Abstract
Healthy aging is accompanied by a decrease in cognitive and motor capacities. In a network associated with movement initiation, we investigated age-related changes of functional connectivity (FC) as well as regional atrophy in a sample of 232 healthy subjects (age range 18–85 years). To this end, voxel-based morphometry and whole-brain resting-state FC were analyzed for the supplementary motor area (SMA), anterior midcingulate cortex (aMCC) and bilateral striatum (Str). To assess the specificity of age-related effects, bilateral primary sensorimotor cortex (S1/M1) closely associated with motor execution was used as control seeds. All regions showed strong reduction of gray matter volume with age. Corrected for this regional atrophy, the FC analysis revealed an age × seed interaction for each of the bilateral Str nodes against S1/M1 with consistent age-related decrease in FC with bilateral caudate nucleus and anterior putamen. Specific age-dependent FC decline of SMA was found in bilateral central insula and the adjacent frontal operculum. aMCC showed exclusive age-related decoupling from the anterior cingulate motor area. The present study demonstrates network as well as node-specific age-dependent FC decline of the SMA and aMCC to highly integrative cortical areas involved in cognitive motor control. FC decrease in addition to gray matter atrophy within the Str may provide a substrate for the declining motor control in elderly. Finally, age-related FC changes in both the network for movement initiation as well as the network for motor execution are not explained by regional atrophy in the healthy aging brain.
Keywords: Gray matter atrophy, Voxel-based morphometry, Functional connectivity, Resting-state fMRI, Aging, Movement initiation
Introduction
An important aspect of physiological aging is a decline in mobility, motor performance and in particular movement coordination, which is consistently found in the elderly (for review see Seidler et al. 2010). One consistent age-related effect is the general slowing of movements including psychomotor slowing (Birren and Fisher 1995). While degenerative processes within the skeleto-muscular system (Clark and Taylor 2011) may contribute to this decline, there is ample evidence that age-related changes in brain networks play a key role in these observations (Tomasi and Volkow 2012). For example, changes of motor control have been related to atrophy in the basal ganglia (BG) system and the prefrontal cortex (Seidler et al. 2010), which simultaneously seem especially vulnerable to age-related structural loss (Jernigan et al. 2001). It has been speculated that both loss of neurons and synapses as well as rarefaction of cortical microvasculature contribute to age-related structural changes in brain tissue (Giorgio et al. 2010). Longitudinal evidence documented the fastest volume reduction rates in the caudate nucleus and dorsal portions of the putamen [Str (Raz et al. 2003)]. Alterations in the BG loops during normal aging likely contribute to age-related decline of motor control up to age-related parkinsonism-like symptoms (Darbin 2012). As the highest correspondence between age-related reduction of gray matter (GM) density and decrease of resting-state functional connectivity (FC) was found for the BG (Segall et al. 2012), functional decoupling of the BG system seems to be part of the neural correlates of deteriorating movement coordination and motor speed with age. Age-related structural decline in the motor system and its possible impact on motor performance are quite well documented (cf. Seidler et al. 2010), and age-dependent changes within the neural network for movement generation remain to be examined. With regard to neural activity, there is evidence for a systematic connection between GM atrophy on one side and task-related under and overactivation in an episodic memory task with age (Kalpouzos et al. 2012). In contrast, it could be shown that there are substantial alterations of resting-state connectivity within the default mode network which are not explained by regional brain atrophy (Onoda et al. 2012; Damoiseaux et al. 2008).
In the last decade, analyses of resting-state fMRI have been proven to be a valuable tool for in vivo detection of brain connectivity based on spontaneous fluctuations of the blood oxygenation level-dependent (BOLD) signal in distinct brain regions (Fox et al. 2007). In their seminal study, Biswal et al. (1995) demonstrated that in a resting-state without any movements, BOLD time series fluctuations of the left and right primary motor cortex are significantly correlated. These correlated fluctuations at rest mirror known similarities in functional properties of the primary motor areas and their tendency to engage in a functional network. Over the years, it has repeatedly been shown that regions functionally connected in a resting-state also co-activate in functional neuroimaging studies and vice versa (e.g., Fox and Raichle 2007) and that these resting-state connectivity networks are robust over time (Shehzad et al. 2009). Furthermore, the comparison between task-free resting-state connectivity and task-based meta-analytic connectivity modeling revealed remarkable similarities between these complementary approaches (Hoffstaedter et al. 2013a; Jakobs et al. 2012; Smith et al. 2009). Moreover, FC analysis of resting-state fMRI data offers great potential for large cohort studies of aging-related FC changes in the human brain owing to the relative simplicity to obtain resting-state fMRI data (Ferreira and Busatto 2013). Andrews-Hanna et al. (2007) for instance could show a marked reduction in FC in elderly subjects linked to both decreasing white matter integrity and broad impairments in cognitive abilities. Furthermore, brain atrophy in later age can be compensated to a certain extend by reorganization of functional brain networks (Ward 2006). In contrast, behavioral dysfunctions like motor decline may also occur despite macroscopically, largely intact neuroanatomy. In the present study, we were interested in age-related alteration of structure and function in a neural network associated with volitional movement initiation, which was assessed using structural MRI and resting-state FC in a large sample of wide age distribution.
Already in 1985, Goldberg distinguished two premotor systems for movement generation based on structural and functional properties, with a lateral system for externally triggered movements and a medial premotor system closely related to internal movement generation. Since then, various studies confirmed the involvement of the supplementary motor area (SMA), the anterior midcingulate cortex (aMCC) and bilateral striatum (Str) comprising anterior putamen and the caudate nucleus in the generation of volitional movements (e.g., Hoffstaedter et al. 2013b; Deiber et al. 1999; Ball et al. 1999; Jenkins et al. 2000). In the current study, we assessed regional GM atrophy as well as whole-brain FC of these four network nodes via voxel-based morphometry (VBM) and seed-based resting-state analysis, respectively. In a first step, an overall FC network of all four seed regions was identified to restrict the following analysis on brain regions engaging in the common functional network associated with motor initiation. It has to be noted, that all of these four regions of interest are involved in a multitude of other aspects of cognitive motor functioning and the generation of volitional behavior in general (for review see Haggard 2008). Subsequently, network-effects were delineated that may underlie the general decline of motor performance with age. To ensure for specificity of these age-related changes, we additionally assessed FC alterations in regions directly associated with motor execution. In particular, FC of the left and right hand representation in the primary sensorimotor cortex (S1/M1) was delineated in the same way to control for unspecific changes of FC with age. We hypothesized the existence of a specific age-dependent alteration of FC within the two functional networks. For volitional movement generation we expected an age-related decrease of FC primarily comprising highly integrative brain areas like the insula, whereas the age-related decrease of FC of the executive network comprises interhemispheric decoupling.
Materials and methods
Subjects
Structural T1 and resting-state fMRI data of 232 healthy subjects (105 female) from two different cohorts [Research Center Jülich/Germany: 100 subjects; Nathan S. Kline Institute (NKI) New York/USA: 132 subjects] with a mean age of 43.5 ± 16.5 years (age range 18–85; Fig. 1) were included in the analysis. All subjects were right handed according to the Edinburgh handedness inventory (Oldfield 1971), without any record of neurological or psychiatric illness. The study protocol has been approved by the local ethics committees of both MRI centers and informed consent was obtained by all participants prior to the examination.
Fig. 1.
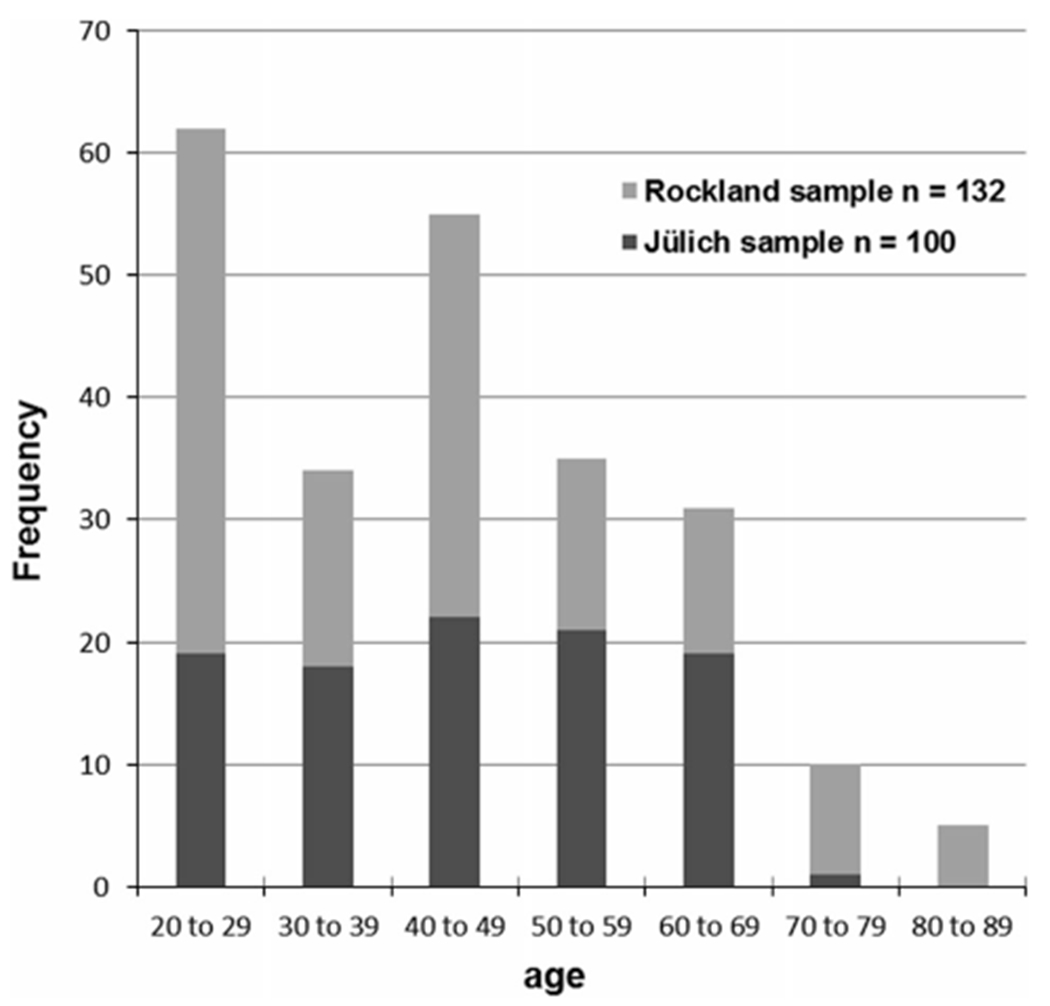
The histogram illustrates the age distribution of the resting-state fMRI sample comprising 232 healthy subjects from the Research Center Jülich (Germany) and from NKI/Rockland (USA; publicly available through the ‘1000 Functional Connectomes Project’)
Regions of interest
The regions used for VBM and seed-based FC analysis were derived from a recent fMRI study on internal movement initiation obtained in an independent sample of 35 healthy subjects (Hoffstaedter et al. 2013b). In this study, self-initiation of voluntary finger movements (relative to well-matched reactive, i.e., cued movements) were associated with enhanced BOLD activity increase in bilateral Str (putamen, caudate nucleus), SMA, and aMCC [areas 32′, a24′; cingulate motor area (Paus 2001; Zilles et al. 1996)] as well as in areas associated with higher ‘cognitive’ processing: inferior parietal and Dorsolateral prefrontal cortex (DLPFC), anterior Insula (aIns) and area 44 (Supplementary Fig. S1). As the current study focused on internal motor initiation, only the first four regions were included in the analysis as part of Goldberg’s (1985) medial premotor system closely related to internal movement generation. We used the group activation clusters from this localizer study [p < 0.05 family-wise error (FWE) corrected for multiple comparisons; (Hoffstaedter et al. 2013b)] as volumes-of-interest (VOIs) for VBM and seed-based resting-state analysis. In addition, functionally defined representations of the left and the right hand in S1/M1 were used as control VOIs, because this region is directly associated with motor execution. The S1/M1 VOIs (areas 3a/b, 4p) were taken from another recent fMRI study (Roski et al. 2013), where 102 subjects performed regular paced finger tapping (Fig. S2). Importantly, the entire volumes of neural activation from the respective fMRI studies were used to define the seeds, rather than considering peak voxel locations only. This approach draws upon a large scale simulation approach to FC (Smith et al. 2011), which recently showed that functional specific seed VOIs provide better results for modeling functional networks than single points or atlas-based VOIs.
Voxel-based morphometry
Structural T1 weighted MR scans were acquired for the 232 subjects on a Siemens MAGNETOM Trio 3 Tesla whole-body scanner (Erlangen, Germany) at the Research Center Jülich in Germany and the Nathan S. Kline Institute (NKI) in New York, respectively (Jülich: TR 2.25 s, TE 3.03 ms, flip angle 9°, resolution 1 mm isotropic; NKI/Rockland: TR 2.5 s, TE 3.5 ms, flip angle 8°, resolution 1 mm isotropic). The scans were processed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) within SPM8 (http://www.fil.ion.ucl.ac.uk/spm) with standard settings for bias-field correction, segmentation of GM, white matter (WM) and CSF, partial volume effect adjustment and spatial normalization into MNI-space within a unified segmentation model (Ashburner and Friston 2005). The segmented images were non-linearly modulated for normalization to the group mean template. The resulting voxel-wise amount of expansion or contraction was used to estimate GM volume of each VOI. Thereby, the VOIs GM volume was corrected for the individual GM volume as they represent the non-linear modulation of the GM of each individual brain in relation to the group template. The individual VOIs GM volumes were corrected for the influence of measurement site (Jülich/NKI) before calculating linear Pearson correlation with age to determine GM changes with age (Bonferroni corrected for multiple comparisons).
Seed-based resting-state analysis
The analysis investigated correlated fluctuations of neural activity as represented by functional MRI measurements of the human brain at rest [‘resting-state fMRI’; (Biswal et al. 1995)]. BOLD signal time series were acquired using echo-planar imaging (EPI) on a Siemens MAGNETOM Trio 3 Tesla whole-body scanner (Erlangen, Germany) at the Research Center Jülich in Germany and the Nathan S. Kline Institute (NKI) in New York, respectively. Resting-state data from the latter institute are freely available in the context of the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). For each subject, whole-brain volumes were acquired using gradient-echo EPI pulse sequence (Jülich: 300 images, TR 2.2 s, TE 30 ms, flip angle 90°, in plane resolution 3.1 × 3.1 mm, 36 axial slices of 3.1 mm thickness; NKI/Rockland: 260 images, TR 2.5 s, TE 30 ms, flip angle 80°, in plane resolution 3.0 × 3.0 mm, 38 axial slices of 3.0 mm thickness). The first four images were discarded to allow for magnetic field saturation, before the remaining images (296 Jülich/256 Rockland) were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). First, images were corrected for head motion by affine registration using a two-pass procedure registering all images to the individual mean of the respective subject. The mean image was then spatially normalized to the MNI single subject template (Holmes et al. 1998) using the ‘unified segmentation’ approach (Ashburner and Friston 2005). The ensuing deformation was then applied to the individual EPI volumes. Finally, images were smoothed by a 5 mm FWHM Gaussian kernel to compensate for residual anatomical variations and to meet requirements of normal distribution of the residuals for Gaussian random field inference to correct for multiple comparisons.
To reduce spurious correlations, variance that could be explained by first- or second-order effects of the following nuisance variables was removed from each voxel’s time series (Satterthwaite et al. 2013; Reetz et al. 2012) applying a GLM approach: (i) the six motion parameters derived from the image realignment; (ii) their first derivative; (iii) mean gray and WM as well as CSF signal intensity; and (iv) coherent signal changes across the whole brain as reflected by the first five components of a PCA decomposition of the whole-brain time series [CompCor denoising (Behzadi et al. 2007; Chai et al. 2012; Jakobs et al. 2012)]. The first three nuisance variables (i–iii) entered the model as first and second-order terms, which were shown to increase specificity and sensitivity of the FC analyses. Finally, the data were band pass filtered between 0.01 and 0.08 Hz (Cordes et al. 2001).
The functionally defined regions in the SMA, aMCC, bilateral Str, and bilateral S1/M1 were used as seed VOIs in the resting-state analysis. The time courses of these regions were extracted for each subject as the first eigenvariate of all GM voxels according to segmentation within the respective VOI. To certain extend, this eigenvariate is robust against inter-individual variance in anatomical localization and distribution of voxels mainly driving the functionally relevant and representative VOI time series used for FC analysis. Notably, previous studies of the effect of aging on functional brain activation found changes in localization as well as in extend and degree of neural activation (Roski et al. 2013; Spreng et al. 2010). As such influence on FC cannot be excluded per se, the representative time series for each region was additionally extracted for enlarged and shrunk VOIs. Therefore, two additional versions of each network node were created via dilatation by two voxels and via erosion by one voxel of every VOI.
To quantify resting-state FC, linear (Pearson) correlation coefficients were computed between each seed time series and those of all other GM voxels in the brain. The voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores, and tested for consistency across subjects by a second-level analysis of variance (ANOVA, including appropriate non-sphericity correction). To specifically analyze the influence of the extend of the seed region on age-related changes in FC, age was introduced as covariate (besides measurement site) in a supplementary group analysis.
To assess age-related changes in FC and its relation to GM atrophy, age and GM volume were introduced as covariate in the ANOVA group analyses of FC. To specifically capture the effect of age in addition to GM atrophy, the effect of GM volume was regressed out of the covariate of interest ‘age’ before it was introduced in the GLM. In addition, the measurement site of the respective cohort (Jülich/Rockland) was included as covariate of no interest to account for effects due to different measurement sites, scanning parameters, or sampling. The results of this random-effects analysis were then FWE corrected for multiple comparisons at the cluster-level (cFWE), and thresholded at pcorrected < 0.05.
As we were primarily interested in the network for movement initiation, common FC of the SMA, aMCC and bilateral Str was identified using a conjunction analysis over the respective FC maps via the minimal statistic approach (Nichols et al. 2005). FC of the network for motor execution was identified similarly over the common FC of left and right S1/M1. To ensure functional specificity of the network for movement initiation, the interaction with the network for motor execution was assessed by computing the conjunction over the contrast between FC of both networks and the common FC of the movement initiation network nodes. The conjunction over all six seed VOIs revealed conjoint FC of both networks. Within the two specific FC networks, linear age-related changes of FC over and above GM atrophy were assessed computing significant covariance of FC with age corrected for GM volume. To additionally test for functional specificity of age-related FC alterations, we calculated the age × seed interaction within both networks.
Anatomical assignment of resulting brain areas of FC was established via the SPM Anatomy toolbox [http://www.fz-juelich.de/ime/spm_anatomy_toolbox, V1.8 (Eickhoff et al. 2007)], which implemented the JuBrain Cytoarchitectonic Atlas as anatomical reference for structure–function relationships (cf. Zilles and Amunts 2010). Details on included regions based on cyto- and receptor architectonics may be found in the following publications reporting on Broca’s region (Amunts et al. 1999), superior temporal cortex (Morosan et al. 2005), premotor cortex (Geyer et al. 1996), primary motor cortex (Geyer et al. 1996), primary sensory cortex (Geyer et al. 2000; Grefkes et al. 2001), inferior frontal junction (Amunts et al. 2010), anterior cingulate and midcingulate cortex (Palomero-Gallagher et al. 2008; Palomero-Gallagher et al. 2009). Regions, which are not yet mapped based on observer-independent histological examination, were labeled macroanatomically via the probabilistic Harvard-Oxford atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html) as implemented in the Anatomy Toolbox.
Results
Age-related decrease of GM volume
The VOIs of both networks showed pronounced negative correlation between GM volume and age with a mean r = −0.50 for the VOIs associated with movement initiation and r = −0.56 for the motor execution nodes (cf. Fig. 2). The aMCC VOI showed the strongest effect of age with r = −0.62 followed by striatal VOIs (left: r = −0.51; right: r = −0.50), while the SMA VOI only featured a moderate age effect (r = −0.37). The hand representations in primary sensorimotor cortex S1/M1 also showed strong age-related decrease of GM in both hemispheres (left: r = −0.57; right: r = −0.55).
Fig. 2.
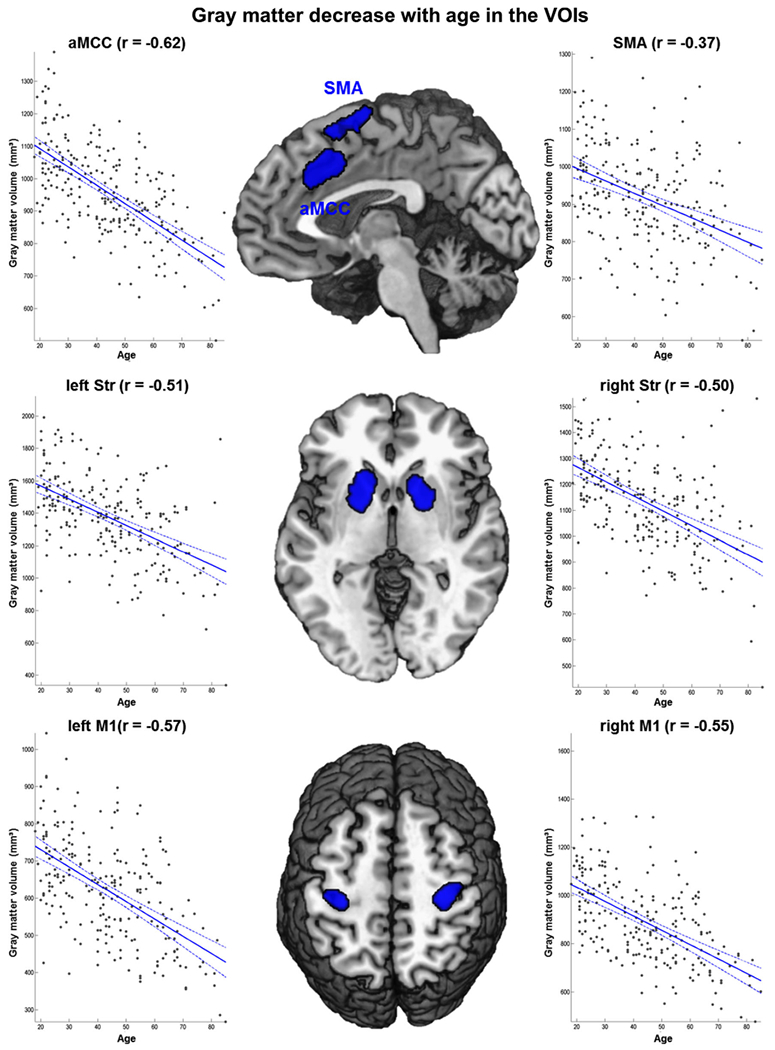
The figure shows Pearson correlations between age and gray matter volume (mm3) for all six VOIs corrected for the influence of measurement site (Bonferroni corrected)
Functional connectivity networks
Contrasting the FC of the four seeds reflecting key areas for movement initiation (SMA, aMCC, bilateral Str) with those of the control seeds (bilateral S1/M1) revealed an extended FC network (Fig. 3). More precisely, FC of the SMA was contrasted with FC of bilateral S1/M1 and entered a conjunction with the FC contrast of aMCC and S1/M1 as well as with each of bilateral Str contrasted to S1/M1. We will refer to this network of regions specifically coupled with the key areas for movement initiation as the extended ‘movement initiation network’ throughout the rest of the manuscript. All four seed regions featured higher functional coupling than bilateral S1/M1 among each other as well as with areas in bilateral DLPFC, dorsal premotor cortex (dPMC), pre-SMA, posterior midcingulate cortex (pMCC: area p24′) and perigenual anterior cingulate cortex (pACC: area p24), central and aIns, inferior frontal junction (IFJ), area 44/45 and frontal operculum. Subcortically, the extended ‘movement initiation network’ comprised the BG including nucleus accumbens, large parts of the thalamus and the cerebellum.
Fig. 3.
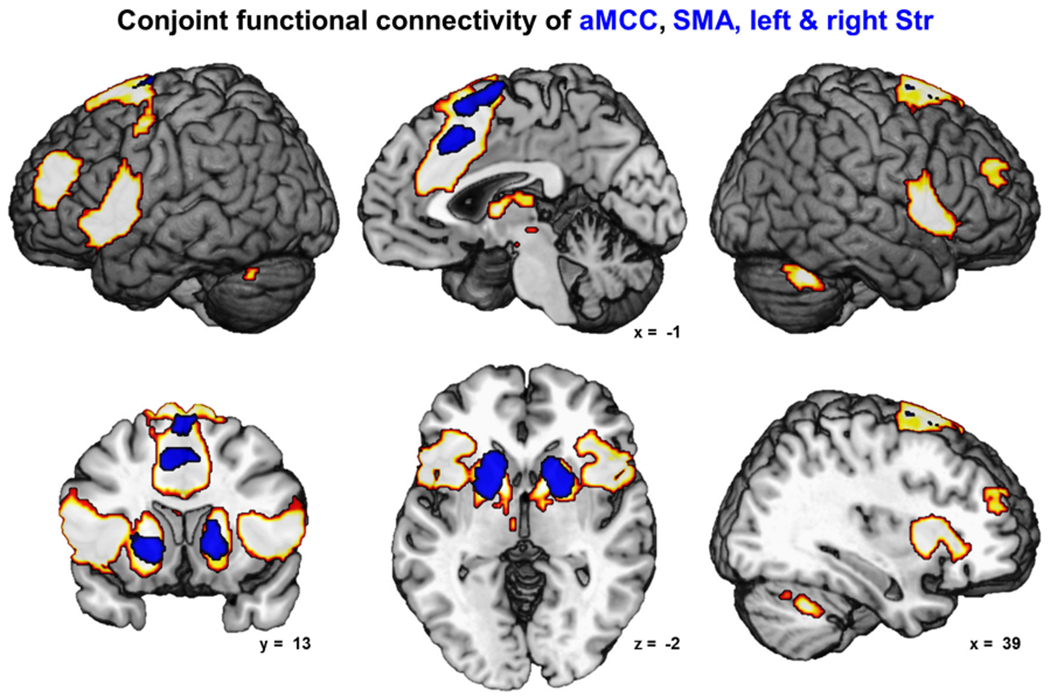
The figure depicts the network of conjoint functional connectivity of the SMA, aMCC and bilateral striatum (all shown in blue) as revealed by seed-based resting-state analysis (cFWE-corrected)
The control VOIs in the left and right S1/M1 were also contrasted against each of the four key nodes of the ‘movement initiation network’. The resulting extended ‘motor execution network’ (Fig. 4) comprised bilateral primary motor (M1: areas 4a/p) and somatosensory cortices (S1: areas 3a/b, 1), bilateral premotor cortex (area 6), SMA, pMCC (area p24′), precuneus, ventro-medial prefrontal cortex and superior temporal cortex (area TE3). Also bilaterally connected were superior prefrontal gyrus, frontal and parietal operculum (areas OP1-6), posterior insula (areas Ig1, Ig2, Id3), amygdala, posterior putamen, hippocampus, cerebellum, inferior parietal lobule (IPL:area PFt and right PFcm, PFop) and superior parietal lobule (SPL: areas 5l, 5m, 7PC) and right intraparietal sulcus (IPS: hIP2/3). Conjoint FC over all regions involved in motor initiation and execution was found bilaterally in posterior dPMC (area 6), SMA, pMCC (area p24′) and left posterior putamen (Fig. S3).
Fig. 4.
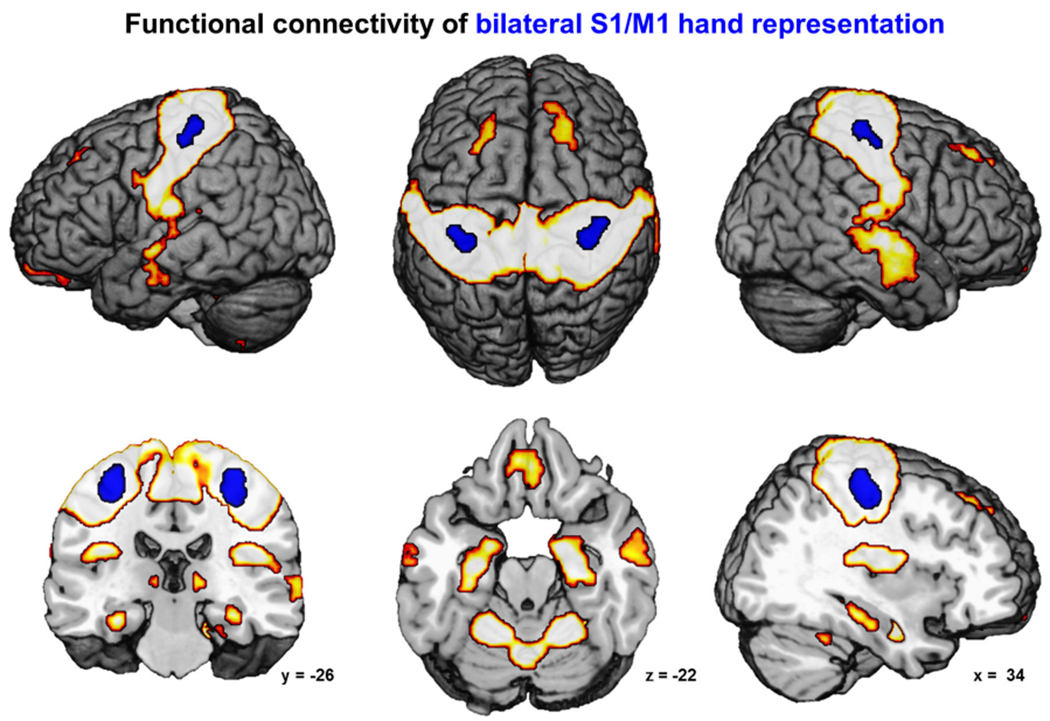
The figure depicts the network of conjoint functional connectivity of bilateral S1/M1 hand representation (shown in blue) as revealed by seed-based resting-state analysis (cFWE-corrected)
Age-related functional connectivity changes
Within the extended ‘movement initiation network’, several regions featured an age-related decrease of FC with different seed VOIs. FC of either Str seed showed an age-dependent decrease of FC with contralateral anterior putamen and bilateral caudate nucleus (Fig. 5). The SMA seed featured age-dependent decrease of FC with adjacent parts of the SMA, bilateral central insula and the adjacent frontal operculum (Fig. 6). Age-dependent FC decline of the aMCC VOI was found for the anterior cingulate motor area situated anteriorly to the seed (area a24′) extending into pACC (area p24). Each S1/M1 control VOI showed age-related decrease of FC with a S1/M1 region on the precentral gyrus superiorly to the respective seed (Fig. 7).
Fig. 5.
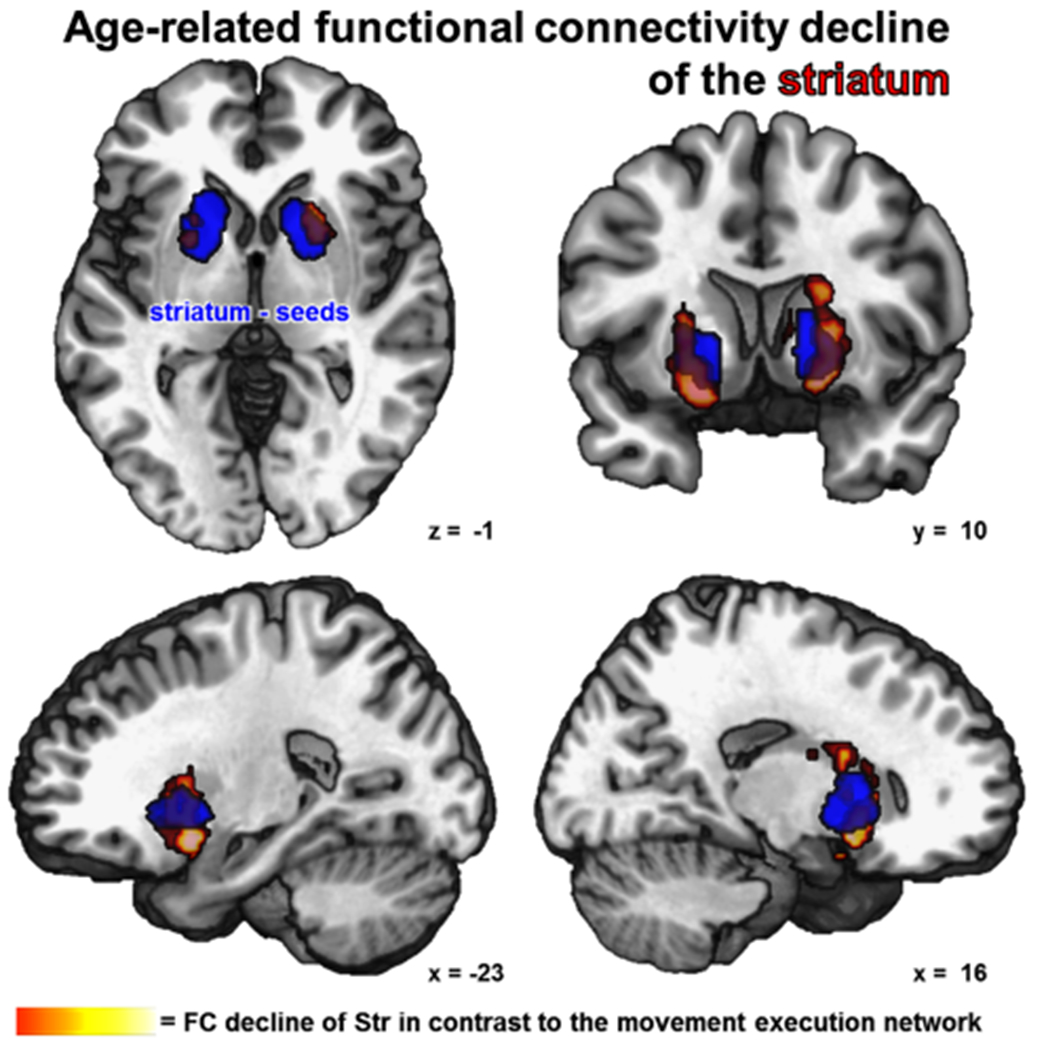
The figure shows a conjunction over age-related decrease of functional connectivity from both striatum seeds (blue) including the age × seed interaction (cFWE-corrected)
Fig. 6.
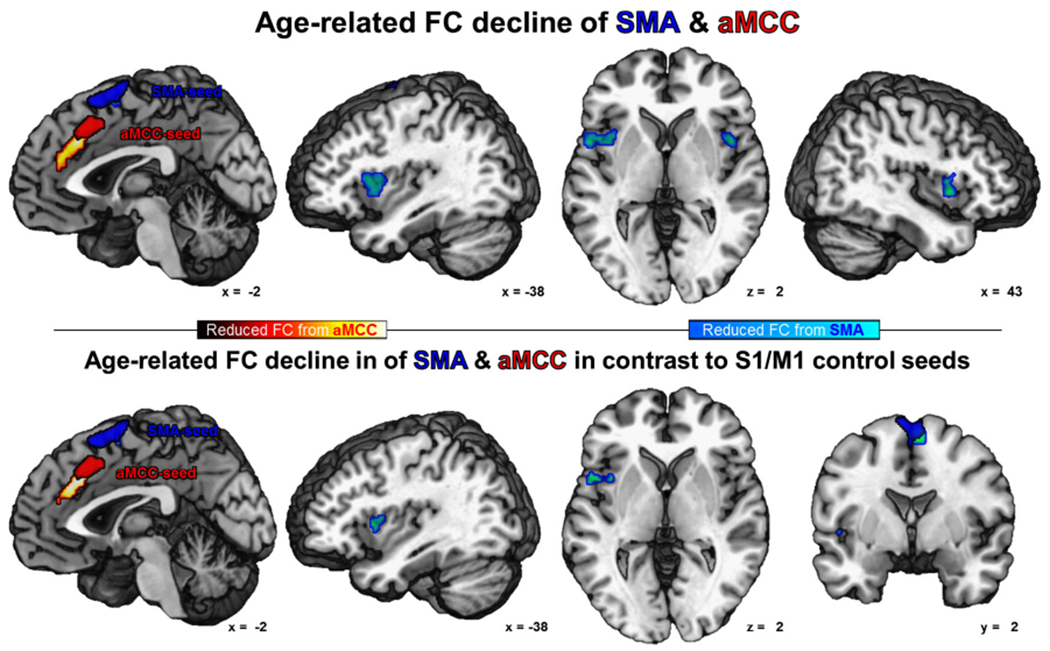
Age-related reduction of functional connectivity of the SMA (in blue) is shown in cold colors and of the aMCC (in red) is shown in warm colors (cFWE-corrected)
Fig. 7.
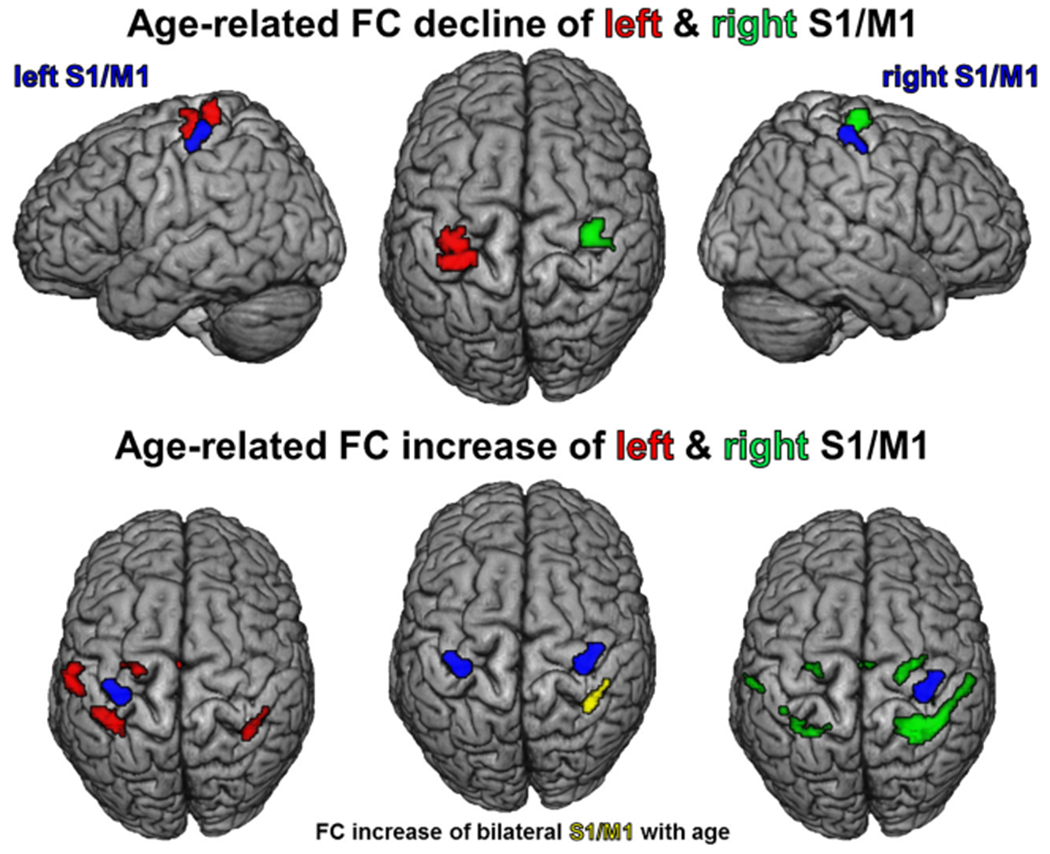
Age-related decline and increase of functional connectivity of left (in red) and right (in green) S1/M1 (seeds in blue) and age-dependent FC increase of bilateral S1/M1 (in yellow; all cFWE-corrected)
No significant age-related increase in FC was found for SMA, aMCC or bilateral Str within the ‘movement initiation network’. In contrast, both S1/M1 control VOIs featured FC increase within the motor execution network, each with a larger increase in the ipsilateral hemisphere (Fig. 7). The left S1/M1 VOI showed age-dependent increase of FC with pMCC (area p24′), left dPMC, bilateral SPL (area 7PC), left IPL (area PFt) and right IPS (area hIP3) within the network for motor execution. The right S1/M1 VOI featured age-dependent increase of FC with pMCC (area p24′), SMA, bilateral dPMC, SPL (areas 7PC, 5l) and IPL (area PFt) as well as right IPS (area hIP3).
To test for strict independence of age-related changes within the networks for movement initiation and motor execution, the interaction of age-dependent FC changes between the nodes of both networks was analyzed (age × seed interaction). To this end, age-related alterations of each VOI’s FC entered a conjunction with the same VOI’s FC changes contrasted against FC changes of the nodes of the respective other network. The FC reduction for both Str VOIs remained significant with bilateral anterior putamen when contrasted to FC decrease of the S1/M1 control seeds in the age × seed interaction (Fig. 5). When contrasted to FC changes of S1/M1, the SMA VOI showed a network specific age-related reduction of FC with the SMA, the left central insula and left frontal operculum (Fig. 6). Also the aMCC VOI showed specific connectivity reduction with the anterior cingulate motor area extending into pACC (area p24). For the age × seed interaction, the control VOIs in S1/M1 did not show network specific age-dependent FC alterations (Fig. 7).
The supplementary analysis testing the influence of VOI extend on age-related FC changes confirmed the overall pattern of both additional analyses with the dilated VOIs resulting in somewhat increased negative correlations of FC with age entailing bigger clusters of FC reduction. The opposite effect was observed for the eroded VOIs, which nevertheless yielded virtually identical results as for the original VOIs (for details see Supplementary Material).
In summary, we observed specific age-related FC changes for all four nodes of the ‘movement initiation network’. In particular, the bilateral anterior putamen, SMA, aMCC and the left central insula showed network specific changes of FC with different nodes of the ‘movement initiation network’ as indicated by a significant age × seed interaction. Importantly, this seed region specific age effect on FC was found despite the fact that all seed regions of the ‘movement initiation network’ were tightly interconnected with each other. This implies that the connectivity from the different nodes of the ‘movement initiation network’ was differentially affected by age, which may imply a differential impairment of specific motoric functions in later age.
Discussion
A prominent neural correlate of voluntary movement initiation is the ‘Bereitschaftspotential’ discovered in 1965 (Kornhuber and Deecke 1965), which relates to an increase in electroencephalographical activity detectable over one second before movement onset. The source of this early negative activity was quite consistently located within the aMCC and SMA using cortical source reconstruction via combinations of EEG and fMRI (Ball et al. 1999; Cunnington et al. 2003) or dipole modeling in MEG (Erdler et al. 2000). The medial premotor system associated with internal movement generation conceived by Goldberg (1985) comprises the SMA-basal ganglia circuit (Haggard 2008) complemented by the aMCC (cf. Hoffstaedter et al. 2013b). Various fMRI studies on internal movement timing found specifically these areas to be involved in intentional motor initiation (Deiber et al. 1999; Jenkins et al. 2000; Ball et al. 1999; Francois-Brosseau et al. 2009). Dissociating self-initiated timing and free selection of movements recently confirmed the specific involvement of the aMCC and SMA together with the Str comprising putamen and caudate nucleus in internal timing of movements (Hoffstaedter et al. 2013b). In concordance with Goldberg’s (1985) medial premotor system, these brain regions represent the core nodes of a neural network crucially involved in the generation of volitional movements. In the current study, we focused on the cognitive aspect of motor functioning in healthy aging and choose the function of movement initiation as basic aspect of cognitive motor control, because it comprises both cognitive and motor processing. To this end, we investigated age-related changes in GM volume and FC connectivity of the functionally defined brain regions as well as the dependence FC alterations on GM atrophy with age. As expected, the VBM analysis revealed marked GM volume in all VOIs. Importantly, age-related alterations of FC were detected over and above GM atrophy in healthy aging.
Age-related gray matter atrophy and cognitive motor control
The key nodes of both networks for movement initiation and for motor execution showed strong reduction of GM volume with age. Such GM atrophy with age was repeatedly found to have regional specificity (Sowell et al. 2003; Good et al. 2001; Smith et al. 2007; Walhovd et al. 2011). Regarding the functional roles associated with each region of the ‘movement initiation network’ showing age-related FC decline, one should expect specific motor functions to be differentially affected in healthy aged individuals. Recently, Rosano et al. (2012) found such relationship for bradykinesia and gait disturbances with GM atrophy in S1/M1 in healthy elderly (n = 307; mean age 83). GM volume of the SMA on the other hand was found to be correlated with individual differences in executive function capacity as assessed by self-report (Sakai et al. 2012). The close relation between atrophy of the putamen and motor impairment was recently demonstrated for Huntington’s disease (Guo et al. 2012), which together with evidence from Parkinson’s may suggest that the Str holds responsible for efficient online motor control on a more automated level. For the aMCC, which is closely related to cognitive motor control, specific and reliable GM loss was previously found in later age (Tisserand et al. 2004; Bergfield et al. 2010). In general, a relationship between regional GM atrophy with age and decline of behavioral performance seems evident and has to be accounted for in the analysis of age-related FC changes.
A network for movement initiation
The extended ‘movement initiation network’, as represented by the FC overlap of SMA, aMCC and bilateral Str FC, embraces lateral and medial premotor cortex, prefrontal and midcingulate regions, Broca’s area, central insula, aIns and frontal operculum as well as the striatum, thalamus, and cerebellum in both hemispheres. All these areas were previously found to be involved in the generation of volitional behavior (Haggard 2008) and, moreover, seem to play specific roles in the realization of self-initiated movements (Hoffstaedter et al. 2013b). The DLPFC, aMCC, area 44 and aIns were associated with preparatory processes like timing of movement initiation, whereas lateral premotor cortex, frontal operculum, striatum, thalamus and cerebellum are involved in sensorimotor processing closer to movement execution. The functional specificity of this ‘movement initiation network’ was controlled for by contrasting each node’s FC with the connectivity of the S1/M1 control seeds, i.e., the ‘motor execution network’. For the age × seed interaction, we found age-related FC decrease from the nodes of the motor initiation network with different regions of the ‘motor initiation network’ including the BG, cingulate cortex, SMA, the central insula and frontal operculum.
Age-related disruption of functional connectivity in the basal ganglia
The striatum VOIs showed an age-related decrease of functional coupling with the Str including interhemispheric disruption. The significant age × seed interaction (using S1/M1 as a control region) for the anterior putamen confirms the functional disruption of the BG system to be highly specific to this subcortical region. As synaptic plasticity in particular in the Str is considered a key neural substrate for adaptive motor control (Kreitzer and Malenka 2008), the observed reduction of FC most likely contributes to the deterioration of motor control in healthy aging. In their recent review on motor control and aging, Seidler et al. (2010) suggested that the degree of nigrostriatal dopamine transporter degeneration in aging may underlie decreased fine movement control and movement slowing. In line with these findings, the specific dysfunction of striatal circuitry associated with Parkinson’s disease (PD) originates in the progressive loss of midbrain dopamine neurons resulting in decreased dopaminergic tone in the motor portions of the putamen (DeLong and Wichmann 2007). Thus, aberrant FC over and above regional atrophy within the BG and especially the Str may be considered a neural correlate of decline in motor control up to pathologic hypokinetic motor deficits. Taken together, the highly specific age-dependent decrease of FC in addition to the structural degeneration of the striatum with age (Raz et al. 2003, 2005) is likely to play a role in age-related decline of automated movement coordination and motor speed.
Loss of functional connectivity of the medial premotor cortex with age
The medial premotor cortex comprising the rostral cingulate motor area, the pre-supplementary and the SMA, can be considered a key structure for motor control (Nachev et al. 2008). Thereof, the aMCC seems to represent a crucial region for cognitive motor control (Paus 2001; Hoffstaedter et al. 2013a), while the pre-SMA is associated with rather specific movement selection (van Eimeren et al. 2006) and inhibition (Swick et al. 2011). The SMA on the other hand is involved in bimanual coordination (Swinnen 2002), motor sequencing (Deiber et al. 1999) and internal movement timing (Hoffstaedter et al. 2013b; Ball et al. 1999). This intermediate relation of the SMA to movement execution is also reflected in the fact that the SMA VOI partly overlaps with the mutual FC of the six seeds for movement initiation and motor execution (Fig. S3). The present resting-state analysis revealed an age-related FC decrease of the SMA VOI within the ‘movement initiation network’ around the seed itself, of which the region below the seed was also significant for the age × seed interaction. The aMCC VOI revealed an age-related decrease of FC with the anterior cingulate motor area expanding into pACC, which as a whole remained significant for the age × seed interaction. These VOI specific changes of FC in the medial premotor cortex may be connected to specific deficiencies in motor functioning in later age. Using pattern classification, Haynes et al. (2007) found the aMCC/pACC region to be specifically informative to classify the content of task preparation while the aMCC was informative for the subsequent task control (both >70 % accuracy). Thus, functional decoupling or the anterior (or rostral) cingulate motor area is likely to be associated with deficits in movement preparation and in particular cognitive control, which is severely the case for lesions in this area (cf. Paus 2001).
Assessing aging-related changes in regional homogeneity of the fMRI signal, Zang et al. (2004) developed different analysis approach assuming that for normal brain function within a functional cluster the hemodynamic characteristics of every voxel should be synchronous to a certain extent. In line with the results of the current analysis, Wu et al. (2007) found a decrease of regional homogeneity in the resting-state signal with age in the medial premotor areas, Str and also M1, which was interpreted as disruption of normal brain function within these regions. In a recent meta-analysis Turner and Spreng (2012) compared the brain activity associated with cognitive control functions in older and younger subjects and found higher activation of the aMCC/SMA region for older subjects. As hypothesized before (e.g., Heuninckx et al. 2010), such age-dependent activation increase within medial premotor areas may be a compensation mechanism to stabilize age-related deteriorations of motor control putatively associated with the specific decrease of aMCC and SMA FC demonstrated here.
Both the aMCC and the SMA showed node-specific reductions of functional coupling with the insular cortex. The aMCC featured age-related FC reduction with aIns, while the SMA decreases in FC with the central insula. This specific pattern of FC reduction is well in line with the results of an ALE meta-analysis of insula activation in the context of various functions represented by behavioral domains in the BrainMap database (Kurth et al. 2010). Comparable to subsequent parcellations of the insula (Cauda et al. 2011; Kelly et al. 2012), functional specific regions of the insula could be differentiated, with the central insular region associated with sensorimotor tasks. The age-related reduction of FC between SMA and central insula may contribute to general impairments of movement coordination in elderly. With regard to network specificity, the age × seed interaction for SMA vs. S1/M1 FC was solely significant for the age-related FC decrease of the SMA with left central insula and the adjacent frontal operculum emphasizing the role of this integrative sensorimotor region in motor control with age. In a similar manner, reduced FC of the SMA with bilateral frontal operculum in advanced age may contribute to observed problems in online movement control in higher age. In line with this reasoning, a recent meta-analysis found SMA to be consistently involved in movement timing over all included studies (Wiener et al. 2010). Similarly, the frontal operculum was shown to be involved in interactive aspects of human movement control (Binkofski et al. 2000) and to exhibit higher activation in elderly (Heuninckx et al. 2005). Taken together, the observed age-dependent alterations of FC from SMA to bilateral insula may represent a neural substrate of deficits in cognitive motor control in later age. Likewise, the demonstrated functional decoupling of SMA from frontal operculum may be a neural correlate of age-related difficulties in online movement control (Seidler et al. 2010).
Unfortunately, in the assessed cohort of 232 subjects, behavioral tests were only available for some subjects and the employed scores varied. Furthermore, for the here investigated function of movement initiation it seems rather difficult to define a specific behavioral measure. Almost all conventional motor performance measures from finger tapping speed to reaction times in simple to complex choice tasks first of all investigate motor execution or inhibition, together with a varying degree of more cognitive abilities like attention, working memory and executive functioning. The ability of flexible and spontaneous generation of movements seems to underlie certain constrains regarding a direct behavioral indicator as a performance measure to be correlated here with individual values of GM volume of FC. On a conceptional level, we would argue that with regard to the networks of interest used here, there is a quite strong connection between behavior and network function, because, the ROIs were directly derived from a study specifically examining movement initiation (Hoffstaedter et al. 2013b). Moreover, the network used here was repeatedly shown to be necessarily associated with internal movement initiation (for review see Haggard 2008) and with motor execution in case of the primary motor cortex.
Alterations of functional connectivity in the motor execution network with age
The age-dependent decrease of FC between primary motor cortices may be associated with the common motor slowing in later age, as an analogous observation is consistently made after stroke with a correlation between the reduction of interhemispheric interaction and the severity of motor impairments (Rehme and Grefkes 2013). Interestingly, and in contrast to the motor initiation network nodes, the S1/M1 motor execution network featured age-related increases in connectivity, especially with dPMC and SPL. As online motor control decreases with age (Seidler et al. 2010), the motor system may shift to a higher degree of anticipatory preparation of motor programs involving premotor and parietal regions (Thoenissen et al. 2002; Toni et al. 2001).
Conclusion
In the present study, we analyzed age-dependent alterations of GM structure and of FC in a network associated with intentional movement initiation. Strong age-related atrophy was found in all examined brain areas, which was controlled for in the subsequent FC analysis. A consistent FC decline with age was found within the putamen, which parallels known deteriorations of motor speed and coordination and seems to happen additional to structural degeneration with age. The age-dependent decreases of FC from the SMA to the central insula may represent a neural correlate of deteriorating movement coordination with age, as the insula is associated with cognitive functioning as well as with sensorimotor and interoceptive awareness. Importantly, age-related FC changes of the nodes for movement initiation (aMCC, SMA, bilateral Str) and for the control seeds (S1/M1) were exclusively found within regions featuring functional coupling with the respective functional network. As confirmed by the age x seed interaction, there are node and network specific patterns of reduced coupling with age supporting our hypothesis of distinct age-related changes within specific FC networks for movement initiation and motor execution, respectively. Finally, age-related FC changes in both the network for movement initiation as well as the network for motor execution are not explained by regional atrophy in the healthy aging brain.
Supplementary Material
Acknowledgements
This study was supported by the Human Brain Project (R01-MH074457-01A1; SBE), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model; KZ, SBE), and the Helmholtz Alliance for Mental Health in an Aging Society (HelMA; KZ). CG was supported by a Grant from the German Research Foundation (Deutsche Forschungsgemeinschaft GR 3285/2-1).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-013-0696-2) contains supplementary material, which is available to authorized users.
Contributor Information
F. Hoffstaedter, Institute of Neuroscience and Medicine (INM-1), Research Centre Jülich, 52425 Jülich, Germany; Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany
C. Grefkes, Max-Planck-Institute for Neurological Research, Neuromodulation and Neurorehabilitation, Cologne, Germany; Department of Neurology, Cologne University, Cologne, Germany
C. Roski, Institute of Neuroscience and Medicine (INM-1), Research Centre Jülich, 52425 Jülich, Germany
S. Caspers, Institute of Neuroscience and Medicine (INM-1), Research Centre Jülich, 52425 Jülich, Germany
K. Zilles, Institute of Neuroscience and Medicine (INM-1), Research Centre Jülich, 52425 Jülich, Germany
S. B. Eickhoff, Institute of Neuroscience and Medicine (INM-1), Research Centre Jülich, 52425 Jülich, Germany; Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and inter-subject variability. J Comp Neurol 412(2):319–341 [DOI] [PubMed] [Google Scholar]
- Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K (2010) Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol 8(9). doi: 10.1371/journal.pbio.1000489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56(5):924–935. doi: 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R (1999) The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage 10(6):682–694 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE (2010) Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage 49(2):1750–1759. doi: 10.1016/j.neuroimage.2009.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, Zilles K, Seitz RJ (2000) Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp 11(4):273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren JE, Fisher LM (1995) Aging and speed of behavior: possible consequences for psychological functioning. Annu Rev Psychol 46:329–353. doi: 10.1146/annurev.ps.46.020195.001553 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541 [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A (2011) Functional connectivity of the insula in the resting brain. Neuroimage 55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049 [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S (2012) Anticorrelations in resting state networks without global signal regression. Neuroimage 59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Taylor JL (2011) Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci 4(3):192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001) Frequencies contributing to functional connectivity in the cerebral cortex in “restingstate” data. Am J Neuroradiol 22(7):1326–1333 [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E (2003) The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage 20(1):404–412. doi: 10.1016/S1053-8119(03)00291-X [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008) Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18(8):1856–1864. doi: 10.1093/cercor/bhm207 [DOI] [PubMed] [Google Scholar]
- Darbin O (2012) The aging striatal dopamine function. Parkinsonism Relat Disord 18(5):426–432. doi: 10.1016/j.parkreldis.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81(6):3065–3077 [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64(1):20–24. doi: 10.1001/archneur.64.1.20 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060 [DOI] [PubMed] [Google Scholar]
- Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windischberger C, Lindinger G, Deecke L (2000) Supplementary motor area activation preceding voluntary movement is detectable with a whole-scalp magnetoencephalography system. Neuroimage 11(6 Pt 1):697–707. doi: 10.1006/nimg.2000.0579 [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF (2013) Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711. doi: 10.1038/Nrn2201 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007) Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56(1):171–184. doi: 10.1016/j.neuron.2007.08.023 [DOI] [PubMed] [Google Scholar]
- Francois-Brosseau FE, Martinu K, Strafella AP, Petrides M, Simard F, Monchi O (2009) Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur J Neurosci 29(6):1277–1286. doi: 10.1111/j.1460-9568.2009.06671.x [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE (1996) Two different areas within the primary motor cortex of man. Nature 382(6594):805–807. doi: 10.1038/382805a0 [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K (2000) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage 11 (6 Pt 1):684–696. doi: 10.1006/nimg.2000.0548 [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H (2010) Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 51(3):943–951. doi: 10.1016/j.neuroimage.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G (1985) Supplementary motor area structure and function–review and hypotheses. Behav Brain Sci 8(4):567–588 [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001) Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14(3):617–631. doi: 10.1006/nimg.2001.0858 [DOI] [PubMed] [Google Scholar]
- Guo Z, Rudow G, Pletnikova O, Codispoti KE, Orr BA, Crain BJ, Duan W, Margolis RL, Rosenblatt A, Ross CA, Troncoso JC (2012) Striatal neuronal loss correlates with clinical motor impairment in Huntington’s disease. Mov Disord Off J Mov Disord Soc 27(11):1379–1386. doi: 10.1002/mds.25159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P (2008) Human volition: towards a neuroscience of will. Nat Rev Neurosci 9(12):934–946. doi: 10.1038/nrn2497 [DOI] [PubMed] [Google Scholar]
- Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE (2007) Reading hidden intentions in the human brain. Curr Biol CB 17(4):323–328. doi: 10.1016/j.cub.2006.11.072 [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005) Neural basis of aging: the penetration of cognition into action control. J Neurosci 25(29):6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2010) Age-related reduction in the differential pathways involved in internal and external movement generation. Neurobiol Aging 31(2):301–314. doi: 10.1016/j.neurobiolaging.2008.03.021 [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB (2013a) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. doi: 10.1002/hbm.22363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB (2013b) The “what” and “when” of self-initiated movements. Cereb Cortex 23(3):520–530. doi: 10.1093/cercor/bhr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22(2):324–333 [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB (2012) Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage 60(4):2389–2398. doi: 10.1016/j.neuroimage.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements II. The effect of movement predictability on regional cerebral blood flow. Brain 123:1216–1228 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22(4):581–594 [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Persson J, Nyberg L (2012) Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging 33 (3):623 e621–623 e613. doi: 10.1016/j.neurobiolaging.2011.02.021 [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP (2012) A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 61(4):1129–1142. doi: 10.1016/j.neuroimage.2012.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L (1965. Hirnpotentialveränderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pfulgers Archiv 284:1–17 [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60(4):543–554. doi: 10.1016/j.neuron.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214(5–6):519–534. doi: 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosan P, Schleicher A, Amunts K, Zilles K (2005) Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol 210(5–6):401–406. doi: 10.1007/s00429-005-0029-1 [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9(11):856–869. doi: 10.1038/Nrn2478 [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005) Valid conjunction inference with the minimum statistic. Neuroimage 25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) Assessment and analysis of handedness–Edinburgh inventory. Neuropsychologia 9(1):97–113 [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S (2012) Decreased functional connectivity by aging is associated with cognitive decline. J Cognit Neurosci 24(11):2186–2198. doi: 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B (2008) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508(6):906–926. doi: 10.1002/Cne.21684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K (2009) Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp 30(8):2336–2355. doi: 10.1002/Hbm.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2(6):417–424. doi: 10.1038/35077500 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD (2003) Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol 24(9): 1849–1856 [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15(11):1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB (2012) Investigating function and connectivity of morphometric findings–exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17). Neuroimage 62(3):1354–1366. doi: 10.1016/j.neuroimage.2012.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Grefkes C (2013) Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol 591(Pt 1):17–31. doi: 10.1113/jphysiol.2012.243469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Bennett DA, Newman AB, Venkatraman V, Yaffe K, Harris T, Kritchevsky S, Aizenstein HJ (2012) Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol Ser A Biol Sci Med Sci 67(9):957–962. doi: 10.1093/gerona/glr262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roski C, Caspers S, Lux S, Hoffstaedter F, Bergs R, Amunts K, Eickhoff SB (2013) Activation shift in elderly subjects across functional systems: an fMRI study. Brain Struct Funct. doi: 10.1007/s00429-013-0530-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Takahara M, Honjo NF, Doi S, Sadato N, Uchiyama Y (2012) Regional frontal gray matter volume associated with executive function capacity as a risk factor for vehicle crashes in normal aging adults. PLoS One 7(9):e45920. doi: 10.1371/journal.pone.0045920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64:240–256. doi: 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JM, Allen EA, Jung RE, Erhardt EB, Arja SK, Kiehl K, Calhoun VD (2012) Correspondence between structure and function in the human brain at rest. Front Neuroinform 6:10. doi: 10.3389/fninf.2012.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP (2009) The resting brain: unconstrained yet reliable. Cereb Cortex 19(10):2209–2229. doi: 10.1093/cercor/bhn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR (2007) Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging 28(7):1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106(31):13040–13045. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW (2011) Network modelling methods for FMRI. Neuroimage 54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063 [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6(3):309–315. doi: 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL (2010) Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev 34(8):1178–1194. doi: 10.1016/j.neubiorev.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U (2011) Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070 [DOI] [PubMed] [Google Scholar]
- Swinnen SP (2002) Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3(5):348–359. doi: 10.1038/nrn807 [DOI] [PubMed] [Google Scholar]
- Thoenissen D, Zilles K, Toni I (2002) Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci 22(20):9024–9034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J (2004) A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex 14(9):966–973. doi: 10.1093/cercor/bhh057 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2012) Aging and functional brain networks. Mol Psychiatry 17(5):471 549–458. doi: 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Thoenissen D, Zilles K (2001) Movement preparation and motor intention. Neuroimage 14(1 Pt 2):S110–S117. doi: 10.1006/nimg.2001.0841 [DOI] [PubMed] [Google Scholar]
- Turner GR, Spreng RN (2012) Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol Aging 33(4):826 e821–813. doi: 10.1016/j.neurobiolaging.2011.06.005 [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Munchau A, Buchel C, Weiller C, Siebner HR (2006) Implementation of visuospatial cues in response selection. Neuroimage 29(1):286–294. doi: 10.1016/j.neuroimage.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM (2011) Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging 32(5):916–932. doi: 10.1016/j.neurobiolaging.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS (2006) Compensatory mechanisms in the aging motor system. Ageing Res Rev 5(3):239–254. doi: 10.1016/j.arr.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB (2010) The image of time: a voxel-wise meta-analysis. Neuroimage 49(2):1728–1740. doi: 10.1016/j.neuroimage.2009.09.064 [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P (2007) Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett 423(3):189–193. doi: 10.1016/j.neulet.2007.06.057 [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K (2010) Centenary of Brodmann’s map–conception and fate. Nat Rev Neurosci 11(2):139–145. doi: 10.1038/nrn2776 [DOI] [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Geyer S, Luppino G, Matelli M, Qu M, Schleicher A, Schormann T (1996) Anatomy and transmitter receptors of the supplementary motor areas in the human and nonhuman primate brain. Adv Neurol 70:29–43 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


