Abstract
Background
Urinary albumin excretion (UAE) is a risk factor for cardiovascular diseases, metabolic syndrome, chronic kidney disease, etc. Only a few genome-wide association studies (GWAS) for UAE have been conducted in the European population, but not in the Asian population. Here we conducted GWAS and identified several candidate genes harboring single nucleotide polymorphisms (SNPs) responsible for UAE in the Japanese population.
Methods
We conducted GWAS for UAE in 7805 individuals of Asian ancestry from health-survey data collected by Tohoku Medical Megabank Organization (ToMMo) and Iwate Tohoku Medical Megabank Organization (IMM). The SNP genotype data were obtained with a SNP microarray. After imputation using a haplotype panel consisting of 2000 genome sequencing, 4,962,728 SNP markers were used for the GWAS.
Results
Eighteen SNPs at 14 loci (GRM7, EXOC1/NMU, LPA, STEAP1B/RAPGEF5, SEMA3D, PRKAG2, TRIQK, SERTM1, TPT1-AS1, OR5AU1, TSHR, FMN1/RYR3, COPRS, and BRD1) were associated with UAE in the Japanese individuals. A locus with particularly strong associations was observed on TSHR, chromosome 14 [rs116622332 (p = 3.99 × 10−10)].
Conclusion
In this study, we successfully identified UAE-associated variant loci in the Japanese population. Further study is required to confirm this association.
Electronic supplementary material
The online version of this article (10.1007/s10157-020-01884-x) contains supplementary material, which is available to authorized users.
Keywords: QTL, GWAS, Albuminuria, Genetics, TSHR, Cohort study
Introduction
Chronic kidney disease (CKD) is one of the most severe global public health problems [1]. The proportion of patients with end-stage renal disease is growing, and thus, the resultant cost poses a big problem in health economics [2]. It is important to diagnose renal failure in the early stages to prevent disease progression. However, it is very difficult to do so as the typical symptoms of renal failure rarely emerge in the earlier stages. The risks of mortality, myocardial infarction, and progression to kidney failure associated with a particular value of estimated glomerular filtration rate (eGFR) are increased independently in patients with moderate to severe urinary albumin excretion (UAE) [3]. Apart from CKD, UAE is known biomarker of cardiovascular diseases, diabetes mellitus, obesity, hypertension, and all-cause mortality [4–8]. Even albuminuria of less than 30 mg/gCr (lower than microalbuminuria) is known as a marker of these diseases [7, 8].
While a few genome-wide association studies (GWAS) on UAE have been conducted in individuals with type 2 diabetes mellitus [9] and type 1 diabetes mellitus [10] in European ancestral cohorts [11–13], there is no such GWAS conducted in the Asian population. Here we have conducted GWAS using health-survey data collected in the Tohoku Medical Megabank Organization (ToMMo) and Iwate Tohoku Medical Megabank Organization (IMM) to identify the several candidate genes harboring single nucleotide polymorphisms (SNPs) responsible for UAE in the Japanese population.
Material and methods
Study subjects
This research was conducted as a part of the residential cohort study of Tohoku Medical Megabank (TMM), a joint organization of the Tohoku University and the Iwate Medical University in Japan, established in 2011 after the Great East Japan Earthquake for creating an advanced medical system.
Over 80,000 almost healthy adult individuals living in the Miyagi and Iwate Prefectures along the Pacific coast of the Tohoku district of northern Japan were recruited from May 2013 to March 2016 for the TMM Project. The participants were of 20–75 years old and completed questionnaires covering a wide range of topics including socio-demographic factors, lifestyle habits, and medical history. Blood and urine tests were performed at baseline survey. The participants living in the Miyagi Prefecture and Iwate Prefecture were recruited by Tohoku University and Iwate Medical University, respectively [14, 15]. We obtained approval from the relevant ethics committees of both the facilities. We obtained written informed consent from each participant when they were enrolled in the TMM cohort study. This study was conducted according to the principals of the Declaration of Helsinki.
From the 10,000 individuals whose data were collected up to 2013, we were able to obtain data of 9,966 individuals after excluding 34 people who withdrew their consent after collection. The data were released as dbToMMo 1.1. Among these 9,966 individuals, 4974 were from the Miyagi prefecture and 4992 were from the Iwate prefecture. Thus, both represented a roughly equal proportion.
Sample quality control
Genotyping was performed for 964,193 SNP markers using Illumina's Human Omni Express Exome- 8 version 1.2 BeadChips. Upon conducting quality control of the samples based on the genotyping data, some people were excluded owing to data loss (n = 1), genotype defect (low call rate: call rate < 0.98, n = 5), or close relationship pairs (identity-by-descent estimates, PI_HAT > 3/32, n = 2155) [16, 17]. Finally, the data sampled from 7805 individuals passed quality control.
Marker quality control
As quality control of the genotyped marker, SNPs with low call rates (< 0.95), and low p values in the Hardy Weinberg equilibrium (HWE) test (p value < 1.0 × 10−4), low minor allele frequencies (MAF < 0.01), and low-quality markers among the duplication markers were filtered out. As a result, 595,171 SNPs remained for the downstream analysis.
Genotype imputation
Genotype imputation was performed using SHAPEIT v2.r837 [18] and IMPUTE2 v2.2.2 [19] software packages with TMM 2KJPN high-quality haplotype reference panel based on the 2049 drafts of the whole genome sequencing and was implemented in the TMM [20]. After genotype imputation, we adjusted the imputation quality (INFO scores) and MAF. The variants with low imputation quality (INFO scores < 0.5) and low minor allele frequency (MAF < 0.03) variants were excluded. Ultimately, 4,962,728 variants were retained for the GWAS.
Phenotype
Phenotype information was obtained from the questionnaires covering age, sex, physical measurement including body mass index (BMI), and systolic blood pressure (SBP). For standardizing the blood pressure estimation, we did not use the antihypertensive medication history because systolic blood pressure strongly influences the glomerular pressure and UAE [21].
Urinary Na (UNa), urinary K (UK), urinary creatinine (UCr), urinary albumin (Ualb), serum creatinine (sCre), serum cystatin C (sCysC), hemoglobin A1c (HbA1c), and eGFR were estimated at baseline. We selected eGFR calculated by serum cystatin C (eGFRcys) for the evaluation of renal functional instead of eGFR calculated by serum creatinine (eGFRcre) because serum cystatin C was a better marker of early-stage CKD than serum creatinine [22]. eGFRcys was calculated by the Japanese equation for eGFR from serum cystatin C as follows [23, 24];
Statistical analyses
After performing a standard linear regression analysis of UAE (PLINK version 1.9 software package) for each SNP, we performed GWAS for UAE. We used UAE corrected by creatinine (continuous variable) as a response variable. The analysis was adjusted for the relevant covariates including age, sex, BMI [25], SBP [26], UNa [27], UK [27], HbA1c [26], eGFRcys [28], and the top significant 26 principal components of the genotypes. These are reported confounding factors for albuminuria.
We constructed a Manhattan plot and Quantile–quantile plot (Q-Q plot) to visually evaluate the analysis result. We used the statistical software R with “qqman” package. We constructed Regional plot to evaluate the linkage disequilibrium (LD) structure around the SNPs with Locus Zoom [URL; https://locuszoom.org]. We evaluated the result of the expression Quantitative Trait Loci (eQTL) analysis for some genome-wide significant SNPs by the Genotype Tissue Expression Project (GTEx) portal [29].
Results
Basic characteristics of the study subjects
The genotype data from 7805 individuals passed quality control and were used for the analysis. The detailed characteristics of the analyzed data are shown in Table 1. There were 60.7% patients of CKD stage 1, 36.6% patients of CDK stage 2, and 2.71% patients of CKD stage ≥ 3. In this setting, cystatin C seemed to be more appropriate for kidney function marker. The mean age of the patients was 61.8 ± 11.2 years, and 34.8% of the patients were male. The average systolic blood pressure of these patients was 127 ± 17.8 mm Hg and the median of UAE was 7.4 mg/gCr [interquartile range (IQR) 8.8]. About one-fourth (24.5%) of the patients were hypertensive [defined as systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg in accordance with The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014)] [30]. In this study, the individuals taking antihypertensive medications were also diagnosed as hypertensive.
Table 1.
Demographic characteristics of the study population
| Characteristics | Total | Ualb – | Ualb + |
|---|---|---|---|
| n = 7805 | n = 6970 | n = 827 | |
| Age, years | 60.8 ± 11.2 | 60.4 ± 11.5 | 64.8 ± 8.64 |
| Sex, male (%) | 2716 (34.8) | 2349 (33.7) | 360 (43.5) |
| BMI | 23.5 ± 3.6 | 23.4 ± 3.52 | 24.6 ± 3.76 |
| SBP, mmHg | 127 ± 17.8 | 126 ± 17.2 | 137 ± 19.9 |
| DBP, mmHg | 75.4 ± 10.8 | 74.9 ± 10.6 | 79.7 ± 11.9 |
| HTN_treat (%) | 211 (2.7) | 275 (2.53) | 31 (3.79) |
| HTN_diag (%) | 1912 (24.5) | 1540 (22.1) | 369 (44.6) |
| Ualb/UCr, mg/gCr (IQR) | 7.4 (8.8)* | 6.7 (6.2)* | 64.7 (99)* |
| < 30 mg/gCr (%) | 6970 (89.3) | ||
| ≧ 30 mg/gCr (%) | 827 (10.6) | ||
| UNa, g/l | 3.02 ± 1.34 | 3.05 ± 1.28 | 2.91 ± 1.23 |
| UK, g/l | 1.63 ± 1.09 | 1.66 ± 1.04 | 1.50 ± 0.91 |
| HbA1c(NGSP), % | 5.56 ± 0.59 | 5.52 ± 0.54 | 5.81 ± 0.85 |
| sCre, mg/dl | 0.69 ± 0.24 | 0.68 ± 0.15 | 0.75 ± 0.43 |
| sCysC, mg/l | 0.77 ± 0.19 | 0.76 ± 0.15 | 0.85 ± 0.15 |
| eGFRcre, ml/min/1.73 m2 | 78.1 ± 15.6 | 78.4 ± 0.15 | 74.8 ± 0.15 |
| eGFRcys, ml/min/1.73 m2 | 97.4 ± 21.9 | 98.4 ± 0.15 | 89.5 ± 0.15 |
| CKD stage 1 (%) | 4737 (60.7) | 4346 (62.4) | 390 (47.2) |
| CKD stage 2 (%) | 2857 (36.6) | 2499 (35.9) | 351 (42.4) |
| CKD stage 3a (%) | 174 (2.23) | 112 (1.60) | 63 (7.58) |
| CKD stage 3b (%) | 27 (0.35) | 12 (0.17) | 15 (1.83) |
| CKD stage 4 (%) | 8 (0.10) | 1 (0.01) | 7 (0.86) |
| CKD stage 5 (%) | 2 (0.03) | 1 (0.01) | 1 (0.12) |
SBP systolic blood pressure, DBP diastolic blood pressure, HTN_treat the person treated as hypertension from questionnaire, HTN_diag the persons diagnosed based on The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014), Ualb/UCr urinary albumin excretion corrected by urinary creatinine, UNa urinary sodium, UK urinary potassium, HbA1c(NGSP) hemoglobin A1c valued as National Glycohemoglobin Standardization Program, sCre serum creatinine, sCysC serum cystatin C, eGFRcre estimated glomerular filtration rate calculated by serum creatinine, eGFRcys estimated glomerular filtration rate calculated by serum cystatin C, CKD stage 1 eGFRcys ≥ 90, CKD stage 2 ≤ 60 eGFRcys < 90, CKD stage 3a 45 ≤ eGFRcys < 60, CKD stage 3b 30 ≤ eGFRcys < 45, CKD stage 4 15 ≤ eGFRcys < 30, CKD stage 5 eGFRcys < 15, Ualb − the group without microalbuminuria nor overt albuminuria, Ualb + the group of microalbuminuria or overt albuminuria, IQR interquartile range
*Median value
About 10% of patients had UAE of > 30 mg/gCr, and hence most of the patients had microalbuminuria. The mean age of the UAE positive patients was 64.4 ± 8.64 years and 43.6% of these patients were males. The mean systolic blood pressure of these patients was 137 ± 19.9 mmHg and their average eGFRcys was 89.5 ± 23.7 ml/min/1.73 m2. The mean age of the UAE negative patients was 60.4 ± 11.5 years and 33.7% of these patients were male. The mean systolic blood pressure of these patients was 126 ± 17.2 mmHg and their average eGFRcys was 98.4 ± 21.3 ml/min/1.73 m2.
When we evaluate correlation between each covariant and urinary albumin excretion, there is no significant correlation (Table S1). When we evaluate correlation between each covariant and other covariant, there are weak correlations between age and eGFRcys (correlation factor 0.56), SBP and BMI (correlation factor 0.48), UNa and UK (correlation factor 0.44) (Table S2).
Genome-wide association study for UAE in the Japanese populations
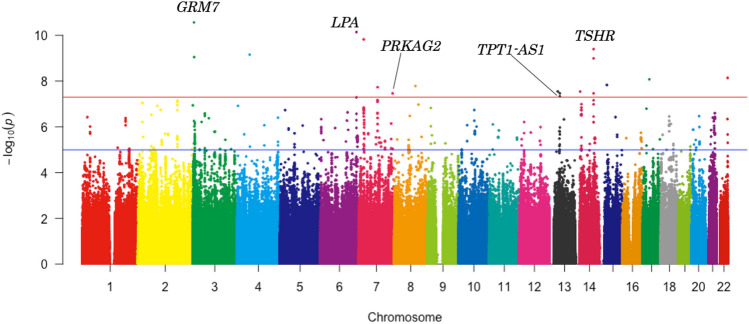
We performed GWAS in 7805 individuals. After genotyping, 595,171 SNPs passed the quality controls and were used for the following imputation analysis. For the imputation analysis, a haplotype reference panel based on the 2049 drafts of the whole genome sequencing was used. Finally, 4,962,728 variants were used for the GWAS. The Q-Q plot is shown in Fig. 1. The genomic inflation factor (λ) showed 0.987 suggesting that the population substructure should not have any substantial effects on the association analysis [14]. Under these conditions, we obtained 18 genome-wide significant SNPs (Table 2). We constructed a Manhattan plot of this GWAS as shown in Fig. 2.
Fig. 1.
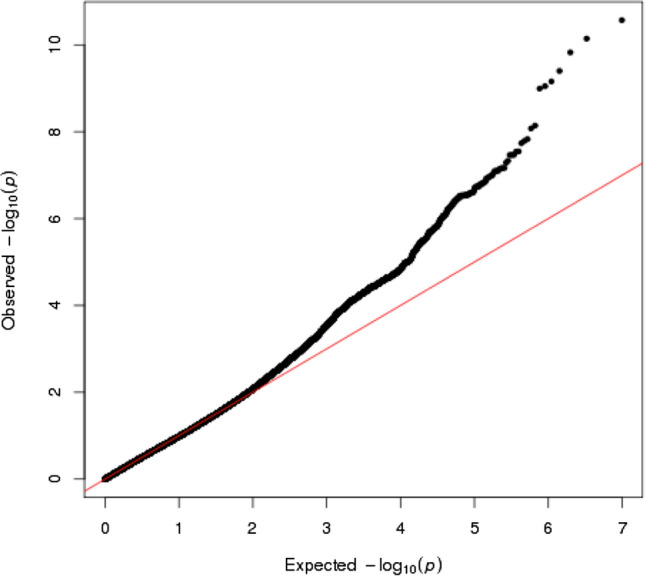
Q-Q plots of GWAS about UAE in the TMM cohort study. The negative logarithm of the observed (y-axis) and the expected (x-axis) p-value was plotted for each SNP (dot), and the red line (y = x) indicates the null hypothesis of no true association. The regression genomic inflation factor (λ score) is 0.987 (SE 6.07 × 10−6) to adequately control the population stratification
Table 2.
UAE associated SNPs reaching a genome-wide significance
| Chr | SNP | Position | Gene(s) | ref | alt | EA | EAF | BETA | SE | INFO | p value | AR2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | rs143146694 | 6,263,450 | GRM7 | G | T | T | 0.035 | 37.81 | 5.667 | 0.931 | 2.69 × 10–11 | 0.034 |
| 3 | rs74971332 | 7,058,507 | GRM7 | C | G | G | 0.055 | 26.43 | 4.307 | 0.97 | 8.91 × 10–10 | 0.034 |
| 4 | rs75938525 | 56,659,946 | EXOC1/NMU | G | C | C | 0.048 | 31.06 | 5.029 | 0.956 | 6.93 × 10–10 | 0.034 |
| 6 | rs146871152 | 160,984,637 | LPA | C | T | T | 0.034 | 37.28 | 5.713 | 0.97 | 7.16 × 10–11 | 0.037 |
| 7 | rs146418897 | 22,440,870 | STEAP1B/RAPGEF5 | T | C | C | 0.033 | 36.85 | 5.743 | 0.941 | 1.49 × 10–10 | 0.034 |
| 7 | rs140221313 | 84,600,098 | SEMA3D | G | A | A | 0.038 | 31.17 | 5.534 | 0.934 | 1.84 × 10–8 | 0.034 |
| 7 | rs118160950 | 151,277,450 | PRKAG2 | C | T | T | 0.036 | 30.48 | 5.517 | 0.975 | 3.43 × 10–8 | 0.036 |
| 8 | rs141491217 | 94,068,096 | TRIQK | A | G | G | 0.039 | 33.01 | 5.84 | 0.932 | 1.63 × 10–8 | 0.035 |
| 13 | rs79163227 | 37,208,221 | SERTM1 | A | G | G | 0.056 | 25.92 | 4.664 | 0.941 | 2.84 × 10–8 | 0.035 |
| 13 | rs142317900 | 45,963,584 | TPT1-AS1 | G | A | A | 0.033 | 31.13 | 5.693 | 0.98 | 4.68 × 10–8 | 0.037 |
| 13 | rs151183316 | 46,021,543 | TPT1-AS1 | C | T | T | 0.035 | 30.6 | 5.54 | 0.981 | 3.43 × 10–8 | 0.035 |
| 14 | chr14:21617499_TCTCA_T | 21,617,499 | OR5AU1 | TCTCA | T | T | 0.052 | 28.48 | 5.126 | 0.871 | 2.87 × 10–8 | 0.034 |
| 14 | rs116622332 | 81,506,821 | TSHR | T | C | C | 0.046 | 30.67 | 4.897 | 0.98 | 3.99 × 10–10 | 0.036 |
| 14 | rs199612558 | 81,508,922 | TSHR | T | TA | TA | 0.046 | 29.57 | 4.835 | 0.983 | 1.00 × 10–9 | 0.037 |
| 14 | rs17111387 | 81,515,680 | TSHR | C | T | T | 0.068 | 22.24 | 4.025 | 0.996 | 3.42 × 10–8 | 0.037 |
| 15 | rs140272046 | 33,494,078 | FMN1/RYR3 | G | A | A | 0.042 | 33.38 | 5.886 | 0.886 | 1.47 × 10–8 | 0.034 |
| 17 | rs148283070 | 30,129,004 | COPRS | A | G | G | 0.034 | 34.15 | 5.922 | 0.972 | 8.42 × 10–9 | 0.036 |
| 22 | chr22:49949123_GA_G | 49,949,123 | BRD1 | GA | G | G | 0.052 | 27.83 | 4.805 | 0.945 | 7.22 × 10–9 | 0.034 |
Chr Chromosome, SNP single-nucleotide polymorphism, Position Chromosome position (GRCh37/hg19), Gene The name of Gene where the SNP is located, ref reference allele, alt alternative allele, EA effective allele, EAF effective allele frequency, BETA regression coefficient, SE Standard error of regression coefficient, INFO INFO score, AR2 Adjusted coefficient of determination. Bold type signifies that the SNPs were located on the gene. Normal type signifies that the SNPs were around the gene
Fig. 2.
Manhattan plot of the GWAS for UAE in the TMM cohort study. The X-axis represents the chromosomal positions and the Y-axis represents the – log10 p-values. The red horizontal line indicates the genome-wide significance threshold of p = 5 × 10−8 and the blue horizontal line indicates the genome-wide suggestive threshold of p = 5 × 10−5. The name of the genes where the SNPs were located is typed in Manhattan plot
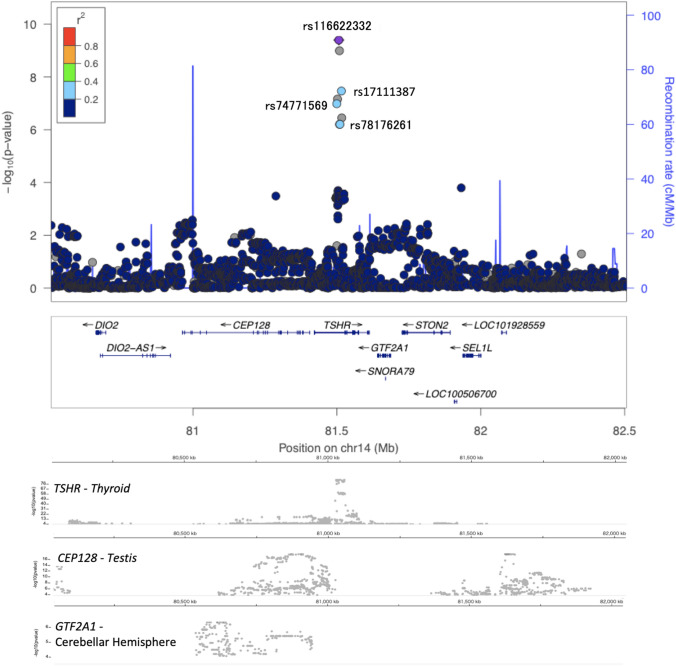
With respect to the SNPs meeting the significance level, we constructed a regional plot to visually examine LD with the surrounding SNPs (around 1 Mbp) and identified the gene in or around which the SNPs were located. We attached the result of eQTL analyses for genome-wide significant SNPs by GTEx (Fig. 3, Fig S1). Three SNPs were located in TSHR on chromosome 14q31 [rs116622332 (p = 3.99 × 10−9), rs199612558 (p = 1.00 × 10−9), and rs17111387 (p = 3.42 × 10−8)] (Fig. 3). Two SNPs were located in GRM7 on chromosome 3p26.1 [rs143146694 (p = 2.69 × 10−11, and rs74971332 (p = 8.91 × 10−10) (Fig S1(a)). One SNP was located in LPA on chromosome 6q25.3 [rs146871152 (p = 7.16 × 10–11)] (Fig S1(b)). One SNP was located in PRKAG2 on chromosome 7q36.1 [rs118160950 (p = 3.43 × 10−8)] (Fig S1(c)). Two SNPs were located in TPT1-AS1 on chromosome 13q14.13 [rs142317900 (p = 4.68 × 10−8) and rs151183316 (p = 3.43 × 10−8)] (Fig S1(d)). One SNP was located in EXOC1/NMU on chromosome 4q12 [rs75938525 (p = 6.93 × 10−10)] (Fig S1(e)). One SNP was located in STEAP1B/RAPGEF5 on chromosome 7p15.3 [rs146418897 (p = 1.49 × 10−10)] (Fig S1(f)). One SNP was located in SEMA3D on chromosome 7q21.11 [rs140221313 (p = 1.84 × 10−8)] (Fig S1(g)). One SNP was located in TRIQK on chromosome 8q22.1 [rs141491217 (p = 1.63 × 10−8)] (Fig S1(h)). One SNP was located in SERTM1 on chromosome 13q13.3 [rs79163227 (p = 2.84 × 10−8)] (Fig S1(i)). One SNP was located in OR5AU1 on chromosome 14q11.2 [chr14:21617499_TCTCA_T (p = 2.87 × 10−8)] (Fig. 3j). One SNP was located in FMN1/RYR3 on chromosome 15q13.3 [rs140272046 (p = 1.47 × 10−8)] (Fig S1(k)). One SNP was located in COPRS on chromosome 17q11.2 [rs148283070 (p = 8.42 × 10−9)] (Fig S1(l)). One SNP was located in BRD1 on chromosome 22q13.33 [chr22:49949123_GA_G (p = 7.22 × 10−9)] (Fig S3(m)).
Fig. 3.
Association signals around the significant loci in TSHR locus. The upper panel is association signals around significant loci. The X-axis represents chromosomal positions (GRC37/hg19) and the Y-axis represents − log10 p -values. The lead variant is shown in purple. Colors represent the degree of LD (r2) between each variant and the lead variant. The LD (r2) was calculated based on the combined dataset of TMM subjects. The lower panels represent the Single-tissue eQTL analyses, where the target was mostly expressed. The data were from GTEx (V8). The X-axis is represents chromosomal positions (GRC38/hg38) and the Y-axis represents − log10 eQTL p -values. The X-axis between the upper and the lower panel is adjusted by calculating with hgLiftOver [https://genome.ucsc.edu/cgi-bin/hgLiftOver].
Discussion
We performed GWAS for UAE in the Japanese general population and identified 18 SNPs, of which, 17 were not reported in any previous report. rs118160950 was already reported as a SNP related to UAE by GWAS performed in the European ancestry [31]. In the previous reports of GWAS for UAE, the study subjects were not a general cohort but consisted of diabetes patients, heart failure patients, or pregnant women with hypertension. In addition, the study subjects were mainly European or African American but not Asians. Our GWAS has profound significance among the Japanese general population.
In previous studies, rs10795433 [9] and rs1801239 [13] were reported as the significant SNPs associated with UAE. They are located on the CUBN gene locus. CUBN encodes cubilin protein acting as a receptor for vitamin B12-intrinsic factor complexes. It was hypothesized that the SNPs found in CUBN on chromosome 10 were significantly (p = 1.0 × 10−11) involved in UAE. However, these loci were not found to be significantly associated in our study. The reasons seem to be related to the difference in the studied population, because the reported significant covariates were not replicated in our population. We showed evidence of genetic differences between the Europeans and the East Asians. The other possibility is that these SNPs on CUBN are associated with UAE only in the diseased condition. There may be big differences in the mechanism between pathophysiological albuminuria and physiological albuminuria.
When we evaluated the functional class of 18 SNPs, 6 SNPs (rs74971332, rs146871152, rs118160950, rs116622332, rs199612558, and rs17111387) were intronic, 11 SNPs (rs143146694, rs75938525, rs146418897, rs140221313, rs141491217, rs79163227, rs151183316, chr14:21617499_TCTCA_T, rs140272046, rs148283070, and chr22:49949123_GA_G) were intergenic, and 1 SNP (rs142317900) was on the non-coding exons. No SNP was located on the coding exons. These candidates may possibly affect the factors regulating the transcription of the genes encoding the proteins involved in UAE.
In our study, the SNPs located in TSHR showed strong peaks. TSHR encodes TSHR (thyroid stimulating hormone receptor), and the TSH receptor is a member of the G protein-coupled receptor superfamily of integral membrane proteins which is coupled to the Gs protein [32, 33]. TSHR expresses mainly on the surface of the thyroid follicular cells and contributes to thyroid hormone secretion. Several pathways are proposed for kidney injury and proteinuria mediated by thyroid dysfunction [34, 35]. In hyperthyroidism, intra-glomerular hypertension, consequent hyperfiltration, increased production of free radicals, and increased renin–angiotensin–aldosterone system are risk factors for albuminuria. In hypothyroidism, GFR and tubular transport capacity are reduced. Hypothyroidism also results in increased glomerular capillary permeability to proteins directly causing proteinuria [36]. The other possibility is that TSHR could have direct effects on albumin re-uptake on the tubular cells of the kidney. TSHR is also expressed in other tissue, for e.g. adipose tissues and fibroblasts. It is known that a small amount of TSHR exists in the kidneys and mainly in the tubules [37–39]. We evaluated the expression of TSHR by immunohistochemistry in the renal biopsy specimens. We found a weak expression of TSHR in the kidney mainly in the tubules. A significant association between the staining level of TSHR and proteinuria was not detected (data not shown) and further study should be required to prove this concept.
PRKAG2 coding 5′-AMP-activated protein kinase subunit gamma-2. AMP-activated protein kinase (AMPK) is a heterotrimeric protein composed of a catalytic alpha subunit, a noncatalytic beta subunit, and a noncatalytic regulatory gamma subunit [40]. AMPK is an important energy-sensing enzyme that monitors the cellular energy status and functions by inactivating the key enzymes involved in regulating the de novo biosynthesis of fatty acids and cholesterol. Mutations in this gene have been associated with WPW (Wolff-Parkinson-White) syndrome [41, 42], familial hypertrophic cardiomyopathy [43, 44], and enlarged kidneys [45]. Studies in transgenic mice indicate that these mutations cause glycogen storage disease of the heart [46]. Several other hereditary glycogen storage diseases present with renal pathologies, such as renal tubular dysfunction [47]. PRKAG2 did not indicate any stronger significance in our GWAS. However, we may consider that renal tubular dysfunction induced by glycol storage can affect the UAE.
We used the GTEx database to examine eQTL of signiicant variants and suggestive variants in each locus. Unfortunately, no eQTL was found in the lead significant variants (Table S3). Then we extended the candidates for including suggestive variants whose p value was less than 1.0 × 10–5, and also that has strong LD against each significant SNP (r2 > 0.2). There are significant eQTL of NMNAT1P1 pseudo-gene on TSHR gene locus in rs17111387, rs74771569, rs78176261, which three SNPs had strong LD against rs116622332 (the lead variant in Chromosome 14). Though NMNAT1P1 itself is a pseudo-gene, many significant eQTL variants are also shared with TSHR and NMNAT1P1. Additionally, about the genes around every lead variant, we can find the consistency between the peak of Manhattan plot and the peak of eQTL information about TSHR in thyroid (Fig. 3). That means there is a probability that rs116622332 allele on chromosome 14, affects TSHR and NMNAT1P1 expression in thyroid. eQTL analysis identified that rs77317344 ,which is in strong LD with rs14237900 (the lead variant in Chromosome 13), affects the expression of COG3. We cannot find other significant information in eQTL analysis about the other genes.
There are some limitations to our study. First, in this study, replication is lacking. Replication studies in other Japanese cohorts and/or other populations are required. Second, many of the individuals analyzed in our study were affected by the Great East Japan Earthquake of 2011. We should consider mental disturbance and stress caused by this big disaster as a confounding factor. To conclude, we investigated the UAE associated SNPs in the Japanese population after adjusting for age, gender, hypertension, and impaired glucose tolerance. The 18 identified SNPs were uncovered to show a statistically significant effect on the UAE. There are limited studies evaluating the association with other candidate genes that we detected. The functional and biological roles exerted by each of the SNPs/genes are required to be elucidated in further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the TMM Project (Special Account for Reconstruction from the Great East Japan Earthquake) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Agency for Medical Research and Development (AMED). The authors sincerely express thanks to all the patients and volunteers who were enrolled in the ToMMo and IMM in the disaster-struck areas affected by the Great East Japan Earthquake. We thank the members of ToMMo and IMM, including GMRCs, office and administrative personnel, and software engineers, for their assistance in the projects. The full list of the members is available at https://www.megabank.tohoku.ac.jp/english/a190601/ for ToMMo and https://iwate-megabank.org/en/about/departments/for IMM.
Data availability
The datasets analyzed in this study are not open to the public for ethical reasons but are available upon request after approval of the Ethical Committee of Tohoku University, the Ethical Committee of Iwate Medical University, and the Materials and Information Distribution Review Committee of the TMM Project.
Compliance with ethical standards
Conflict of interest
The author(s) received no specific funding for this work.
Human and animal rights
The study was conducted in accordance with the guidelines written in the Declaration of Helsinki. We obtained approval from the relevant ethics committees at Tohoku Medical. Megabank Organization. The assignment number is 2018-4-034.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
The original version of this article was revised due to a Retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/27/2021
A Correction to this paper has been published: 10.1007/s10157-021-02053-4
References
- 1.Collaborators GMaCoD. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544. doi: 10.1016/S0140–6736(16)31012–1. [DOI] [PMC free article] [PubMed]
- 2.Ozieh MN, Bishu KG, Dismuke CE, Egede LE. Trends in healthcare expenditure in United States adults with chronic kidney disease: 2002–2011. BMC Health Serv Res. 2017;17(1):368. doi: 10.1186/s12913-017-2303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 5.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT, et al. Urinary albumin excretion and its relation with C-reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care. 2005;28(10):2525–2530. doi: 10.2337/diacare.28.10.2525. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.CIR.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 8.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 9.Teumer A, Tin A, Sorice R, Gorski M, Yeo NC, Chu AY, et al. Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes. 2016;65(3):803–817. doi: 10.2337/db15-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandholm N, Forsblom C, Mäkinen VP, McKnight AJ, Osterholm AM, He B, et al. Genome-wide association study of urinary albumin excretion rate in patients with type 1 diabetes. Diabetologia. 2014;57(6):1143–1153. doi: 10.1007/s00125-014-3202-3. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SJ, Yang Q, Meigs JB, Pearce EN, Fox CS. A genome-wide association for kidney function and endocrine-related traits in the NHLBI's Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S10. doi: 10.1186/1471-2350-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis JW, Chen MH, Foster MC, Liu CT, Larson MG, de Boer I, et al. Validated SNPs for eGFR and their associations with albuminuria. Hum Mol Genet. 2012;21(14):3293–3298. doi: 10.1093/hmg/dds138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böger CA, Chen MH, Tin A, Olden M, Köttgen A, de Boer IH, et al. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22(3):555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hachiya T, Komaki S, Hasegawa Y, Ohmomo H, Tanno K, Hozawa A, et al. Genome-wide meta-analysis in Japanese populations identifies novel variants at the TMC6-TMC8 and SIX3-SIX2 loci associated with HbA. Sci Rep. 2017;7(1):16147. doi: 10.1038/s41598-017-16493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuriyama S, Yaegashi N, Nagami F, Arai T, Kawaguchi Y, Osumi N, et al. The Tohoku Medical Megabank Project: Design and Mission. J Epidemiol. 2016;26(9):493–511. doi: 10.2188/jea.JE20150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka F, Yamamoto K, Suzuki S, Inoue H, Tsurumaru M, Kajiyama Y, et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut. 2010;59(11):1457–1464. doi: 10.1136/gut.2009.205724. [DOI] [PubMed] [Google Scholar]
- 18.Delaneau O, Howie B, Cox AJ, Zagury JF, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93(4):687–696. doi: 10.1016/j.ajhg.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasaki M, Yasuda J, Katsuoka F, Nariai N, Kojima K, Kawai Y, et al. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat Commun. 2015;6:8018. doi: 10.1038/ncomms9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveras A, Armario P, Martell-Clarós N, Ruilope LM, de la Sierra A, Registry SSoH-RH Urinary albumin excretion is associated with nocturnal systolic blood pressure in resistant hypertensives. Hypertension. 2011;57(3):556–560. doi: 10.1161/HYPERTENSIONAHA.110.165563. [DOI] [PubMed] [Google Scholar]
- 22.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 23.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S, GFR CDtJEfE GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61(2):197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Liu Y, Chen Y, Li Y, Shao X, Liang Y, et al. Body mass index (BMI) is associated with microalbuminuria in Chinese hypertensive patients. Int J Environ Res Public Health. 2015;12(2):1998–2008. doi: 10.3390/ijerph120201998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005;54(10):2983–2987. doi: 10.2337/diabetes.54.10.2983. [DOI] [PubMed] [Google Scholar]
- 27.Aaron KJ, Campbell RC, Judd SE, Sanders PW, Muntner P. Association of dietary sodium and potassium intakes with albuminuria in normal-weight, overweight, and obese participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Clin Nutr. 2011;94(4):1071–1078. doi: 10.3945/ajcn.111.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta-analysis of the jackson heart study, the Multi-Ethnic study of atherosclerosis, and the cardiovascular health study. Clin J Am Soc Nephrol. 2017;12(9):1386–1398. doi: 10.2215/CJN.01860217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A novel approach to high-quality postmortem tissue procurement: The GTEx project. Biopreserv Biobank. 2015;13(5):311–319. doi: 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37(4):253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 31.Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, Glazer NL, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42(5):376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farid NR, Szkudlinski MW. Minireview: structural and functional evolution of the thyrotropin receptor. Endocrinology. 2004;145(9):4048–4057. doi: 10.1210/en.2004-0437. [DOI] [PubMed] [Google Scholar]
- 33.Calebiro D, Nikolaev VO, Lohse MJ. Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J Mol Endocrinol. 2010;45(1):1–8. doi: 10.1677/JME-10-0014. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160(4):503–515. doi: 10.1530/EJE-08-0837. [DOI] [PubMed] [Google Scholar]
- 35.Vargas F, Moreno JM, Rodríguez-Gómez I, Wangensteen R, Osuna A, Alvarez-Guerra M, et al. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol. 2006;154(2):197–212. doi: 10.1530/eje.1.02093. [DOI] [PubMed] [Google Scholar]
- 36.Wheatley T, Edwards OM. Mild hypothyroidism and oedema: evidence for increased capillary permeability to protein. Clin Endocrinol (Oxf) 1983;18(6):627–635. doi: 10.1111/j.1365-2265.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams GR. Extrathyroidal expression of TSH receptor. Ann Endocrinol (Paris). 2011;72(2):68–73. doi: 10.1016/j.ando.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS. Thyrotropin receptor expression in adrenal, kidney, and thymus. Thyroid. 1997;7(6):879–884. doi: 10.1089/thy.1997.7.879. [DOI] [PubMed] [Google Scholar]
- 39.Sellitti DF, Akamizu T, Doi SQ, Kim GH, Kariyil JT, Kopchik JJ, et al. Renal expression of two 'thyroid-specific' genes: thyrotropin receptor and thyroglobulin. Exp Nephrol. 2000;8(4–5):235–243. doi: 10.1159/000020674. [DOI] [PubMed] [Google Scholar]
- 40.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–669. doi: 10.1042/bj3460659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaughan CJ, Hom Y, Okin DA, McDermott DA, Lerman BB, Basson CT. Molecular genetic analysis of PRKAG2 in sporadic Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2003;14(3):263–268. doi: 10.1046/j.1540-8167.2003.02394.x. [DOI] [PubMed] [Google Scholar]
- 42.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344(24):1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 43.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10(11):1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 44.Gollob MH. Modulating phenotypic expression of the PRKAG2 cardiac syndrome. Circulation. 2008;117(2):134–135. doi: 10.1161/CIRCULATIONAHA.107.747345. [DOI] [PubMed] [Google Scholar]
- 45.Burwinkel B, Scott JW, Bührer C, van Landeghem FK, Cox GF, Wilson CJ, et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76(6):1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109(3):357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozen H. Glycogen storage diseases: new perspectives. World J Gastroenterol. 2007;13(18):2541–2553. doi: 10.3748/wjg.v13.i18.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are not open to the public for ethical reasons but are available upon request after approval of the Ethical Committee of Tohoku University, the Ethical Committee of Iwate Medical University, and the Materials and Information Distribution Review Committee of the TMM Project.