Abstract
Dyspnoea is a cardinal symptom of fibrotic interstitial lung disease (ILD), with a lack of proven effective therapies. With emerging evidence of the role of facial and nasal airflow for relieving breathlessness, this pilot study was conducted to examine the feasibility of conducting a clinical trial of a handheld fan (HHF) for dyspnoea management in patients with fibrotic ILD. In this mixed-methods, randomised, assessor-blinded, controlled trial, 30 participants with fibrotic ILD who were dyspnoeic with a modified Medical Research Council Dyspnoea grade ≥ 2 were randomised to a HHF for symptom control or no intervention for 2 weeks. Primary outcomes were trial feasibility, change in Dyspnoea-12 scores at Week 2, and participants’ perspectives on using a HHF for dyspnoea management. Study recruitment was completed within nine months at a single site. Successful assessor blinding was achieved in the fan group [Bang’s Blinding Index − 0.08 (95% CI − 0.45, 0.30)] but not the control group [0.47 (0.12, 0.81)]. There were no significant between-group differences for the change in Dyspnoea-12 or secondary efficacy outcomes. During qualitative interviews, participants reported that using the HHF relieved breathlessness and provided relaxation, despite initial scepticism about its therapeutic benefit. Oxygen-experienced participants described the HHF being easier to use, but not as effective for symptomatic relief, compared to oxygen therapy. Our results confirmed the feasibility of a clinical trial of a HHF in fibrotic ILD. There was a high level of patient acceptance of a HHF for managing dyspnoea, with patients reporting both symptomatic benefits and ease of use.
Subject terms: Medical research, Signs and symptoms
Introduction
Patients with fibrotic interstitial lung disease (ILD) experience distressing breathlessness and reduced exercise tolerance that significantly impact quality of life. At presentation, 44–83% of patients with fibrotic ILD walk slower than people of similar age or need to stop when walking at their own pace on the level due to dyspnoea1,2. Despite recent advances in anti-fibrotic therapies that slow progression of fibrotic ILD3–7, these drugs are not effective in relieving symptoms or in improving health-related quality of life (HRQoL).
There is emerging evidence that facial and nasal airflow can reduce the sensation of breathlessness8–10. Handheld fans (HHFs) are inexpensive, portable, and readily available. More importantly, the use of a HHF may enhance self-efficacy for symptom management. In patients with dyspnoea secondary to other conditions, including chronic obstructive pulmonary disease (COPD) and malignancy, symptomatic benefits have been reported with the use of a HHF11–13.
The effects of a HHF for dyspnoea management in patients with fibrotic ILD have not been explored. This pilot study aimed to explore the feasibility and acceptability of a randomised controlled trial evaluating the use of a HHF for dyspnoea management in patients with fibrotic ILD.
Methods
Study design and participants
This mixed-methods, randomised, single-blinded, controlled trial was conducted at a quaternary hospital in Melbourne, Australia. Eligible participants were aged ≥ 18 years with fibrotic ILD who were dyspnoeic with a modified Medical Research Council (mMRC) Dyspnoea grade ≥ 2 and were not currently using a HHF. Fibrotic ILD was defined as a multidisciplinary diagnosis of chronic ILD of any aetiology14, with features of diffuse fibrosing lung disease of > 10% extent on high-resolution CT chest. Exclusion criteria included significant concurrent COPD (defined as FEV1/FVC < 60% on the most recent lung spirometry or extent of emphysema greater than extent of fibrosis on the most recent CT chest), and hospitalisation within the 4 weeks before screening. The study was approved by the Austin Health Research Ethics Board (HREC/46012/Austin-2018) and conducted according to the National Health and Medical Research Council’s National Statement on Ethical Conduct In Human Research (2007), and all subsequent updates, and in accordance with the Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95), the Health Privacy Principles described in the Health Records Act 2001 (Vic) and Section 95A of the Privacy Act 1988 (and subsequent Guidelines). Individual informed consents were obtained for all participants. This study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618001949279, date of trial registration: 30/11/2018).
Interventions, randomisation and blinding
Thirty participants were randomised in a 1:1 ratio to either the intervention group (HHF for symptom control according to standardised instructions (Supplementary file 1: Standardised instructions for intervention) or the control group (no intervention) for 2 weeks, using a computer-generated sequence. Allocation concealment was achieved using sequentially numbered, opaque, sealed envelopes and was performed by an investigator without patient contact (A.E.H.). Given the lack of a plausible placebo, participants and clinicians were unblinded to the group assignments. Single blinding was achieved by ensuring the trial assessor (K.S.) was unaware of the allocation of study groups until the completion of study evaluation. This was accomplished by having a separate trial visit with an investigator (Y.H.K.) for intervention allocation and education, between 7 and 10 days from baseline assessment, in the absence of the trial assessor. The success of assessor blinding was measured at Week 2 using the Bang’s Blinding Index, with the assessor being asked to guess participants’ intervention allocation after the completion of trial assessment. Using the responses, the index is scaled to a continuum of − 1 to 1, with 1 being complete lack of blinding, 0 being perfect blinding, and − 1 being opposite guessing15.
Study assessments
Assessments were performed by the blinded assessor at baseline (after eligibility screening) and at Week 2. At screening, the mMRC was used to assess potential participants’ dyspnoea-related functional disability for trial eligibility. The Dyspnoea-12, a 12-item validated assessment tool with both physical and affective components16, was used to evaluate the severity of dyspnoea. Health-related quality of life was examined using the King’s Brief Interstitial Lung Disease Questionnaire, a validated 15-item ILD-specific questionnaire that covers three domains: breathlessness and activities, chest symptoms, and psychological symptoms17. The Self-efficacy for Managing Chronic Disease 6-item Scale18 was used to measure participants’ confidence in self-management. Functional performance was measured using validated questionnaires to quantify physical disability for activities of daily living (Manchester Respiratory Activities of Daily Living Questionnaire)19 and life-space mobility (UAB Study of Aging Life-Space Assessment)20, and objectively using a multisensor activity monitor, the SenseWear Armband (Bodymedia Inc., Pittsburgh, PA, USA).
Individual semi-structured interviews were conducted with all participants who completed the study to evaluate participants’ perspectives regarding use of a HHF for dyspnoea management and their trial experiences. An interview topic guide was devised from the investigators’ clinical experience as well as a comprehensive literature review (Supplementary file 2: Interview topic guide)13,21. HHF usage frequency and patterns were evaluated through self-report during semi-structured interviews. All interviews were recorded digitally, transcribed verbatim, and anonymised for analysis.
Study outcomes
Primary outcomes were trial feasibility, change in Dyspnoea-12 scores between baseline and Week 2, and participants' perspectives regarding the use of a HHF for managing their symptoms. Evaluation of trial feasibility included study recruitment, participant withdrawal, data completeness for outcome measures, trial assessor blinding, and participants’ trial experience. Secondary outcomes were changes in exploratory efficacy measurements at Week 2, including HRQoL, self-efficacy, functional performance (activities of daily living and life-space mobility assessments, physical activity levels), and participants’ self-reported usage of the HHF.
Sample size
It was considered that a sample of 30 would be sufficient to inform trial feasibility for a Phase III randomised controlled trial, as well as to qualitatively evaluate participants’ perceptions.
Statistical analysis
Quantitative analysis
Statistical analyses were performed using Stata (v16 StataCorp, USA). Descriptive analyses were conducted to evaluate trial feasibility outcomes. Between-group comparisons were performed using linear mixed models with intervention groups and time, adjusted for potential confounders (including age, sex, and lung function measurements). For comparisons of enrolled and non-enrolled patients, t tests or Mann–Whitney tests were used for parametric and non-parametric data, respectively, with data distribution being evaluated using the Shapiro–Wilk normality test. Level of statistical significance was set at p < 0.05.
Qualitative analysis
Methodological principles of grounded theory underpinned qualitative analysis of the interview transcripts, which were conducted independently by two investigators (Y.H.K. and J.L.). The transcripts were initially analysed using open coding, where they were read line-by-line and fragmented into descriptive codes to represent the data22,23. Codes were then organized hierarchically to form themes and the original transcripts were searched to refine the relationship between themes and codes. The final themes were agreed through iterative discussion between the two investigators.
Results
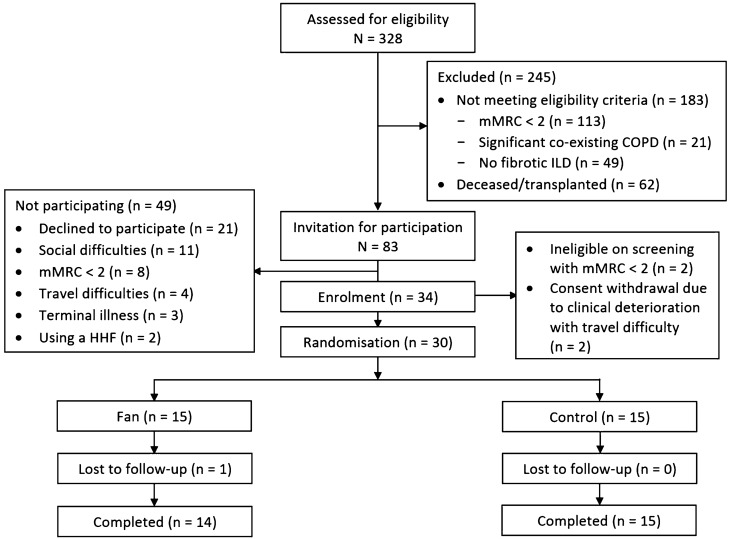
Of 328 patients whose medical records were screened, 83 met the inclusion criteria and were invited for trial participation (Fig. 1). A total of 34 (41%) were enrolled in the study, with no significant differences in baseline characteristics, diagnoses, and lung function, compared to those who did not enrol (Supplementary file 3: Table E1). Of these, 30 were randomised with two not meeting the inclusion criterion of mMRC Dyspnoea grade ≥ 2 and two withdrawing their consent due to clinical deterioration requiring escalation of medical therapy. Baseline participant characteristics were similar in the two groups (Table 1). The most common diagnoses were idiopathic pulmonary fibrosis and connective tissue disease-related ILD. Seven participants were using domiciliary oxygen therapy at study enrolment. There were seven participants who were using a combination of inhaler therapies for management of asthma or mild COPD.
Figure 1.
Study flow diagram.
Table 1.
Baseline participant characteristics.
| Intervention group (N = 15) | Control group (N = 15) | |
|---|---|---|
| Age, years | 73.7 ± 10.5 | 71.7 ± 7.3 |
| Gender, female:male | 8:7 | 6:9 |
| BMI, kg/m2 | 28.9 ± 5.2 | 29.2 ± 5.3 |
| Ever smoker, n (%) | 9 (60) | 10 (67) |
| Duration of diagnosis, years | 3 (2, 8) | 3 (2, 6) |
| Diagnosis, n (%) | ||
| IPF | 5 (33) | 5 (33) |
| Non-IPF | 10 (67) | 10 (67) |
| Asbestosis | – | 1 |
| CTD-ILD | 6 | 3 |
| Drug-induced ILD | 1 | – |
| Fibrotic HP | 1 | 3 |
| Non-specific interstitial pneumonia | 1 | 1 |
| Unclassifiable ILD | 1 | 2 |
| FEV1/FVC | 77.6 ± 6.0 | 79.3 ± 8.0 |
| FEV1 (% pred) | 78.2 ± 18.2 | 71.3 ± 15.0 |
| FVC (% pred) | 77.6 ± 18.0 | 68.2 ± 15.3 |
| DLCO (% pred) | 42.1 ± 11.5 | 41.7 ± 12.2 |
| mMRC dyspnoea score | 2 (2, 3) | 2 (2, 3) |
| Domiciliary oxygen therapy, n (%) | 2 (13) | 5 (33) |
| ILD therapies, n (%) | ||
| Anti-fibrotic therapy | 4 (27) | 4(27) |
| Immunosuppressant therapy | 6 (40) | 7 (47) |
| Inhaler therapies, n (%) | ||
| Inhaled corticosteroid + long-acting beta-agonist | 3 (20) | 1 (7) |
| Long-acting muscarinic antagonist | 1 (7) | 1 (7) |
| Short-acting beta-agonist | 5 (33) | 2 (13) |
| Pulmonary rehabilitation in the previous 6 months, n (%) | 7 (47) | 8 (53) |
Data are expressed as mean ± standard deviation or median (interquartile range), except where indicated.
BMI body mass index, CTD-ILD connective tissues disease-related interstitial lung disease, DLCO diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in one second, FVC forced vital capacity, HP hypersensitivity pneumonitis, ILD interstitial lung disease, IPF idiopathic pulmonary fibrosis, mMRC modified Medical Research Council.
Primary outcomes
Trial feasibility
Study recruitment was completed within nine months, between February and November 2019, with an enrolment to randomisation ratio of 1.1:1. Only one participant (the fan group) withdrew from the study after randomisation due to social stresses with difficulty continuing study participation. Data completion rates were satisfactory, with 77% for SenseWear armband monitoring and 100% for all other outcome measures. Data incompleteness for SenseWear armband monitoring was due to insufficient device wearing time.
Evaluation of assessor blinding using the Bang’s blinding index revealed random guessing for the fan group [Index = −0.08 (95% confidence interval: − 0.45, 0.30)] and unsuccessful blinding for the control group [Index = 0.47 (95% confidence interval: 0.12, 0.81)].
All participants who completed the study reported positive experiences with this trial and stated they would recommend it to others if appropriate. Participants perceived minimal burden with their involvement in this trial. Most were motivated to advance research for symptom management in ILD and inspired to help others. At study completion, all participants, except for two in the control group, stated they would like to continue or start using a HHF.
Dyspnoea
There was no significant difference in the change of Dyspnoea-12 scores at Week 2 between groups [mean difference of − 2.2 (95% CI − 6.4, 1.9), p = 0.29; Table 2].
Table 2.
Patient-reported outcome measures between baseline and Week 2.
| Fan groupa | Control groupa | Treatment effectb | p value | |||
|---|---|---|---|---|---|---|
| Baseline | Week 2 | Baseline | Week 2 | |||
| Dyspnoea-12 | 16.1 ± 2.2 | 13.4 ± 2.3 | 13.3 ± 2.2 | 12.8 ± 2.2 | − 2.2 (− 6.4, 1.9) | 0.29 |
| K-BILD Breathlessness & Activities | 29.2 ± 3.9 | 32.2 ± 3.9 | 32.5 ± 3.7 | 37.0 ± 3.7 | − 1.5 (− 8.9, 5.9) | 0.69 |
| K-BILD Chest Symptoms | 47.0 ± 5.2 | 56.1 ± 5.4 | 59.6 ± 5.0 | 58.8 ± 5.2 | 10 (− 5.1, 25.1) | 0.19 |
| K-BILD Psychological Symptoms | 51.7 ± 4.2 | 53.4 ± 4.2 | 56.9 ± 4.1 | 57.5 ± 4.1 | 1.1 (− 4.1, 6.3) | 0.40 |
| K-BILD Total | 48.0 ± 2.5 | 50.3 ± 2.5 | 52.3 ± 2.4 | 54.0 ± 2.4 | 0.7 (− 3.3, 4.7) | 0.33 |
| Self-efficacyc | 5.4 ± 0.6 | 5.7 ± 0.7 | 5.5 ± 0.6 | 5.7 ± 0.6 | 0.07 (− 1.9, 2.0) | 0.94 |
| Activities of daily livingd | 14.4 ± 0.9 | 14.6 ± 1.0 | 15.1 ± 0.9 | 16.9 ± 0.9 | − 2.5 (− 4.8, 0.3) | 0.08 |
| Life-spacee | 58.6 ± 6.3 | 58.0 ± 6.4 | 66.6 ± 6.1 | 63.7 ± 6.1 | 2.4 (− 10.4, 15.2) | 0.72 |
Measured using: cSelf-efficacy for Managing Chronic Disease 6-item Scale; dManchester Respiratory Activities of Daily Living Questionnaire; eUniversity of Alabama at Birmingham Study of Aging Life-Space Assessment.
Data are expressed as amean ± standard error or bmean difference (95% confidence interval). P values are between group comparison for mean difference.
K-BILD King’s Brief Interstitial Lung Disease.
Participants’ qualitative interviews
Three major and one minor theme emerged from the semi-structured interviews conducted with all completed participants (Table 3).
Table 3.
Major and minor interview themes with illustrative quotes.
| Major themes |
| Varying initial attitudes towards using a handheld fan as an intervention |
| I thought it was very unusual yeah, “I wonder how a fan would work,” so that’s what interested me the most. I couldn’t quite understand the concept of how a fan would work. (P8) |
| At the beginning, I was thinking, that’s a gimmick. (P27) |
| I thought about past experiences, riding the motorbike, keeping your face cool—you can breathe better. Or driving with the window down in the car. So would imagine having a fan would be beneficial. (P29) |
| Benefits of using a handheld fan |
| Just seemed to supply me with more air. Yeah, to get some air into my lungs. (P1) |
| I feel, I feel like uh, example, when you sweat and have cold drinks I feel better. When I have this one, I am like uh, hard with breathing, when I put this one, after I feel I breathe easy and uh, body is more comfortable. (P10) |
| It made me feel relaxed a bit more. (P17) |
| I think that the fan is something that is, secure. It’s something that you can put in your brain that is secure for you. It’s like a backup kind of thing. (P18) |
| I can use the fan in almost any place as long as it’s not noisy or intrusive. (P24) |
| Relative effects of handheld fans, oxygen, and inhaler therapies for symptom management |
| The fan helps a little bit more with it, you know, to help me breathe a bit better quicker (compared to inhaler therapies). Use my puffer a little bit less actually. (P2) |
| Not the same. The fan, it helps a little bit but, when I got really breathless, only the oxygen, oxygen helps more than the fan. I use the fan because it’s easier. (P4) |
| I believe it (the fan) helped more when I didn’t have the oxygen connected to me than when I did. Certainly it helped me in both cases, whether I had the oxygen or not. (P9) |
| Minor theme |
| Challenges of using a handheld fan |
| I’ve been to a lot of shops and, just can’t find any of these. All they have are those manual ones, that needs pumping. (P1) |
| I didn’t want to be in the public using something like that. It’s just embarrassing. (P15) |
Major themes
Varying initial attitudes towards using a HHF as an intervention: Participants expressed different responses (12 positive, 12 uncertain, and 5 negative), when they were first introduced to the concept of using a HHF for symptom management. Some reported their scepticism and uncertainties with regards to the potential effects of using a HHF, while others anticipated beneficial effects based on their personal experiences, including airflow from overhead fans or riding in a vehicle.
Benefits of using a HHF: Most participants described symptomatic relief with using a HHF. In addition to providing facial airflow to relieve the sensation of dyspnoea, some participants expressed a sense of security and relaxation with using a HHF. They reported the ease of integrating the use of a HHF into their daily life.
Relative effects of HHF, oxygen, and inhaler therapies for dyspnoea management: Participants reported differential effects on breathlessness of HHF and existing interventions of oxygen and inhaler therapies. Oxygen-experienced participants described oxygen therapy provided greater relieving effects on breathlessness than the HHF, with potential additive effects when both were used together. Some participants favoured use of the HHF over inhaler therapies for symptom control, as the HHF resulted in a timelier improvement.
Minor theme
Challenges of using a HHF: Despite most participants describing the ease of using a HHF, a minority reported feeling embarrassed with using a HHF in public. Difficulties with sourcing a HHF were also raised by some participants who had previously attempted to get one.
Secondary outcomes
Exploratory efficacy measurements
There were no significant differences between groups in HRQoL, self-efficacy, physical difficulties with activities of daily living, life-space mobility, or physical activity levels measured by SenseWear Armbands at Week 2 (Tables 2, 4).
Table 4.
Physical activity levels measured using SenseWear armbands between baseline and Week 2.
| Fan groupa | Control groupa | Treatment effectb | p value | |||
|---|---|---|---|---|---|---|
| Baseline | Week 2 | Baseline | Week 2 | |||
| Steps per day | 3423 ± 540 | 3620 ± 527 | 3082 ± 453 | 3206 ± 488 | 74 (− 807, 956) | 0.87 |
| Total energy expenditure (kCal/day) | 8269 ± 343 | 8383 ± 340 | 8470 ± 288 | 8481 ± 287 | 104 (− 197, 404) | 0.50 |
| Total METs | 1.10 ± 0.04 | 1.12 ± 0.04 | 1.05 ± 0.03 | 1.06 ± 0.03 | 0.003 (− 0.04, 0.44) | 0.88 |
| Duration of sedentary time per day (mins) | 1129 ± 38 | 1139 ± 36 | 1205 ± 32 | 1149 ± 31 | 66 (− 30, 162) | 0.18 |
| Duration of time ≥ 3 METs per daya (mins) | 221 ± 27 | 243 ± 27 | 187 ± 23 | 183 ± 23 | 26 (− 16.1, 68.3) | 0.23 |
Data are expressed as amean ± standard error or bmean difference (95% confidence interval). p values are between group comparison.
METs metabolic equivalents of task.
Handheld fan usage
Most participants (71%) used the HHF for recovery after activities. They used it both in the home environment and for outdoor activities. Nine participants (64%) used their HHF daily, with the rest using it either weekly or for a few days a week. There was wide variability in the self-reported HHF usage duration for each episode, ranging from less than one minute to 20 min.
Discussion
This pilot study demonstrated that a randomised controlled trial of HHF for dyspnoea management in patients with fibrotic ILD is feasible, although adequate assessor blinding was not achieved. There were no significant differences in patient-reported outcomes between groups, likely due to the limited intervention duration and small sample size in this pilot study. At study completion, participants expressed their willingness to use a HHF for self-management, although some were sceptical of its value initially. Participants described the HHF as being a helpful intervention for dyspnoea management, which could be easily adapted into daily life.
There is currently no established intervention that effectively controls dyspnoea in patients with fibrotic ILD. There is limited efficacy of various pharmacological agents for relieving dyspnoea in patients with ILD and other conditions, including opioids, benzodiazepines, and sertraline24–27. Recent studies have shown that ambulatory oxygen may improve quality of life and symptoms in patients with ILD and exertional desaturation28,29, although its longer-term impacts remain unknown. Our qualitative data and successful study recruitment highlight that patients with ILD are willing to participate in research evaluating non-pharmacological symptom management strategies. This is the first study that evaluated the possibility of using a HHF as an intervention for dyspnoea management in fibrotic ILD. Given the lack of feasibility in participant blinding for a HHF, we incorporated assessor blinding in this study, which was only achieved in the fan group. This confirms the challenge of blinding of group allocation for a non-pharmacological intervention such as the HHF, since participants may accidentally disclose their intervention allocation during assessments. Nevertheless, our study design and selected outcome measures using questionnaires and physical activity monitors were acceptable to participants, with high levels of data completeness.
The exact mechanisms by which the HHF aids relief of breathlessness remain unclear. A HHF may exert its effects by providing cooling of the facial skin and stimulation of oral and nasal mucosal flow receptors, which are innervated by the second and third branches of the trigeminal nerve8,30,31. Preliminary functional neuroimaging data suggest that facial airflow can alter neural activities within the brain and modulate the central perception of breathlessness32. A recent systematic review showed improved breathlessness both at rest and during exertion with facial and nasal airflow delivered by medical air or HHF33. In this study, participants described such sensations when using a HHF, with the additional benefits of relaxation and sense of security. Similar effects were reported in previous qualitative studies of patients with malignancy and COPD that evaluated the effects of a HHF for dyspnoea management, either as a sole intervention or in combination with a multi-component chronic breathlessness intervention program13,20. Our findings extend previous results, with some participants reporting differential effects and preference for using a HHF in comparison to oxygen and inhaler therapies for symptom control. The HHF provides an alternative option for dyspnoea management. However, the optimal timing and usage duration of a HHF, and how or whether it should be co-administered with other therapies, are unknown.
Dyspnoea perception is a result of complex interactions of physiological, psychosocial, and social and environmental factors34,35. Thus, it would seem likely that optimal management of breathlessness in advanced lung disease would require a combination of pharmacological and non-pharmacological interventions36. However, in general, the utilisation of non-pharmacological interventions is lower than pharmacological agents in medical care, with patients’ acceptance being an important factor37–39. Given the simplicity of a HHF, many participants disbelieved its therapeutic potential at the outset of this study. This highlights the importance of providing adequate explanation regarding the purported scientific rationale of using a HHF with instructions and/or demonstration of how to use the HHF in the appropriate facial region, when prescribing one for managing breathlessness in clinical practice. Patients’ negative expectations of an intervention can have undesirable effects on treatment outcomes and adherence, the so-called nocebo effect40. An effective patient-clinician relationship may improve patients’ acceptance of such an intervention41.
This study has several limitations. In addition to inadequate blinding of assessors, there was a lack of an appropriate control intervention and participant blinding in this study. Both selecting a control intervention and blinding are crucial fundamental methodological components in clinical trials, in order to minimise differential management or assessment and prevent biased estimates of treatment effects. However, blinding of participants and appropriately matching the control intervention of a HHF would be very challenging. This study was not designed to assess intervention efficacy, but to evaluate the appropriateness of different outcome measures and patients’ acceptability of the intervention. Although participants reported benefits from using a HHF, its true efficacy and the magnitude of its effects, if any, are unclear. Anxiety and psychological well-being should be evaluated in future studies, given participants’ perception of increased sensation of security with using a HHF. There was wide variability in HHF usage patterns among participants, which could impact on the evaluation of its intervention effects. Given differences in participants’ lifestyles and home environments, the instructions for HHF usage suggested that participants should use the HHF according to their daily needs, but optimal strategies for using a HHF for dyspnoea management remains unknown. Nevertheless, a low HHF usage level may dampen its potential intervention effects. Usage of the HHF was not objectively measured, so it was not possible to evaluate any relationship between greater hours of use and perceived efficacy. Electronic measurement of usage could be considered for future trials. While there was a small proportion of participants with co-existing obstructive lung disease, we ensured that these participants had primarily fibrotic ILD by excluding those with significant obstructive ventilatory defects or predominant emphysematous changes on CT chest. The control group had a lower mean FVC and more participants on domiciliary oxygen therapy than the intervention group, which may have influenced the efficacy outcomes. Patients with different severities of dyspnoea and lung disease may have varying responses to therapeutic effects of the HHF. Previous positive studies of HHF had predominantly evaluated patients with severe COPD or advanced malignancy associated with high symptom burden11,33.
Conclusion
This mixed-methods pilot study confirmed the feasibility of conducting a randomised controlled trial to evaluate the use of a HHF for dyspnoea management in patients with fibrotic ILD, although adequate trial assessor blinding was not achieved. This is the first study to suggest that a HHF can be used for dyspnoea management in fibrotic ILD, with adequate patient education needed at the intervention initiation to ensure patient understanding and acceptance of the device.
Supplementary Information
Acknowledgements
This study was funded by the Lung Foundation Australia Eleanor Greenwood Memorial Grant-in-Aid for Interstitial Lung Disease Research. Boehringer Ingelheim provided research support to fund the handheld fans. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author contributions
Y.H.K., A.E.H., C.J.R., C.F.M. and N.S.L.G. were responsible for trial conception and development of the study protocol. Y.H.K., K.S. and N.S.L.G. were responsible for patient recruitment and data collection. Y.H.K., K.S. and J.Y.T.L. were responsible for data analysis. All authors were involved in writing and final approval of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
Y.H.K. and N.S.L.G. report non-financial support from Air Liquide Healthcare, grants and personal fees from Boehringer Ingelheim, personal fees from Roche, outside the submitted work. A.E.H. reports grants from Roche, personal fees from Boehringer Ingelheim, non-financial support from BOC Healthcare and Air Liquide Healthcare, outside the submitted work. C.F.M. reports personal fees from GSK and Novartis, other from Menarini and Air Liquide Healthcare, and grants from Boehringer Ingelheim, outside the submitted work. C.J.R. reports grants and personal fees from Boehringer Ingelheim and Hoffmann-La Roche, outside the submitted work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86326-8.
References
- 1.Nishiyama O, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010;36:1067–1072. doi: 10.1183/09031936.00152609. [DOI] [PubMed] [Google Scholar]
- 2.Khadawardi H, Mura M. A simple dyspnoea scale as part of the assessment to predict outcome across chronic interstitial lung disease. Respirology. 2017;22:501–507. doi: 10.1111/resp.12945. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Distler O, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KR, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 7.Maher TM, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2019;8:147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 8.Schwartzstein RM, Lahive K, Pope A, Weinberger SE, Weiss JW. Cold facial stimulation reduces breathlessness induced in normal subjects. Am. Rev. Respir. Dis. 1987;136:58–61. doi: 10.1164/ajrccm/136.1.58. [DOI] [PubMed] [Google Scholar]
- 9.Liss HP, Grant BJ. The effect of nasal flow on breathlessness in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1988;137:1285–1288. doi: 10.1164/ajrccm/137.6.1285. [DOI] [PubMed] [Google Scholar]
- 10.Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J. Pain. Symptom. Manag. 2010;39:831–838. doi: 10.1016/j.jpainsymman.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Farquhar MC, Higginson IJ, Fagan P, Booth S. The feasibility of a single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. BMC Palliat. Care. 2009;8:9. doi: 10.1186/1472-684X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farquhar MC, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med. 2014;12:194. doi: 10.1186/s12916-014-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luckett T, et al. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur. Respir. J. 2017;50:1700262. doi: 10.1183/13993003.00262-2017. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, et al. An Official American Thoracic Society/European Respiratory Society Statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin. Trials. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Yorke J, et al. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest. 2011;139:159–164. doi: 10.1378/chest.10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AS, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax. 2012;67:804–810. doi: 10.1136/thoraxjnl-2012-201581. [DOI] [PubMed] [Google Scholar]
- 18.Ritter PL, Lorig K. The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. J. Clin. Epidemiol. 2014;67:1265–1273. doi: 10.1016/j.jclinepi.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Yohannes AM, Roomi J, Winn S, Connolly MJ. The Manchester Respiratory Activities of Daily Living questionnaire: development, reliability, validity, and responsiveness to pulmonary rehabilitation. J. Am. Geriatr. Soc. 2000;48:1496–1500. doi: 10.1111/jgs.2000.48.11.1496. [DOI] [PubMed] [Google Scholar]
- 20.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J. Am. Geriatr. Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MJ, Booth S, Currow DC, Lam LT, Phillips JL. A mixed-methods, randomized, controlled feasibility trial to inform the design of a phase III trial to test the effect of the handheld fan on physical activity and carer anxiety in patients with refractory breathlessness. J. Pain Symptom Manag. 2016;51:807–815. doi: 10.1016/j.jpainsymman.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Braun V, Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 23.Kuper A, Reeves S, Levinson W. An introduction to reading and appraising qualitative research. BMJ. 2008;337:a288. doi: 10.1136/bmj.a288. [DOI] [PubMed] [Google Scholar]
- 24.Kohberg C, Andersen CU, Bendstrup E. Opioids: an unexplored option for treatment of dyspnea in IPF. Eur. Clin. Respir. J. 2016;3:30629. doi: 10.3402/ecrj.v3.30629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon ST, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst. Rev. 2016;10:CD007354. doi: 10.1002/14651858.CD007354.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currow DC, et al. Sertraline in symptomatic chronic breathlessness: a double blind, randomised trial. Eur. Respir. J. 2018;53:1801270. doi: 10.1183/13993003.01270-2018. [DOI] [PubMed] [Google Scholar]
- 27.Currow D, et al. Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75:50–56. doi: 10.1136/thoraxjnl-2019-213681. [DOI] [PubMed] [Google Scholar]
- 28.Visca D, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir. Med. 2018;6:759–770. doi: 10.1016/S2213-2600(18)30289-3. [DOI] [PubMed] [Google Scholar]
- 29.Khor YH, et al. Ambulatory oxygen in fibrotic ILD: a pilot, randomised, triple-blinded, sham-controlled trial. Chest. 2020;158:234–244. doi: 10.1016/j.chest.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Burgess KR, Whitelaw WA. Effects of nasal cold receptors on pattern of breathing. J. Appl. Physiol. 1988;64:371–376. doi: 10.1152/jappl.1988.64.1.371. [DOI] [PubMed] [Google Scholar]
- 31.Simon PM, et al. Oral mucosal stimulation modulates intensity of breathlessness induced in normal subjects. Am. Rev. Respir. Dis. 1991;144:419–422. doi: 10.1164/ajrccm/144.2.419. [DOI] [PubMed] [Google Scholar]
- 32.Johnson MJ, et al. Magnetoencephalography to investigate central perception of exercise-induced breathlessness in people with chronic lung disease: a feasibility pilot. BMJ Open. 2015;5:e007535. doi: 10.1136/bmjopen-2014-007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swan F, et al. Airflow relieves chronic breathlessness in people with advanced disease: an exploratory systematic review and meta-analyses. Palliat. Med. 2019;33:618–633. doi: 10.1177/0269216319835393. [DOI] [PubMed] [Google Scholar]
- 34.Parshall MB, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012;185:432–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubé BP, Vermeulen F, Laveneziana P. Exertional dyspnoea in chronic respiratory diseases: from physiology to clinical application. Arch. Bronconeumol. 2017;53:62–70. doi: 10.1016/j.arbres.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Chin C, Booth S. Managing breathlessness: a palliative care approach. Postgrad. Med. J. 2016;92:393–400. doi: 10.1136/postgradmedj-2015-133578. [DOI] [PubMed] [Google Scholar]
- 37.Krebs EE, et al. Comparative mortality among Department of Veterans Affairs patients prescribed methadone or long-acting morphine for chronic pain. Pain. 2011;152:1789–1795. doi: 10.1016/j.pain.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Staedtler AV, Nunez D. Nonpharmacological therapy for the management of neuropsychiatric symptoms of Alzheimer's disease: linking evidence to practice. Worldviews Evid. Based Nurs. 2015;12:108–115. doi: 10.1111/wvn.12086. [DOI] [PubMed] [Google Scholar]
- 39.Becker WC, et al. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam. Pract. 2017;18:41. doi: 10.1186/s12875-017-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pouillon L, et al. The nocebo effect: a clinical challenge in the era of biosimilars. Expert Rev. Clin. Immunol. 2018;14:739–749. doi: 10.1080/1744666X.2018.1512406. [DOI] [PubMed] [Google Scholar]
- 41.Data-Franco J, Berk M. The nocebo effect: a clinicians guide. Aust N. Z. J. Psychiatry. 2013;47:617–623. doi: 10.1177/0004867412464717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.