed RNA extraction and bioanalysis, obtained funding, and wrote the manuscript. All authors critically read and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102232.
Supplemental information
Fisher exact test (FDR<0.05).
The interactions were obtained from the RNA Interactome database (https://www.rna-society.org/rnainter/home.html). The score incorporates experimental evidence and computational prediction.
References
- Agrawal V., Lahm T., Hansmann G., Hemnes A.R. Molecular mechanisms of right ventricular dysfunction in pulmonary arterial hypertension: focus on the coronary vasculature, sex hormones, and glucose/lipid metabolism. Cardiovasc. Diagn. Ther. 2020;10:1522–1540. doi: 10.21037/cdt-20-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A., van der Feen D.E., Andersen S., Schultz J.G., Hansmann G., Bogaard H.J. Animal models of right heart failure. Cardiovasc. Diagn. Ther. 2020;10:1561–1579. doi: 10.21037/cdt-20-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S., Nielsen-Kudsk J.E., Vonk Noordegraaf A., de Man F.S. Right ventricular fibrosis. Circulation. 2019;139:269–285. doi: 10.1161/CIRCULATIONAHA.118.035326. [DOI] [PubMed] [Google Scholar]
- Bernardo R.J., Haddad F., Couture E.J., Hansmann G., de Jesus Perez V.A., Denault A.Y., de Man F.S., Amsallem M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc. Diagn. Ther. 2020;10:1580–1603. doi: 10.21037/cdt-20-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel D.C., Butler M.G., Kibiryeva N., Marshall J.A., Chen J., Lofland G.K., O'Brien J.E., Jr. Gene expression in cardiac tissues from infants with idiopathic conotruncal defects. BMC Med. Genomics. 2011;4:1. doi: 10.1186/1755-8794-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel D.C., Kibiryeva N., Marshall J.A., O'Brien J.E. MicroRNA-421 dysregulation is associated with tetralogy of fallot. Cells. 2014;3:713–723. doi: 10.3390/cells3030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouvarine P., Geldner J., Giagnorio R., Legchenko E., Bertram H., Hansmann G. Trans-right-ventricle and transpulmonary microRNA gradients in human pulmonary arterial hypertension. Pediatr. Crit. Care Med. 2020;21:340–349. doi: 10.1097/PCC.0000000000002207. [DOI] [PubMed] [Google Scholar]
- Dorn G.W., 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi T.A., Juranek S.A., Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–1214. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Ruehle F., Stoll M. Evolutionary patterns of non-coding rna in cardiovascular biology. Noncoding RNA. 2019;5(1):15. doi: 10.3390/ncrna5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil N., Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 2020;21:102–117. doi: 10.1038/s41576-019-0184-5. [DOI] [PubMed] [Google Scholar]
- Hansmann G. Pulmonary hypertension in infants, children, and young adults. J. Am. Coll. Cardiol. 2017;69:2551–2569. doi: 10.1016/j.jacc.2017.03.575. [DOI] [PubMed] [Google Scholar]
- Hansmann G., Koestenberger M., Alastalo T.P., Apitz C., Austin E.D., Bonnet D., Budts W., D'Alto M., Gatzoulis M.A., Hasan B.S. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019;38:879–901. doi: 10.1016/j.healun.2019.06.022. [DOI] [PubMed] [Google Scholar]
- Hsu S., Kokkonen-Simon K.M., Kirk J.A., Kolb T.M., Damico R.L., Mathai S.C., Mukherjee M., Shah A.A., Wigley F.M., Margulies K.B. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137:2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobi T., Siede D., Eschenbach J., Heumuller A.W., Busch M., Nietsch R., Meder B., Most P., Dimmeler S., Backs J. Deep Characterization of circular RNAs from human cardiovascular cell models and cardiac tissue. Cells. 2020;9(7):1616. doi: 10.3390/cells9071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legchenko E., Chouvarine P., Borchert P., Fernandez-Gonzalez A., Snay E., Meier M., Maegel L., Mitsialis S.A., Rog-Zielinska E.A., Kourembanas S. PPARgamma agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018;10:eaao0303. doi: 10.1126/scitranslmed.aao0303. [DOI] [PubMed] [Google Scholar]
- Lin Y., Liu T., Cui T., Wang Z., Zhang Y., Tan P., Huang Y., Yu J., Wang D. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 2020;48:D189–D197. doi: 10.1093/nar/gkz804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.F., Tang W.H.W. Epigenetics in cardiac hypertrophy and heart failure. JACC Basic Transl. Sci. 2019;4:976–993. doi: 10.1016/j.jacbts.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou T., Cieslak D., Gaitanaki C., Lazou A. Differential roles of MAPKs and MSK1 signalling pathways in the regulation of c-Jun during phenylephrine-induced cardiac myocyte hypertrophy. Mol. Cell Biochem. 2009;322:103–112. doi: 10.1007/s11010-008-9945-8. [DOI] [PubMed] [Google Scholar]
- Mohan N., Kumar V., Kandala D.T., Kartha C.C., Laishram R.S. A splicing-Independent function of RBM10 controls specific 3' UTR processing to regulate cardiac hypertrophy. Cell Rep. 2018;24:3539–3553. doi: 10.1016/j.celrep.2018.08.077. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- Ni Y.G., Berenji K., Wang N., Oh M., Sachan N., Dey A., Cheng J., Lu G., Morris D.J., Castrillon D.H. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J.E., Kibiryeva N., Zhou X.G., Marshall J.A., Lofland G.K., Artman M., Chen J., Bittel D.C. Noncoding RNA expression in myocardium from infants with tetralogy of fallot. Circ. Cardiovasc. Genet. 2012;5:279–286. doi: 10.1161/CIRCGENETICS.111.961474. [DOI] [PubMed] [Google Scholar]
- Omura J., Habbout K., Shimauchi T., Wu W.H., Breuils-Bonnet S., Tremblay E., Martineau S., Nadeau V., Gagnon K., Mazoyer F. Identification of long noncoding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020;142:1464–1484. doi: 10.1161/CIRCULATIONAHA.120.047626. [DOI] [PubMed] [Google Scholar]
- Rau C.D., Romay M.C., Tuteryan M., Wang J.J., Santolini M., Ren S., Karma A., Weiss J.N., Wang Y., Lusis A.J. Systems genetics approach identifies gene pathways and Adamts2 as drivers of isoproterenol-induced cardiac hypertrophy and cardiomyopathy in mice. Cell Syst. 2017;4:121–128.e4. doi: 10.1016/j.cels.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S., Bernstein D. Molecular mechanisms of right ventricular failure. Circulation. 2015;132:1734–1742. doi: 10.1161/CIRCULATIONAHA.114.012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S., Zhao M., Hu D.Q., Fajardo G., Hu S., Ghosh Z., Rajagopalan V., Wu J.C., Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol. Genomics. 2012;44:562–575. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santens B., Van De Bruaene A., De Meester P., D'Alto M., Reddy S., Bernstein D., Koestenberger M., Hansmann G., Budts W. Diagnosis and treatment of right ventricular dysfunction in congenital heart disease. Cardiovasc. Diagn. Ther. 2020;10:1625–1645. doi: 10.21037/cdt-20-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.K., Kim J., Lee J.S., Nho K.J., Jeong H.C., Kim J., Ahn Y., Park W.J., Kim D.H. Pik3ip1 modulates cardiac hypertrophy by inhibiting PI3K pathway. PLoS One. 2015;10:e0122251. doi: 10.1371/journal.pone.0122251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesniak M.W., Makalowska I. lncRNA-RNA interactions across the human transcriptome. PLoS One. 2016;11:e0150353. doi: 10.1371/journal.pone.0150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T., Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series) Pulm. Circ. 2014;4:185–190. doi: 10.1086/675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen C.E.E., Tedford R.J., Handoko M.L., van der Velden J., de Man F.S. RV pressure overload: from hypertrophy to failure. Cardiovasc. Res. 2017;113:1423–1432. doi: 10.1093/cvr/cvx145. [DOI] [PubMed] [Google Scholar]
- Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wu C., Ren L., Bao Y., Han Y., Li C., Li Y. MiR-30e-5p is sponged by Kcnq1ot1 and represses Angiotensin II-induced hypertrophic phenotypes in cardiomyocytes by targeting ADAM9. Exp. Cell Res. 2020;394:112140. doi: 10.1016/j.yexcr.2020.112140. [DOI] [PubMed] [Google Scholar]
- Wang X.M., Zhang K., Li Y., Shi K., Liu Y.L., Yang Y.F., Fang Y., Mao M. Screening miRNA and their target genes related to tetralogy of Fallot with microarray. Cardiol. Young. 2014;24:442–446. doi: 10.1017/S104795111300053X. [DOI] [PubMed] [Google Scholar]
- Wehbe N., Nasser S.A., Pintus G., Badran A., Eid A.H., Baydoun E. MicroRNAs in cardiac hypertrophy. Int. J. Mol. Sci. 2019;20(19):4714. doi: 10.3390/ijms20194714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z.Q., Li S.H., Shui X., Tang L.L., Zheng J.R., Chen L. LncRNA PEG10 aggravates cardiac hypertrophy through regulating HOXA9. Eur. Rev. Med. Pharmacol. Sci. 2019;23:281–286. doi: 10.26355/eurrev_201908_18658. [DOI] [PubMed] [Google Scholar]
- Yang D., Li J., Yuan Z. Gene expression analysis in cardiac tissues from infants identifies candidate agents for Tetralogy of Fallot. Pediatr. Cardiol. 2013;34:1637–1644. doi: 10.1007/s00246-013-0689-1. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chang J.J., Xu F., Ma X.J., Wu Y., Li W.C., Wang H.J., Huang G.Y., Ma D. MicroRNA deregulation in right ventricular outflow tract myocardium in nonsyndromic tetralogy of fallot. Can J. Cardiol. 2013;29:1695–1703. doi: 10.1016/j.cjca.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang J., Muise E., Han S., Kutchukian P., Costet P., Zhu Y., Kan Y., Zhou H., Shah V., Huang Y. Molecular profiling reveals a common metabolic signature of tissue fibrosis. Cell Rep. Med. 2020;1:100056. doi: 10.1016/j.xcrm.2020.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Qiao C., Chang L., Guo Y., Fan Y., Villacorta L., Chen Y.E., Zhang J. Cardiomyocyte overexpression of FABP4 aggravates pressure overload-induced heart hypertrophy. PLoS One. 2016;11:e0157372. doi: 10.1371/journal.pone.0157372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Li C., Feng J., Zhang J., Fang Y. lncRNA UCA1 is a novel regulator in cardiomyocyte hypertrophy through targeting the miR-184/HOXA9 axis. Cardiorenal. Med. 2018;8:130–139. doi: 10.1159/000487204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wang B., Yang Y.X., Jia Q.J., Zhang A., Qi Z.W., Zhang J.P. Long noncoding RNAs in pathological cardiac remodeling: a review of the update literature. Biomed. Res. Int. 2019;2019:7159592. doi: 10.1155/2019/7159592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fisher exact test (FDR<0.05).
The interactions were obtained from the RNA Interactome database (https://www.rna-society.org/rnainter/home.html). The score incorporates experimental evidence and computational prediction.
Data Availability Statement
This study did not generate any software, except for Perl/bash scripts for subtracting circRNA read counts from the total RNA read count data, which are deposited to https://github.com/pch-code/circ-scripts. Sequencing data are not publicly available due to consent restrictions. Upon request, the data can be made available via controlled access (National Register for Congenital Heart Defects, Berlin, Germany).