Abstract
Background:
Phthalates are endocrine-disrupting chemicals that are widely present in consumer products. In the United States, Black women are more highly exposed to phthalates than other racial/ethnic groups, yet information on predictors of phthalate exposure among Black women is limited.
Objective:
We evaluated the association of demographics, lifestyle, reproductive history, and personal care product use with urinary concentrations of phthalate and phthalate alternative metabolites, using cross-sectional data from 754 Black women from Detroit, Michigan (2010–2012).
Methods:
Women completed questionnaires and provided urine specimens which were analyzed for 16 phthalate and phthalate alternative metabolites. We used linear regression models to estimate mean percentage differences and 95% confidence intervals (CIs) in concentrations across levels of correlates.
Results:
Monoethyl (MEP) and mono-n-butyl phthalate concentrations were positively associated with personal care product use, particularly nail products. Educational attainment was positively associated with high molecular weight phthalate concentrations but inversely associated with monobenzyl phthalate (MBzP) concentrations. Parity was positively associated with MBzP concentrations and inversely associated with concentrations of MEP and high molecular weight phthalates.
Significance:
We found that sociodemographics, reproductive characteristics, and use of certain personal care products were associated with urinary phthalate concentrations among Black women. Our results emphasize the importance of examining exposure determinants among multiply-marginalized populations.
Keywords: Black women, DINCH, phthalates, reproductive-aged
INTRODUCTION
Phthalates are ubiquitous endocrine-disrupting chemicals used as solvents and to increase the flexibility of plastics.1 They are present in a wide variety of consumer products. Low molecular weight phthalates such as diethyl phthalate (DEP) are found in personal care products (e.g., as a plasticizer in nail polishes to reduce chipping and as a solvent and fixative in fragrances).2 High molecular weight phthalates such as di-2-ethylhexyl phthalate (DEHP) help increase the flexibility of polyvinyl chloride plastics, and are found in medical supplies, vinyl flooring, and building materials.2 Phthalates are not chemically bound to the plastics matrix and can easily leach into the environment. Routes of human exposure to phthalates include inhalation, ingestion, and dermal uptake.3 In 2002, the non-phthalate plasticizer 1,2-cyclohexane dicarboxylic acid-diisononyl ester (DINCH) was introduced to the market as a purportedly safer alternative to phthalates.4 DINCH is used as replacement chemical primarily for DEHP and diisononyl phthalate (DINP), and is found in children’s toys and food packaging.5
Phthalates have half-lives on the order of hours; therefore, measures of phthalate metabolites in urine represent recent exposures. Over 75% of the United States population has detectable concentrations of phthalate metabolites in their urine,6 suggesting that exposure to the parent compounds is highly prevalent. In addition, data from the United States and Germany indicate that urinary DINCH concentrations are increasing in industrialized nations.5, 7 Phthalate exposure varies by demographic characteristics: females tend to have higher concentrations than males,6 and Black individuals tend to have higher concentrations than White individuals.6, 8, 9 Comparisons with other racial/ethnic groups are challenging given the dearth of data on exposure to phthalates. Differential patterns of personal care product use across racial groups,10, 11 due largely to discrimination based on skin tone12–14 and hair texture;15–17 persistence of European beauty norms; misinformation about the risks and benefits of use of particular products (e.g., feminine douching products);18 and targeted advertising of specific beauty products to Black women (including hair relaxers, skin lightening cream, and feminine hygiene products),19–21 have likely contributed to racial disparities in phthalate exposure.22–24 These patterns of use may differ by socioeconomic status.25 This is particularly problematic given that women of color and poor women have both higher cumulative exposure to chemical hazards and heightened vulnerability to the health effects of both chemical and non-chemical exposures,26, 27 which has implications for racial and socioeconomic health disparities.28 For example, phthalate exposure has been associated with several health outcomes that disproportionately affect Black women, including elevated body mass index (BMI),29–31 diabetes,32 adverse birth outcomes,33 endometriosis,34 uterine leiomyomata (UL),35, 36 and breast cancer.37 Likewise, although health effects of DINCH are largely unstudied, urinary DINCH metabolite concentrations have been related to poorer markers of ovarian response among a population of predominantly White women undergoing fertility treatment at a Massachusetts hospital38 and increased inflammation in racially-diverse cohort of pregnant women recruited from four academic medical centers across the United States.39 These results have implications for racial disparities in health outcomes.
Although higher urinary phthalate metabolite concentrations have been associated with specific components of the diet (e.g., higher poultry intake),40, 41 more frequent personal care product use,40, 42 recent use of medications with a sustained-release coating,43, 44 and higher BMI,45, 46 correlates of phthalate exposure among Black women are understudied, despite evidence of racial/ethnic disparities in exposure6, 8, 9 and the associated potential adverse health effects.29–37 There has been limited study of correlates of exposure to DINCH. To address these knowledge gaps, we examined demographic, lifestyle, reproductive, and personal care product correlates of urinary phthalate and DINCH metabolite concentrations in a cohort of 754 reproductive-aged Black women.
MATERIALS AND METHODS
Study design.
The Study of Environment, Lifestyle, and Fibroids (SELF) is a prospective cohort designed to identify risk factors for UL. The study design has been described in detail elsewhere.47 Briefly, during 2010–2012, we enrolled women who were aged 23–35 years, self-identified as Black or African American, and resided in the greater Detroit, Michigan metropolitan area. Recruitment took place initially through the Henry Ford Health System, a large integrated healthcare system serving a diverse patient population in the Detroit area. Additional recruitment used a coordinated media strategy across the metro area, including a website, fliers, brochures at healthcare clinics, local advertising, and information booths at community events. Exclusion criteria included a history of diagnosed UL, cancer, or autoimmune disease. At baseline, participants completed a series of computer- and telephone-assisted questionnaires, a validated food frequency questionnaire, and a study visit at the Henry Ford Health System in Detroit. At the study visit, women underwent transvaginal ultrasound for detection of UL and standardized measurements of weight, height, and blood pressure. They brought in their first-morning urine samples (except for 2.3% who provided urine at the clinic), and blood samples were collected. Women attended follow-up visits using similar procedures every 20 months over a five year period. The study was approved by the Institutional Review Boards at the Henry Ford Health System, the National Institute of Environmental Health Sciences (NIEHS), and Boston University Medical Campus. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects’ research. All participants provided written informed consent.
Within the SELF cohort, urine samples for quantification of phthalate and DINCH metabolites were selected using a case-cohort study design, with the overall goal of examining the association between endocrine-disrupting chemicals and UL incidence. A random subset of 592 women free of UL at baseline and all incident cases of UL detected over the five year follow-up period were selected for analysis of urinary phthalate and DINCH metabolite concentrations (Supplemental Figure 1). We quantified phthalate and DINCH metabolites in urine samples from baseline through the 40-month follow-up for non-cases and from baseline through the follow-up before UL detection for cases. In the present work, we examine the distribution of urinary phthalate and DINCH metabolite concentrations at baseline and during follow-up among the case-cohort subset (n=754 baseline samples, 554 20-month samples, and 425 40-month samples). We additionally investigated factors potentially correlated with baseline urinary concentrations of metabolites. The case-cohort participants did not differ substantially from the overall cohort with respect to key baseline characteristics.48
Assessment of metabolites of phthalates and phthalate alternatives.
Urine samples collected at baseline, 20 months, and 40 months were stored at −80 degrees Celsius at the NIEHS biorepository, then shipped on dry ice to the CDC where they were analyzed for 14 phthalate and two DINCH metabolites using a modification of previously-described online solid-phase extraction coupled with isotope dilution high-performance liquid chromatography tandem mass spectrometry method.49 Specific metabolites measured included: mono-n-butyl phthalate (MBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-hydroxyisobutyl phthalate (MHiBP), monoethyl phthalate (MEP), monobenzyl phthalate (MBzP), mono-carboxyisononyl phthalate (MCNP), mono-isononyl phthalate (MNP), mono-carboxyisooctyl phthalate (MCOP), mono-3-carboxypropyl phthalate (MCPP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), 1,2-cyclohexane dicarboxylic acid-monohydroxy isononyl ester (MHiNCH), and 1,2-cyclohexane dicarboxylic acid-monocarboxy isooctyl ester (MCOCH). Analytic measurements were performed following strict quality control guidelines, including analysis of proficiency testing samples. Each analytic run included high- and low-concentration quality control materials and reagent blanks. Blind duplicates were incorporated both within and across batches. Inter-batch precision was high, with coefficients of variation ranging from 1.8–13.2%, depending on the metabolite and concentration. Urinary creatinine was measured at the NIEHS using a Beckman Coulter clinical analyzer AU400e.
Assessment of correlates.
We measured potential correlates of urinary concentrations of phthalates and phthalate alternatives from data collected by self-report and by research staff at the clinic visit. Height and weight were measured by a trained technician and used to calculate BMI (kg/m2). We assessed socio-demographic characteristics (age, educational attainment, annual household income), lifestyle factors (cigarette smoking, alcohol intake), reproductive history (parity, contraceptive use [including current use of oral contraceptive pills, hormonal and non-hormonal intrauterine devices (IUD), patches, implants, vaginal ring, and shots]), and personal care product use in the previous 24 hours (exposure of the genital area to feminine hygiene powder, vaginal douches, feminine hygiene deodorant, or condoms; and use of perfume/cologne, creams/lotions before bed, makeup, solvents, hairspray/gel/oil/mousse, chemical hair treatments, and nail polish). For personal care product use in the past 24 hours, we created a “total number of products” variable by summing the number of products used in the past 24 hours (range: 0–7).
Statistical analysis.
For urine samples with metabolite concentrations below the limit of detection (LOD), we used reported instrument values provided by the CDC. We adjusted for urinary dilution by dividing all urinary metabolite concentrations by urinary creatinine concentrations, and report all metabolite concentrations on a creatinine-adjusted basis (μg/g creatinine). We present the median and 90th percentile of creatinine-adjusted concentrations for each metabolite at baseline, 20 months, and 40 months and compared with concentrations from non-Hispanic Black females aged 23–35 years from the 2011–2012 National Health and Nutrition Examination Survey (NHANES) cycle.50 We calculated intraclass correlation coefficients (ICC) and 95% confidence intervals (CIs) to examine within-person variability in metabolite concentrations over time. We used Spearman correlation coefficients to measure correlations between individual metabolite concentrations. In addition to examining individual metabolites, we calculated molar sums of DINP and DEHP by dividing each metabolite concentration by its molecular weight and summing across metabolites.
We used multiple imputation to account for missing data. Missingness was infrequent in the data set: we imputed income for 7 women, personal care product use in the past 24 hours for 1 woman, and concentrations of MHBP, MHiBP, MNP, and MCOCH for 20 women whose urine was analyzed in earlier batches before the CDC included these metabolites in the method panel. We generated five imputed data sets and statistically combined the estimates across data sets for the analysis using Rubin’s rule.51
We used linear regression models to estimate associations between each potential correlate and individual and summed phthalate metabolite concentrations. We calculated the mean percentage differences and 95% confidence intervals (CIs) by regressing log-transformed creatinine-adjusted metabolite concentrations on categorical or continuous (when appropriate) correlates, and transforming the exponentiated regression coefficients into percentage differences (percentage difference=100*(eβ−1)). We ran multivariable models adjusted for all other correlates. For DINCH metabolites where detection frequencies were relatively low, we used log-binomial regression to estimate prevalence ratios (PRs) for having a detectable vs. non-detectable concentration for each metabolite. We ran regression models for all phthalate and phthalate alternative metabolites unadjusted, adjusted for age and education only, and adjusted for all other potential correlates.
To assess the influence of including incident UL cases who were not part of the baseline random subcohort (n=162) on the results, we conducted an additional analysis restricted to the 592 women who were randomly selected at baseline.
RESULTS
At baseline, the 754 women included in this analysis (Table 1) had a mean age of 28.6 years (standard deviation [SD]=3.5). Twenty-one percent had 12 or fewer years of education (≤high school/GED) and 46% had an annual household income of less than $20,000, which is below the U.S. federal poverty level for families of three or more52 and below the median household income in Detroit ($29,000).53 Nineteen percent of participants smoked cigarettes and 17% drank seven or more alcoholic drinks per week. The mean BMI in the cohort was 33.7 kg/m2 (SD=9.6) and most women were parous (62%). Of the 232 women using non-barrier contraceptive methods at baseline, 37% used oral contraceptive pills, 20% used depo medroxyprogesterone acetate (DMPA) injections, 5% used the vaginal ring, 36% used any IUD, and 3% used a hormonal patch or implant. The proportions of women with genital area exposure to products in the past 24 hours were 13% for genital powder, 2% for vaginal douches, 10% for feminine hygiene deodorant, and 5% for condoms. The personal care products used by the largest proportion of women in the past 24 hours were makeup (74%) and perfume (65%). More than one-third of women also reported use of creams or lotions before bed (34%) and hair products (hairspray, gel, oil, or mousse; 33%) in the past 24 hours. Chemical hair treatment in the past 24 hours was rare (2%).
Table 1.
Distribution of selected correlates at baseline among SELF study participants (n=754)
| Correlate | N (%) |
|---|---|
| Age at enrollment (years) | |
| 23–25 | 184 (24.4) |
| 26–28 | 187 (24.8) |
| 29–31 | 200 (26.5) |
| 32–35 | 183 (24.3) |
| Educational attainment | |
| <High school | 47 (6.2) |
| High school/GED | 117 (15.5) |
| Some college/Associate’s/Technical degree | 381 (50.5) |
| Bachelor’s degree | 147 (19.5) |
| Advanced degree | 62 (8.2) |
| Annual household income | |
| <$20,000 | 342 (45.4) |
| $20,000–$50,000 | 284 (37.7) |
| >$50,000 | 128 (17.0) |
| Body mass index (kg/m2) | |
| <25 | 142 (18.8) |
| 25–29 | 157 (20.8) |
| 30–34 | 151 (20.0) |
| 35–39 | 127 (16.8) |
| ≥40 | 177 (23.5) |
| Cigarette smoking | |
| Never | 553 (73.3) |
| Former | 58 (7.7) |
| Current | 143 (19.0) |
| Alcohol intake (drinks/week) | |
| 0 | 204 (27.1) |
| 1–6 | 421 (55.8) |
| 7–13 | 69 (9.2) |
| ≥14 | 60 (8.0) |
| Parity | |
| Nulliparous | 285 (37.8) |
| 1 birth | 205 (27.2) |
| 2 births | 129 (17.1) |
| ≥3 births | 135 (17.9) |
| Current contraception use | |
| Oral contraceptive pills | 86 (11.4) |
| Intrauterine device (hormonal + non-hormonal) | 82 (10.9) |
| Depo medroxyprogesterone acetate | 47 (6.2) |
| Vaginal ring | 12 (1.6) |
| Hormonal implant | 3 (0.4) |
| Hormonal patch | 2 (0.3) |
| Exposures to genital area in past 24 hours | |
| Powder | 97 (12.9) |
| Douching | 18 (2.4) |
| Feminine hygiene deodorant | 75 (10.0) |
| Condoms | 36 (4.8) |
| Hair product use in past 24 hours | |
| Hairspray, gel, oil, or mousse | 252 (33.4) |
| Chemical hair treatments | 17 (2.3) |
| Nail product use in past 24 hours | |
| Nail polish | 79 (10.5) |
| Solvents | 79 (10.5) |
| Other personal care product use in past 24 hours | |
| Perfume or cologne | 489 (64.9) |
| Creams or lotions | 260 (34.5) |
| Makeup | 557 (73.9) |
GED=General Educational Development; SELF=Study of the Environment, Lifestyle and Fibroids
All phthalate metabolites were detected among more than 90% of SELF participants, with the exception of MNP, which was detected in 71.3% of participants (Table 2). Concentrations were generally higher among SELF participants than similarly-aged non-Hispanic Black females from NHANES. For example, median MBP concentrations were 16.0 μg/g creatinine among SELF participants and 9.5 μg/g creatinine among non-Hispanic Black females from NHANES. MEP concentrations were lower in SELF than in NHANES (59.4 vs. 79.7 μg/g creatinine). DINCH metabolites were detected among relatively few SELF participants (24.3% for MHiNCH and 9.0% for MCOCH), consistent with NHANES data. Phthalate metabolite concentrations generally declined over follow-up; on the other hand, DINCH metabolite concentrations increased over follow-up (Supplemental Table 1). As evidenced by the ICCs (ranging from 0.07 to 0.47), reproducibility across the three follow-up points was low for all individual metabolites (Supplemental Table 1).
Table 2.
Distribution of urinary concentrations of metabolites of phthalates and phthalate alternatives (μg/g creatinine), Study of Environment, Lifestyle and Fibroids, and National Health and Nutrition Examination Survey.
| Metabolite | Parent compound | Sources | SELF (2010–2012) N=754 | NHANES (2011–2012) N=38 | ||||
|---|---|---|---|---|---|---|---|---|
| LODC | % detected | Median | 90th p-tile | Non-Hispanic Black Females Age 23–35 Years | ||||
| Median | 90th p-tile | |||||||
| Mono-n-butyl phthalate (MBP) | Di-n-butyl phthalate (DBP) | Cosmetics, fragrances, construction | 0.4 | 99.9 | 16.0 | 38.7 | 9.5 | 32.4 |
| Mono-hydroxybutyl phthalate (MHBPa) | 0.4 | 93.6 | 1.2 | 2.8 | 0.7 | 1.5 | ||
| Mono-isobutyl phthalate (MiBP) | Diisobutyl phthalate (DiBP) | Cosmetics, fragrances, construction | 0.8 | 99.3 | 11.2 | 26.7 | 6.9 | 18.9 |
| Mono-hydroxyisobutyl phthalate (MHiBPa) | 0.4 | 99.2 | 3.0 | 7.8 | 3.4 | 11.0 | ||
| Monoethyl phthalate (MEP) | Diethyl phthalate (DEP) | Fragrances | 1.2 | 100.0 | 59.4 | 251.6 | 79.7 | 402.2 |
| Monobenzyl phthalate (MBzP) | Benzylbutyl phthalate (BzBP) | Flooring, construction, clothing, household | 0.3 | 99.7 | 6.5 | 20.7 | 5.0 | 23.3 |
| Mono-carboxyisononyl phthalate (MCNP) | Diisodecyl phthalate (DIDP) | Flooring, construction, clothing, household, wire/cable | 0.2 | 99.7 | 2.8 | 8.6 | 1.9 | 5.7 |
| Mono-isononyl phthalate (MNP) | Diisononyl phthalate (DINP) | Flooring, automotive trim, construction, clothing, household, wire/cable coatings | 0.9 | 71.3 | 1.1 | 9.3 | 1.0 | 6.0 |
| Mono-carboxyisooctyl phthalate (MCOP) | 0.3 | 100.0 | 19.9 | 116.8 | 12.7 | 71.5 | ||
| Mono-3-carboxypropyl phthalate (MCPP) | Dioctyl phthalate (DOP), DBP | Flooring, vinyl gloves, wire and cable insulation, adhesives; cosmetics, fragrances | 0.4 | 98.0 | 2.2 | 17.3 | 1.8 | 15.4 |
| Mono(2-ethyl-5-carboxypentyl phthalate (MECPP) | Di(2-ethylhexyl) phthalate (DEHP) | Vinyl tile, automotive trim, paint, medical uses, clothing, garden hoses, wire/cable coatings | 0.4 | 99.9 | 15.1 | 41.2 | 11.7 | 51.8 |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | 0.4 | 99.7 | 11.8 | 31.2 | 8.0 | 36.4 | ||
| Mono(2-ethylhexyl) phthalate (MEHP) | 0.8 | 91.5 | 2.3 | 7.4 | 1.2 | 7.5 | ||
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 0.2 | 99.7 | 7.3 | 20.2 | 5.4 | 18.8 | ||
| 1,2-Cyclohexane dicarboxylic acid-monohydroxy isononyl ester (MHiNCH) | 1,2-Cyclohexane dicarboxylic acid diisononyl ester (DINCH) | Replacement for DEHP and DINP | 0.4 | 24.3 | 0.2 | 0.6 | 0.2 | 0.8 |
| 1,2-Cyclohexane dicarboxylic acid-monocarboxy isooctyl ester (MCOCHb) | 0.5 | 9.0 | 0.2 | 0.5 | 0.5 | 1.7 | ||
Data from 2013–2014 NHANES cycle (not available in 2011–2012).
Data from 2015–2016 NHANES cycle (not available in 2011–2012 or 2013–2014 cycles).
Limit of detection units are in μg/L.
As expected, baseline concentrations of metabolites from the same parent phthalate were highly correlated (Supplemental Figure 2). For example, the Spearman correlation between MBP and MHBP, metabolites of di-n-butyl phthalate, was 0.84, as was the correlation between MiBP and MHiBP, metabolites of diisobutyl phthalate. The correlation between DINP metabolites (MNP and MCOP) was 0.75. Correlation between metabolites of DEHP ranged from 0.63 (MECPP and MEHP) to 0.94 (MEHHP and MEOHP). Correlations between metabolites derived from different parent compounds ranged considerably from −0.08 to 0.75, although low molecular weight phthalates (e.g., MBP and MiBP) tended to have stronger correlations with other low molecular weight phthalates than with high molecular weight phthalates (e.g., MCOP and MCPP), and vice versa (Supplemental Figure 2).
Multivariable-adjusted associations between age and urinary metabolite concentrations varied by metabolite (Figure 1, Supplemental Table 2). MBzP was inversely associated with age (percentage difference per 5-year increase in age: −12.3% [95% CI: −20.4%, −3.4%]) in the fully-adjusted model, whereas the sum of DINP metabolites and MCPP was positively associated with age (percentage differences in concentration for a 5-year increase of 10.3% [95% CI: −3.1%, 25.6%] and 20.6% [95% CI: 5.1%, 38.6%], respectively). Higher concentrations of DEHP metabolites were generally associated with slightly older age (percentage differences for individual metabolites ranged from 3.6%–8.3% for a 5-year increase in age). Having detectable urinary concentrations of DINCH metabolites was not strongly associated with age (Table 3, Supplemental Table 3). When we restricted our analytic population to the random subcohort, associations with age were generally stronger (Supplemental Table 2).
Figure 1.
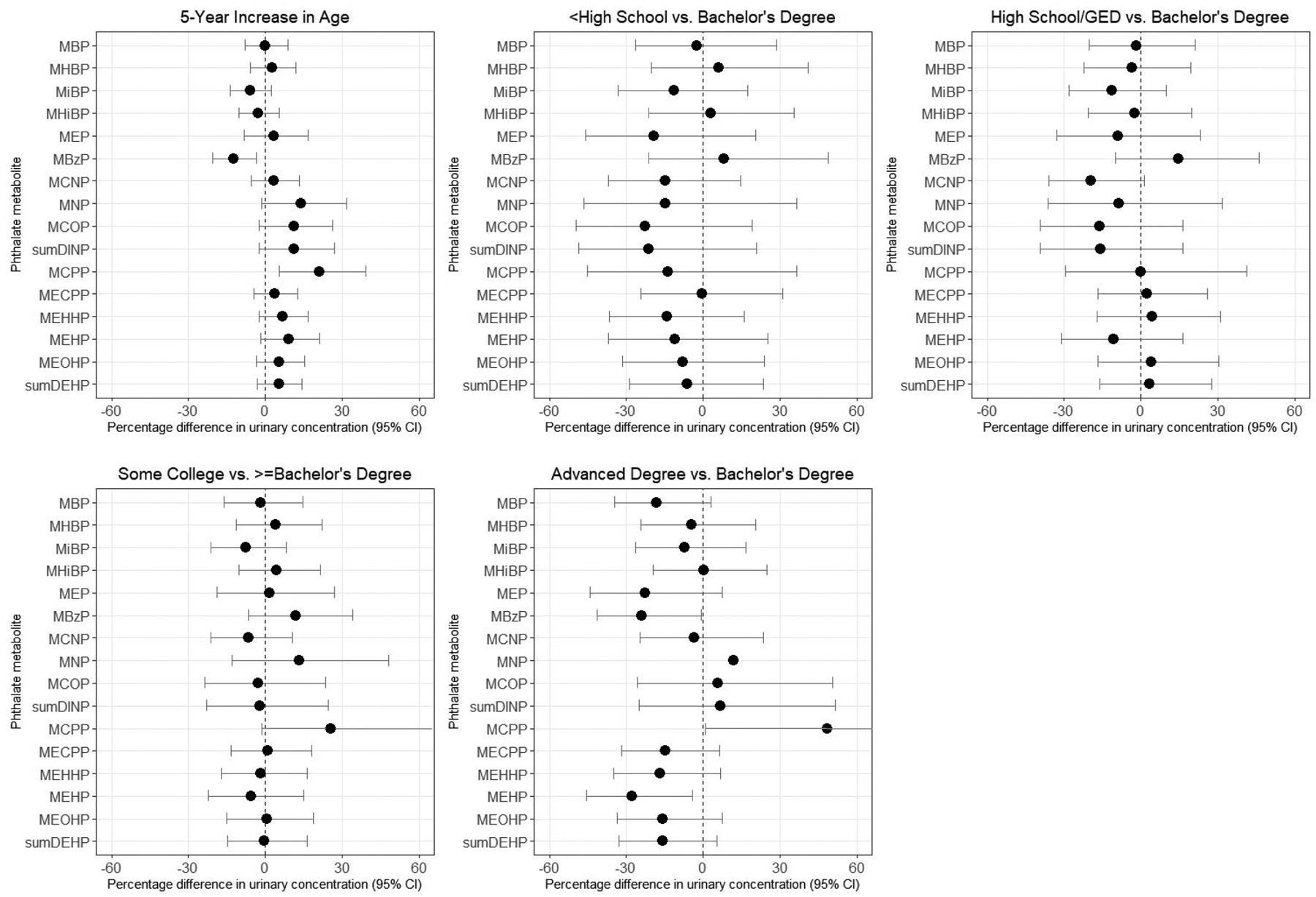
Percentage difference in baseline urinary phthalate concentrations by demographic variables, SELF. Estimates are adjusted for age, education, income, body mass index, smoking, alcohol intake, parity, current contraceptive use, and personal care product use in the past 24 hours. Note: confidence intervals are truncated at 60% in the graphs for education. Upper bounds for MCPP were 62.6% for some college vs. Bachelor’s degree and 116.4% for advanced degree vs. Bachelor’s degree.
Table 3.
Prevalence ratios (PR) comparing the prevalence of detectable concentrations of urinary 1,2-cyclohexane dicarboxylic acid-diisononyl ester (DINCH) metabolites across categories of demographic, lifestyle, and reproductive characteristics, and personal care product use.
| Correlate | MHiNCH PR (95% CI)a |
MCOCH PR (95% CI)a |
|---|---|---|
| Age (5-year increase) | 0.95 (0.78, 1.15) | 0.91 (0.67, 1.23) |
| Education (vs. Bachelor’s degree) | ||
| <High school | 1.26 (0.69, 2.32) | 0.93 (0.69, 1.25) |
| High school/GED | 1.29 (0.80, 2.09) | 1.66 (0.59, 4.65) |
| Some college/Associate’s/Technical | 1.18 (0.81, 1.70) | 2.43 (1.13, 5.25) |
| Advanced degree | 0.34 (0.14, 0.85) | 0.43 (0.10, 1.90) |
| Annual household income (vs. >$50,000) | ||
| <$20,000 | 1.09 (0.71, 1.66) | 0.98 (0.49, 1.97) |
| $20,000–$50,000 | 0.84 (0.55, 1.28) | 0.80 (0.39, 1.64) |
| Body mass index (5-kg/m2 increase) | 1.00 (0.98, 1.01) | 1.01 (0.99, 1.03) |
| Smoking (1 cigarette/day increase) | 0.98 (0.94, 1.02) | 0.96 (0.90, 1.03) |
| Alcohol (per each additional drink/day) | 0.99 (0.97, 1.01) | 1.00 (0.97, 1.02) |
| Parous vs. nulliparous | 1.19 (0.88, 1.61) | 1.11 (0.71, 1.74) |
| Current contraceptive use (vs. non-use of each product) | ||
| Vaginal ring use vs. not current | 1.07 (0.40, 2.91) | --b |
| Oral contraceptive use vs. not current | 1.15 (0.77, 1.71) | 1.10 (0.59, 2.05) |
| Depo medroxyprogesterone acetate use vs. not current | 0.67 (0.36, 1.24) | 1.04 (0.50, 2.19) |
| Intrauterine device use vs. not current | 1.04 (0.69, 1.56) | 0.83 (0.40, 1.70) |
| Product use in past 24 hours (vs. non-use of each product) | ||
| Vaginal product use | 1.17 (0.89, 1.56) | 1.31 (0.86, 1.99) |
| Hair product use | 0.91 (0.69, 1.20) | 0.91 (0.60, 1.38) |
| Nail product use | 0.90 (0.61, 1.33) | 0.96 (0.54, 1.70) |
| Make up, perfume, or cream use | 1.06 (0.69, 1.64) | 0.97 (0.51, 1.84) |
| Total personal care product use (per each additional product) | 0.98 (0.89, 1.07) | 1.04 (0.91, 1.19) |
DINCH=1,2-cyclohexane dicarboxylic acid-diisononyl ester; GED=General Educational Development; MCOCH=1,2-cyclohexane dicarboxylic acid-monocarboxy isooctyl ester; MHiNCH=1,2-cyclohexane dicarboxylic acid-monohydroxy isononyl ester; PR=prevalence ratio
Adjusted for all other correlates.
Not estimable because there were no vaginal ring users with detectable concentrations of MCOCH.
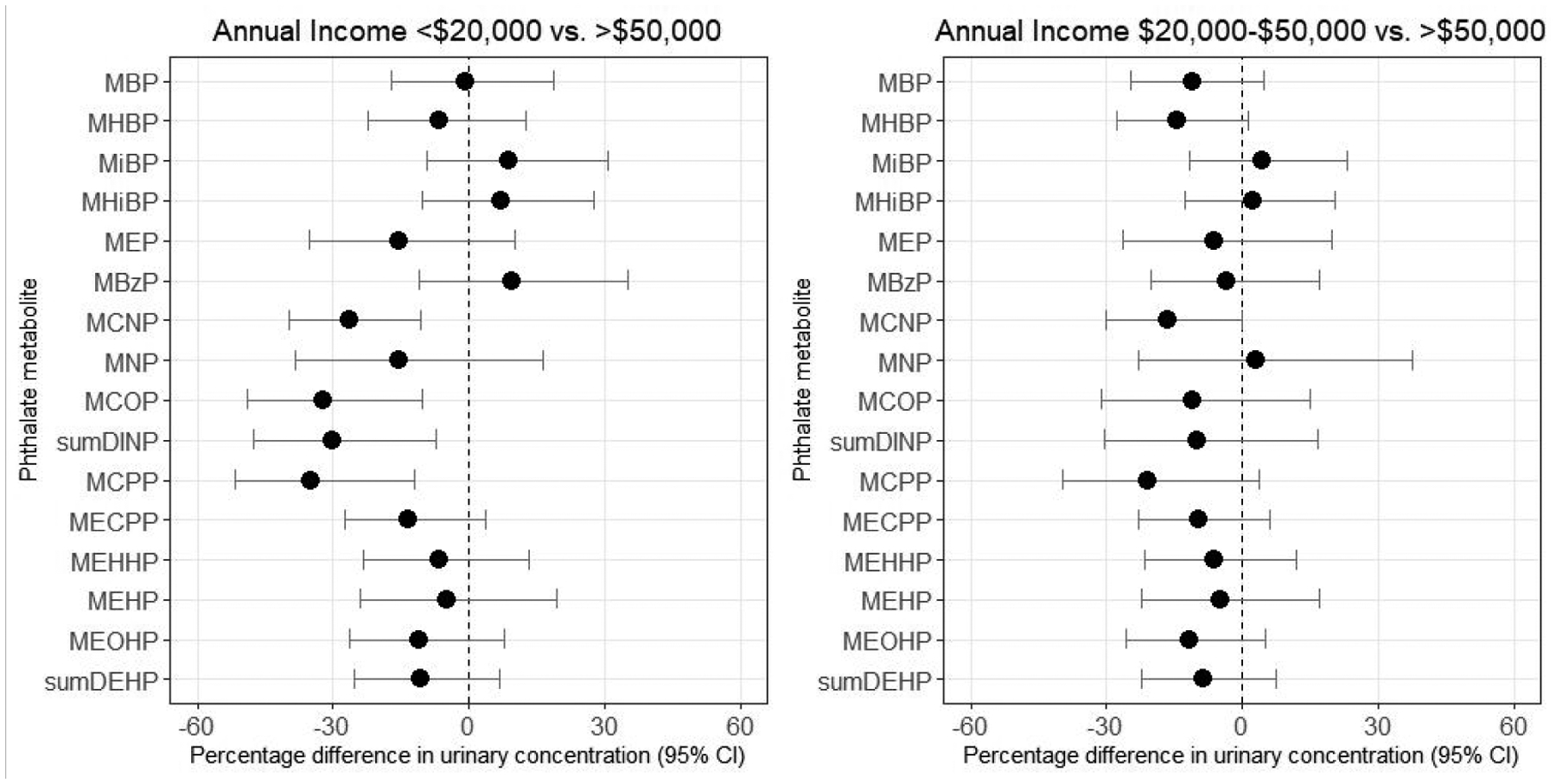
Educational attainment was inversely associated with urinary concentrations of MBzP and positively associated with urinary concentrations of MCNP, DINP metabolites, and MCPP (Figure 1, Supplemental Table 2). Other metabolites varied across categories of educational attainment, but did not show evidence of a dose-response association. Income was also positively associated with MCNP, DINP metabolites, and MCPP concentrations, but was not appreciably associated with MBzP concentrations. Women with advanced degrees were less likely to have detectable concentrations of MHiNCH (probability ratio (PR)=0.34 [95% CI: 0.14, 0.85]) and MCOCH (PR=0.43 [95% CI: 0.10, 1.90]) than women with Bachelor’s degrees, but income was not appreciably associated with DINCH metabolites (Table 3, Supplemental Table 3). In the random subcohort (Supplemental Table 2), associations for education tended to be slightly stronger and associations for income tended to be slightly weaker, although differences were not substantial enough to change our interpretation of the results.
Urinary concentrations of several phthalate metabolites, including MBP, MEP, DINP metabolites, MCPP, and all DEHP metabolites except for MEHP, were positively associated with BMI (percentage differences for a 1-kg/m2 increase in BMI ranged from 0.4%–1.8% for these metabolites; Figure 2, Supplemental Table 2). Conversely, MHiBP and MEHP were inversely associated with BMI (percentage differences were −0.4% and −0.8%, respectively). Smoking was positively associated with urinary concentrations of MEP (percentage difference for a 1 cigarette per day increase was 2.7% [95% CI: 0.3%, 5.3%]). We also observed some evidence that smoking was positively associated with higher urinary concentrations of MNP, MCOP, and MEHP, but associations were weak. Alcohol intake was associated with lower urinary concentrations of MCOP (percentage difference for a 1 drink per week increase was −1.0% [95% CI: −2.3%, 0.2%]) and MCPP (−1.2% [95% CI: −2.4%, 0.1%]). BMI, cigarette smoking, and alcohol intake were not strongly associated with having detectable concentrations of MHiNCH or MCOCH. Associations for BMI, smoking, and alcohol were similar when restricting to the random subcohort (Supplemental Table 2).
Figure 2.
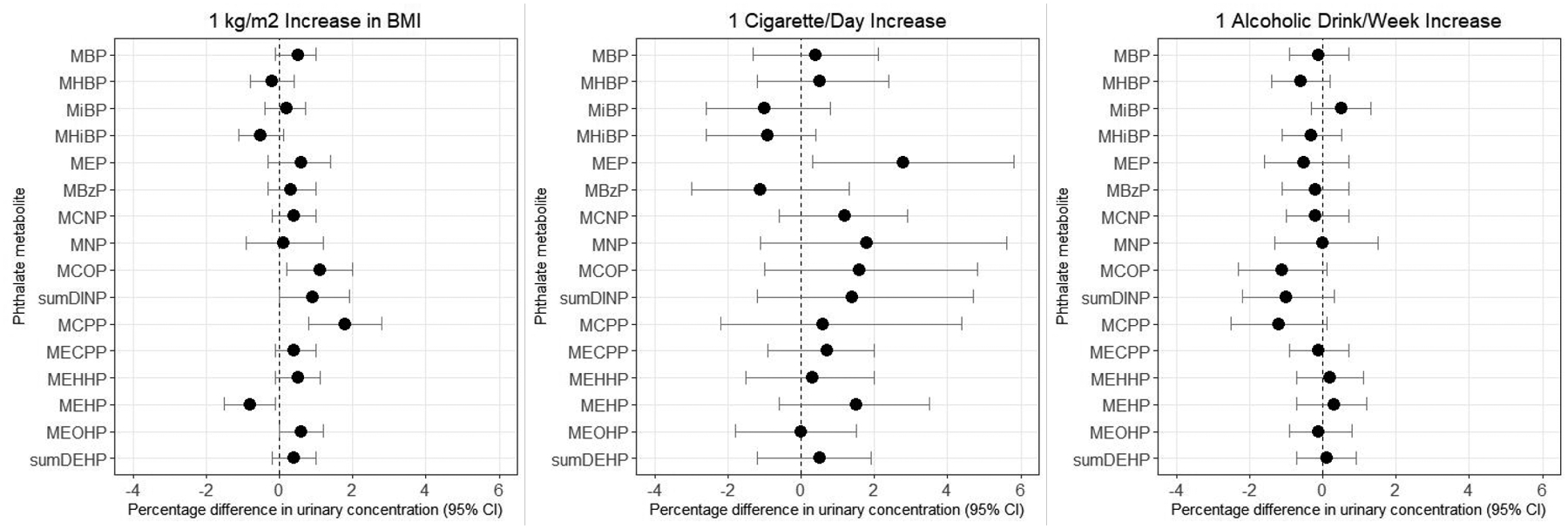
Percentage difference in baseline urinary phthalate concentrations by lifestyle factors, SELF. Estimates are adjusted for age, education, income, body mass index, smoking, alcohol intake, parity, current contraceptive use, and personal care product use in the past 24 hours. BMI=body mass index
Compared with nulliparous women, parous women had higher urinary concentrations of MiBP (percentage difference=13.9% [95% CI: 0.3%, 29.4%]), MHiBP (percentage difference=14.2% [95% CI: 1.2%, 29.0%]), MBzP (percentage difference=15.8% [95% CI: 0.0%, 34.1%]), and MCNP (percentage difference=20.3% [95% CI: 4.8%, 38.1%]), but lower concentrations of MEP (percentage difference=−17.4% [95% CI: −31.2%, −0.8%]), the sum of DINP metabolites (percentage difference=−14.3% [95% CI: −29.6%, 4.2%]), and MCPP (percentage difference=−17.6% [95% CI: −33.2%, 1.6%]; Figure 3, Supplemental Table 2). Parous women were also more likely to have detectable concentrations of MHiNCH (PR=1.19 [95% CI: 0.88, 1.61]) and MCOCH (PR=1.11 [95% CI: 0.71, 1.74]), but results were imprecise (Table 3, Supplemental Table 3).
Figure 3.
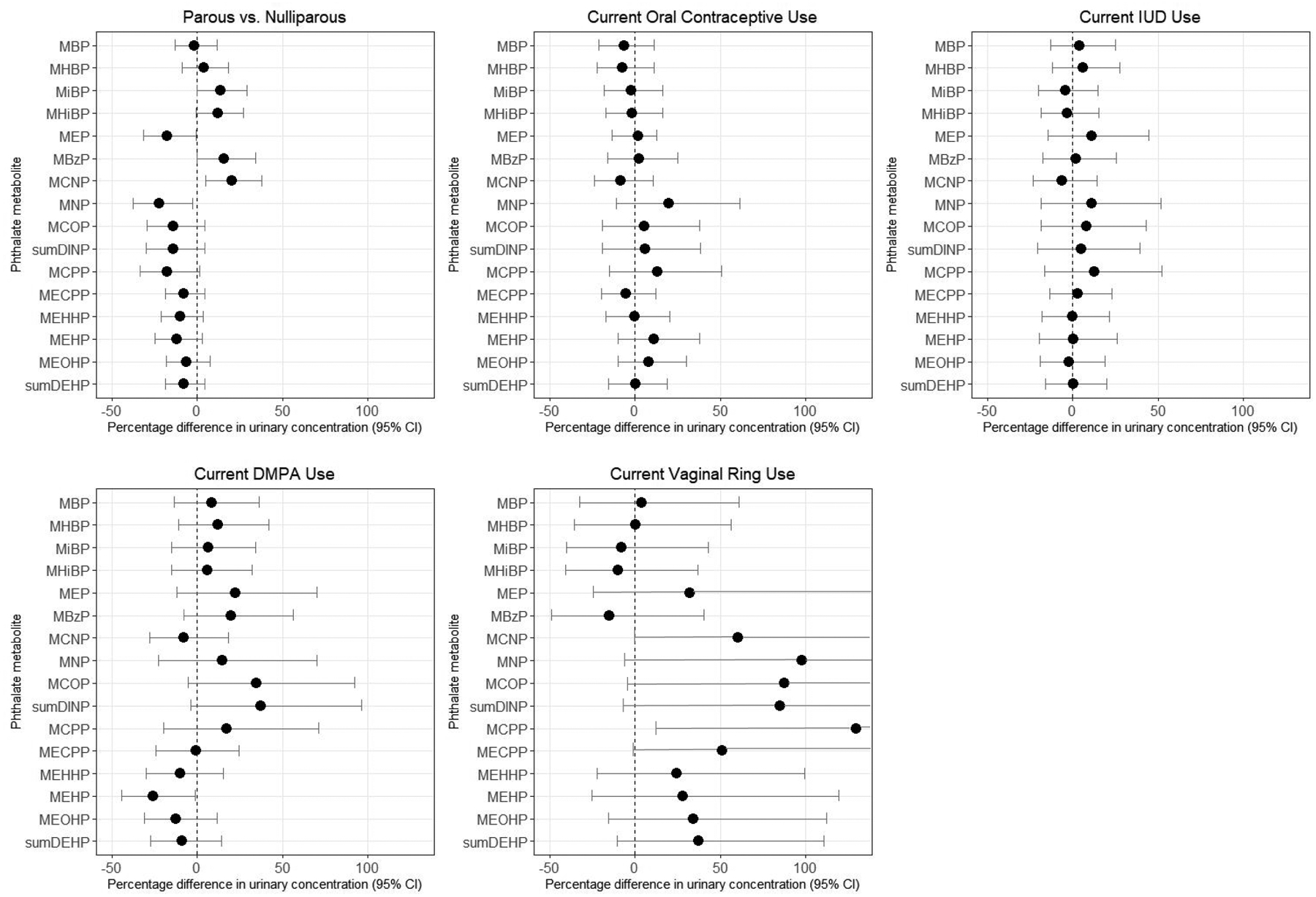
Percentage difference in baseline urinary phthalate concentrations by parity and contraceptive methods, SELF. Estimates are adjusted for age, education, income, body mass index, smoking, alcohol intake, parity, current contraceptive use, and personal care product use in the past 24 hours. IUD=Intrauterine Device; DMPA=depo medroxyprogesterone acetate. Note: confidence intervals are truncated at 130% in the graph for vaginal ring. Upper bounds were 146.7% for MEP, 155.5% for MCNP, 314.3% for MNP, 265.8% for MCOP, 262.1% for the sum of DINP metabolites, 367.7% for MCPP and 130.0% for MECPP.
Current oral contraceptive and IUD use (hormonal and non-hormonal combined) were not appreciably associated with phthalate or DINCH metabolite concentrations compared with non-use of these methods (Figure 3, Supplemental Table 2). However, current DMPA use was associated with 38.1% higher concentrations of the sum of DINP metabolites (95% CI: −2.8%, 96.3%) and 25.7% lower concentrations of MEHP (95% CI: −44.0%, −1.3%). Current vaginal ring use was associated with large, although imprecise, increases in urinary concentrations of several high molecular weight phthalate metabolites, including MCNP [percentage difference=59.7% [95% CI: −0.2%, 155.5%]), MNP (percentage difference=97.6% [95% CI: −5.8%, 314.3%]), MCOP (percentage difference=86.6% [95% CI: −4.6%, 265.8%]), MCPP (percentage difference=128.1% [95% CI: 11.3%, 367.7%]), and MECPP (percentage difference=50.6% [95% CI: −1.3%, 130.0%]). However, when we restricted our analytic sample to the random subcohort (Supplemental Table 2), associations were substantially attenuated. Urinary concentrations of DINCH metabolites were not appreciably associated with current contraceptive use (Table 3, Supplemental Table 3). Numbers of current users of hormonal implants (n=3) and patches (n=2) were too small to assess the relation between these contraceptive methods and urinary metabolite concentrations.
Vaginal product use in the 24 hours before urine collection was associated with 22.6% higher concentrations of MCNP (95% CI: 6.8%, 40.7%) and 16.4% higher concentrations of MEHP (95% CI: −0.6%, 36.3%; Figure 4 and Supplemental Table 2). Use of hair products, including hairspray, gel, oil, mousse, or chemical hair treatments, in the past 24 hours was not appreciably associated with urinary concentrations of metabolites of low molecular weight phthalates, and in fact, appeared to be inversely correlated with concentrations of some high molecular weight phthalate metabolites, including MCNP (percentage difference=−18.7%), the sum of DINP metabolites (−13.2%), MCPP (−11.2%), and the sum of DEHP metabolites (−10.8%). However, nail product use, including both nail polish and solvents in the past 24 hours, was associated with higher urinary concentrations of MBP, MHBP, MEP, and MCNP (percentage differences of 19.3%, 19.8%, 18.8%, and 18.4%, respectively). Use of other personal care products in the past 24 hours, including makeup, creams/lotions, and perfumes/colognes were associated with 15.0% higher urinary concentrations of MiBP (95% CI: −5.1%, 39.3%) and 17.6% higher concentrations of MEP (95% CI: −10.7%, 54.8%). When personal care products were summed together to evaluate the total number of products used during the past 24 hours, for use of each additional personal care product, there was an associated 10.6% (95% CI: 4.7%, 16.8%) increase in urinary concentrations of MEP, but not other phthalate metabolites. Use of vaginal products in the past 24 hours was associated with slightly higher risk of having detectable concentrations of DINCH metabolites, but results were imprecise (Table 3, Supplemental Table 3). Use of other personal care products was not meaningfully associated with the risk of detectable DINCH metabolite concentrations. Associations between urinary metabolites and personal care product use in the past 24 hours did not vary consistently or substantially when restricting to the random subcohort (Supplemental Table 2), with the exception of the vaginal ring results mentioned above.
Figure 4.
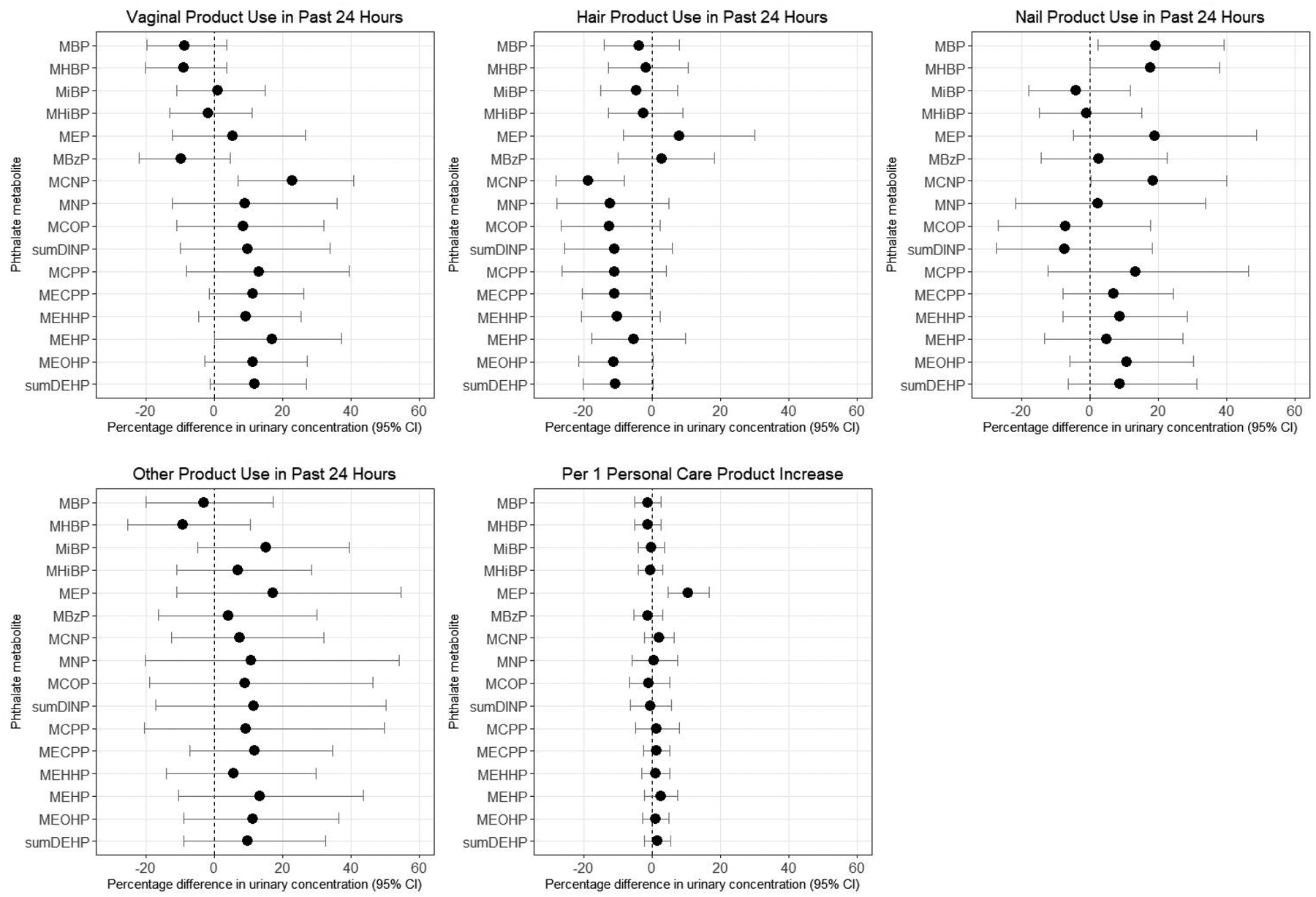
Percentage difference in baseline urinary phthalate concentrations by personal care product use in past 24 hours, SELF. Vaginal products include genital powder, douching liquids, feminine hygiene deodorant, and condoms. Hair products include hairspray, gel, oil, mousse, and chemical hair treatments. Nail products include nail polish and nail polish remover (solvents). Other personal care products includes makeup, cream or lotion before bed, and perfume or cologne. Estimates are adjusted for age, education, income, body mass index, smoking, alcohol intake, parity, current contraceptive use, and personal care product use in the past 24 hours.
DISCUSSION
Previous literature on correlates of phthalate biomarkers have focused on specific sources of exposure, for example, diet,40, 41 medication use,43, 44 and personal care product use.40, 42 There have been few studies examining socio-demographic, lifestyle, and reproductive correlates of exposure, particularly among populations at higher risk of exposure, such as Black women. The associations reported in the present work provide information on potential sources of exposure, including established (i.e., personal care products) and novel (i.e., contraceptive vaginal ring) sources. Our results also document inequitable exposure to phthalates through differential patterns of product use among Black women and emphasize the importance of identifying exposure sources among populations in which multiple sources of oppression - i.e., racism, sexism, and classism - intersect and interact to shape risk of exposure to multiple environmental chemicals.54, 55 Identification of sources of exposure, specifically those that are differentially distributed across women, allows for development of specific interventions - for example, improved regulation and/or disclosure of chemicals in beauty products or advertising campaigns that promote more diverse beauty norms - to reduce health disparities.
The literature is inconsistent regarding the relation between age and phthalate metabolite concentrations. Because phthalates have short biological half-lives (on the order of hours), they do not persist in the body or bioaccumulate with age. Older reproductive-aged women (maximum age=35 years) in our cohort had lower concentrations of MBzP and higher concentrations of other high molecular weight phthalates including MNP, MCOP, and MCPP. These findings are consistent with some studies,40 but not others.42 Women may change their personal care product use, diet, medication use, and use of other phthalate-containing products as they age, and different levels of adjustment for these variables in previous studies could explain the conflicting findings.
Many sources of phthalates - including diet, medication use, personal care product use, housing characteristics, and occupation - vary with socioeconomic status. In the SELF cohort, women with lower educational attainment had higher concentrations of MBzP and DINCH metabolites; however, income was not appreciably associated with these metabolites. Our findings for MBzP are consistent with several previous analyses using NHANES data.6, 56, 57 Education may better serve as a predictor of socioeconomic status, social circumstances, and the built environment than income among Black Americans,27, 58 which could explain the different results for income and education. However, both educational attainment and income were associated with lower concentrations of certain high molecular weight phthalate metabolites (including MCNP, MNP, MCOP, and MCPP). The difference in the directions of association with socioeconomic status across these metabolites is unexpected, given that general categories of exposure sources (vinyl tiling, adhesives, and PVC plastics) tend to be similar between MBzP, MCNP, MNP, MCOP, and MCPP. Our findings indicate that the relationship between socioeconomic status and phthalate exposure varies considerably across metabolites. Our work supports prior research demonstrating higher MBzP concentrations among populations of lower socioeconomic position. However, other relationships are less clear, and we may not have collected specific enough information on exposure sources to elucidate those with social patterning.
We found that urinary concentrations of the low molecular weight phthalate metabolites MEP and MBP were positively associated with BMI, which is consistent with some40, 59–61 but not all62 previous studies. We also observed higher concentrations of metabolites of high molecular weight phthalates (including DINP and DEHP) among women with higher BMI. The literature on this topic is mixed: for example, DEHP metabolites have been associated with both lower60 and higher40, 59, 61, 62 BMI. Given the cross-sectional design of our study, it is unclear whether these associations reflect the potential obesogenic actions of phthalates,45, 63, 64 differential exposure sources, or altered metabolism in individuals with higher levels of body fat.65 The observation that three of the four DEHP metabolites (MECPP, MEHHP, and MEOHP) were positively associated with BMI, yet the fourth DEHP metabolite (MEHP) was inversely associated with BMI indicates that the associations may not be due to differences in exposure sources but perhaps to the metabolism of DEHP. MEHP concentrations were also associated with lower BMI among females from the 1999–2002 NHANES data; this same study found positive associations with other DEHP metabolites.45
Cigarette smoking was associated with higher urinary concentrations of MEP, which is consistent with results from studies of pregnant women in Spain,61 Canada,60 and the Netherlands,59 as well as men participating in a semen quality study in Massachusetts.42 These results are expected, given that diethyl phthalate, the parent compound of MEP, is a component of some cigarette filters.66 We did not observe positive associations between alcohol intake and urinary concentrations of any of the measured metabolites, despite prior research demonstrating that phthalates can migrate from plastic contact materials in processing, shipping, and storage of alcoholic beverages.67
Parous women had lower concentrations of MEP than nulliparous women, as has been observed in previous studies.40, 60 On the other hand, parous women had higher concentrations of MiBP, MHiBP, MBzP, and MCNP compared with nulliparous women. The explanation for these findings is unclear. It could stem in part from increasing exposure to plastics in children’s toys, as well as changes in personal care product use and diet after having children.
Interestingly, we found strong, although imprecise, associations between concentrations of several high molecular weight phthalates and current use of the vaginal ring. To our knowledge, this is the first report of such an association. The vaginal ring is reportedly made from ethylene vinylacetate copolymers,68 which should not contain ortho-phthalates. However, exposure may result from phthalate contamination during manufacturing or packaging of the vaginal ring. Though unstudied, use of hormonal contraception could also alter metabolism of phthalates. However, this finding could also result from chance, as there were only 12 women currently using a vaginal ring at baseline and results were attenuated when we restricted to the random subcohort.
As expected, personal care product use in the past 24 hours was associated with higher concentrations of phthalate metabolites, and associations varied by type of product. Use of nail polish or solvents in the past 24 hours was associated with higher concentrations of MBP, MHBP, and MEP, consistent with previous studies.69–71 Use of makeup and creams or lotions were associated with higher concentrations of MiBP and MEP. However, not all associations were in the expected direction. Previous studies have documented higher use of feminine hygiene products among Black women due to targeted advertising of these products19–21 and misinformation regarding their benefits and risks,18 and have shown that increased vaginal product use is associated with higher concentrations of MEP and MBP.22 However, we did not find that exposure of the genital area to products, particularly genital powder, vaginal douches, and feminine hygiene deodorant in the past 24 hours was associated with higher concentrations of these phthalate biomarkers in SELF. The majority of SELF participants who reported douching used water and vinegar rather than scented products that were likely to contain phthalates such as DEP or DBP, which could explain the lack of association. Likewise, previous studies demonstrate that hair products such as hairspray, mousse, and gel may contain DEP and DBP,21, 23, 42, 69 yet we found little evidence of an association between metabolites of these phthalates and use of hair products in the past 24 hours. Observed discrepancies could stem from our lack of information on the specific brand, type, or quantity of product used, which would have resulted in some misclassification of exposures. In addition, we did not collect comprehensive data on all personal care products. Because use of specific products can correlate strongly with use of other products, there may be some residual confounding by other products not assessed on our questionnaires.
With the exception of income and education, DINCH metabolite concentrations were not strongly correlated with any of the variables assessed in this study. DINCH is primarily used as a substitute for DEHP, yet we did not find consistencies between correlates of DINCH and DEHP metabolites, perhaps because we did not have sufficient detail on sources of DINCH to be able to examine correlates of exposure and/or too few women in our cohort had detectable concentrations of DINCH metabolites.
The primary limitation of this study is that we only collected one first morning urine specimen, which only reflects exposures from the previous few hours and likely misses exposures from morning product use, given that over 97% of urines were first morning urines collected at home. In addition, we queried women about their use of products in the previous 24 hours only, rather than frequent or regular use of products. Lastly, our assessment is limited by the cross-sectional study design.
This study also has several notable strengths. Importantly, this is one of the only studies of correlates of phthalate exposure among Black women, who are more likely to have higher exposure to some phthalates,6, 8, 9 are at greater risk of several diseases potentially linked to phthalates,29–37 and are historically underrepresented in environmental health research. Our results, which demonstrate an association between product use and biomarkers of phthalate exposure in Black women, support research emphasizing the importance of framing of product use as an environmental justice issue with implications for racial disparities in reproductive health throughout the life course.10, 28 In addition, our study has a relatively large sample size and we measured metabolites of both phthalates and phthalate alternatives, which are increasing in prevalence in industrialized regions and whose health effects are understudied.
In this population of reproductive-aged Black women residing in the Detroit metropolitan area, urinary concentrations of metabolites of phthalates and phthalate alternatives were similar to, or often higher than, concentrations measured in a nationally-representative sample of Black women. Several sociodemographic, behavioral, reproductive, and personal care product variables were correlated with urinary concentrations of specific metabolites, often supporting observations from previous literature. We also reported a novel association between current use of the vaginal ring and several high molecular weight phthalate metabolites, which were strong, but imprecise, and should be examined in future work. These results support further examination of the ways in which structural racism has contributed to differential exposure to endocrine-disrupting chemicals among Black women, and the relevance of inequitable exposure to phthalates and other chemicals in explaining racial health disparities.
Supplementary Material
ACKNOWLEDGMENTS
We thank Quaker E. Harmon for facilitating use of the SELF data and two anonymous reviewers for their insightful comments on the manuscript.
FUNDING
This research was funded primarily by the extramural program of the National Institute of Environmental Health Sciences (R01-ES024749). In addition, the research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences and in part by funds allocated for health research by the American Recovery and Reinvestment Act. The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
CONFLICTS OF INTEREST
In the last three years, Lauren Wise has served as a consultant for AbbVie, Inc. and has accepted in-kind donations from Swiss Precision Diagnostics, Sandstone Diagnostics, FertilityFriend.com, and Kindara for primary data collection in Pregnancy Study Online. The remaining authors declare they have no actual or potential competing financial interests.
REFERENCES
- 1.Koch HM, Calafat AM Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009; 364: 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry. Phthalates. In. Atlanta, GA, 2008. [Google Scholar]
- 3.Phthalates NRCCotHRo. Phthalate Exposure Assessment in Humans. In: Phthalates and Cumulative Risk Assessment: The Tasks Ahead. National Academies Press: Washington, DC, 2008. [PubMed] [Google Scholar]
- 4.European Food Safety Authority Opinion of the Scientific Panel on food additives, flavourings, processing aids, and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials. EFSA J 2006; 395–401: 1–21. [Google Scholar]
- 5.Silva MJ, Jia T, Samandar E, Preau JL Jr., Calafat AM Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012). Environmental research 2013; 126: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental health perspectives 2004; 112: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll(R) DINCH(R) in 24 h urine samples from the German Environmental Specimen Bank. International journal of hygiene and environmental health 2014; 217: 421–426. [DOI] [PubMed] [Google Scholar]
- 8.CDC US. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 9.Varshavsky JR, Zota AR, Woodruff TJ A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environmental science & technology 2016; 50: 10616–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zota AR, Shamasunder B The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol 2017; 217: 418 e411–418 e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor KW, Baird DD, Herring AH, Engel LS, Nichols HB, Sandler DP et al. Associations among personal care product use patterns and exogenous hormone use in the NIEHS Sister Study. J Expo Sci Environ Epidemiol 2017; 27: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon AR, Telles EE Skin color and colorism: global research, concepts, and measurement. Ann Rev Sociol 2017; 43: 405–424. [Google Scholar]
- 13.Hunter ML “If you’re light you’re alright”: light skin color as social capital for women of color. Gender Society 2002; 16: 175–193. [Google Scholar]
- 14.Thompson MS, Keith VM The blacker the berry: gender, skin tone, self-esteem, and self-efficacy. Gender Society 2001; 15: 336–357. [Google Scholar]
- 15.Banks I Hair Matters: Beauty, Power, and Black Women’s Consciousness. NYU Press: New York, NY, 2000. [Google Scholar]
- 16.Robinson CL Hair as race: why “good hair” may be bad for Black females. Howard J Comm 2011; 22: 358–376. [Google Scholar]
- 17.Johnson AM, Godsil R, MacFarlane J, Tropp L, Goff PA. The “Good Hair” Study: Explicit and Implicit Attitudes Toward Black Women’s Hair. The Perception Institute, 2017. [Google Scholar]
- 18.Gazmararian JA, Bruce FC, Kendrick JS, Grace CC, Wynn S Why do women douche? Results from a qualitative study. Matern Child Health J 2001; 5: 153–160. [DOI] [PubMed] [Google Scholar]
- 19.Ferranti M An odor of racism: vaginal deoderants in African-American beauty culture and advertising. Advert Society Rev 2011; 11. [Google Scholar]
- 20.Charles C The derogatory representations of the skin bleaching products sold in Harlem. J Pan Afr Stud 2011; 4. [Google Scholar]
- 21.James-Todd T, Senie R, Terry MB Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health 2012; 14: 506–511. [DOI] [PubMed] [Google Scholar]
- 22.Branch F, Woodruff TJ, Mitro SD, Zota AR Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environmental health : a global access science source 2015; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environmental research 2018; 165: 448–458. [DOI] [PubMed] [Google Scholar]
- 24.Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R et al. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J Expo Sci Environ Epidemiol 2010; 20: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaston SA, James-Todd T, Harmon Q, Taylor KW, Baird D, Jackson CL Chemical/straightening and other hair product usage during childhood, adolescence, and adulthood among African-American women: potential implications for health. J Expo Sci Environ Epidemiol 2020; 30: 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017; 389: 1453–1463. [DOI] [PubMed] [Google Scholar]
- 27.Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff (Millwood) 2011; 30: 879–887. [DOI] [PubMed] [Google Scholar]
- 28.James-Todd TM, Chiu YH, Zota AR Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep 2016; 3: 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro C, Mendes V, Peleteiro B, Delgado I, Araujo J, Aggerbeck M et al. Association between the exposure to phthalates and adiposity: A meta-analysis in children and adults. Environmental research 2019; 179: 108780. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Carmona Y, Cantoral A, Trejo-Valdivia B, Tellez-Rojo MM, Svensson K, Peterson KE et al. Phthalate exposure during pregnancy and long-term weight gain in women. Environmental research 2019; 169: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz Santana MV, Hankinson SE, Bigelow C, Sturgeon SR, Zoeller RT, Tinker L et al. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environmental health : a global access science source 2019; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radke EG, Galizia A, Thayer KA, Cooper GS Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environment international 2019; 132: 104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamai EM, McElrath TF, Ferguson KK Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environmental health : a global access science source 2019; 18: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai W, Yang J, Liu Y, Bi Y, Wang H Association between Phthalate Metabolites and Risk of Endometriosis: A Meta-Analysis. Int J Environ Res Public Health 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environmental health perspectives 2010; 118: 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zota AR, Geller RJ, Calafat AM, Marfori CQ, Baccarelli AA, Moawad GN Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril 2019; 111: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahern TP, Broe A, Lash TL, Cronin-Fenton DP, Ulrichsen SP, Christiansen PM et al. Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. J Clin Oncol 2019; 37: 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minguez-Alarcon L, Souter I, Chiu YH, Williams PL, Ford JB, Ye X et al. Urinary concentrations of cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center. Environmental research 2016; 151: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van TETJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL et al. Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environmental science & technology 2019; 53: 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environment international 2014; 62: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colacino JA, Harris TR, Schecter A Dietary intake is associated with phthalate body burden in a nationally representative sample. Environmental health perspectives 2010; 118: 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duty SM, Ackerman RM, Calafat AM, Hauser R Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental health perspectives 2005; 113: 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM Medications as a source of human exposure to phthalates. Environmental health perspectives 2004; 112: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez-Diaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol 2013; 37: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environmental health : a global access science source 2008; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lind PM, Roos V, Ronn M, Johansson L, Ahlstrom H, Kullberg J et al. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environmental health : a global access science source 2012; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baird DD, Harmon QE, Upson K, Moore KR, Barker-Cummings C, Baker S et al. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J Womens Health (Larchmt) 2015; 24: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bethea TN, Wesselink AK, Weuve J, McClean MD, Hauser R, Williams PL et al. Correlates of exposure to phenols, parabens, and triclocarban in the Study of Environment, Lifestyle and Fibroids. J Expo Sci Environ Epidemiol 2019; e-pub ahead of print 2019/01/30; doi 10.1038/s41370-019-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 860: 106–112. [DOI] [PubMed] [Google Scholar]
- 50.National Center for Health Statistics. NHANES Laboratory Data. In. Atlanta, GA, 2011–2012. [Google Scholar]
- 51.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons: Hoboken, NJ, 2004. [Google Scholar]
- 52.Office of the Assistant Secretary for Planning and Evaluation. Poverty Guidelines. In: U.S. Department of Health and Human Services, (ed). Washington, D.C., 2020. [Google Scholar]
- 53.United States Census Bureau. QuickFacts: Detroit City, Michigan. In, 2020. [Google Scholar]
- 54.Krenshaw K Demarginalizing the Intersection of Race and Sex: A Black Feminist Critique of Antidiscrimination Doctrine, Feminist Theory, and Antiracist Politics. University of Chicago Legal Forum; 1989. [Google Scholar]
- 55.Malin SA, Ryder SS Developing a deeply intersectional environmental justice scholarship. Environmental Sociology 2018; 4: 1–7. [Google Scholar]
- 56.Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environmental research 2012; 115: 11–17. [DOI] [PubMed] [Google Scholar]
- 57.Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL et al. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environmental health perspectives 2002; 110: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith A Educational attainment as a determinant of social class among black americans. J Negro Educ 1989; 58: 416–429. [Google Scholar]
- 59.Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S et al. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environmental research 2018; 161: 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A et al. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environment international 2014; 68: 55–65. [DOI] [PubMed] [Google Scholar]
- 61.Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J et al. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. International journal of hygiene and environmental health 2015; 218: 220–231. [DOI] [PubMed] [Google Scholar]
- 62.Reeves KW, Santana MD, Manson JE, Hankinson SE, Zoeller RT, Bigelow C et al. Predictors of urinary phthalate biomarker concentrations in postmenopausal women. Environmental research 2019; 169: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buser MC, Murray HE, Scinicariello F Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. International journal of hygiene and environmental health 2014; 217: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heggeseth BC, Holland N, Eskenazi B, Kogut K, Harley KG Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environmental research 2019; 175: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Rongen A, Valitalo PAJ, Peeters MYM, Boerma D, Huisman FW, van Ramshorst B et al. Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin Pharmacokinet 2016; 55: 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips Morris USA. PM USA Cigarette Non-Tobacco Ingredients. In, 2015.
- 67.Fan Y, Liu S, Xie Q Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Talanta 2014; 119: 291–298. [DOI] [PubMed] [Google Scholar]
- 68.drugs.com. NuvaRing. In, 2019. [Google Scholar]
- 69.Koniecki D, Wang R, Moody RP, Zhu J Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environmental research 2011; 111: 329–336. [DOI] [PubMed] [Google Scholar]
- 70.Parlett LE, Calafat AM, Swan SH Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 2013; 23: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsieh CJ, Chang YH, Hu A, Chen ML, Sun CW, Situmorang RF et al. Personal care products use and phthalate exposure levels among pregnant women. The Science of the total environment 2019; 648: 135–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


