Highlights
-
•
Measles virus replication in lymphoid tissue uses CD150 as a B and T cell receptor.
-
•
Persistent RNA in lymphoid tissue is associated with generation of durable immunity.
-
•
Germinal centers and numbers of peripheral Tfh cells and antibody-secreting cells increase for months.
-
•
Increased susceptibility to other infections continues for years after apparent recovery.
-
•
Memory and naïve B cells are depleted and diversity of pre-existing antibodies is reduced.
Abstract
Effects of measles on the immune system are only partially understood. Lymphoid tissue is a primary site of measles virus (MeV) replication where CD150 is the receptor for infection of both B and T cells. Lymphocyte depletion occurs during the acute phase of infection, but initiation of the adaptive immune response leads to extensive lymphocyte proliferation, production of MeV-specific antibody and T cells, the rash and clearance of infectious virus. Viral RNA persists in lymphoid tissue accompanied by ongoing germinal center proliferation, production of antibody-secreting cells, functionally distinct populations of T cells and antibody avidity maturation to establish life-long immunity. However, at the same time diversity of pre-existing antibodies and numbers of memory and naive B cells are reduced and susceptibility to other infections is increased.
Current Opinion in Virology 2021, 46:9–14
This review comes from a themed issue on Viral immunology
Edited by Allan J Zajac and Annette Oxenius
For complete overview of the section, please refer the article collection – Viral Immunology (2021) and https://www.sciencedirect.com/topics/medicine-and-dentistry/viral-immunology
Available online 4th September 2020
https://doi.org/10.1016/j.coviro.2020.08.002
1879-6257/© 2020 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Measles is a systemic rash disease that is an increasing cause of morbidity and mortality worldwide despite the availability of a safe and effective live attenuated virus vaccine [1,2]. Infection with measles virus (MeV), the causative agent of measles, is initiated in the respiratory tract, rapidly spreads to lymphoid tissue and has profound short and long-term effects on the immune system. For instance, MeV infection increases susceptibility to other infections that are responsible for most of the measles-associated acute mortality [3,4,5•], can trigger autoimmune encephalomyelitis [6] and induces lifelong protective immunity [7,8]. Because macaques develop a disease very similar to human measles, investigations aimed at understanding the immunopathogenisis of measles have focused on experimentally infected macaques as well as naturally infected children [9, 10, 11].
MeV is an enveloped negative sense RNA virus that belongs to the Morbillivirus genus of Paramyxoviridae. MeV encodes two surface glycoproteins, hemagglutinin (H) and fusion (F); four internal proteins, matrix (M), nucleocapsid (N), phosphoprotein (P) and large polymerase (L); and two nonstructural regulatory proteins, C and V. H is on the surface of the virion as a dimer of dimers in non-covalent association with F and is the attachment protein for cell entry and target for neutralizing antibody. H can interact with several receptors: CD46 is a complement regulatory protein expressed on all nucleated cells, but used mainly by vaccine strains of MeV. Nectin 4 is an adherens junction protein expressed on the basolateral surface of epithelial cells and used by both vaccine and wild type (WT) strains of MeV. CD150/signaling lymphocyte activation molecule (SLAM) is an immunoregulatory protein expressed on activated lymphocytes, monocytes and dendritic cells used by both vaccine and WT viruses and is the primary receptor for WT MeV infection of lymphoid tissue and systemic virus spread [10,12].
Measles virus replication in lymphoid tissue
After initiation of infection in the respiratory tract, MeV is transported to the draining lymph node (LN) where it infects CD150/SLAMF1-expressing cells. CD150 is a glycosylated immunoglobulin superfamily protein that was the first member (F1) of the six-member SLAM subfamily of CD2 transmembrane proteins. Most SLAM family proteins have an extracellular segment that consists of two immunoglobuin-like domains (one variable (V)-like and one constant (C)-like), a transmembrane and a cytoplasmic domain. The V-like extracellular domain is a self ligand that binds SLAM proteins on other cells in homotypic or heterotypic cell interactions [13, 14, 15]. The cytoplasmic domain has immunoreceptor tyrosine-based switch motifs (ITSMs) that are docking sites for SH2 domain-containing adaptor, tyrosine kinase and tyrosine and inositol phosphatase proteins that mediate and regulate SLAM signaling [16,17].
The MeV receptor SLAMF1 is constitutively expressed on immature thymocytes, naïve and memory B cells, memory T cells and is induced during activation of naïve T cells [18,19]. In dendritic cells and macrophages SLAMF1 is localized to endocytic recycling vesicles and trafficked to the surface with Raf-1 and ERK-induced activation of acidic sphingomyelinase in the vesicles [15,20]. SLAMF1 is a dual function co-stimulatory molecule and interaction of the ITSM in the cytoplasmic domain with SLAM adaptor protein (SAP) determines whether SLAM transmits a positive or negative signal in T cells [16,21]. SAP, first identified as a T cell protein mutated in X-linked lymphoproliferative disease, binds both phosphorylated and unphosphorylated ITSMs to regulate recruitment of phosphatases SHP-1 and SHP-2 and control signaling [16]. SLAM promotes TCR-induced CD28-independent proliferation of memory T cells, Th1 differentiation and IFN-γ production of naïve T cells, IL-17 production, proliferation and cytotoxicity of CD8+ T cells, and proliferation and antibody secretion by B cells [21, 22, 23, 24].
H interaction with SLAMF1 or other cellular receptors induces a conformational change in F that leads to fusion of the viral envelope with the plasma membrane and initiation of infection with delivery of the genome into the cell cytoplasm. H also interacts with toll-like receptor (TLR) 2, a pathogen recognition receptor expressed on most immune cells and epithelial cells that signals through adaptor proteins MyD88 and IRAK4 to activate NF-κB, induce transcription of mRNAs for IL-6 family member proteins, IL-1β and TNFα and increase expression of SLAM [25]. The effect of H-induced SLAMF1 or TLR2 signaling on virus replication in lymphocytes or on immune responses to MeV is not known.
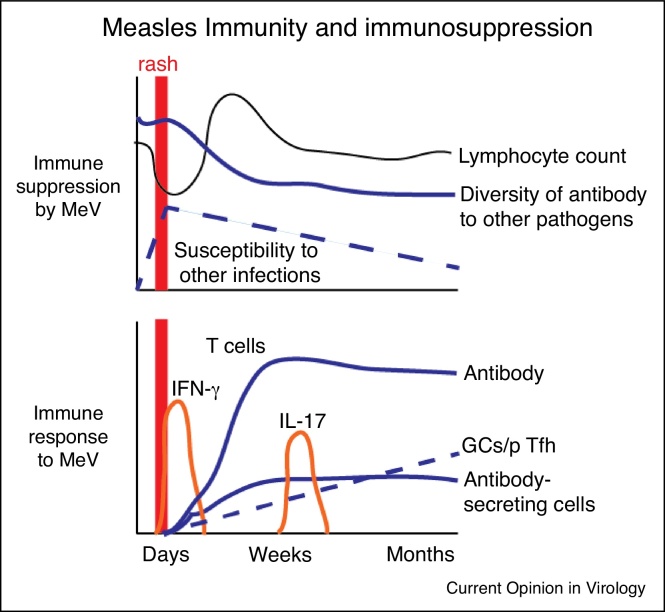
Immune cells expressing SLAMF1 in lymphoid tissue are the main sites of virus amplification and fuel the viremia and systemic spread of MeV [10]. Virus replication in lymphoid cells has been studied ex vivo in thymus organ cultures, tonsil explants and peripheral blood mononuclear cells (PBMCs) and has shown preferential replication in double-positive thymocytes, B cells and memory T cells consistent with SLAMF1 expression [26, 27, 28, 29]. In vivo, there is extensive replication of MeV in B and T cells in blood and lymphoid tissues with a higher percentage of MeV-positive B cells than T cells. Replication occurs in both naïve and memory B cells, but primarily in memory T cells [29,30,31•,32••]. MeV infection can induce lymphocyte cell death and leukopenia commonly accompanies the viremia [29,33,34]. Lymphocyte depletion is followed by a rapid rebound in cell numbers with immune-mediated lymphocyte activation and proliferation as well as an increase in output of cells from the thymus [35,36]. In LNs, this phase of B and T cell depletion is followed at the time of the rash by LN enlargement due to repopulation with proliferating lymphocytes [29]. B cell follicles expand and germinal centers increase in number and continue to produce MeV-specific antibody-secreting cells (ASCs) for several months after recovery [37••] (Figure 1).
Figure 1.
Diagrammatic representation of the dual effects of measles virus (MeV) infection on the immune system: immune suppression with a prolonged increase in susceptibility to other infections (top panel) and induction of a vigorous immune response to MeV that results in life-long immunity to re-infection (lower panel). GC – germinal center; pTfh – peripheral T follicular helper cells.
The effects of cell depletion and proliferation change the relative representation of subtypes of immune cells in circulation over time [31•,38]. In general, naïve T cells and memory B cells are decreased while activated and memory T cells and regulatory T cells are increased after recovery compared to before infection [31•]. Several waves of functionally distinct MeV-specific T cells appear in circulation during recovery and cytokine production shifts from interferon (IFN)-γ to IL-4, IL-10 and IL-17 [39,40]. MeV-specific ASCs are found in blood as the rash fades and then continue to be produced for several months after recovery [37••] (Figure 1).
Immune-mediated clearance
MeV infection is clinically inapparent during the incubation period when virus is actively replicating in lymphoid tissue and spreading systemically. Innate responses are not well defined with evidence primarily of inflammasome (IL-1β, IL-18) and NF-κB (IL-6), rather than type I IFN pathway activation, but these responses do not prevent virus replication and dissemination [11,41]. Clearance is dependent on the adaptive immune response. The maculopapular rash that appears 10–14 days after infection is a manifestation of the cellular immune response to infection with lymphocyte infiltration into sites of virus replication in skin epithelial cells [42]. MeV-specific IFN-γ-producing T cells and IgM antibodies are detectable in blood as the rash is fading and infectious virus is cleared within a week after appearance of the rash. MeV-specific IgM antibodies provide the primary diagnostic test for confirmation of a diagnosis of measles. Although antibody is likely to contribute, MeV-specific T cell responses are required for virus clearance [43,44].
Clinical evidence of the importance of cellular immunity to MeV for virus clearance comes from observations of the outcome of infection in immunocompromised children. Congenital inability to produce antibodies allows recovery from measles, while defects in T-lymphocyte function can lead to fatal progressive pulmonary or neurologic disease [45, 46, 47, 48]. The cellular immune response to MeV infection includes activation of CD4+ and CD8+ T lymphocytes that evolve functionally during and after recovery [38]. The predominant initial cellular response important for control and clearance of infectious virus is characterized by appearance of MeV-specific IFN-γ-producing CD4+ T cells and cytotoxic CD8 + T cells [40,43,49,50]. However, MeV RNA can be detected in samples from several sites for at least 3 months in naturally infected children and in the lymphoid tissue of experimentally infected macaques for at least six months [37••,43,51,52]. The later shift from MeV-specific Th1 to Th2, Th17 and Tfh CD4+ T cell responses may be important for the production and maturation of MeV-specific antibodies.
Maturation of the immune response and development of protective immunity
Protective immunity following WT MeV infection is lifelong [7] as is the production of MeV-specific antibody [8]. Several lines of evidence suggest a primary role for antibody in protection: Vaccine-induced neutralizing antibody levels correlate with protection from clinical measles, passively acquired maternal antibodies protect infants from infection and anti-MeV immune globulin protects exposed, susceptible individuals from disease [53,54]. However, analysis of protection provided by a variety of experimental vaccines in macaques indicates that neutralizing antibody alone generally protects from disease (rash), but not necessarily from infection [55,56]. Vaccine-induced T cell responses alone do not prevent infection or affect the level of viremia or development of a rash, but do lead to more rapid clearance of viral RNA from PBMCs [57].
The mechanism(s) involved in sustaining high levels of neutralizing antibody to MeV are not completely understood, but persistent MeV RNA in lymphoid tissue is associated with continued development of germinal centers, production of ASCs that traffic to the bone marrow and progressive avidity maturation suggesting that prolonged immune stimulation may be an important factor [37••]. Immunologic memory to MeV includes both continued circulation of MeV-specific CD4+ and CD8+ T lymphocytes so long-lasting cellular immunity may also play an important supportive role in protection from infection and disease.
Immune suppression and effects on pre-existing immune responses
Measles poses an interesting paradox in the juxtaposition of a robust MeV-specific immune response with prominent suppression of immunity to other pathogens and increase in susceptibility to other infectious diseases [58,59•]. Most measles deaths are due to other infections [3] and there is an epidemiologically detectable long-term increase in susceptibility to infectious diseases in those who survive [4,5•,59•]. In addition to infection of B and T cells and a transient lymphopenia during the acute phase of infection, MeV acts both directly on T cells to inhibit activation-induced proliferation and produces a long-term generalized effect on pathogen recognition.
MeV affects T cell function directly by interaction of MeV H and proteolytically activated F with lipid rafts on the cell surface of lymphocytes to block cell cycle progression independent of interaction with SLAMF1. This process involves activation of sphingomyelinases to produce ceramide and inhibit cytoskeletal reorganization required for T cell responses to receptor crosslinking [60, 61, 62, 63].
Measles also increases long-term susceptibility to infection by altering the repertoire of pathogen-specific antibody responses and types of cells in circulation. Numbers of memory B cells are reduced and there is an incomplete reconstitution of naïve B cells [31•,64••]. In addition, diversity of anti-pathogen antibodies in plasma is decreased presumably due to MeV-induced loss of long-lived plasma cells that produce most of the antibody found in circulation, either due directly to MeV-induced death or eviction from bone marrow niches during the response to MeV [65••]. Myeloma cells are susceptible to MeV-induced apoptosis [66], but bone marrow is not a major site of MeV replication [10,37••], so the mechanism of plasma cell functional loss during measles requires further study.
Conclusions
Measles has a complex interaction with the immune system through direct infection of B and T lymphocytes expressing CD150 that results in transient lymphocyte depletion followed by immune activation that generates life-long immunity to reinfection. Over months after apparent recovery and clearance of infectious virus, MeV RNA persists in lymphoid tissue and drives continued stimulation of MeV-specific B and T cell responses. T cells change from IFN-γ production to IL-17 production and numbers of peripheral Tfh cells increase. Germinal centers proliferate and produce antibody-secreting cells that home to the bone marrow and sustain plasma levels of MeV antibody for life. However, levels of antibody to other pathogens decrease and this likely contributes to a long-term increase in susceptibility to other infections.
Conflict of interest statement
Diane Griffin is a member of the GlaxoSmithKline Vaccines Research Advisory Board and Takeda Pharmaceuticals Zika Vaccine Data Monitoring Committee.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
Acknowledgements
The contributions to this work of Debra Hauer and Drs. Wen-Hsuan Lin, Ashley Nelson, Nicole Putnam, William Moss, Sallie Permar and Robert Adams are gratefully acknowledged. Work from the author’s laboratory was supported by research grants from the Bill and Melinda Gates Foundation and the US National Institutes of Health (R01 AI131228; R21 AI095981). Funders had no role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report or in the decision to submit the article for publication.
References
- 1.Dabbagh A., Laws R.L., Steulet C., Dumolard L., Mulders M.N., Kretsinger K., Alexander J.P., Rota P.A., Goodson J.L. Progress toward regional measles elimination - worldwide, 2000-2017. Morb Mortal Wkly Rep. 2018;67:1323–1329. doi: 10.15585/mmwr.mm6747a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Measles in 2019 - going backward. N Engl J Med. 2019;380:2185–2187. doi: 10.1056/NEJMp1905099. [DOI] [PubMed] [Google Scholar]
- 3.Beckford A.P., Kaschula R.O., Stephen C. Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr Med J. 1985;68:858–863. [PubMed] [Google Scholar]
- 4.Mina M.J., Metcalf C.J., de Swart R.L., Osterhaus A.D., Grenfell B.T. Vaccines. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348:694–699. doi: 10.1126/science.aaa3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Gadroen K., Dodd C.N., Masclee G.M.C., de Ridder M.A.J., Weibel D., Mina M.J., Grenfell B.T., Sturkenboom M., van de Vijver D., de Swart R.L. Impact and longevity of measles-associated immune suppression: a matched cohort study using data from the THIN general practice database in the UK. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-021465. [DOI] [PMC free article] [PubMed] [Google Scholar]; A population-based cohort study showing that the incidence rate for non-measles infectious disease was increased for up to five years after measles.
- 6.Johnson R.T., Griffin D.E., Hirsch R.L., Wolinsky J.S., Roedenbeck S., Lindo de Soriano I., Vaisberg A. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med. 1984;310:137–141. doi: 10.1056/NEJM198401193100301. [DOI] [PubMed] [Google Scholar]
- 7.Panum P. Observations made during the epidemic of measles on the Faroe Islands in the year 1846. Med Classics. 1938;3:829–886. [Google Scholar]
- 8.Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 9.Auwaerter P.G., Rota P.A., Elkins W.R., Adams R.J., DeLozier T., Shi Y., Bellini W.J., Murphy B.R., Griffin D.E. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- 10.de Swart R.L., Ludlow M., de Witte L., Yanagi Y., van Amerongen G., McQuaid S., Yuksel S., Geijtenbeek T.B., Duprex W.P., Osterhaus A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W.W., Nelson A.N., Ryon J.J., Moss W.J., Griffin D.E. Plasma cytokines and chemokines in Zambian children with measles: innate responses and association with HIV-1 coinfection and in-hospital mortality. J Infect Dis. 2017;215:830–839. doi: 10.1093/infdis/jix012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 13.Punnonen J., Cocks B.G., Carballido J.M., Bennett B., Peterson D., Aversa G., de Vries J.E. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Driel B.J., Liao G., Engel P., Terhorst C. Responses to microbial challenges by SLAMF receptors. Front Immunol. 2016;7:4. doi: 10.3389/fimmu.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannons J.L., Schwartzberg P.L. SAP and lessons learned from a primary immunodeficiency. J Immunol. 2017;199:1531–1533. doi: 10.4049/jimmunol.1701007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordiienko I., Shlapatska L., Kovalevska L., Sidorenko S.P. SLAMF1/CD150 in hematologic malignancies: silent marker or active player? Clin Immunol. 2019;204:14–22. doi: 10.1016/j.clim.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Cocks B.G., Chang C.C., Carballido J.M., Yssel H., de Vries J.E., Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 19.Kruse M., Meinl E., Henning G., Kuhnt C., Berchtold S., Berger T., Schuler G., Steinkasserer A. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1 beta. J Immunol. 2001;167:1989–1995. doi: 10.4049/jimmunol.167.4.1989. [DOI] [PubMed] [Google Scholar]
- 20.Schneider-Schaulies J., Schneider-Schaulies S. Sphingolipids in viral infection. Biol Chem. 2015;396:585–595. doi: 10.1515/hsz-2014-0273. [DOI] [PubMed] [Google Scholar]
- 21.Sidorenko S.P., Clark E.A. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.H., Tsai K., Ma C., Vallance B.A., Priatel J.J., Tan R. SLAM-SAP signaling promotes differentiation of IL-17-producing T cells and progression of experimental autoimmune encephalomyelitis. J Immunol. 2014;193:5841–5853. doi: 10.4049/jimmunol.1301435. [DOI] [PubMed] [Google Scholar]
- 23.Cannons J.L., Tangye S.G., Schwartzberg P.L. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 24.Yurchenko M., Shlapatska L.M., Romanets O.L., Ganshevskiy D., Kashuba E., Zamoshnikova A., Ushenin Y.V., Snopok B.A., Sidorenko S.P. CD150-mediated Akt signalling pathway in normal and malignant B cells. Exp Oncol. 2011;33:9–18. [PubMed] [Google Scholar]
- 25.Bieback K., Lien E., Klagge I.M., Avota E., Schneider-Schaulies J., Duprex W.P., Wagner H., Kirschning C.J., Ter Meulen V., Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto Y., Vricella L.A., Moss W.J., Griffin D.E. Immature CD4+CD8+ thymocytes are preferentially infected by measles virus in human thymic organ cultures. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condack C., Grivel J.C., Devaux P., Margolis L., Cattaneo R. Measles virus vaccine attenuation: suboptimal infection of lymphatic tissue and tropism alteration. J Infect Dis. 2007;196:541–549. doi: 10.1086/519689. [DOI] [PubMed] [Google Scholar]
- 28.Grivel J.C., Garcia M., Moss W.J., Margolis L.B. Inhibition of HIV-1 replication in human lymphoid tissues ex vivo by measles virus. J Infect Dis. 2005;192:71–78. doi: 10.1086/430743. [DOI] [PubMed] [Google Scholar]
- 29.de Vries R.D., McQuaid S., van Amerongen G., Yuksel S., Verburgh R.J., Osterhaus A.D., Duprex W.P., de Swart R.L. Measles immune suppression: lessons from the macaque model. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laksono B.M., Grosserichter-Wagener C., de Vries R.D., Langeveld S.A.G., Brem M.D., van Dongen J.J.M., Katsikis P.D., Koopmans M.P.G., van Zelm M.C., de Swart R.L. In vitro measles virus infection of human lymphocyte subsets demonstrates high susceptibility and permissiveness of both naive and memory B cells. J Virol. 2018;92:e00131–18. doi: 10.1128/JVI.00131-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Laksono B.M., de Vries R.D., Verburgh R.J., Visser E.G., de Jong A., Fraaij P.L.A., Ruijs W.L.M., Nieuwenhuijse D.F., van den Ham H.J., Koopmans M.P.G. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun. 2018;9 doi: 10.1038/s41467-018-07515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the PBMCs from children before and after measles and showed decreased frequency of memory B cells and increased frequency of regulatory T cells after measles.
- 32••.Lin W.W., Moran E., Adams R.J., Sievers R.E., Hauer D., Godin S., Griffin D.E. A durable protective immune response to wild-type measles virus infection of macaques is due to viral replication and spread in lymphoid tissues. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aax7799. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared the immune responses to vaccine and WT MeV and showed that a limited ability of vaccine strain MeV to replicate in lymphocytes was associated with lower antibody responses but similar T cell responses compared to WT MeV.
- 33.Iwasa T., Suga S., Qi L., Komada Y. Apoptosis of human peripheral blood mononuclear cells by wild-type measles virus infection is induced by interaction of hemagglutinin protein and cellular receptor, SLAM via caspase-dependent pathway. Microbiol Immunol. 2010;54:405–416. doi: 10.1111/j.1348-0421.2010.00231.x. [DOI] [PubMed] [Google Scholar]
- 34.White R.G., Boyd J.F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973;13:343–357. [PMC free article] [PubMed] [Google Scholar]
- 35.Ward B.J., Johnson R.T., Vaisberg A., Jauregui E., Griffin D.E. Spontaneous proliferation of peripheral mononuclear cells in natural measles virus infection: identification of dividing cells and correlation with mitogen responsiveness. Clin Immunol Immunopathol. 1990;55:315–326. doi: 10.1016/0090-1229(90)90107-2. [DOI] [PubMed] [Google Scholar]
- 36.Permar S.R., Moss W.J., Ryon J.J., Douek D.C., Monze M., Griffin D.E. Increased thymic output during acute measles virus infection. J Virol. 2003;77:7872–7879. doi: 10.1128/JVI.77.14.7872-7879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Nelson A.N., Lin W.W., Shivakoti R., Putnam N.E., Mangus L.M., Adams R.J., Hauer D., Baxter V.K., Griffin D.E. Association of persistent wild-type measles virus RNA with long-term humoral immunity in rhesus macaques. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134992. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of MeV-infected monkeys for 6 months showed persistence of MeV RNA in lymphoid tissue associated with increasing numbers of germinal centers in lymph nodes, continued appearance of antibody-secreting cells and Tfh cells in circulation and accumulation of plasma cells in bone marrow.
- 38.Ryon J.J., Moss W.J., Monze M., Griffin D.E. Functional and phenotypic changes in circulating lymphocytes from hospitalized zambian children with measles. Clin Diagn Lab Immunol. 2002;9:994–1003. doi: 10.1128/CDLI.9.5.994-1003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss W.J., Ryon J.J., Monze M., Griffin D.E. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J Infect Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- 40.Nelson A.N., Putnam N., Hauer D., Baxter V.K., Adams R.J., Griffin D.E. Evolution of T cell responses during measles virus infection and RNA clearance. Sci Rep. 2017;7 doi: 10.1038/s41598-017-10965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivakoti R., Hauer D., Adams R.J., Lin W.H., Duprex W.P., de Swart R.L., Griffin D.E. Limited in vivo production of type I or type III interferon after infection of macaques with vaccine or wild-type strains of measles virus. J Interferon Cytokine Res. 2015;35:292–301. doi: 10.1089/jir.2014.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polack F.P., Auwaerter P.G., Lee S.H., Nousari H.C., Valsamakis A., Leiferman K.M., Diwan A., Adams R.J., Griffin D.E. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat Med. 1999;5:629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- 43.Lin W.H., Kouyos R.D., Adams R.J., Grenfell B.T., Griffin D.E. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc Natl Acad Sci U S A. 2012;109:14989–14994. doi: 10.1073/pnas.1211138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Permar S.R., Klumpp S.A., Mansfield K.G., Carville A.A., Gorgone D.A., Lifton M.A., Schmitz J.E., Reimann K.A., Polack F.P., Griffin D.E. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J Infect Dis. 2004;190:998–1005. doi: 10.1086/422846. [DOI] [PubMed] [Google Scholar]
- 45.Okamura A., Itakura O., Yoshioka M., Kubota M., Kikuta H., Kobayashi K. Unusual presentation of measles giant cell pneumonia in a patient with acquired immunodeficiency syndrome. Clin Infect Dis. 2001;32:E57–58. doi: 10.1086/318499. [DOI] [PubMed] [Google Scholar]
- 46.Albertyn C., van der Plas H., Hardie D., Candy S., Tomoka T., Leepan E.B., Heckmann J.M. Silent casualties from the measles outbreak in South Africa. S Afr Med J. 2011;101:313–314. doi: 10.7196/samj.4616. 316-317. [DOI] [PubMed] [Google Scholar]
- 47.Enders J.F., Mc C.K., Mitus A., Cheatham W.J. Isolation of measles virus at autopsy in cases of giant-cell pneumonia without rash. N Engl J Med. 1959;261:875–881. doi: 10.1056/NEJM195910292611801. [DOI] [PubMed] [Google Scholar]
- 48.Good R.A., Zak S.J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18:109–149. [PubMed] [Google Scholar]
- 49.Permar S.R., Klumpp S.A., Mansfield K.G., Kim W.K., Gorgone D.A., Lifton M.A., Williams K.C., Schmitz J.E., Reimann K.A., Axthelm M.K. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol. 2003;77:4396–4400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaye A., Magnusen A.F., Sadiq A.D., Corrah T., Whittle H.C. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J Clin Invest. 1998;102:1969–1977. doi: 10.1172/JCI3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Permar S.R., Moss W.J., Ryon J.J., Monze M., Cutts F., Quinn T.C., Griffin D.E. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183:532–538. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- 52.Riddell M.A., Moss W.J., Hauer D., Monze M., Griffin D.E. Slow clearance of measles virus RNA after acute infection. J Clin Virol. 2007;39:312–317. doi: 10.1016/j.jcv.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Chen R.T., Markowitz L.E., Albrecht P., Stewart J.A., Mofenson L.M., Preblud S.R., Orenstein W.A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 54.Black F.L., Yannet H. Inapparent measles after gamma globulin administration. JAMA. 1960;173:1183–1188. doi: 10.1001/jama.1960.03020290009002. [DOI] [PubMed] [Google Scholar]
- 55.Pan C.H., Greer C.E., Hauer D., Legg H.S., Lee E.Y., Bergen M.J., Lau B., Adams R.J., Polo J.M., Griffin D.E. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J Virol. 2010;84:3798–3807. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan C.H., Valsamakis A., Colella T., Nair N., Adams R.J., Polack F.P., Greer C.E., Perri S., Polo J.M., Griffin D.E. Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc Natl Acad Sci U S A. 2005;102:11581–11588. doi: 10.1073/pnas.0504592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin W.H., Pan C.H., Adams R.J., Laube B.L., Griffin D.E. Vaccine-induced measles virus-specific T cells do not prevent infection or disease but facilitate subsequent clearance of viral RNA. mBio. 2014;5 doi: 10.1128/mBio.01047-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamashiro V.G., Perez H.H., Griffin D.E. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 59•.Behrens L., Cherry J.D., Heininger U., the Swiss Measles Immune Amnesia Study G The susceptibility to other infectious diseases following measles during a three year observation period in Switzerland. Pediatr Infect Dis J. 2020;39:478–482. doi: 10.1097/INF.0000000000002599. [DOI] [PubMed] [Google Scholar]; This study confirmed the sustained increase in susceptibility to other infectious diseases after apparent recovery from measles
- 60.Mueller N., Avota E., Collenburg L., Grassme H., Schneider-Schaulies S. Neutral sphingomyelinase in physiological and measles virus induced T cell suppression. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weidmann A., Maisner A., Garten W., Seufert M., ter Meulen V., Schneider-Schaulies S. Proteolytic cleavage of the fusion protein but not membrane fusion is required for measles virus-induced immunosuppression in vitro. J Virol. 2000;74:1985–1993. doi: 10.1128/jvi.74.4.1985-1993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erlenhoefer C., Wurzer W.J., Loffler S., Schneider-Schaulies S., ter Meulen V., Schneider-Schaulies J. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J Virol. 2001;75:4499–4505. doi: 10.1128/JVI.75.10.4499-4505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller N., Avota E., Schneider-Schaulies J., Harms H., Krohne G., Schneider-Schaulies S. Measles virus contact with T cells impedes cytoskeletal remodeling associated with spreading, polarization, and CD3 clustering. Traffic. 2006;7:849–858. doi: 10.1111/j.1600-0854.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 64••.Petrova V.N., Sawatsky B., Han A.X., Laksono B.M., Walz L., Parker E., Pieper K., Anderson C.A., de Vries R.D., Lanzavecchia A. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aay6125. [DOI] [PubMed] [Google Scholar]; This study used B cell receptor sequencing of PBMCs from children before and after measles to show failure to reconstitute naïve B cells and depletion of memory B cells after measles.
- 65••.Mina M.J., Kula T., Leng Y., Li M., de Vries R.D., Knip M., Siljander H., Rewers M., Choy D.F., Wilson M.S. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366:599–606. doi: 10.1126/science.aay6485. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of plasma samples before and after MeV infection of humans and macaques showed a loss of diversity of pre-existing anti-pathogen antibody.
- 66.Dispenzieri A., Tong C., LaPlant B., Lacy M.Q., Laumann K., Dingli D., Zhou Y., Federspiel M.J., Gertz M.A., Hayman S. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia. 2017;31:2791–2798. doi: 10.1038/leu.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]